Abstract
BACKGROUND AND OBJECTIVES:
Childhood metabolic syndrome (MetS) is a risk factor for adverse outcomes later in life. Our goal was to identify temporal trends among US adolescents in the severity of MetS, its individual components, and factors related to diet and physical activity.
METHODS:
We analyzed 5117 participants aged 12 to 19 from NHANES. We used regression analysis of individual waves of data, 1999 to 2012. MetS severity was calculated using a gender- and race/ethnicity-specific MetS severity z score.
RESULTS:
There was a linear trend of decreasing MetS severity in US adolescents from 1999 to 2012 (P = .030). This occurred despite a trend of increasing BMI z score (P = .005); instead, the decrease in MetS severity appeared to be due to trends in increasing high-density lipoprotein (HDL; P < .0001) and decreasing triglyceride (P = .0001) levels. In considering lifestyle factors, there was no change in physical activity over the time period. Regarding dietary patterns, total calorie consumption and carbohydrate consumption were positively associated with triglyceride levels and negatively associated with HDL levels, whereas unsaturated fat consumption exhibited the opposite associations. Consistent with these associations, there was a trend of decreasing total calorie consumption (P < .0001), decreasing carbohydrate consumption (P < .0001), and increasing unsaturated fat consumption (P = .002).
CONCLUSIONS:
The healthier trend of declining MetS severity in adolescents appeared to be due to favorable increases in HDL and decreases in fasting triglyceride measurements. These were in turn associated with favorable changes in dietary patterns among US adolescents. Future studies should investigate the causality of dietary differences on changes in MetS severity in adolescents.
What’s Known on This Subject:
The prevalence of the metabolic syndrome (MetS) has recently appeared to be decreasing in adults. There has been a plateauing of obesity in adolescents, but temporal trends of the MetS prevalence and severity in this age range are unclear.
What This Study Adds:
The severity of MetS decreased in adolescents from 1999 to 2012 because of decreases in abnormalities in high-density lipoprotein cholesterol and triglycerides. Over the same time frame, there were improvements in dietary intake, potentially related to these improvements.
The metabolic syndrome (MetS) is characterized by central obesity, high fasting glucose, high fasting triglycerides, high blood pressure (BP), and low high-density lipoprotein (HDL). Traditional MetS criteria, such as those developed for adults by the National Cholesterol Education Program Adult Treatment Panel (ATP-III)1 or adolescent adaptations,2 define MetS diagnosis as presence of at least 3 abnormalities among these 5 clinical components. MetS is a priority to characterize because it is a significant risk factor for cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM).3–7
However, the binary nature of the ATP-III MetS diagnostic criteria has limitations in epidemiologic studies. The ATP-III criteria give no indication of disease severity, limiting their ability to track MetS severity over time.8 The ATP-III criteria also do not account for differences in MetS by race/ethnicity and gender, resulting in MetS underdiagnosis in specific groups, such as non-Hispanic blacks.9–14 This is a concern because non-Hispanic-black individuals have higher rates of T2DM and CVD despite low rates of ATP-III MetS diagnosis.15–19
The recently developed MetS Severity Score (MetS z score) calculator addresses the epidemiologic limitations of the ATP-III criteria.20,21 This tool accounts for differences in MetS by race/ethnicity and gender. It is reflective of severity, with a higher score correlating to more severe MetS and increased odds for associated risk factors.20,21 Childhood MetS z score elevations are associated with risk for adult T2DM and CVD.22,23
Recent reports have shown a decrease in the prevalence of MetS among US adults, attributed to increased awareness and pharmaceutical treatment of the individual components of MetS.24,25 The goal of the current study was to investigate for trends in MetS prevalence (by using modified ATP-III criteria) and severity (using MetS z scores20) in US adolescents. Our hypothesis was that the decreasing trends observed in adults would not be observed in US adolescents because of the persisting childhood obesity epidemic.26,27 Our secondary goal was to evaluate for trends in physical activity and overall dietary factors that may be related to any changes in MetS. These studies may help gauge how the United States is responding to childhood MetS.
Methods
We examined participant data from the Centers for Disease Control and Prevention (CDC) NHANES (1999–2012). NHANES is a cross-sectional, national, stratified, multistage probability survey conducted in 2-year waves with randomly selected noninstitutionalized US civilians. NHANES oversampled racial/ethnic minority groups as well as those at or below 130% of the federal poverty level. Calculated sample weights accounted for this oversampling as well as different response rates among groups to ensure nationally representative estimates. This study was approved by the National Center for Health Statistics ethics review board. Participants provided informed consent. Laboratory and clinical measurements were obtained by using controlled equipment and protocols.24,28,29 Fasting blood samples were obtained from participants who attended morning sessions at CDC NHANES mobile examination centers. HDL was measured by direct immunoassay. Fasting glucose was measured by using an enzyme hexokinase method. Triglycerides were measured by using a timed-end point method. BMI z scores were calculated according to the US CDC 2000 growth reference adjusting for age and gender.30 High BP was defined as systolic or diastolic BP exceeding the 90th percentile for gender, age, and height.31
We examined data from 5117 adolescents aged 12 to 19 with complete data regarding MetS and not meeting exclusion criteria. Participants were excluded for the following conditions that may affect participants’ laboratory measures in a way that is unlikely to be associated with the lifestyle factors we were evaluating: nonfasting status, pregnancy, active hepatitis B infection (n = 8), physician-diagnosed diabetes (n = 66), or current use of antidiabetic or antihyperlipidemic medication (n = 42). Participants taking antihypertensives were classified as having elevated BP (n = 69).
There are no official criteria for evaluating MetS in children and adolescents. Multiple sets of criteria have been proposed and used.32,33 We elected to use the adolescent adaptation of the ATP-III criteria because these are based on the NCEP ATP-III definition of MetS in adults.1 MetS was defined as having at least 3 of the following: BMI z score ≥1.645, fasting glucose ≥100 mg/dL, fasting triglycerides ≥110 mg/dL, HDL ≤40 mg/dL, and systolic or diastolic BP exceeding the 90th percentile for height, age, and gender.2
MetS severity was calculated by using the Pediatric MetS z Score (http://mets.health-outcomes-policy.ufl.edu/calculator).20 This set of scores was formulated previously by using data from non-Hispanic white, non-Hispanic black, and Hispanic adolescents aged 12 to 19 years participating in NHANES 1999 to 2010. We used confirmatory factor analysis on individual gender- and racial/ethnic group, generating equations to calculate a z score estimating the severity of MetS. These scores thus use the same clinical measurements as the ATP-III criteria, but with race/ethnicity- and gender-specific loading factors to account for the unequal contributions to MetS of each clinical factor across different groups.20 These scores highly correlate with other surrogate markers of MetS risk, including high-sensitivity C-reactive protein, uric acid, and the homeostasis model of insulin resistance.20,21 In a longitudinal study of 629 children and adolescents followed into adulthood, MetS z score correlated with 27- and 37-year later risk of developing CVD and T2DM.22,23
Dietary intake was determined from a 24-hour food recall administered by a trained dietary interviewer by using a 4-step multipass approach in the mobile examination center on the examination day. Interview data were processed and coded based on individual foods and portion sizes to determine specific nutrient intake based on US Department of Agriculture’s National Nutrient Database for Standard Reference.34 Specific food group consumption was reported as percentage of calories accounted by specific food group.29 The equations were as follows: % total energy from carbohydrates = (4 * grams of carbohydrates)/total calories, % total energy from fats = (9 * grams of fat)/total calories, % total energy from protein = (7 * grams of protein)/total calories.
From 2007 to 2012, physical activity was assessed as minutes per week of moderate to vigorous physical recreational activity per day. Participants were first asked if they did any vigorous/moderate-intensity sports, fitness, or recreational activities that cause large increase in breathing or heart rate, such as running or basketball for at least 10 minutes continuously. If they answered yes, they were further questioned for how many minutes per typical day they do these activities.
Statistical significance was defined as P < .05. Statistical analysis was performed using SAS (version 9.4, Cary, NC). SAS survey procedures were used to account for the complex survey design to obtain population-based estimates. Linear regression was used to estimate means of quantitative measurements across each NHANES period and assess for temporal linear trends. Fasting plasma triglyceride data were log transformed for regression analysis and back-transformed for data presentation. Temporal linear trends in prevalence of clinical abnormalities were assessed with logistic regression analyses.
Results
Prevalence and Trends in MetS
We analyzed data from 5117 adolescents with complete MetS data, including 1490 non-Hispanic whites, 1655 non-Hispanic blacks, and 2018 Hispanics (Table 1). The overall prevalence of ATP-III MetS in this time period was 9.83%. This overall prevalence varied by gender (males 10.90%, females 6.29%, P < .0001) and race/ethnicity (Hispanics 9.97%, non-Hispanic whites 9.04%, and non-Hispanic blacks 4.45%, P < .0001).
TABLE 1.
Participant Characteristics
| Total n | Non-Hispanic White n (%) | Non-Hispanic Black n (%) | Hispanic n (%) | Mean Age, y (95% CI) | Male, % | Uptake, % | |
|---|---|---|---|---|---|---|---|
| 1999–2000 | 937 | 212 (62) | 255 (15) | 470 (22) | 15.9 (15.5–16.3) | 50 | 85 |
| 2001–2002 | 960 | 306 (70) | 315 (15) | 339 (15) | 16.1 (15.7–16.4) | 50 | 87 |
| 2003–2004 | 928 | 248 (67) | 371 (16) | 309 (17) | 16.1 (15.7–16.6) | 52 | 85 |
| 2005–2006 | 869 | 233 (68) | 301 (16) | 335 (16) | 16.0 (15.7–16.2) | 50 | 82 |
| 2007–2008 | 459 | 154 (65) | 117 (15) | 188 (19) | 16.0 (15.8–16.3) | 52 | 84 |
| 2009–2010 | 547 | 195 (63) | 113 (16) | 239 (21) | 16.0 (15.8–16.3) | 52 | 86 |
| 2011–2012 | 463 | 142 (60) | 183 (18) | 138 (22) | 15.9 (15.6–16.1) | 51 | 76 |
| Total | 5117 | 1490 (68) | 1655 (18) | 2018 (22) | 16.0 (15.9–16.1) | 51 | 84 |
Numbers of participants in each racial/ethnic category are reported along with weighted percentages. Uptake response rate for NHANES is shown by wave for adolescents who participated in the examination. CI, confidence interval.
Overall mean (SD) MetS z score in this time period was –0.05 (0.02). Mean MetS z score varied by race: Hispanics 0.04 (0.03), non-Hispanic whites –0.04 (0.02), and non-Hispanic blacks –0.19 (0.03).
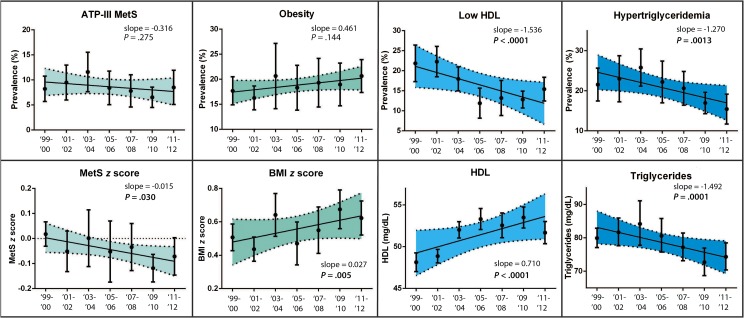
Tables 2 and 3 and Fig 1 display overall temporal trends in MetS and its individual components, along with variations by gender and race/ethnicity. Overall, there were temporal trends of decreasing MetS z score (P = .030) and fasting triglyceride measurements (P = .0001). There were further temporal trends of increasing BMI z score (P = .005) and HDL levels (P < .0001). There were no significant trends in overall fasting glucose and mean systolic BP measurements. There were no associated trends in the prevalence of ATP-III MetS and obesity. There were decreasing trends in the proportion of children with high fasting triglycerides (P < .0013) and low HDL prevalence (P < .0001).
TABLE 2.
Changes in Risk Factor Prevalence Over Time (Odds Ratios)
| ATP III MetS | Obesity (BMI z Score ≥1.645) | High Fasting Blood Glucose (≥100 mg/dL) | High Fasting Blood Triglycerides (≥110 mg/dL) | Low HDL (≤40 mg/dL) | High BPa | |
|---|---|---|---|---|---|---|
| Overall | 0.95 (0.88–1.03) | 1.03 (0.99–1.07) | 0.97 (0.93–1.02) | 0.92 (0.87–0.96)* | 0.89 (0.85–0.94)* | 0.94 (0.88–1.02) |
| Males | 0.92 (0.84–1.01) | 1.02 (0.96–1.09) | 0.94 (0.89–1.00) | 0.92 (0.87–0.97)* | 0.90 (0.85–0.97)* | 0.93 (0.85–1.03) |
| Females | 1.00 (0.88–1.14) | 1.040 (0.96–1.12) | 1.04 (0.95–1.14) | 0.91 (0.84–0.99)* | 0.86 (0.79–0.94)* | 0.96 (0.83–1.11) |
| Non-Hispanic white | 0.93 (0.84–1.09) | 1.02 (0.95–1.09) | 0.95 (0.88–1.03) | 0.89 (0.82–0.95)* | 0.88 (0.82–0.95)* | 0.88 (0.77–1.00) |
| Non-Hispanic black | 0.95 (0.82–1.04) | 1.02 (0.94–1.10) | 1.02 (0.92–1.12) | 0.97 (0.88–1.07) | 0.93 (0.84–1.02) | 0.93 (0.85–1.02) |
| Hispanic | 1.03 (0.94–1.12) | 1.07 (1.00–1.15)* | 1.04 (0.97–1.11) | 1.00 (0.94–1.06) | 0.90 (0.83–0.97)* | 1.11 (0.97–1.26) |
For binary risk factor prevalences, odds ratio estimates reported with 95% confidence intervals in parentheses.
P < .05.
Systolic or diastolic BP >90th percentile for height, age, and gender.
TABLE 3.
Changes in Laboratory Values Over Time (Slopes)
| MetS z Score | BMI z Score | Fasting Blood Glucose | Fasting Blood Triglycerides (Log Transformed) | HDL | Systolic BP | |
|---|---|---|---|---|---|---|
| Overall | –0.02 (–0.03 to 0.00)* | 0.02 (0.00 to 0.04)* | −0.03 (–0.21 to 0.13) | –1.79 (–2.96 to –0.63)* | 0.71 (0.48 to 0.94)* | −0.11 (–0.41 to 0.19) |
| Males | –0.02 (–0.05 to 0.00)* | 0.02 (0.00 to 0.05) | −0.10 (–0.31 to 0.11) | –1.98 (–3.84 to –0.13)* | 0.73 (0.42 to 1.03) | −0.18 (–0.65 to 0.27) |
| Females | 0.00 (–0.02 to 0.01) | 0.02 (0.00 to 0.05)* | 0.02 (–0.21 to 0.26) | –1.61 (–3.07 to –0.15)* | 0.69 (0.36 to 1.03) | −0.06 (–0.31 to 0.19) |
| Non-Hispanic White | −0.02 (–0.04 to 0.00) | 0.03 (0.01 to 0.06)* | −0.09 (–0.31 to 0.12) | –0.02 (–0.03 to 0.00)* | 0.83 (0.54 to 1.12) | −0.22 (–0.60 to 0.16) |
| Non-Hispanic Black | −0.01 (–0.04 to 0.01) | 0.01 (–0.02 to 0.05) | −0.03 (–0.26 to 0.20) | –0.02 (–0.03 to –0.00)* | 0.48 (0.08 to 0.87)* | 0.14 (–0.23 to 0.51) |
| Hispanic | 0.00 (–0.03 to 0.02) | 0.02 (–0.01 to 0.06) | 0.30 (–0.05 to 0.67) | 0.00 (–0.02 to 0.01) | 0.61 (0.17 to 1.05) | 0.01 (–0.4 to 0.50) |
Slope estimates are reported with 95% confidence intervals in parentheses. All reported values were obtained from survey procedures based on weighted numbers.
P < .05.
FIGURE 1.
Trends in prevalence and measurements of MetS and its components versus time. Individual means and 95% confidence interval bars are shown for each sampling period. Regression lines are shown with 95% confidence bands shaded. P values are reported for nonzero trends. For prevalence values, slope is reported as change in percentage per sampling period. For measurements, slope is reported as change in measurement unit per sampling period. There were significant decreasing trends of low HDL and hypertriglyceridemia prevalence. There were significant decreasing trends in MetS z score and fasting plasma triglycerides. There were significant increasing trends in BMI z score and HDL.
In a sensitivity analysis, we repeated the linear regression analyses assessing trends in MetS z score and the laboratory values versus time in a sample that included adolescents with diagnosed diabetes or taking antidiabetic or antihyperlipidemic agents. There were no significant differences with the previous analyses when these previously excluded adolescents were included (data not shown).
Lifestyle Trends Among US Adolescents
Given the changes in fasting triglycerides, HDL, BMI z score, and improvements in overall MetS severity among adolescents, we next evaluated for potentially related trends in dietary intake and physical activity.
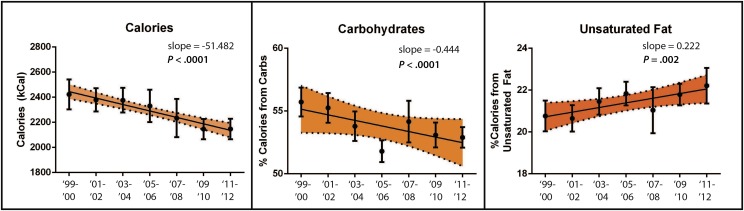
Overall temporal trends in calorie consumption and food group intake and their variations by gender and race/ethnicity are reported in Table 4 and Fig 2. Overall, there were temporal trends of decreasing total calorie consumption, decreasing carbohydrate consumption, and increasing unsaturated fat consumption.
TABLE 4.
Dietary Trends Over Time
| Total Calories (kCal) | % Energy From Unsaturated Fats | % Energy From Saturated Fats | % Energy From Carbohydrates | % Energy From Protein | |
|---|---|---|---|---|---|
| Overall | –51 (–69 to –33)* | 0.22 (0.08 to 0.36)* | –0.00 (–0.08 to 0.06) | –0.43 (−0.63 to –0.24)* | 0.23 (0.16 to 0.31)* |
| Males | –68 (–98 to –39)* | 0.15 (0.01 to 0.29)* | −0.00 (–0.10 to 0.09) | –0.29 (−0.54 to –0.03)* | 0.18 (0.07 to 0.30)* |
| Females | –33 (–57 to –10)* | 0.29 (0.07 to 0.52)* | −0.01 (–0.10 to 0.08) | –0.59 (−0.86 to –0.31)* | 0.28 (0.15 to 0.40)* |
| Non-Hispanic White | –64 (–89 to 38)* | 0.21 (–0.00 to 0.43) | 0.00 (–0.11 to 0.12) | –0.48 (−0.81 to –0.15)* | 0.26 (0.13 to 0.38)* |
| Non-Hispanic Black | –41 (–78 to –4.3)* | 0.24 (0.02 to 0.45)* | −0.03 (–0.14 to 0.08) | −0.35 (–0.71 to 0.01) | 0.13 (0.01 to 0.26)* |
| Hispanic | −7.0 (–35 to 21) | 0.24 (0.03 to 0.45)* | 0.01 (–0.13 to 0.16) | 0.35 (–0.72 to 0.01) | 0.17 (0.03 to 0.31)* |
Changes in specific nutrient intake are reported as changes in percentage of calorie intake derived from specific nutrient group over total calorie consumption; 95% confidence intervals are reported in parentheses. All reported values were obtained from survey procedures based on weighted numbers.
P < .05.
FIGURE 2.
Trends in dietary factors. Individual means and 95% confidence interval bars are shown for each sampling period. Regression lines are shown with 95% confidence bands shaded. P values are reported for nonzero trends. For total calorie consumption, slope is reported as change in calories consumed per sampling period. For carbohydrate and unsaturated fat intake, slope is reported as change in percentage per sampling period. There were significant decreasing trends in total calorie consumption and percentage of calories accounted for by carbohydrates. There was a significant increasing trend in percentage of calories accounted for by unsaturated fats.
Mean calorie consumption was positively associated with fasting triglyceride levels (P = .003) and inversely associated with BMI z score (P < .0001) and HDL (P = .0009).
Carbohydrate intake was directly associated with fasting triglyceride levels (P = .0004) and inversely associated with HDL levels (P < .0001). There was no association between carbohydrate intake and BMI z score.
Unsaturated fat intake was inversely associated with fasting triglyceride levels (P < .0001) and directly associated with HDL levels (P < .0001). There was no association between unsaturated fat intake and BMI z score.
Percentage of total calories from saturated fats did not change over time (data not shown). Saturated fat intake was directly associated with HDL levels (P = .015). There was no association between saturated fat intake and fasting triglyceride levels or BMI z score.
Percentage of total calories from protein was 13.2% in 1999–2000 and 14.7% 2011–2012, representing a significant increasing trend over time (P < .0001). Protein intake was directly associated with BMI z score (P = .005). There was no association between protein intake and fasting triglyceride or HDL levels.
Physical activity data were only available in a consistent format from 2007–2012. During this time period, there were no trends in minutes per week of moderate to vigorous physical recreational activity. There was a significant decreasing trend in minutes per week of moderate to vigorous physical recreational activity in non-Hispanic black adolescents (P = .036). Physical activity was not associated with MetS z score (P = .377).
Discussion
In evaluating temporal trends regarding MetS, previous studies have reported on changes in the prevalence of MetS. In using ATP-III criteria, the prevalence of MetS appears to be stable or decreasing among US adults.24,25 We found in using an adolescent modification of these criteria that there was no change in MetS prevalence over this time period. Although MetS criteria have advantages in identifying future risk for disease in the presence of MetS,35,36 simple binary criteria have disadvantages in being unable to follow for changes in these metabolic derangements over time. We additionally used a MetS severity z score to assess for these changes, revealing a decline in MetS severity in US adolescents from 1999–2012. Given that MetS severity in childhood has been linked to risk for future T2DM23 and CVD,22 these trends may represent a turning point in future disease risk in this age group.
We sought to identify which clinical components of MetS could account for the decreasing trend in MetS z score. Interestingly, the decreasing trend in MetS z score was observed despite a significant increasing trend in BMI z score. The overall decreasing trend in MetS z score is likely secondary to the increasing trend in HDL measurements and decreasing trend in fasting triglycerides measurements, which is consistent with previous findings.37 These individual MetS components trends were also observed in US adults and attributed to increased usage of antidiabetics and antihyperlipidemics.24 However, because we excluded all adolescent participants using antidiabetics and antihyperlipidemics, the trends observed in US adolescents must be attributable to other factors.
It is important to understand what may be contributing to this US adolescent population improvement in MetS severity because identifying the contributing factors could aid in ensuring their continued effectiveness, with potential beneficial implications for upcoming generations of children with respect to long-term health outcomes. We sought to identify trends in lifestyle factors that could be associated with the trends in MetS and its individual components. Percentage of total calories accounted for by carbohydrate or unsaturated fat intake were correlated with fasting triglyceride and HDL measurements. The increasing trend in HDL and decreasing fasting triglyceride measurements could be attributable in part to the trends of decreasing carbohydrate intake and increasing unsaturated fat intake. The Lyon Diet Heart Study, a randomized interventional trial, has shown that adherence to a Mediterranean diet, which emphasizes lower carbohydrate intake and greater unsaturated fat intake, resulted in significant reductions in CVD risks among adults <70 years old.38
Carbohydrate and unsaturated fat consumption were not significantly correlated with BMI z score. It was curious that total calorie consumption was significantly negatively correlated with BMI z score. Recent findings suggest that participants with higher BMI tended to underreport their calorie consumption.39 It could also potentially relate to reverse causation in which heavier children sought to reduce their total calorie consumption.40 Finally, despite the fact that we were limited in our ability to assess for changes in physical activity due to alterations in the assessment technique over the time period, data from the Youth Risk Behavior Surveillance system showed that there is increasing sedentary behaviors among US adolescents.41 The lower calorie requirements associated with sedentary activities could offer an explanation for our data that obesity prevalence increased over time despite a significant decrease in total calorie consumption. This explanation would also corroborate our finding that total calorie consumption is indeed decreasing temporally in US adolescents.
As alluded to earlier, we were unable to attribute the trends in MetS severity and its individual factors to physical activity factors. Our analysis of physical activity data was limited because NHANES did not have a consistent variable assessing physical activity from 1999 to 2012. Additionally, there are questions about the validity of quantifying adolescent physical activity data. There have been findings that adolescent self-reported physical activity data are significantly correlated to accelerometer data.42 However, adolescent accelerometry data also has limitations with respect to bias and generizability.43
There were additional limitations to our study. The ATP-III criteria lead to underdiagnosis of MetS in specific racial/ethnic groups.9 This could result in an overall underestimate of MetS prevalence in the US adolescent population, particularly among African American adolescents. However, other sets of MetS criteria, which differ largely in individual component cutoffs, have also had racial/ethnic discrepancies noted.15 We do not believe the use of a different set of MetS criteria would have had a significant effect on our results regarding trends in MetS diagnosis. Instead, we attempted to overcome the epidemiologic limitations by use of a MetS severity score that uniquely addresses differences in MetS by race/ethnicity. These MetS severity z scores have been validated, although they currently lack absolute cutoffs that correspond to particular risk for outcomes. There are current and future research efforts directed at achieving this goal. We were unable to assess causality due to the cross-sectional nature of NHANES. We correlated trends in MetS with lifestyle factors, but this can only be interpreted as association with unclear links to causality. Future studies should be directed at investigating the causality of lifestyle factors in improvements of MetS severity. Diet quality assessment was limited by its dependence on participant accurate reporting and bias.
Conclusions
This study has importance in that it is nationally representative for the US adolescent population, is novel in its temporal assessment of MetS severity, and explores possible lifestyle factors contributing to population health trends. These data further confirm the need for research, public health, and clinical collaboration in combatting childhood MetS.
Glossary
- ATP-III
Adult Treatment Panel
- BP
blood pressure
- CDC
Centers for Disease Control and Prevention
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- MetS
metabolic syndrome
- T2DM
type 2 diabetes mellitus
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was supported by National Institutes of Health grant 1R01HL120960 (MJG and MDD). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Dr Lee participated in the design and analysis of the research and was responsible for the write-up; Dr Gurka participated in the design and analysis of the research; Dr DeBoer participated in the design, analysis, and write-up of the research and had primary responsibility for the final content; and all authors approved of the final manuscript as submitted.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, et al. ; American Heart Association; National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752 [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115(19):2526–2532 [DOI] [PubMed] [Google Scholar]
- 3.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250 [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Eckel RH, Grundy SM, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645 [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Ajani UA, Mokdad AH; National Health and Nutrition Examination . The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care. 2005;28(4):878–881 [DOI] [PubMed] [Google Scholar]
- 6.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173(2):309–314 [DOI] [PubMed] [Google Scholar]
- 7.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428 [DOI] [PubMed] [Google Scholar]
- 8.Kahn R, Buse J, Ferrannini E, Stern M; American Diabetes Association; European Association for the Study of Diabetes . The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28(9):2289–2304 [DOI] [PubMed] [Google Scholar]
- 9.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr. 2009;155(3 S7):7.e7–7.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the metabolic syndrome is associated with disproportionately high levels of high-sensitivity C-reactive protein in non-Hispanic black adolescents: an analysis of NHANES 1999–2008. Diabetes Care. 2011;34(3):734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the ability of metabolic syndrome criteria to predict elevations in fasting insulin levels in adolescents. J Pediatr. 2011;159(6):975–981.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBoer MD, Gurka MJ. Low sensitivity for the metabolic syndrome to detect uric acid elevations in females and non-Hispanic-black male adolescents: an analysis of NHANES 1999–2006. Atherosclerosis. 2012;220(2):575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deboer MD, Wiener RC, Barnes BH, Gurka MJ. Ethnic differences in the link between insulin resistance and elevated ALT. Pediatrics. 2013;132(3). Available at: www.pediatrics.org/cgi/content/full/132/3/e718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deboer MD. Ethnicity, obesity and the metabolic syndrome: implications on assessing risk and targeting intervention. Expert Rev Endocrinol Metab. 2011;6(2):279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2012;22(2):141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241 [DOI] [PubMed] [Google Scholar]
- 17.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163(4):427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ukegbu UJ, Castillo DC, Knight MG, et al. Metabolic syndrome does not detect metabolic risk in African men living in the U.S. Diabetes Care. 2011;34(10):2297–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arslanian S, Suprasongsin C, Janosky JE. Insulin secretion and sensitivity in black versus white prepubertal healthy children. J Clin Endocrinol Metab. 1997;82(6):1923–1927 [DOI] [PubMed] [Google Scholar]
- 20.Gurka MJ, Ice CL, Sun SS, Deboer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol. 2012;11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurka MJ, Lilly CL, Oliver MN, DeBoer MD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism. 2014;63(2):218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of metabolic syndrome as a predictor of cardiovascular disease between childhood and adulthood: the Princeton Lipid Research Cohort Study. J Am Coll Cardiol. 2015;66(6):755–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeBoer MDG, Gurka MJ, Woo JG, Morrison JA. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the Princeton Lipid Research Cohort Study. Diabetologia. 2015;58(12):2745–2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol. 2013;62(8):697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–1974 [DOI] [PubMed] [Google Scholar]
- 26.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Center for Health Statistics. National Health and Nutrition Examination Survey protocol. Available at: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm . Accessed July 10, 2015
- 29.Reedy J, Krebs-Smith SM. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J Am Diet Assoc. 2010;110(10):1477–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27 [PubMed] [Google Scholar]
- 31.Kaplan N. Kaplan’s Clinical Hypertension. Philadelphia, PA: Lippincott Williams & Wilkins; 2010 [Google Scholar]
- 32.Reinehr T, de Sousa G, Toschke AM, Andler W. Comparison of metabolic syndrome prevalence using eight different definitions: a critical approach. Arch Dis Child. 2007;92(12):1067–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmet P, Alberti G, Kaufman F, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention of Diabetes . The metabolic syndrome in children and adolescents. Lancet. 2007;369(9579):2059–2061 [DOI] [PubMed] [Google Scholar]
- 34.US Department of Agriculture National Nutrient Database for Standard Reference. Available at: http://ars.usda.gov/ba/bhncr/ndl. Accessed August 14, 2015
- 35.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120(2):340–345 [DOI] [PubMed] [Google Scholar]
- 36.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152(2):201–206 [DOI] [PubMed] [Google Scholar]
- 37.Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr. 2015;169(3):272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99(6):779–785 [DOI] [PubMed] [Google Scholar]
- 39.Freedman LS, Commins JM, Moler JE, et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. Am J Epidemiol. 2015;181(7):473–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackenzie T, Brooks B, O’Connor G. Beverage intake, diabetes, and glucose control of adults in America. Ann Epidemiol. 2006;16(9):688–691 [DOI] [PubMed] [Google Scholar]
- 41.Kann L, Kinchen S, Shanklin SL, et al. ; Centers for Disease Control and Prevention (CDC) . Youth risk behavior surveillance—United States, 2013. MMWR Surveill Summ. 2014;63(suppl 4):1–168 [PubMed] [Google Scholar]
- 42.Chaumeton N, Duncan SC, Duncan TE, Strycker LA. A measurement model of youth physical activity using pedometer and self, parent, and peer reports. Int J Behav Med. 2011;18(3):209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loprinzi PD, Smit E, Cardinal BJ, Crespo C, Brodowicz G, Andersen R. Valid and invalid accelerometry data among children and adolescents: comparison across demographic, behavioral, and biological variables. Am J Health Promot. 2014;28(3):155–158 [DOI] [PubMed] [Google Scholar]