Abstract
Oxidative stress plays an important role in the pathogenesis of various liver diseases. Safflower yellow B (SYB) has been reported to protect the brain against damage induced by oxidative stress; however, whether SYB can also protect hepatocytes from oxidative stress remains unknown. In the present study, to determine whether pre-treatment with SYB reduces hydrogen peroxide (H2O2)-induced oxidative stress in HepG2 cells, we investigated H2O2-induced oxidative damage to HepG2 cells treated with or without SYB. Cell viability was measured by MTT assay and cytotoxicity was evaluated by lactate dehydrogenase (LDH) assay. The activities of the antioxidant enzymes, glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) were determined using respective kits. Intracellular reactive oxygen species (ROS) accumulation in the HepG2 cells was monitored using the fluorescent marker, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA). Cell apoptosis was evaluated by determining the activity of caspase-3 and by Annexin V/propidium iodide (PI) double staining. Protein expression levels were measured by western blot analysis, and the levels of related cellular kinases were also determined. H2O2 induced pronounced injury to the HepG2 cells, as evidenced by increased levels of malondialdehyde (MDA) and ROS, the decreased activity of SOD and GSH-Px, the increased activitation of caspase-3 and cell apoptosis, and the loss of mitochondrial membrane potential. SYB significantly inhibited the damaging effects of H2O2, indicating that it protected the cells against H2O2-induced oxidative damage. Moreover, pre-treatment with SYB increased the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase 1 (HO-1) and NAD(P)H dehydrogenase, quinone 1 (NQO1) which are peroxiredoxins. SYB also significantly increased the phosphorylation of AKT. However, this inductive effect was blunted in the presence of the AKT inhibitor, LY294002. The findings of our study suggest that the activation of the AKT/Nrf2 pathway is involved in the cytoprotective effects of SYB against oxidative stress. Our findings provide new insight into the cytoprotective effects of SYB and the possible mechanisms underlying these effects. Thus, SYB may prove to be of therapeutic value for the treatment of various liver diseases.
Keywords: safflower yellow B, oxidative stress, HepG2 cells, Akt, nuclear factor erythroid 2-related factor 2
Introduction
Reactive oxygen species (ROS) and other free radicals are produced during normal cell metabolism, a necessary and normal process, and they play important physiological roles (1,2). However, under pathological conditions, the uncontrolled generation of ROS results in oxidative stress in cells, which subsequently leads to damage to different cellular structures, such as proteins, DNA and lipids (3). A proper balance between the formation of ROS and the antioxidant network is known to be essential for the regulation of biological processes. Clinical and experimental studies have suggested that oxidative stress is involved in the pathogenesis of a number of diseases (4,5). The liver, due to its high metabolic activity and its anatomical positioning to receive blood from the gastrointestinal tract, is vulnerable to toxicity from a variety of drugs and environmental contaminants. ROS-induced mechanisms have, for instance, been related to different chronic liver diseases and hepatocellular carcinoma (HCC), and are induced by various risk factors for liver cancer, such as hepatitis B and C or aflatoxin-B, and thus may be a possible driving force in hepatocarcinogenesis (6,7). Previous research has demonstrated that antioxidants are efficacious in preventing oxidative stress-related liver diseases and protecting cells from toxic insults (8). Therefore, there is increasing interest in the potential preventive/protective effects of exogenous antioxidants on oxidative stress-related hepatocellular disorders.
Carthamus tinctorius L (safflower), which belongs to the Compositae family, has long been used in Chinese medicine in clinical settings for the treatment of various diseases due to its antioxidant properties. The chemical constituents in safflower have been reported to include lignans, flavonoids, triterpene alcohols and polysaccharides (9–12). Previous research has indicated that the main effective constituent of safflower is safflower yellow (SY), which is a flavonoid. SY consists of hydroxysafflower yellow A (HSYA), safflower yellow A (SYA) and safflower yellow B (SYB), as well as other chemicals (13).
Safflower has long been used in the treatment of cardiovascular diseases, and its anti-myocardial ischemic effects are well known. Safflower also exerts other pharmacological effects, including anticoagulant, antioxidant, neuroprotective and calcium antagonist effects (14). However, which component is responsible for these protective effects remains largely unknown. As one of the main components of safflower, SYB has been extensively used in the treatment of cardiocerebro-vascular diseases in traditional Chinese medicine and has been shown to exert neuroprotective effects following permanent middle cerebral artery occlusion in rats (15). However, to the best of our knowledge, little research on the effects of SYB on liver preservation has been undertaken as of yet. Thus, the aim of the present study was to determine whether SYB is an effective component of safflower, and whether it can protect hepatocytes from oxidative damage. In our experiments, we used a control group treated with N-acetylcysteine (NAC, 200 µM, a frequently used antioxidant in clinical settings), to establish whether SYB has antioxidant potential.
Materials and methods
Materials
SYB (purity >98%) was purchased from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Dulbecco's modified Eagle's medium (DMEM) was obtained from Gibco Life Technologies (Grand Island, NY, USA) and the fetal bovine serum (FBS) we used was provided by Hangzhou Sijiqing Biological Engineering Materials Co., Ltd. (Hangzhou, China). NAC, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), type I collagenase and western blot reagents were purchased from Sigma (St. Louis, MO, USA). The kits for the determination of lactate dehydrogenase (LDH), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and caspase-3 activity were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Anti-β-actin primary (sc-7210), anti-AKT (sc-8312), anti-phosphorylated (p-)AKT (sc-33437), anti-heme oxygenase 1 (HO-1; sc-10789), anti-nuclear factor erythroid 2-related factor 2 (Nrf2; sc-7943) and anti-NAD(P)H dehydrogenase, quinone 1 (NQO1; sc-25591) antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The caspase-3 assay kit was purchased from Chemicon International, Inc. (Temecula, CA, USA). 9-[4-[Bis[2-[(acetyloxy)methoxy]-2-oxoethyl]amino]-3-[2-[2-[bis[2-[(acetyloxy)methoxy]-2-oxoethyl]amino]phenoxy] ethoxy]phenyl]-3,6-bis(dimethylamino)xanthylium bromide (Rhod-2 AM), DAPI, JC-1 and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) were purchased from Molecular Probes/Invitrogen (Carlsbad, CA, USA).
Cell lines and cell culture
The human hepatoma cell line, HepG2, was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in DMEM supplemented with 10% FBS, glucose, penicillin and streptomycin. The HepG2 cells were grown in 10-cm cell culture dishes and incubated in a humidified atmosphere containing 5% CO2 at 37°C. The cells were seeded at a density of 4×104 cells/ml in 96-well microplates for MTT assay, or 3×105 cells/ml in 6-well microplates for MDA activity assay, antioxidant enzyme assays and other biochemical indicator determinations. The culture medium was changed every second day.
Cell treatments
The HepG2 cells were pre-treated with SYB (50, 100 and 150 nmol/l) for 24 h and then exposed to hydrogen peroxide (H2O2; 200 µmol/l) for a further 6 h. Cells not exposed to H2O2 served as the controls, and cells exposed to H2O2 and not subjected to any treatments, served as the model group. In order to determine the ability of SYB to protect the cells from damage, the cells were also treated with NAC (200 µM), a well-characterized chemoprotective compound. In addition, in order to determine the role of AKT in the effects of SYB, the cells were treated with the AKT inhibitor, LY294002 (10 µM; purchased from Sigma).
Cell viability and cytotoxicity assays
Cell viability was determined by MTT assay. The cells were seeded at a density of 1×104 cells/well in 96-well plates. After the cells were subjected to the different treatments, 20 µl MTT solution (5 mg/ml) was added to each well and the final concentration at 5 mg/ml was maintained for 4 h at 37°C. Subsequently, the medium was removed and DMSO (150 ml) was added to each well. The optical density (OD) was determined spectrophotometrically at 490 nm using a microplate reader (Infinite M200 PRO; Tecan, Männedorf, Switzerland), and the cell survival ratio was expressed as a percentage of the control.
Cytotoxicity was evaluated by LDH leakage assay after collecting the culture medium, and the cells were scraped in phosphate-buffered saline (PBS) after the cells were subjected to the different treatments. The cells were first sonicated to ensure the cell membrane broke down to release the total amount of LDH; subsequently, centrifugation (1,000 × g for 15 min) to clear up the cell sample was undertaken. LDH leakage was estimated from the ratio between the LDH activity in the culture medium and that of the whole cell content.
Determination of intracellular ROS accumulation
Intracellular ROS accumulation in the HepG2 cells was monitored using the fluorescent marker, H2DCF-DA. Briefly, the HepG2 cells (1×105 cells/well) were seeded into 6-well plates and pre-treated with or without NAC or increasing concentrations of SYB (50, 100 and 150 nmol/l) for 24 h. Oxidative stress was induced by the addition of H2O2 to the culture medium for 30 min. At the end of the incubation period, the culture supernatant was removed and the cells were washed twice with PBS. H2DCF-DA (10 µM) was mixed with 500 µl DMEM and added to the culture plate. Following incubation for 30 min, the relative fluorescence intensity was quantified using a fluorescence spectrophotometer (Hidex Oy, Turku, Finland) at 485/535 nm (A485/535). The percentage of ROS generation was calculated as follows: (A485/535 of treated cells/A485/535 of untreated cells) ×100.
Measurement of GSH-Px and SOD activities
The activities of antioxidant enzymes were determined according to the instructions provided with the assay kits (Nanjing Jiancheng Bioengineering Institute). Briefly, after the cells were subjected to the different treatments, they were washed twice with PBS, resuspended in 1 ml 0.1 M phosphate buffer (pH 7.4) and homogenized. The homogenate was centrifuged at 2,200 × g for 10 min at 4°C, and the supernatants were collected following centrifugation for the following analyses. SOD activity was assayed at 560 nm on the basis of its ability to inhibit the oxidation of hydroxylamine via the superoxide anion from the xanthine oxidase system. GSH-Px activity was measured at 412 nm on the basis of the rate of oxidation of the reduced glutathione to oxidized glutathione by H2O2 under the catalyst, GSH-Px. The protein content in the cell lysate was determined using Coomassie blue staining solution (from Nanjing Jiancheng Bioengineering Institute).
Apoptosis
Apoptosis was evaluated by Annexin V/propidium iodide (PI) double staining assay. Briefly, the cells were trypsinized following a wash in PBS and resuspended in 400 µl binding buffer. Subsequently, 5 µl Annexin V-FITC and 5 µl PI (50 µM) working solution were added to every 200 µl cell suspension. The cells were incubated at room temperature for 20 min in the dark and analyzed by flow cytometry. Annexin V and PI emissions were detected in the FL1-H and FL2-H channels of a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) using emission filters of 525 and 575 nm, respectively. The number of each type of cells was expressed as percentages of the number of total stained cells. Data were acquired using CellQuest software and analyzed by ModFit software.
Apoptosis was also evaluated by examining the activation of caspase-3. The cells were lysed in buffer containing 5 mM Tris, pH 8, 20 mM ethylenediaminetetraacetic acid (EDTA) and 0.5% Triton X-100 (both from Sigma). The reaction mixture contained 20 mM HEPES, pH 7, 10% glycerol, 2 mM dithiothreitol and 30 µg protein per well, as well as 20 µM Ac-DEVD-AMC as substrate. The enzymatic activity was determined by measuring fluorescence at an excitation wavelength of 380 nm and an emission wavelength of 440 nm (BioTek Instruments, Inc., Winooski, VT, USA).
Protein extraction and western blot analysis
The cells were lysed with either mammalian protein extraction reagent or nuclear and cytoplasmic extraction reagent kits (Pierce Biotechnology, Rockford, IL, USA). Protein concentrations were determined using Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of protein samples (30 µg) were separated by 10% SDS-PAGE, and the separated proteins were transferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories) overnight. The transferred protein membranes were blocked with 5% non-fat dried milk for 30 min, followed by incubation with specific primary anti-bodies (β-actin, AKT, p-AKT, HO-1, Nfr2 and NQO1) overnight at 4°C, and either horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse antibodies for 1 h at 37°C. The blots were detected using VL Chemi-Smart 3000 (Viogene-Biotek, Sunnyvale, CA, USA) with enhanced chemiluminescence (ECL) western blotting reagent (Millipore, Billerica, MA, USA). β-actin was used as a loading control.
Measurement of mitochondrial membrane potential (ΔΨm)
ΔΨm in the HepG2 cells was evaluated by 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1) staining. The fluorescent dye, JC-1, exhibits potential dependent accumulation in mitochondria by a fluorescence emission shift from green (monomeric form, indicating depolarized/low ΔΨm) to red (aggregated form, indicating polarized/normal ΔΨm). In this study, the HepG2 cells (1×105 cells/well) were seeded in 35-mm dishes and pre-treated with or without increasing concentrations of SYB or NAC for 24 h. Oxidative stress was induced by the addition of H2O2 into the culture medium. At the end of the incubation period, the culture supernatant was removed and the cells were washed twice with PBS. The cells were incubated with JC-1 (Qcbio Science & Technologies Co., Ltd., Shanghai, China) staining liquid for 20 min at 37°C, washed 3 times with JC-1 staining buffer and examined under a confocal microscope (SP5-FCS; Leica Microsystems, Wetzlar, Germany).
Measurement of mitochondrial calcium
To follow H2O2-induced intramitochondrial calcium trafficking, mitochondrial calcium was estimated by co-incubating the cells with a mitochondria-permeant calcium fluorophore, Rhod-2 AM (2 µM). For confocal images, 2×104 cells were seeded onto a glass coverslip, pre-treated with SYB for 24 h, and then subjected to H2O2. Subsequently, the cells were stained with Rhod-2 AM (2 µM), fixed with 4% buffered paraformaldehyde, washed 3 times with PBS and mounted with antifade on a glass slide for image acquisition using a confocal microscope (SP5-FCS; Leica Microsystems). The untreated unstained cells served as the negative controls and untreated fluorophore-loaded cells served as the controls for background correction for the confocal experiments.
Statistical analysis
All values are expressed as the means ± SD. A one-way analysis of variance test, followed by Bonferoni's correction, was carried out to test for any differences between the mean values of all groups. A P-value <0.05 was considered to indicate a statistically significant difference.
Results
SYB protects cells against H2O2-induced cell damage
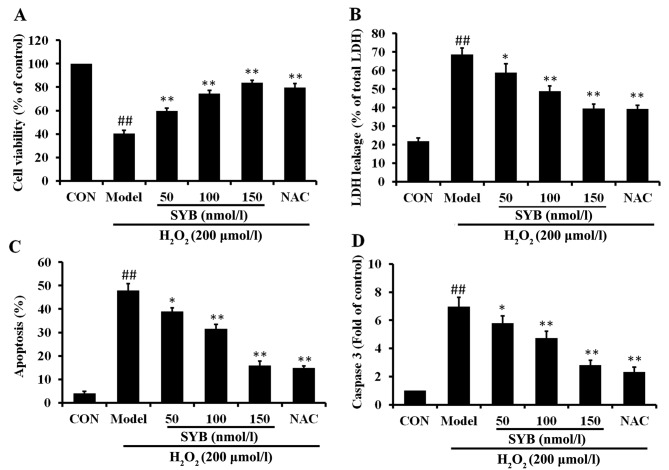
In order to elucidate the mechanisms responsible for the protective effects of SYB against H2O2-induced cell damage, the human hepatoma HepG2 cells were pre-treated with SYB (50, 100 and 150 nmol/l) for 24 h and then exposed to H2O2 (200 µmol/l) for a further 6 h. Concentrations of SYB which did not cause any measurable adverse effects to the cells (data not shown) were selected. The effects of pre-treatment with SYB on cell viability and cell integrity following exposure to H2O2 were determined by MTT assay and LDH leakage assay, respectively, and the ability of SYB to protect the cells from damage was compared to a well-characterized chemoprotective compound, NAC (200 µM). The results revealed that pre-treatment with SYB for 24 h protected the HepG2 cells from subsequent H2O2-induced damage in a concentration-dependent manner, as shown by the measurement of cell viability by MTT assay (Fig. 1A). Similarly, the LDH leakage assay revealed that treatment with SYB for 24 h prior to exposure to H2O2 decreased LDH leakage from the cells, indicating that SYB enabled the HepG2 cells to maintain cell integrity. The protective effects of SYB against LDH leakage also occurred in dose-dependent manner (Fig. 1B). The protective effects of SYB (150 nmol/l) were equal or greater to those exerted by NAC (200 µM).
Figure 1.
Effects of safflower yellow B (SYB) on H2O2-induced cell injury. (A) Cells were treated with SYB (50, 100 and 150 nmol/l) for 24 h before being treated with H2O2 (200 µmol/l), and cell viability was assayed by MTT assay. The results are expressed as percentages of the control. (B) HepG2 cells were incubated with or without SYB (50, 100 and 150 nmol/l) for 24 h before being treated with H2O2 (200 µmol/l), and lactate dehydrogenase (LDH) leakage (% of total LDH) was assayed as described in the Materials and methods. (C) Cell apoptosis was determined by Annexin V-FITC/propidium iodide (PI) double staining. (D) SYB attenuated the H2O2-induced changes in the caspase-3 levels. Data represent the means ± SD. ##P<0.01, statistically significant difference between model vs. control group; *P<0.05 and **P<0.01, statistically significant difference between SYB vs. model group.
To determine whether SYB exerts anti-apoptotic effects, Annexin V-FITC/PI double staining and caspase-3 assay were performed. The results of flow cytometric detection revealed that H2O2 induced a significant increase in the apoptosis of the HepG2 cells compared with the control group, and that apoptosis was markedly decreased by pre-treatment with SYB (Fig. 1C). As the sequential activation of caspases plays a central role in the execution phase of cell apoptosis (16), the activation of caspase-3 was examined in this study. Exposure of the HepG2 cells to H2O2 induced a significant increase in the level of caspase-3 compared to the control group; however, pre-treatment with SYB or NAC significantly decreased the level of caspase-3 (Fig. 1D). These results suggested that SYB protected the HepG2 cells against apoptosis induced by H2O2.
Effects of SYB on ΔΨm
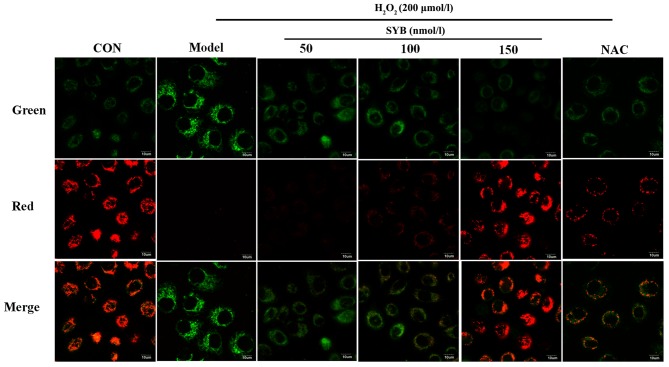
In many systems, apoptosis is associated with the loss of ΔΨm, which may be regarded as a limiting factor in the apoptotic pathway (17). Excessive ROS production, which leads to a decrease in ΔΨm, may also induce apoptotic cell death (18). In light of these facts, in this study, we assessed whether there was any reduction in ΔΨm in the H2O2-exposed cells using the potential-sensitive dye, JC-1. The exposure of the HepG2 cells to H2O2 for 6 h resulted in a decrease in the ratio of red to green fluorescence, as detected by confocal microscopy when compared to the control cells, and treatment with SYB or NAC restored this ratio (Fig. 2).
Figure 2.
Effects of safflower yellow B (SYB) on mitochondrial membrane potential. Morphology of HepG2 cells following incubation with H2O2 for 6 h with or without pre-treatment with SYB (50, 100 and 150 nmol/l) or N-acetylcysteine (NAC; 200 µM) for 24 h.
SYB reduces H2O2-dependent intracellular ROS production and MDA formation and increases antioxidant enzyme activity
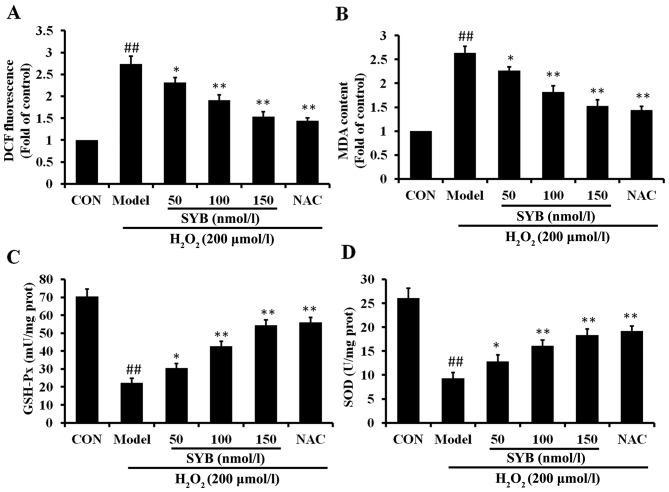
To investigate the mechanisms through which SYB protects cells against H2O2-induced damage, the effects of pre-treatment with SYB on the intracellular ROS levels were determined. The HepG2 cells were pre-treated with SYB for 24 h, and then exposed to 200 µmol/l H2O2 for 6 h, and the ROS levels were measured by DCF fluorescence. The results revealed that exposure to H2O2 induced a rapid and significant increase in the intracellular ROS levels, when compared to the untreated controls (Fig. 3A). Pre-treatment of the cells with SYB for 24 h significantly decreased the H2O2-induced production of intra-cellular ROS compared with the H2O2-exposed cells not treated with SYB (model group; P<0.05). To examine the effects of SYB on H2O2-induced lipid peroxidation, the level of MDA in the cells was measured. Exposure of the HepG2 cells to H2O2 markedly increased the intracellular MDA levels (Fig. 3B). When the cells were pre-treated with SYB, the increase in the MDA levels induced by H2O2 was significantly prevented in a dose-dependent manner.
Figure 3.
Safflower yellow B (SYB) reduces H2O2-dependent intracellular reactive oxygen species (ROS) production and malondialdehyde (MDA) formation and increases antioxidant enzyme activity. (A) Cells were treated with SYB (50, 100 and 150 nmol/l) or N-acetylcysteine (NAC; 200 µM) for 24 h before being treated with H2O2 (200 µmol/l), and then 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA; 10 µM) was mixed with 500 µl DMEM and added to the culture plate. Following incubation for 30 min, the relative fluorescence intensity was quantified using a fluorescence spectrophotometer. (B) SYB attenuated the H2O2-induced changes in the MDA content. Effects of SYB on the activities of (C) GSH-Px and (D) superoxide dismutase (SOD) in HepG2 cells. Data represent the means ± SD. ##P<0.01, statistically significant difference between model vs. control group; *P<0.05 and **P<0.01, statistically significant difference between SYB vs. model group.
GSH-Px and SOD activity was measured as an index of the enzymatic antioxidant defense system in HepG2 cells during oxidative stress. The HepG2 cells were exposed to 200 µmol/l H2O2 and either pre-treated or not with SYB or NAC. The results revealed that exposure to H2O2 markedly decreased the GSH-Px and SOD levels compared to the control group. More significantly, the results revealed that pre-treatment with SYB increased the GSH-Px and SOD levels, which were decreased following exposure to H2O2 (Fig. 3C and D). These data suggest that SYB exerts its protective effects by enhancing the the anti-oxidant capability of the cells.
Effects of SYB on Nrf2-mediated antioxidant defense system proteins in HepG2 cells
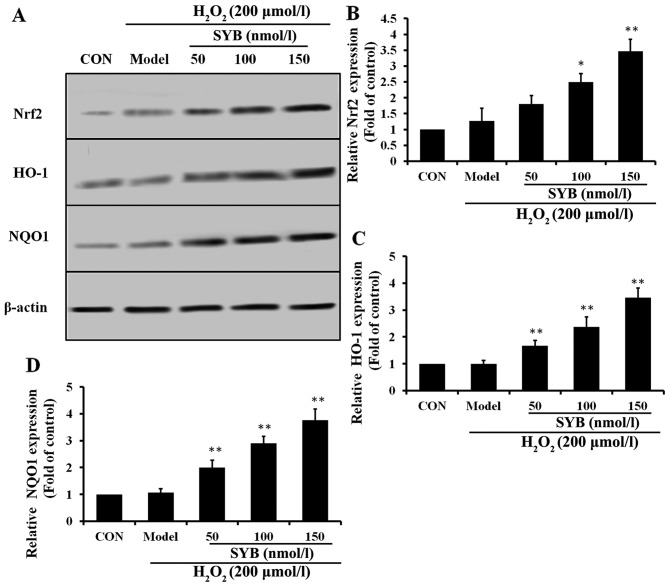
To examine the effects of SYB on Nrf2 activity, we first treated the HepG2 cells with various concentrations of SYB for 24 h and then examined the dose-response effects of SYB on the expression of Nrf2. Pre-treatment with SYB gradually increased cytosolic Nrf2 expression in a dose-dependent manner (Fig. 4A and B). Activated Nrf2 binds to antioxidant response element (ARE) sites and causes the upregulation of its target genes (19). It is well known that HO-1 and NQO1 are two major antioxidant enzymes that play an important role in H2O2-induced antioxidant defense in hepatic cells (20). Thus, we hypothesized that the inhibitory effects of SYB on H2O2-induced hepatic enzyme leakage and/or the augmentation of GSH-Px and SOD levels result from the induction of antioxidant genes, such as HO-1 and NQO1. As expected, we observed that SYB significantly increased the HO-1 and NQO1 expression levels in the H2O2-exposed HepG2 cells in a dose-dependent manner (Fig. 4C and D). These data clearly indicate that the cytoprotective effects of SYB are mediated through Nrf2.
Figure 4.
Effects of safflower yellow B (SYB) on nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated antioxidant defense proteins in HepG2 cells. Cells were treated with SYB (50, 100 and 150 nmol/l) for 24 h prior to treatment with H2O2 (200 µmol/l). Total cell lysates were prepared and subjected to western blot analysis to monitor the expression levels of antioxidant proteins, namely Nrf2, heme oxygenase 1 (HO-1) and NAD(P)H dehydrogenase, quinone 1 (NQO1). β-actin served as an internal control. (A) Western blot analysis of Nrf2, HO-1 and NQO1 expression. Quantitative representations of western blot analysis of (B) Nrf2, (C) HO-1 and (D) NQ01. Data represent the means ± SD. *P<0.05 and **P<0.01, statistically significant difference between SYB vs. model group.
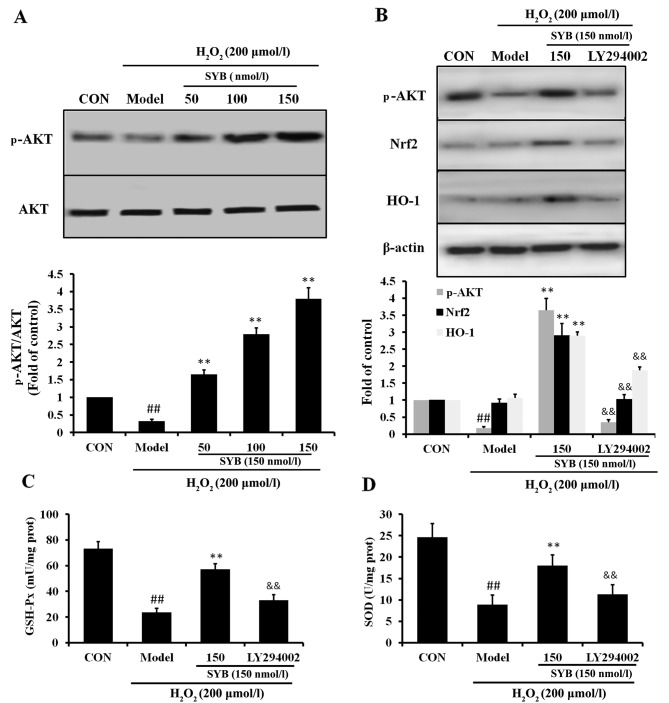
Role of AKT in the SYB-induced upregulation of Nrf2 and HO-1
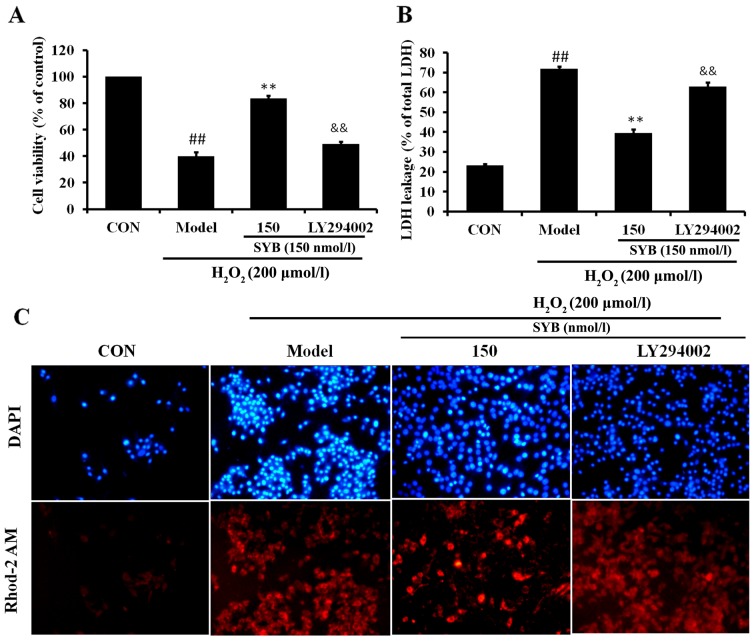
It has previously been noted that AKT is involved in the induction of Nrf2/ARE-driven gene expression (21). Thus, in order to identify which signaling cascade controls Nrf2 activation and the induction of HO-1 expression in the SYB-treated HepG2 cells, the activation of the AKT pathway was investigated. As shown in Fig. 5A, in the HepG2 cells exposed to H2O2 (model group), the reduced activation of AKT was observed; however, treatment with SYB significantly increased the phosphorylation of AKT in a dose-dependent manner. Subsequently, we examined whether SYB enhances Nrf2 expression through the AKT pathway. Treatment of the cells with the AKT inhibitor, LY294002, suppressed the SYB-induced phosphorylation of AKT in the HepG2 cells exposed to H2O2. Moreover, the inhibition of the AKT pathway by LY294002 markedly reduced the capacity of SYB to increase Nrf2 and HO-1 expression (Fig. 5B), as well as the GSH-Px and SOD levels (Fig. 5C and D). Consistently, the inhibition of AKT by LY294002 also eliminated the SYB-induced cytoprotective effects against H2O2-induced cell death; the cell viability rate decreased, LDH leakage increased and calcium overload was noted in the mitochondria following treatment with LY294002 (Fig. 6). These results suggest that SYB induces the activation of Nrf2/HO-1 and the antioxidant defense machinery by activating the AKT pathway.
Figure 5.
Role of AKT in safflower yellow B (SYB)-induced nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase 1 (HO-1) upregulation. (A) Effects of SYB (50, 100 and 150 nmol/l) on AKT protein expression in HepG2 cells exposed to H2O2 (200 µmol/l). (B) Role of AKT in SYB-induced Nrf2 expression. Cells were pre-treated with SYB (150 nmol/l) or LY294002 (10 µM) for 24 h, then exposed to H2O2 (200 µmol/l). Total cell lysates were prepared and subjected to western blot analysis. β-actin was used as a loading control. (C) GSH-Px and (D) superoxide dismutase (SOD) levels were measured using respective kits. Data represent the means ± SD. ##P<0.01, statistically significant difference between model vs. control group; **P<0.01, statistically significant difference between SYB vs. model group; &&P<0.01, statistically significant difference between LY294002 vs. SYB group.
Figure 6.
Effects of safflower yellow B (SYB) and LY294002 on cell viability, cell integrity and calcium overload in HepG2 cells. Cells were pre-treated with SYB (150 nmol/l) or LY294002 (10 µM) for 24 h, then exposed to H2O2 (200 µmol/l). (A) Cell viability was assayed by MTT assay. (B) Lactate dehydrogenase (LDH) leakage (% of total LDH) was assayed as described in the Materials and methods. (C) The representative staining for Rhod-2 AM (red signal, specific for mito-chondrial calcium) and DAPI (blue signal) are shown by confocal microscopy. The image panels are representative of the best of 3 independent image acquisitions. ##P<0.01, statistically significant difference between model vs. control group; **P<0.01, statistically significant difference between SYB vs. model group; &&P<0.01, statistically significant difference between LY294002 vs. SYB group.
Discussion
Oxidative stress is an abnormal phenomenon that occurs inside the human body when the production of free radicals exceeds the antioxidant capacity. The excessive production of free radicals and other ROS damages essential macromolecules of the cell, leading to the development of a number of human diseases, including atherosclerosis, rheumatoid arthritis, inflammation, cancer and neurodegenerative diseases (22). Oxidative stress associated with the formation of ROS plays an important role in the pathogenesis of hepatic ischaemia/reperfusion (I/R) injury. Previous research has indicated that although ROS are generated mainly as by-products of mitochondrial respiration (as the primary cellular consumer of oxygen, together with multiple electron carriers and redox enzymes), they are themselves extremely susceptible to oxidative damage (23). The overproduction of ROS leads to lipid peroxidation, damage to the mitochondrial membrane, the release of mitochondrial apoptogenic factors into the cytoplasm, followed by caspase activation and finally, cell apoptosis (24). Natural antioxidants that inhibit ROS generation are considered essential in terms of protecting the liver from chemical- or I/R-induced damage. Consequently, previous research has focused on natural anti-oxidants that have chemopreventive properties and on their mechanisms of action (25).
In a previous study, it was demonstrated that SYB is able to significantly attenuate brain injury induced by ischemia, and it was pointed out that SYB exerts neuroprotective effects against brain injury induced by ischemia by increasing the activities of antioxidant enzymes in the brain tissue, thus decreasing free radical generation and improving mitochondrial function (15). However, to the best of our knowledge, there are no studies available to date on the effects of SYB on I/R- or H2O2-induced oxidative stress in liver cells. Thus, in the present study, we examined the inhibitory effects of SYB on H2O2-induced injury to HepG2 cells. The results revealed that the exposure of HepG2 cells to H2O2 significantly decreased cell viability and increased the release of LDH, and pre-treatment of the cells with SYB increased cell viability and reduced the release of LDH, implying that SYB has the ability to protect HepG2 cells from H2O2-induced injury. In addition, we established that the ability of SYB to modulate important proteins of the cell signaling cascades is directly involved in its protective effects.
Oxidative stress-induced cell injury is associated with increases in ROS, such as H2O2, hydroxyl radicals, superoxide anion, singlet oxygen, nitric oxide and peroxynitrites (26). Lipid peroxidation of polyunsaturated fatty acid produces ROS and toxic aldehydes, such as 4-hydroxy-2-nonenal (4-HNE) and MDA (27). Therefore, the concentration of MDA in cells or tissue lysates is considered to be a major cause of lipid peroxidation. In this study, we demonstrated that exposure to H2O2 induced the overproduction of ROS in hepatic cells and led to oxidative stress; however, treatment with SYB significantly suppressed H2O2-induced ROS generation and lipid peroxidation, possibly due to the powerful antioxidant and free radical scavenging activity of SYB.
It has previously been noted that oxidants not only stimulate inflammatory cytokines in HepG2 cells and cause cellular senescence, but also induce the apoptosis of HepG2 cells, and certain apoptotic agents increase the production of ROS in mitochondria (28), and antioxidants such as NAC and vitamin E prevent cell apoptosis. Apoptosis is programmed cell death and it plays an important role in embryogenesis, metamorphosis and cellular homeostasis (29). In this study, we discovered that H2O2 induced the apoptosis of HepG2 cells using flow cytometry. SYB markedly decreased the apoptosis induced by H2O2 in a dose-dependent manner, and these results were verified by determining the caspase-3 levels.
Mitochondria are an important source of ROS within the majority of mammalian cells. Excessive ROS production contributes to mitochondrial damage in a wide range of pathologies (30). Damaged mitochondria can then cause defects in lipid homeostasis, bioenergetics and ROS production, leading to lipid accumulation and oxidative stress (31). Increasing evidence suggests that ΔΨm assay can be used as a more specific test for early mitochondrial injury (32). In the present study, using JC-1, we revealed that H2O2 induced the loss of ΔΨm in HepG2 cells. Pre-treatment with SYB for 24 h restored the ΔΨm to the basal level. These findings implied that the ability of SYB to attenuate oxidative stress partly depends on inhibiting mitochondrial-related apoptosis.
Oxidative stress caused by ROS is responsible for a wide variety of cellular damage and is the most validated mechanism of secondary injury (33). Following oxidative stress, the overproduction of ROS, and subsequently the depletion of antioxidants, results in the total breakdown of the endogenous antioxidant defense mechanisms, culminating in failure to protect cells from damage induced by oxidative stress. SOD is an oxygen radical scavenger that scavenges superoxide radicals by converting them into H2O2, which is then converted to water by catalase and GSH-Px (34). In our study, exposure of the HepG2 cells to H2O2 induced a marked decrease in the concentration of SOD and GSH-Px, which was reversed by pre-treatment with SYB for 24 h.
Nrf2, a basic leucine zipper (bZIP) transcription factor that belongs to the cap'n'collar (CNC) family, plays an important role in the transcriptional regulation of phase II enzymes (35). The Nrf2-activating pathway provides a rapid feedback triggered mechanism through which the cell is challenged by oxidative stress (36). Upon stimulation, Nrf2 disassociates from its cytosolic inhibitor, Keap-1, and translocates into the nucleus, then binds to the ARE in the promoter regions of many phase II enzymes, including HO-1 and NQO1 (37). As the Nrf2/ARE pathway has been shown to provide protection against several oxidative impacts in different organs, the induction of HO-1 and NQO1 under the regulation of Nrf2/ARE may provide a therapeutic option for liver diseases in cases of severe oxidative stress (38). We hypothesized that the increased protein expression level of HO-1 and NQO1 was due to activation of the Nrf-2 signaling pathway. As expected, SYB had the effect of activating Nrf2, which led to the increased protein expression of HO-1 and NQO1.
Previous research has demonstrated that a wide variety of phytochemicals from natural products, such as but in and 3′,4′-dide-methylnobiletin protect against oxidative stress-induced cell damage through the PI3K/AKT/Nrf2-dependent pathway (39). Therefore, we investigated whether that pathway contributes to the protective effects of SYB against H2O2-induced oxidative stress. Importantly, the phosphorylation of AKT was significantly increased in the SYB-treated cells, and the inhibition of AKT signaling by the AKT inhibitor, LY294002, blocked the SYB-induced protein expression of Nrf2 and HO-1 and reduced the expression level of GSK-Px and SOD. Further analysis also indicated that LY294002 abolished the ability of SYB to control the calcium levels which were significantly increased by H2O2. These results indicate that AKT/Nrf2 signaling is involved in the cytoprotective effects of SYB.
In conclusion, the findings of this study demonstrate that pre-treatment with SYB leads to the protection of hepatic cells against H2O2-induced oxidative stress through a mechanism that involves Nrf2 activation and the upregulation of the expression of its downstream antioxidant genes, mediated by AKT. These results provide a scientific basis for the hepatoprotective effects of pure compound SYB derived from Safflower and suggest that it may be of therapeutic value in the treatment of various liver diseases associated with oxidative stress.
Acknowledgments
This study was supported by the National Science Foundation of China (nos. 81173514 and 81302695).
References
- 1.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44:275–295. doi: 10.1080/10408690490468489. [DOI] [PubMed] [Google Scholar]
- 2.Cui K, Luo X, Xu K, Ven Murthy MR. Role of oxidative stress in neurodegeneration: Recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:771–799. doi: 10.1016/j.pnpbp.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 3.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitar MS, Al-Saleh E, Al-Mulla F. Oxidative stress - mediated alterations in glucose dynamics in a genetic animal model of type II diabetes. Life Sci. 2005;77:2552–2573. doi: 10.1016/j.lfs.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Coskun O, Ocakci A, Bayraktaroglu T, Kanter M. Exercise training prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Tohoku J Exp Med. 2004;203:145–154. doi: 10.1620/tjem.203.145. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 7.Tien Kuo M, Savaraj N. Roles of reactive oxygen species in hepatocarcinogenesis and drug resistance gene expression in liver cancers. Mol Carcinog. 2006;45:701–709. doi: 10.1002/mc.20240. [DOI] [PubMed] [Google Scholar]
- 8.Zhu R, Wang Y, Zhang L, Guo Q. Oxidative stress and liver disease. Hepatol Res. 2012;42:741–749. doi: 10.1111/j.1872-034X.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 9.Palter R, Lundin RE, Haddon WF. A cathartic lignan glycoside isolated from Carthamus tinctorius. Phytochemistry. 1972;11:2871–2874. doi: 10.1016/S0031-9422(00)86527-9. [DOI] [Google Scholar]
- 10.Kazuma K, Takahashi T, Sato K, Takeuchi H, Matsumoto T, Okuno T. Quinochalcones and flavonoids from fresh florets in different cultivars of Carthamus tinctorius L. Biosci Biotechnol Biochem. 2000;64:1588–1599. doi: 10.1271/bbb.64.1588. [DOI] [PubMed] [Google Scholar]
- 11.Akihisa T, Yasukawa K, Oinuma H, Kasahara Y, Yamanouchi S, Takido M, Kumaki K, Tamura T. Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. Phytochemistry. 1996;43:1255–1260. doi: 10.1016/S0031-9422(96)00343-3. [DOI] [PubMed] [Google Scholar]
- 12.Wakabayashi T, Hirokawa S, Yamauchi N, Kataoka T, Woo JT, Nagai K. Immunomodulating activities of polysaccharide fractions from dried safflower petals. Cytotechnology. 1997;25:205–211. doi: 10.1023/A:1007947329496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan JL, Wang JW, Guan Y, Yin Y, Wei G, Cui J, Zhou D, Zhu YR, Quan W, Xi MM, Wen AD. Safflor yellow A protects neonatal rat cardiomyocytes against anoxia/reoxygenation injury in vitro. Acta Pharmacol Sin. 2013;34:487–495. doi: 10.1038/aps.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng WC, Chen DB, Li B, Zhang L. The preventive effects of safflor yellow on myocardium injury of myocardium ischemic reperfusion in rats. Chin Pharmacol Bull. 2003;19:1032–1034. [Google Scholar]
- 15.Wang C, Zhang D, Li G, Liu J, Tian J, Fu F, Liu K. Neuroprotective effects of safflor yellow B on brain ischemic injury. Exp Brain Res. 2007;177:533–539. doi: 10.1007/s00221-006-0705-2. [DOI] [PubMed] [Google Scholar]
- 16.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwong JQ, Henning MS, Starkov AA, Manfredi G. The mitochondrial respiratory chain is a modulator of apoptosis. J Cell Biol. 2007;179:1163–1177. doi: 10.1083/jcb.200704059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639–645. doi: 10.1016/j.tips.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Campbell MR, Karaca M, Adamski KN, Chorley BN, Wang X, Bell DA. Novel hematopoietic target genes in the NRF2-mediated transcriptional pathway. Oxid Med Cell Longev. 2013;2013:120305. doi: 10.1155/2013/120305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu D, Hu L, Xia X, Song J, Li L, Song E, Song Y. Tetrachloro-benzoquinone induces acute liver injury, up-regulates HO-1 and NQO1 expression in mice model: the protective role of chlorogenic acid. Environ Toxicol Pharmacol. 2014;37:1212–1220. doi: 10.1016/j.etap.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Lee YJ, Jeong HY, Kim YB, Lee YJ, Won SY, Shim JH, Cho MK, Nam HS, Lee SH. Reactive oxygen species and PI3K/Akt signaling play key roles in the induction of Nrf2-driven heme oxygenase-1 expression in sulforaphane-treated human meso-thelioma MSTO-211H cells. Food Chem Toxicol. 2012;50:116–123. doi: 10.1016/j.fct.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Okezie IA. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc. 1998;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr. 2004;36:347–352. doi: 10.1023/B:JOBB.0000041766.71376.81. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh M, Manna P, Sil PC. Protective role of a coumarin-derived schiff base scaffold against tertiary butyl hydroperoxide (TBHP)-induced oxidative impairment and cell death via MAPKs, NF-κB and mitochondria-dependent pathways. Free Radic Res. 2011;45:620–637. doi: 10.3109/10715762.2011.564166. [DOI] [PubMed] [Google Scholar]
- 25.Masella R, Di Benedetto R, Varì R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16:577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 29.Renehan AG, Booth C, Potten CS. What is apoptosis, and why is it important? BMJ. 2001;322:1536–1538. doi: 10.1136/bmj.322.7301.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serviddio G, Bellanti F, Sastre J, Vendemiale G, Altomare E. Targeting mitochondria: a new promising approach for the treatment of liver diseases. Curr Med Chem. 2010;17:2325–2337. doi: 10.2174/092986710791698530. [DOI] [PubMed] [Google Scholar]
- 32.Jiang J, Yu S, Jiang Z, Liang C, Yu W, Li J, Du X, Wang H, Gao X, Wang X. N-acetyl-serotonin protects HepG2 cells from oxidative stress injury induced by hydrogen peroxide. Oxid Med Cell Longev. 2014;2014:310504. doi: 10.1155/2014/310504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang CC, Fang KM, Yang CS, Tzeng SF. Reactive oxygen species-induced cell death of rat primary astrocytes through mitochondria-mediated mechanism. J Cell Biochem. 2009;107:933–943. doi: 10.1002/jcb.22196. [DOI] [PubMed] [Google Scholar]
- 34.Kondo T, Higashiyama Y, Goto S, Iida T, Cho S, Iwanaga M, Mori K, Tani M, Urata Y. Regulation of gamma-glutamyl-cysteine synthetase expression in response to oxidative stress. Free Radic Res. 1999;31:325–334. doi: 10.1080/10715769900300891. [DOI] [PubMed] [Google Scholar]
- 35.Ryter SW, Alam J, Choi AMK. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 36.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339:79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 37.Kay HY, Won Yang J, Kim TH, Lee da Y, Kang B, Ryu JH, Jeon R, Kim SG. Ajoene, a stable garlic by-product, has an antioxidant effect through Nrf2-mediated glutamate-cysteine ligase induction in HepG2 cells and primary hepatocytes. J Nutr. 2010;140:1211–1219. doi: 10.3945/jn.110.121277. [DOI] [PubMed] [Google Scholar]
- 38.Na HK, Kim EH, Jung JH, Lee HH, Hyun JW, Surh YJ. (−)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch Biochem Biophys. 2008;476:171–177. doi: 10.1016/j.abb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Su JD, Yen JH, Li S, Weng CY, Lin MH, Ho CT, Wu MJ. 3′,4′-Didemethylnobiletin induces phase II detoxification gene expression and modulates PI3K/Akt signaling in PC12 cells. Free Radic Biol Med. 2012;52:126–141. doi: 10.1016/j.freeradbiomed.2011.10.002. [DOI] [PubMed] [Google Scholar]