Abstract
Acute liver failure, i.e., the fatal deterioration of liver function, is the most common indication that emergency liver transplantation is necessary. Moreover, in the USA, drug-induced liver injury (DILI), including acetaminophen (APAP)-induced hepatotoxicity, is the main cause of acute liver failure. Matching a donor for liver transplantation is extremely difficult, and thus the development of a novel therapy for DILI is urgently needed. Following recent approval by the FDA of the proteasomal inhibitor bortezomib, its therapeutic effects on various human diseases, including solid and hematologic malignancies, have been validated. However, the specific action of proteasomal inhibition in cases of DILI had not been elucidated prior to this study. To examine the effects of proteasomal inhibition in DILI experimentally, male C56Bl/6 mice were injected with 1 mg bortezomib/kg before APAP treatment. Bortezomib not only alleviated APAP-induced hepatotoxicity in a time- and dose-dependent manner, it also alleviated CCl4- and thioacetamide-induced hepatotoxicity. We also noted that bortezomib significantly reduced cytochrome P450 2E1 (CYP2E1) expression and activity in the liver, which was accompanied by the induction of endoplasmic reticulum (ER) stress. In addition, bortezomib decreased hepatocyte nuclear factor-1α-induced promoter activation of CYP2E1 in Hep3B cells. By contrast, another proteasome inhibitor, MG132, did not cause ER stress and did not markedly affect CYP2E1 enzyme activity. Liver injury induced by APAP was aggravated by MG132, possibly via elevation of connexin 32 expression. This study suggests that proteasome inhibition has different effects in cases of DILI depending on the specific inhibitor being used. Furthermore, results from the mouse model indicated that bortezomib, but not MG132, was effective in alleviating DILI. ER stress induced by proteasome inhibition has previously been shown to exert various effects on DILI patients, and thus each available proteasomal inhibitor should be evaluated individually in order to determine its potential for clinical application.
Keywords: bortezomib, liver, cytochrome P450 2E1, toxicity, acetaminophen, proteasome
Introduction
Drug-induced liver injury (DILI) is the most frequent cause of acute liver failure (1); of various drugs, acetaminophen (APAP) overdose accounts for approximately one-half of all cases of acute liver failure in the USA and the UK (2). Acute liver failure suddenly affects young, otherwise healthy individuals, and is associated with an extremely high rate of mortality, and is the most frequent cause of emergency liver transplantation (1). Drug-induced hepatotoxicity is usually caused by the formation of drug metabolites, which are often formed by the action of cytochrome P450 2E1 (CYP2E1; EC 1.14.13.n7), which transforms various chemicals into reactive metabolites. For example, APAP is converted by CYP2E1 into N-acetyl-p-benzoquinone imine (NAPQI), an electrophilic metabolite that binds to cysteine groups in proteins (3), depletes glutathione (GSH), and causes respiratory dysfunction and reactive oxidative stress (4). Since finding a matching donor for liver transplantation is not always easy, novel therapies for treating DILI are urgently needed.
The proteasome is a multisubunit enzyme complex that degrades ubiquitin-tagged proteins; it plays a critical role in the regulation of proteins that control cell-cycle progression and apoptosis. Consequently, it has become an important target for anticancer therapy (5). In in vitro experiments and in animal studies, the inhibition of the proteasome, either alone or in combination with conventional chemotherapeutic agents, demonstrated antitumour effects against numerous tumour types (5). In 2008, bortezomib (VELCADE; formerly, PS-341, LDP-341, and MLN341) was approved by the US Food and Drug Administration (FDA) as a therapeutic agent for multiple myeloma (6). Recently, the therapeutic potential of bortezomib has been re-evaluated, and it has been reported that the compound is therapeutically effective for various diseases, such as tumours (7), congenital erythropoietic porphyria (8), and graft-versus-host disease (9).
To investigate the role of the proteasome and the effects of its inhibition on DILI, we examined the effects of two proteasome inhibitors, bortezomib and MG132, on drug- and chemical-induced hepatotoxicity. Interestingly, bortezomib alleviated APAP-induced hepatotoxicity, whereas MG132 had the opposite effect. In addition, bortezomib treatment decreased liver damage induced by CCl4 or thioacetamide (TAA) and significantly decreased hepatic CYP2E1 transcription, leading to diminished enzyme activity. Results of the present study suggest that clinical treatment with bortezomib may be useful for alleviating DILI and possibly other forms of acute liver disease.
Materials and methods
Materials
Bortezomib was purchased from Biovision (Mountain View, CA, USA). APAP, CCl4, TAA, MG132, sodium 4-phenylbutyrate (4-PBA), and 2-aminoethyl diphenylborinate (2-APB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The primary antibodies used in this study were anti-CYP2E1 (AB1252; Millipore, Bedford, MA, USA), anti-connexin 32 (CX32; 35-8900; Invitrogen, Carlsbad, CA, USA), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; MAB374) (Millipore, Billerica, MA, USA), anti-binding immunoglobulin protein (BiP; 3177; Cell Signaling Technology, Beverly, MA, USA), anti-CCAAT-enhancer-binding protein homologous protein (CHOP; 5554; Cell Signaling Technology) and anti-β-actin (A5316; Sigma-Aldrich).
Animals
Male C57BL/6J mice, which were 6–8 weeks of age, were purchased from Orient Bio, Inc. (Seoul, Korea) and housed under special pathogen-free conditions. All animals were treated in accordance with the Animal Care Guidelines of Ewha Womans University. To induce hepatotoxicity, mice were injected intraperitoneally with the following: APAP (350 or 500 mg/kg), TAA (200 mg/kg), or CCl4 (2 ml/kg), as described previously (10). Before being injected, mice were fasted overnight. To inhibit the proteasome, bortezomib was injected twice: first at 12 h (1 mg/kg) and then at 1 h (1 mg/kg) prior to the injection of APAP, as previously described (11). The inhibitor MG132 was injected (5 mg/kg) twice: first at 12 h and then at 1 h prior to the administration of APAP, as previously described (12,13). In some cases, different doses of bortezomib (0-1 mg/kg) were injected twice: first at 12 h and then at 1 h prior to the administration of APAP, and 1 mg/kg bortezomib was injected at different times prior to the administration of APAP. For the inhibition of gap junctions, 2-APB (20 mg/kg) was injected 2 h prior to the administration of APAP. DMSO-treated mice were used as the relevant control group. Each group consisted of 4–6 mice. For liver extraction, the mice were sacrificed at 0, 2, 4 6 h after the APAP injection and, at 24 h after the TAA or CCl4 injection. The livers were then perfused with PBS to remove the blood via portal vein.
Cell culture
Human hepatocarcinoma Hep3B cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) heat-inactivated foetal bovine serum, penicillin, and streptomycin (Gibco, Carlsbad, CA, USA). Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. Prior to RNA extraction for expression studies or luciferase assays, cells were treated with bortezomib (10-250 nM) (14,15) in serum-free medium for 15 h. To reduce ER stress, 4-PBA (1–5 mM) was added 1 h before bortezomib treatment.
Serum enzyme marker measurement
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured by the Korean Animal Clinical Research Center (Guri, Korea) using a Hitachi 7020 automatic biochemical analyser (Hitachi, Tokyo, Japan).
Reverse transcription-quantitative PCR (RT-qPCR)
Total mRNA from the liver tissues was extracted using an RNeasy Mini kit (Qiagen, Valencia, CA, USA), and cDNA was prepared from the mRNA using a Verso cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. Primer sets are described in Table I. Relative gene expression was calculated as 2−ΔΔCt by quantitative PCR, using the SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) and an ABI PRISM 7500 Sequence Detection system (Applied Biosystems) (16,17).
Table I.
Primers used for quantitative PCR.
| Species | Gene | Primer sequences (5′→3′) | Refs. |
|---|---|---|---|
| Mouse | CYP2E1 | F: GTTGCCTTGCTTGTCTGGAT | |
| R: AGGAATTGGGAAAGGTCCTG | |||
| BiP | F: TCATCGGACGCACTTGGAA | (50,51) | |
| R: CAACCACCTTGAATGGCAAGA | |||
| CHOP | F: GTCCCTAGCTTGGCTGACAGA | (50,51) | |
| R: TGGAGAGCGAGGGCTTTG | |||
| CX32 | F: TGGTCCCTGCAGCTTATCTT | (10) | |
| R: CCTCAAGCCGTAGCATTTTC | |||
| CX43 | F: ATCCAAAGACTGCGGATCTC | (10) | |
| R: GACCAGCTTGTACCCAGGAG | |||
| GAPDH | F: CACTCTTCCACCTTCGATGC | ||
| R: CCCTGTTGCTGTAGCCGTAT |
CYP2E1, cytochrome P450 2E1; BiP, binding immunoglobulin protein; CHOP, CCAAT-enhancer-binding protein homologous protein; CX32, connexin 32; CX43, connexin 43.
Western blot analysis
Liver tissues were lysed in radioimmu-noprecipitation assay buffer [50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% (v/v) Nonidet P-40, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v) SDS] containing 50 mM NaF, 2 mM Na3VO4, and protease and phosphatase inhibitors (Sigma-Aldrich). Protein concentration was quantified using Bradford assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). Protein samples (40 µg) were subjected to SDS-polyacrylamide gel [8% (w/v)] electrophoresis and then transferred to nitrocellulose membranes. Membranes were incubated with primary antibodies followed by peroxidase-conjugated secondary antibodies (111-035-003 and 115-035-003; Jackson ImmunoResearch, West Grove, PA, USA) after blocking with 5% (w/v) BSA in 20 mM Tris-HCl at pH 7.5, 500 mM NaCl, and 0.1% (v/v) Tween-20. The immunocomplexes were detected by chemiluminescence using SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific) and detected with a Bio-Imaging Analyzer (LAS-4000; Fuji, Tokyo, Japan).
Hematoxylin and eosin (H&E) staining
Liver tissues were fixed in 4% (w/v) paraformaldehyde, embedded in paraffin, sectioned at 4-µm thickness, and then stained with H&E using standard methods. Briefly, following deparafinization, the sections were stained with hematoxylin (Sigma-Aldrich) and then destained with acid ethanol (1 ml hydrochloric acid + 99 ml 70% ethanol). After washing with tap water, sections were stained with eosin (Sigma-Aldrich).
Measurement of GSH levels
Levels of GSH in the fresh liver were measured using a GSH assay kit (Biovision) according to the manufacturer's instructions. Briefly, 40 mg of liver tissues were homogenized with cold glutathione assay buffer, and 6 N perchloric acid was added. The samples were then precipitated with 3 N potassium hydroxide, and then centrifuged for 2 min at 13,000 x g. The neutralized samples were incubated with the o-phthalaldehyde probe and glutathione assay buffer for 40 min to detect reduced GSH. Fluorescence was deteced using a microplate fluorescence reader (BioTek Synergy H1; BioTek, Winooski, VT, USA).
Measurement of CYP2E1 enzyme activity
In order to measure CYP2E1 activity, the rate of transformation of p-nitrophenol to p-nitrocatechol was analysed with isolated hepatic microsomes, as described previously (18,19). Briefly, the liver microsomes were incubated at 37°C in assay buffer containing potassium phosphate buffer (50 mM, pH 7.4), NADPH (1 mM) and p-nitrophenol (0.1 mM), at the final concentrations indicated. After 20 min, the reactions were terminated by the addition of 0.6 N perchloric acid, and centrifuged at 10,000 × g for 5 min. Subsequently, 6 N sodium hydroxide was added to the supernatant, and the absorbance was measured at 546 nM using a microplate reader (BioTek Synergy H1; BioTek).
Reporter plasmid construct containing CYP2E1 gene promoter
In the present study, a 540-bp human CYP2E1 gene promoter using 5′-TAGGTACCCAGAAGTGAGATTCCTGTTCT-3′ and 5′-CCCAAGCTTTGCCGATGGGGCTCCACTCT-3′ as primers was subcloned into the corresponding restriction sites of the luciferase reporter pGL3-basic vector (Promega, San Luis Obispo, CA, USA), as described in a previous study (20). The underlined letters denote the corresponding restriction sites, KpnI and HindIII The subcloned sequences were verified by DNA sequence analyses.
Luciferase assay
The luciferase assay was performed with the Dual-Luciferase Reporter assay system (Promega) as previously described (21). Reporter plasmid containing the CYP2E1 promoter region was transfected into Hep3B cells using Metafectene transfection reagent (Biontex, San Diego, CA, USA USA), and pRL-cytomegalovirus promoter (Renilla luciferase vector; Promega) was co-transfected to normalise the transfection efficiency. If necessary, pcDNA5/FRT/HNF-1α, obtained from Addgene (Addgene plasmid 31104), or its control vector pcDNA5/FRT/TO was transfected. After 24 h, each well was treated with bortezomib or 4-PBA, as indicated in the figure legends. Cells were lysed using Promega lysis buffer (Promega). Firefly and Renilla luciferase activities were measured sequentially in the same sample using a GloMax™ 20/20 Luminometer (Promega). Luciferase activities were normalised by dividing the firefly luciferase activity by the Renilla luciferase activity.
Statistical analysis
Values are expressed as the means ± SEM. Statistical significance was determined using the Student's t-test, and a p-value <0.05 was considered to indicate a statistically significant difference.
Results
Bortezomib treatment alleviates APAP-induced hepatotoxicity in a time- and dose-dependent manner
In order to determine the effect of bortezomib on DILI induced by APAP, we injected bortezomib intraperitoneally twice: first at different time points (1 mg/kg) (as indicated in Fig. 1A) and then at 1 h (1 mg/kg) prior to the administration of APAP. Injecting 500 mg/kg APAP markedly elevated serum AST and ALT (Fig. 1). When bortezomib was injected 2 h before APAP administration, serum AST and ALT levels were greatly elevated, similar to the DMSO-treated group (Fig. 1A). Treatment with bortezomib for more than 3 h was needed to diminish the APAP-induced hepatotoxicity, as indicated by decreased serum AST and ALT levels and H&E staining (Fig. 1A and C). In addition, bortezomib decreased APAP-induced hepatotoxicity in a dose-dependent manner, and this protective effect was noted at concentrations greater than 0.063 mg bortezomib/kg (Fig. 1B and D).
Figure 1.
Bortezomib alleviates acetaminophen (APAP)-induced hepatotoxicity in a time- and dose-dependent manner. Bortezomib (1 mg/kg) was intraperitoneally injected twice: first at the indicated time points and then at 1 h prior to the administration of APAP (500 mg/kg). The mice were sacrificed 6 h after the APAP injection. (A) Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels and (C) H&E staining of liver sections are shown, and arrows indicate necrotic areas. Various doses of bortezomib (0–1 mg/kg) were injected twice: first at 12 h and then at 1 h prior to the administration of APAP, and (B) serum AST and ALT levels and (D) H&E-stained liver sections are shown. Data are expressed as the means ± SEM (n=6). Image magnification, ×100. The image is representative of six independent experiments. *p<0.05, **p<0.01.
Bortezomib treatment diminishes hepatotoxicity induced by other chemicals
Since the toxic mechanism differs between various drugs and chemicals, we investigated whether bortezomib treatment also protects against liver damage caused by other chemicals. Surprisingly, bortezomib treatment also alleviated CCl4- and TAA-induced hepatotoxicity (Fig. 2). Thus, we posit that the common toxic mechanism of all three chemicals involves CYP2E1 (22–24) or gap junction function (25).
Figure 2.
Protective effects of bortezomib on CCl4- and thioacetamide (TAA)-induced hepatotoxicity. Bortezomib (1 mg/kg) was intraperitoneally injected twice: first at 12 h and then at 1 h before CCl4 (2 ml/kg) or TAA (200 mg/kg) treatment. The mice were sacrificed 24 h after the CCl4 or TAA injection. (A) Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) levels, and H&E-stained sections of liver are shown for the CCl4-injected group. (B) Serum AST, ALT levels, and H&E-stained liver sections are shown for the group injected with TAA. Data are expressed as the means ± SEM (n=4). *p<0.05, **p<0.01, ***p<0.001. Image magnification, ×100. Arrows indicate necrotic areas. The image is representative of four independent experiments.
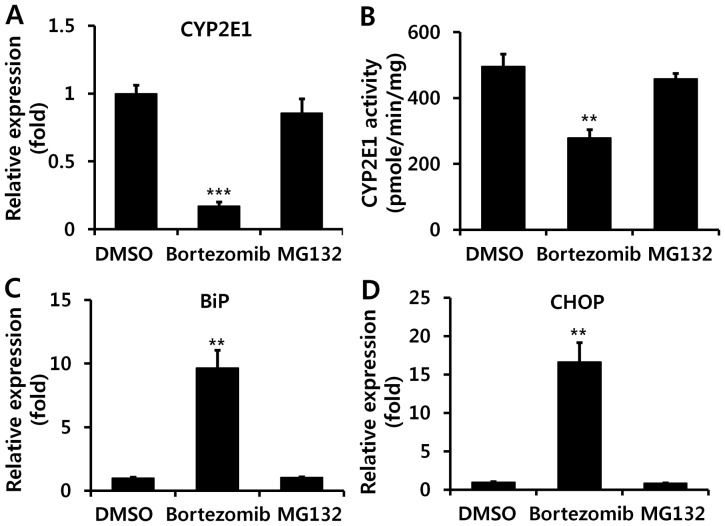
Bortezomib decreases CYP2E1 enzyme expression and activity
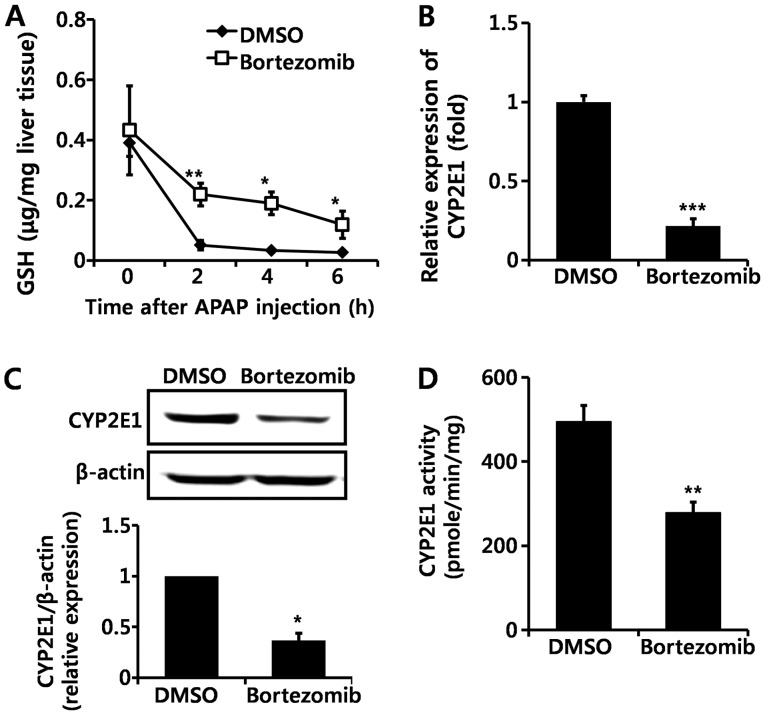
GSH is an important antioxidant that detoxifies NAPQI by conjugation, and GSH depletion leads to toxic cascades of NAPQI (3,4). Basal levels of GSH did not differ significantly between DMSO- and bortezomib-treated mice (Fig. 3A). Although GSH was markedly decreased in both DMSO- and bortezomib-treated groups following APAP administration, GSH levels remained higher at all time-points in bortezomib-versus DMSO-treated animals (Fig. 3A). Lower GSH depletion in bortezomib-treated mice implies the decreased formation of NAPQI. Since NAPQI is transformed by CYP2E1, we subsequently measured CYP2E1 expression by both RT-qPCR (Fig. 3B) and western blot analysis (Fig. 3C). Bortezomib treatment significantly decreased CYP2E1 transcription (Fig. 3B), which resulted in lower CYP2E1 protein levels (Fig. 3C). In addition, CYP2E1 enzymatic activity was significantly lower in the livers of bortezomib-treated versus control mice (Fig. 3D). Therefore, we conclude that the decreased CYP2E1 enzyme activity caused by bortezomib administration alleviates liver damage induced by APAP, CCl4, and TAA exposure. This is in agreement with results of previous studies, in which a CYP2E1 deficiency prevented hepatotoxicity induced by these three chemicals (22–24).
Figure 3.
Bortezomib decreases cytochrome P450 2E1 (CYP2E1) expression and enzyme activity. (A) Glutathione (GSH) levels were measured at the indicated times after acetaminophen (APAP) (500 mg/kg) injection. (B) RT-qPCR of hepatic CYP2E1 gene expression and (C) representative western blot analysis (top panel) and quantification (bottom panel) (n=6) of CYP2E1 levels were examined 12 h after bortezomib (1 mg/kg) administration. (D) Hepatic CYP2E1 enzyme activity was measured 12 h after bortezomib (1 mg/kg) treatment (n=6). Values are expressed as the means ± SEM (n=6). *p<0.05, **p<0.01, ***p<0.001. Data are representative of six independent experiments that provided similar results.
Bortezomib diminishes hepatocyte nuclear factor (HNF)-1α-induced CYP2E1 transcription
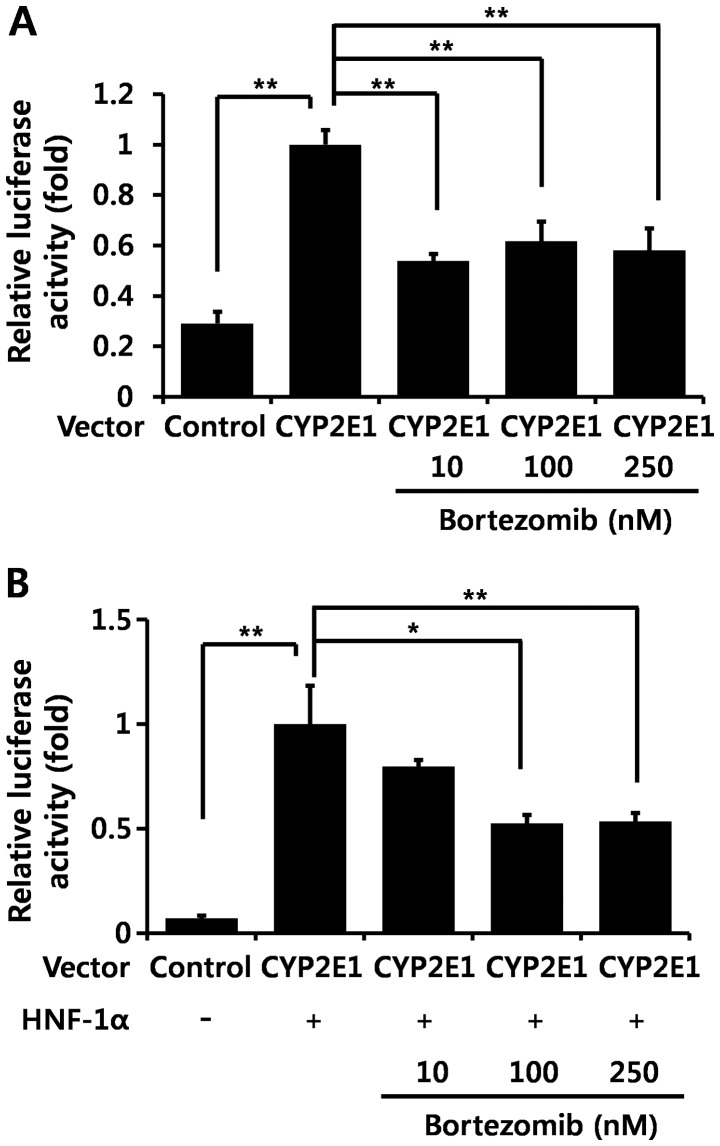
In order to confirm whether bortezomib regulates CYP2E1 transcription, we transfected a hepatocyte cell line (Hep3B cells) with the reporter plasmid containing the CYP2E1 gene promoter region, and performed luciferase assays. Bortezomib treatment significantly decreased CYP2E1 promoter activity by itself (Fig. 4A) as well as HNF-1α-induced CYP2E1 promoter activity (Fig. 4B). HNF-1α is the main transcription factor which positively regulates CYP2E1 gene transcription (26–28).
Figure 4.
Bortezomib diminishes cytochrome P450 2E1 (CYP2E1) promoter activity in vitro. (A and B) Hep3B cells were transfected with a reporter plasmid containing the CYP2E1 promoter region with or without hepatocyte nuclear factor-1α (HNF-1α), and bortezomib at the indicated concentrations was added for 15 h. Relative luciferase activity was calculated as the ratio of firefly luciferase activity to Renilla luciferase activity in the same sample. Values are expressed as the means ± SEM (n=4). Data are representative of four independent experiments that provided similar results. *p<0.05, **p<0.01.
Bortezomib induces endoplasmic reticulum (ER) stress in the liver, which partially contributes to decreased CYP2E1 levels
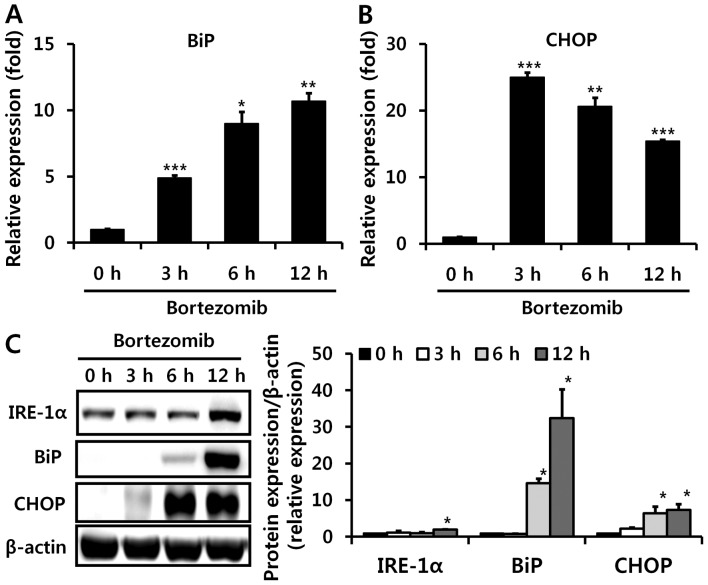
Since bortezomib can lead to ER stress in vitro (29,30), which can induce decreased CYP2E1 mRNA levels (31), we next investigated whether bortezomib administration also causes ER stress in the liver in vivo. We examined altered levels of several proteins that were reported to be induced during ER stress. BiP, also referred to as glucose-regulated protein 78, is a central regulator of ER stress (32), and CHOP is one of the most highly induced transcription factors during ER stress (33). Bortezomib injections markedly elevated hepatic mRNA and protein levels of BiP and CHOP, indicating the in vivo induction of ER stress (Fig. 5). IRE-1α protein expression was also increased 12 h after bortezomib treatment (Fig. 5C). Subsequently, we used 4-PBA to confirm whether ER stress is directly involved in bortezomib-induced CYP2E1 decrement. Treatment with 4-PBA (5 mM), which is reported to decrease ER stress (34), partially recovered CYP2E1 luciferase activity decreased by bortezomib treatment (Fig. 6). Therefore, bortezomib-induced ER stress partially contributes to decreased CYP2E1 levels.
Figure 5.
Bortezomib induces endoplasmic reticulum (ER) stress in vivo. Hepatic mRNA levels of (A) binding immunoglobulin protein (BiP) and (B) CCAAT-enhancer-binding protein homologous protein (CHOP) were measured using RT-qPCR at the indicated times after bortezomib (1 mg/kg) administration. (C) Elevated protein levels of inositol-requiring protein 1 (IRE-1)α, BiP, and CHOP by bortezomib administration are shown by western blot analysis (left panel), with results quantified in the right panel (n=6). Values are expressed as the means ± SEM (n=6). Data are representative of six independent experiments that provided similar results. *p<0.05, **p<0.01, ***p<0.001.
Figure 6.
Sodium 4-phenylbutyrate (4-PBA) partially recovered diminished cytochrome P450 2E1 (CYP2E1) promoter activity caused by bortezomib in vitro. Hep3B cells were transfected with a reporter plasmid containing the CYP2E1 promoter region with or without hepatocyte nuclear factor-1α (HNF-1α), and 4-PBA at the indicated concentrations was added for 1 h before bortezomib treatment. Relative luciferase activity was calculated as the ratio of firefly luciferase activity to Renilla luciferase activity in the same sample. Values are expressed as the means ± SEM (n=4). Data are representative of four independent experiments that provided similar results. *p<0.05.
Protective effect of bortezomib on APAP-induced hepatotoxicity is not derived from direct inhibition of the proteasome
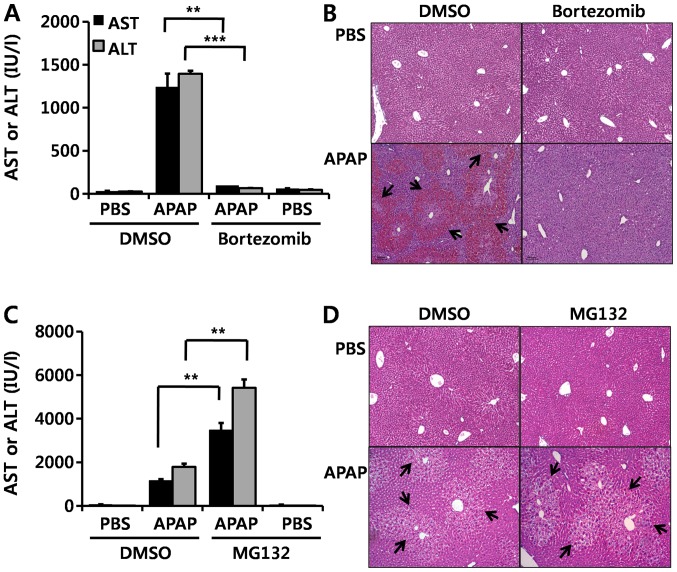
In the present study, in order to investigate whether this effect of bortezomib is due to proteasome inhibition, we injected MG132, another well-known proteasome inhibitor, before APAP administration. By contrast to bortezomib treatment (Fig. 7A and B), MG132 aggravated APAP-induced hepatotoxicity, as demonstrated by elevated serum AST and ALT levels (Fig. 7C) and by enhanced H&E staining (Fig. 7D). These results suggest that the protective effect of bortezomib on APAP-induced hepatotoxicity is not derived from direct inhibition of the proteasome.
Figure 7.
Protective effects of bortezomib on acetaminophen (APAP)-induced hepatotoxicity. Mice were intraperitoneally injected with bortezomib twice: first at 12 h (1 mg/kg) and then at 1 h (1 mg/kg) before APAP treatment (350 mg/kg). The mice were sacrificed 6 h after the APAP injection. (A) Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured, and (B) hematoxylin and eosin (H&E)-stained slides of liver sections are shown. Arrows indicate necrotic areas. Similar to bortezomib administration, mice were intraperitoneally injected with MG132 (5 mg/kg) twice: first at 12 h and then at and 1 h prior to APAP administration (350 mg/kg). The mice were sacrificed 6 h after the APAP injection. (C) Serum AST and ALT levels were measured, and (D) liver sections were stained with H&E, and arrows indicate necrotic areas. Data are expressed as the means ± SEM (n=5–6). **p<0.01, ***p<0.001. Image magnification, ×100. The image is representative of at least five independent experiments.
Proteasome inhibitor MG132 does not induce hepatic ER stress
Since we noted that MG132, a second proteasome inhibitor, aggravated APAP-induced liver injury, by contrast with bortezomib (Fig. 1), we subsequently examined the effect of MG132 on CYP2E1 expression and ER stress. Unlike treatment with bortezomib, hepatic mRNA levels of CYP2E1 were not diminished upon MG132 administration (Fig. 8A), and we also noted relatively unaltered CYP2E1 enzyme activity upon administration of MG132 (Fig. 8B). In addition, it was demonstrated that MG132 administration did not induce ER stress, as indicated by unaltered mRNA levels of BiP and CHOP (Fig. 8C and D).
Figure 8.
The proteasome inhibitor MG132 does not influence cytochrome P450 2E1 (CYP2E1) enzyme activity. The compound MG132 (5 mg/kg) or bortezomib (1 mg/kg) was injected at 12 h and 1 h before liver extraction. (A) Hepatic mRNA levels of CYP2E1 and (B) CYP2E1 enzyme activity were examined 12 h after bortezomib (1 mg/kg) or MG132 administration. RT-qPCR analysis of (C) binding immunoglobulin protein (BiP) and (D) CCAAT-enhancer-binding protein homologous protein (CHOP) expression was performed. Values are expressed as the means ± SEM (n=5–6). **p<0.01, ***p<0.001.
MG132, but not bortezomib, increases CX32 levels, which increases hepatotoxicity
In addition to CYP2E1 enzyme activity, it has previously been suggested that the common toxic mechanism of all three chemicals (APAP, TAA, and CCl4) may also involve gap junctions (10,25,35,36), since gap junctions have been reported to play a critical role in the propagation of hepatotoxicity of all three chemicals used in the present study; ablation of CX32 (a key protein in hepatic gap junctions) significantly protects against DILI (10,25,35,36). Thus, we examined CX32 levels. Notably, MG132 administration significantly increased CX32 protein expression (Fig. 9A), possibly by inhibiting its degradation, and 2-APB, a gap junction inhibitor, abolished MG132-aggravated APAP-induced liver damage (Fig. 9B). Unexpectedly, bortezomib decreased CX32 expression, as well as CX43 mRNA levels (Fig. 9C and D). Therefore, the opposite effects of bortezomib and MG132 on CX32 levels may contribute to the different impacts of bortezomib and MG132 on DILI.
Figure 9.
Proteasome inhibitors affect hepatic connexin (CX) 32 levels differently, depending on the drug used. The inhibitor MG132 (5 mg/kg) was injected twice: first at 12 h and then at 1 h prior to the administration of APAP. (A) Elevated CX32 protein levels after 12 h of MG132 treatment are demonstrated by western blot analysis (upper panel), with results quantified in the lower panel (n=6). 2-Aminoethyl diphenylborinate (2-APB) (20 mg/kg), a gap junction inhibitor, was injected 1 h before a second MG132 administration, and APAP was then intraperitoneally injected after 2 h. The mice were sacrificed at 6 h after the APAP injection. (B) Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured (n=6). (C) Decreased CX32 protein levels after bortezomib (1 mg/kg) treatment for 12 h are illustrated by western blot analysis (upper panel), and results are quantified in the lower panel (n=6). (D) RT-qPCR analysis of CX32 and CX43 mRNA was performed at the indicated times after bortezomib administration (n=6). Values are expressed as the means ± SEM. Data are representative of six independent experiments that provided similar results. *p<0.05, **p<0.01 and ***p<0.001.
Discussion
Protein metabolism, including both synthesis and degradation, is crucial for cellular homeostasis. Eukaryotic cells possess three different systems which are necessary for protein degradation: mitochondrial proteases, which degrade the majority of mitochondrial proteins; lysosomal proteases, which degrade membrane and endocytosed proteins; and the ubiquitin-proteasome system, which degrades the vast majority (80–90%) of intracellular proteins (37). The proteasome system has emerged as a master regulator of diverse cellular processes, including cell cycle, survival, and apoptosis, and plays a critical role in many diseases, such as cancer (38) and neurodegenerative (39) and cardiovascular diseases (37). In the present study, we demonstrated that the FDA-approved proteasome inhibitor bortezomib is effective at preventing drug- and chemical-induced acute liver injury via the regulation of CYP2E1 expression.
Hepatic CYP2E1 levels were markedly decreased upon bortezomib treatment. Since CYP2E1 degradation involves the ubiquitin-proteasome pathway (40,41), the protective effect of bortezomib on drug- and chemical-induced liver injury seems attributable to a different mechanism than proteasome inhibition. Although bortezomib was originally developed as a proteasome inhibitor, other distinct mechanisms of bortezomib have been uncovered. One of these is ER stress (29,30), which, in turn, induces IRE-1α-mediated degradation of CYP2E1 mRNA (31). In the present study, ER stress induced by bortezomib treatment also played a role in decreased CYP2E1 transcription, which may alleviate DILI. The main governing factor of CYP2E1 transcriptional regulation is HNF-1α (26–28), and we noted that bortezomib significantly decreased HNF-1α-induced promoter activation of CYP2E1, and 4-PBA treatment partially recovered bortezomib's inhibition of CYP2E1 promoter activity. Therefore, ER stress plays a role in bortezomib-induced CYP2E1 decrement. Although MG132 was reported to induce ER stress in vitro (42), its administration in vivo did not cause ER stress. Although questions such as whether the relatively long half-life of bortezomib (43) contributes to the different phenotype in vivo compared with MG132, or, rather, a distinct mechanism of bortezomib other than proteasome inhibition may exist, require further study, the protective effect of bortezomib on DILI is noteworthy, considering its current clinical use.
Interestingly, the effects of two well-known proteasome inhibitors, bortezomib and MG132, exerted opposite effects on APAP-induced liver injury. Since proteasome inhibition potentiates CYP2E1-mediated toxicity in HepG2 cells by elevating CYP2E1 levels (44), the aggravating effects of MG132 on APAP-induced liver damage are predictable. However, since no marked change was observed in CYP2E1 enzyme activity, the aggravating effect of MG132 on APAP-induced liver damage appears to be due to some other mechanism, such as gap junction elevation; this was confirmed by elevation of CX32 levels. Gap junctions have recently been implicated as important players in amplifying DILI (25,36). Gap junction channels are composed of CX proteins and play an important role in intercellular communication and in the propagation of liver toxicity and inflammation (10,25,36). Ablation or decreased levels of CX32, a major hepatic gap junction protein, protected against DILI which was induced by the same three hepatotoxic agents used in the present study (10,25,36); thus, diminished CX32 levels caused by bortezomib treatment protect against DILI, and, on the contrary, the increased CX32 levels caused by MG132 are expected to exert opposite effects on DILI. Gap junction proteins are degraded by the proteasome system; thus, proteasome inhibition usually increases CX levels (45–47). On the other hand, ER stress decreases CX43 expression at both the protein and mRNA levels by inhibiting CX43 promoter activity and accelerating the degradation of CX43 (48). Therefore, we suggest that the balance between proteasome inhibition and induction of ER stress determines the effect of bortezomib on CX levels. Decreased bortezomib-induced CX32 levels also contribute to the protective effect on APAP-induced liver injury in addition to decreasing levels of CYP2E1 enzymatic activity.
Since bortezomib is currently being used in clinical settings, the protective effect of bortezomib on APAP-induced liver injury has clinical importance despite its unclear mechanism. Unfortunately, our study shows that bortezomib in mice was only effective when treatments longer than 3 h were used before APAP administration. Considering that the half-life of CYP2E1 protein is 6–7 h (41), the time needed for bortezomib to transcriptionally diminish CYP2E1 expression makes sense, but this finding weakens the practical usefulness of bortezomib as a treatment for DILI, since many patients need medical support when liver damage is already present (49). Still, it is possible for bortezomib to be used to prevent DILI, as the use of high doses of some drugs known to be metabolised by CYP2E1 is clinically inevitable. In addition, bortezomib may help to increase the range of drug dosage, which may otherwise be limited due to DILI.
Proteasome inhibition has recently emerged as an effective therapeutic target in several human diseases. The present study suggests that proteasome inhibition has different effects with respect to DILI, depending on the specific drug employed. Bortezomib, but not MG132, was effectively used for alleviating drug- and chemical-induced liver injury in mice. Since ER stress, induced by proteasome inhibition, has distinct effects, each proteasome inhibitor should be individually scrutinised for its therapeutic applicability in future studies.
Acknowledgments
This study was supported by a Gachon University Research Grant from 2014 (GCU 2014-5101) and awards from the National Research Foundation of Korea funded by the Korean government (Ministry of Education, Science and Technology) (NRF-2013R1A1A1057912).
Abbreviations
- DILI
drug-induced liver injury
- ALT
alanine aminotransferase
- APAP
acetaminophen
- 2-APB
2-aminoethyl diphenylborinate
- AST
aspartate aminotransferase
- BiP
binding immunoglobulin protein
- CHOP
CCAAT-enhancer-binding protein homologous protein
- CX
connexin
- DMEM
Dulbecco's modified Eagle's medium
- ER
endoplasmic reticulum
- FDA
US Food and Drug Administration
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GSH
glutathione
- HNF
hepatocyte nuclear factor
- NAPQI
N-acetyl-p-benzoquinone imine
- 4-PBA
sodium 4-phenyl-butyrate
- TAA
thioacetamide
References
- 1.Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 2.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James LP, Alonso EM, Hynan LS, Hinson JA, Davern TJ, Lee WM, Squires RH, Pediatric Acute, Liver Failure, Study Group. Detection of acetaminophen protein adducts in children with acute liver failure of indeterminate cause. Pediatrics. 2006;118:e676–e681. doi: 10.1542/peds.2006-0069. [DOI] [PubMed] [Google Scholar]
- 4.Burcham PC, Harman AW. Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem. 1991;266:5049–5054. [PubMed] [Google Scholar]
- 5.Adams J. The proteasome: structure, function, and role in the cell. Cancer Treat Rev. 2003;29(Suppl 1):3–9. doi: 10.1016/S0305-7372(03)00081-1. [DOI] [PubMed] [Google Scholar]
- 6.Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu Rev Med. 2006;57:33–47. doi: 10.1146/annurev.med.57.042905.122625. [DOI] [PubMed] [Google Scholar]
- 7.Deming DA, Ninan J, Bailey HH, Kolesar JM, Eickhoff J, Reid JM, Ames MM, McGovern RM, Alberti D, Marnocha R, et al. A Phase I study of intermittently dosed vorinostat in combination with bortezomib in patients with advanced solid tumors. Invest New Drugs. 2014;32:323–329. doi: 10.1007/s10637-013-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blouin JM, Duchartre Y, Costet P, Lalanne M, Ged C, Lain A, Millet O, de Verneuil H, Richard E. Therapeutic potential of proteasome inhibitors in congenital erythropoietic porphyria. Proc Natl Acad Sci USA. 2013;110:18238–18243. doi: 10.1073/pnas.1314177110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Wu Q, Yan Z, Li D, Lu G, Mou W, Wu S, Pan X, Lu Q, Xu K. The protection and therapy effects of bortezomib in murine acute graft-versus-host disease. Transplant Proc. 2013;45:2527–2535. doi: 10.1016/j.transproceed.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 10.Park WJ, Park JW, Erez-Roman R, Kogot-Levin A, Bame JR, Tirosh B, Saada A, Merrill AH, Jr, Pewzner-Jung Y, Futerman AH. Protection of a ceramide synthase 2 null mouse from drug-induced liver injury: role of gap junction dysfunction and connexin 32 mislocalization. J Biol Chem. 2013;288:30904–30916. doi: 10.1074/jbc.M112.448852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner-Ballon O, Pisani DF, Gastinne T, Tulliez M, Chaligné R, Lacout C, Auradé F, Villeval JL, Gonin P, Vainchenker W, Giraudier S. Proteasome inhibitor bortezomib impairs both myelofibrosis and osteosclerosis induced by high thrombopoietin levels in mice. Blood. 2007;110:345–353. doi: 10.1182/blood-2006-10-054502. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho AN, Marques C, Rodrigues E, Henderson CJ, Wolf CR, Pereira P, Gama MJ. Ubiquitin-proteasome system impairment and MPTP-induced oxidative stress in the brain of C57BL/6 wild-type and GSTP knockout mice. Mol Neurobiol. 2013;47:662–672. doi: 10.1007/s12035-012-8368-4. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Kosaras B, Klein PM, Jensen FE. Mammalian target of rapamycin complex 1 activation negatively regulates Polo-like kinase 2-mediated homeostatic compensation following neonatal seizures. Proc Natl Acad Sci USA. 2013;110:5199–5204. doi: 10.1073/pnas.1208010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oerlemans R, Franke NE, Assaraf YG, Cloos J, van Zantwijk I, Berkers CR, Scheffer GL, Debipersad K, Vojtekova K, Lemos C, et al. Molecular basis of bortezomib resistance: proteasome subunit beta5 (PSMB5) gene mutation and overexpression of PSMB5 protein. Blood. 2008;112:2489–2499. doi: 10.1182/blood-2007-08-104950. [DOI] [PubMed] [Google Scholar]
- 15.Markovina S, Callander NS, O'Connor SL, Kim J, Werndli JE, Raschko M, Leith CP, Kahl BS, Kim K, Miyamoto S. Bortezomib-resistant nuclear factor-kappaB activity in multiple myeloma cells. Mol Cancer Res. 2008;6:1356–1364. doi: 10.1158/1541-7786.MCR-08-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JW, Park ES, Choi EN, Park HY, Jung SC. Altered brain gene expression profiles associated with the pathogenesis of phenylketonuria in a mouse model. Clin Chim Acta. 2009;401:90–99. doi: 10.1016/j.cca.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Phillips IR, Shephard EA, editors. Cytochrome P450 Protocols. Humana Press; Totowa, NJ: 2006. [Google Scholar]
- 19.Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos. 1985;13:548–552. [PubMed] [Google Scholar]
- 20.Matsunaga N, Ikeda M, Takiguchi T, Koyanagi S, Ohdo S. The molecular mechanism regulating 24-hour rhythm of CYP2E1 expression in the mouse liver. Hepatology. 2008;48:240–251. doi: 10.1002/hep.22304. [DOI] [PubMed] [Google Scholar]
- 21.Park JW, Lee MH, Choi JO, Park HY, Jung SC. Tissue-specific activation of mitogen-activated protein kinases for expression of transthyretin by phenylalanine and its metabolite, phenylpyruvic acid. Exp Mol Med. 2010;42:105–115. doi: 10.3858/emm.2010.42.2.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong FW, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153:109–118. doi: 10.1006/taap.1998.8547. [DOI] [PubMed] [Google Scholar]
- 23.Kang JS, Wanibuchi H, Morimura K, Wongpoomchai R, Chusiri Y, Gonzalez FJ, Fukushima S. Role of CYP2E1 in thioacetamide-induced mouse hepatotoxicity. Toxicol Appl Pharmacol. 2008;228:295–300. doi: 10.1016/j.taap.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 25.Patel SJ, Milwid JM, King KR, Bohr S, Iracheta-Vellve A, Li M, Vitalo A, Parekkadan B, Jindal R, Yarmush ML. Gap junction inhibition prevents drug-induced liver toxicity and fulminant hepatic failure. Nat Biotechnol. 2012;30:179–183. doi: 10.1038/nbt.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno T, Gonzalez FJ. Transcriptional control of the rat hepatic CYP2E1 gene. Mol Cell Biol. 1990;10:4495–4505. doi: 10.1128/MCB.10.9.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu SY, Gonzalez FJ. Role of the liver-enriched transcription factor HNF-1 alpha in expression of the CYP2E1 gene. DNA Cell Biol. 1995;14:285–293. doi: 10.1089/dna.1995.14.285. [DOI] [PubMed] [Google Scholar]
- 28.Cheung C, Akiyama TE, Kudo G, Gonzalez FJ. Hepatic expression of cytochrome P450s in hepatocyte nuclear factor 1-alpha (HNF1alpha)-deficient mice. Biochem Pharmacol. 2003;66:2011–2020. doi: 10.1016/S0006-2952(03)00586-0. [DOI] [PubMed] [Google Scholar]
- 29.Nawrocki ST, Carew JS, Dunner K, Jr, Boise LH, Chiao PJ, Huang P, Abbruzzese JL, McConkey DJ. Bortezomib inhibits PKR-like endoplasmic reticulum (ER) kinase and induces apoptosis via ER stress in human pancreatic cancer cells. Cancer Res. 2005;65:11510–11519. doi: 10.1158/0008-5472.CAN-05-2394. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong JL, Flockhart R, Veal GJ, Lovat PE, Redfern CP. Regulation of endoplasmic reticulum stress-induced cell death by ATF4 in neuroectodermal tumor cells. J Biol Chem. 2010;285:6091–6100. doi: 10.1074/jbc.M109.014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hur KY, So JS, Ruda V, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Iwawaki T, Glimcher LH, Lee AH. IRE1α activation protects mice against acetaminophen-induced hepatotoxicity. J Exp Med. 2012;209:307–318. doi: 10.1084/jem.20111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 34.Basseri S, Lhoták S, Sharma AM, Austin RC. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J Lipid Res. 2009;50:2486–2501. doi: 10.1194/jlr.M900216-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong X, Dong S, Yu M, Wang Q, Tao L. Role of heteromeric gap junctions in the cytotoxicity of cisplatin. Toxicology. 2013;310:53–60. doi: 10.1016/j.tox.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Asamoto M, Hokaiwado N, Murasaki T, Shirai T. Connexin 32 dominant-negative mutant transgenic rats are resistant to hepatic damage by chemicals. Hepatology. 2004;40:205–210. doi: 10.1002/hep.20256. [DOI] [PubMed] [Google Scholar]
- 37.Herrmann J, Ciechanover A, Lerman LO, Lerman A. The ubiquitin-proteasome system in cardiovascular diseases - a hypothesis extended. Cardiovasc Res. 2004;61:11–21. doi: 10.1016/j.cardiores.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 38.Mani A, Gelmann EP. The ubiquitin-proteasome pathway and its role in cancer. J Clin Oncol. 2005;23:4776–4789. doi: 10.1200/JCO.2005.05.081. [DOI] [PubMed] [Google Scholar]
- 39.Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. doi: 10.1016/S0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- 40.Goasduff T, Cederbaum AI. NADPH-dependent microsomal electron transfer increases degradation of CYP2E1 by the proteasome complex: role of reactive oxygen species. Arch Biochem Biophys. 1999;370:258–270. doi: 10.1006/abbi.1999.1399. [DOI] [PubMed] [Google Scholar]
- 41.Roberts BJ. Evidence of proteasome-mediated cytochrome P-450 degradation. J Biol Chem. 1997;272:9771–9778. doi: 10.1074/jbc.272.15.9771. [DOI] [PubMed] [Google Scholar]
- 42.Park HS, Jun Y, Han CR, Woo HJ, Kim YH. Proteasome inhibitor MG132-induced apoptosis via ER stress-mediated apoptotic pathway and its potentiation by protein tyrosine kinase p56lck in human Jurkat T cells. Biochem Pharmacol. 2011;82:1110–1125. doi: 10.1016/j.bcp.2011.07.085. [DOI] [PubMed] [Google Scholar]
- 43.Levêque D, Carvalho MC, Maloisel F. Review. Clinical pharmacokinetics of bortezomib. In Vivo. 2007;21:273–278. [PubMed] [Google Scholar]
- 44.Pérez MJ, Cederbaum AI. Proteasome inhibition potentiates CYP2E1-mediated toxicity in HepG2 cells. Hepatology. 2003;37:1395–1404. doi: 10.1053/jhep.2003.50228. [DOI] [PubMed] [Google Scholar]
- 45.Fernandes R, Girão H, Pereira P. High glucose down-regulates intercellular communication in retinal endothelial cells by enhancing degradation of connexin 43 by a proteasome-dependent mechanism. J Biol Chem. 2004;279:27219–27224. doi: 10.1074/jbc.M400446200. [DOI] [PubMed] [Google Scholar]
- 46.Minogue PJ, Beyer EC, Berthoud VM. A connexin50 mutant, CX50fs, that causes cataracts is unstable, but is rescued by a proteasomal inhibitor. J Biol Chem. 2013;288:20427–20434. doi: 10.1074/jbc.M113.452847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laing JG, Tadros PN, Westphale EM, Beyer EC. Degradation of connexin43 gap junctions involves both the proteasome and the lysosome. Exp Cell Res. 1997;236:482–492. doi: 10.1006/excr.1997.3747. [DOI] [PubMed] [Google Scholar]
- 48.Huang T, Wan Y, Zhu Y, Fang X, Hiramatsu N, Hayakawa K, Paton AW, Paton JC, Kitamura M, Yao J. Downregulation of gap junction expression and function by endoplasmic reticulum stress. J Cell Biochem. 2009;107:973–983. doi: 10.1002/jcb.22202. [DOI] [PubMed] [Google Scholar]
- 49.Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–548. vi. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Farfel-Becker T, Vitner E, Dekel H, Leshem N, Enquist IB, Karlsson S, Futerman AH. No evidence for activation of the unfolded protein response in neuronopathic models of Gaucher disease. Hum Mol Genet. 2009;18:1482–1488. doi: 10.1093/hmg/ddp061. [DOI] [PubMed] [Google Scholar]
- 51.Hetz C, Lee AH, Gonzalez-Romero D, Thielen P, Castilla J, Soto C, Glimcher LH. Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc Natl Acad Sci USA. 2008;105:757–762. doi: 10.1073/pnas.0711094105. [DOI] [PMC free article] [PubMed] [Google Scholar]