Abstract
Purpose
Subretinal drusenoid deposits (SDD) have been associated with the progression to late age-related macular degeneration (AMD). To determine whether SDD in eyes in normal macular health increases risk for early AMD, this study examined the association between presence of SDD at baseline in a cohort of older adults in normal macular health and incident AMD 3 years later.
Methods
Subjects enrolled in the Alabama Study on Early Age-Related Macular Degeneration (ALSTAR) were assessed for the presence of SDD using color fundus photos, infrared reflectance and fundus autofluorescence images, and spectral-domain optical coherence tomography volumes. The study sample included 799 eyes from 455 participants in normal macular health per grading of color fundus photographs using the 9-step Age-Related Eye Disease Study (AREDS) classification system. Age-related macular degeneration was defined as eyes having an AREDS grade ≥2 at the 3-year follow-up.
Results
Twenty-five percent of participants had SDD in one or both eyes at baseline. At follow-up visit, 11.9% of eyes in the sample developed AMD. Compared to eyes without SDD, those with SDD were 2.24 (95% confidence interval [CI] 1.36–3.70) times more likely to have AMD at follow-up. After adjusting for age, C-reactive protein quartile, and family history of AMD, the association persisted.
Conclusions
Results suggest that SDD in older eyes with normal macular health as defined by the AREDS scale is a risk factor for the development of early AMD. Older adults in seemingly normal macular health yet having SDD may warrant closer clinical monitoring for the possible onset of early AMD.
Keywords: age-related macular degeneration, normal macular health, subretinal drusenoid deposits
Age-related macular degeneration (AMD) is a progressive disease and the leading cause of irreversible vision loss among older adults in industrialized countries.1 An estimated 10 million individuals in the United States alone have AMD, and the vast majority of these individuals (90%) have early disease.2 Because aging is the largest risk factor for AMD, more information about the transition from normal retinal aging to early AMD would help direct research to important biologic mechanisms and eventually therapies.3 It is known that large areas of small hard drusen increase the risk of developing AMD (Klein R, et al. IOVS 2015;56:ARVO E-Abstract 2571) and developing larger soft drusen, which are considered an early sign of AMD and a well-recognized risk factor for progression to advanced AMD.4,5 Decades of histopathology have demonstrated that hard and soft drusen alike are located posterior to the retinal pigment epithelium (RPE), between the RPE basal lamina and the inner collagenous layer of Bruch's membrane.6,7
Another type of extracellular lesion, subretinal drusenoid deposits (SDD), has different characteristics compared to conventional soft drusen.7 A biomicroscopic sign seen in en face fundus imaging called reticular pseudodrusen was originally ascribed to choroidal fibrosis.8 Later, investigators using spectral-domain optical coherence tomography (SD-OCT) found hyperreflectivity located anterior to the RPE and attributed it to SDD,9 which had been described independently in histology.10,11 Evidence that reticular pseudodrusen and SDD are one and the same is accruing.12–15 First thought to consist of the same material as soft drusen (lipoprotein-derived debris),11,16,17 recent evidence suggests that the lipid composition of the subretinal lesions differs markedly from their sub-RPE counterparts while several proteins are common to both.18,19
Prospective population-based studies using color fundus photography, long considered the gold standard for drusen detection based on the Wisconsin Age-Related Maculopathy Grading System, reported that reticular pseudodrusen confer a high risk for development of late stage AMD,4,20 visual impairment,4 and poorer survival.4 However, the detection of reticular pseudodrusen in color fundus photography may have also included soft drusen,9 so risk for AMD was likely to be an aggregate risk of reticular pseudodrusen and soft drusen combined. Therefore the AMD risk conferred by reticular pseudodrusen alone, herein called SDD, remains uncertain.9
Several longitudinal studies utilizing newer imaging methods (e.g., SD-OCT, near-infrared reflectance, alone and in combination) concluded that SDD is associated with progression to both geographic atrophy and choroidal neovascularization.9,21–24 In a retrospective study of 200 patients with unilateral neovascularization, SDD detected by near-infrared reflectance and SD-OCT was associated with development of geographic atrophy (hazard ratio [HR] = 4.93), but not neovascularization (HR = 1.19), in the fellow eye.21 However, in a prospective study of patients with unilateral neovascularization, SDD detected by color photography and fluorescein angiograms was associated with development of both geography atrophy (risk ratio [RR] = 2.0) and neovascularization (RR = 1.7) in the fellow eye.22 Similarly, in another prospective study of participants with unilateral neovascular AMD, all participants who developed geographic atrophy had SDD at baseline based on SD-OCT, and presence of SDD was associated with development of neovascular AMD (odds ratio [OR] = 5.05) in the fellow eye.23 Subretinal drusenoid deposits have been associated with progression of geographic atrophy as well.25 In a retrospective study of patients who were diagnosed with geographic atrophy in at least one eye and were imaged with fundus autofluorescence and near-infrared reflectance, geographic atrophy progression was more frequent in fields with SDD compared to those without (74.2% vs. 41.7%, respectively).25 Only one study thus far has reported on SDD in early AMD, finding in 88 eyes that SDD was cross-sectionally associated with early AMD features including drusen >63 μm in diameter, hypopigmentation, and hyperpigmentation.23
To our knowledge no study has examined whether SDD in eyes in normal macular health are associated with the transition from normal retinal aging to incident early AMD. Our purpose was to examine whether SDD is associated with AMD 3 years later in a large sample of eyes in normal macular health at baseline.
Methods
Study Sample
This study included eyes from individuals who participated in the Alabama Study on Early Age-Related Macular Degeneration (ALSTAR) cohort.26,27 The recruitment process, characteristics, and study methods for the sample have been described.26 Briefly, the cohort was recruited from two primary care ophthalmology practices and included adults aged 60 years and older living in north central Alabama. Eligible persons had normal macular health in at least one eye based on three-field digital color stereo fundus photos (CFP) (450 Plus camera; Carl Zeiss Meditec, Dublin CA, USA) assessed by an experienced grader masked to other study variables. The 9-step Age-Related Eye Disease Study (AREDS) classification system28 was used to determine disease presence and severity. For the purposes of this study, normal macular health is defined as step 1 in the AREDS 9-step classification system,28 corresponding to a macula where drusen diameter is <125 μm and decreased or increased pigmentation and geographic atrophy are absent. Eyes with early AMD (steps 2–4), intermediate (steps 5–8), or late-stage AMD (steps 9–11) at baseline were excluded from the analysis. Persons with glaucoma, other retinal conditions, optic nerve disease, corneal disease, diabetes, or neurological or psychiatric conditions were not eligible. Patients were enrolled between May 2009 and December 2011 and were followed up 3 years later.
The present analysis included all eyes with an AREDS step 1 at the baseline visit. Other inclusion criteria included those who completed the 3-year follow-up visit and those who had gradable images at the baseline and 3-year follow-up visit. This study was approved by the Institutional Review Board of the University of Alabama at Birmingham and followed the tenets of the Declaration of Helsinki.
Data Collection
After informed consent was obtained, the baseline visit included interviewer-administered questionnaires covering demographic characteristics (age, sex, race, education completed), smoking status,29 and alcohol use.30 General health was assessed by asking about the presence or absence of 15 chronic medical conditions.31 The number of comorbid conditions was summed and presented as categories. Height (cm) and weight (kg) were measured so that body mass index (BMI) could be computed. Participants were asked whether they were aware of any family history of AMD among their biological parents, siblings, or children. Best-corrected visual acuity was assessed via the Electronic Visual Acuity test (EVA) under photopic conditions (100 cd/m2) and expressed as the logarithm of the minimum angle resolvable (logMAR).32 Blood (4–8 mL) was collected by phlebotomy and the resultant heparinized plasma used for analysis. Because previous research indicated that elevated C-reactive protein (CRP) is an independent risk factor for AMD and its progression,33–35 CRP concentration was measured by ELISA as described previously.36
All participants underwent imaging at baseline with SD-OCT, infrared reflectance (IR) and fundus autofluorescence (FAF), and CFP. Spectral-domain OCT, IR, and FAF were obtained using a Spectralis HRA+SD-OCT (Heidelberg Engineering, Heidelberg, Germany). We use the terminology of Staurenghi et al.37 for the outer retinal hyperreflective bands. Spectral-domain OCT was considered gradable when it included both a macular volume with 73 horizontal B-scans at 60-μm intervals and an optic nerve head (ONH) volume with 48 radial B-scans. Spectral-domain OCT was gradable if the grader could distinguish the area between (and inclusive of) the external limiting membrane and RPE-Bruch's membrane complex on both volumes. The presence or absence of SDD was noted on SD-OCT, IR, AF, and CFP based on recognition of characteristic features defined in a separate report.38 Briefly, SDD was defined as present and discernible (PAD) on SD-OCT images if ≥1 dome-shaped or oval hyperreflective material of any size internal to and adjacent to the RPE with or without disturbance of the ellipsoid zone was present. In IR, AF, and color fundus images, SDD were defined as PAD if discrete foci correlated with SD-OCT findings or formed an arcuate pattern along the superotemporal arcades or perifovea superior and nasal to the macula. In addition, SDD in normal and early AMD eyes tend to fall outside the macular and ONH SD-OCT volumes; these can be recognized with practice if compared to lesions within the volume where subretinal reflectivity can be confirmed. For the current analysis, SDD was defined as present at the eye level if there was ≥1 SDD judged as PAD on SD-OCT and at least one en face modality; or at least ≥1 SDD judged as PAD on two or more en face modalities in the absence of SD-OCT findings; otherwise SDD was defined as not present at the eye level. Images were graded by an ophthalmologist with vitroretinal surgery experience who was masked to all other study variables. A subset of images was regraded approximately 2 weeks later without reference to previous grades. Intragrader agreement was moderately high at 0.73.39–41
Age-related macular degeneration status at follow-up was determined based on CFP and the 9-step AREDS classification system,28 as described above. Presence of AMD at follow-up was defined as eyes having an AREDS grade of ≥2.
Statistical Analysis
Baseline demographic and behavioral characteristics were compared between individuals with and without SDD using χ2 and t-tests for categorical and continuous variables, respectively. Wilcoxon rank-sum tests were used to compare medians for variables that were not normally distributed. Visual acuity was compared between eyes with and without SDD using generalized estimating equations (GEE) to account for the within-person correlation. The presence of AMD was defined as having an AREDS grade of ≥2 in the eye at the 3-year follow-up visit. To determine the association between presence of SDD at baseline and AMD 3 years later (at the eye level), crude and adjusted logistic regression models using GEE were used. Variables associated with the primary exposure of interest (i.e., SDD) in the univariate analysis were included as potential confounders in the adjusted models. As a secondary analysis, the sample was limited to individuals with normal macular health in both eyes in order to evaluate risk of AMD onset associated with SDD in a relatively homogenous sample of healthy eyes.
Results
Of the 1302 eyes from 651 participants who were enrolled, 283 eyes had an AREDS grade of ≥2 at baseline, 48 had missing or ungradable images, 7 had other medical conditions that did not meet study inclusion criteria, and an additional 165 did not complete the follow-up visit. Therefore, the final sample included 799 eyes with AREDS grade of 1 from 455 participants.
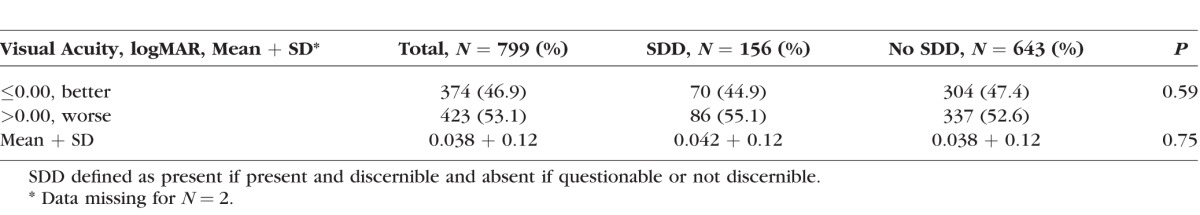
Twenty-five percent of these participants (n = 116 of 455) had SDD in one or both eyes at baseline. Of those 116 participants with SDD, 34% (n = 40 of 116) were affected in both eyes. On average, participants with SDD were slightly older (69.5 vs. 68.3, P = 0.062) and had higher median levels of CRP (2.9 vs. 2.2, P = 0.069) than those without SDD, although these associations did not reach statistical significance (Table 1). In addition, reporting a family history of AMD was approximately twice as common among those with SDD compared to those without SDD (11.2% vs. 5.3%; P = 0.030). There were no significant differences in sex, race, education, smoking status, alcohol consumption, number of medical conditions reported, or BMI. Visual acuity in eyes with and without SDD was similar (Table 2).
Table 1.
Baseline Characteristics by SDD Status at the Person Level
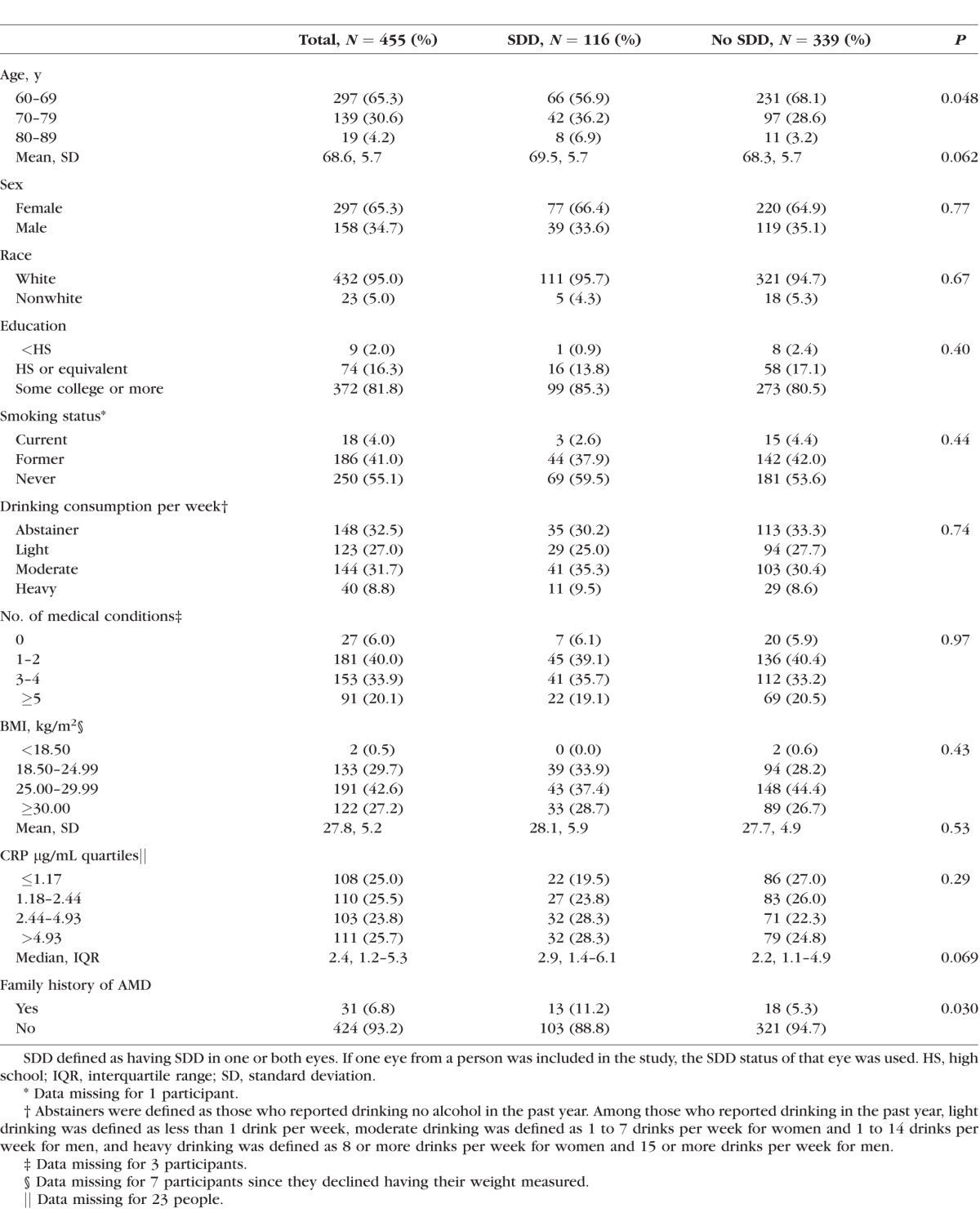
Table 2.
Visual Acuity by SDD Status at the Eye Level
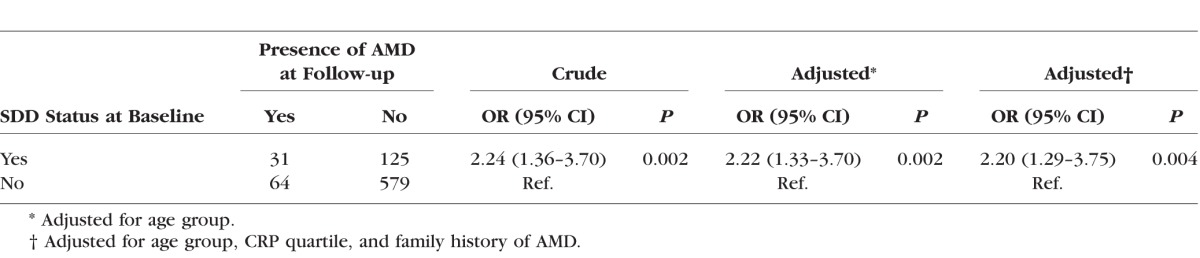
At the 3-year follow-up visit, there were 95 eyes with AMD, corresponding to a cumulative incidence of 11.9% (n = 95 of 799 eyes). Ninety were early AMD and five were intermediate AMD. No eyes developed advanced AMD (AREDS grade ≥ 9) by the 3-year follow-up visit. Of the eyes with SDD at baseline, 19.8% developed AMD (n = 31 of 156). Of the eyes without SDD at baseline, 10.0% developed AMD (n = 64 of 643). Eyes with SDD at baseline were 2.24 (95% confidence interval [CI] 1.36–3.70) times more likely to have AMD at the follow-up visit compared to eyes without SDD (Table 3). After adjusting for age, CRP quartile, and family history of AMD, the association persisted (OR 2.20, 95% CI 1.29–3.75), suggesting minimal confounding.
Table 3.
Crude and Adjusted Odds Ratio and Corresponding 95% Confidence Interval for Incident AMD (n = 799 Eyes From 455 People)
Because the existence of AMD in one eye is associated with increased risk for AMD in the other eye,42–44 our next step was to limit the sample of eyes to individuals with normal macular health in both eyes at baseline (n = 697 eyes from 353 participants). Based on this sample, the cumulative incidence of AMD 3 years later was 9.5% (n = 66 of 697 eyes). Sixty-three were early AMD and three were intermediate AMD. Of the eyes with SDD at baseline, 15.0% developed AMD (n = 18 of 120). Of the eyes without SDD at baseline, 8.3% developed AMD 3 years later (n = 48 of 577). From this subsample with an AREDS grade of 1 in both eyes at baseline, those with SDD at baseline were nearly two times (adjusted OR 1.97, 95% CI 1.00–3.87, P = 0.049) more likely to have AMD at the follow-up visit compared to eyes without SDD at baseline after adjusting for age, CRP quartiles, and family history.
Discussion
Motivated by growing interest in the significance of SDD in AMD pathogenesis, this prospective study examined whether the presence of SDD in eyes in normal macular health at baseline, using a multimodal imaging approach, is associated with presence or absence of AMD 3 years later. We found that early AMD at follow-up was 2.2 times more likely among eyes with normal macular health having SDD compared to those without SDD even after adjusting for confounding factors. It is also notable that the association persisted when both eyes, not just one eye, were in normal macular health at baseline. To our knowledge this is the first report to show that SDD in otherwise healthy eyes is a risk factor for the development of early AMD. Our findings imply that patients who have seemingly normal maculas yet have SDD may require closer follow-up to monitor for emergent early AMD. This will become increasingly relevant as pharmaceutical companies develop treatments for the earliest phases of AMD, or AMD prevention strategies while the macula still appears healthy. Furthermore, being at increased risk for AMD development makes this subgroup with SDD of great interest for research on AMD precursors and the transition from normal aging to early AMD.
We found that SDD was present in 25% of eyes in normal macular health, which is lower than that estimated for patients with early AMD (49%),38 fellow eyes of patients with geographic atrophy (94%),25 and fellow eyes in patients with neovascular AMD (35–58%).21–23 Differences in prevalence estimates across studies likely stem from differences in the presence and severity of AMD in the sample, the definition of SDD used, imaging modalities used to identify SDD, retinal ascertainment area, and person- versus eye-level characteristics. For example, in a retinal clinic population, Zweifel et al.45 enforced a five-lesion criterion for SDD presence on SD-OCT and various en face imaging modalities compared to our one-lesion criterion. Further, we used SD-OCT scan volumes centered on the ONH as well as a separate scan volume centered on the fovea, thus expanding the area that was checked for lesions.
Information on a number of factors was compared between those participants with and without SDD. Consistent with previous work, the presence of SDD was associated with older age and higher levels of plasma CRP.22,46 The present study found no sex differences by SDD status, agreeing with some studies21 but disagreeing with others noting a higher prevalence of SDD among women.22,23 In addition, there were no differences in lifestyle factors such as BMI, alcohol use, and smoking status.23,45 Adjusting for age, CRP, and family history of AMD did not appreciably change the point estimate for the association between SDD and presence of AMD, suggesting that these factors were not confounding the association.
Since SDD is a characteristic lesion of AMD yet is present in eyes that meet criteria for normal macular health based on color fundus photography, this implies that early AMD pathogenesis is underway even though not clinically visible by widely used diagnostic methods. How SDD-mediated disease begins may be informed by our knowledge of drusen biogenesis. In brief, ∼60-nm-diameter apolipoprotein B- and E-containing lipoprotein particles rich in cholesterol accumulate throughout adulthood in Bruch's membrane.47 These particles, of apparent RPE origin, eventually form basal linear deposit and/or soft drusen between the inner collagenous layer and the basal lamina of the RPE in individuals that progress to AMD.17 Events analogous to those in Bruch's membrane are hypothesized to occur in the subretinal space, where the cholesterol identified in SDD may also be secreted by RPE as part of lipoproteins as part of outer retinal lipid recycling and even retinoid transport.9,12,18 For both drusen and SDD, lesions contacting RPE and photoreceptors, as well as increased diffusion distance for essential molecules of choroidal and retinal origin, can negatively impact the health of these key cells. How SDD develop is an important biologic question requiring future research using ultrastructural methods to identify SDD precursors.
Our definition of normal macular health was based on the 9-step AREDS classification system, which at the time of study enrollment in 2009 was a widely implemented grading system in the research community with strong empirical support. It was fortuitous that the AREDS system incorporated a means to record pseudodrusen presence, even if CFP generally reveals fewer lesions than other imaging modalities.9,38,48 In contrast, the newly designed 2013 Clinical Classification of AMD based on CFP and popularly known as the Beckman classification49 does not mention pseudodrusen. It does, however, separate eyes with the small conventional drusen (63-μm diameter) from eyes with no detectable drusen. The association between SDD and incident AMD among low-risk patients as defined by the Beckman system, which uses a stricter definition of “normal,” is therefore of interest. We recomputed associations for AREDS step 1 eyes, which encompasses the first three categories of the Beckman system (i.e., no aging changes, normal aging changes including drusen ≤63-μm diameter, and early AMD including drusen ≤125 μm). We found that the association between SDD and incident AMD persists for those with no apparent aging changes, normal aging changes, and early AMD (OR = 1.6, 2.3, and 2.5, respectively). Future studies are needed to understand whether a greater risk for AMD is conferred by SDD in eyes with larger drusen.
A strength of this study is the use of multimodal retinal imaging to identify SDD. While there is currently no consensus on the best imaging approach for SDD detection, employing at least SD-OCT and IR is recommended,50 though these modalities can be supplemented by CFP, AF, indocyanine green angiography, and red-free light.7,24 For studies involving retina clinic patients at more advanced stages of disease, SDD are generally considered present when lesions form a pattern of dots or ribbons visible with various en face imaging modalities. By reference to histology18 and to adaptive optics assisted imaging,13 the current study also included small solitary lesions and sought to limit potential overestimation by requiring detection by at least two modalities. In addition, multimodal imaging with SD-OCT allowed for differentiating small solitary SDD lesions from drusen. As detailed in our separate publication,38 it is possible to cross-validate SD-OCT and en face images for sparse and out-of-volume subretinal lesions. Finally, as also detailed separately,38 SDD tend to appear first outside macular OCT volumes, many in the peripapillary area, which is a topography that is consistent with that of rod photoreceptors and a model of lesion biogenesis involving rod cholesterol homeostasis.18 Another strength of this study is a very large number of eyes in normal macular health (n = 799) analyzed with multimodal imaging, unprecedented in the literature. That SDD can be detected in a primary eye care clinic sample, as this study shows, may be helpful for primary care clinicians who typically manage the care of older adults in good eye health. Finally, this analysis was based on a prospective cohort study with a good follow-up rate, limiting the potential for bias due to differential loss to follow-up.
Limitations must also be acknowledged. Misclassification of SDD presence or absence is also possible given that identification methods are still evolving. Yet eyes were graded by a masked observer without knowledge of other clinical characteristics, so we would not expect that the misclassification was differential according to AMD status, thus biasing the results toward the null. In addition, assessment of SDD is a challenging subjective task; and while our intrarater agreement indicates substantial agreement and is consistent with previously reported intragrader agreement for macular lesions in AMD, future studies on SDD will investigate interrater agreement results as well. Because this was an observational study, we cannot rule out residual or unmeasured confounding for unmeasured covariates, such as genetics, though information on many other factors known to affect AMD was included in the analysis. While previous work has reported a link between SDD in early and advanced AMD and visual dysfunction (e.g., impaired contrast sensitivity and light sensitivity, multifocal electroretinogram changes, delayed dark adaptation)51–54 (Neely D, et al. IOVS 2015:56;ARVO E-Abstract 2777), the question of whether SDD in normal macular health are associated with visual dysfunction including its relationship to incident AMD was beyond the scope of this study and is an issue for further investigation.
In conclusion, this study suggests that SDD in older eyes in normal macular health as defined by the AREDS grading system is a risk factor for developing early AMD. Consideration should be given to closer clinical monitoring of these individuals for the possible onset of AMD.
Acknowledgments
Supported by the National Institutes of Health (NIH R01AG04212, R01EY06109, R01EY024378); the EyeSight Foundation of Alabama; Alfreda J. Schueler Trust; and Research to Prevent Blindness, Inc.
Disclosure: C. Huisingh, None; G. McGwin Jr, None; D. Neely, None; A. Zarubina, None; M. Clark, None; Y. Zhang, None; C.A. Curcio, None; C. Owsley, None
References
- 1. Wong WL,, Su X,, Li X,, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014; 2: e106–e116. [DOI] [PubMed] [Google Scholar]
- 2. Klein R,, Chou C,, Klein B,, Zhang X,, Meuer S,, Saaddine J. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011; 129: 75–80. [DOI] [PubMed] [Google Scholar]
- 3. Tomany SC,, Wang JJ,, van Leeuwen R,, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004; 111: 1280–1287. [DOI] [PubMed] [Google Scholar]
- 4. Klein R,, Meuer SM,, Knudtson MD,, Iyengar SK,, Klein BEK. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008; 145: 317–326, e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klein ML,, Ferris FL,, Armstrong J,, et al. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008; 115: 1026–1031. [DOI] [PubMed] [Google Scholar]
- 6. Sarks J,, Sarks S,, Killingsworth M. Evolution of soft drusen in age-related macular degeneration. Eye. 1994; 8: 269–283. [DOI] [PubMed] [Google Scholar]
- 7. Spaide RF,, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010; 30: 1441–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arnold JJ,, Sarks SH,, Killingsworth MC,, Sarks JP. Reticular pseudodrusen: a risk factor in age-related maculopathy. Retina. 1995; 15: 183–191. [PubMed] [Google Scholar]
- 9. Zweifel SA,, Spaide RF,, Curcio CA,, Malek G,, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010; 117: 303–312 e301. [DOI] [PubMed] [Google Scholar]
- 10. Curcio CA,, Presley JB,, Malek G,, Medeiros NE,, Avery DV,, Kruth HS. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res. 2005; 81: 731–741. [DOI] [PubMed] [Google Scholar]
- 11. Rudolf M,, Malek G,, Messinger JD,, Clark ME,, Wang L,, Curcio CA. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp Eye Res. 2008; 87: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki M,, Sato T,, Spaide RF. Pseudodrusen subtypes as delineated by multimodal imaging of the fundus. Am J Ophthalmol. 2014; 157: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y,, Wang X,, Rivero EB,, et al. Photoreceptor perturbation around subretinal drusenoid deposits as revealed by adaptive optics scanning laser ophthalmoscopy. Am J Ophthalmol. 2014; 158: 584–596, e581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spaide RF. Colocalization of pseudodrusen and subretinal drusenoid deposits using high-density en face spectral domain optical coherence tomography. Retina. 2014; 34: 2336–2345. [DOI] [PubMed] [Google Scholar]
- 15. Schaal K,, Legarreta A,, Gregori G,, et al. Widefield en face optical coherence tomography imaging of subretinal drusenoid deposits. Ophthalmic Surg Lasers Imaging Retina. 2015; 46: 550–559. [DOI] [PubMed] [Google Scholar]
- 16. Sarks J,, Sarks S,, Killingsworth M. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988; 2: 552–577. [DOI] [PubMed] [Google Scholar]
- 17. Curcio CA,, Johnson M,, Huang J-D,, Rudolf M. Age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Retin Eye Res. 2009; 28: 393–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Curcio CA,, Messinger JD,, Sloan KR,, McGwin G,, Medeiros NE,, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013; 33: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oak AS,, Messinger JD,, Curcio CA. Subretinal drusenoid deposits: further characterization by lipid histochemistry. Retina. 2014; 34: 825–826. [DOI] [PubMed] [Google Scholar]
- 20. Joachim N,, Mitchell P,, Rochtchina E,, Tan AG,, Wang JJ. Incidence and progression of reticular drusen in age-related macular degeneration: findings from an older Australian cohort. Ophthalmology. 2014; 121: 917–925. [DOI] [PubMed] [Google Scholar]
- 21. Finger RP,, Wu Z,, Luu CD,, et al. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology. 2014; 121: 1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pumariega NM,, Smith RT,, Sohrab MA,, LeTien V,, Souied EH. A prospective study of reticular macular disease. Ophthalmology. 2011; 118: 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hogg RE,, Silva R,, Staurenghi G,, et al. Clinical characteristics of reticular pseudodrusen in the fellow eye of patients with unilateral neovascular age-related macular degeneration. Ophthalmology. 2014; 121: 1748–1755. [DOI] [PubMed] [Google Scholar]
- 24. Ueda-Arakawa N,, Ooto S,, Tsujikawa A,, Yamashiro K,, Oishi A,, Yoshimura N. Sensitivity and specificity of detecting reticular pseudodrusen in multimodal imaging in Japanese patients. Retina. 2013; 33: 490–497. [DOI] [PubMed] [Google Scholar]
- 25. Marsiglia M,, Boddu S,, Bearelly S,, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration: association between GA progression and RPD in dry AMD. Invest Ophthalmol Vis Sci. 2013; 54: 7362–7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Owsley C,, Huisingh C,, Jackson GR,, et al. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Invest Ophthalmol Vis Sci. 2014; 55: 4776–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Owsley C,, McGwin Jr,, G, Clark ME,, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. In press. [DOI] [PMC free article] [PubMed]
- 28. Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS report No. 17. Arch Ophthalmol. 2005; 123: 1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Health Interview Survey. Questionnaires, datasets, and related documentation 1997 to the present. Available at: http://www.cdc.gov/nchs/nhis/quest_data_related_1997_forward.htm. Accessed February 13, 2012.
- 30. West S,, Munoz B,, Rubin G,, et al. Function and visual impairment in a population-based study of older adults. Invest Ophthalmol Vis Sci. 1997; 38: 72–82. [PubMed] [Google Scholar]
- 31. Owsley C,, McGwin Jr,, G, Sloane M,, Wells J,, Stalvey B,, Gauthreaux S. Impact of cataract surgery on motor vehicle crash involvement by older adults. JAMA. 2002; 288: 841–849. [DOI] [PubMed] [Google Scholar]
- 32. Beck R,, Moke P,, Turpin A,, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003; 135: 194–205. [DOI] [PubMed] [Google Scholar]
- 33. Seddon J,, Gensler G,, Milton R,, Klein M,, Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004; 291: 704–710. [DOI] [PubMed] [Google Scholar]
- 34. Seddon J,, George S,, Rosner B,, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. 2005; 123: 774–782. [DOI] [PubMed] [Google Scholar]
- 35. Klein R,, Myers C,, Cruickshanks K,, et al. Markers of inflammation, oxidative stress, and endothelial dysfunction and the 20-year cumulative incidence of early age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol. 2014;132:446–455. [DOI] [PMC free article] [PubMed]
- 36. Szalai A,, van Ginkel F,, Wang Y,, McGhee J,, Volanakis J. Complement-dependent acute-phase expression of C-reactive protein and serum amyloid P-component. J Immunol. 2000; 165: 1030–1035. [DOI] [PubMed] [Google Scholar]
- 37. Staurenghi G,, Sadda S,, Chakravarthy U,, Spaide RF. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN•OCT consensus. Ophthalmology. 2014; 121: 1572–1578. [DOI] [PubMed] [Google Scholar]
- 38. Zarubina AV,, Neely D,, Clark ME,, et al. Prevalence of subretinal drusenoid deposits in older persons with and without age-related macular degeneration, by multimodal imaging. Ophthalmology. 2016; In press. [DOI] [PMC free article] [PubMed]
- 39. Klein R,, Davis MD,, Magli YL,, Segal P,, Klein BE,, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991; 98: 1128–1134. [DOI] [PubMed] [Google Scholar]
- 40. Bird A,, Bressler N,, Bressler S,, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995; 39: 367–374. [DOI] [PubMed] [Google Scholar]
- 41. Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study report No. 6. Am J Ophthalmol. 2001; 132: 668–681. [DOI] [PubMed] [Google Scholar]
- 42. Klein R,, Klein BE,, Jensen SC,, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997; 104: 7–21. [DOI] [PubMed] [Google Scholar]
- 43. Klein R,, Klein BE,, Tomany SC,, Meuer SM,, Huang G-H. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2002; 109: 1767–1779. [DOI] [PubMed] [Google Scholar]
- 44. Klein R,, Klein B,, Knudtson M,, Meuer S,, Swift M,, Gangnon R. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007; 114: 253–262. [DOI] [PubMed] [Google Scholar]
- 45. Zweifel SA,, Imamura Y,, Spaide TC,, Fujiwara T,, Spaide RF. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010; 117: 1775–1781. [DOI] [PubMed] [Google Scholar]
- 46. Bhutto IA,, Baba T,, Merges C,, Juriasinghani V,, McLeod DS,, Lutty GA. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Br J Ophthalmol. 2011; 95: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pikuleva IA,, Curcio CA. Cholesterol in the retina: the best is yet to come. Prog Retin Eye Res. 2014; 41: 64–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schmitz-Valckenberg S,, Alten F,, Steinberg JS,, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 5009–5015. [DOI] [PubMed] [Google Scholar]
- 49. Ferris F,, III,, Wilkinson C,, Bird A,, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013; 120: 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saade C,, Smith RT. Reticular macular lesions: a review of the phenotypic hallmarks and their clinical significance. Clin Exp Ophthalmol. 2014; 42: 865–874. [DOI] [PubMed] [Google Scholar]
- 51. Ooto S,, Suzuki M,, Vongkulsiri S,, Sato T,, Spaide RF. Multimodal visual function testing in eyes with nonexudating age-related macular degeneration. Retina. 2015; 35: 1726–1734. [DOI] [PubMed] [Google Scholar]
- 52. Querques G,, Massamba N,, Srour M,, Boulanger E,, Georges A,, Souied EH. Impact of reticular pseudodrusen on macular function. Retina. 2014; 34: 321–329. [DOI] [PubMed] [Google Scholar]
- 53. Alten F,, Heiduschka P,, Clemens CR,, Eter N. Longitudinal structure/function analysis in reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2014; 55: 6073–6081. [DOI] [PubMed] [Google Scholar]
- 54. Flamendorf J,, Agrón E,, Wong WT,, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015; 122: 2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]


