Abstract
The aim of this study was to determine the effects of two anti-inflammatory agents on the abnormalities in colonic endocrine cells in dextran sodium sulfate (DSS)-induced colitis. Colitis was induced in male Wistar rats (n=45) using DSS; a further 15 rats without colitis were included in a healthy control group. The animals with DSS-induced colitis were randomly divided into 3 treatment groups as follows: i) DSS group, rats were treated with 0.5 ml of 0.5% carboxymethyl cellulose (CMC); ii) DSS-G group, rats were treated with 3-[(dodecyl thiocarbonyl)-methyl]-glutarimide (DTCM-G), a novel activator protein 1 (AP-1) inhibitor, 20 mg/kg in CMC; and iii) DSS-Q group, rats were treated with dehydroxymethylepoxyquinomicin, a nuclear factor κB (NF-κB) inhibitor, 15 mg/kg in CMC. The treatments were administered intraperitoneally, twice daily for 5 days, after which the animals were sacrificed and tissue samples from the colon were immunostained for chromogranin A (CgA), serotonin, peptide YY (PYY), enteroglucagon, pancreatic polypeptide (PP), somatostatin, leukocytes, B/T lymphocytes, B lymphocytes, T lymphocytes, macrophages/monocytes and mast cells. The densities of these endocrine and immune cells were quantified by computer-aided image analysis. The densities of CgA-, serotonin-, PYY- and enteroglucagon-producing cells were significantly higher, and those of PP- and somatostatin-producing cells were significantly lower in the DSS-G, DSS-Q and control groups than in the DSS group. The densities of all the immune cells were lower in the DSS-G, DSS-Q and control groups than in the DSS group. The densities of all endocrine cell types and immune cells in both the DSS groups treated with anti-inflammatory agents were restored to control levels. In conclusion, our data demonstrate that there is an interaction between endocrine and immune cells during inflammation. This interaction with subsequent changes in endocrine cells is responsible for the clinical manifestation of colitis symptoms.
Keywords: chromogranin A, dextran sodium sulfate-induced colitis, peptide YY, somatostatin, serotonin
Introduction
The intestinal endocrine cells have been reported to be abnormal in patients with inflammatory bowel disease (IBD) and in animal models of IBD (1–20). Recent discussion on the interaction between the hormones secreted by gut endocrine cells and the immune system has led to the hypothesis that this interaction plays an important role in the pathophysiology of IBD (21,22, unpublished data). One recent study found that the densities of all colonic endocrine cell types were affected in rats with dextran sodium sulfate (DSS)-induced colitis, and that this change strongly correlated with changes in the densities of mucosal immune cells caused by the inflammation (unpublished data). These findings confirmed the likelihood of an interaction between intestinal hormones and the immune cells.
3-[(Dodecylthiocarbonyl)-methyl]-glutarimide (DTCM-G) is a synthetic derivative of 9-methylstreptimidone, which is a potent anti-inflammatory agent that suppresses activator protein 1 (AP-1), and dehydroxymethylepoxyquinomicin (DHME-Q) is a low-molecular-weight nuclear factor κB (NF-κB) inhibitor that also exhibits potent anti-inflammatory activity. Both of these anti-inflammatory agents have been shown to be effective in animal models of IBD (23–26). The aim of this study was to determine whether the anti-inflammatory effects of these two agents restore the densities of colonic endocrine cells to normal levels in rats with DSS-induced colitis.
Materials and methods
Rats
Sixty male Wistar rats (Hannover GALAS; Taconic Farms, Inc., Lille Skensved, Denmark) with a mean body weight of 290 g (range, 241–395 g) were housed in Makrolon III cages with ad libitum access to water and food. They were fed a standard diet (B&K Universal AS, Nittedal, Norway) and maintained in an environment at 21±1°C, a relative humidity of 55±5% and a 12/12 h light/dark cycle. The animals were allowed to acclimatize in the animal house for 8 days prior to the experiments, and were then divided into 4 groups of 15 animals each.
The animals in the control group were provided with normal drinking water for 7 days, and colitis was induced in the rats in the remaining 3 groups by providing the rats with distilled water containing 5% DSS (molecular weight 40 kDa; TdB Consultancy, Uppsala, Sweden), which was prepared daily, for 7 days, as previously described (27,28). The 3 DSS-treated groups were then randomized to receive the vehicle [0.5 ml of 0.5% carboxymethyl cellulose (CMC; DSS group)], DTCM-G at 20 mg/kg body weight in 0.5% CMC (DSS-G group), and DHME-Q at 15 mg/kg body weight in 0.5% CMC (DSS-Q group), intraperitoneally, twice daily for 5 days. The synthesis of DTCM-G and DHME-Q is described elsewhere (23,27–31). The animals were monitored twice daily, and any animals exhibiting signs of pain were administered a subcutaneous injection of 1 ml of Temgesic solution (containing 0.3 g/ml Temgesic; Merck Pharmaceutical).
At the end of the 5-day treatment period, all the animals were sacrificed by CO2 inhalation, and a post-mortem laparotomies were carried out. The colon was dissected out, and tissue samples were taken from the lower part of the colon for histological examinations.
The local ethics committee for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes approved the study protocols.
Histopathology and immunohistochemistry
The tissue samples were fixed overnight in 4% buffered paraformalde-hyde, embedded in paraffin and then sectioned at a thickness of 5 µm. The sections were deparaffinized and then stained with hematoxylin and eosin, or immunostained using the ultraView Universal DAB Detection kit (version 1.02.0018) and the BenchMark Ultra IHC/ISH staining module (both from Ventana Medical Systems, Basel, Switzerland). The sections were immunostained by incubating them with one of the primary antibodies for 32 min at 37°C. The primary antibodies used are summarized in Table I.
Table I.
Summary of the primary antibodies used in this study.
| Antibodies raised against | Type of antibody | Source | Code no. | Detects |
|---|---|---|---|---|
| N-terminal of purified CgA | Monoclonal, raised in mouse | Dako, Glostrup, Denmark | M869 | CgA |
| Serotonin | Monoclonal, raised in mouse | Dako, Glostrup, Denmark | 5HT-209 | Serotonin |
| PYY | Polyclonal, raised in rabbit | Alpha-Diagnostica, San Antonio, TX, USA | PYY 11A | PYY |
| Porcine glicentin/glucagon | Polyclonal, raised in rabbit | Acris Antibodies, Herford, Germany | BP508 | Enteroglucagon (oxyntomodulin) |
| Synthetic human PP | Polyclonal, raised in rabbit | Diagnostic Biosystems,Pleasanton, CA, USA | #114 | PP |
| Synthetic human somatostatin | Polyclonal, raised in rabbit | Dako, Glostrup, Denmark | A566 | Somatostatin |
| Human CD45 | Monoclonal, raised in mouse | Dako, Glostrup, Denmark | M0701 | CD45 is considered a common leukocyte antigen and is expressed exclusively on cells of the hematopoietic system and their progenitors |
| Human CD5 | Monoclonal, raised in mouse | Dako, Glostrup, Denmark | IS082 | B and T lymphocytes |
| Human CD57 | Monoclonal, raised in mouse | Dako, Glostrup, Denmark | IS647 | Subsets of natural killer of cells and CD8+ lymphocytes, and by a small proportion CD4+/CD45R0+ T lymphocytes |
| Human CD23 | Monoclonal, raised in mouse | Dako, Glostrup, Denmark | IS781 | B lymphocytes |
| Human CD68 | Monoclonal, raised in mouse | Dako, Glostrup, Denmark | M0814 | Monocytes, macrophages, and myeloid cells |
| Human mast cell tryptase | Monoclonal, raised in mouse | Dako, Glostrup, Denmark | M7052 | Mast cells |
CgA, chromogranin A; PYY, peptide YY; PP, pancreatic polypeptide.
Quantification of endocrine and immune cells
The endocrine and immune cells were quantified using Olympus cellSens imaging software (version 1.7) as described elsewhere (32,33). A ×40 objective was used, which meant that each frame (field) displayed on the monitor represented a tissue area of 0.035 mm2. The data are presented as the number of cells/mm2 of epithelium for endocrine cells, and the number of cells per field for immune cells. The sections were coded, and the measurements were made by the same person (M.E.-S.) who was unaware of the identity of the sections.
Statistical analysis
The Kruskal-Wallis non-parametric test, with Dunn's test as a post-test was used to compare the findings obtained form the animals in the control, DSS, DSS-G and DSS-Q groups. The data are presented as the mean ± SEM values, and the cut-off for statistical significance was set at P<0.05.
Results
The examination of the hematoxylin and eosin-stained sections of the colonic tissues revealed normal histological results in the control, DSS-G, and DSS-Q groups, while a disrupted mucosal architecture, edema, bleeding, crypt abscesses and immune cell infiltration into the mucosa and submucosa were observed in the DSS group.
Endocrine cells
The cell densities of various endocrine cell types in the control, DSS, DSS-G, and DSS-Q groups are summarized in Table II and illustrated in Figs. 1–3.
Table II.
Densities of the different endocrine cell types (number/mm2 of epithelium) in the control animals, and in animals with DSS-induced colitis treated with the vehicle (CgA, DSS group), DTCM-G (DSS-G group) and DHME-Q (DSS-Q group).
| Animal group | Endocrine cell type
|
|||||
|---|---|---|---|---|---|---|
| CgA | Serotonin | PYY | Enteroglucagon | PP | Somatostatin | |
| Control | 119.0±16.5 | 39.9±4.4 | 96.0±1.2 | 43.1±3.2 | 60.7±2.8 | 43.1±2.8 |
| DSS | 300.6±27.7a | 62.6±5.8a | 120.5±5.9b | 87.2±7.2a | 41.5±2.8a | 28.3±2.2c |
| DSS-G | 146.7±22.4 | 35.9±4.9 | 98.2±7.0 | 51.3±3.6 | 65.8±4.4 | 40.7±2.7 |
| DSS-Q | 136.6±12.2 | 36.2±4.3 | 84.8±2.0 | 49.5±2.9 | 59.1±3.5 | 46.7±3.8 |
Data are the mean ± SEM values DSS, dextran sodium sulfate; DTCM-G, 3-[(dodecylthiocarbonyl)-methyl]-glutarimide; DHME-Q, dehydroxy-methylepoxyquinomicin; CgA, chromogranin A; PYY, peptide YY; PP, pancreatic polypeptide.
P<0.0001,
P<0.01 and
P<0.05 vs. controls.
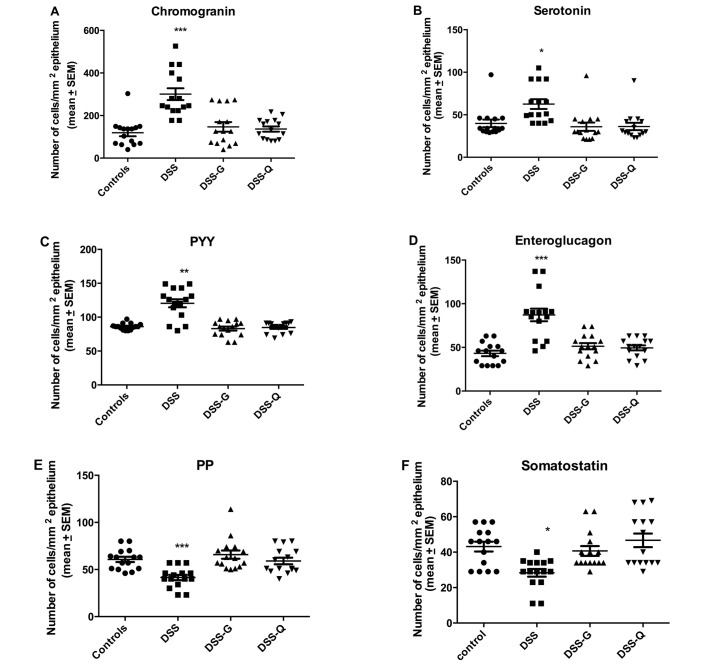
Figure 1.
Densities of different colonic endocrine cells in control rats and in rats with dextran-sulfate-sodium-induced colitis (DSS), rats with DSS-induced colitis and treated with DTCM-G (DSS-G), and rats with DSS-induced colitis and treated with DHME-Q (DSS-Q). ***P<0.0001, **P<0.01, *P<0.05 vs. controls.
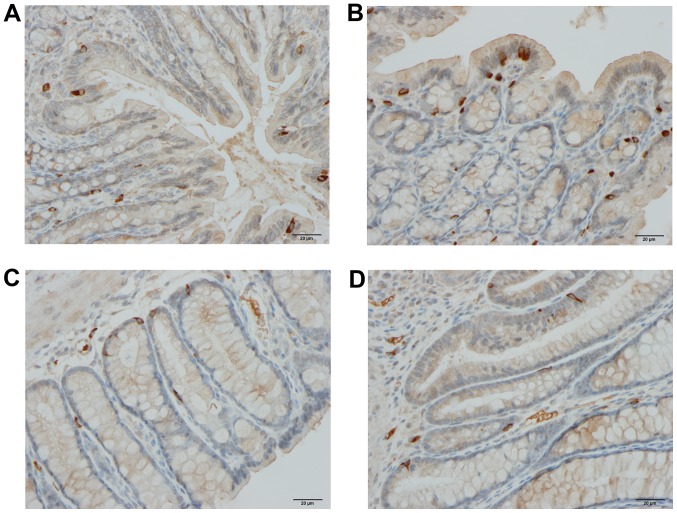
Figure 2.
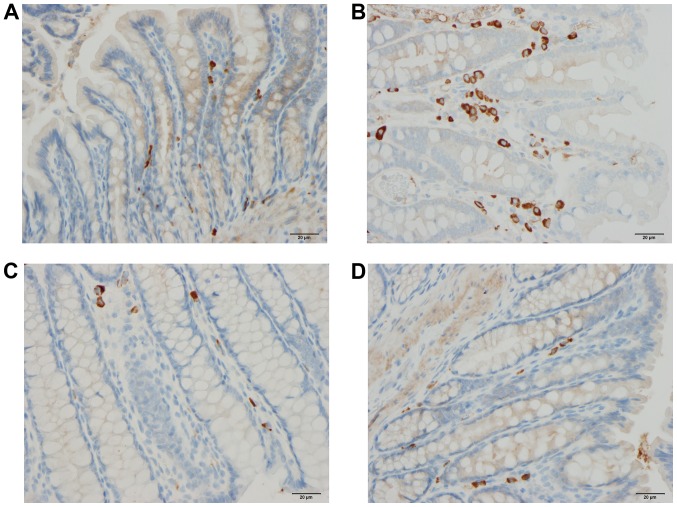
Chromogranin A-producing cells in the colons of rats from the control (A), DSS (B), DSS-G (C) and DSS-Q (D) groups.
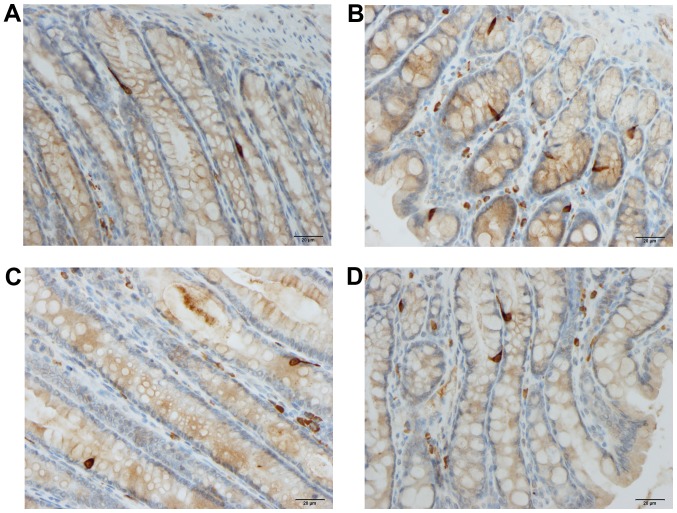
Figure 3.
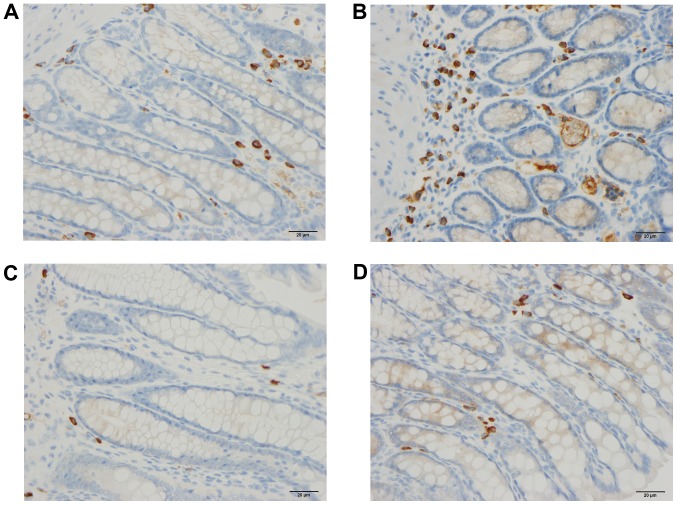
Colonic peptide YY-producing cells in rats from the control (A), DSS (B), DSS-G (C), and DSS-Q (D) groups.
Chromogranin A (CgA)-producing cells
The density CgA-producing cells was significantly higher in the DSS group than in the control, DSS-G, and DSS-Q groups (P<0.0001, 0.0009 and <0.0001, respectively). There was no significant difference observed between the control group and the DSS-G and DSS-Q groups (P=0.6 and 0.2, respectively).
Serotonin-producing cells
The density serotonin-producing cells was significantly higher in the DSS group than in the control, DSS-G and DSS-Q groups (P=0.0006, 0.0006 and 0.0003, respectively), and it did not differ significantly between the control group and the DSS-G and DSS-Q groups (P=0.1 for both).
Peptide YY (PYY)- and enteroglucagon-producing cells
The densities of the PYY- and enteroglucagon- producing cells were significantly higher in the DSS group than in the control, DSS-G and DSS-Q groups (PYY, P=0.005, 0.04 and 0.005, respectively; and enteroglucagon, P<0.0001, 0.0004 and 0.0004, respectively). The cell density did not differ significantly between the control group and the DSS-G and DSS-Q groups for either PYY (P=0.2 and 0.9) or enteroglucagon (P=0.1 for both).
Pancreatic polypeptide (PP)- and somatostatin-producing cells
Both the densities of PP- and somatostatin-producing cells were significantly lower in the DSS group than in the control, DSS-G, and DSS-Q groups (PP, P=0.0001, <0.0001 and 0.0008, respectively; and somatostatin, 0.02, 0.003 and 0.001, respectively). The cell density did not differ significantly between the control group and the DSS-G and DSS-Q groups for either PP (P=0.5 and 0.6, respectively) or somatostatin (P=0.6 and 0.5, respectively).
Immune cells
The densities of the immune cells in the control, DSS, DSS-G, and DSS-Q groups are presented in Table III and Figs. 4–6.
Table III.
Densities of the various immune cells (number/field) in the control group and various experimental treatment groups with DSS-induced colitis.
| Animal group | Immune cell type
|
|||||
|---|---|---|---|---|---|---|
| Leukocytes | B/T lymphocytes | B lymphocytes | T lymphocytes | Macrophages/monocytes | Mast cells | |
| Control | 5.9±0.3 | 7.6±0.7 | 9.5±0.5 | 5.9±0.5 | 6.9±0.3 | 5.5±0.5 |
| DSS | 22.4±1.9a | 23.5±1.4a | 23.7±1.9a | 26.2±2.6a | 22.1±1.8a | 27.7±1.9a |
| DSS-G | 6.8±0.8 | 7.6±0.6 | 8.8±0.7 | 7.6±0.9 | 6.9±0.5 | 7.1±0.8 |
| DSS-Q | 5.8±0.6 | 7.3±0.6 | 8.7±0.6 | 7.7±0.6 | 6.4±0.7 | 6.5±0.7 |
Data are the mean ± SEM values DSS, dextran sodium sulfate.
P<0.0001 vs. controls.
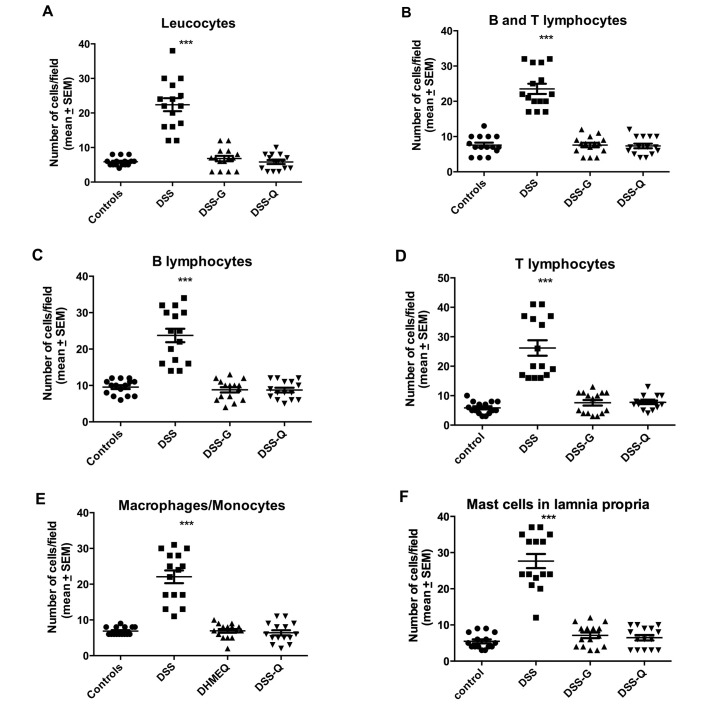
Figure 4.
Densities of various immune cells in the colonic lamina propria of control, DSS, DSS-G and DSS-Q group rats. ***P<0.0001, **P<0.01, *P<0.05 vs. controls.
Figure 5.
B/T lymphocytes in rats from the control (A), DSS (B), DSS-G (C) and DSS-Q (D) groups.
Figure 6.
Mast cells in rats from the control (A), DSS (B), DSS-G (C) and DSS-Q (D) groups.
Leukocytes
The density of leukocytes was significantly higher in the DSS group than in the control, DSS-G, and DSS-Q groups (P<0.0001 for all), and it did not differ significantly between the control group and the DSS-G and DSS-Q groups (P=0.3 and 0.9, respectively).
Lymphocytes
The density of B/T lymphocytes (Fig. 5) in the DSS group was increased relative to the control, DSS-G, and DSS-Q groups (P<0.0001 for all), as were the densities of the B lymphocytes (P<0.0001 for all) and T lymphocytes (P<0.0001 for all). There were no differences observed between the control group and the DSS-G and DSS-Q groups with respect to the densities of B/T lymphocytes (P=0.9 and 0.7, respectively), B lymphocytes (P=0.9 and 0.7, respectively) and T lymphocytes (P=0.2, and 0.2, respectively).
Macrophages/monocytes
The density of macrophages/monocytes was higher in the DSS group than in the control, DSS-G, and DSS-Q groups (P<0.0001 for all), and did not differ significantly between the control group and the DSS-G and DSS-Q groups (P=0.7 and 0.4, respectively).
Mast cells
The density of mast cells (Fig. 6) was significantly higher in the DSS group than in the control, DSS-G, and DSS-Q groups (P<0.0001 for all), and did not differ significantly between the control group and the DSS-G and DSS-Q groups (P=0.2 and 0.4, respectively).
Discussion
DSS-induced colitis is an animal model that closely replicates both the clinical and morphological characteristics of human ulcerative colitis (UC) (34). Thus, animals with DSS-induced colitis suffer from bloody diarrhea, dehydration and the loss of body weight (34). Furthermore, the morphological features resemble human UC both macroscopically and microscopically (35). However, this model lacks the chronicity (i.e., disease relapse and remission) observed in human UC (35). Therefore, care should be taken when drawing conclusions about human UC based on this model.
In the present study, 5 days of treatment with either DTCM-G or DHME-Q ameliorated the inflammation caused by DSS, as indicated by the restored mucosal architecture and normal histological appearance in the animals with DSS-induced colitis treated with these agents compared to the untreated controls with DSS-induced colitis. This finding is in line with the results of previously published studies on colitis induced by trinitrobenzene sulfonic acid (TNBS) and DSS in rats and mice (25,26, unpublished data). This resolution of inflammation was associated with the restoration of the normal densities of all colonic endocrine cell types to normal levels. DTCM-G and DHME-Q are anti-inflammatory agents with different modes of action: the former acts by inhibiting AP-1, while the latter inhibits NF-κB. Thus, the effects of these substances on colonic endocrine cell densities cannot be attributed to a direct effect on the endocrine cells; rather, they are more likely to be attributable to their effects on inflammation.
There is a growing body of evidence indicating that some of the hormones produced by the colonic endocrine cells investigated herein interact with the immune cells during the inflammation process. Thus, CgA peptides reduce the release of interleukin (IL)-16 and IL-5, with the reduction of the number of lymphocytes at the sites inflammation and hence the pro-inflammatory action of lymphocytes and monocytes (36–38). Moreover, CgA inhibits the vascular leakage caused by tumor necrosis factor α (TNF-α) (39). The serotonin cell number has been reported to be reduced in mice lacking the T lymphocyte receptors (36), IL-13 receptors have been shown to be localized on serotonin-producing cells (40) and serotonin receptors have been found in lymphocytes, monocytes, machrophages and dendritic cells (41). Furthermore, serotonin the inhibits apoptosis of immune cells and promotes the recruitment of T cells, and affects the proliferation of lymphocytes, protects natural killer cells (42–45). The present observation that the immune cell densities were normalized to the same extent as the colonic endocrine cells following treatment with DTCM-G and DHME-Q, and the previous finding of a strong correlation between the changes in immune cells and endocrine cells in rats with DSS-induced colitis support the suggested interaction between the two types of cell during the inflammatory process (unpublished data).
It has been proposed that changes in the endocrine cell density in response to or as a result of inflammation play a major role in the manifestation of clinical symptoms such as accelerated intestinal motility, decreased intestinal absorption of water and electrolytes, and diarrhea (unpublished data). Treatment with either DTCM-G or DHME-Q, administered under the same regimen as that used in the present study, was also found to improve these clinical symptoms in DSS-induced colitis in rats (unpublished data). The present finding that treatment with these anti-inflammatory agents restores the densities of the endocrine cells supports the assumption regarding the importance of changes in the endocrine cell populations in the development of the clinical symptoms during colitis.
Acknowledgments
The present study was supported by grants from Helse-Vest and Helse-Fonna.
References
- 1.El-Salhy M, Danielsson A, Stenling R, Grimelius L. Colonic endocrine cells in inflammatory bowel disease. J Intern Med. 1997;242:413–419. doi: 10.1046/j.1365-2796.1997.00237.x. [DOI] [PubMed] [Google Scholar]
- 2.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Chromogranin A cell density as a diagnostic marker for lymphocytic colitis. Dig Dis Sci. 2012;57:3154–3159. doi: 10.1007/s10620-012-2249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. High densities of serotonin and peptide YY cells in the colon of patients with lymphocytic colitis. World J Gastroenterol. 2012;18:6070–6075. doi: 10.3748/wjg.v18.i42.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Salhy M, Lomholt-Beck B, Gundersen TD. High chromogranin A cell density in the colon of patients with lymphocytic colitis. Mol Med Rep. 2011;4:603–605. doi: 10.3892/mmr.2011.492. [DOI] [PubMed] [Google Scholar]
- 5.Moran GW, Pennock J, McLaughlin JT. Enteroendocrine cells in terminal ileal Crohn's disease. J Crohn's Colitis. 2012;6:871–880. doi: 10.1016/j.crohns.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Moran GW, Leslie FC, McLaughlin JT. Crohn's disease affecting the small bowel is associated with reduced appetite and elevated levels of circulating gut peptides. Clin Nutr. 2013;32:404–411. doi: 10.1016/j.clnu.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Besterman HS, Mallinson CN, Modigliani R, Christofides ND, Pera A, Ponti V, Sarson DL, Bloom SR. Gut hormones in inflammatory bowel disease. Scand J Gastroenterol. 1983;18:845–852. doi: 10.3109/00365528309182104. [DOI] [PubMed] [Google Scholar]
- 8.El-Salhy M, Mazzawi T, Gundersen D, Hatlebakk JG, Hausken T. The role of peptide YY in gastrointestinal diseases and disorders (review) Int J Mol Med. 2013;31:275–282. doi: 10.3892/ijmm.2012.1222. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirotani Y, Mikajiri K, Ikeda K, Myotoku M, Kurokawa N. Changes of the peptide YY levels in the intestinal tissue of rats with experimental colitis following oral administration of mesalazine and prednisolone. Yakugaku Zasshi. 2008;128:1347–1353. doi: 10.1248/yakushi.128.1347. [DOI] [PubMed] [Google Scholar]
- 10.Vona-Davis LC, McFadden DW. NPY family of hormones: clinical relevance and potential use in gastrointestinal disease. Curr Top Med Chem. 2007;7:1710–1720. doi: 10.2174/156802607782340966. [DOI] [PubMed] [Google Scholar]
- 11.El-Salhy M, Suhr O, Danielsson A. Peptide YY in gastrointestinal disorders. Peptides. 2002;23:397–402. doi: 10.1016/S0196-9781(01)00617-9. [DOI] [PubMed] [Google Scholar]
- 12.Tari A, Teshima H, Sumii K, Haruma K, Ohgoshi H, Yoshihara M, Kajiyama G, Miyachi Y. Peptide YY abnormalities in patients with ulcerative colitis. Jpn J Med. 1988;27:49–55. doi: 10.2169/internalmedicine1962.27.49. [DOI] [PubMed] [Google Scholar]
- 13.Sciola V, Massironi S, Conte D, Caprioli F, Ferrero S, Ciafardini C, Peracchi M, Bardella MT, Piodi L. Plasma chromogranin a in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:867–871. doi: 10.1002/ibd.20851. [DOI] [PubMed] [Google Scholar]
- 14.Bishop AE, Pietroletti R, Taat CW, Brummelkamp WH, Polak JM. Increased populations of endocrine cells in Crohn's ileitis. Virchows Arch A Pathol Anat Histopathol. 1987;410:391–396. doi: 10.1007/BF00712758. [DOI] [PubMed] [Google Scholar]
- 15.Manocha M, Khan WI. Serotonin and GI Disorders: an update on clinical and experimental studies. Clin Transl Gastroenterol. 2012;3:e13. doi: 10.1038/ctg.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoyanova II, Gulubova MV. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem. 2002;104:185–192. doi: 10.1078/0065-1281-00641. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto H, Morise K, Kusugami K, Furusawa A, Konagaya T, Nishio Y, Kaneko H, Uchida K, Nagai H, Mitsuma T, et al. Abnormal neuropeptide concentration in rectal mucosa of patients with inflammatory bowel disease. J Gastroenterol. 1996;31:525–532. doi: 10.1007/BF02355052. [DOI] [PubMed] [Google Scholar]
- 18.Payer J, Huorka M, Duris I, Mikulecky M, Kratochvílová H, Ondrejka P, Lukác L. Plasma somatostatin levels in ulcerative colitis. Hepatogastroenterology. 1994;41:552–553. [PubMed] [Google Scholar]
- 19.Watanabe T, Kubota Y, Sawada T, Muto T. Distribution and quantification of somatostatin in inflammatory disease. Dis Colon Rectum. 1992;35:488–494. doi: 10.1007/BF02049408. [DOI] [PubMed] [Google Scholar]
- 20.Koch TR, Carney JA, Morris VA, Go VL. Somatostatin in the idiopathic inflammatory bowel diseases. Dis Colon Rectum. 1988;31:198–203. doi: 10.1007/BF02552546. [DOI] [PubMed] [Google Scholar]
- 21.Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161:19–27. doi: 10.1111/j.1365-2249.2010.04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margolis KG, Gershon MD. Neuropeptides and inflammatory bowel disease. Curr Opin Gastroenterol. 2009;25:503–511. doi: 10.1097/MOG.0b013e328331b69e. [DOI] [PubMed] [Google Scholar]
- 23.Ota E, Takeiri M, Tachibana M, Ishikawa Y, Umezawa K, Nishiyama S. Synthesis and biological evaluation of molecular probes based on the 9-methylstreptimidone derivative DTCM-glutarimide. Bioorg Med Chem Lett. 2012;22:164–167. doi: 10.1016/j.bmcl.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 24.Shibasaki S, Yamashita K, Goto R, Wakayama K, Tsunetoshi Y, Zaitsu M, Igarashi R, Haga S, Ozaki M, Umezawa K, Todo S. Immunosuppressive effects of DTCM-G, a novel inhibitor of the mTOR downstream signaling pathway. Transplantation. 2013;95:542–550. doi: 10.1097/TP.0b013e31827b3d90. [DOI] [PubMed] [Google Scholar]
- 25.Funakoshi T, Yamashita K, Ichikawa N, Fukai M, Suzuki T, Goto R, Oura T, Kobayashi N, Katsurada T, Ichihara S, et al. A novel NF-κB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic injury in mice. J Crohn's Colitis. 2012;6:215–225. doi: 10.1016/j.crohns.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 26.El-Salhy M, Umezawa K, Gilja OH, Hatlebakk JG, Gundersen D, Hausken T. Amelioration of severe TNBS induced colitis by novel AP-1 and NF-κB inhibitors in rats. Scientific World Journal. 2014;2014:1–8. doi: 10.1155/2014/813804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeiri M, Tachibana M, Kaneda A, Ito A, Ishikawa Y, Nishiyama S, Goto R, Yamashita K, Shibasaki S, Hirokata G, et al. Inhibition of macrophage activation and suppression of graft rejection by DTCM-Glutarimide, a novel piperidine derived from the antibiotic 9-methylstreptimidone. Inflamm Res. 2011;60:879–888. doi: 10.1007/s00011-011-0348-z. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa Y, Tachibana M, Matsui C, Obata R, Umezawa K, Nishiyama S. Synthesis and biological evaluation on novel analogs of 9-methylstreptimidone, an inhibitor of NF-kappaB. Bioorg Med Chem Lett. 2009;19:1726–1728. doi: 10.1016/j.bmcl.2009.01.107. [DOI] [PubMed] [Google Scholar]
- 29.Ueki S, Yamashita K, Aoyagi T, Haga S, Suzuki T, Itoh T, Taniguchi M, Shimamura T, Furukawa H, Ozaki M, et al. Control of allograft rejection by applying a novel nuclear factor-kappaB inhibitor, dehydroxymethylepoxyquinomicin. Transplantation. 2006;82:1720–1727. doi: 10.1097/01.tp.0000250548.13063.44. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto N, Ariga A, To-e S, Nakamura H, Agata N, Hirano S, Inoue J, Umezawa K. Synthesis of NF-kappaB activation inhibitors derived from epoxyquinomicin C. Bioorg Med Chem Lett. 2000;10:865–869. doi: 10.1016/S0960-894X(00)00114-1. [DOI] [PubMed] [Google Scholar]
- 31.Umezawa N, Matsumoto N, Iwama S, Kato N, Higuchi T. Facile synthesis of peptide-porphyrin conjugates: towards artificial catalase. Bioorg Med Chem. 2010;18:6340–6350. doi: 10.1016/j.bmc.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 32.El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Endocrine cells in the ileum of patients with irritable bowel syndrome. World J Gastroenterol. 2014;20:2383–2391. doi: 10.3748/wjg.v20.i9.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Low-Grade inflammation in the rectum of patients with sporadic irritable bowel syndrome. Mol Med Rep. 2013;7:1081–1085. doi: 10.3892/mmr.2013.1320. [DOI] [PubMed] [Google Scholar]
- 34.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 35.Low D, Nguyen DD, Mizoguchi E. Animal models of ulcerative colitis and their application in drug research. Drug Des Devel Ther. 2013;7:1341–1357. doi: 10.2147/DDDT.S40107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiller R. Serotonin and GI clinical disorders. Neuropharmacology. 2008;55:1072–1080. doi: 10.1016/j.neuropharm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Egger M, Beer AG, Theurl M, Schgoer W, Hotter B, Tatarczyk T, Vasiljevic D, Frauscher S, Marksteiner J, Patsch JR, et al. Monocyte migration: a novel effect and signaling pathways of catestatin. Eur J Pharmacol. 2008;598:104–111. doi: 10.1016/j.ejphar.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Feistritzer C, Mosheimer BA, Colleselli D, Wiedermann CJ, Kähler CM. Effects of the neuropeptide secretoneurin on natural killer cell migration and cytokine release. Regul Pept. 2005;126:195–201. doi: 10.1016/j.regpep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Ferrero E, Magni E, Curnis F, Villa A, Ferrero ME, Corti A. Regulation of endothelial cell shape and barrier function by chromogranin A. Ann N Y Acad Sci. 2002;971:355–358. doi: 10.1111/j.1749-6632.2002.tb04495.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Steeds J, Motomura Y, Deng Y, Verma-Gandhu M, El-Sharkawy RT, McLaughlin JT, Grencis RK, Khan WI. CD4+ T cell-mediated immunological control of enterochro-maffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut. 2007;56:949–957. doi: 10.1136/gut.2006.103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cloëz-Tayarani I, Changeux JP. Nicotine and serotonin in immune regulation and inflammatory processes: a perspective. J Leukoc Biol. 2007;81:599–606. doi: 10.1189/jlb.0906544. [DOI] [PubMed] [Google Scholar]
- 42.Stefulj J, Cicin-Sain L, Schauenstein K, Jernej B. Serotonin and immune response: effect of the amine on in vitro proliferation of rat lymphocytes. Neuroimmunomodulation. 2001;9:103–108. doi: 10.1159/000049013. [DOI] [PubMed] [Google Scholar]
- 43.Betten A, Dahlgren C, Hermodsson S, Hellstrand K. Serotonin protects NK cells against oxidatively induced functional inhibition and apoptosis. J Leukoc Biol. 2001;70:65–72. [PubMed] [Google Scholar]
- 44.Laberge S, Cruikshank WW, Beer DJ, Center DM. Secretion of IL-16 (lymphocyte chemoattractant factor) from serotonin-stimulated CD8+ T cells in vitro. J Immunol. 1996;156:310–315. [PubMed] [Google Scholar]
- 45.Soga F, Katoh N, Inoue T, Kishimoto S. Serotonin activates human monocytes and prevents apoptosis. J Invest Dermatol. 2007;127:1947–1955. doi: 10.1038/sj.jid.5700824. [DOI] [PubMed] [Google Scholar]