We assessed rising CO2 effects on metabolism and development of a nesting wrasse by reciprocal transplant experiments in the field. Offspring brooded under different CO2 conditions exhibited similar responses. However, embryos from High-CO2 site were resilient to a wider range of pCO2 levels than those belonging to current-day conditions.
Keywords: Early development, global change, physiological performance, Symphodus ocellatus, temperate fish
Abstract
Volcanic CO2 seeps provide opportunities to investigate the effects of ocean acidification on organisms in the wild. To understand the influence of increasing CO2 concentrations on the metabolic rate (oxygen consumption) and the development of ocellated wrasse early life stages, we ran two field experiments, collecting embryos from nesting sites with different partial pressures of CO2 [pCO2; ambient (∼400 µatm) and high (800–1000 µatm)] and reciprocally transplanting embryos from ambient- to high-CO2 sites for 30 h. Ocellated wrasse offspring brooded in different CO2 conditions had similar responses, but after transplanting portions of nests to the high-CO2 site, embryos from parents that spawned in ambient conditions had higher metabolic rates. Although metabolic phenotypic plasticity may show a positive response to high CO2, it often comes at a cost, in this case as a smaller size at hatching. This can have adverse effects because smaller larvae often exhibit a lower survival in the wild. However, the adverse effects of increased CO2 on metabolism and development did not occur when embryos from the high-CO2 nesting site were exposed to ambient conditions, suggesting that offspring from the high-CO2 nesting site could be resilient to a wider range of pCO2 values than those belonging to the site with present-day pCO2 levels. Our study identifies a crucial need to increase the number of studies dealing with these processes under global change trajectories and to expand these to naturally high-CO2 environments, in order to assess further the adaptive plasticity mechanism that encompasses non-genetic inheritance (epigenetics) through parental exposure and other downstream consequences, such as survival of larvae.
Introduction
There is widespread concern that the increase of dissolved CO2 concentrations in the oceans and the consequent alteration of the water chemistry (ocean acidification, OA) may severely affect a wide range of marine organisms (Kroeker et al., 2010, 2013; Wittmann and Pörtner, 2013). Modelling projections based on CO2 emission scenarios proposed by the Intergovernmental Panel on Climate Change (IPCC) suggest that if global emissions will not be reduced, ocean CO2 partial pressure (pCO2) will continue to increase up to ∼1000 µatm by the end of this century, and the average surface ocean pH will drop by up to 0.4 units compared with present-day levels (Pörtner et al., 2014).
In the last decade, the number of studies dealing with the effects of OA on the marine biota has constantly increased, suggesting OA as one of the most serious threats for marine organisms and ecosystem functions (Kroeker et al., 2010). However, a wide variability of responses to OA have been observed among different taxonomic groups and ontogenetic stages, with calcifying organisms considered the most threatened taxa (Kroeker et al., 2013).
Being able to regulate their internal acid–base balance actively, fish have been long considered to be less sensitive to changes in the water carbonate chemistry (e.g. Melzner et al., 2009b). However, sublethal effects were displayed by fish exposed to high pCO2 levels, especially during early life stages. It has been suggested that fish exposed to high CO2 concentrations have to adjust the internal HCO3− to maintain homeostasis, with this having consequences on ionic regulation processes (e.g. Brauner and Baker, 2009; Esbaugh et al., 2012; Heuer and Grosell, 2014). This regulation in response to high CO2 concentrations is predicted to be energetically costly (Pörtner et al., 2004; Munday et al., 2009a) and may lead to altered survival rates (Baumann et al., 2012; Chambers et al., 2014), reproduction (Miller et al., 2013), development (Frommel et al., 2012; Miller et al., 2012; Forsgren et al., 2013) and calcification (Checkley et al., 2009; Munday et al., 2011; Pimentel et al., 2014a) in early life stages of some fish species. In addition, an increasing number of studies have documented effects of altered pCO2 levels on the behaviour and neurosensory functions of different fish species, such as impaired learning, loss of behavioural lateralization and altered auditory and olfactory abilities (Munday et al., 2009b, 2010, 2014; Simpson et al., 2011; Domenici et al., 2012; Nilsson et al., 2012; Jutfelt et al., 2013; Chivers et al., 2014; Heuer and Grosell, 2014). The molecular mechanism underpinning such responses has been elucidated by Nilsson et al. (2012), who demonstrated how the function of the GABAA receptor, an inhibitory neurotransmitter of the vertebrate brain, is affected by higher CO2 concentrations.
Physiological studies revealed that adult fish display an apparently high tolerance even at very high pCO2 levels that will not be reached in any worst-case OA scenario (Ishimatsu et al., 2008), whereas early life stages seem to be threatened at pCO2 levels expected by the end of this century (i.e. ∼1000 µatm) or beyond (Baumann et al., 2012; Frommel et al., 2012; Forsgren et al., 2013; Chambers et al., 2014; Pimentel et al., 2014a). The higher sensitivity of early life stages has been linked to their high volume-to-surface ratio affecting the diffusive processes and to an acid–base balance system not yet fully developed, with ionic exchanges occurring across the skin and the yolk (Ishimatsu et al., 2008). It has also been suggested that the energetic cost of maintenance of acid–base balance in high-CO2 conditions would increase the metabolic rate, with potential consequences for development, performance and survival of subsequent ontogenetic stages (Sokolova et al., 2012). To date, studies on fish metabolic responses to altered levels of pCO2 were mainly carried out on larval and post-larval stages and often showed mixed responses (Melzner et al., 2009a; Munday et al., 2009a, 2014; Miller et al., 2012; Couturier et al., 2013; Enzor et al., 2013; Rummer et al., 2013; Pimentel et al., 2014b; Pope et al., 2014). Only a few studies addressed the combined effects of CO2 and temperature on potential alterations of fish embryonic physiology, such as the metabolic response of the little skate, Leucoraja erinacea (Di Santo, 2015), and the Antarctic dragonfish, Gymnodraco acuticeps (Flynn et al., 2015).
More importantly, most of the knowledge about the effects of OA is derived from studies performed in laboratory conditions, whereas the responses of organisms in their natural habitats have seldom been verified, although this could better reflect their adaptation to natural variability in pH and pCO2 (Munday et al., 2014) and to long-term selective pressure. Volcanic CO2 seeps represent a suitable situation in which to disclose these responses and have been used recently to address fish behavioural responses and potential transgenerational effects on populations chronically exposed to high-CO2 conditions (Munday et al., 2014).
Here, we performed two experiments in a natural CO2 seep off Vulcano Island (Italy) to assess the effects of different pCO2 levels on the early development of the temperate ocellated wrasse, Symphodus ocellatus (Forsskål, 1775). This species was chosen because it was previously observed spawning along the CO2 gradient and its eggs are laid on benthic nests. In the first experiment, we used a factorial design to assess the oxygen consumption of three embryonic stages (i.e. initial, middle and late) reared in nesting sites in present-day ambient conditions (ambient, A) and end-of-century high-CO2 conditions (high CO2, H). Embryos were also transplanted from the high-CO2 nesting site to the ambient site and vice versa (HA and AH, respectively) or replaced into the original nesting site (HH and AA) to control the translocation effect, and then exposed for 30 h before testing. This enabled us to assess the effects of elevated CO2 on egg size and oxygen consumption of the S. ocellatus embryos and their responses when developed in different CO2 conditions from the original nesting site. In the second experiment, using the same factorial design, we assessed how different pCO2 levels may affect the yolk consumption and length of newly hatched larvae.
Materials and methods
Study species
Symphodus ocellatus is a Mediterranean wrasse widespread in shallow rocky areas. This species is a benthic spawner, and the females lay eggs in nests built by territorial males (nesting males or nest-builders; Taborsky et al., 1987). The breeding season usually occurs from April to July, probably according to seawater temperature (Lejeune, 1985). During the reproductive period, the nesting males become strictly territorial and brightly coloured and build nests with fragments of algae, where they attract females for mating. The sexual activity of this species is recurrent, and each nesting male completes several nesting cycles over the breeding season (Taborsky et al., 1987). Females visit different nests and lay tens of small eggs (approximately 10–40 eggs) for each spawning event (Lejeune, 1985). The eggs are immediately fertilized, and nesting males provide care for the embryos until hatching, moving the pectoral fins above the nest surface. Development of embryos takes up to 80 h at 21°C (Lejeune, 1985), whereas the subsequent pelagic larval phase lasts from 9 to 13 days (with average planktonic larval duration of 10 days as documented by settlement marks in otoliths; Raventos and McPherson, 2001).
Study site
The experiments were carried out in two different breeding seasons (early and late May 2012 and June 2013) in the Baia di Levante of Vulcano Island (Aeolian Archipelago, Northeastern Sicily, Italy). In this area, a submersed CO2 seep system generates a CO2/pH gradient that runs parallel to the coast (Boatta et al., 2013). The main submersed seep is located along southern and western shores of Baia di Levante (38°25′03.07″N, 14°57′35.90″E) at 1 m depth, and the gas composition is mainly CO2 (>99%). Other gases potentially toxic for cell respiration (e.g. H2S) are found at the main seeping site but do not extend to the nesting sites we considered in the present study, which are located at a distance of >400 m from the main degassing area (Boatta et al., 2013). Oxygen concentrations reach ambient conditions at a few tens of metres from the main seeps (Boatta et al., 2013). Two nesting sites with different pCO2 and pH levels were identified for the experiments: ambient CO2 (A) in present-day conditions (∼400 µatm, pCO2) and high CO2 (H), mimicking projected end-of-century CO2 conditions (∼1000 µatm pCO2 for the first experiment and ∼800 µatm pCO2 for the second experiment; see online supplementary material Table S1 and Fig. S1; RCP8.5 scenario; IPCC, 2013).
Experiment 1: effects of different CO2 concentrations on oxygen consumption of embryos
In each nesting site exposed to different CO2 conditions, we identified 10 nests where the dominant nesting male was in the spawning phase, and we collected portions of the nests for subsequent 30 h exposure. Specifically, fragments of nests with eggs were moved from the high-CO2 nesting site to the ambient-CO2 site and vice versa (treatments HA and AH, respectively; five nests for each condition) or replaced into the original nesting site (treatments HH and AA; five nests for each condition) to control the translocation effect. Translocations were performed by placing the portions of each nest in 100 ml plastic containers with four sides opened and covered with a mesh (0.1 mm) to ensure water flow-through and oxygenation of the embryos. The containers were fixed to the bottom for 30 h at the same depth as the nests of provenience (3–4 m). After the 30 h exposure, eggs from the A and H nesting sites were also collected from eight additional nests.
Embryos belonging from the six different treatments (A, H, AH, AA, HA and HH) were classified on the basis of their development stages, as follows: the first stage (initial) included embryos at the end of epiboly phase (∼6–8 h after fertilization); the second stage (middle) included embryos from the end of the epiboly phase to the formation of the last somite (∼10–30 h after fertilization); and the last stage (late) started with the exhibition of body and eye pigmentation and progressed until the pre-hatching phase (∼60–80 h after fertilization). As expected, only embryos belonging to the middle and late stage of development were found in the AH, AA, HA and HH treatments after the 30 h exposure.
The oxygen consumption rate (OCR) was measured for groups of 10 embryos from each treatment and developmental stage. To measure the OCR, we used the electrode microrespiration System, MRS-8 (Unisense, Aarhus, Denmark), provided with eight microrespiration glass chambers containing ∼0.5 ml (Bartolini et al., 2013; Simoni et al., 2013). To ensure the constant homogeneity of the water samples, each chamber was stirred with a glass-embedded micromagnet (separated from the eggs through a fine mesh of stainless steel) and an individual stirring device. Respirometric chambers were filled with filtered (0.2 µm) seawater to reduce the confounding effect of microorganismal respiration. The temperature of the system was maintained at the same temperature recorded in the field during the first experiment (see supplementary Fig. S2). When the system stabilized at the experimental temperature, 10 eggs were introduced to the glass chambers and oxygen concentration was measured. An end-point measurement was performed after 2 min, and the OCR was extrapolated from the difference between the two measurements (i.e. oxygen depletion/time) and was reported as the absolute value. The approximate single embryo OCR was estimated by dividing the oxygen consumption by the number of embryos in the respirometric chamber. After measuring the oxygen consumption, we placed the eggs on a Petri dish with a 1 mm grid with 0.05 mm scale bars and we photographed them with a Leica camera attached to a stereo microscope (Leica MZ APO). Egg surface area was obtained from digital photographs using ImageJ software by tracing around each egg, and the area was estimated to the nearest 0.001 mm2. As the difference in size of eggs could influence the amount and the capacity of gas exchange of the embryos tested for the respirometry, we standardized the oxygen consumption to the average egg surface calculated for each replicate (10 embryos) and thus reported oxygen consumption in micromoles per hour per square millimetre.
Experiment 2: effects of different CO2 concentrations on yolk area and length at hatching
In June 2013, a second experiment was performed to evaluate whether exposure to different CO2 conditions may affect the yolk consumption (i.e. yolk area at hatching) and the length of larvae at hatching. We used the same experimental design and the same methods as in the first experiment. Portions of nests were collected from A and H nesting sites (five nests per site), and portions of five nests each containing some hundreds (appoximately 500–700) of embryos were exposed to each of the translocation treatments (AH, HA, HH and AA; 20 portions of nests in total). In this case, we chose to focus collections on nests in the last stage of the nesting cycle (when the nesting male provides parental care by fanning) to be sure to obtain enough hatchlings for subsequent measurements. After the 30 h exposure, the nest portions were collected from each CO2 treatment and transferred to a laboratory facility close to the field site, where they were placed in six different rearing aquaria (10 l each) and maintained in aerated seawater from the site of origin. The temperature was monitored using a 556 MPS YSI (Yellow Springs, OH, USA) multiprobe.
Hatching is synchronous in this species and occurs at dusk, probably to avoid predation (Lejeune, 1985). Hatchlings were collected from each aquarium within 2 h, photographed under a binocular microscope (Leica, MZ-APO) and then released in the nesting sites of origin. Yolk area and total length of the larvae were estimated to the nearest 0.001 mm2 and 0.01 mm, respectively, using the digital photographs and the image analysis software ImageJ.
Seawater carbonate chemistry
For the first experiment, salinity and pH expressed as total scale (pHT) were measured in situ for 3 days consecutively in early and late May 2012, using a 556 MPS YSI multiprobe positioned at 2 m depth and previously calibrated using Tris–HCl and 2-aminopyridine–HCl buffer solutions (Dickson et al., 2007). Hobo Onset loggers were also deployed in the two nesting sites to monitor seawater temperatures (in degrees Celsius) continually at 1 h intervals for the whole duration of the first experiment (supplementary Fig. S2). Dissolved oxygen (DO, in milligrams per litre) was measured only in late May (n = 6 for each nesting site). At both sites, 100 ml water samples (n = 3) were also collected, passed through a Whatman GF/F, poisoned with 0.05 ml of 50% HgCl2 (Merck, Analar) and stored in the dark at 4°C for subsequent analyses of total alkalinity (TA). Three replicate 20 ml sub-samples were analysed at 25°C using a titration system composed of a pH meter with a Methrom pH electrode and a 1 ml automatic burette (Methrom). The pH was measured at 0.02 ml increments of 0.1 N HCl. Total alkalinity (in micromoles per kilogram) was calculated from the Gran function applied to pH from 4.2 to 3.0, as milliequivalents per litre from the slope of the curve for pH vs. HCl volume. Results were corrected against TA standards provided by A.G. Dickson (batch 99 and 102). For the first experiments, parameters of the carbonate system [pCO2, CO32 and HCO3−] were calculated from pHT, average TA, temperature and salinity using the free-access CO2SYS package (Pierrot and Wallace, 2006). Means of pHT were calculated from hydrogen ion concentrations of each measurement and then reconverted back to pH.
For the second experiment, pCO2 (in microatmospheres) was continuously measured by deploying a HidroCtmCO2 II sensor for dissolved CO2 (Contros System & Solutions GmbH, Germany) at 2 m depth at each nesting site for 20 h, whilst salinity and seawater temperature (in degrees Celsius) were monitored on several visits using a 556 MPS YSI.
The seawater carbonate chemistry of the first experiment and the pCO2 measured during the second experiment are presented as online supplementary material (supplementary Tables S1 and S2 and Fig. S1).
Experimental design and data analyses
Potential differences in egg size, OCR, yolk area and length at hatching were tested by permutational univariate analysis of variance (PERMANOVA; Anderson et al., 2008) using PRIMER v. 6.1 (PRIMER-E, Ivybridge, UK). Differences in egg size were tested using ‘nesting site’ as a fixed factor with two levels (H and A) to assess potential effects of different pCO2 levels on eggs laid at different nesting sites, or ‘period’ as a fixed factor with two levels (early May and late May) to assess potential differences across the breeding season.
Differences in the overall oxygen consumption of embryos among the three developmental stages were then determined by a two-factor design, with ‘embryonic stage’ as a fixed factor with three levels (initial, middle and late) and ‘nesting site’ as a fixed orthogonal factor with two levels (H and A). Within each embryonic stage, differences in OCR were tested using ‘CO2 treatment’ as a fixed factor with two levels (H and A) for the initial developmental stage, five levels for the middle developmental stage (H, A, AH, HA and AA; as we were not able to obtain enough middle-stage embryos for the HH treatments) and with six levels (H, A, AH, HA, HH and AA) for the late developmental stage. Differences in length at hatching were determined using ‘treatment’ as a fixed factor with six levels (A, H, AH, HA, AA and HH). Data for each experimental trials were not transformed, and a triangular matrix based on Euclidean distance was calculated for each data set. One-way and two-way PERMANOVAs were run using 9999 unrestricted permutations of the raw data. Pairwise t-test comparisons of significant terms in PERMANOVAs were used to assess differences between levels. A linear regression analysis was also run to assess the relationships between the three development stages of S. ocellatus embryos from ambient- and high-CO2 nesting sites and their oxygen consumption (in micromoles per hour per square millimetre).
Results
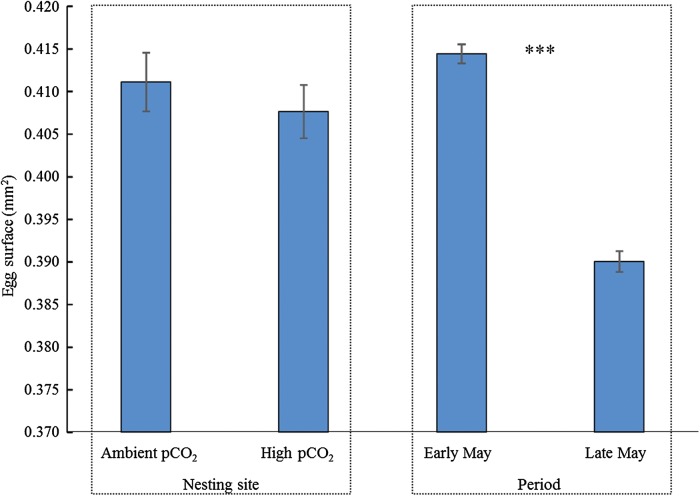
In the first experiment, the average size of newly fertilized eggs did not differ between A and H conditions (means ± SEM: A, 0.411 ± 0.003 mm2; H, 0.408 ± 0.003 mm2; PERMANOVA, pseudo-F1,174 = 0.55209, P = 0.4476; Fig. 1). The eggs collected in late May were significantly smaller than eggs collected in early May in both ambient- and high-CO2 sites (means ± SEM: early May, 0.414 ± 0.001 mm2; late May, 0.390 ± 0.001 mm2; PERMANOVA, pseudo-F1,1224 = 202.99, P < 0.001; Fig. 1).
Figure 1:
Mean (±SEM) surface area of newly fertilized eggs collected in the first experiment from nesting sites with ambient (A) and high (H) CO2 (left panel) and overall mean (±SEM) surface area of eggs regardless of the stage of development in the two periods (early and late May; right panel). In this latter case, eggs belonged to both CO2 nesting sites. ***P = 0.001. n = 88 (A), n = 87 (H), n = 769 (early May) and n = 456 (late May).
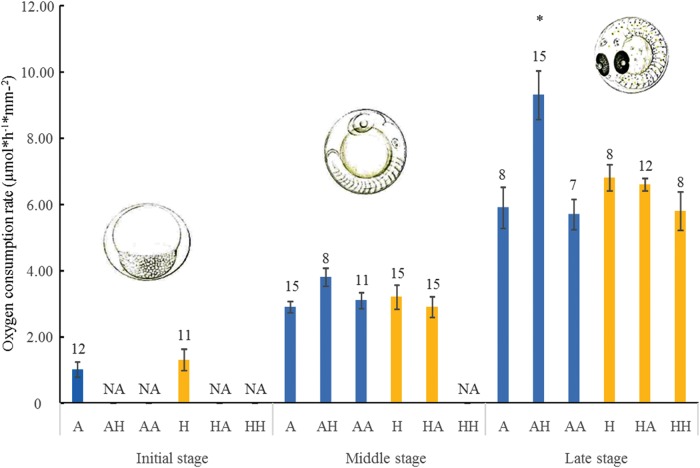
The OCRs of embryos collected from high- and ambient-CO2 nesting sites increased linearly as development progressed, from the initial to the late stage (ambient CO2, r2 = 0.7559, P < 0.001; high CO2, r2 = 0.7234, P < 0.001; supplementary Fig. S3). At each developmental stage, we found no differences in the OCR between embryos collected in A and H conditions (Fig. 2; PERMANOVA: pseudo-F1,68 = 2.959, P = 0.0903; supplementary Table S3). Also, these treatments were not different from the translocation controls, HH and AA, in the middle and late developmental stages and from embryos belonging to high-CO2 and moved to ambient-CO2 nesting sites (HA) for 30 h exposure (Fig. 2; supplementary Table S4). Embryos collected in A and exposed to H conditions (i.e. AH treatment) showed an increasing trend of oxygen consumption in the middle stage and a significantly higher OCR for embryos in the late developmental stage (Fig. 2 and supplementary Table S4).
Figure 2:
Oxygen consumption rate of embryos standardized to egg surface area (in micromoles per hour per square millimetre). Embryos were reared in nesting sites at ambient conditions (A) and end-of-century high-CO2 conditions (H), transplanted from the high-CO2 to the ambient nesting site and vice versa (HA and AH, respectively), or replaced into the original nesting site (HH and AA) to control the translocation effect. Graph shows the average (±SEM) O2 consumption in each treatment and developmental stage (results for HH treatments in the middle stage were not available). Numbers above bars indicate the number of replicates. Asterisk (*) indicates the presence of significant differences (at P < 0.05) (see also pairwise tests in supplementary Table S4). NA, not available.
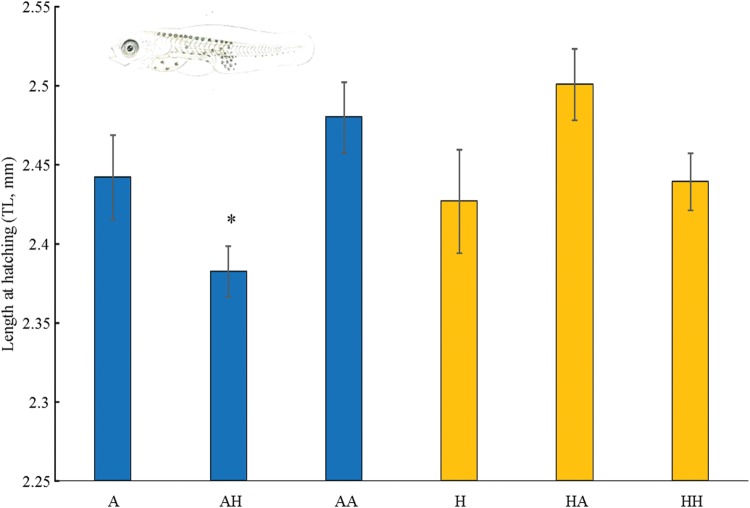
In the second experiment, the length of newly hatched larvae did not differ between A and H nesting sites (PERMANOVA: pseudo-F5,413 = 3.3936, P = 0.0052; supplementary Table S5), where the total length of the larvae were on average 2.44 ± 0.03 and 2.42 ± 0.03 mm, respectively (means ± SEM; Fig. 3). Larvae hatched from AA and HH treatments showed no significant differences from A and H treatments, as total length was on average 2.48 ± 0.02 mm in the AA treatment and 2.43 ± 0.01 mm in the HH treatment (Fig. 3 and supplementary Table S5). Larvae hatched from the AH treatment were significantly smaller (2.38 ± 0.01 mm) than those in all the other treatments (supplementary Table S5).
Figure 3:
Mean (±SEM) total length (in millmetres) of larvae hatched from embryos exposed to the six treatments. Asterisk (*) indicates the presence of significant differences (at P < 0.05). n = 48 (A), n = 72 (AH), n = 84 (AA), n = 62 (H), n = 66 (HA) and n = 82 (HH).
The yolk area of the newly hatched larvae showed a high variability among the treatments (PERMANOVA: pseudo-F5,413 = 34.485, P = 0.001; supplementary Fig. S4). The yolk area in all the treatments differed significantly from each other except those belonging to the HA and HH treatments (supplementary Table S6). On average, the largest yolk area was recorded in the AH treatment (0.109 ± 0.003 mm2) and the smallest in the H treatment (0.065 ± 0.001 mm2; supplementary Fig. S4).
Discussion
We show that embryos of the ocellated wrasse (S. ocellatus) living at a temperate CO2 seep are tolerant to CO2 concentrations expected by the end of this century. Specifically, the oxygen consumption of embryos and their size at hatching were similar between ambient- and high-CO2 nesting sites. However, we found an altered respiration rate in embryos collected from an ambient-CO2 and moved to a high-CO2 nesting site for a 30 h exposure, implying that embryos from ambient-CO2 conditions could be less able to cope with higher pCO2 levels. Likewise, hatchlings showed an impaired development in these conditions. On the contrary, embryos belonging to the high-CO2 nesting site showed no such response when translocated to the ambient-CO2 treatment, therefore suggesting that offspring from high-CO2 nesting sites could be resilient to a wider range of pCO2 values than those belonging to the site with present-day pCO2 levels.
Embryos reared in ambient-CO2 conditions experienced a lower variability in pH and pCO2 than embryos reared in high-CO2 conditions off the Vulcano Island seep in both experiments. It has been suggested that through maternal provisioning females may adjust egg characteristics (such as optimal egg size; Einum et al., 2002) to environmental conditions (Chambers, 1997). However, we found that the size of S. ocellatus eggs laid in the two nesting sites did not differ, whereas we documented a decrease in egg size during the breeding season. Egg surface is often used as indicator of egg quality in fish and represents a fundamental life-history trait for marine fish that influences the size of embryos and their physiology, growth rate and survival at hatching, with cascading effects on species performances and fitness (Chambers, 1997). Our findings suggest that S. ocellatus could modulate their investment in ways other than egg dimension. Likewise, no differences were found in the size of eggs laid from parents of the cinnamon anemonefish (Amphiprion melanopus) reared in aquaria at pCO2 levels comparable with those considered in the present study (Miller et al., 2013). The observed differences in egg size throughout the breeding season have already been documented for several temperate spring-spawning species (e.g. Chambers, 1997; Mazzoldi et al., 2002), including the co-generic wrasse Symphodus roissali (Raventos and Planes, 2008), and have been related to the body condition and the size of the females (Marshall et al., 2010).
As fish primarily use metabolic adjustments to cope with acid–base disturbances (Heuer and Grosell, 2014), metabolic responses measured as OCR may give a clear indication of the physiological performance and potential resilience of organisms exposed to different pCO2 levels (Sokolova et al., 2012). As suggested by some authors, when the pCO2 increases and pH drops, respiration rate increases in order to exhale CO2 and restore homeostasis, affecting the whole energy budget of the organisms (Ishimatsu et al., 2008; Pörtner, 2008; Munday et al., 2009a). Other authors reported metabolic depression in fish exposed to high pCO2 levels (Rummer et al., 2013; Pimentel et al., 2014b), probably because of a mechanism of protection of the body fluids from excessive acidification (Rummer et al., 2013). As expected, we recorded an increased oxygen consumption throughout the embryonic development. Given that the oxygen consumption of embryos in initial, middle and late developmental stages did not show any difference between ambient- and high-CO2 sites, we suggest that offspring of S. ocellatus from parents living in altered CO2 conditions could be resilient to high pCO2. This result could indicate that parental effects (i.e. when parents and offspring are exposed to the same CO2 levels) can compensate for the effects of high CO2 on embryonic metabolic performance by conditioning the offspring to specific CO2 levels through epigenetic transgenerational plasticity, allowing offspring to develop efficient physiological pathways for the high-CO2 environment (e.g. Miller et al., 2012). As S. ocellatus is highly territorial during the breeding season and embryos develop in benthic nests (Taborsky et al., 1987), both parents and offspring were exposed to the same environmental conditions off Vulcano Island CO2 gradient. However, the 30 h exposure of embryos collected at ambient-CO2 site and moved to higher levels of pCO2 (high-CO2 nesting site) led to an increase of OCR, which was more evident during the late developmental stage (with embryos showing an average 33.8% increase in OCR relative to embryos exposed to other treatments). As oxygen consumption may be considered a proxy of energetic demand for basal maintenance and development, our results could suggest that embryos from parents living in ambient-CO2 conditions increase their oxygen requirement when they are exposed to high-CO2 conditions in order to support the increased acid–base regulatory activity (Heuer and Grosell, 2014). On the contrary, there is no sign of metabolic impairment in embryos moved from high-CO2 to ambient-CO2 nesting sites, indicating that this translocation does not represent a stressful event for developing embryos. Laboratory experiments revealed that Juveniles of the tropical anemonefish Amphyprion melanopus exposed to altered pCO2 levels display increased routine metabolic rate (Miller et al., 2012). However, this adverse effect of increased CO2 on metabolic rate did not occur when juveniles were reared in the same CO2 conditions as their parents, suggesting that the conditions experienced by adults may lead to improved capacity to cope with CO2 stress (Miller et al., 2012).
Similar to what has been observed for the metabolic response, the length of larvae hatched from nests at ambient and high CO2 did not differ between nesting sites, whereas hatchlings from embryos moved from ambient- to high-CO2 nesting sites were significantly smaller than those hatched in all other treatments. In the absence of an efficient regulatory system, exposure to high levels of CO2 might lead to an increase of internal pCO2 levels and acidification of internal fluid compartments (Heuer and Grosell, 2014). This might cause direct acid–base imbalances in the organism, which can lead to larval tissue damage (Frommel et al., 2012), and reallocation of energy resources away from growth or development (Baumann et al., 2012). Some studies assessing aspects of embryonic and larval development have shown increased or no differences in the length at hatching of larvae after exposure to high pCO2 levels (Munday et al., 2009c; Franke and Clemmesen, 2011; Frommel et al., 2013; Hurst et al., 2013; Miller et al., 2013; Chambers et al., 2014). This implies that the observed increment in the metabolic activity of embryos from the AH treatment could be a short-term response of transplanted embryos to support the increased energy demand for acid–base regulation in increased CO2 levels (Pörtner, 2012; Sokolova et al., 2012), potentially leading to a decreased growth rate (reduced size at hatching). A recent experiment showed that larvae of Seriola lalandi hatched from eggs exposed to moderate and high pCO2 levels (880 and 1700 µatm, respectively) were significantly smaller than larvae hatched from the control treatment, suggesting an additional energetic cost to cope with altered pCO2 levels (Munday et al., 2015). Smaller larvae may exhibit a lower performance and survival in the wild, because it has been documented that large offspring are advantaged from enhanced swimming ability and more efficient predator avoidance (Miller et al., 1988). However, our second experiment failed to find a consistent response when considering the yolk size at hatching. Yolk areas of larvae hatching from ambient-CO2 and moved to high-CO2 conditions were bigger than yolks in the other treatments. Further research is need to highlight potential trade-off mechanisms involving yolk consumption.
In conclusion, our study reveals that S. ocellatus offspring brooded in different CO2 conditions exhibited similar responses, but after the 30 h exposure to higher CO2 levels the embryos and larvae from parents that spawned in the ambient-CO2 nesting site showed metabolic and size differences, unlike those from parents exposed to high-pCO2 conditions. Indeed, it is undoubted that the role of acclimatization and adaptation processes will have significant consequences for our understanding of how fish will respond to a future high-CO2 ocean (Sunday et al., 2014). In this context, our study identifies a crucial need to increase the number of studies dealing with these processes under climate change trajectories and to expand these to naturally high-CO2 environments to assess further the adaptive plasticity mechanism that encompasses non-genetic inheritance (epigenetics) through parental exposure.
Supplementary material
Supplementary material is available at Conservation Physiology online.
Funding
This work was supported by FFR-A (2012) from the University of Palermo to M.M. and C.C., and contributes to the COST action ‘Conservation Physiology of Marine Fishes’ (FA1004).
Supplementary Material
Acknowledgements
We thank Davide Spatafora, Francesco D′Amore and Maria Vassallo for helping in the field and Riccardo Rodolfo-Metalpa for TA analyses. This study has been conducted in accordance with institutional and national guidelines concerning the use of animals in research (Italian Legislative decree no. 116/1992).
References
- Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Plymouth. [Google Scholar]
- Bartolini F, Barausse A, Pörtner HO, Giomi F (2013) Climate change reduces offspring fitness in littoral spawners: a study integrating organismic response and long-term time-series. Glob Change Biol 19: 373–386. [DOI] [PubMed] [Google Scholar]
- Baumann H, Talmage SC, Gobler CJ (2012) Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat Clim Change 2: 38–41. [Google Scholar]
- Boatta F, D’Alessandro W, Gagliano L, Liotta M, Milazzo M, Rodolfo-Metalpa R, Spencer HJM, Parello F (2013) Geochemical survey of Levante Bay, Vulcano Island (Italy), a natural laboratory for the study of ocean acidification. Mar Pollut Bull 73: 485–494. [DOI] [PubMed] [Google Scholar]
- Brauner CJ, Baker DW (2009) Patterns of acid-base regulation during exposure to hypercarbia in fishes. In Glass ML, Wood SC, eds, Cardio-Respiratory Control in Vertebrates: Comparative and Evolutionary Aspects. Springer, Berlin, Heidelberg, pp 43–63. [Google Scholar]
- Chambers RC. (1997) Environmental influences on egg and propagule sizes in marine fishes. In Chambers RC, Trippel EA, eds, Early Life History and Recruitment in Fish Populations. Chapman & Hall, London, pp 63–102. [Google Scholar]
- Chambers RC, Candelmo AC, Habeck EA, Poach ME, Wieczorek D, Cooper KR, Greenfield CE, Phelan BA (2014) Effects of elevated CO2 in the early life stages of summer flounder, Paralichthys dentatus, and potential consequences of ocean acidification. Biogeosciences 11: 1613–1626. [Google Scholar]
- Checkley DM Jr, Dickson AG, Takahashi M, Radich JA, Eisenkolb N, Asch R (2009) Elevated CO2 enhances otolith growth in young fish. Science 324: 1683. [DOI] [PubMed] [Google Scholar]
- Chivers DP, McCormick MI, Nilsson GE, Munday PL, Watson SA, Meekan MG, Mitchell MD, Corkill KC, Ferrari MCO (2014) Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Glob Change Biol 20: 515–522. [DOI] [PubMed] [Google Scholar]
- Couturier CS, Stecyk JW, Rummer JL, Munday PL, Nilsson GE (2013) Species-specific effects of near-future CO2 on the respiratory performance of two tropical prey fish and their predator. Comp Biochem Physiol A Mol Integr Physiol 166: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean CO2 measurements. PICES Special Publication 3: 191. [Google Scholar]
- Di Santo V. (2015) Ocean acidification exacerbates the impacts of global warming on embryonic little skate, Leucoraja erinacea (Mitchill). J Exp Mar Biol Ecol 463: 72–78. [Google Scholar]
- Domenici P, Allan B, McCormick MI, Munday PL (2012) Elevated carbon dioxide affects behavioural lateralization in a coral reef fish. Biol Lett 8: 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einum S, Hendry AP, Fleming IA (2002) Egg-size evolution in aquatic environments: does oxygen availability constrain size? Proc Biol Sci 269: 2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzor L, Zippay ML, Place SP (2013) High latitude fish in a high CO2 world: synergistic effects of elevated temperature and carbon dioxide on the metabolic rates of Antarctic notothenioids. Comp Biochem Physiol A Mol Integr Physiol 164: 154–161. [DOI] [PubMed] [Google Scholar]
- Esbaugh AJ, Heuer R, Grosell M (2012) Impacts of ocean acidification on respiratory gas exchange and acid–base balance in a marine teleost, Opsanus beta. J Comp Physiol B 182: 921–934. [DOI] [PubMed] [Google Scholar]
- Flynn EE, Bjelde BE, Miller NA, Todgham AE (2015) Ocean acidification exerts negative effects during warming conditions in a developing Antarctic fish. Conserv Physiol 3: doi:10.1093/conphys/cov033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren E, Dupont S, Jutfelt F, Amundsen T (2013) Elevated CO2 affects embryonic development and larval phototaxis in a temperate marine fish. Ecol Evol 3: 3637–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, Clemmesen C (2011) Effect of ocean acidification on early life stages of Atlantic herring (Clupea harengus L.). Biogeosciences 8: 3697–3707. [Google Scholar]
- Frommel AY, Maneja R, Lowe D, Malzahn AM, Geffen AJ, Folkvord A, Piatkowski U, Reusch TBH, Clemmesen C (2012) Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nat Clim Change 2: 42–46. [Google Scholar]
- Frommel AY, Schubert A, Piatkowski U, Clemmesen C (2013) Egg and early larval stages of Baltic cod, Gadus morhua, are robust to high levels of ocean acidification. Mar Biol 160: 1825–1834. [Google Scholar]
- Heuer RM, Grosell M (2014) Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am J Physiol Regul Integr Comp Physiol 307: R1061–R1084. [DOI] [PubMed] [Google Scholar]
- Hurst TP, Fernandez ER, Mathis JT (2013) Effects of ocean acidification on hatch size and larval growth of walleye pollock (Theragra chalcogramma). ICES J Mar Sci 70: 812–822. [Google Scholar]
- IPCC (2013) Climate Change 2013: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK and New York, NY, USA.
- Ishimatsu A, Hayashi M, Kikkawa T (2008) Fishes in high-CO2, acidified oceans. Mar Ecol Prog Ser 373: 295–302. [Google Scholar]
- Jutfelt F, Bresolin de Souza K, Vuylsteke A, Sturve J (2013) Behavioural disturbances in a temperate fish exposed to sustained high-CO2 levels. PLoS ONE 8: e65825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker KJ, Kordas RL, Crim RN, Singh GG (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett 13: 1419–1434. [DOI] [PubMed] [Google Scholar]
- Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso J-P (2013) Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob Change Biol 19: 1884–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejeune P. (1985) Etude écoéthologique des comportements reproducteurs et sociaux des Labridés méditerranéens des genres Symphodus (Rafinesque 1810) et Coris (Lacepede 1802). Cah Ethol Appl 5: 1–208. [Google Scholar]
- Marshall DJ, Heppell SS, Munch SB, Warner RR (2010) The relationship between maternal phenotype and offspring quality: do older mothers really produce the best offspring? Ecology 91: 2862–2873. [DOI] [PubMed] [Google Scholar]
- Mazzoldi C, Poltronieri C, Rasotto M (2002) Egg size variability and mating system in the marbled goby Pomatoschistus marmoratus (Pisces: Gobiidae). Mar Ecol Prog Ser 233: 231–239. [Google Scholar]
- Melzner F, Göbel S, Langenbuch M, Gutowska MA, Pörtner H-O, Lucassen M (2009. a) Swimming performance in Atlantic cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater pCO2. Aquat Toxicol 92: 30–37. [DOI] [PubMed] [Google Scholar]
- Melzner F, Gutowska M, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Pörtner H-O (2009. b) Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6: 2313–2331. [Google Scholar]
- Miller GM, Watson SA, Donelson JM, McCormick MI, Munday PL (2012) Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat Clim Change 2: 858–861. [Google Scholar]
- Miller GM, Watson SA, McCormick MI, Munday PL (2013) Increased CO2 stimulates reproduction in a coral reef fish. Glob Change Biol 19: 3037–3045. [DOI] [PubMed] [Google Scholar]
- Miller TJ, Crowder LB, Rice JA, Marchall EA (1988) Larval size and recruitment mechanisms in fishes: toward a conceptual framework. Can J Fish Aquat Sci 45: 1657–1670. [Google Scholar]
- Munday PL, Crawley NE, Nilsson GE (2009. a) Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar Ecol Prog Ser 388: 235–242. [Google Scholar]
- Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Døving KB (2009. b) Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci USA 106: 1848–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Donelson JM, Dixson DL, Endo GG (2009. c) Effects of ocean acidification on the early life history of a tropical marine fish. Proc Biol Sci 276: 3275–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MCO, Chivers DP (2010) Replenishment of fish populations is threatened by ocean acidification. Proc Natl Acad Sci USA 107: 12930–12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL, Hernaman V, Dixson DL, Thorrold SR (2011) Effect of ocean acidification on otolith development in larvae of a tropical marine fish. Biogeosciences 8: 1631–1641. [Google Scholar]
- Munday PL, Cheal AJ, Dixson DL, Rummer JL, Fabricius KE (2014) Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nat Clim Change 4: 487–492. [Google Scholar]
- Munday PL, Watson S-A, Parsons DM, King A, Barr NG, McLeod IM, Allan BJM, Pether SMJ (2015) Effects of elevated CO2 on early life history development of the yellowtail kingfish, Seriola lalandi, a large pelagic fish. ICES J Mar Sci doi:10.1093/icesjms/fsv210. [Google Scholar]
- Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sørensen C, Watson SA, Munday PL (2012) Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat Clim Change 2: 201–204. [Google Scholar]
- Pierrot DE, Wallace DWR (2006) MS Excel Program Developed for CO2 System Calculations. ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN. [Google Scholar]
- Pimentel MS, Faleiro F, Dionísio G, Repolho T, Pousão-Ferreira P, Machado J, Rosa R (2014. a) Defective skeletogenesis and oversized otoliths in fish early stages in a changing ocean. J Exp Biol 217: 2062–2070. [DOI] [PubMed] [Google Scholar]
- Pimentel M, Pegado M, Repolho T, Rosa R (2014. b) Impact of ocean acidification in the metabolism and swimming behavior of the dolphinfish (Coryphaena hippurus) early larvae. Mar Biol 161: 725–729. [Google Scholar]
- Pope EC, Ellis RP, Scolamacchia M, Scolding JWS, Keay A, Chingombe P, Shields RJ, Wilcox R, Speirs DC, Wilson RW et al. (2014) European sea bass, Dicentrarchus labrax, in a changing ocean. Biogeosciences 11: 2519–2530. [Google Scholar]
- Pörtner HO. (2008) Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar Ecol Prog Series 373: 203–217. [Google Scholar]
- Pörtner HO. (2012) Integrating climate-related stressor effects on marine organisms: unifying principles linking molecule to ecosystem-level changes. Mar Ecol Prog Ser 470: 273–290. [Google Scholar]
- Pörtner H-O, Langenbuch M, Reipschläger A (2004) Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J Oceanogr 60: 705–718. [Google Scholar]
- Pörtner HO, Karl DM, Boyd PW, Cheung WL, Lluch-Cota SE, Nojiri Y, Schmidt DN, Zavialov PO (2014) Ocean systems. In Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC et al. eds, Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK: and New York, NY, USA, pp 411–484. [Google Scholar]
- Raventos N, McPherson E (2001) Planktonic larval duration and settlement marks on the otoliths of Mediterranean littoral fishes. Mar Biol 138: 1115–1120. [Google Scholar]
- Raventos N, Planes S (2008) Maternal size effects on early life traits of the temperate fish Symphodus roissali. Aquat Biol 4: 1–6. [Google Scholar]
- Rummer JL, Stecyk JAW, Couturier CS, Watson S, Nilsson GE, Munday PL (2013) Elevated CO2 enhances aerobic scope of a coral reef fish. Conserv Physiol 1: doi:10.1093/conphys/cot023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R, Giomi F, Spigoli D, Pörtner HO, Cannicci S (2013) Adaptations to semi-terrestrial life in embryos of East African mangrove crabs: a comparative approach. Mar Biol 160: 2483–2492. [Google Scholar]
- Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY (2011) Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol Lett 7: 917–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA (2012) Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar Environ Res 79: 1–15. [DOI] [PubMed] [Google Scholar]
- Sunday JM, Calosi P, Dupont S, Munday PL, Stillman JH, Reusch TB (2014) Evolution in an acidifying ocean. Trends Ecol Evol 29: 117–125. [DOI] [PubMed] [Google Scholar]
- Taborsky M, Hudde B, Wirtz P (1987) Reproductive behaviour and ecology of Symphodus (Crenilabrus) ocellatus, a European wrasse with four types of male behaviour. Behaviour 102: 82–118. [Google Scholar]
- Wittmann AC, Pörtner H-O (2013) Sensitivities of extant animal taxa to ocean acidification. Nat Clim Change 3: 995–1001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.