Abstract
Background
The aim of this study was to explore the potential role of TRPC6 in the pathophysiology of HK-2 cell injury following ischemia reperfusion (I/R).
Material/Methods
TRPC6 expression was analyzed by immunofluorescence staining. siRNA was transfected to knockout of TRPC6 in HK-2 cells, and in vitro I/R was then induced. Cell apoptosis and necrosis were determined by Annexin V-FITC/PI staining. Necroptosis was determined by necrostatin-1 and expressions of necroptosis-related proteins were evaluated. OAG, SKF96365, or KN-93 was further used to interfere with TRPC6 expression.
Results
Cytoplasmic TRPC6 expression was demonstrated. I/R induced TRPC6 expression in normal or NC siRNA-transfected cells but not in TRPC6 siRNA-knockout ones. There was a progressive increase in apoptotic and necrotic cells with increasing reoxygenation time in all 3 groups, while necrosis in TRPC6 siRNA-transfected cells was comparatively higher than that of the other 2 groups (p<0.05). Expressions of necroptosis-related proteins were interfered with following I/R and these effects were enhanced by TRPC6 siRNA. Application of OAG, SKF96365, or KN93 further affected necroptosis following I/R.
Conclusions
This study described the expression and functional relevance of TRPC6 in the pathophysiology of HK-2 cell following I/R. Our results regarding the ability of TRPC6 to specifically interrupt necroptosis may shed new light on its role in prevention and control of ischemic kidney injury.
MeSH Keywords: Acute Kidney Injury, Reperfusion Injury, Transient Receptor Potential Channels
Background
The kidneys are easily influenced by ischemia-reperfusion (I/R) injury due to their physical structure and function. Renal I/R is a common clinical problem associated with acute kidney injury (AKI), which may progress to chronic kidney disease and lead to high mortality. It is typically characterized by oliguria, a severe reduction in glomerular filtration rate, a variable fall in renal blood flow, and renal tubular epithelial cell injury [1]. The pathophysiology of I/R is complex. Following I/R, renal cells may undergo apoptosis, necrosis, and/or necroptosis, also known as programmed necrosis [2,3]. A previous study of AKI suggested that apoptosis was more involved than necrosis in the process of cell injury following acute renal ischemia (4). Several hypothesis, including oxygen abnormality, calcium abnormality, PH abnormality, and activation of complement system, have been proposed [1]. However, the detailed molecular mechanisms involved in renal I/R injury have not been well-established.
As a member of the transient receptor potential family of calcium channels, transient receptor potential channel 6 (TRPC6) is a receptor-activated nonselective cation channel that is homogeneously expressed throughout the central nervous system and peripheral tissues, including the kidneys [5]. It has been suggested to play a significant role in I/R injury of the lungs, retinas, and brain [6–8]. Our previous bioinformatics study identified TRPC6 as an up-regulated differentially expressed gene in the pathogenesis of I/R injury, suggesting that it may be a potential novel target for therapy [9]. However, the potential role of TRPC6 expression in renal I/R injury has not been investigated. The primary target of renal I/R injury is renal tubular epithelial cells [10]. TRPC6 expression has been reported in the cytoplasm of renal tubular epithelia cells [11]. Therefore, in the present study, the potential role of TRPC6 in cell injury following I/R was investigated by transfection of TRPC6 siRNA into renal tubular epithelial HK-2 cells. The cell apoptosis and necrosis and expression of related proteins following I/R injury were investigated.
Material and Methods
HK-2 cell culture
Human renal tubular epithelial HK-2 cells were obtained from the Kunming Cell Bank of the Chinese Academy of Sciences (Kunming, China). Cells were cultured in DMEM/F12 medium (HyClone, Logan, UT, USA) supplemented with 5 ng/mL epidermal growth factor (EGF; Sigma, USA), 10% fetal bovine serum (FBS; Gibco, Grand Island, NY), 10 μg/mL penicillin (Invitrogen), and 10 μg/mL streptomycin (Invitrogen) at 37°C in 5% CO2 atmosphere. Cells were sub-cultured when 70–80% confluence was reached.
To observe the TRPC6 expression in HK-2 cells, cells were passaged onto a confocal laser dish for 24 h and then fixed in 4% paraformaldehyde at 4°C for 20 min, and permeabilized in 1% Triton X-100 for 10 min. The cells were then incubated with rabbit anti-TRPC6 polyclonal antibody (1:50, Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight, followed by fluorescein isothiocyanate (FITC)-labeled secondary antibody (1:50) for 1 h. Nuclei were counterstained with Hoechst 33258 (Sigma, USA). Fluorescence signals were visualized by a confocal laser scanning microscopy (Leica Microsystems, Heidelberg, Germany).
TRPC6 siRNA transfection
To obtain suitable siRNA to knockdown TRPC6 expression, 3 fragments of siRNA against human TRPC6 were designed and synthesized by Guangzhou RiboBio Co., Ltd (Guangzhou, China). A non-specific siRNA served as a negative control (NC siRNA, Sense: 5′-UAAGGCUAUGAAGAGAUAC-3′, Anti-sense: 3′-AUUCCGAUACUUCUCUA UG-5′). The sequences for TRPC6 siRNAs were: TRPC6 siRNA-1 Sense: 5′-GGACCAGCAUACAUGUUUAdTdT-3′, Anti-sense: 3′-dTdTCCUGGUCGUAUGUAC AAAU-5′; TRPC6 siRNA-2 Sense: 5′-GUUCAAGAAUGACUACAAA dTdT-3′, Anti-sense: 3′-dTdTCAAGUUCUUACUGAUGUUU-5′; TRPC6 siRNA-3 Sense: 5′-GCUUCUAGCUA UUAGUAAAdTdT-3′, Anti-sense: 3′-dTdTCGAAGAUCGAUAAU CAUUU-5′. HK-2 cells were transfected with TRPC6 siRNAs or NC siRNA using lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
To determine the transfection efficiency of TRPC6 siRNA, Western blot assay was performed. Briefly, cells were transfection with TRPC6 siRNA for 48 h, washed 3 times with cold phosphate-buffered saline (PBS), and then lysed in RIPA lysis buffer. The total protein concentrations were determined using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Protein samples were separated using 4% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride membranes, and blocked with nonfat dry milk. The sections were then incubated with rabbit anti-TRPC6 polyantibody (1:300, Santa Cruz Biotechnology, Santa Cruz, CA) or mouse anti-β-actin antibody (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by the respective horseradish peroxidase (HRP)-conjugated secondary antibodies. The immunoreaction products were visualized with diaminobenzidine (DAB kit, Zhongshan Bio-Tech Co., Zhongshan Bio-Tech Co., Ltd., Beijing, China). TRPC6 siRNA with the best knockdown effect was used for further study.
Determination of in vitro ischemia-reperfusion model in HK-2 cells
An In vitro I/R model was induced by adapting the method described previously [12]. Briefly, the HK-2 cells (NC siRNA or TRPC6 siRNA-1 transfected, or without transfection as normal control) were washed with PBS and re-suspended in PBS supplemented with 1.5 mmol/L CaCl2 and 2.0 mmol/L MgCl2. A layer of mineral oil (Sigma, USA) was deposited onto the surface to induce ischemia for 6 h. Cells were then separated and washed 5 times with PBS before transfer to culture medium. Cells were reoxygenated for 2, 6, or 12 h to induce the I/R model. Cell morphology was observed with an inverted fluorescence microscope.
To further confirm the suitable reoxygenation condition for this study, apoptosis and necrosis of HK-2 cells were determined by Annexin V-FITC/PI double staining and quantified by flow cytometry (FACSCalibur, Becton Dickinson, Franklin Lakes, USA) after reoxygenation for 0, 2, 6, and 12 h. After being reoxygenated for specific times, cells were harvested, resuspended in PBS, and were then successively stained with 5 μL Annexin V-FITC (Beyotime Biotechnology, Shanghai, China) and 10 μL propidium iodide (PI, Beyotime Biotechnology, Shanghai, China) for 5 and 15 min in the dark, respectively. Flow cytometry was used to quantify cell apoptosis and necrosis. The results are shown as quadrant dot plots with intact cells (Annexin V−/PI−), apoptotic cells (Annexin V+/PI−), necrotic cells (Annexin V+/PI+), and mechanically damaged cells (Annexin V-/PI+).
Confirmation of necroptosis using necrostatin-1
Based on the model mentioned above, we used necrostatin-1 (Sigma, USA), an inhibitor of necroptosis, to determine whether necroptosis existed in the HK-2 cells during I/R injury. Cells (normal control, NC siRNA, and TRPC6 siRNA) were subdivided into 3 groups depending on whether they underwent reoxygenation or addition of necrostatin-1 (20 μmol/L) before I/R insult, respectively. Cell apoptosis and necrosis was determined by flow cytometry as mentioned above.
Western blot analysis of necroptosis-related proteins
The expression of necroptosis-related proteins was analyzed as mentioned above using the antibodies against sirtuin-2 (Abcam, Cambridge, MA, USA), receptor-interacting protein kinase 1 (RIP1) (Santa Cruz Biotechnology, Santa Cruz, CA), poly (ADP-ribose) polymerase 1 (PARP-1) (Santa Cruz Biotechnology, Santa Cruz, CA), and apoptosis-inducing factor (AIF) (Santa Cruz Biotechnology, Santa Cruz, CA).
Intervention effects of OAG, SKF96365, or KN-93 during I/R injury
To further investigate the role of TRPC6 on the cell apoptosis and necrosis following I/R injury we used: OAG (Sigma, USA), a TRPC6 activator; SKF96365 (Sigma, USA), a TRPC6 inhibitor; and KN-93 (Sigma, USA), a CaMKII inhibitor. Cells (normal control, NC siRNA, and TRPC6 siRNA) were divided into 5 groups according to whether reoxygenation was performed or OAG (20 μmol/L), SKF96365 (10 μmol/L), or KN-93 (10 μmol/L) was applied before the I/R insult, respectively. Cell apoptosis and necrosis and protein expression of RIP1 and PARP-1 were analyzed using the above methods.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). Statistical analyses were performed with SPSS version17.0 (SPSS Inc., Chicago, IL, USA). Data were subjected to a one-way analysis of variance (ANOVA). Differences were considered significant at P<0.05.
Results
Cells morphology and TRPC6 expression of HK-2 cells
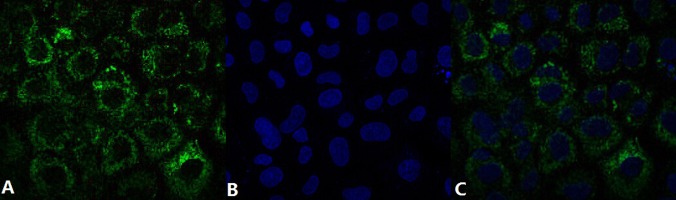
HK-2 cells exhibited a typical epithelial cuboidal shape with the cobblestone morphology (Data not shown). Double immunofluorescence staining showed intense cytoplasmic expression of TRPC6 in HK-2 cells (Figure 1).
Figure 1.
Double immunofluorescence staining of HK-2 cells showed intense cytoplasmic expression of TRPC6. (A) TRPC6 expression was detected by FITC-labeled antibody; (B) Nuclei were counterstained with Hoechst 33258; (C) The images were merged to localize the TRPC6 expression in HK-2 cells.
siRNA inhibited TRPC6 expression in HK-2 cells
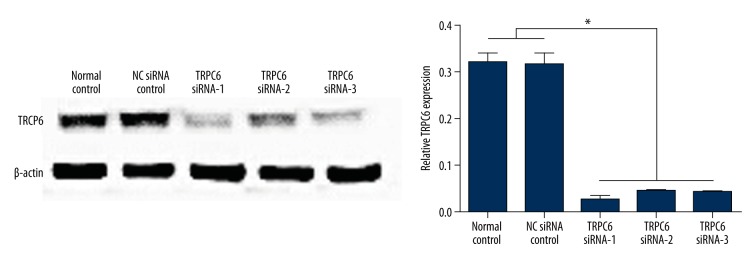
Transfection of TRPC6 siRNAs significantly inhibited the protein expression of TRPC6 in HK-2 cells, and the TRPC6 siRNA-1 showed the best effect on TRPC6 knockout (Figure 2). Therefore, TRPC6 siRNA-1 transfected HK-2 cells were used for the following study.
Figure 2.
Western blot analysis showed the significantly downregulated expression of TRPC6 in HK-2 cells following the transfection of TRPC6 siRNAs. * p<0.05 vs. the HK-2 cells transfected with NC siRNA or without transfection.
Six hours was chosen to be the reoxygenation time to induce I/R model
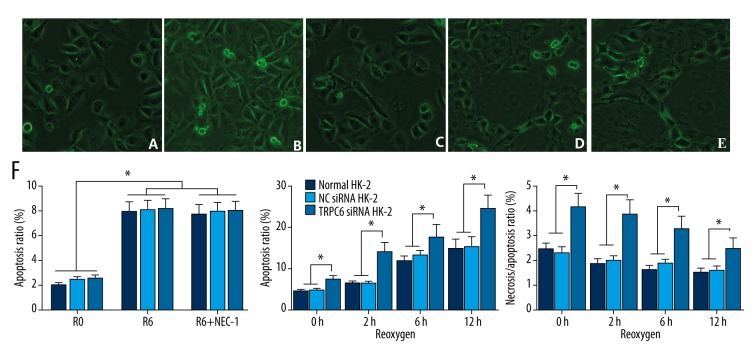
Cell morphology following I/R insult was determined. HK-2 cells were plump and showed typical cobblestone morphology under the normal condition (Figure 3A). After ischemia for 6 h, cells were thinner, shrunken, and lacked normal morphology (Figure 3B). Many thinner cells that lacked normal morphology were observed spread out on the surface, and sporadic dead cells were also noted after reoxygenation for 2 h (Figure 3C). Further reoxygenation for 6 and 12 h resulted in many floating dead cells (Figure 3D, 3E).
Figure 3.
Cell morphology and TRPC6 expression following I/R insult. Morphology of HK-2 cells (A) with 6-h ischemia (B) followed by 2-h (C), 6-h (D), or 12-h reoxygenation (E). Flow cytometric analysis of cell apoptosis and necrosis following I/R insult (F). Necrosis of the TRPC6 siRNA transfected cells was comparatively higher than that of NC siRNA-transfected or -non-transfected cells, while apoptosis was significantly different among the groups, which resulted in a significantly higher apoptosis/necrosis ratio in TRPC6 siRNA-transfected cells. * p<0.05 vs. the NC siRNA-transfected or -non-transfected groups, or vs. the corresponding group at the different reoxygenation times.
Flow cytometric analysis showed a progressive increase in apoptotic and necrotic cells with increasing reoxygenation time in cells of all 3 groups (p<0.05). However, as shown in Figure 3F, necrosis of the TRPC6 siRNA transfected cells was comparatively higher than that of the other 2 groups (p<0.05), while apoptosis of HK-2 cells was not significantly different among the groups. This resulted in a significantly higher apoptosis/necrosis ratio in HK-2 cells transfected with TRPC6 siRNA compared to NC siRNA transfected or non-transfected cells (p<0.05). In combination of cell morphology and apoptosis and necrosis of HK-2 cells following I/R, a reoxygenation time of 6 h was used for further study.
TRPC6 expression and necroptosis in the HK-2 cells during I/R injury
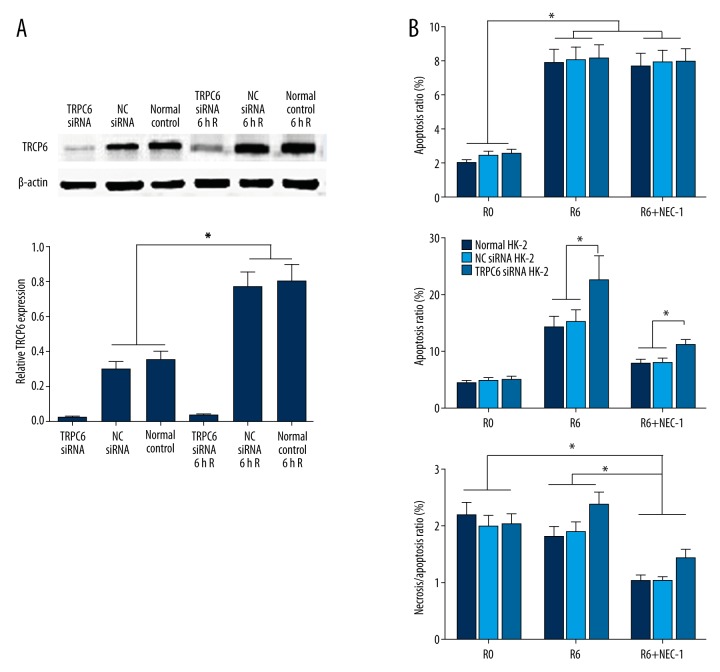
Western blot analysis showed that I/R could induce the TRPC6 expression in HK-2 cells. Protein expression of TRPC6 was significantly upregulated in HK-2 cells transfected with NC siRNA or without transfection after reoxygenation for 6 h (p<0.05). However, TRPC6 expression in TRPC6 siRNA transfected cells only showed a slight increase compared with the cells without reoxygenation (Figure 4A).
Figure 4.
Western blot analysis showed the upregulated expression of TRPC6 following the I/R in HK-2 cells transfected with NC siRNA or without transfection, while no significant TRPC-6 expression was identified following I/R in the TRPC-6 siRNA-transfected HK-2 cells (A). Administration of necrostatin-1 suppressed the I/R-induced necrosis, although in TRPC6 siRNA-transfected cells it was still significantly higher than in the other 2 groups (B). * p<0.05 vs. the NC siRNA-transfected or -non-transfected groups, or vs. the corresponding group at the different reoxygenation times.
Treatment of cells with necrostatin-1, an inhibitor for necroptosis, suppressed the I/R-induced necrosis, although apoptosis of HK-2 cells transfected with TRPC6 siRNA was still significantly higher than that of the other 2 groups (Figure 4B).
Elevated expressions of necroptosis-related proteins following I/R injury
Protein expressions of the necroptotic pathway following I/R insult were evaluated. Western blot results showed that I/R induced significant upregulation of necroptosis-related proteins (RIP1, PARP-1, and sirtuin-2) in HK-2 cells transfected with NC siRNA or without transfection (Figure 5; p<0.05). There was no obvious change in protein expression of AIF following I/R in any of the groups (data not shown). In cells transfected with TRPC6 siRNA, however, PARP-1 expression was not significantly induced by I/R, and RIP1 expression was markedly upregulated when compared with the other two groups, while protein expression of sirtuin-2 showed a pattern similar to that of cells of the other 2 groups. These results suggest that TRPC6 may participate in the necroptosis of cells following I/R injury.
Figure 5.
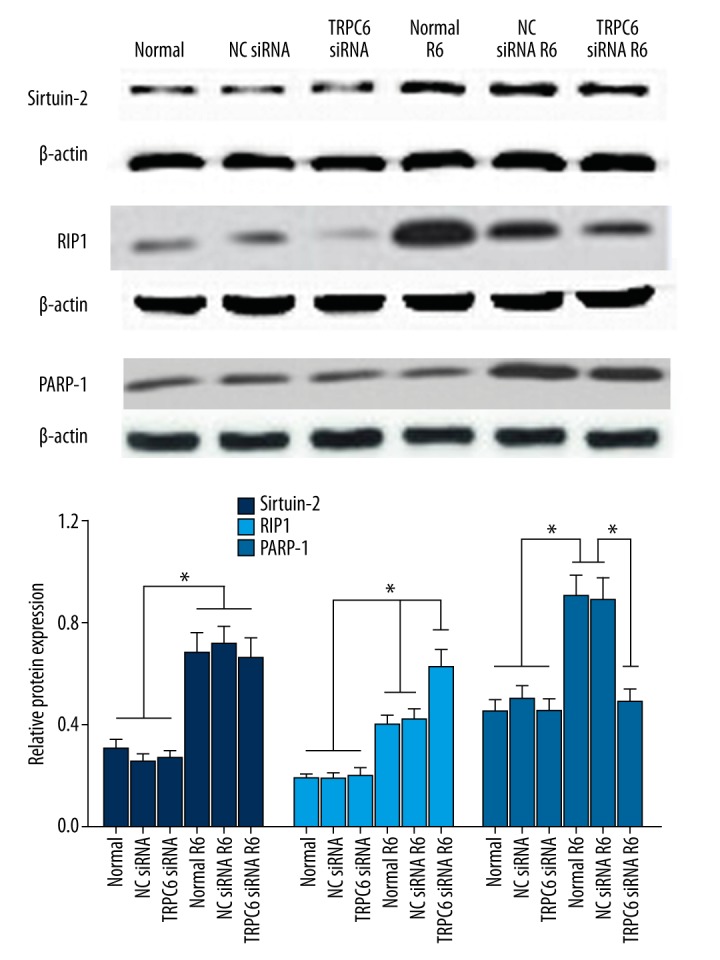
Western blot analysis of expression of necroptosis-related proteins (Sirtuin-2, RIP1, and PARP-1) following I/R insult of HK-2 cells transfected with TRPC6 siRNA, NC siRNA, or without transfection. * p<0.05 vs. the NC siRNA-transfected or -non-transfected groups, or vs. the corresponding group undergoing different treatments.
OAG, SKF96365, and KN93 further affected necrosis and necroptosis-related protein following I/R insult
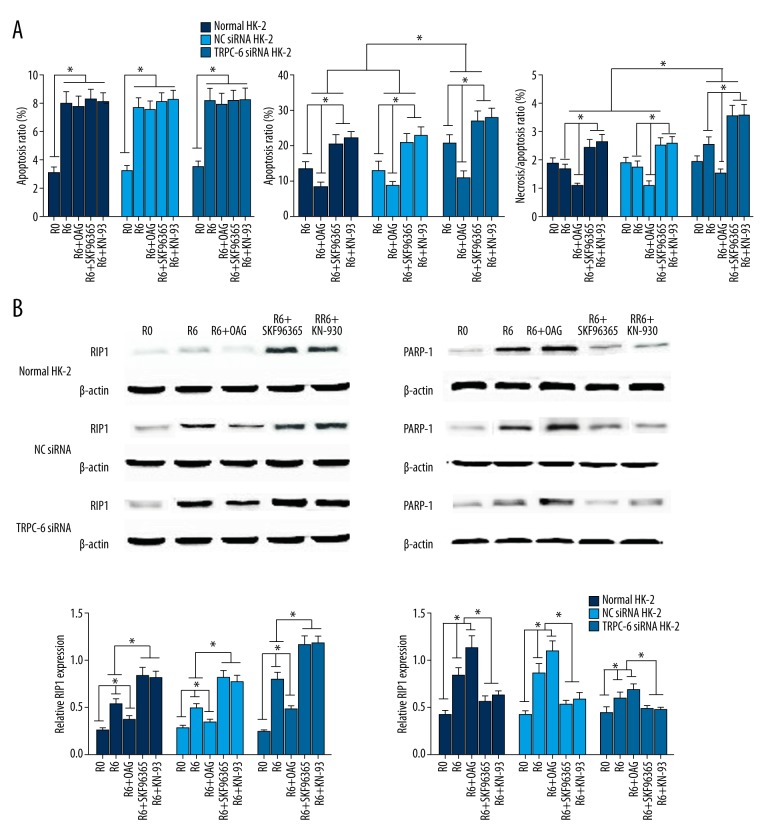
As shown in Figure 6A, OAG treatment markedly reversed the I/R-induced necrosis, while application of SKF96365 or KN93 resulted in the further enhanced cell necrosis in all 3 groups after reoxygenation for 6 h (p<0.05). However, necrosis of cells transfected with TRPC6 siRNA was always significantly higher than in the other 2 cell groups (p<0.05).
Figure 6.
Effect of OAG, SKF96365, or KN-93 application on necrosis and necroptosis-related protein expression following I/R insult. (A) OAG, SKF96365, and KN-93 further affected cell apoptosis and necrosis following I/R as determined by flow cytometry analysis; (B) Western blot analysis showed the remarkably downregulated RIP1 and upregulated PARP-1 expressions following I/R in presence of OAG, while the opposite effects were observed when either SKF96365 or KN93 were used. * p<0.05 vs. the NC siRNA-transfected or -non-transfected groups, or vs. the corresponding group undergoing different treatments.
The expression of the necroptosis-related proteins was further investigated. Western blot analysis showed remarkably downregulated RIP1 and upregulated PARP-1 expressions following I/R in the presence of OAG, while the opposite effects were observed when either SKF96365 or KN93 were used (Figure 6B).
Discussion
The Ca2+-permeable ion channel protein TRPC6 is known as an intrinsic constituent of receptor-operated cation entry, and is involved in numerous physiological processes. In the kidney, TRPC6 has been reported to express throughout the kidney, specifically in podocytes [13]. However, the previous published reports on TRPC6 expression in the renal tissues/cells were discordant. TRPC6 expression has been reported in the cytoplasm of renal tubular epithelia cells and HK-2 cells [11,14,15], while the study by Riccio et al. indicated that TRPC6 was homogeneously expressed throughout the different tissues, yet no TRPC6 transcripts were detected in the HK-2 cell lines [5]. Therefore, in the present study, protein expression of TRPC6 in HK-2 cells was investigated. Our results showed that TRPC6 was intensively expression in the cytoplasm of HK-2 cells, consistent with the results reported by Liu and Ji [11]. TRPC6 is an intrinsic constituent of receptor-operated cation entry, which may participate in numerous physiological functions [16]. There is growing evidence that TRPC6 may be involved in cell injury following I/R in target organs [6–8]. In the kidney, TRPC6 has been thought to be enriched in the podocyte foot process and to be an essential component of the glomerular slit diaphragm, which is required for normal renal function [17]. Upregulation of TRPC6 channels has been reported following renal I/R injury and was suggested to be involved in podocyte response to ischemic injury [18]. However, another study of the roles of TRPC6 in other cells also indicated that TRPC6 signals were reduced in the collecting ducts following acute renal tubular injury [19]. The renal tubular epithelial cells were reported to be the primary target of renal I/R injury [10]. However, the potential role of TRPC6 expression in the response of renal tubular epithelial cells to I/R insult has not been investigated. Therefore, in our study, TRPC6 expression following I/R insult of HK-2 cells was detected. The findings of our study show that I/R insult induced a significant upregulation of TRPC6 expression in HK-2 cells, consistent with the results of previous studies, which indicated a significantly increased expression of TRPC6 following I/R injury [6,8,18]. These results were dramatically different from the other studies, which showed a markedly downregulated TRPC6 expression induced by I/R insult [19–21]. Our study also showed that apoptosis and necrosis of HK-2 cells were significantly enhanced following I/R, while TRPC6 knockdown by siRNA resulted in increased necrosis but not apoptosis. Treatment of cells with necrostatin-1, an inhibitor for necroptosis, significantly suppressed the I/R-induced cell necrosis. The detection of necroptosis in our study gave rise to the hypothesis that TRPC6 may play a role in I/R injury through inhibition of necroptosis in HK-2 cells.
Necroptosis is a newly discovered form of cell death characterized by morphological features resembling non-regulated necrosis. Receptor-interacting protein kinase (RIP)-dependent necroptosis has been identified and suggested to play a more important role in the pathophysiological course of ischemic kidney injury than apoptosis [3,22]. In the setting of I/R injury, RIP1 has been reported to be deacetylated in a sirtuin 2-dependent manner [23], and was associated with PARP-1 activation and AIF translocation [24,25]. Therefore, the expression of necroptosis-related protein (RIP1, PARP-1, sirtuin-2, and AIF) was determined. The result showed that protein expressions of RIP1, PARP-1, and sirtuin-2 were significantly induced following I/R insult of HK-2 cells, while siRNA knockout of TRPC6 further enhanced I/R-induced upregulation of RIP1 expression and reversed the I/R-induced PARP1 expression, but showed no effect on sirtuin-2 expression following I/R. All these results provided further evidence that TRPC6 may be involved in necroptosis during I/R injury. The OAG, SKF96365, and KN93 were then further used to investigate the effect of TRPC6 expression on I/R-induced necroptosis. Our findings showed that OAG, a TRPC6 agonist, significantly reversed the necrotic effect of the TRPC6 siRNA knockout following I/R. However, utilization of SKF96365, which is a TRP channel inhibitor, and KN93, which is a specific inhibitor of Ca2+/calmodulin (CaM)-dependent kinase II (CaMKII) that regulates TRPC6 channel activity, were found to further enhance the effect of TRPC6 siRNA knockout in cell necrosis following I/R insult of HK-2 cells. All these results suggest that TRPC6 may play a protective role in I/R injury through blocking I/R-induced necroptosis.
Conclusions
Our study described the expression and functional relevance of TRPC6 in the pathophysiology of HK-2 cells following I/R injury. Our results on the ability of TRPC6 to specifically interrupt necroptosis may shed new light on its role in prevention and control of ischemic kidney injury.
Footnotes
Source of support: This work was financially supported by the National Natural Science Foundation of China (No. 81570610)
Competing interests
The authors declare no competing interests.
References
- 1.Hussein AA, El-Dken Z, Barakat N, Abol-Enein H. Renal ischaemia/reperfusion injury: possible role of aquaporins. Acta Physiol. 2012;204(3):308–16. doi: 10.1111/j.1748-1716.2011.02372.x. [DOI] [PubMed] [Google Scholar]
- 2.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80(1):29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linkermann A, De Zen F, Weinberg J, et al. Programmed necrosis in acute kidney injury. Nephrol Dial Transplant. 2012;27(9):3412–19. doi: 10.1093/ndt/gfs373. [DOI] [PubMed] [Google Scholar]
- 4.Price PM, Hodeify R. A possible mechanism of renal cell death after ischemia/reperfusion. Kidney Int. 2012;81(8):720–21. doi: 10.1038/ki.2011.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riccio A, Medhurst AD, Mattei C, et al. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Mol Brain Res. 2002;109(1):95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- 6.Weissmann N, Sydykov A, Kalwa H, et al. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat Commun. 2012;3:649. doi: 10.1038/ncomms1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du W, Huang J, Yao H, et al. Inhibition of TRPC6 degradation suppresses ischemic brain damage in rats. J Clin Invest. 2010;120(10):3480. doi: 10.1172/JCI43165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Teng L, Li A, et al. TRPC6 channel protects retinal ganglion cells in a rat model of retinal ischemia/reperfusion-induced cell death. Invest Ophthalmol Vis Sci. 2010;51(11):5751–58. doi: 10.1167/iovs.10-5451. [DOI] [PubMed] [Google Scholar]
- 9.Shen B, Zhou S, He Y, et al. Revealing the underlying mechanism of ischemia reperfusion injury using bioinformatics approach. Kidney Blood Press Res. 2013;38(1):99–108. doi: 10.1159/000355759. [DOI] [PubMed] [Google Scholar]
- 10.Lieberthal W, Levine JS. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol Renal Physiol. 1996;271(3):F477–88. doi: 10.1152/ajprenal.1996.271.3.F477. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Ji Z. FK506 alleviates proteinuria in rats with adriamycin-induced nephropathy by down-regulating TRPC6 and CaN expression. J Nephrol. 2012;25(6):918–25. doi: 10.5301/jn.5000192. [DOI] [PubMed] [Google Scholar]
- 12.Covington MD, Burghardt RC, Parrish AR. Ischemia-induced cleavage of cadherins in NRK cells requires MT1-MMP (MMP-14) Am J Physiol Renal Physiol. 2006;290(1):F43–51. doi: 10.1152/ajprenal.00179.2005. [DOI] [PubMed] [Google Scholar]
- 13.Winn MP, Daskalakis N, Spurney RF, Middleton JP. Unexpected role of TRPC6 channel in familial nephrotic syndrome: does it have clinical implications? J Am Soc Nephrol. 2006;17(2):378–87. doi: 10.1681/ASN.2005090962. [DOI] [PubMed] [Google Scholar]
- 14.Rampino T, Maggio M, Broggini M, et al., editors. Gene transcription induced by Hepatocyte Growth Factor (HGF) and Macrophage Stimulating Protein (MSP) in renal tubular cells. A c-DNA microarray study. J Am Soc Nephrol. 2003 [Google Scholar]
- 15.Rampino T, Gregorini M, Guidetti C, et al. KCNA1 and TRPC6 ion channels and NHE1 exchanger operate the biological outcome of HGF/scatter factor in renal tubular cells. Growth Factors. 2007;25(6):382–91. doi: 10.1080/08977190801892184. [DOI] [PubMed] [Google Scholar]
- 16.Flockerzi V, Nilius B. Transient receptor potential (TRP) channels. Springer Science & Business Media; 2007. [Google Scholar]
- 17.Reiser J, Polu KR, Möller CC, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37(7):739–44. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao B, Yang H, Zhang R, et al. The role of TRPC6 in oxidative stress-induced podocyte ischemic injury. Biochem Biophys Res Commun. 2015;461(2):413–20. doi: 10.1016/j.bbrc.2015.04.054. [DOI] [PubMed] [Google Scholar]
- 19.Jin Y, Li W, Liu X, et al. Recombinant human erythropoietin pretreatment attenuates acute renal tubular injury against ischemia-reperfusion by restoring transient receptor potential channel-6 expression and function in collecting ducts. Crit Care Med. 2014;42(10):e663–72. doi: 10.1097/CCM.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 20.Yao C, Zhang J, Chen F, Lin Y. Neuroprotectin D1 attenuates brain damage induced by transient middle cerebral artery occlusion in rats through TRPC6/CREB pathways. Mol Med Report. 2013;8(2):543–50. doi: 10.3892/mmr.2013.1543. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Chen F, Zhang J, et al. Neuroprotective effect of resveratrol on ischemia/reperfusion injury in rats through TRPC6/CREB pathways. J Mol Neurosci. 2013;50(3):504–13. doi: 10.1007/s12031-013-9977-8. [DOI] [PubMed] [Google Scholar]
- 22.Linkermann A, Bräsen JH, Himmerkus N, et al. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81(8):751–61. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- 23.Narayan N, Lee IH, Borenstein R, et al. The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature. 2012;492(7428):199–204. doi: 10.1038/nature11700. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Chua CC, Kong J, et al. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J Neurochem. 2007;103(5):2004–14. doi: 10.1111/j.1471-4159.2007.04884.x. [DOI] [PubMed] [Google Scholar]
- 25.Jouan-Lanhouet S, Arshad M, Piquet-Pellorce C, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19(12):2003–14. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]