Abstract
BACKGROUND
Clinical trials report improvements in function and perfusion with direct injection of bone marrow cells into the hearts of patients with ischemic cardiomyopathy. Preclinical data suggest these cells improve vascular density, which would be expected to decrease fibrosis and inflammation.
OBJECTIVES
We tested the hypothesis that bone marrow stem cells (CD34+) will improve histologic measurements of vascularity, fibrosis, and inflammation in human subjects undergoing left ventricular assist device (LVAD) placement as a bridge to cardiac transplantation.
METHODS
Subjects with ischemic cardiomyopathy who were scheduled for placement of an LVAD as a bridge to transplantation underwent bone marrow aspiration the day prior to surgery; the bone marrow was processed into cell fractions (bone marrow mononuclear cells, CD34+, and CD34−). At LVAD implantation, all fractions and a saline control were injected epicardially into predetermined areas and each injection site marked. At transplant, injected areas were collected. Data were analyzed by paired Student t test comparing the effect of cell fractions injected within each subject.
RESULTS
Six subjects completed the study. There were no statistically significant differences in complications with the procedure versus control subjects. Histologic analysis indicated that myocardium injected with CD34+ cells had decreased density of endothelial cells compared to saline-injected myocardium. There were no significant differences in fibrosis or inflammation between groups; however, density of activated fibroblasts was decreased in both CD34+ and CD34− injected areas.
CONCLUSIONS
Tissue analysis does not support the hypothesis that bone marrow-derived CD34+ cells promote increased vascular tissue in humans with ischemic cardiomyopathy via direct injection.
Keywords: angiogenesis, cell therapy, heart failure, ischemia
Ischemic heart disease is characterized by a loss of myocardial cells and vasculature leading to end-stage heart failure. Cell therapy offers an appealing solution to replace these components and restore normal structure and function. Bone marrow cells are an easily accessible source of cells that have been shown to differentiate into cardiac components that potentially provide paracrine factors to improve healing of injured myocardium.
More than a decade ago, studies in small animal models showed that injection of bone marrow-derived stem cells improved cardiac remodeling following myocardial infarction (MI) (1). Specifically, injection of either bone marrow stem cells (CD34+) or bone marrow mononuclear cells (BMMC) improved vascularity, decreased scar burden, and resulted in improved cardiac function compared to noninjected animals (2,3). Follow-up studies using large animal models of bone marrow cell injection following MI showed similar results (4,5).
In vitro studies of human bone marrow cells produced tantalizing data that these findings could be easily translated. Stem cells isolated from bone marrow or circulated blood carrying the markers CD34 and/or CD133 can differentiate into endothelial cells and form vascular structures (6,7). Furthermore, in vitro studies have demonstrated that other bone marrow cell components, including CD34+ cells and mesenchymal stem cells (MSC), can provide paracrine factors to promote angiogenesis and cardiomyocyte cell survival; the same is seen when these cells are transplanted into immune-deficient rodents (8–11).
Clinical trials of bone marrow cell therapy in humans with ischemic heart disease have not yielded as dramatic results as in animals. Direct injection of bone marrow cells into ischemic myocardium has shown the most benefit in a review of these trials (12). Some subjects with chronic ischemic cardiomyopathy undergoing intra-myocardial injection with BMMCs have improved function and perfusion on noninvasive imaging studies compared to untreated subjects (13–18). However, other trials, in similar populations, have failed to show a significant benefit to this therapy (19). Furthermore, a trial in subjects with nonischemic cardiomyopathy showed similar benefit (20), implying that the in vivo mechanisms responsible for improvements in animal models remain to be validated in humans.
We sought to analyze the potential mechanisms by which bone marrow cells could improve cardiac function in ischemic cardiomyopathy. We developed a novel model in which tissue from humans with severe ischemic heart disease could be analyzed for structural changes following injection of bone marrow-derived cell fractions. Our studies revealed that animal studies do not necessarily predict the effects of cell therapy in humans, but do point to potential therapeutic beneficial effects. Furthermore, these studies shed light on the dynamic nature of repair even in the presence of severe ischemic cardiomyopathy.
METHODS
Twenty-six subjects with ischemic cardiomyopathy scheduled for placement of a left ventricular assist device (LVAD) as a bridge to transplantation (BTT) were screened for the trial (Figure 1). Inclusion criteria included age >18 years, ischemic cardiomyopathy, and need for LVAD implantations as a BTT. Exclusion criteria included presence of an intra-aortic balloon pump, emergent LVAD, and inability of the subject to consent. Eight subjects consented to the study. One subject withdrew and a second was too unstable for injection. Of the 18 subjects who refused consent or met exclusion criteria, 13 consented to safety data collection as controls.
FIGURE 1. Study Enrollment.
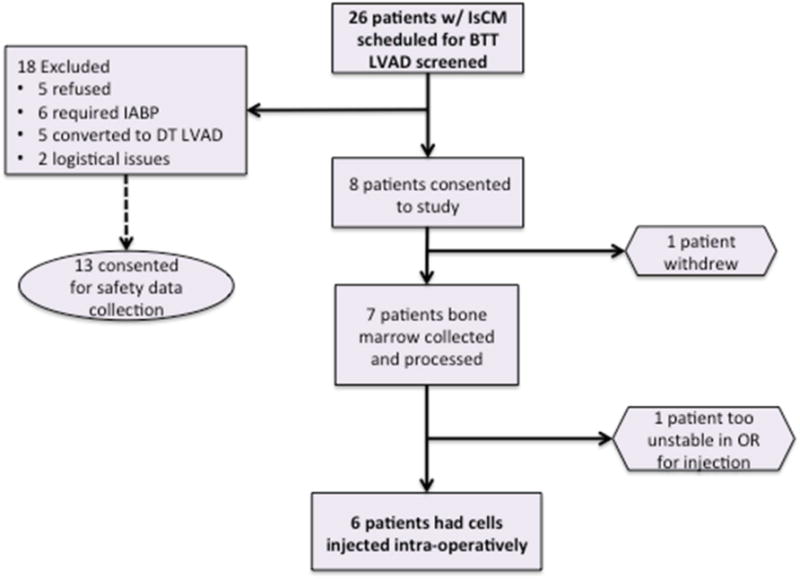
Of the 18 originally excluded patients, 13 consented to safety data collection, serving as controls.
BTT = bridge to transplantation LVAD; DT = destination therapy LVAD; IABP = intra-aortic balloon pump; IsCM = ischemic cardiomyopathy; LVAD = left ventricular assist device; OR = operating room.
The day prior to LVAD implantation, 15 to 20 cc of marrow was collected from the iliac crest and transported to the Fred Hutchinson Cell Therapeutics facility. Marrow was processed into 3 fractions for injection: 0.5 × 106 CD34+ cells, 1.0 × 106 CD34− cells, and 1.0 × 106 BMMC cells, and placed in numbered vials. An additional vial was loaded with 1 cc of Plasmalyte as a control. The contents of all vials were blinded to the investigators (Online Figure 1). The doses of cells correspond to the average cell number per injection site reported in earlier clinical trials (Online Table 1).
Preoperatively, technetium single photon emission computed tomography rest scans were reviewed and areas of 50% to 75% perfusion identified for injection. In the operating room, the contents of each vial were drawn up into a sterile syringe fitted with a sterile 25 gauge needle. Pilot sheering studies indicated minimal cell death (viability >98% by Trypan Blue stain) with this diameter. Areas to be injected were identified on the heart’s surface and a 1 cm diameter marker sewn on. The cells for each fraction and for saline were injected separately into the area’s 4 quadrants. Thus, each area received 4 250 ul injections of a single cell fraction or saline. Following injection, the LVAD implantation proceeded per routine.
Case report forms were completed 24 hours and 7 days following the LVAD implantation. Usage of blood products [packed red blood cells (PRBC), fresh frozen plasma (FFP), and platelets (PLT)] was recorded at each time point. Chart notes, 12-lead electrocardiograms (ECG), and telemetry were reviewed to define episodes of ventricular tachycardia in the postoperative period. Safety data were reviewed by the principal investigator and the safety monitor following each subject enrollment to assess for safety endpoints that would preclude enrollment of additional subjects.
TISSUE HARVEST AND ANALYSIS
At the time of transplant, the explanted heart was obtained and the markers identified. The area below each marker was excised from the epicardial to endocardial surface. Following excision, this cube was cut into 4 sections to correspond to each injected region. Two sections were processed for histology and 2 were frozen in liquid nitrogen. One histologic sample was placed in sucrose formaldhyde and embedded in paraffin. The second histologic sample was flash frozen in OCT. For each endpoint, analysis was performed on 5 50 micron step sections. Antibodies or staining was used as follows for histologic endpoints: picrosirius red for fibrillar collagen, CD31 (1A10, Leica Microsystems Inc., Buffalo Grove, Illinois) for endothelial cells, CD68 (M0876, Dako North America, Inc., Carpinteria, California) for macrophages, alpha-smooth muscle actin (alpha-SMA) (1A4, Dako) for activated fibroblasts, and Ki-67 (M7240, Dako) for proliferating cells. Quantification of picrosirius red, CD31, and alpha-SMA was performed by an automated image analysis system. To quantify activated fibroblast content, alpha-SMA+ cells in blood vessel walls (smooth muscle cells) were manually excluded. Quantification of macrophage density was performed by manual count of CD68+ cells. Quantification of blood vessels was performed by manual count of circular CD31+ structures. Vessels with 2 or fewer nuclei and no smooth muscle media were counted as micro-vessels. Vessels with >2 nuclei or containing a smooth muscle rich media were counted as macro-vessels. All analyses were performed by blinded observers.
STATISTICAL ANALYSIS
To account for the effect of injection on tissue structure, a saline injected segment was used as the control. Since each subject had their own control measurement within 1 of the 4 quadrants, we were able to examine the difference of each treatment from control within each subject. Outcome measurements were averaged across samples for each subject, so each outcome measure has 1 value per treatment for each subject. The comparison of each treatment to the control is a distinct biologic question of interest. For each outcome measure, we computed a 2-tailed paired Student t test to compare each treatment to control. No adjustments for multiple comparisons were applied. Analysis of safety endpoints was performed using independent sample Student t tests and Fisher’s exact test to compare patients in the study group and the control group. Exploratory endpoints were examined using Pearson correlations to compare results from CD34+ and CD34-depleted treatments.
RESULTS
Six patients completed both bone marrow aspiration and cell injection at the time of LVAD placement; their clinical characteristics are seen in Table 1. Age and incidence of diabetes was similar to previous populations of patients with ischemic heart disease eligible for cardiac transplantation. Eleven subjects were on HMG-CoA reductase inhibitors. Content of CD34+ cells varied between subjects; however, all subjects had robust representation of CD133+ cells in the CD34+ cell fraction. One subject had insufficient CD34+ cells for injection, but received injection of BMMC fraction and saline. Harvest of injected tissue occurred at cardiac transplant, and time between LVAD placement and transplant ranged between 47 and 374 days.
TABLE 1.
Subject Characteristics
| Subject | Sex | Age (yrs) | Injection to Transplant (days) | Diabetes Pre-LVAD | Insulin Pre-LVAD | % CD34+ in Bone Marrow | % CD133+ in CD34+ Fraction |
|---|---|---|---|---|---|---|---|
| A1 | M | 70 | 177 | N | N | 3.5 | 57.3 |
| A2 | M | 57 | 241 | Y | Y | 0.6* | 46.9 |
| A3 | M | 63 | 87 | Y | N | 2.9 | 65.2 |
| A4 | M | 60 | 374 | Y | Y | 10.8 | 71.6 |
| A5 | F | 54 | 42 | Y | Y | 5.2 | 44.6 |
| A6 | M | 60 | 53 | Y | N | 3.2 | 75.6 |
| All† | M:83% | 61 ± 6 | 162 ± 129 | Y:83% | Y:50% | 4.4 ± 3.5 | 60.2 ± 13 |
Insufficient CD34+ cells (<0.5 × 106) could be isolated for injection. Value included in calculation of mean and SD.
Values are mean ± SD or %.
LVAD = left ventricular assist device.
Comparison of safety endpoints between injected patients and controls who underwent LVAD implantation without injection showed no statistically significant differences in bleeding as quantified by blood product use at 1 and 7 days postoperatively (PRBC 4 ± 2.2 vs. 4.6 ± 3.8 units; p = 0.66; FFP 6 ± 7.8 vs. 6 ± 3.2 units; p > 0.9; PLT 1.3 ± 1.2 vs. 2.3 ± 2.4 6-pack units; p = 0.25 by independent samples t-test; Online Figure 2). Furthermore, there were no statistically significant differences in the incidence of ventricular arrhythmias in the first week post-LVAD between injected subjects and un-injected controls (4/6 vs. 8/13; p > 0.9 by Fisher exact test). There were no deaths or re-operations in either group.
PRIMARY ENDPOINTS
To test the hypothesis that injection of bone marrow-derived CD34+ stem cells would improve vascularity, we quantified the density of endothelial cells as measured by CD31 staining using automated image analysis. Surprisingly, the areas injected with CD34+ cells had significantly fewer endothelial cells in comparison to saline injected areas (0.0058 ± 0.0032 vs. 0.013 ± 0.0065 % CD31+ area; n = 5; p = 0.02 by paired Student t test; Figure 2). Although not statistically significant, there was a trend toward a reduction in endothelial density in regions injected with both the BMMC and CD34-depleted cell fractions (BMMC 0.0068 ± 0.0043 vs. saline 0.011 ± 0.0079, n = 6; p = 0.2; CD34-depleted 0.0085 ± 0.0071 vs. saline 0.013 ± 0.0065 % CD31-positive area, n = 5; p = 0.2). Measurement of microvascular density showed no statistically significant difference between saline and CD34+ cell injected areas (55.7 ± 4.7 vs. 59.8 ±16.7 vessels/mm2; p = 0.6).
FIGURE 2. Injection of CD34+ Cells: No Increased Endothelial Density.
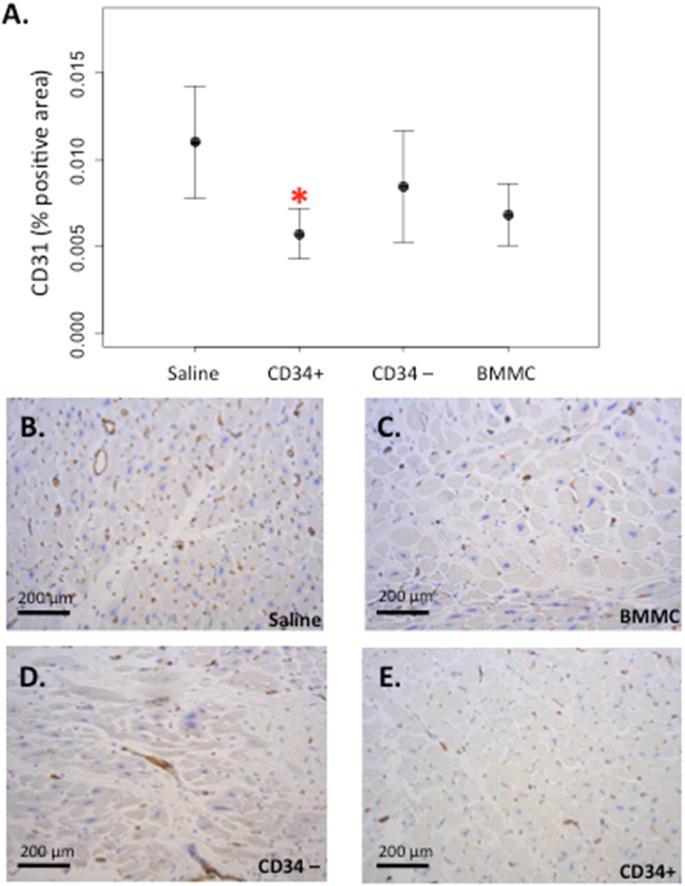
(A) Endothelial density quantified as percent positive area stained for CD31 (mean ± SE); *p = 0.02 versus saline (B–E) Representative sections of CD31 immunohistochemistry from a single subject. BMMC = bone marrow mononuclear cells.
Because stem cells may modulate cardiomyocyte survival, we measured fibrosis as a common endpoint for a beneficial effects of bone marrow cells on cardiac structure. Overall, there was no statistically significant improvement in fibrosis between tissue injected with CD34+, CD34-depleted, or BMMC compared to saline injected tissue (32.4 ± 11.4, n = 5; 31.7 ± 15.8, n = 5; 33.3 ± 16.1, n = 6; 28.0 ± 10.6, n = 6, % picrosirius red-positive area, respectively; p > 0.5 for each treatment compared to saline; Figure 3A). Analysis of individual results showed a marked variability in response of fibrosis to cell therapy (Figure 3B) although histology showed significant fibrosis remained in treated subjects independent of response (Figure 3C–F).
FIGURE 3. Injection of Bone Marrow-derived Cells: No Decreased Fibrosis.
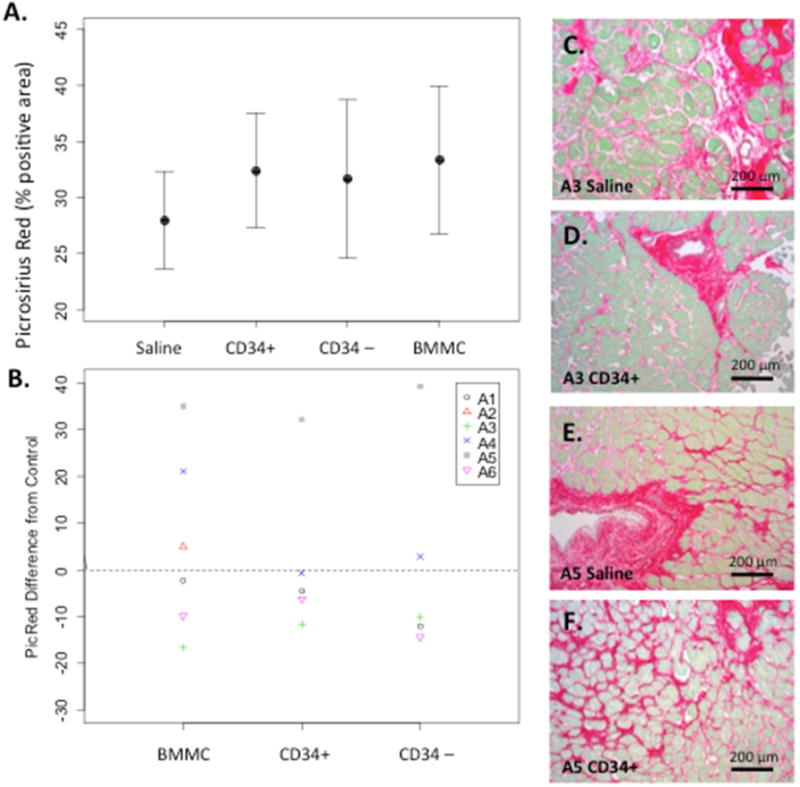
(A) Cardiac fibrosis quantified as percent positive picrosirius red area (mean ± SE). (B) Changes in fibrosis in individual subjects A1-A6 by treatment group. Subject A5 is the only female subject. (C–F) Representative sections showing fibrosis for subjects A3 and A5 in saline and CD34+ injected tissue. Abbreviations as in Figure 2.
SECONDARY ENDPOINTS
To determine if injection of specific bone marrow cell fractions modulated inflammation, activation of myofibroblasts, or cell proliferation, we quantified the density of CD68 (a marker of macrophages), alpha-SMA (a marker of activated fibroblasts and vascular smooth muscle cells), and Ki-67 (present in the nuclei of dividing cells). There were no statistically significant differences in macrophage density between saline-injected tissues and tissues injected with any cell type (208.6 ± 36.3, n = 5; 206.2 ± 30.7, n = 5; 201.3 ± 50.2, n = 6; 197.9 ± 95.5, n = 6; cells/mm2 for CD34+, CD34-depleted, BMMC, and saline respectively; p ≥ 0.5 for each treatment comparison to saline; Figure 4A). On the other hand, there were significantly fewer activated myofibroblasts (defined as blood vessel-independent alpha-SMA positive) in tissues injected with either CD34+ or CD34-depleted cells in comparison to saline-injected areas (CD34+ 4.6 ± 1.2 vs. saline 5.6 ± 0.5, n = 5; p = 0.04; CD34-depleted 4.8 ± 0.9 vs. 5.6 ± 0.5, n = 5; p = 0.014, % alpha-SMA positive area; Figure 4B).
FIGURE 4. Effects of Bone Marrow-derived Cell Injection on Beneficial Remodeling.
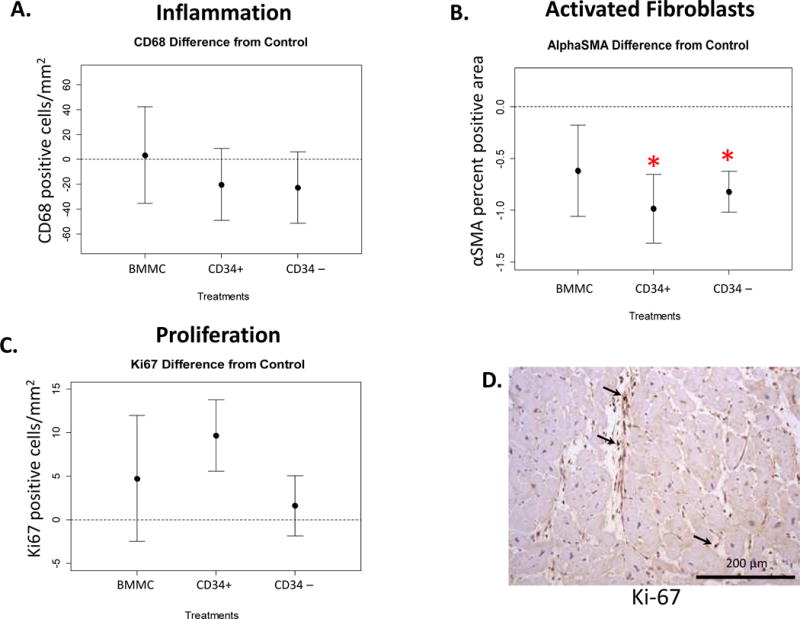
Plots represent the difference from saline-injected sample (mean ± SE) for (A) macrophages (CD68 positive); (B) activated fibroblasts (alpha-smooth muscle actin [αSMA] positive, exclusive of blood vessels; *p = 0.04 for CD34+ versus saline and p = 0.01 for CD34-depleted versus saline); and (C) proliferating cells (Ki-67 positive; *p = 0.08 versus saline). (D) Representative micrograph of Ki-67 immunohistochemistry in CD34+ cell injected tissue.
Proliferating cells were seen in the interstitial space in all segments as measured by Ki-67 positivity. However, there were no differences in proliferating cells detected in tissue injected with CD34+ stem cells compared to saline-injected tissue (24.4 ± 6.4 vs. 14.7 ± 6.7 Ki-67 positive cells/mm2, n = 5; p = 0.08; Figure 4C, 4D). Ki-67 positivity was not seen in nuclei contained within myofibrils.
To determine if bone marrow cells can transdifferentiate into cardiac cell components, our protocol included incubation of a fraction of the BMMC with Feridex, a solution of iron nanoparticles. Our labeling protocol resulted in 82.5% iron positivity in BMMC indicating that multiple cell populations were labeled. The first 2 subjects in the protocol underwent injection of Feridex-labeled BMMC cell fractions. However, soon after their enrollment, Feridex was taken out of clinical production and further subjects were unable to undergo cell labeling. Analysis of tissues for iron-labeled cells using Prussian blue stain and matching with adjacent sections with various immunohistochemistry cell phenotypes showed that most iron labeled cells were also positive for the macrophage marker CD68 (Figure 5A, B). No Prussian blue positive, CD31+ cells were detected.
FIGURE 5. Effects of Bone Marrow-derived Cells and Cell Phenotype.
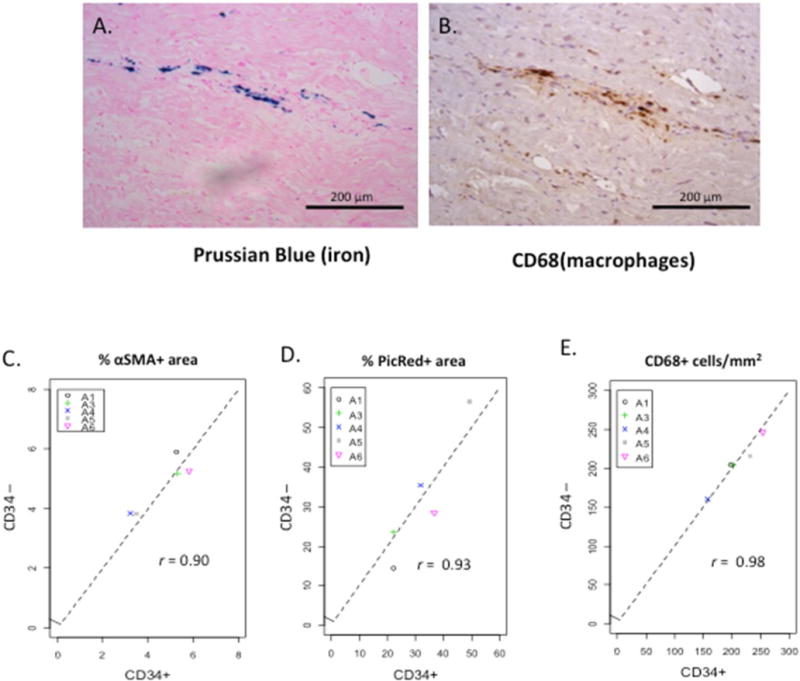
The effects of bone marrow-derived cells may be independent of the injected cell phenotype as evidenced by (A and B) iron-positive cells in injected tissue, and (C–E) Pearson correlation coefficient between tissue injected with CD34+ versus CD34-depleted cell populations. Abbreviations as in Figure 4.
EXPLORATORY ENDPOINTS
Because our results were contrary to the published literature and human subjects have great genetic variability, we performed several exploratory studies to determine if analysis of individual patient results would be illuminating. Analysis of the relationship of the time between cell injection and tissue harvest revealed no differences in our primary endpoints of endothelial density or fibrosis between subjects harvested early (<100 days) versus late (>170 days) following injection of cells. To test whether cell effects were specific to CD34+ stem cells, we correlated results from areas injected with CD34+ and CD34-depleted areas. Intriguingly, there was a tight correlation in the density of macrophages, activated myofibroblasts, and fibrosis between tissue injected with the stem cell versus the nonstem cell fraction (Pearson correlation of 0.98, 0.90, 0.93 and p = 0.004, 0.035, 0.023 respectively, Figure 5C, D, E).
DISCUSSION
In this unique trial we demonstrated the safety and feasibility of direct injection of cell therapeutic products into the hearts of human subjects with ischemic cardiomyopathy undergoing LVAD implantation (Central Illustration). Furthermore, we show this strategy can be used in a bridge-to-transplant population to determine the mechanisms by which these cells alter cardiac structure. Our data support the importance of these mechanistic studies, as the effects of the cell fractions in humans with advanced heart disease following LVAD implantation were not as predicted from other models. Specifically, injection of CD34+ stem cells did not result in a statistically significant increase in vascularity in ischemic myocardium as defined by 2 separate measures: density of CD31+ endothelial cells and number of manually counted blood vessels. Furthermore, there were no statistically significant differences in fibrosis or macrophage density in cell- versus saline-injected segments. Although the study lacked the power to detect small changes, the lack of dramatic improvements in histologic structure makes it unlikely that these cell therapeutics would result in meaningful functional improvements in this patient population.
On the other hand, several of our results support the hypothesis that cell therapy may modulate some elements of cardiac biology in subjects following LVAD implantation and provide targets for future studies. Areas injected with either CD34+ or CD34-depleted cells had a significantly decreased density of myofibroblasts. Although this finding may represent a Type 2 error, it was intriguing that the measurements of myofibroblasts, fibrosis, and macrophage density were highly correlated between areas injected with CD34+ and CD34-depleted fractions, raising the hypothesis that changes in myofibroblast number are insufficient to alter fibrosis and inflammation in this population. Furthermore, our data demonstrating the persistence of iron particles from labeled cells imply that most injected cells eventually died, consistent with animal studies (21). However, as only 2 subjects received labeled cells it does not rule out the possibility of persistence or trans-differentiation of small numbers of injected cells.
A substantial literature in animal models and ex vivo human cell culture has demonstrated the ability of bone marrow-derived CD34+ stem cells to both differentiate into vascular cells and promote vessel formation via paracrine factors (2,22–24). Thus, use of these cells to therapeutically increase perfusion in patients with ischemic heart disease was rapidly translated to clinical trials. These trials have targeted different patient populations (post-MI, refractory angina, and ischemic cardiomyopathy); used a variety of cell fractions (BMMC, CD34+, CD133+, and MSC); and delivered cells via intracoronary, direct epicardial injections, and catheter-delivered endocardial injections. A meta-analysis and Cochrane Review of published trials to date has shown modest improvements in ventricular function with these cells at both early and late time-points following injection (12,25). Moreover, many of these trials demonstrated improvements in noninvasive measurements of myocardial perfusion supporting the hypothesis that bone marrow cell therapy directly increases vascularity (26).
Patients with ischemic cardiomyopathy represent a growing population and increasingly many of these patients are undergoing LVAD placement (27). There are reports of myocardial recovery following LVAD implantation; however, these cases have been predominantly in subjects with idiopathic cardiomyopathy (28,29). It is presumed that in patients with ischemic disease, both the presence of fibrosis and decreased myocardial perfusion contribute to a process that is irreversible. We sought to determine if recovery could be augmented in this population via injection of cells to improve vascularity with subsequent decrease in fibrosis. Unfortunately, our study failed to demonstrate dramatic improvements in vascularity or decreases in fibrosis on direct histologic assessment.
There are several possibilities why our data may be discordant with other preclinical and clinical studies. This is the first study to test the angiogenic properties of bone marrow cells in unloaded myocardium. Myocardial unloading by the LVAD results in decreased oxygen utilization by the heart (30) and other changes that may independently alter the angiogenic properties of the cardiac tissue (31). Furthermore, the aortic valve is closed during the majority of cardiac cycles and flow is nonpulsatile in LVAD patients, which may modulate flow-mediated signals for angiogenesis (32,33). On the other hand, in comparison to other clinical populations, the consistent unloading of the LVAD provided a relatively homogenous environment for the cells that may obviate the need to control for changes in filling pressures or new ischemic events in our population.
Second, the quality of the cell product could have contributed to the lack of effect. Several studies have noted a correlation between patient characteristics and angiogenic activity of stem cells. Stem cells from older subjects or those with comorbid risk factors, such as hypertension and diabetes, may be less robust at promoting angiogenesis by both trans-differentiation or paracrine mechanisms (34). All of our subjects were older, and 5 of our 6 subjects had diabetes potentially impacting the angiogenic capacity of the bone marrow cell fractions (35–37). Due to the small amount of bone marrow collected, we had insufficient cells to test angiogenic properties of the cell fractions. However, we did confirm significant enrichment of CD133+ cells in the CD34+ fractions in all subjects. Furthermore, when analyzed as cells injected per volume of tissue, our doses were close to other studies in patients with similar clinical characteristics (Online Table 1), if extrapolated to cells injected per cubic centimeter (13,16). On the other hand, these doses are less than doses in more recent studies focused on the antianginal effect of cells in patients with similar risk factors (38,39).
A final explanation for the discrepancy between our data and other models is the significant difference in the pathophysiology of advanced human cardiomyopathy versus acute MI in both human subjects and animal models. Unlike models in which a discrete infarction is generated and cell therapeutics given soon afterward, our human subjects had longstanding neurohormonal activation with chronic remodeling of even normally perfused areas of myocardium. This fibro-inflammatory mileu may alter tissue response to cell therapy. Furthermore, as opposed to other studies, all of our subjects had been exposed to many years of medical therapy, which may alter the response of their myocardium to cell therapies.
STUDY LIMITATIONS
Limitations of the study include: 1) left ventricle is unloaded during LVAD and remodeling and effect of cell injection may not be the same as in a nonunloaded heart; 2) small numbers increase risk of both Type I and Type II statistical errors; and 3) Older age of donor cells may have affected capacity for regeneration.
CONCLUSIONS
Injection of bone marrow-derived cells at the time of LVAD implantation is safe and feasible and may provide mechanistic insights into cell therapy. CD34+ stem cells do not increase vascularity in the unloaded, ischemic ventricle. Injection of cells may induce cellular changes that are independent of the origin of the cell therapeutic delivered. However, these data suggest that preclinical studies of cardiovascular cell therapies are insufficient to predict results in human subjects with chronic cardiomyopathy and LVADs. Similar mechanistic studies should be performed prior to large-scale clinical trials of other therapeutic cell products.
Supplementary Material
CENTRAL ILLUSTRATION Cell Therapy in the Human Heart.
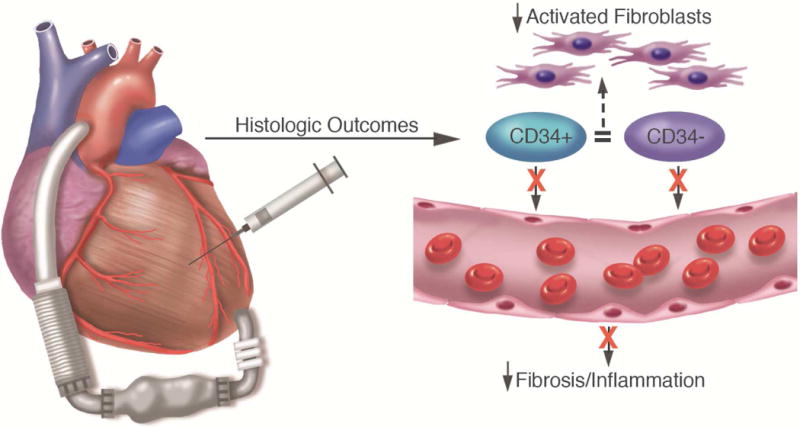
Injection of bone marrow fractions into the heart does not increase vascularity but may decrease activated fibroblasts and induce proliferation of resident cardiac cells in human subjects with end-stage heart disease and left ventricular assist device support.
PERSPECTIVES.
Competency in Medical Knowledge
Fibrosis and inflammation are common features of the myocardium of patients with end-stage heart failure. Although bone marrow derived stem cell therapy promotes vascularity in preclinical models of acute myocardial injury, this approach does not translate into histological benefit in patients with chronic ischemic heart disease supported with left ventricular assist devices who are awaiting cardiac transplantation.
Translational Outlook
Better mechanistic models that more closely mimic chronic human heart disease are needed to inform the design of clinical trials of stem cell therapy.
Acknowledgments
The authors would like to acknowledge Gordon Cohen, MD, who served as the safety monitor for the study, Creighton Don for assistance with data collection, Susanne Steffes for research coordination and Veronica Poppas and Eileen Friedman for assistance with histology.
Funding Sources: NIH R01 HL094384 (to ASO), UL1TR000423 (UW Institute for Translational Health Sciences), and the Tall Family Foundation, Seattle Washington
ABBREVIATIONS
- BMMC
bone marrow mononuclear cells
- FFC
fresh frozen plasma
- LVAD
left ventricular assist device
- MI
myocardial infarction
- PRBC
packed red blood cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts: none
References
- 1.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 2.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 3.Thompson RB, van den Bos EJ, Davis BH, et al. Intracardiac transplantation of a mixed population of bone marrow cells improves both regional systolic contractility and diastolic relaxation. J Heart Lung Transplant. 2005;24:205–14. doi: 10.1016/j.healun.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Tse HF, Siu CW, Zhu SG, et al. Paracrine effects of direct intramyocardial implantation of bone marrow derived cells to enhance neovascularization in chronic ischaemic myocardium. Eur J Heart Fail. 2007;9:747–53. doi: 10.1016/j.ejheart.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–52. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 7.Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, Deliliers GL. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol. 2001;115:186–94. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- 8.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol. 2007;42:441–8. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawamoto A, Tkebuchava T, Yamaguchi J, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–8. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 10.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–8. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 11.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 12.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–68. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel AN, Geffner L, Vina RF, et al. Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: a prospective randomized study. J Thorac Cardiovasc Surg. 2005;130:1631–8. doi: 10.1016/j.jtcvs.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 14.van Ramshorst J, Bax JJ, Beeres SL, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 15.Perin EC, Dohmann HF, Borojevic R, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 16.Stamm C, Kleine HD, Choi YH, et al. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–25. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 17.Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792–6. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717–26. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seth S, Narang R, Bhargava B, et al. Percutaneous intracoronary cellular cardiomyoplasty for nonischemic cardiomyopathy: clinical and histopathological results: the first-in-man ABCD (Autologous Bone Marrow Cells in Dilated Cardiomyopathy) trial. J Am Coll Cardiol. 2006;48:2350–1. doi: 10.1016/j.jacc.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 21.Terrovitis J, Stuber M, Youssef A, et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555–62. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- 22.Barclay GR, Tura O, Samuel K, et al. Systematic assessment in an animal model of the angiogenic potential of different human cell sources for therapeutic revascularization. Stem Cell Res Ther. 2012;3:23. doi: 10.1186/scrt114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Zhang S, Rabinovich B, et al. Human CD34+ cells in experimental myocardial infarction: long-term survival, sustained functional improvement, and mechanism of action. Circ Res. 2010;106:1904–11. doi: 10.1161/CIRCRESAHA.110.221762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawamoto A, Iwasaki H, Kusano K, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–9. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 25.Clifford D, Fisher S, Brunskill S, et al. Stem cell treatment of acute myocardial infarction. Cochrane Database of Systemic Reviews. 2012;2:CD006536. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 26.Premer C, Hare JM. Can endothelial progenitor cells treat patients with refractory angina? Circ Res. 2014;115:904–7. doi: 10.1161/CIRCRESAHA.114.305284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirklin JK, Naftel DC, Kormos RL, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–56. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Maybaum S, Mancini D, Xydas S, et al. Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD Working Group. Circulation. 2007;115:2497–505. doi: 10.1161/CIRCULATIONAHA.106.633180. [DOI] [PubMed] [Google Scholar]
- 29.Drakos SG, Kfoury AG, Stehlik J, et al. Bridge to recovery: understanding the disconnect between clinical and biological outcomes. Circulation. 2012;126:230–41. doi: 10.1161/CIRCULATIONAHA.111.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xydas S, Rosen RS, Pinney S, et al. Reduced myocardial blood flow during left ventricular assist device support: a possible cause of premature bypass graft closure. J Heart Lung Transplant. 2005;24:1976–9. doi: 10.1016/j.healun.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Hall JL, Grindle S, Han X, et al. Genomic profiling of the human heart before and after mechanical support with a ventricular assist device reveals alterations in vascular signaling networks. Physiol Genomics. 2004;17:283–91. doi: 10.1152/physiolgenomics.00004.2004. [DOI] [PubMed] [Google Scholar]
- 32.Galie PA, Nguyen DH, Choi CK, Cohen DM, Janmey PA, Chen CS. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci U S A. 2014;111:7968–73. doi: 10.1073/pnas.1310842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cullen JP, Sayeed S, Sawai RS, et al. Pulsatile flow-induced angiogenesis: role of G(i) subunits. Arterioscler Thromb Vasc Biol. 2002;22:1610–6. doi: 10.1161/01.atv.0000034470.37007.58. [DOI] [PubMed] [Google Scholar]
- 34.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 35.Li TS, Kubo M, Ueda K, et al. Identification of risk factors related to poor angiogenic potency of bone marrow cells from different patients. Circulation. 2009;120:S255–61. doi: 10.1161/CIRCULATIONAHA.108.837039. [DOI] [PubMed] [Google Scholar]
- 36.Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res. 2002;90:E89–93. doi: 10.1161/01.res.0000020861.20064.7e. [DOI] [PubMed] [Google Scholar]
- 37.Jarajapu YP, Hazra S, Segal M, et al. Vasoreparative Dysfunction of CD34+ Cells in Diabetic Individuals Involves Hypoxic Desensitization and Impaired Autocrine/Paracrine Mechanisms. PloS One. 2014;9:e93965. doi: 10.1371/journal.pone.0093965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Povsic TJ, Junge C, Nada A, et al. A phase 3, randomized, double-blinded, active-controlled, unblinded standard of care study assessing the efficacy and safety of intramyocardial autologous CD34+ cell administration in patients with refractory angina: design of the RENEW study. Am Heart J. 2013;165:854–861 e2. doi: 10.1016/j.ahj.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Jimenez-Quevedo P, Gonzalez-Ferrer JJ, Sabate M, et al. Selected CD133+ Progenitor Cells to Promote Angiogenesis in Patients With Refractory Angina: Final Results of the PROGENITOR Randomized Trial. Circ Res. 2014;115:950–60. doi: 10.1161/CIRCRESAHA.115.303463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


