Abstract
BACKGROUND:
We previously reported improved neurodevelopmental outcomes at 2 years among infants treated with the erythropoiesis-stimulating agents (ESAs) darbepoetin alfa (darbepoetin) or erythropoietin. Here we characterize 4-year outcomes.
METHODS:
Former preterm infants randomly assigned to receive darbepoetin (10 μg/kg, once per week), erythropoietin (400 U/kg, 3 times/week), or placebo through 35 weeks’ postconceptual age were evaluated at 3.5 to 4 years of age. For comparison, healthy children formerly delivered full term (term controls [TCs]) were also recruited. All participants were assessed by using measures of full-scale IQ (FSIQ) and general language from the Wechsler Preschool and Primary Scale of Intelligence, Third Edition, and an overall measure of executive function, on the basis of tests evaluating inhibitory control and spatial working memory. Rates of neurodevelopmental impairment were compared across groups.
RESULTS:
Multivariate analysis of variance compared children randomly assigned to ESAs (n = 39), placebo (n =14), and TCs (n = 24). FSIQ and performance IQ were significantly higher in the ESA group than in the placebo group (FSIQ: 91.1 ± 17.5 vs 79.2 ± 18.5, P = .036; performance IQ: 93.0 ± 17.0 vs 79.5 ± 19.5, P = .018). Follow-up analyses revealed that the children receiving ESAs performed better than those who received placebo on executive function tasks. The ESA group’s performance was below that of TCs, but the results did not reach significance on executive function. The incidence of neurodevelopmental impairment was greater in the placebo group than in the ESA group.
CONCLUSIONS:
ESA-treated infants had better cognitive outcomes and less developmental impairment at 3.5 to 4 years of age compared with placebo-treated infants. ESAs show promise in improving long-term cognitive outcomes of infants born prematurely.
What’s Known on This Subject:
We and others previously reported that erythropoietin administration to preterm infants improves short-term developmental outcome. There are limited data on outcomes in preschoolers, and the effects of the longer-acting erythropoietic agent, darbepoetin, have not been evaluated.
What This Study Adds:
Children previously randomly assigned to either darbepoetin or erythropoietin treatment as preterm infants had significantly higher cognitive scores and improved executive function compared with children previously randomly assigned to placebo at 3.5 to 4 years of age.
Although major morbidity such as cerebral palsy, intellectual disability, and learning and attention deficits during school age are common outcomes of extremely low birth weight infants, successful neuroprotective interventions have yet to be developed. Recent studies in animals and humans evaluating the nonhematopoietic effects of erythropoiesis-stimulating agents (ESAs) suggest a neuroprotective potential via such mechanisms as increased oligodendrogenesis, decreased inflammation, decreased oxidative injury, and decreased apoptosis.1–9
We previously reported 18- to 22-month outcomes on our prospective, randomized, masked study in preterm infants randomly assigned to receive ESAs or placebo10 and hypothesized that ESA recipients would have higher composite cognitive scores measured by using the Bayley Scales of Infant Development III (BSID-III).11 Indeed, those very low birth weight infants receiving erythropoietin or darbepoetin alfa (hereafter “darbepoetin”) had significantly higher composite cognitive scores, including higher scores on a scale of executive function.12 Moreover, neurodevelopmental impairment (NDI) was significantly lower in ESA recipients than in placebo recipients, and cerebral palsy was only identified in placebo recipients.
In the current study we sought to determine if neurocognitive improvement persisted into preschool age (3.5–4 years). We compared full-scale IQ (FSIQ) and the General Language Composite (GLC) scores measured by the Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III), along with a composite measure of executive function, in former preterm children randomly assigned to receive ESAs or placebo. In addition, we enrolled children previously born healthy at term to serve as typically developing term controls (TCs). We hypothesized that former preterm children who received ESA therapy would perform significantly better than former preterm children who received placebo on measures of general cognitive ability, language, and executive function at 3.5 to 4 years of age. We further hypothesized that TCs would perform better than both the preterm-treated and untreated groups.
Methods
Initial Study
Preterm infants <32 weeks’ gestation and with a birth weight of 500 to 1250 g were enrolled at ≤48 hours of age. Infants with trisomies, significant congenital anomalies (including known neurologic anomalies), hypertension, seizures, thromboses, or hemolytic disease, or who were already receiving erythropoietin, were ineligible for study. Randomization stratified by center (New Mexico, Utah, and Colorado) was performed by using a computer-generated permuted block method. Twins were randomly assigned to the same treatment group. All caregivers and investigators (except for the research pharmacists and coordinators administering the study medicine) were masked to the treatment assignment. An investigational new drug application was approved by the Food and Drug Administration (IND100138), and the study was registered at clinicaltrials.gov (NCT00334737).
Dosing of Study Drug and Supplements
Infants were randomly assigned to 1 of 3 groups: erythropoietin (400 U/kg, given subcutaneously 3 times/week), darbepoetin (10 μg/kg, given subcutaneously once per week, with sham dosing 2 other times/week), or placebo (consisting of 3 sham doses/week). Dosing continued until 35 completed weeks’ gestation, discharge, transfer to another hospital, or death. All infants received supplemental iron, folate, and vitamin E.
Present Study
Children previously enrolled in the above study were eligible for the BRain Imaging and Developmental Follow-up of Infants Treated with Erythropoietin (BRITE) Study (NCT01207778), performed at the Utah and New Mexico sites. In addition, healthy children previously born term (TC) without hospital complications (hypoglycemia requiring glucose, hyperbilirubinemia requiring phototherapy, sepsis evaluation, or in utero drug exposure) were enrolled at the New Mexico site. Children were evaluated at 3.5 to 4 years of age by certified examiners (J.L. and M.S.) blinded to the treatment group. The study was approved by institutional review boards at the University of Utah and University of New Mexico, and informed consent was obtained from parents.
Demographic information and medical history were obtained from the family. Anthropometric measurements were obtained and a detailed neurologic evaluation was performed. Assessments of hearing impairment (characterized functionally as requirement of a hearing aid or unilateral or bilateral deafness) and visual impairment (defined as blindness or vision requiring significant correction) were based on examination and parental report.
The WPPSI-III, a widely used scale of general cognitive abilities, was administered.13 The WPPSI-III is standardized for children aged 2 years 6 months through 7 years and yields FSIQ, verbal IQ (VIQ), performance IQ (PIQ), and GLC scores. Our primary analysis focused on the summary variables of the FSIQ and GLC. Two other developmental measures were performed to assess executive function. The Memory for Location Test14 was used as a measure of spatial working memory. Children had to locate a toy hidden under 1 of 6 cups after 1-, 5-, and 10-second delays. A principal components analysis revealed that scores from the 3 conditions loaded on a single, large factor (75.46% of variance). The Gift Delay Test15 was administered as a measure of inhibition. This test requires a child to hear a “gift” being wrapped out of their view, and the child is timed to see how long they can wait before touching or opening the gift placed in front of them. Four variables captured performance: seconds before peeking, seconds before touching, number of peeks, and the number of reminders needed. Principal components analysis revealed a single, large factor accounted for test performance (68% of variance). Thus, the first principal component emerging from analysis of each test was used to represent performance in these 2 specific domains of executive function. Finally, an overall measure of executive function expressed as a standard score was derived by summing and averaging performance from these correlated (r = 0.23, P < .05) tests of spatial working memory and inhibitory control. All cognitive data are presented as standard scores.
NDI was defined as ≥1 of the following: bilateral blindness, severe hearing loss, any cerebral palsy, or WPPSI-III FSIQ <70. A board-certified child neurologist (JP) or developmental pediatrician (S.Winter, MD) performed detailed neurologic examinations.
Statistical Analysis
After planned analyses revealed no significant differences between erythropoietin and darbepoetin recipients they were combined as a single ESA group. Baseline characteristics, growth, and medical outcomes were compared among the 3 study arms (ESA, placebo, TC), and the significance of any differences was determined by using analysis of variance or Kruskal-Wallis (K-W) rank-sum tests (depending on distributional characteristics) for quantitative dependent variables and χ2 tests for nominal and categorical dependent variables. When significant differences were discovered (at an α level of 0.05) among the 3 study arms, we performed post hoc pairwise tests by using 2-tailed Fisher's exact tests for nominal and categorical dependent variables and 2-tailed t tests or K-W tests for quantitative dependent variables. Our primary hypothesis was that general cognition (FSIQ), language (GLC), and executive function (as described above) would be greater among subjects in the ESA-treated group than in the placebo group. To evaluate this hypothesis, we used a multivariate general linear model. Significant cognitive effects were followed up with more detailed analyses.
Results
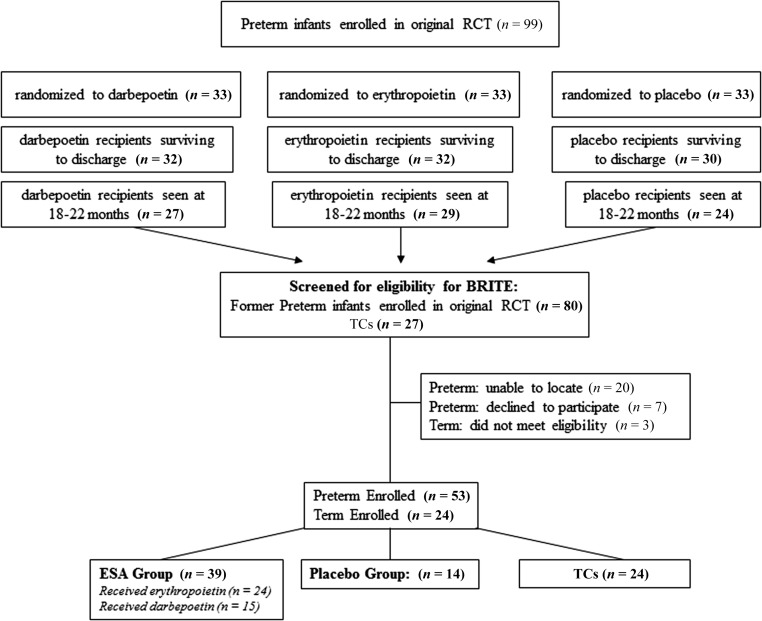
The number of children eligible for the study and evaluated at 3.5 to 4 years of age are shown in Fig 1. Reasons for nonenrollment included parent refusal (7 subjects) or lost to contact (20 subjects). Of the 80 preterm infants evaluated at 18 to 22 months in the original randomized study, outcomes were determined for 53 (66%) at 3.5 to 4 years.
FIGURE 1.
Numbers of infants screened for eligibility for the BRITE study, were eligible for the study, and evaluated at 3.5 to 4 years. RCT, randomized controlled trial.
Characteristics of the 77 children (n = 14 placebo, 39 ESA-treated, and 24 TCs) comprising the BRITE study are shown in Table 1. Analyses using χ2 tests revealed no significant differences in gender (P = .31), maternal ethnicity (P = .24), or maternal racial background (0.58) among the 3 groups. Maternal education was significantly different between placebo and ESA groups (P = .019). Eighty-nine percent of the ESA group mothers completed high school, compared with 71% of the placebo group. Fifty percent of the placebo group mothers had graduate degrees, compared with 15% of the ESA group mothers. All analyses were adjusted for maternal education.
TABLE 1.
Characteristics of the Children and Their Families
| Group | P | ||||
|---|---|---|---|---|---|
| ESA | Placebo | TC | ESA-Treated Versus Placebo | ESA-Treated Versus TCs | |
| Subjects, n | 39 | 14 | 24 | ||
| New Mexico,a n (%) | 20 (51) | 6 (43) | 24 (100) | .76 | <.001 |
| Utah, n (%) | 19 (49) | 8 (57) | 0 (0) | .76 | <.001 |
| Female, n (%) | 17 (44) | 6 (43) | 14 (58) | .99 | .31 |
| Age, median (IQR), mo | 47.0 (3.0) | 48.5 (3.0) | 45.5 (4.0) | .64 | <.001 |
| Birth weight, median (IQR), g | 946.0 (351.0) | 1005.0 (220.5) | 3317.0 (412.0) | .90 | <.001 |
| Gestation, median (IQR), wk | 28.0 (2.0) | 28.0 (1.2) | 39.1 (2.0) | .51 | <.001 |
| Mother's ethnicity, n (%) | .99 | .19 | |||
| Hispanic | 15 (38) | 5 (36) | 14 (58) | ||
| Non-Hispanic | 24 (62) | 9 (64) | 10 (42) | ||
| Mother's racial background, n (%) | .90 | .44 | |||
| American Indian | 8 (21) | 3 (21) | 3 (12) | ||
| Asian | 1 (3) | 0 (0) | 1 (4) | ||
| Black | 3 (8) | 0 (0) | 0 (0) | ||
| Pacific Islander | 0 (0) | 0 (0) | 0 (0) | ||
| White | 26 (68) | 11 (79) | 20 (83) | ||
| Mother's highest grade, n (%) | .019 | .12 | |||
| Some high school | 4 (11) | 4 (29) | 2 (8) | ||
| High school degree | 13 (33) | 2 (14) | 7 (29) | ||
| Some college | 2 (5) | 0 (0) | 6 (25) | ||
| College degree | 14 (36) | 1 (7) | 3 (12) | ||
| Graduate degree | 6 (15) | 7 (50) | 6 (25) | ||
| Household income, n (%) | .45 | .41 | |||
| <$5000 | 2 (5) | 0 (0) | 0 (0) | ||
| $5000–$9999 | 6 (15) | 3 (21) | 2 (8) | ||
| $10 000–$19 999 | 6 (15) | 5 (36) | 2 (8) | ||
| $20 000–$29 999 | 5 (13) | 2 (14) | 6 (25) | ||
| $30 000–$39 999 | 7 (18) | 0 (0) | 4 (17) | ||
| $40 000–$49 999 | 1 (3) | 1 (7) | 3 (12) | ||
| ≥$50 000 | 10 (26) | 3 (21) | 7 (29) | ||
| Not stated | 2 (5) | 0 (0) | 0 (0) | ||
| Morbidities (initial hospitalization), n (%) | |||||
| Intracranial hemorrhage ≥ grade 3 | 2 (5) | 2 (14) | — | .29 | |
| Bronchopulmonary dysplasia | 26 (68) | 7 (50) | — | .33 | |
| Necrotizing enterocolitis | 1 (3) | 1 (7) | — | .47 | |
| Growth and medical outcomes | |||||
| Weight, median (IQR), kg | 15.2 (2.3) | 15.7 (3.0) | 16.4 (3.1) | .65 | .008 |
| Weight <10th percentile, n (%) | 12 (31) | 5 (36) | 1 (4) | .75 | .012 |
| Height, median (IQR), cm | 101.5 (6.5) | 101.5 (8.0) | 104.0 (7.0) | .83 | .018 |
| Height <10th percentile, n (%) | 11 (28) | 5 (36) | 0 (0) | .74 | .004 |
| Head circumference, median (IQR), cm | 50.0 (2.0) | 49.0 (2.7) | 51.0 (2.2) | .19 | .097 |
| Head circumference <10th percentile, n (%) | 2 (5) | 4 (29) | 1 (4) | .036 | .99 |
| Systolic blood pressure, median (IQR), mm Hg | 93.0 (11.0) | 95.0 (12.8) | 90.0 (4.0) | .98 | .13 |
| Diastolic blood pressure, median (IQR), mm Hg | 56.0 (12.0) | 56.5 (15.8) | 58.0 (6.0) | .45 | .22 |
IQR, interquartile range; —, term control children were not hospitalized and were not analyzed for the preterm morbidities listed in the table.
Two subjects evaluated at the New Mexico Site were from Colorado.
TCs had greater birth weight, gestational age, weight at follow-up, and height at follow-up than either placebo or treated children, and these differences were found to be significant with the use of using K-W tests. TCs were also younger at follow-up (median = 45.5 months) than treated (median = 47 months) and placebo (median = 48.5 months) children, and this difference was significant (P < .001, K-W test). At follow-up there were no significant differences between ESA and placebo groups in weight, height, or head circumference; however, a greater proportion of children in the placebo group had a head circumference less than the 10th percentile (5% in the ESA group versus 29% in the placebo group; P = .036).
Of the 80 former preterm subjects seen at the 18- to 22-month follow-up,12 53 enrolled in BRITE (n = 14 placebo; n = 39 treated, of whom 24 were erythropoietin recipients and 15 were darbepoetin recipients), whereas 27 did not (10 placebo, 17 treated). The characteristics of the children evaluated at 3.5 to 4 years were similar to those not evaluated. Of those not evaluated, 13 (48%) were female, compared with 23 (76.6%) who were evaluated (P = .81). Children not evaluated had a mean gestational age at birth of 28.0 ± 1.9 weeks, compared with 27.8 ± 1.7 weeks for children who were evaluated (P = .45). Children not evaluated had a mean birth weight of 947 ± 191 g compared with 946 ± 203 g for children who were evaluated (P = .94).
Table 2 provides detailed information on cognitive performance. As mentioned previously, analyses of darbepoetin and erythropoietin groups revealed no differences in developmental testing (Table 3), so the groups were combined into 1 ESA group. Children in the ESA group scored significantly better than the placebo group in FSIQ (P = .011), PIQ (P = .006), and overall executive function (P = .035). To test our primary hypothesis we conducted a multivariate analysis of variance with 3 dependent variables (FSIQ, GLC, and executive function) with group (placebo, ESA, TC), gender, and maternal education as factors, along with the gender by group and maternal education by group interaction terms. The multivariate effect of group was significant across the set of cognitive measures (F[6, 112] = 4.87, P < .001), as were the univariate effects for FSIQ (P < .001), GLC (P < .001), and executive function (P = .003). We followed up these analyses with specific group comparisons. For the critical comparison of ESA versus placebo, the overall effect was significant (F[3, 38] = 3.98, P = .015), as were univariate effects for FSIQ (P = .017) and executive function (P = .045) but not GLC (P = .26). Comparison of the ESA group with the TC group revealed significantly better FSIQ among the TC group (P = .011; Table 2), although differences did not reach significance in tests of executive function (P = .083; Table 2). Thus, ESA-treated children had better cognitive performance than placebo-treated children but still had lower cognitive performance than the TC group. No interactions of group with gender or of group with maternal education were observed in any analysis. The overall pattern of results for cognitive abilities across groups is shown in Fig 2.
TABLE 2.
Cognitive Performance Among Groups
| Group | P | ||||
|---|---|---|---|---|---|
| ESA-Treated | Placebo | TC | ESA-Treated Versus Placebo | ESA-Treated Versus TCs | |
| Subjects, n | 39 | 14 | 24 | ||
| FSIQ | 91.1 (17.5) | 79.2 (18.5) | 102.58 (12.78) | 0.036 | 0.011 |
| VIQ | 91.7 (18.3) | 82.9 (16.9) | 100.8 (15.2) | 0.091 | 0.071 |
| PIQ | 93.0 (17.0) | 79.5 (19.5) | 103.9 (10.6) | 0.018 | 0.006 |
| GLC | 89.82 (16.98) | 84.79 (17.11) | 101.83 (15.11) | 0.35 | 0.006 |
| Executive function | 99.88 (11.65) | 91.52 (13.26) | 105.15 (7.79) | 0.035 | 0.083 |
| Working memory | 100.63 (14.58) | 91.57 (17.24) | 103.92 (12.44) | 0.047 | 0.35 |
| Inhibition | 99.13 (15.21) | 91.53 (17.61) | 106.36 (9.95) | 0.11 | 0.066 |
Data are presented as means (SDs) unless otherwise indicated.
TABLE 3.
Cognitive Performance in Erythropoietin and Darbepoetin Recipients
| Erythropoietin | Darbepoetin | Pa | |
|---|---|---|---|
| Subjects, n | 24 | 15 | |
| FSIQ | 89.62 (19.24) | 94.47 (16.14) | .42 |
| VIQ | 93.54 (17.85) | 92.07 (16.11) | .80 |
| PIQ | 89.62 (19.24) | 94.47 (16.14) | .12 |
| GLC | 87.91 (17.96) | 92.73 (16.12) | .41 |
| Executive function | 98.34 (13.47) | 102.33 (7.75) | .49 |
| Working memory | 98.31 (16.98) | 104.34 (8.90) | .42 |
| Inhibition | 98.39 (16.81) | 100.30 (12.70) | .91 |
Data are presented as means (SDs) unless otherwise indicated.
Erythropoietin compared with darbepoetin.
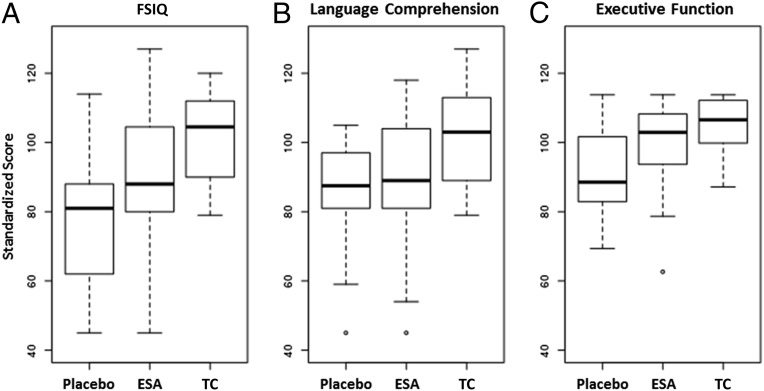
FIGURE 2.
Comparison of FSIQ (A), language comprehension (B), and executive function (C) in placebo, ESA-treated, and TC children. Data shown are box and whisker plots of medians (first and third quartiles). ESA-treated children scored significantly higher in FSIQ (P = .036) and executive function (P = .031).
Because our primary interest was the comparison of placebo versus ESA-treated children, we conducted a more detailed analysis of performance difference between the 2 groups. The FSIQ is composed of both verbal and nonverbal tasks, as reflected in VIQ and PIQ. Analyses of variance (with group and gender as fixed factors) revealed a significant effect for PIQ (F[1, 52] = 5.03, P = .029) but not for VIQ (F[1, 52] = 2.41, not significant). We next examined the 2 components of executive function. Neither component was significantly different (spatial working memory: F[1, 52] = 3.43, P = .07; inhibition: F[1, 52] = 2.31, P = .13).
The incidence of NDI was significantly greater in the placebo group than in the ESA group. Four of 14 children (29%) in the placebo group had an FSIQ of <70, compared with 3 of 39 children (8%) in the ESA group (P = .07). Three children (21%) in the placebo group had mild cerebral palsy, compared with 0 children (0%) in the ESA group (P = .016). No children in the study were deaf or blind at follow-up. No disabilities were identified in the TC group.
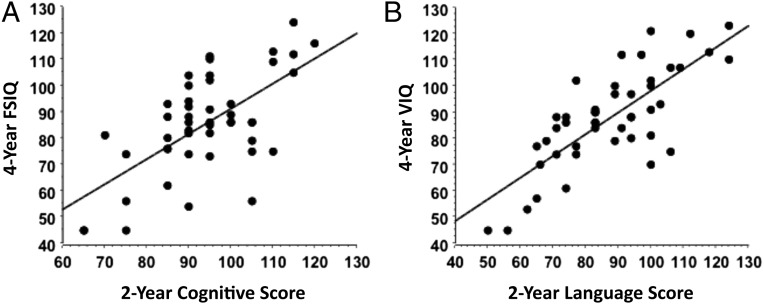
Finally, we compared cognitive test results at 2 years versus 4 years and found a significant correlation between BSID-III composite language score and VIQ (R2 = 0.592, P < .0001) and between BSID-III composite score and FSIQ (R2 = 0.414, P < .0001; Fig 3).
FIGURE 3.
Relationship between BSID-III cognitive scores at 2 years and FSIQ at 4 years (R2 = 0.414, P < .0001) (A) and BSID-III composite language scores at 2 years and VIQ at 4 years (R2 = 0.592, P < .0001) (B) in former preterm children. Both cognitive and language scores correlated significantly between 2- and 4-year assessment time points.
Discussion
This study is the first, to our knowledge, to report neurocognitive outcomes of darbepoetin and erythropoietin treatment in former preterm children at preschool age. Using a double-blind, placebo-controlled design, we showed that darbepoetin and erythropoietin improve neurocognitive outcomes at 3.5 to 4 years of age in former preterm infants. Similar to 2-year outcomes,12 children formerly born preterm who were randomly assigned to receive ESAs during their initial hospital stay scored significantly better than children formerly born preterm randomly assigned to placebo in scores of FSIQ, PIQ, and measures of executive function at 4 years of age.
Although overall growth among former preterm children was similar, we did identify a greater percentage of children in the placebo group with a head circumference below the 10th percentile. This percentage increased in the placebo group from the 18- to 22-month assessment, from 20.8% to 29%, whereas the percentage of children with a head circumference less than the 10th percentile actually decreased in the ESA group, from 10.7% to 5%. This finding requires further evaluation. In addition, differences in maternal education between ESA and placebo groups require further inquiry. Although a greater number of placebo group mothers had graduate degrees, a larger number of mothers in the placebo group did not complete high school. There were no differences in maternal education between TC mothers and ESA or placebo group mothers. As expected, former term-born healthy children scored better overall than former preterm children, although the statistical comparison fell just short of significance when comparing executive function between TCs and ESA recipients. ESAs closed the gap between preterm and term children.
This study is the first, to our knowledge, to report outcomes that support the use of ESAs to improve developmental outcome in preterm children at preschool age. A number of clinical trials evaluating ESAs for neuroprotection are ongoing. Fauchère et al16 in Switzerland recently completed a study that used high-dose erythropoietin for neuroprotection in preterm infants. Although the primary outcome (neurodevelopment at 2 years) is still forthcoming, imaging results in a subset of the infants are encouraging. Leuchter et al17 reported decreased gray and white matter injury in 77 of the erythropoietin-treated infants compared with 88 placebo-treated infants, and O’Gorman et al18 reported improved white matter development as assessed by diffusion tensor imaging and tract-based spatial statistics in 24 of the erythropoietin-treated infants compared with 34 placebo-treated infants. A US multicenter trial of high-dose erythropoietin for neuroprotection in 960 extremely low gestational age infants (PENUT [Preterm Epo Neuroprotection Trial]; NCT01534481) is ongoing, and neurodevelopmental outcomes are expected in ∼3 years.
Randomized studies of either erythropoietin19,20 or darbepoetin21 for treatment of hypoxic ischemic encephalopathy have reported pharmacokinetic evaluations that suggest neuroprotection, and safety profiles showed no adverse effects of either ESA in term infants. Doses in the DANCE (Darbepoetin Administration in Neonates undergoing Cooling for Encephalopathy) study were similar to the darbepoetin doses administered in our original randomized controlled trial,8 although term infants only received 2 doses 1 week apart in the DANCE study, compared with up to 10 weekly doses in our study. Long-term developmental assessments of infants enrolled in these term neuroprotection studies are ongoing. Our study coupled with previously published studies in preterm infants22–25 provide growing evidence for ESAs to be neuroprotective in term and preterm neonates.
In animal models, ESAs are protective in the developing brain, suggesting the possibility that they might be of benefit to very premature infants who are at risk of intraventricular hemorrhage, hypoxic-ischemic injury, and developmental delay. The neuroprotective mechanisms of ESAs include decreased neuronal apoptosis, decreased inflammation, promotion of oligodendrocyte differentiation and maturation, and improved white matter survival.1–9 These biological mechanisms of neuroprotection may be revealed through MRI.26 All children enrolled in the BRITE study underwent MRI, and analyses of total and regional brain volume, cortical thickness, surface area, spectroscopy, total and regional cerebral blood flow, and diffusion tensor imaging are nearing completion. All children are being seen again at 5.5 to 6 years for developmental testing and MRI to provide longitudinal assessments. Regardless of neuroimaging findings, we found significant and clinically important improvement in cognitive function with ESAs and believe these findings would be of great clinical importance to both families and care providers.
Diminished executive function skills (encompassing working memory, inhibition, and cognitive flexibility) have contributed to academic failure and behavioral problems in children born preterm, over and above the impact of FSIQ.27 Executive function deficits are commonly observed at school age28 and may be found as early as 18 to 22 months.29,30 These findings are consistent with our previous study in this same cohort, which found the ESA group (darbepoetin recipients in particular) had better early working memory skills at 18 to 22 months.12 In a longitudinal study from 4 to 12 years of age, children born preterm were found to have difficulty in verbal fluency, inhibition, and cognitive flexibility or shifting.31 Our current study showed that the ESA group at 3.5 to 4 years continued to score higher on intellectual and executive function skills, as they did at 18 to 22 months,12 and in fact were similar to TC subjects in tests of executive function. The higher executive function skills we saw in the ESA group were promising with regard to future reasoning and coping skills, which are obviously important to school success. We are hopeful that this pattern will persist as we continue to follow this cohort of children into early school age.
We conclude that ESA administration to preterm infants resulted in higher cognition at 3.5 to 4 years of age. Given its significantly longer half-life,32–34 darbepoetin may be the more attractive and practical choice of ESA for preterm infants. We speculate that ESAs may serve as a beneficial therapy for preterm infants, not only in acute hospitalization where the risk of anemia exists but also as possible neuroprotective agents to improve long-term neurodevelopmental outcomes.
Acknowledgments
We thank the research coordinators and bedside nurses involved in the original randomized study and are indebted to the parents for their willingness to allow their children to participate in this study. We wish to acknowledge US taxpayers for providing the funding to support the National Institutes of Health and this study.
Glossary
- BRITE
BRain Imaging and Developmental Follow-up of Infants Treated with Erythropoietin
- BSID-III
Bayley Scales of Infant Development III
- ESA
erythropoiesis-stimulating agent
- FSIQ
full-scale IQ
- GLC
General Language Composite
- K-W
Kruskal-Wallace
- NDI
neurodevelopmental impairment
- PIQ
performance IQ
- TC
term control
- VIQ
verbal IQ
- WPPSI-III
Wechsler Preschool and Primary Scale of Intelligence, Third Edition
Footnotes
Dr Ohls conceptualized and designed the study, performed analyses, and drafted the initial manuscript; Mr. Cannon performed the statistical analyses and critically reviewed the final manuscript; Drs Lowe and Caprihan conceptualized and designed the study, performed statistical analyses, and critically reviewed and revised the manuscript; Dr Wiedmeier was the initial Utah site principal investigator and supervised data collection and reviewed the final manuscript; Dr Patel is the current Utah site principal investigator and supervised data collection at the Salt Lake City site and reviewed the final manuscript; Dr Phillips conceptualized and designed the study, performed neurologic examinations at the New Mexico site, performed statistical analyses, and critically reviewed and revised the manuscript; Dr Winter performed the neurologic exams at the Utah site and reviewed and revised the manuscript; Mr Steffen performed all of the developmental tests at the Utah site and reviewed and revised the manuscript; Drs Yeo and Campbell performed statistical analyses and critically reviewed and revised the manuscript; Ms Baker coordinated the study and performed data collection at the Utah site and critically reviewed and revised the manuscript; Mr Gonzales coordinated the study and performed data collection at the New Mexico site and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifiers NCT01207778, NCT00334737, IND100138).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD059856), the University of New Mexico Clinical Translational Science Center (UL1 TR000041), the University of Utah Center for Clinical and Translational Sciences (UL1TR001067), and the University of New Mexico Department of Pediatrics. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Zhang L, Chopp M, Zhang RL, et al. Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. 2010. 5(6):e11016:1-8 [DOI] [PMC free article] [PubMed]
- 2.Iwai M, Stetler RA, Xing J, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41(5):1032–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juul SE, Beyer RP, Bammler TK, McPherson RJ, Wilkerson J, Farin FM. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatr Res. 2009;65(5):485–492 [DOI] [PubMed] [Google Scholar]
- 4.Chattopadhyay A, Choudhury TD, Bandyopadhyay D, Datta AG. Protective effect of erythropoietin on the oxidative damage of erythrocyte membrane by hydroxyl radical. Biochem Pharmacol. 2000;59(4):419–425 [DOI] [PubMed] [Google Scholar]
- 5.Vairano M, Dello Russo C, Pozzoli G, et al. Erythropoietin exerts anti-apoptotic effects on rat microglial cells in vitro. Eur J Neurosci. 2002;16(4):584–592 [DOI] [PubMed] [Google Scholar]
- 6.Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Effects of erythropoietin on glial cell development: oligodendrocyte maturation and astrocyte proliferation. Neurosci Res. 2002;44(4):391–403 [DOI] [PubMed] [Google Scholar]
- 7.Jantzie LL, Miller RH, Robinson S. Erythropoietin signaling promotes oligodendrocyte development following prenatal systemic hypoxic-ischemic brain injury. Pediatr Res. 2013;74(6):658–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jantzie LL, Getsy PM, Firl DJ, Wilson CG, Miller RH, Robinson S. Erythropoietin attenuates loss of potassium chloride co-transporters following prenatal brain injury. Mol Cell Neurosci. 2014;61(Jul):152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jantzie LL, Corbett CJ, Firl DJ, Robinson S. Postnatal erythropoietin mitigates impaired cerebral cortical development following subplate loss from prenatal hypoxia-ischemia. Cereb Cortex. 2015;25(9):2683–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohls RK, Christensen RD, Kamath-Rayne BD, et al. A randomized, masked, placebo controlled study of darbepoetin administered to preterm infants. Pediatrics. 2013;132(1). Available at: www.pediatrics.org/cgi/content/full/132/1/e119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio, TX: Harcourt Assessment; 2006 [Google Scholar]
- 12.Ohls RK, Kamath-Rayne BD, Christensen RD, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics. 2014;133(6):1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wechsler D. The Wechsler Preschool and Primary Scale of Intelligence. 3rd ed. San Antonio, TX: The Psychological Corporation; 2002 [Google Scholar]
- 14.Vicari S, Caravale B, Carlesimo GA, Casadei AM, Allemand F. Spatial working memory deficits in children at ages 3-4 who were low birth weight. Neuropsychology. 2004;18(4):673–678 [DOI] [PubMed] [Google Scholar]
- 15.Carlson SM. Developmentally sensitive measures of executive function in preschool children. Dev Neuropsychol. 2005;28(2):595–616 [DOI] [PubMed] [Google Scholar]
- 16.Fauchère JC, Koller BM, Tschopp A, Dame C, Ruegger C, Bucher HU; Swiss Erythropoietin Neuroprotection Trial Group . Safety of early high-dose recombinant erythropoietin for neuroprotection in very preterm infants. J Pediatr. 2015;167(1):52–7 [DOI] [PubMed] [Google Scholar]
- 17.Leuchter RH, Gui L, Poncet A, et al. Association between early administration of high-dose erythropoietin in preterm infants and brain MRI abnormality at term-equivalent age. JAMA. 2014;312(8):817–824 [DOI] [PubMed] [Google Scholar]
- 18.O’Gorman RL, Bucher HU, Held U, Koller BM, Hüppi PS, Hagmann CF; Swiss EPO Neuroprotection Trial Group . Tract-based spatial statistics to assess the neuroprotective effect of early erythropoietin on white matter development in preterm infants. Brain. 2015;138(pt 2):388–397 [DOI] [PubMed] [Google Scholar]
- 19.Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012;130(4):683–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers EE, Bonifacio SL, Glass HC, et al. Erythropoietin and hypothermia for hypoxic-ischemic encephalopathy. Pediatr Neurol. 2014;51(5):657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baserga MC, Beachy JC, Roberts JK, et al. Darbepoetin administration to neonates undergoing cooling for encephalopathy: a safety and pharmacokinetic trial. Pediatr Res. 2015;78(3):315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bierer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics. 2006;118(3). Available at: www.pediatrics.org/cgi/content/full/118/3/e635 [DOI] [PubMed] [Google Scholar]
- 23.Brown MS, Eichorst D, Lala-Black B, Gonzalez R. Higher cumulative doses of erythropoietin and developmental outcomes in preterm infants. Pediatrics. 2009;124(4). Available at: www.pediatrics.org/cgi/content/full/124/4/e681 [DOI] [PubMed] [Google Scholar]
- 24.Neubauer AP, Voss W, Wachtendorf M, Jungmann T. Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol. 2010;67(5):657–666 [DOI] [PubMed] [Google Scholar]
- 25.McAdams RM, McPherson RJ, Mayock DE, Juul SE. Outcomes of extremely low birth weight infants given early high-dose erythropoietin. J Perinatol. 2013;33(3):226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips JP, Montague EQ, Aragon M, et al. Prematurity affects cortical maturation in early childhood. Pediatr Neurol. 2011;45(4):213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32(1):51–58 [DOI] [PubMed] [Google Scholar]
- 28.Mulder H, Pitchford NJ, Hagger MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol. 2009;34(4):393–421 [DOI] [PubMed] [Google Scholar]
- 29.Woodward LJ, Clark CA, Pritchard VE, Anderson PJ, Inder TE. Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Dev Neuropsychol. 2011;36(1):22–41 [DOI] [PubMed] [Google Scholar]
- 30.Lowe JR, Duncan AF, Bann CM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Early working memory as a racially and ethnically neutral measure of outcome in extremely preterm children at 18-22 months. Early Hum Dev. 2013;89(12):1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aarnoudse-Moens CS, Oosterlaan J, Duivenvoorden HJ, van Goudoever JB, Weisglas-Kuperus N. Development of preschool and academic skills in children born very preterm. J Pediatr. 2011;158(1):51–56 [DOI] [PubMed] [Google Scholar]
- 32.Warwood TL, Ohls RK, Lambert DK, et al. Uninary excretion of darbepoetin after intravenous vs subcutaneous administration to preterm infants. J Perinatol. 2006;26(10):636–639 [DOI] [PubMed] [Google Scholar]
- 33.Warwood TL, Ohls RK, Lambert DK, et al. Intravenous administration of darbepoetin to NICU patients. J Perinatol. 2006;26(5):296–300 [DOI] [PubMed] [Google Scholar]
- 34.Patel S, Ohls RK. Darbepoetin administration in term and preterm neonates. Clin Perinatol. 2015;42(3):557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]