Abstract
Gilbert's syndrome is a mild genetic liver disorder characterized by unconjugated hyperbilirubinemia due to defects in the UDP-glucuronosyltransferase 1A1 (UGT1A1) gene. The T-3279G mutation in the phenobarbital responsive enhancer module (PBREM), the TA-insertion in the TATA box, creating the A(TA)7TAA motif instead of A(TA)6TAA and the G211A mutation in coding exon 1, particularly in Asian populations, of the human UGT1A1 gene are the three common genotypes found in patients with Gilbert's syndrome. Different approaches for detecting the T-3279G, A(TA)6/7TAA and G211A mutations of the UGT1A1 gene have been described. In this study, to the best of our knowledge, we established a three-dimensional polyacrylamide gel-based DNA microarray method for the first time, in order to study UGT1A1 gene polymorphisms. This method, based on a step-by-step three-dimensional polyacrylamide gel-based DNA microarray protocol, successfully identified all possible genotypes of T-3279G, A(TA)6/7TAA and G211A in 20 patients with hyperbilirubinemia. In addition, sequencing was performed to confirm these results. The data from the current study demonstrate that the three-dimensional polyacrylamide gel microarray method has the potential to be applied as a useful, reliable and cost-effective tool to detect the T-3279G, the A(TA)6/7TAA and the G211A mutations of the UGT1A1 gene in patients with hyperbilirubinemia and thereby aid in the diagnosis of Gilbert's syndrome.
Keywords: Gilbert's syndrome, UDP-glucuronosyltransferase 1A1, hyperbilirubinemia, three-dimensional polyacrylamide gel-based DNA microarray, genotype
Introduction
Gilbert's syndrome, which was first reported by Augustin Nicolas Gilbert in 1901, is a mild genetic liver disorder characterized by unconjugated hyperbilirubinemia without overt signs of hemolysis or structural liver disease (1). The clinical manifestation of Gilbert's syndrome is an elevated level of serum bilirubin (2). Moreover, some patients may present with weakness, indigestion, abdominal pain in the liver area and an intolerance to fat (1).
With the development of molecular biology, Gilbert's syndrome has been investigated extensively. It has been found that UDP-glucuronosyltransferase 1A1 (UGT1A1) plays a critical role in the elimination pathway of bilirubin and defects in UGT1A1 result in the development of Gilbert's syndrome (3–5). Numerous mutations of the UGT1A1 gene, in the regulatory region and the coding region among others, have been detected to confirm the diagnosis of Gilbert's syndrome (6–10,12,21). According to previous studies, there are currently three common genotypes found in patients with Gilbert's syndrome: the T-3279G mutation in the phenobarbital responsive enhancer module (PBREM), the TA-insertion in the TATA box, creating the A(TA)7TAA motif instead of A(TA)6TAA and the G211A mutation in coding exon 1 of the UGT1A1 gene (11–13).
At present, the direct sequencing method is the principal approach used to detect Gilbert's syndrome in the clinical laboratory; however, the price of sequencing is expensive. In order to diagnose Gilbert's syndrome, it is important to establish a simple, effective and low cost method to detect mutations of the UGT1A1 gene. In this study, to the best of our knowledge, we applied the three-dimensional polyacrylamide gel-based DNA microarray method for the first time, in order to detect the T-3279G, A(TA)6/7TAA and G211A mutations of the UGT1A1 gene to confirm the diagnosis of Gilbert's syndrome.
Three-dimensional polyacrylamide gel-based DNA microarray hybridized with dual-color fluorescent probes is a rapid, simple and low coast approach used for gene mutation analysis. This method relies on the co-polymerization of acrylamide-modified PCR products with acrylamide monomers and acryl-modified slides to prepare the gel-based microarray. Acrylamide-modified PCR products from genomic DNA specimens are spotted and immobilized onto acrylamide-modified glass slides to fabricate a microarray. The slide is then transferred to a vacuum chamber with N,N,N',N'-tetramethyl-ethylenediamine (TEMED), so that TEMED is vaporized and diffuses into the spots to induce polymerization. Following hybridization with the specific probes labeled with Cy3 or Cy5, electrophoresis is performed to remove the non-specifically bound targets and mismatches. Through two-color fluorescent (green and red) scanning, images are captured to determine the genotype of each sample (14).
In order to correctly diagnose Gilbert's syndrome and to avoid side-effects from the adminstration of unecessary therapeutic agents, in this study, we established a novel technique (three-dimensional polyacrylamide gel-based DNA microarray) for the first time, to the best of our knowledge, in order to identify UGT1A1 gene mutations in 20 patients with hyperbilirubinemia from the Chinese population.
Patients and methods
Study participants and DNA isolation
Twenty Chinese patients with hyperbilirubinemia were recruited at the Second Hospital of Nanjing, Affiliated to the Medical School of Southeast University, (Nanjing, China). Peripheral blood samples were collected from all participants in the morning following an overnight fast. Total DNA was extracted using the QIAamp DNA Blood Midi kit (Qiagen, Hilden, Germany) according to the standard protocol. All the participants provided written informed consent prior to enrollment and all research procedures were approved by the Ethics Committee of the Second Hospital of Nanjing, Affiliated to the Medical School of Southeast University.
PCR amplification
A pair of primers F1, 5′-CACCTCCTCCTTATTCTCTT-3′ and R1, 5′-acrylamide-CTCATTCCTCCTCTCTAGCC-3′, whose design was based on published DNA sequences (GenBank no. AF297093.1), was used for PCR to obtain the PBREM region of the UGT1A1 gene. The cycling conditions were as follows: 94°C for 3 min, 32 cycles of 94°C for 30 sec, 54.2°C for 45 sec, and 72°C for 45 sec, and then 72°C for 10 min. The region containing the TATA-box and the 211 site of the UGT1A1 gene was generated by PCR using Ex Taq (Takara, Otsu, Japan) with two primers F2, 5′-CCCTGCTACCTTTGTGGACT-3′ and R2, 5′-acrylamide-CATTATGCCCGAGACTAACAAA-3′. The reaction conditions were as follows: 94°C for 3 min followed by 32 cycles of 94°C for 30 sec, 57°C for 45 sec, and 72°C for 45 sec, and then a final elongation step at 72°C for 10 min. Following agarose gel electrophoresis, the acrylamide-modified PCR products were processed by ethanol precipitation overnight at −20°C. The acrylamide-modified PCR products were subsquently harvested by centrifugation at 14,000 × g for 20 min and diluted in water.
Immobilization of acrylamide-modified PCR products
Preparation of the acrylamide-modified slides is the first step of PCR product immobilization. The protocol of acryl-modified slides fabrication was performed as previously described (15). Solutions containing acrylamide-modified PCR products, acrylamide monomer (29:1, acrylamide:bis-acrylamide), glycerol and ammonium persulfate (APS) were then prepared at the desired concentrations and spotted on the modified glass slide. After spotting, the slide was placed into a humid sealed chamber in which a well containing TEMED had been deposited in advance. The pressure in the sealed chamber was reduced to approximately 1,000 Pascal (Pa), and this pressure was maintained for 30 min at room temperature. Under this pressure, TEMED was vaporized and diffused into the spots and onto the slide surfaces to induce the co-polymerization of the acrylamide groups and the acryl groups.
Hybridization with the corresponding probes
Following the immobilization of the acrylamide-modified PCR products, double-stranded DNA (dsDNA) on the slide was denatured in 0.1 M sodium hydroxide solution for 10 min to obtain single-stranded DNA (ssDNA), and then subjected to electrophoresis in 1X TBE buffer for 10 min to remove sodium hydroxide. Finally, hybridization was performed in a humid glass chamber with the corresponding probes (Table I) at 37°C for 2 h. A schematic outline of the gel immobilization micro-array approach for high-throughput genotyping is illustrated in Fig. 1.
Table I.
Probe sequences used in this study.
| Probe | Probe sequences |
|---|---|
| −3279T | 5′-Cy3-TTCAGTTTGAACA-3′ |
| −3279G | 5′-Cy5-TTCAGTGTGAACA-3′ |
| A(TA)6TAA | 5′-Cy3-GCCATATATATATATATAAG-3′ |
| A(TA)7TAA | 5′-Cy5-GCCATATATATATATATATAAG-3′ |
| 211G | 5′-Cy3-AGAGACGGAGCAT-3′ |
| 211A | 5′-Cy5-AGAGACAGAGCAT-3′ |
Bold underlined characters indicate the test loci. Probe-3279T and probe-3279G are perfectly matched with the homozygous wild-type and the homozygous mutant of −3279 loci, respectively. Probe 211G and probe 211A are perfectly matched with the homozygous wild-type and the homozygous mutant of 211 loci respectively. Probe A(TA)6TAA and probe A(TA)7TAA are perfectly matched with homozygote A(TA)6TAA and homozygote A(TA)7TAA, respectively.
Figure 1.
A schematic outline of genotyping approach using dual-color fluorescence hybridization. (A) T-3279T homozygous wild-type; (B) T-3279G heterozygote; (C) G-3279G homozygous mutant.
Image scanning
Following hybridization, in order to remove the non-specifically bound targets and mismatches, the slide was subjected to electrophoresis under 38 V/cm for 25 min in 1X TBE buffer at room temperature. The slide was then rinsed in water and dried under a stream of nitrogen. Images of the hybridization slide were scanned using a confocal scanner (LuxScan-10K/A; CapitalBio Corp., Beijing, China) and analyzed with LuxScan 3.0 software.
Sequencing the PBREM region and the region containing the TATA-box and the 211 site of the UGT1A1 gene
Two pairs of primers F1 and R3, 5′-CTCATTCCTCCTCTCTAGCC-3′, and F2 and R4, 5′-CATTATGCCCGAGACTAACAAA-3′, were used for PCR to obtain the PBREM region and the region containing the TATA-box and the 211 site of the UGT1A1 gene, respectively. The PCR products were sequenced directly with the use of an BigDye Terminator v1.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA, USA) with the appropriate primers.
Results
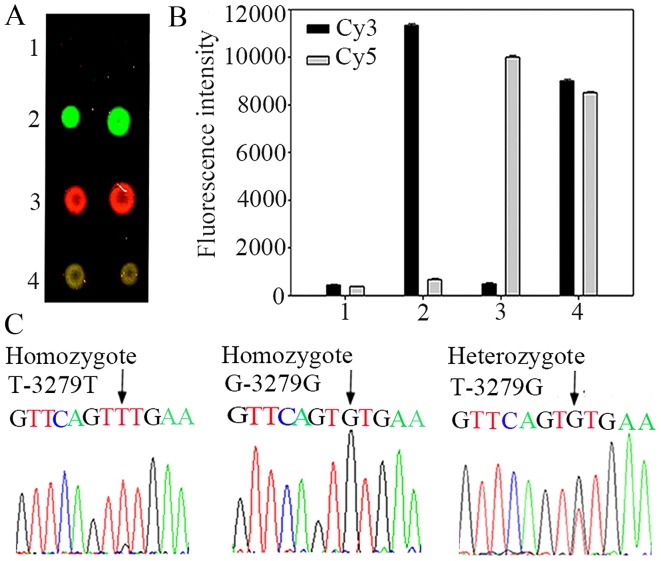
Firstly, the acrylamide-modified PCR products of different sizes were obtained and analyzed by electrophoresis on a 1% agarose gel (Fig. 2). In principle, the homozygous wild-type yielded strongly fluorescent Cy3 spots (green fluorescence), while the homozygous mutant yielded strongly fluorescent Cy5 spots (red fluorescence). Moreover, fluorescent Cy3 and Cy5 spots were shown for the heterozygote, and after overlap-ping, a strong 'yellow' fluorescence was shown.
Figure 2.

Agarose gel electrophoresis of PCR products. M, DNA marker; lane 1, the region containing TATA-box and 211 site of UGT1A1 gene; lane 2, gtPBREM region of the UGT1A1 gene. bp, base pairs; UGT1A1, UDP-glucuronosyltransferase 1A1; PBREM, phenobarbital responsive enhancer module.
For the T-3279T homozygote, the probe-3279T labeled with Cy3 was perfectly matched with the immobilized ssDNA, while the probe-3279G labeled with Cy5 had a mismatched base in the middle of the sequence to the ssDNA. Thus, only a Cy3 fluorescent signal (green fluorescence) was obtained in the dual-color fluorescence hybridization (Fig. 3A, line 2). The fluorescence scores of Cy3 and Cy5 in the homozygote T-3279T were 11,328 and 682, respectively, and Cy3/Cy5 was 17 (second row in Fig. 3B). In the same way, for the G-3279G homozygote, only the Cy5 fluorescent signal (red fluorescence) was shown (Fig. 3A, line 3). The fluorescence scores of Cy5 and Cy3 in the homozygote G-3279G were 10,027 and 515, respectively, and Cy5/Cy3 was 20 (Fig. 3B, third row). Moreover, for the T-3279G heterozygote, both the Cy3 and Cy5 fluorescent signals (green fluorescence and red fluorescence) were detected, and after overlapping, a strong 'yellow' fluorescence was shown (Fig. 3A, line 4). The fluorescence scores of Cy3 and Cy5 in the heterozygote T-3279G, were 9,106 and 8,544, respectively, and Cy3/Cy5 was 1.07 (Fig. 3B, fourth row). In order to evaluate the reliability of this technique, we compared the results obtained by sequencing (Fig. 3C).
Figure 3.
Hybridization results of samples with three genotypes (T-3279G) in UGT1A1 gene. (A) Line 1, blank control; line 2, homozygote T-3279T (green); line 3, homozygote G-3279G (red); line 4, heterozygote T-3279G (yellow). Each sample was spotted two times in a line. (B) Average relative fluorescence intensities. Row 1, blank control; row 2, homozygote T-3279T; row 3, homozygote G-3279G; row 4, heterozygote T-3279G. (C) Sequencing result of the PCR products with ABI Prism 377. The arrows indicate the tested loci. UGT1A1, UDP-glucuronosyltransferase 1A1.
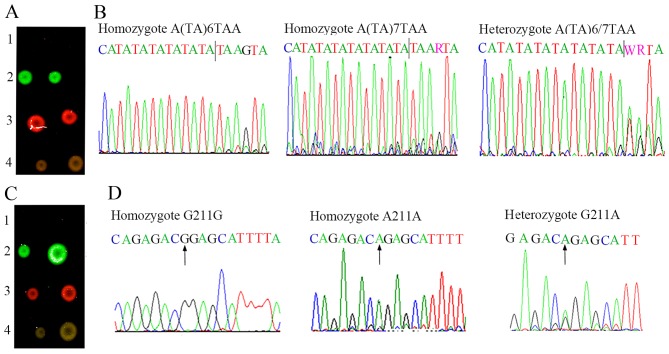
In the same way, for the A(TA)6TAA homozygote and G211G homozygote, only the Cy3 fluorescent signal (green fluorescence) was obtained in the dual-color fluorescence hybridization (Fig. 4A and C, line 2). For the A(TA)7TAA homozygote and A211A homozygote, only the Cy5 fluorescent signal (red fluorescence) was shown (Fig. 4A and C, line 3). Moreover, for the A(TA)6/7TAA heterozygote and G211A heterozygote, both the Cy3 and Cy5 fluorescent signals (green fluorescence and red fluorescence) were detected, and after overlapping, a strong 'yellow' fluorescence was shown (Fig. 4A and C, line 4). The above-mentioned results were further validated by sequencing (Fig. 4B and D).
Figure 4.
Hybridization results of A(TA)6/7TAA and G211A in UGT1A1 gene. (A) Line 1, blank control; line 2, homozygote A(TA)6TAA (green); line 3, homozygote A(TA)7TAA (red); line 4, heterozygote A(TA)6/7TAA (yellow). Each sample was spotted two times in a line. (B) Sequencing result of the PCR products with ABI Prism 377. (C) Line 1, blank control; line 2, homozygote G211G (green); line 3, homozygote A211A (red); line 4, heterozygote G211A (yellow). Each sample was spotted two times in a line. (D) Sequencing result of the PCR products with ABI Prism 377. The arrows indicate the tested loci. UGT1A1, UDP-glucuronosyltransferase 1A1.
Samples from 20 patients with hyperbilirubinemia were analyzed for the presence of the T-3279G locus, the TA-insertion locus (A(TA)6/7TAA) and the G211A locus. All possible genotypes of the 20 samples from the patients enlisted were successfully identified and are shown in Fig. 5. In addition, all results obtained by three-dimensional polyacrylamide gel-based DNA microarray method were further validated by sequencing.
Figure 5.
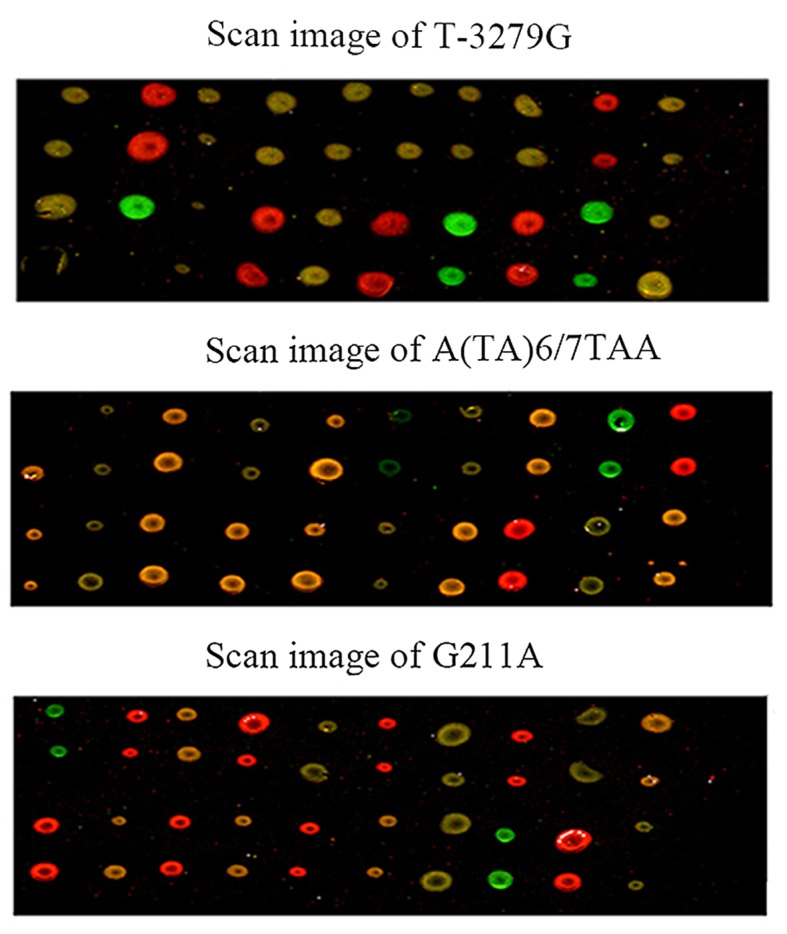
Microarray images from 20 samples assayed for the T-3279G, A(TA)6/7TAA and G211A of the UGT1A1 gene. Each sample was spotted two times in a line. The green spots indicate wild homozygous, the red spots indicate mutant homozygous, and the yellowspots indicate heterozygote. UGT1A1, UDP-glucu ronosyltransferase 1A1.
Discussion
Gilbert's syndrome is a mild genetic liver disorder characterized by unconjugated hyperbilirubinemia without overt signs of hemolysis or structural liver disease (1). Its estimated prevalence is approximately 3–7% in the general population (16). In general, Gilbert's syndrome is considered a benign condition and does not require therapy since it does not cause chronic liver dysfunction or fibrosis (17,18). However, this mild hyperbilirubinemia may be mistaken for hepatic jaundice, hemolytic jaundice or obstructive jaundice. Thus, patients may suffer from unwarranted anxiety and unexpected toxicity from therapeutic agents. For these reasons, it is important to make the correct diagnosis in time.
Currently, the direct sequencing method, the TaqMan MGB SNP genotyping assay, DNA melting curve analysis and the restriction fragment length polymorphism (RFLP) method have been used to detect mutations of the UGT1A1 gene and thereby diagnose Gilbert's syndrome (19–21). In this study, we established a novel method (three-dimensional polyacrylamide gel-based DNA microarray) for the first time, to the best of our knowledge, in order to detect UGT1A1 gene mutations in 20 patients with hyperbilirubinemia from the Chinese population.
The three-dimensional polyacrylamide gel-based DNA microarray method is a rapid, simple and low cost approach with which to carry out gene mutation analysis. It has been widely used in the genotyping of a number of genes, such as the oxidized low-density lipoprotein receptor 1 (OLR-1) gene, the brain-derived neurotrophic factor (BDNF) gene, and the gamma-aminobutyric acid receptor beta 3 subunit (GABRB3) gene (14,22,23). Three-dimensional polyacrylamide gel-based DNA microarray only requires a small quantity of expensive fluorescent-labeled probes which can be used for genotyping an unlimited number of samples. Furthermore, this method is time-saving and increases efficiency by assaying thousands of samples in one experiment.
Immobilization and electrophoresis are two critical steps in the three-dimensional polyacrylamide gel-based DNA microarray method. Firstly, immobilization relies on the co-polymerization of acrylamide-modified PCR products with acrylamide monomers and acryl-modified slides to prepare the gel-based microarray. Thus, in the present study, reverse primers (R1 and R2) were modified with an acrylamide group at the 5′-terminal in order to covalently attach to the polyacryl-amide gel. TEMED is a volatile alkali and is easily vaporized at room temperature. When the pressure in the sealed chamber was reduced to approximately 1,000 Pa, TEMED was vaporized and diffused into the spots and onto the slide surfaces to induce co-polymerization of the acrylamide groups and the acryl groups. Subsequently, the array was hybridized with specific fluorescent-labeled probes. The removal of the non-specifically bound targets and mismatches is the most important procedure. However, polyacrylamide gel has a porous structure which intensively adsorbs the non-specifically labeled probes during hybridization. Thus, the conventional washing steps fail to remove the non-specifically adsorbed probes, resulting in high background signals. As nucleic acids in PCR products carry the negative charges, electrophoresis is an effective method with which to effectively remove the non-specifically adsorbed probes. If the voltage is too high or the duration of electrophoresis is too long, specifical probes will be removed. Through repeated tests, the slide was subjected to electrophoresis under 38 V/cm for 25 min in 1X TBE buffer at room temperature to remove the non-specifically bound targets and mismatches. Finally, genotyping was based on the images captured through two-color fluorescent scanning. The T-3279T homozygote, the A(TA)6TAA homozygote and the G211G homozygote all yielded strong fluorescent Cy3 spots (green fluorescence), while the G-3279G homozygote, the A(TA)7TAA homozygote and the A211A homozygote all yielded strong fluorescent Cy5 spots (red fluorescence) (Figs. 3A and 4A and C). Moreover, both fluorescing the Cy3 and Cy5 spots were shown for the T-3279G heterozygote, the A(TA)6/7TAA heterozygote and the G211A heterozygote, and after overlapping, a strong 'yellow' fluorescence was shown.
In conclusion, in the present study, we successfully detected the UGT1A1 gene mutations in 20 Chinese patients with hyperbilirubinemia with the use of the three-dimensional polyacrylamide gel-based DNA microarray method. This method holds significant promise for future applications in the diagnosis of Gilbert's syndrome.
Acknowledgments
The present study was supported by the Medical Science and Technology Development Foundation, Nanjing Department of Health (no. JQX14007) and by the National Natural Science Foundation of China (no. 81301938).
References
- 1.Fretzayas A, Moustaki M, Liapi O, Karpathios T. Gilbert syndrome. Eur J Pediatr. 2012;171:11–15. doi: 10.1007/s00431-011-1641-0. [DOI] [PubMed] [Google Scholar]
- 2.Teich N, Lehmann I, Rosendahl J, Tröltzsch M, Mössner J, Schiefke I. The inverse starving test is not a suitable provocation test for Gilbert's syndrome. BMC Res Notes. 2008;1:35. doi: 10.1186/1756-0500-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosma PJ, Seppen J, Goldhoorn B, Bakker C, Oude Elferink RP, Chowdhury JR, Chowdhury NR, Jansen PL. Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J Biol Chem. 1994;269:17960–17964. [PubMed] [Google Scholar]
- 4.Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. N Engl J Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 5.Raijmakers MT, Jansen PL, Steegers EA, Peters WH. Association of human liver bilirubin UDP-glucuronyltransferase activity with a polymorphism in the promoter region of the UGT1A1 gene. J Hepatol. 2000;33:348–351. doi: 10.1016/S0168-8278(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 6.Sato H, Adachi Y, Koiwai O. The genetic basis of Gilbert's syndrome. Lancet. 1996;34:557–558. doi: 10.1016/S0140-6736(96)91266-0. [DOI] [PubMed] [Google Scholar]
- 7.Sugatani J, Yamakawa K, Yoshinari K, Machida T, Takagi H, Mori M, Kakizaki S, Sueyoshi T, Negishi M, Miwa M. Identification of a defect in the UGT1A1 gene promoter and its association with hyperbilirubinemia. Biochem Biophys Res Commun. 2002;292:492–497. doi: 10.1006/bbrc.2002.6683. [DOI] [PubMed] [Google Scholar]
- 8.Maruo Y, Sato H, Yamano T, Doida Y, Shimada M. Gilbert syndrome caused by a homozygous missense mutation (Tyr486Asp) of bilirubin UDP-glucuronosyltransferase gene. J Pediatr. 1998;132:1045–1047. doi: 10.1016/S0022-3476(98)70408-1. [DOI] [PubMed] [Google Scholar]
- 9.Maruo Y, Nishizawa K, Sato H, Doida Y, Shimada M. Association of neonatal hyperbilirubinemia with bilirubin UDP-glucuronosyltransferase polymorphism. Pediatrics. 1999;103:1224–1227. doi: 10.1542/peds.103.6.1224. [DOI] [PubMed] [Google Scholar]
- 10.Canu G, Minucci A, Zuppi C, Capoluongo E. Gilbert and Crigler Najjar syndromes: An update of the UDP-glucuronosyltransferase1A1 (UGT1A1) gene mutation database. Blood Cells Mol Dis. 2013;50:273–280. doi: 10.1016/j.bcmd.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Maruo Y, D'Addario C, Mori A, Iwai M, Takahashi H, Sato H, Takeuchi Y. Two linked polymorphic mutations (A(TA)7TAA and T-3279G) of UGT1A1 as the principal cause of Gilbert syndrome. Hum Genet. 2004;115:525–526. doi: 10.1007/s00439-004-1183-x. [DOI] [PubMed] [Google Scholar]
- 12.Matsui K, Maruo Y, Sato H, Takeuchi Y. Combined effect of regulatory polymorphisms on transcription of UGT1A1 as a cause of Gilbert syndrome. BMC Gastroenterol. 2010;10:57. doi: 10.1186/1471-230X-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalotychou V, Karakosta M, Tzanetea R, Stamoulakatou A, Konstantopoulos K, Rombos Y. Contribution of G71R mutation to Gilbert's syndrome phenotype in a Greek patient: a case report. World J Gastrointest Pharmacol Ther. 2011;2:42–45. doi: 10.4292/wjgpt.v2.i5.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao PF, Cheng L, Wan Y, Sun BL, Chen ZZ, Zhang SY, Zhang CZ, Zhou GH, Lu ZH. An improved gel-based DNA microarray method for detecting single nucleotide mismatch. Electrophoresis. 2006;27:3904–3915. doi: 10.1002/elps.200500918. [DOI] [PubMed] [Google Scholar]
- 15.Rehman FN, Audeh M, Abrams ES, Hammond PW, Kenney M, Boles TC. Immobilization of acrylamide-modified oligonu-cleotides by co-polymerization. Nucleic Acids Res. 1999;27:649–655. doi: 10.1093/nar/27.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YH, Yeon JE, Jung GM, Kim HJ, Kim JS, Byun KS, Bak YT, Lee CH. A study of polymorphism in UDP-glucuronosyltransferase 1 (UGT-1A1) promoter gene in Korean patients with Gilbert's syndrome. Taehan Kan Hakhoe Chi. 2002;8:132–138. In Korean. [PubMed] [Google Scholar]
- 17.Tukey RH, Strassburg CP. Human UDP-glucurono-syltransferases: Metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 18.Minucci A, Concolino P, Giardina B, Zuppi C, Capoluongo E. Rapid UGT1A1 (TA)(n) genotyping by high resolution melting curve analysis for Gilbert's syndrome diagnosis. Clin Chim Acta. 2010;411:246–249. doi: 10.1016/j.cca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Wong FL, Wang MK, Boo NY, Hamidah NH, Ainoon BO. Rapid detection of the UGT1A1 single nucleotide polymorphism G211A using real-time PCR with Taqman minor groove binder probes. J Clin Lab Anal. 2007;21:167–172. doi: 10.1002/jcla.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsieh TY, Shiu TY, Chu NF, Chao TY, Chu HC, Chang WK, Chao YC, Huang HH. Rapid molecular diagnosis of the Gilbert's syndrome-associated exon 1 mutation within the UGT1A1 gene. Genet Mol Res. 2014;13:670–679. doi: 10.4238/2014.January.28.12. [DOI] [PubMed] [Google Scholar]
- 21.Shiu TY, Huang HH, Lin HH, Shih YL, Chu HC, Chang WK, Hsieh TY. Restriction fragment length polymorphism effectively identifies exon 1 mutation of UGT1A1 gene in patients with Gilbert's syndrome. Liver Int. 2015;35:2050–2056. doi: 10.1111/liv.12785. [DOI] [PubMed] [Google Scholar]
- 22.Cheng L, Ge Q, Xiao P, Sun B, Ke X, Bai Y, Lu Z. Association study between BDNF gene polymorphisms and autism by three-dimensional gel-based microarray. Int J Mol Sci. 2009;10:2487–2500. doi: 10.3390/ijms10062487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang J, Xiao P. Polymerizing immobilization of acrylamide-modified nucleic acids and its application. Biosens Bioelectron. 2009;24:1817–1824. doi: 10.1016/j.bios.2008.09.018. [DOI] [PubMed] [Google Scholar]