Abstract
The roles of mast cells in health and disease remain incompletely understood. While the evidence that mast cells are critical effector cells in IgE-dependent anaphylaxis and other acute IgE-mediated allergic reactions seems unassailable, studies employing various mice deficient in mast cells or mast cell-associated proteases have yielded divergent conclusions about the roles of mast cells or their proteases in certain other immunological responses. Such “controversial” results call into question the relative utility of various older versus newer approaches to ascertain the roles of mast cells and mast cell proteases in vivo. This review discusses how both older and more recent mouse models have been used to investigate the functions of mast cells and their proteases in health and disease. We particularly focus on settings in which divergent conclusions about the importance of mast cells and their proteases have been supported by studies that employed different models of mast cell or mast cell protease deficiency. We think that two major conclusions can be drawn from such findings: (1) no matter which models of mast cell or mast cell protease deficiency one employs, the conclusions drawn from the experiments always should take into account the potential limitations of the models (particularly abnormalities affecting cell types other than mast cells) and (2) even when analyzing a biological response using a single model of mast cell or mast cell protease deficiency, details of experimental design are critical in efforts to define those conditions under which important contributions of mast cells or their proteases can be identified.
1. MAST CELL BIOLOGY
1.1. Origin and tissue distribution of mast cells
Mast cells (MCs) are long-lived granulated cells derived from hematopoietic precursors; such MC progenitors ordinarily are found only in small numbers in the blood and complete their differentiation and maturation in the microenvironments of almost all vascularized tissues (Douaiher et al., 2014; Galli, Grimbaldeston, & Tsai, 2008; Gurish & Austen, 2012; Moon et al., 2010). Like cells in the monocyte lineage, mature MCs located in the tissues can proliferate after appropriate stimulation (Galli, Borregaard, & Wynn, 2011). In addition, increased recruitment, survival, and maturation of MC progenitors may also contribute to the local expansion of MC populations (Galli et al., 2008; Gurish & Austen, 2012). Stem cell factor (SCF), the ligand for Kit, is produced by structural cells in the tissues (and also by MCs) and plays a crucial role in MC development, survival, migration, and function (Douaiher et al., 2014; Galli, Zsebo, & Geissler, 1994; Gurish & Austen, 2012; Moon et al., 2010). Other growth factors (Galli et al., 2008; Gurish & Austen, 2012) that have been shown to influence MC growth and survival include interleukin (IL)-3, IL-4, IL-9, IL-10, IL-33, and TGF-β. MCs are distributed throughout nearly all tissues, and often in close proximity to potential targets of their mediators such as epithelia and glands, smooth muscle and cardiac muscle cells, fibroblasts, blood and lymphatic vessels, and nerves. Mature MCs are particularly abundant in tissues and organs exposed to the external environment, such as the skin, the lung, and the gut (Galli et al., 2008).
1.2. The spectrum of mast cell-derived mediators
MCs can store and release upon degranulation and/or secrete de novo a wide spectrum of biologically active mediators, many of which also can be produced by other cell types. During IgE-associated biologic responses, the antigen-dependent cross-linking of antigen-specific IgE bound to FcεRI on the plasma membrane of MCs induces the aggregation of FcεRI, thereby activating downstream signaling events that lead to the secretion of biologically active products implicated in allergic reactions (Blank & Rivera, 2004; Boyce, 2007; Galli & Tsai, 2012; Metcalfe, Peavy, & Gilfillan, 2009; Rivera, Fierro, Olivera, & Suzuki, 2008). Following antigen binding, MCs very rapidly release into the extracellular space mediators pre-stored in their cytoplasmic granules, for example, vasoactive amines (histamine and serotonin), neutral proteases (tryptases, chymases, and carboxypeptidase A3 [CPA3]), proteoglycans (e.g., heparin), and some cytokines and growth factors by a process called degranulation. A second class of secreted products is generated by de novo synthesis of proinflammatory lipid mediators, such as prostaglandins and leukotrienes. Finally, MCs are also able to synthesize and secrete a large number of growth factors, cytokines, and chemokines, e.g., IL-1, IL-6, IL-10, and TNF-α, VEGF, angiopoietin-1, TGF-β, and many others, with the types and amounts of such products that are released being influenced by factors such as the type and species of origin of the MCs, the nature of the stimulus inducing MC activation (Galli, Kalesnikoff, et al., 2005; Galli, Nakae and Tsai, 2005; Moon et al., 2010), and, in the case of IgE-dependent MC activation, whether the activation is by low- or high-affinity stimuli (Suzuki et al., 2014).
Notably, MCs can be activated to secrete biologically active products not only by IgE and specific antigen, but by a long list of other stimuli including physical agents, products of diverse pathogens (Abraham & St John, 2010), many innate danger signals (Supajatura et al., 2002), certain endogenous peptides and structurally similar peptides found in invertebrate and vertebrate venoms (Akahoshi et al., 2011; Metz et al., 2006; Schneider, Schlenner, Feyerabend, Wunderlin, & Rodewald, 2007), and products of innate and adaptive immune responses including products of complement activation (Schäfer et al., 2012), certain chemokines and cytokines (including IL-33; Enoksson et al., 2011; Lunderius-Andersson, Enoksson, & Nilsson, 2012), and immune complexes of IgG. The ability of MCs to secrete biologically active mediators can be modulated by many factors, including interactions with other granulocytes (Fantozzi et al., 1985), regulatory T cells (Gri et al., 2008), or lymphocytes (Gaudenzio et al., 2009), and certain cytokines, including the main MC development and survival growth factor, the Kit ligand, SCF (Galli, Kalesnikoff, et al., 2005; Galli, Nakae, et al., 2005; Galli, Zsebo, et al., 1994; Hill et al., 1996; Ito et al., 2012), as well as IL-33 (Komai-Koma et al., 2012) and interferon-γ (Okayama, Kirshenbaum, & Metcalfe, 2000). Many mediators which can be produced by MCs have been shown to have various positive or negative effects on the function of diverse immune or structural cells, findings which indicate that MCs at least have the potential to influence inflammation, hemostasis, tissue remodeling, cancer, metabolism, reproduction, behavior, sleep, homeostasis, and many other biological responses (Galli et al., 2008; Gilfillan & Beaven, 2011; Kennelly, Conneely, Bouchier-Hayes, & Winter, 2011; Ribatti & Crivellato, 2011).
1.3. Phenotypic heterogeneity and functional plasticity
Many phenotypic and functional characteristics of MCs, such as proliferation, survival, and ability to store and/or secrete various products, as well as the magnitude and nature of their secretory responses to particular activation signals, can be modulated or “tuned” by many environmental and genetic factors (Galli, Kalesnikoff, et al., 2005; Galli, Nakae, et al., 2005). The properties of individual MCs thus may differ depending on the genetic background of the host and/or the local or systemic levels of factors that affect various aspects of MC biology. This “plasticity” of multiple aspects of MC phenotype can result in the development of phenotypically distinct populations of MCs in various anatomic sites and in different animal species. Such altered expression of MC phenotypes can also be induced during particular biologic responses in vivo.
The extent to which it is useful to subclassify MCs into distinct subtypes based on differences in the phenotype of the cells, and the extent to which such phenotypic differences are “fixed” as opposed to malleable, have been a matter of discussion and debate. However, MCs in some animal species can be placed into “subpopulations” based on readily identifiable features such as differences in the ability of the MCs to synthesize and store various proteases or proteoglycans. In humans, MCs have been classified into those containing mainly tryptase and those containing both tryptase and chymase (Craig & Schwartz, 1989) [although human MCs containing chymase but little or no tryptase also have been described, it seems likely that this is an uncommon population compared to those that contain tryptase with little or no chymase or both tryptase and chymase (Weidner & Austen, 1993; Welle, 1997)].
However, the potential malleability of multiple aspects of MC phenotype makes the classification of MCs into “subsets” challenging, as features of the cells may differ at baseline as opposed to in the settings of immune responses or disease. For example, chymase+/tryptase+ human MCs can have either abundant or negligible amounts of CPA3 in their granules, and tryptasehichymaselowCPA3hi intraepithelial MCs have been detected in the lungs of patients with asthma (Douaiher et al., 2014). In mice, so-called connective tissue-type MCs (CTMCs, which include MCs found in serosal cavities that are sometimes called “serosal MCs”) are distinguished from mucosal MCs (MMCs, a population that is more dependent on T-cell-derived factors than are CTMCs) according to their anatomic localization, morphology, and content of heparin and proteases (Galli, Kalesnikoff, et al., 2005; Galli, Nakae, et al., 2005; Gurish & Austen, 2012; Moon et al., 2010). Notably, as discussed in Section 1.4, the protease content of mouse MCs can change when the cells are transferred to new microenvironments or during the course of certain parasite infections (Friend et al., 1996; Godfraind et al., 1998; Jippo et al., 2001; Lee et al., 1998). Accordingly, no matter which criteria are used to identify the subpopulations of MCs that are present at a particular time in an individual anatomic location under baseline conditions, one should keep in mind the possibility that aspects of MC phenotype (and therefore function) may be influenced by the tissue microenvironment and may change in important ways during ongoing innate or adaptive immune responses or diseases (Galli et al., 2011; Galli, Nakae, et al., 2005).
1.4. Mast cell-associated proteases and their cellular distribution
Various proteases that are largely restricted to MCs occupy a dominant position among the diverse products that are stored within MC secretory granules (Douaiher et al., 2014; Pejler, Åbrink, Ringvall, & Wernersson, 2007; Wernersson & Pejler, 2014) (Fig. 1). These include serine proteases of tryptase and chymase type, which have trypsin-like (i.e., cleave after Lys/Arg residues) and chymotrypsin-like (i.e., cleave after aromatic amino acid resides) substrate specificity, respectively. In addition, MCs can express high levels of CPA3, a Zn-containing exopeptidase that cleaves off amino acid residues (preferentially aromatic amino acid residues) from the C-terminal end of its substrates. In addition to these MC-restricted proteases, it is known that MCs can express and store in their secretory granules a number of proteases whose expression is not restricted to cells of the MC lineage. These include granzyme B, lysosomal cathepsins, active caspase-3, neuropsin/Prss19, cathepsin G, matrix metalloprotease 9 and renin [reviewed in Douaiher et al., 2014; Pejler et al., 2007; Wernersson & Pejler, 2014].
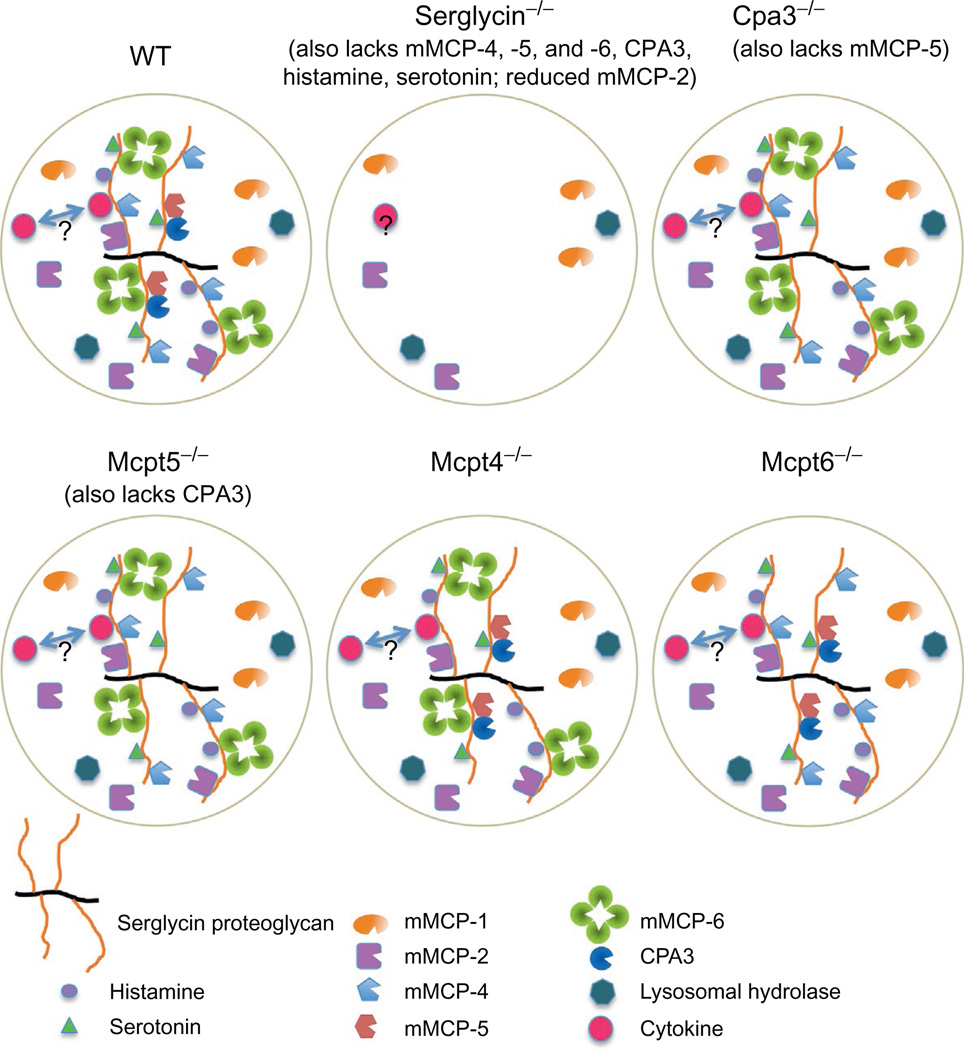
Figure 1. The effect of various gene knockouts on the storage of mast cell (MC) granule compounds.
The figure depicts the granule contents of MCs from wild-type mice of C57BL/6 genetic background. MC granules can contain several preformed compounds, including serglycin proteoglycan, chymases (mMCP-1, mMCP-2, mMCP-4, mMCP-5), tryptases (mMCP-6; mMCP-7 is absent in C57BL/6 mice), CPA3, bioactive amines (histamine, serotonin), various lysosomal hydrolases (such as β-hexosaminidase), and certain cytokines. For simplicity, a hypothetical granule of mixed “CTMC” (expressing mMCP-4, mMCP-5, mMCP-6, CPA3)/“MMC” (expressing mMCP-1, mMCP-2) phenotype is shown. As indicated, many of the granule compounds are stored in complex with serglycin proteoglycan and the absence of serglycin results in impaired storage of such compounds. However, note that several granule constituents (such as mMCP-1) are stored independently of serglycin, whereas others (such as mMCP-2) depend only partially on serglycin for storage. It is not yet established whether any cytokines which can be found in granules depend on serglycin for storage (indicated by “?” in the figure). Note that the absence of CPA3 leads to a secondary defect in the storage of mMCP-5 and vice versa; that is, the absence of mMCP-5 results in impaired CPA3 storage. In contrast, the absence of mMCP-4 or mMCP-6 does not induce pronounced effects on the storage of other granule mediators.
Human MCs express α- and β-tryptase, of which β-tryptase is enzymatically active whereas α-tryptase is essentially devoid of catalytic activity (Caughey, 2011; Douaiher et al., 2014). β-Tryptases are further subdivided into βI-, βII-, and βIII-tryptase, of which βII- and βIII-tryptase are alleles at one locus (TPSB2) and βΙ- and α-tryptase are alleles at a neighboring locus (TPSAB1) (Caughey, 2006; Douaiher et al., 2014). As reviewed by Douaiher et al. (2014), several point mutations have been identified in the human TPSAB1 and TPSB2 genes, and different isoforms of human β-tryptase have been identified which reflect differential splicing of the precursor transcripts, but the functional significance of these observations largely remains to be determined.
Mouse MCs can express two major types of tryptases, mMCP-6 and -7, of which mMCP-6 most likely represents the counterpart to human β-tryptase. A unique feature of all of these tryptases is their tetrameric organization, with the active sites facing inwards toward a narrow central pore (Pereira et al., 1998). Due to this tetrameric organization, tryptases are resistant to all endogenous protease inhibitors and have a relatively narrow substrate cleavage profile. In addition to the tetrameric tryptases, MCs express a monomeric transmembrane tryptase (γ-tryptase; Prss31; TPSG1) and an additional, enzymatically inactive tryptase denoted δ-tryptase (TPSD1) (Hellman & Thorpe, 2014).
In humans, only one MC chymase gene, belonging to the α-chymase family, is expressed (CMA1). In contrast, the corresponding chymase locus in mice has undergone extensive expansion, and encompasses several different MC chymase genes, including one α-chymase (mMCP-5) but also several β-chymases: MC protease (Mcpt)1, Mcpt2, Mcpt4, Mcpt9, and Mcpt10 (Hellman & Thorpe, 2014; Pejler et al., 2007) (the corresponding proteins are denoted mMCP-1, -2, -4, -5, -9, and -10, respectively). Based on amino acid sequence similarities, mMCP-5 may be regarded as the homologue to human chymase, which would suggest that Mcpt5−/− animals might represent the most relevant model for studies of human chymase function. However, mMCP-5 and human chymase have fundamentally divergent substrate cleavage profiles, with mMCP-5 having elastase-like rather than chymotrypsin-like specificity (i.e., cleaves after aliphatic amino acid residues) (Karlson, Pejler, Tomasini-Johansson, & Hellman, 2003). For this reason, mMCP-5 is most likely not the functional counterpart to human chymase. Of the remaining mouse chymases, mMCP-4 has a similar substrate cleavage profile as human chymase, has a similar tissue distribution and also has similar proteoglycan-binding properties (Hellman & Thorpe, 2014; Pejler et al., 2007). In contrast, mMCP-1, -2, and -9 each has a different expression pattern and proteoglycan-binding properties as compared with human chymase. Hence, among the mouse chymases, mMCP-4 may be regarded as a close functional homologue to human chymase, and studies conducted with mMCP-4-deficient animals may thus provide important insights into the functions of human chymase. In contrast to the tryptases and chymases, only one Cpa3 gene is expressed in MCs of any species yet studied.
Typically, MCs express remarkably high levels of the various proteases described above, mRNA levels often approaching and even exceeding those of standard house-keeping genes. It is also noteworthy that all of these proteases are expressed in a constitutive fashion, with MC activation by various stimuli having little or no effect on the corresponding mRNA levels (Pejler et al., 2007). As noted above, the expression of chymases, tryptases, and CPA3 is to a large extent (albeit not wholly) MC-restricted. Early evidence for this came from studies comparing the mRNA levels for these proteases in skin tissues from WT versus MC-deficient mice, where it was shown that the absence of MCs resulted in a decrease in the mRNAs for these proteases down to nondetectable levels (Stevens et al., 1994). More recently, it was confirmed by using deep-CAGE sequencing of human skin MCs that the expression of tryptase (TPSAB1/TPSAB2) and, in particular, CMA1 was highly confined to cells of the MC lineage in comparison with all other cell types covered by the FANTOM5 project (Motakis et al., 2014). However, it was noted that low levels of tryptase mRNA expression could be seen in macrophages, a finding that is in line with a previous observation (Huang et al., 1993). There is also some evidence suggesting that low levels of tryptase, both at the protein and mRNA level, can be found in human basophils (Jogie-Brahim, Min, Fukuoka, Xia, & Schwartz, 2004; Li et al., 1998).
CPA3 also is highly expressed in MCs in comparison with other cell types, although the selectivity for MCs in comparison with other cell types was not as high as observed for CMA1 and tryptase (Motakis et al., 2014). Indeed, there is evidence that CPA3 (protein) can be found at low levels in basophil-like cells in the blood of human allergic patients (Li et al., 1998). In further agreement with this notion, it has recently been shown that cell toxicity driven by the Cpa3 promoter in mice results in ablation of a large fraction of the basophil population (in addition to ablating MCs) (Feyerabend et al., 2011; Lilla et al., 2011), indicating that the Cpa3 promoter is active in a fraction of mature basophils and/or their progenitors. By contrast, in accordance with the highly MC-restricted expression of chymase, cell toxicity driven by a mouse chymase promoter (Mcpt5) resulted in efficient ablation of MCs, with little if any effects on other cell types (including bone marrow basophils) (Dudeck et al., 2011).
As discussed in Section 1.3, mouse MCs can be classified as either CTMCs or MMCs based on their anatomical locations and protease expression profiles, although several studies indicate that the pattern of protease expression in MC subpopulation is not fixed but can change under the influence of the tissue microenvironment, including during certain inflammatory responses (Friend et al., 1996; Godfraind et al., 1998; Jippo et al., 2001; Kanakura et al., 1988; Lee et al., 1998; Otsu et al., 1987). Under baseline conditions, CTMCs in the skin and other connective tissues express CPA3, the chymases mMCP-4 and -5 as well as the tryptases mMCP-6 and -7, while MMCs express the chymases mMCP-1 and -2 [Mcpt2 mRNA is expressed in MMCs in the stomach of WBB6F1, but not C57BL/6, mice (Jippo et al., 1997)] but little/no tryptase nor CPA3 (Pejler et al., 2007). However, in seeming discordance with the proposed lack of CPA3 expression in MMCs, cell toxicity driven by the Cpa3 promoter ablates MCs of both CTMC and MMC subtypes in mice (Feyerabend et al., 2011; Lilla et al., 2011), suggesting that MMCs or their precursors, at least during certain stages of their development, express significant amounts of Cpa3. By contrast, in agreement with the notion that Mcpt5 expression is confined to the CTMC subtype, Mcpt5-driven toxicity resulted in ablation of CTMCs, whereas MMCs were not detectably affected (Dudeck et al., 2011).
2. NONGENETIC APPROACHES FOR ANALYZING THE FUNCTIONS OF MAST CELLS AND MAST CELL-ASSOCIATED PROTEASES IN VIVO
What kinds of experimental approaches can permit one to identify the actual contributions of MCs when investigating their potential roles in particular biological settings? The simplest would be to be able to ablate MCs selectively in vivo, e.g., with a drug or an antibody, or (in experimental animals) genetically. Moreover, one ideally would be able to ablate selectively either all MCs (producing a fully MC-deficient host, in which potential local and/or systemic effects of MCs could be tested) or only the MC populations of interest (e.g., those in the skin, lungs, joints, gut, etc.). Once it has been established that MCs have a detectable role in a biological response, it is useful then to define how that MC role is expressed in that setting. To address this question, one ideally would be able to delete selectively elements of MC activation pathways, or MC products, or to block specifically those MC-derived products by which MCs might express that function.
2.1. Pharmacological approaches
2.1.1 Mast cell stabilizers
So-called MC stabilizers comprise a family of compounds which are thought to inhibit MC activation by stabilizing membranes. The most commonly used of these stabilizers are cromolyn and nedocromil (Howell & Altounyan, 1967; Lal, Malhotra, Gribben, & Hodder, 1984). Cromolyn has been used by many groups to assess MC functions in rodents in diverse inflammatory models (Kim, Lim, & Kim, 2012; Kneilling et al., 2007; Liu et al., 2009; Ramos, Pena, Cai, Deitch, & Ulloa, 2010; Soucek et al., 2007; Wyss, Bonneau, & Trifilieff, 2005). This approach can appear tempting since it does not require use of genetically modified animals and has the potential to generate experimental results which might translate into the clinic. However, cromolyn (and most probably other “MC stabilizers”) affects the functions of other types of cells as well, including granulocytes and B cells (Arumugam, Ramachandran, & Logsdon, 2006; Norris, 1996). Moreover, we recently reported that cromolyn treatment inhibits MC-dependent IgE-mediated passive cutaneous anaphylaxis (PCA) reactions in rats but not in mice (Oka, Kalesnikoff, Starkl, Tsai, & Galli, 2012). We also showed that cromolyn can inhibit LPS-induced TNF production in both wild-type (WT) and KitW−sh/W−sh MC-deficient mice, indicating that such effect is MC-independent (Oka et al., 2012).
2.1.2 Mast cell activators
“MC activators” comprise a family of structurally diverse cationic peptides and polymeric compounds that can induce MC degranulation in a G protein-dependent manner (Aridor, Rajmilevich, Beaven, & Sagi-Eisenberg, 1993; Ferry, Brehin, Kamel, & Landry, 2002). These include compound 48/80 (c48/80) (Fawcett, 1954; Paton, 1951; Rothschild, 1970) and a variety of peptide toxins, such as MC-degranulating peptide, found in honeybee venom (Gushchin, Miroshnikov, Martynov, & Sviridov, 1981), and mastoparan, found in wasp venom (Hirai et al., 1979). These MC activators have been widely used as tools to induce MC degranulation both in vitro and in vivo (Ferry et al., 2002). Interestingly, c48/80 can function as a potent adjuvant, and evidence obtained using the so-called MC knockin approach in MC-deficient KitW/W−v mice suggests that such an adjuvant effect is largely mediated by MCs (McLachlan et al., 2008) (see Section 3.1 for details about the use and potential limitations of the MC knockin model). However, c48/80 can also have direct effects on other cell types, as recently demonstrated for excitation of cultured enteric neurons (Schemann et al., 2012).
2.1.3 Purified or recombinant mast cell proteases
One approach for studying the biological functions of MC proteases has been to administer the corresponding purified or recombinant enzymes at various sites in experimental animals. The general conclusion of such studies has been that MC proteases can have proinflammatory properties. As examples, human β-tryptase and γ-tryptase have been shown to induce airway hyper-responsiveness when administered to the lungs of sheep (Molinari et al., 1996) or mice (Wong et al., 2002), respectively. Moreover, human and mouse (mMCP-6 and mMCP-7) tryptases have been shown to recruit neutrophils and/or eosinophils into the peritoneum of mice and guinea pigs (Hallgren, Karlson, Poorafshar, Hellman, & Pejler, 2000; He, Peng, & Walls, 1997; Huang et al., 2001, 1998). Furthermore, human chymase was shown to elicit neutrophil- and eosinophil- containing inflammatory responses in guinea pigs and mice (He & Walls, 1998a), a process that was associated with increased vascular permeability (He & Walls, 1998b). In agreement with these studies, it has been shown that mouse chymase (i.e., mMCP-4) can induce substantial inflammation when administered to mice (Watanabe et al., 2002). Notably, to our knowledge there have been no corresponding studies in which the effects of exogenously administered CPA3 have been studied in vivo.
2.1.4 Tryptase and chymase inhibitors
Another approach for studying the function of MC proteases is to assess various inhibitors of the MC proteases in experimental disease models. By using a first generation, slow-acting tryptase inhibitor of low selectivity for tryptase over other trypsin-like proteases (APC-366), it was demonstrated that tryptase inhibition may reduce allergen-induced airway responses in sheep (Clark et al., 1995) and also can diminish tryptase-induced cutaneous inflammatory responses (Molinari et al., 1995). Based on these findings, APC-366 underwent a clinical trial for the indication of asthma but the outcome of this trial was relatively disappointing (Krishna et al., 2001). Since then, several tryptase inhibitors of higher efficacy and with higher selectivity for tryptase have been developed and have been shown to have efficacy in models of airway inflammation (Costanzo et al., 2008; Oh et al., 2002; Wright et al., 1999). Moreover, studies in humans have shown that a highly selective tryptase inhibitor (APC-2059) can ameliorate ulcerative colitis to some extent (Tremaine et al., 2002) and that a dual inhibitor of tryptase and pancreatic trypsin (RWJ-58643) can diminish nasal allergic responses (Erin et al., 2006).
A large number of chymase inhibitors have also been developed and have been evaluated in numerous experimental models of disease. In line with the proinflammatory role of exogenously administered chymase, chymase inhibitors have been shown to have anti-inflammatory properties, as indicated by reductions in eosinophilic inflammation in mice (Watanabe et al., 2002). It also has been demonstrated that chymase inhibition can ameliorate allergic conjunctivitis in guinea pigs (Nabe et al., 2013) and a large body of evidence indicates that chymase inhibition can diminish the development of fibrosis in various rodent models (Sakaguchi et al., 2004; Takato et al., 2011; Tomimori et al., 2003) and ameliorate aspects of various cardiac and other circulatory disorders in animal models (Bot et al., 2011; Inoue et al., 2009; Oyamada, Bianchi, Takai, Chu, & Sellke, 2011; Tsunemi et al., 2004).
When testing MC protease inhibitors in experimentally induced disease models in animals, it should be recognized that all such inhibitors have been developed to inhibit the human enzymes. Therefore, one cannot exclude the possibility that the particular inhibitor, although being efficient in inhibiting the human protease, may be considerably less active on the corresponding endogenous MC protease (in most cases, such data have not been reported). It also is possible that an inhibitor developed to target a human MC protease may in addition interact efficiently with an unknown endogenous target that is not necessarily a protease expressed by MCs. Another potential problem is that many of the evaluated MC protease inhibitors show low selectivity for the target MC protease. For example, APC-366 is approximately equally effective toward human tryptase and pancreatic trypsin, and many of the chymase inhibitors that have been developed are poorly selective for chymase over neutrophil cathepsin G (Pejler et al., 2007). Taken together, these findings indicate that one should be cautious when interpreting data obtained by using inhibitors of human MC proteases in experimental animal models. Indeed, in many cases it may not be possible to ascertain to what extent the effects of a particular MC protease inhibitor reflect its interaction with the putative target MC protease, as opposed to reflecting, in whole or in part, off-target effects.
2.1.5 Tyrosine kinase inhibitors
Some tyrosine kinase inhibitors such as imatinib (STI 571) or masitinib (AB1010) are potent inhibitors of Kit-dependent MC activation and can induce MC apoptosis (Dubreuil et al., 2009; Juurikivi et al., 2005; Takeuchi et al., 2003). Imatinib has been shown to reduce inflammation in certain mouse models of autoimmune diseases, including antibody-dependent arthritis (Paniagua et al., 2006). Imatinib also alleviates diarrhea in a mouse model of intestinal allergy (Vaali et al., 2012). However, these agents do not affect solely MCs. Kit has pleiotropic functions unrelated to MCs, including regulation of hematopoietic stem cell (HSC) survival, self-renewal, and differentiation (Bowie, Kent, Copley, & Eaves, 2007; Czechowicz, Kraft, Weissman, & Bhattacharya, 2007; Ikuta & Weissman, 1992; Ogawa et al., 1991). Kit expression has also been described in purified eosinophils from mice infected with Schistosoma mansoni (Oliveira et al., 2002) and in mouse DCs after stimulation with cholera toxin or house dust mite extract (Krishnamoorthy et al., 2008). Kit is also expressed in some cell types outside the immune system, including melanocytes and germ cells (Manova & Bachvarova, 1991; Manova, Nocka, Besmer, & Bachvarova, 1990), interstitial cells of Cajal (ICC) in the gastrointestinal tract (Huizinga et al., 1995), sensory neurons (Milenkovic et al., 2007), certain nerves in the CNS (Takagi et al., 2008), keratinocytes (Peters et al., 2003), and tubular epithelial cells in the kidney (Stokman et al., 2010). Moreover, imatinib and related molecules are also potent inhibitors of some other tyrosine kinases such as PDGFR or Bcr-Abl (Heinrich et al., 2000).
2.2. Antibody-based approaches
Depletion of MCs from mice by conventional techniques, such as the injection of depleting antibodies, is limited by the lack of surface markers that have been shown to be unique to MCs. Several groups have used blocking antibodies against SCF or Kit to interfere with MC functions in vivo. Treatment with anti-SCF antibodies has been shown to reduce eosinophil numbers and histamine levels in mouse models of allergic airway inflammation (Berlin, Hogaboam, & Lukacs, 2006; Berlin, Lincoln, Tomkinson, & Lukacs, 2004; Lukacs et al., 1996) and multiple injections of mice with a blocking anti-Kit antibody resulted in MC depletion and diminished oral allergen-induced diarrhea (Brandt et al., 2003). Treatment of mice with anti-SCF or anti-Kit blocking antibodies also abrogated MC hyperplasia induced by the parasite Trichinella spiralis and resulted in delayed worm expulsion (Donaldson, Schmitt, Huntley, Newlands, & Grencis, 1996). By contrast, while anti-SCF treatment diminished intestinal MMC hyperplasia in rats infected with Nippostrongylus brasiliensis (or T. spiralis), such treatment decreased parasite egg production during N. brasiliensis infection (Newlands, Miller, MacKellar, & Galli, 1995). This result raised the possibility that some effects of SCF and/or MCs (perhaps MC-dependent enhancement of local vascular permeability at sites of parasite infection), actually favored parasite fecundity in this setting.
As with tyrosine kinase inhibitors, such antibody-based approaches are interesting since they potentially could be used to block MC functions in humans. However, one has to keep in mind that many cell types express Kit (as described in Section 2.1.5). Therefore, the effects of anti-SCF and anti-Kit blocking antibodies do not necessarily reflect solely their actions on MCs. For example, while repeated treatment with antibodies that neutralize SCF (Newlands et al., 1995) or block Kit (Brandt et al., 2003; Gekara & Weiss, 2008) can result in the depletion of MCs in vivo, such treatments also have potential effects on many other cell types, including HSCs (Czechowicz et al., 2007).
3. GENETIC APPROACHES FOR ANALYZING THE FUNCTIONS OF MAST CELLS IN VIVO
Much progress has been made, particularly over the last few years, in devising genetic approaches to investigate specific functions of MCs or MC-associated products. However, each of the new approaches (as well as older models that have been widely used for many years) have known or potential limitations that must be kept in mind when interpreting the results of such work.
3.1. Mice with mutations affecting c-kit structure or expression and “MC knockin mice”
As agents that can solely and specifically suppress MC activation are not yet available, genetic approaches now represent a more definitive way to identify and characterize MC functions in mice in vivo. To date, mutant mice whose only abnormality is a specific lack of all populations of MCs have not been reported. For many years, we and others have used c-kit mutant, MC-deficient mice to analyze the functions of MCs in vivo (Dawicki & Marshall, 2007; Galli, Kalesnikoff, et al., 2005; Galli, Nakae, et al., 2005; Grimbaldeston et al., 2005; Kitamura, 1989; Piliponsky et al., 2010). Kit, the receptor for the main MC growth and survival factor, SCF (Oliveira & Lukacs, 2003; Reber, Da Silva, & Frossard, 2006), is also highly expressed in HSCs and certain other, nonhematopoietic lineages. While most hematopoietic/immune cells lose detectable Kit expression upon cell differentiation, MCs remain Kit+ throughout their life span. Activation of Kit by SCF contributes to regulation of the self-renewal, survival, and differentiation of HSCs (Bowie et al., 2007; Czechowicz et al., 2007; Ikuta & Weissman, 1992; Ogawa et al., 1991) as well as maturation, survival, proliferation, migration, and functional responses of MCs (Galli, Tsai, Wershil, Tam, & Costa, 1995; Galli, Zsebo, et al., 1994; Okayama & Kawakami, 2006; Tsai, Shih, et al., 1991; Tsai, Takeishi, et al., 1991). In addition, as noted above (in Section 2.1.5), Kit expression has also been detected in eosinophils from mice infected with S. mansoni (Oliveira et al., 2002), in mouse DCs after stimulation with cholera toxin or house dust mite extract (Krishnamoorthy et al., 2008) and in lung DCs after respiratory virus infection (Grayson et al., 2007), as well as in melanocytes, germ cells (Manova & Bachvarova, 1991; Manova et al., 1990) and ICC in the gastrointestinal tract c-kit mutant (Huizinga et al., 1995). As noted above, Kit expression has been detected in many other structural cell types in mice, including subpopulations of sensory neurons (Milenkovic et al., 2007), certain nerves in the CNS (Takagi et al., 2008), keratinocytes (Peters et al., 2003), and renal tubular epithelial cells (Stokman et al., 2010). Moreover, KitW/W−v mice backcrossed on the A/JxB6 F1 background display reduced naïve airway hyper-responsiveness (AHR) to methacholine as compared to Kit+/+ littermate controls in a MC-independent manner, suggesting the existence of a yet unidentified nonhematopoietic Kit+ cell type which accounts for this phenotype (Cozzi et al., 2011). Constitutive disruption of Kit or SCF expression or function in mice thus hinders development of the MC lineage and other Kit expressing cell types whose development is also critically dependent on Kit/SCF interactions.
WBB6F1-KitW/W−v and C57BL/6-KitW−sh/W−sh mice are the most commonly used kit mutant mice for studies of MC functions in vivo (Dawicki & Marshall, 2007; Galli, Kalesnikoff, et al., 2005; Galli, Nakae, et al., 2005; Grimbaldeston et al., 2005; Grimbaldeston, Metz, Yu, Tsai, & Galli, 2006; Grimbaldeston, Nakae, Kalesnikoff, Tsai, & Galli, 2007; Piliponsky et al., 2010; Zhou, Xing, Friend, Austen, & Katz, 2007). KitW is a point mutation that produces a truncated Kit that is not expressed on the cell surface (Hayashi, Kunisada, Ogawa, Yamaguchi, & Nishikawa, 1991), KitW−v is a mutation in the c-kit tyrosine kinase domain that substantially reduces the kinase activity of the receptor (Nocka et al., 1990), and KitW−sh is an inversion mutation that affects the transcriptional regulatory elements upstream of the c-kit transcription start site on mouse chromosome 5 (Nagle, Kozak, Mano, Chapman, & Bucan, 1995; Nigrovic et al., 2008). Both KitW/W−v and KitW−sh/W−sh mice are profoundly deficient in MCs and melanocytes, but only KitW/W−v mice are sterile (Grimbaldeston et al., 2005; Tsai, Grimbaldeston, Yu, Tam, & Galli, 2005). Both WBB6F1-KitW/W−v and C57BL/6-KitW−sh/W−sh mice have several other phenotypic abnormalities that, as described above, reflect the biological distribution and functions of Kit in cells within and outside the immune system of these mice, including some abnormalities affecting hematopoietic cells other than MCs (Chervenick & Boggs, 1969; Grimbaldeston et al., 2005; Nigrovic et al., 2008; Piliponsky et al., 2010; Tsai et al., 2005; Zhou et al., 2007). However, some of these “non-MC” phenotypic abnormalities differ between the two most commonly used types of c-kit mutant MC-deficient mice. For example, WBB6F1-KitW/W−v mice are anemic, have reduced numbers of neutrophils (Chervenick & Boggs, 1969; Nigrovic et al., 2008; Piliponsky et al., 2010; Zhou et al., 2007) and basophils (Akahoshi et al., 2011; Lantz et al., 1998; Piliponsky et al., 2010), and are sterile (Galli, Kalesnikoff, et al., 2005; Galli, Nakae, et al., 2005; Grimbaldeston et al., 2005). By contrast, C57BL/6-KitW−sh/W−sh mice are neither anemic nor sterile, but have increased numbers of neutrophils (Grimbaldeston et al., 2005; Nigrovic et al., 2008; Piliponsky et al., 2010; Zhou et al., 2007) and basophils (Piliponsky et al., 2010).
Differences in the biological responses in c-kit mutant mice compared with WT mice of course may reflect any one (or more) of the abnormalities that result from the alterations of c-kit structure or expression in these animals, in any of the directly or indirectly affected cell lineages, and may not be due solely or even partly to their deficiency in MCs. However, at many anatomical sites, the deficiency in MCs in c-kit mutant mice can be selectively “repaired” by the adoptive transfer of genetically compatible, in vitro-derived WT or mutant MCs (Galli, Kalesnikoff, et al., 2005; Galli, Nakae, et al., 2005; Grimbaldeston et al., 2005, 2006; Kitamura, 1989; Nakano et al., 1985). Such in vitro-derived MCs, for example, bone marrow-derived cultured MCs (BMCMCs), can be administrated intravenously (i.v.), intraperitoneally (i.p.), intradermally (i.d.), intra-articularly (i.a.) (Reber, Marichal, et al., 2014), or intra-cranially (Arac et al., 2014; Christy, Walker, Hessner, & Brown, 2013) to create so-called MC knockin mice. Since their description in 1985 (Nakano et al., 1985), such MC knockin mice have been widely employed to assess the importance of MCs in regulating the expression of biological responses in vivo.
However, it has long been known that, depending on the route of injection of MCs and/or the numbers of MCs injected, the numbers and/or anatomical distribution of the adoptively transferred MCs in the recipient c-kit mutant mice can differ from those of the corresponding native MC populations in the corresponding WT mice (Grimbaldeston et al., 2005; Martin et al., 1993; Tanzola, Robbie-Ryan, Gutekunst, & Brown, 2003; Tsai et al., 2005). With direct injection of BMCMCs into the skin or peritoneal cavity of WBB6F1-KitW/W−v or C57BL/6-KitW−sh/W−sh mice, the numbers and anatomic distribution of adoptively transferred MCs in the dermis or in the peritoneal cavity and mesentery, respectively, when assessed 4–8 weeks after MC transfer, can be similar to those of native MCs in WT mice (Grimbaldeston et al., 2005; Tsai et al., 2005). By contrast, at 4–28 weeks after injection of BMCMCs i.v. into WBB6F1-KitW/W−v or C57BL/6-KitW−sh/W−sh mice, few or no MCs are detectable in the trachea of the mice (and numbers are much less than those in the corresponding WT mice), whereas the numbers of MCs in the periphery of the lung typically are substantially greater than, and the numbers of MCs around the bronchi can be similar to, those in the corresponding WT mice (Grimbaldeston et al., 2005; Martin et al., 1993; Tsai et al., 2005; Wolters et al., 2005). Such differences in MC numbers and anatomical distribution of adoptively transferred versus corresponding WT MC populations should be taken into account when considering the results obtained in MC knockin versus corresponding WT mice.
One must also consider the possibility that the native and adoptively transferred MC populations differ in certain aspects of phenotype. Although direct comparisons of such populations have in general shown that, over time, the phenotype of the adoptively transferred MCs comes to closely resemble that of the corresponding native MC populations (Nakano et al., 1985; Otsu et al., 1987), there have been relatively few studies of that type. Moreover, it is not currently possible to define every aspect of the phenotype of either native or adoptively transferred MC populations in situ. Therefore, one cannot formally rule out the possibility that the two MC populations might express phenotypic differences that in turn might influence the results obtained in a particular biological response.
Kit has pleiotropic functions unrelated to MCs. Therefore, even when MC engraftment results in MC numbers, anatomical distributions, and phenotypes in the recipient c-kit mutant mice that are very similar to those of the corresponding WT mice, it is possible that such adoptively transferred MCs can “normalize” some of the biological responses that are abnormal in c-kit mutant mice because the transferred MCs compensate in the mutant mice for abnormalities in lineages other than the MC—abnormalities that do not exist in the corresponding WT mice. Put differently, MCs may play more critical roles in some biological responses in c-kit mutant mice than in WT mice because that biological response has less redundancy in the c-kit mutant mice than is present in the WT animals.
3.2. MC-deficient mice with normal c-kit
Because of the potential complexities and caveats inherent in interpreting findings based on work employing c-kit mutant MC-deficient mice, several groups sought to develop mice that are MC-deficient but which lack abnormalities related to c-kit structure or expression. A common approach has been to generate mice in which Cre recombinase (Cre) is expressed under the control of promoters thought to be “MC-specific” or at least “MC-associated” (Feyerabend et al., 2009; Lilla et al., 2011; Musch, Wege, Mannel, & Hehlgans, 2008; Scholten et al., 2008). To date, three new strains of mutant mice with marked constitutive deficiencies in MCs have been reported (Dudeck et al., 2011; Feyerabend et al., 2011; Lilla et al., 2011) (Table 1).
Table 1.
Characteristics of newly described mast cell (MC)-deficient mice
| Deficiency | Mice | Construct | MC numbers | IgE-dependent MC function |
Basophil numbers/ function |
References |
|---|---|---|---|---|---|---|
| Constitutive | Mcpt5-Cre; R-DTA Tg(Cma1-cre) ARoer; B6.129P2-Gt(ROSA) 26Sortm1(DTA) Lky/J | Cross between R-DTA floxed mice and transgenic mice expressing Cre under the control of the Mcpt5 promoter | Steady-state: marked reductions in peritoneal (98%) and skin (89–96.5%) MCs, mucosal MCs (MMCs) unlikely to be depleted Inflammatory conditions: deficient in peritoneal MCs 4 h, 1 and 3 days following i.p. S. aureus infection | Not assessed | Not assessed (basophils thought not to express Mcpt5) | Dudeck et al. (2011) and Ronnberg et al. (2014) |
| “Cre-Master” Cpa3Cre/+ Cpa3tm3(icre) Hrr | Gene targeting: Cre expression under the control of the Cpa3 promoter while deleting 28 nucleotides of the first exon of Cpa3 locus | Steady-state: absence of connective-tissue and mucosal MCs (in skin, peritoneum, intestine) Inflammatory conditions: remain deficient in skin MCs after PMA-induced dermatitis and in intestinal MMCs following helminth infection | Do not develop IgE-dependent models of PSA or PCA; PSA response restored by systemic engraftment of WT BMCMCs | 60% reduction in spleen basophil numbers, basophil function not assessed | Feyerabend et al. (2011) | |
| “Hello Kitty” Cpa3-Cre; Mcl-1fl/fl Tg(Cpa3-cre) 3Glli; B6;129-Mcl1tm3sjkJ | Cross between Mcl-1 floxed mice and transgenic mice expressing Cre under the control of a Cpa3 promoter fragment | Steady-state: marked reductions (92–100%) in connective-tissue and mucosal MCs in the skin, trachea, lung, peritoneum, digestive tract, etc., but no reduction in small numbers of splenic MCs | Markedly reduced features of IgE-dependent models of PSA and PCA; PCA response restored by intradermal engraftment of WT BMCMCs | Reductions in basophil numbers in spleen (58%), blood (74%), and bone marrow (75%); markedly reduced IgE- and basophil-dependent chronic allergic inflammation of skin | Lilla et al. (2011) | |
| Inducible | Mcpt5-Cre;iDTR Tg(Cma1-cre) ARoer; C57BL/6-Gt(ROSA) 26Sortm1(HBEGF) Awai/J | Cross between inducible DTR floxed mice and transgenic mice expressing Cre under the control of the Mcpt5 promoter | Steady-state: One week after 4 weekly i.p. and 2 s.c. DT treatments: deficient in peritoneal and skin MCs (97.5%); stomach and intestinal MMCs present Repopulation: 10% of pre-treatment skin and peritoneal MC numbers 3 weeks after the last DT treatment | Not assessed | Bone marrow basophils not affected 1 week after 4 weekly i.p. treatments with DT | Dudeck et al. (2011) |
| “Mas-TRECK� | Transgenic mice expressing human DTR under the control of an intronic enhancer of the Il-4 gene | Steady-state: Three days after 5 daily i.p. DT treatments: deficient in peritoneal, skin, stomach, and mesenteric window MCs Repopulation: Skin MCs undetectable 12 days after the last DT treatment | Markedly reduced features of IgE-dependent models of PSA and PCA 2 days after 5 daily i.p. treatments with DT | Transient >95% reduction in blood basophil numbers 5 days after start of DT treatment and recovery 12 days after the last DT treatment; markedly reduced features of IgE- and basophil-dependent chronic allergic inflammation of skin (induced 2 days after 5 daily i.p. treatments with DT) | Otsuka et al. (2011) and Sawaguchi et al. (2012) | |
| Cpa3-Cre;iDTR Tg(Cpa3-cre) 3Glli; C57BL/6-Gt(ROSA) 26Sortm1(HBEGF) Awai/J | Cross between inducible DTR floxed mice and transgenic mice expressing Cre under the control of a Cpa3 promoter fragment | Steady-state: One week after 2 weekly intra-articular injections of low dose DT (50 ng): deficient in synovial MCs in the ankle joints. No effect on blood basophils 48 h after i.p. injection of 500 ng DT; deficient in peritoneal MCs, but no effect on ear skin MCs Repopulation: Not assessed | Markedly reduced IgE-dependent tissue swelling in the joints after intra-articular MC depletion (Reber et al., unpublished data) | Reduced blood basophils after i.p. treatments with DT, but basophils are not affected after i.a. DT injection | Reber, Marichal, et al. (2014) | |
| KitCreERT2/+ R26-GFPStopFDTA | Cross between R26-GFPStopFDTA mice, in which expression of DTA is induced upon the removal of a loxP-flanked STOP cassette, and transgenic KitCreERT2/+ mice expressing a tamoxifen-inducible Cre recombinase (CreER) under the control of one allele of the endogenous c-kit locus | Steady-state: Fourteen days following treatment with a tamoxifen-containing diet (400 mg/kg tamoxifen citrate): deficient in peritoneal, ear skin, back skin, and glandular stomach MCs Inflammatory conditions: following tamoxifen regimen and upon IL-3 injection, no increase in Mcpt1 and Mcpt2 expression in the small intestine, as well as no detectable mMCP-1+ cells in the large intestine Repopulation: Ear skin and peritoneal MCs undetectable 4 weeks after the tamoxifen treatment | Markedly reduced features of IgE-dependent models of PSA and PCA 14 days after the beginning of tamoxifen-containing diet | Splenic basophil number slightly reduced 14 days following tamoxifen regimen, restored when analyzed 4 weeks after the cessation of the treatment | Heger, Seidler, et al. (2014) |
This is an updated version of Table 1 from Reber, Marichal, and Galli (2012).
3.2.1 Mcpt5-Cre;R-DTA mice
Dudeck et al. (2011) crossed MC protease (Mcpt)5-Cre transgenic mice with R-DTAfl/fl mice (Voehringer, Liang, & Locksley, 2008) to generate a mouse strain in which the diphtheria toxin alpha (DTA) chain is produced only in Cre-expressing cells, thereby driving Cre-specific ablation of such cells (Dudeck et al., 2011). Naive Mcpt5-Cre;R-DTA mice displayed a constitutive lack of peritoneal and ear skin MCs as well as >90% reductions in the numbers of abdominal and back skin MCs in comparison to the Cre− counterparts (Dudeck et al., 2011). It will be of interest to determine whether there are any effects of Cre-mediated DTA expression on MMCs, which are thought not to express mMCP-5, or other hematopoietic cell types in steady-state or inflammatory conditions, as well as to assess the efficiency of DTA-induced deletion of CTMCs during various inflammatory responses associated with increased numbers of MCs.
3.2.2 Cpa3Cre/+—“Cre-Master” mice
“Cre-Master” stands for “Cre-mediated mast cell eradication.” Feyerabend et al. (2011) used an elegant knockin strategy to induce Cre expression under the control of the Cpa3 promoter while deleting 28 nucleotides of the first exon of Cpa3, which encodes for the MC-associated protease CPA3. Unexpectedly, heterozygous Cpa3Cre/+ mice exhibited a virtually complete lack of MCs, multiple MC-associated proteases, and a MC gene expression signature, as assessed in the peritoneal cavity and skin. Notably, skin MCs were still undetectable under inflammatory conditions that can be associated with the development of skin MCs in WBB6F1-KitW/W−v mice (Gordon& Galli, 1990b) and MMCs remained absent in the intestine after helminth infection (Feyerabend et al., 2011). In addition, Cpa3Cre/+ mice did not detectably respond in an IgE-dependent model of PCA and exhibited neither reduced body temperature nor mortality when subjected to an IgE-dependent model of passive systemic anaphylaxis (PSA) (Ando, Martin, & Galli, 1993), unless they were engrafted with WT BMCMCs.
This profound depletion of MCs appears to be mediated by Cre-induced genotoxicity (Schmidt-Supprian & Rajewsky, 2007). However, although CPA3 is highly expressed in MCs (Serafin, Dayton, Gravallese, Austen, & Stevens, 1987), it is also expressed in basophils (Voehringer, Shinkai, & Locksley, 2004) and some populations of T-cell progenitors and thymic T cells (Feyerabend et al., 2009; Taghon, Yui, & Rothenberg, 2007), and in certain hematopoietic progenitor cells (Franco, Chen, Drukker, Weissman, & Galli, 2010). Consistent with this, the authors also detected some Cre activity in T cells (Feyerabend et al., 2009), as well as a 60% reduction in numbers of spleen basophils (Feyerabend et al., 2011). While Cre expression in basophils was not sufficient to ablate the entire population, it must be kept in mind that the residual basophils may not be fully functional.
3.2.3 Cpa3-Cre;Mcl-1fl/fl—“Hello Kitty” mice
Our group generated transgenic mice expressing Cre under the control of a Cpa3 promoter fragment (Lilla et al., 2011) and crossed them with mice in which the gene coding the antiapoptotic factor myeloid cell leukemia sequence 1 (Mcl-1) (Steimer et al., 2009; Zhou et al., 1998) was floxed (Lilla et al., 2011). The resulting Cpa3-Cre;Mcl-1fl/fl mice exhibited a marked kit-independent constitutive reduction in numbers of MCs (92–100% reduction in all anatomical sites tested except the spleen, that, like the spleen of the corresponding control mice, contained small numbers of MCs); Cpa3-Cre;Mcl-1fl/fl mice also exhibited a substantial reduction in bone marrow, blood, and spleen basophils (reductions of 78%, 74%, and 58%, respectively, in comparison to the Cpa3-Cre controls). Because these phenotypes are seen in the absence of mutations affecting c-kit structure or expression, these mice are informally called “Hello Kitty” MC- (and basophil)- deficient mice.
Assessing the responses of these markedly MC-deficient mice in three models of IgE-dependent inflammation revealed, as expected, that they were markedly deficient in two responses that previously had been characterized (in c-kit mutant MC-deficient mice) as IgE- and MC-dependent (Miyajima et al., 1997; Takeishi, Martin, Katona, Finkelman, & Galli, 1991; Wershil, Mekori, Murakami, & Galli, 1987), specifically, IgE-dependent PCA (except at sites engrafted with WT MCs) (Wershil et al., 1987) and IgE-dependent PSA (Miyajima et al., 1997). However, these studies also revealed that the reduction in numbers of basophils in Hello Kitty mice, although relatively modest compared to the deficiency in tissue MCs, was associated with a profound impairment in the animals’ ability to orchestrate a response that is IgE- and basophil-dependent, but MC-independent (Lilla et al., 2011; Mukai et al., 2005). The latter finding illustrates that mutant mice with less than full ablation of a certain type of effector cell (in this case, the basophil) may nevertheless exhibit a marked abnormality in a biological response that is particularly dependent on that cell type.
These three new types of MC-deficient mice represent welcome new tools for investigating the role of MCs in biological responses in vivo. Nevertheless, in designing experiments employing such mice (or the older models), and in interpreting the results of such work, we recommend keeping in mind the potential limitations of these models (both those already recognized and others which may yet to be revealed); limitations that may turn out to be more important in some types of biological responses than in others. One potential problem common to each of the three new strains, as well as to the c-kit mutant MC-deficient mice, is that the effects on certain biological responses of a constitutive deficiency of MCs may be different than those observed when the MCs are ablated just before or during the response. The latter situation generally has more clinical relevance than the former, as in most cases one would not attempt to reduce MC numbers or functions in human subjects unless there was compelling clinical evidence that MCs are important in the pathology associated with a particular disorder.
3.3. Inducible models of mast cell deficiency
Employing mouse models to test the hypothesis that MCs represent an important therapeutic target in a particular setting should ideally be performed using mice in which inducible and selective MCs ablation can be achieved. One promising approach for achieving the selective and efficient depletion of a particular cell population is the injection of diphtheria toxin (DT) into transgenic mice bearing the DT receptor (DTR) only in that particular cell type (Buch et al., 2005). This approach was recently used by three different groups to deplete MCs in adult mice (Dudeck et al., 2011; Otsuka et al., 2011; Reber, Marichal, et al., 2014; Sawaguchi et al., 2012) (Table 1). Another group generated a mouse strain in which a tamoxifen-inducible Cre is expressed under the control of the endogenous c-kit locus (Heger, Seidler, et al., 2014). By crossing these mice with mice bearing a Cre-inducible DTA transgene (R-DTAfl/fl mice; Voehringer et al., 2008), they generated a mouse strain in which tamoxifen treatment can ablate MCs (and other Kit+ cells, including ICC) (Heger, Seidler, et al., 2014).
3.3.1 Mcpt5-Cre;iDTR mice
Dudeck et al. (2011) mated Mcpt5-Cre mice with iDTRfl/fl mice expressing a simian DTR transgene with a floxed stop codon inserted into the Gt(ROSA)26Sor (ROSA26) locus, to achieve Cre-dependent expression of DTR in MCs. The authors reported that a single i.p. injection of DT leads to nearly complete ablation of peritoneal MCs in Mcpt5-Cre+;iDTR mice after 24 h, however they did not comment on MC numbers in other organs or whether there were any effects on other cell types. Repeated i.p. injections of DT (once a week for 4 weeks) led to complete ablation of MCs in the peritoneal cavity and abdominal skin of Mcpt5-Cre+;iDTR mice as compared to Mcpt5-Cre− mice, when assessed 1 week after the last DT injection. Achieving complete deletion of ear skin MCs required combining repeated i.p. and subcutaneous treatment with DT (Dudeck et al., 2011). Moreover, analysis of the small intestine and stomach of DT-treated Mcpt5-Cre+;iDTR mice showed depletion of subepithelial CTMCs but not intraepithelial MMCs, most likely reflecting a lack of Mcpt5-Cre transgene expression in MCs of the mucosal type (Dudeck et al., 2011). Nevertheless, these animals should represent a valuable tool for studying the effects of a local depletion of MCs in various acute biological processes. These mice may even be used to study the role of MCs in more chronic settings since only about 10% of peritoneal and skin MCs reappeared 3 weeks after cessation of treatment with DT under steady-state conditions. The authors reported that numbers of other major hematopoietic cells, including bone marrow basophils, were not affected by DT treatment. However, this analysis was performed 1 week after the last DT injection, and it would be of interest to know whether DT injections resulted in any transient depletion of other cell types.
3.3.2 “Mas-TRECK” mice
Otsuka et al. (2011) and Sawaguchi et al. (2012) described a new transgenic strain, named “Mas-TRECK” (for Mast cell-specific enhancer-mediated Toxin Receptor-mediated Conditional cell Knockout), in which expression of the human DTR gene is under the control of an intronic enhancer (IE) element of the Il-4 gene. They previously reported that this IE element was essential for IL-4 expression in MCs but not basophils, natural killer (NK) T cells or TH2 cells (Yagi, Tanaka, Motomura, & Kubo, 2007). Repeated i.p. treatment of Mas-TRECK mice with DT for 5 consecutive days completely depleted MCs in the skin, peritoneal cavity, stomach and mesenteric windows, as assessed 3 days after the last injection, and abrogated IgE-dependent PCA and PSA reactions (Sawaguchi et al., 2012). They also showed that skin MCs remain depleted for at least 12 days after cessation of DT treatment (Otsuka et al., 2011). However, DT treatment in these mice also leads to a transient depletion of blood basophils and virtually completely inhibited the development of a model of basophil-dependent, IgE-mediated chronic allergic inflammation of the skin (Mukai et al., 2005; Sawaguchi et al., 2012). Other major types of leukocytes (DCs, B cells, T cells, NKT cells, eosinophils, and neutrophils) did not express DTR mRNA and were not affected by DT treatment, although numbers of these cells were reported only for analyses done 12 days after the end of DT treatment (Otsuka et al., 2011; Sawaguchi et al., 2012).
3.3.3 Cpa3-Cre;iDTR mice
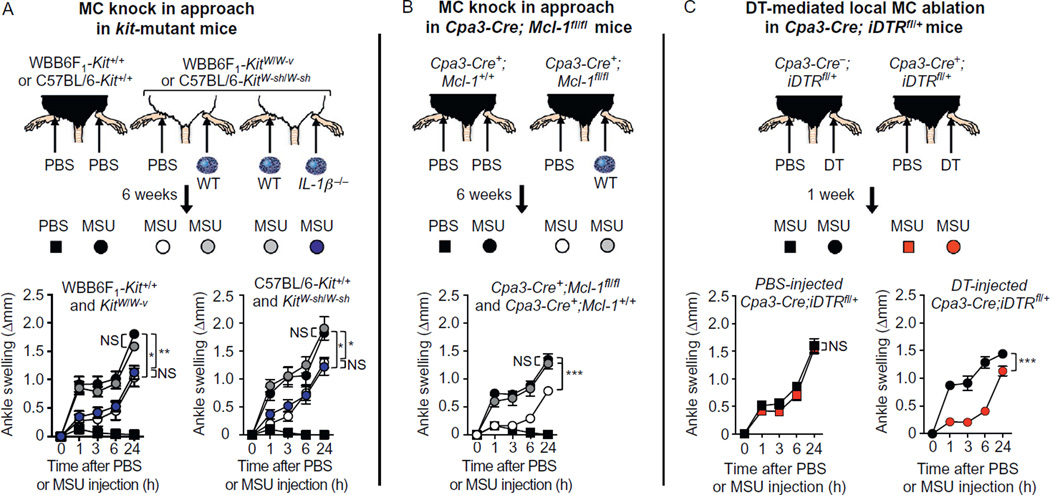
We mated Cpa3-Cre mice with iDTRfl/fl mice, to achieve Cre-dependent expression of DTR in MCs and basophils (Reber, Marichal, et al., 2014). We found that a single i.p. injection of 500 ng DT leads to nearly complete ablation of peritoneal MCs in Cpa3-Cre+;iDTR mice at 48 h without affecting MC numbers in the ear skin or ankle synovium (Reber et al., unpublished data). Such treatment with DT significantly reduced levels of blood basophils without significantly influencing levels of blood neutrophils, eosinophils, monocytes, T cells, or B cells at 48 h. Importantly, we observed that a single i.p. injection of DT induced signs of toxicity in Cpa3-Cre+; iDTR mice (but not in Cpa3-Cre−;iDTR mice), as reflected in a significant reduction in body weight at 48 h after DT injection, therefore precluding the use of this approach to achieve systemic ablation of MCs (Reber et al., unpublished data). However, we found that intra-articular (i.a.) injection of a low dose of DT (50 ng once a week for 2 weeks) resulted in ablation of MCs in the ankle joint treated with DT but not in the contra-lateral joint (which had been injected with PBS), peritoneal cavity or ear skin, nor did the animals exhibit evidence of systemic toxicity. This local (i.a.) injection of DT did not affect blood levels of basophils, monocytes, neutrophils, and eosinophils as assessed 1 week after the last injection of DT (Reber, Marichal, et al., 2014). We think that these animals should represent a valuable tool for studying the consequences of a local depletion of MCs in various acute biological processes.
3.3.4 KitCreERT2 and KitCreERT2/+R26-GFPStopFDTA mice
Heger, Seidler, et al. (2014) recently generated KitCreERT2 mice, which express a tamoxifen-inducible Cre recombinase (CreERT2) under the control of the endogenous c-kit locus. Kit levels and MC numbers in the peritoneal cavity are significantly reduced in KitCreERT2/+ mice due to disruption of one of the two c-kit alleles, while the numbers and distribution of MCs at other locations, as well as other hematopoietic cell types, appear to be largely unaffected in these KitCreERT2/+ mice. This KitCreERT2 mouse strain, when crossed to a Cre activity reporter strain, exhibited efficient stable induction of reporter protein in MCs purified from skin and peritoneal cavity (Heger, Seidler, et al., 2014) and in ICC (Klein et al., 2013), but only minimal recombination is detected in other immune cells, indicating that the KitCreERT2 strain can be used to efficiently delete MCs or MC-specific products in the skin and peritoneal cavity. KitCreERT2/+R26-GFPStopFDTA mice are created by crossing KitCreERT2 mice with a R26 knockin strain, in which expression of DTA is induced upon the removal of a loxP-flanked STOP cassette (R26-GFPStopFDTA). Feeding KitCreERT2/+R26-GFPStopFDTA mice with tamoxifen for 2 weeks did not alter numbers of hematopoietic progenitors or innate or adaptive immune cells, except for a small reductions in numbers of splenic BMCPs (which were defined as Lin−Kit+CD16/32 + Integrinβ7+), eosinophils, and basophils. MCs are essentially absent in tamoxifen-treated KitCreERT2/+R26-GFPStopFDTA mice, including MCs at mucosal surfaces. Moreover, KitCreERT2/+R26-GFPStopFDTA mice remain MC deficient when analyzed 4 weeks post-tamoxifen-induced ablation. Although MCs can be efficiently ablated at multiple anatomical locations at a defined time point in KitCreERT2/+R26-GFPStopFDTA mice after administration of tamoxifen, this treatment also depletes ICC in these mice and therefore does not specifically delete MCs (Klein et al., 2013).
3.4. Specific deletion of mast cell-associated products by Cre-lox approaches
Analyzing to what extent MCs represent important sources of products that can also be derived from other cell types, such as leukotrienes, prostaglandins, cytokines, chemokines, and growth factors, would require deletion of that product specifically in MCs. In this regard, the newly developed “MC-specific Cre” mice (Feyerabend et al., 2009; Furumoto et al., 2011; Heger, Seidler, et al., 2014; Lilla et al., 2011; Musch et al., 2008; Scholten et al., 2008) may allow for specific deletion of “floxed” genes in MCs. To our knowledge, Dudeck et al. were the first to take advantage of this system in order to reduce secretion of MC-derived IL-10 in vivo by crossing Mcpt5-Cre transgenic mice (Scholten et al., 2008) with Il-10fl/fl mice (Dudeck et al., 2011). Other researchers used this approach with Mcpt5-Cre mice in order to drive expression of a gain-of-function mutation of c-kit (KitD814V) (Gerbaulet et al., 2011) or to specifically delete the SH2 domain-containing phosphatase-2 (SHP2) gene (Sharma et al., 2012), Stat5 (Ando et al., 2014), SHP-1 (Ando et al., 2014), or the ubiquitin-editing enzyme and NF-κB negative regulator A20 (aka, Tnfaip3) (Heger, Fierens, et al., 2014) in CTMCs. Recently, a new mouse strain expressing Cre under the control of the high-affinity receptor for IgE, β chain promoter (FcεRI-β Cre) was generated and was used to delete the phosphatase and tensin homolog (Pten) gene in the MC compartment (Furumoto et al., 2011).
Two critical issues have to be taken into consideration when interpreting the results obtained using a Cre/lox approach. First, Cre activity must be efficient in, and ideally selectively restricted to, MCs, both in naive animals and under inflammatory conditions, since MC promoter-driven Cre expression may vary depending on the conditions and models tested. In this regard, using a reporter mouse is a valuable tool for the assessment of Cre-mediated recombination under different conditions in vivo. In the study by Dudeck et al., Mcpt5-Cre+ mice were crossed to the Cre excision reporter mice ROSA26 Stopflox EYFP (R26Y) and EYFP expression was assessed by flow cytometry in naive animals, confirming a highly efficient recombination in peritoneal and skin MCs but also revealing an unexpected recombination in a small population of blood NK cells. Lilla et al. crossed transgenic Cpa3-Cre mice (Lilla et al., 2011) with a mT/mG reporter line (Muzumdar, Tasic, Miyamichi, Li, & Luo, 2007), thus revealing the steady-state detection of Cre expression in a small population of basophils, eosinophils, and neutrophils in addition to MCs (Lilla et al., 2011). The breeding of Cpa3-Cre mice with particular “floxed” mice might therefore result in gene inactivation in certain populations of granulocytes (as well as in MCs) in double transgenic mice and this might limit the ability of this approach to reveal specific roles of MC-derived products in settings in which such other cells also may have important roles. Transgenic mice expressing Cre under the control of the conserved baboon alpha-chymase promoter (Chm:Cre) (Musch et al., 2008) displayed Cre expression specifically in lung and colon tissues by using Chm:Cre/ROSA26R reporter mice. However, in the lung of Chm:Cre/ROSA26R naive mice, 26% of Cre-positive cells were Kit negative, strongly suggesting that Cre activity might not be fully MC-specific.
Second, the Cre-mediated gene inactivation should be demonstrated in MCs and only in MCs. Dudeck et al. used an elegant, sensitive method of single-cell PCR in order to assess the specificity and efficiency of Cre-mediated Il-10 gene inactivation in several cell types using nested primers (Dudeck et al., 2011; Haff, 1994). The authors showed thereby an efficient inactivation of the functional Il-10 locus in peritoneal and skin MCs, but not in peritoneal B cells, macrophages or skin T cells from Mcpt5-Cre+;Il-10fl/fl mice, or any cell type tested in Mcpt5-Cre−;Il-10fl/fl mice. However, blood NK cells, which exhibited some Cre-mediated recombination, apparently have not been tested for Il-10 gene inactivation (Dudeck et al., 2011).
4. GENETIC APPROACHES FOR ANALYZING THE FUNCTIONS OF MAST CELL-ASSOCIATED PROTEASES IN VIVO
If a mediator is selectively expressed by MCs (and to prove this, expression needs to be analyzed in MCs and other cell types under both baseline conditions and during biological responses of interest, including disease models), its role can be investigated in vivo by testing animals in which that mediator has been knocked out. However, many of these highly MC-associated (if not truly MC-selective) mediators (such as MC-associated proteases) show strong interdependence in terms of proper storage in the cytoplasmic granules (Fig. 1); this clearly must be kept in mind when interpreting results obtained with mice genetically deficient in such mediators.
To date, constitutive knockouts for a majority of the various MC-restricted proteases have been generated (Table 2). The first one of these reported was the knockout for the MMC protease, mMCP-1, by Miller’s group (Wastling et al., 1998). Subsequently, knockouts for mMCP-4 (Tchougounova et al., 2003), CPA3 (Feyerabend et al., 2005), mMCP-6 (Shin et al., 2008; Thakurdas et al., 2007), and mMCP-5 (Younan et al., 2010) each have been reported, as well as a triple knockout of mMCP-4, mMCP-6, and CPA3 (Grujic et al., 2013). A knockout for γ-tryptase has also been generated recently (Hansbro et al., 2014), whereas genetically engineered mouse strains lacking mMCP-2 or mMCP-7 have not yet been reported (however, it has been reported that some mouse strains, including C57BL/6, lack mMCP-7; Hunt et al., 1996).
Table 2.
Genetic deletion of mast cell (MC)-associated products
| Mutant mice | Gene name | Phenotype and/or limitations | References |
|---|---|---|---|
| Hdc−/− | Histidine decarboxylase | No histamine produced (mice should be maintained on a histamine free diet, since histamine can also be acquired through ingestion) Decreased MC numbers Altered storage of various proteases in MC cytoplasmic granules Histamine can also be produced by other cell types, including some other hematopoietic cells such as basophils or neutrophils | Ohtsu et al. (2001) and Wiener et al. (2001) |
| Mcpt1−/− | MC protease 1 | Markedly reduced esterase activity in intestinal mucosal MCs Histochemical and ultrastructural changes in cytoplasmic granules of mucosal MCs | Wastling et al. (1998) |
| Mcpt4−/− | MC protease 4 (chymase) | Does not affect the number or morphology of MCs in multiple anatomical sites tested Increased tryptase activity in peritoneal MCs Abolished chymotrypsin-like activity in connective tissue-type MCs | Tchougounova, Pejler, and Abrink (2003) and Younan et al. (2010) |
| Mcpt5−/− | MC protease 5 | Markedly reduced storage of CPA3 and CPA activity in peritoneal MCs Increased tryptase activity in peritoneal MCs Decreased chymase activity in peritoneal MCs | Abonia et al. (2005), Stevens et al. (1996), and Younan et al. (2010) |
| Mcpt6−/− | MC protease 6 | Does not affect the number or morphology of MCs in multiple anatomical sites tested Does not affect histamine and mMCP-4 levels in peritoneal MCs | Shin et al. (2008) and Thakurdas et al. (2007) |
| Mcpt7−/− (=C57BL/6) | MC protease 7 | C57BL/6 mice are unable to express mMCP-7 because of a point mutation in the exon/intron 2 splice of the Mcpt7 gene Does not affect the expression of mMCP-6 Does not affect the number or morphology of MCs in multiple anatomical sites tested | Hunt et al. (1996) |
| Cpa3−/− | Carboxypeptidase A3 | Markedly reduced storage of mMCP-5 in MC cytoplasmic granules Reduced staining of cytoplasmic granules | Feyerabend et al. (2005) |
| Mcpt4−/−Mcpt6−/−Cpa3−/− | MC proteases 4, 6 and carboxypeptidase A3 | Markedly reduced storage of mMCP-5 in MC cytoplasmic granules Reduced staining and proteoglycan content of cytoplasmic granules | Grujic et al. (2013) |
| Cpa3Y356L, E378A | Carboxypeptidase A3 | Inactive CPA3 due to two point mutations in the catalytic domain Does not affect storage of mMCP-5 in MC cytoplasmic granules | Akahoshi et al. (2011) and Schneider et al. (2007) |
| Ndst-2−/− | N-deacetylase/N-sulfotransferase-2 | MCs are unable to synthesize heparin MCs are unable to store histamine Decreased numbers of connective tissue-type MCs Reduced storage of proteases (mMCP-4, mMCP-5, mMCP-6, CPA3) in MC cytoplasmic granules | Forsberg et al. (1999) and Humphries et al. (1999) |
| Srgn−/− | Serglycin | MCs are unable to store histamine and serotonin Reduced storage of proteases (mMCP-4, mMCP-5, mMCP-6, CPA3) in MC cytoplasmic granules | Åbrink, Grujic, and Pejler (2004) and Braga et al. (2007) |
| Prss31−/− | Protease serine member S31/transmembrane tryptase/tryptase γ | MCs lack transmembrane tryptase Does not affect the number or morphology of peritoneal or ear skin MCs Normal expression of mMCP-4, mMCP-5, and mMCP-6 | Hansbro et al. (2014) |
This is an updated version of Table 2 from Reber et al. (2012).
An interesting finding from studies of the CPA3 and mMCP-5 knockout strains was that these two proteases show a strong interdependence at the protein (but not mRNA) level, i.e., when CPA3 is absent there is also a lack of mMCP-5 protein, and vice versa (Feyerabend et al., 2005; Younan et al., 2010) (Fig. 1). Hence, it is not possible to ascertain whether effects of either CPA3 or mMCP-5 deficiency are the result of a lack of the respective genetically targeted enzyme, as opposed to secondary effects related to the absence of its binding partner. To surmount this obstacle, Rodewald and colleagues generated a mouse strain in which the active site of CPA3 was mutated to render it catalytically inactive and showed that mMCP-5 storage was intact in MCs from this mouse (Schneider et al., 2007).
As an additional example of posttranslational effects influencing MC proteases, it has been shown that the storage of mMCP-4, mMCP-5, mMCP-6, and CPA3 is strongly dependent on their electrostatic interaction with anionic proteoglycans of serglycin type, i.e., the absence of serglycin or reduction of the anionic charge of serglycin (the latter due to the knockout of Ndst2) causes a profound reduction in the ability of MCs to store these proteases (Åbrink et al., 2004; Forsberg et al., 1999; Humphries et al., 1999) (Fig. 1). Serglycin and NDST2 null MCs can thus be used to study the concerted actions of the affected MC proteases. However, since the expression of serglycin is not restricted to MCs, it cannot be ascertained that effects of serglycin deficiency are related to serglycin expression within MCs as opposed to its expression by other cell types. To date, no mouse strain with an inducible deficiency of any MC-restricted protease has been reported.
5. USING MAST CELL-DEFICIENT OR MAST CELL-ASSOCIATED PROTEASE-DEFICIENT MICE TO ANALYZE FUNCTIONS OF MAST CELLS OR THEIR PROTEASES IN VIVO
Having identified a role for MCs in a given pathological setting, a major task is to determine the exact molecular mechanism(s) by which MCs exert such activity. In this respect, the MC-restricted proteases have been regarded as attractive candidate compounds, based both on their high expression in MCs and also because various in vitro findings have suggested that MC proteases can exert activities that may be of relevance in pathological contexts (reviewed in Pejler et al., 2007). As elaborated below, effects of MC protease deficiencies have in most cases been in line with the global effect of MC deficiency in a given setting in which that protease has been implicated; that is, the respective MC protease is likely to account for the effect of MCs. In some cases, the respective proteases may nearly fully account for the global effect of MCs (i.e., the same phenotype occurs when either MCs or the particular MC protease of interest are absent), whereas in other settings that particular protease may only partially account for the global impact of MCs. In at least two settings, described below, there is evidence that a MC-associated protease has effects on a disease model which actually counteract those of the MC itself.
5.1. Settings in which similar results have been obtained using multiple models of mast cell deficiency and/or deficiencies in mast cell-associated proteases
5.1.1 IgE-dependent local and systemic anaphylaxis reactions
The nonredundant function of MCs in IgE-dependent local and systemic anaphylaxis reactions (e.g., those elicited in mice after passive transfer of antigen-specific IgE antibodies) has been consistently demonstrated in several strains of MC-deficient mice with either abnormal (KitW/W−v, KitW−sh/W−sh, KitCreERT2/+ R26-GFPStopFDTA) or normal (Cpa3Cre/+, Cpa3-Cre;Mcl-1fl/fl, and Mas-TRECK) expression of Kit. In addition, analysis of mouse models of peanut allergy elicited in KitW/W−v mice, KitW−sh/W−sh mice, and MC-depleted-Mcpt5-Cre;iDTR mice (Arias et al., 2011; Reber et al., 2013; Smit et al., 2011; Sun et al., 2007), models in which IgE antibodies to peanut allergens can be detected (Arias et al., 2011; Reber et al., 2013; Smit et al., 2011; Sun et al., 2007), are in accord in revealing a significant contribution of MCs to such peanut allergen-induced systemic anaphylactic responses in mice.
By contrast, MCs are not required for the expression of several features of active anaphylaxis responses involving antigen-specific IgG1 antibodies (Dombrowicz et al., 1997; Miyajima et al., 1997; Strait, Morris, Yang, Qu, & Finkelman, 2002; Takeishi et al., 1991), which typically require larger amounts of antigen for their induction than do IgE-dependent PSA reactions (Finkelman, 2007; Strait et al., 2002; Tsujimura et al., 2008), or for the elicitation of PSA responses in mice injected with antigen-specific IgG1 antibodies (Finkelman, 2007; Miyajima et al., 1997; Strait et al., 2002; Tsujimura et al., 2008). However, immune complexes of IgG1 and specific antigen can activate mouse MCs via their FcγRIII receptors (Latour, Bonnerot, Fridman, & Daeron, 1992), and work comparing responses in KitW/W−v versus the corresponding WT mice suggests that MCs might be able to contribute to the magnitude of certain features of IgG1-dependent PSA responses or 2.4G2-antibody (i.e., anti-FcγRII/FcγRIII)-induced reactions in mice, including the hypothemia, cardiopulmonary changes, and mortality associated with these responses (Dombrowicz et al., 1997; Miyajima et al., 1997).
5.1.2 Intestinal nematode infections
MCs have long been considered as major sentinels in host defense against bacteria and parasites. Experiments employing KitW/W−v and/or KitW−sh/W−sh mice have suggested that MCs can be important for intestinal nematode expulsion after primary infection with N. brasiliensis (Ohnmacht & Voehringer, 2010) (although the effects attributed to MCs in this study were small, and Crowle et al. reported no effect of MCs in the primary response to N. brasiliensis based on studies in KitW/W−v mice; Crowle, 1983), Strongyloides ratti (Abe & Nawa, 1987), Strongyloides venezuelensis (Khan, Horii, Tiuria, Sato, & Nawa, 1993; Lantz et al., 1998; Sasaki et al., 2005), T. spiralis (Ha et al., 1983; Oku, Itayama, & Kamiya, 1984), and Trichinella muris (Hepworth et al., 2012; Koyama & Ito, 2000). However, the delay in intestinal worm clearance observed in c-kit mutant mice may not be fully explained by their lack of intestinal MCs because these mice also have abnormal gut motility due to their deficiency in the ICC network. It also has been difficult to repopulate MMCs in c-kit mutant mice by adoptive transfer of MCs, therefore, the role of MCs in intestinal parasite resistance has not yet been supported by evidence derived from using the MC knockin approach. By contrast, the fact that the expulsion of T. spiralis is significantly delayed in Mcpt1−/− mice supports an important contribution of intestinal MMCs and mMCP-1 in the clearance of this infection (Knight, Wright, Lawrence, Paterson, & Miller, 2000). The kinetics of T. spiralis expulsion from the small intestine are similar between mMCP-6-deficient and WT mice, but the mMCP-6-deficient mice have significantly diminished eosinophil recruitment in skeletal muscle after infection (Shin et al., 2008). Blankenhaus et al. (2014) recently showed that BALB/c-Cpa3Cre/+ mice inoculated with S. ratti exhibited increased parasite burden in the small intestine at day 6 after infection, supporting a role for MCs in host defense against this parasite in this setting. Their work also provided evidence that IL-9-mediated MC activation is a key mechanism mediating S. ratti repulsion, a process that is suppressed by Foxp3+ Treg cells in the BALB/c, but not the C57BL/6, strain.
Finally, one should keep in mind the possibility that some effects of MCs in parasite infections might favor the parasite. We noted above that anti-SCF treatment diminished intestinal MMC hyperplasia in rats infected with N. brasiliensis or T. spiralis, but such anti-SCF treatment decreased parasite egg production during N. brasiliensis infection (Newlands et al., 1995). These findings were in accord with results from prior work reporting that, during a primary infection with N. brasiliensis, c-kit mutant MC-deficient Ws/Ws rats exhibited significantly less egg output in the feces at day 8 of infection than did the corresponding WT rats (Arizono et al., 1993). However, neither the anti-SCF treatment nor the mutations in Ws/Ws rats exclusively affected MCs, so neither of these studies proves that the positive effects on parasite fecundity observed in animals with reduced numbers of MCs necessarily reflected an effect of MCs on the infection.
5.1.3 Resistance to animal venoms
Using MC knockin in KitW/W−v and KitW−sh/W−sh mice, we reported evidence that MCs can enhance the resistance of mice to diverse animal venoms and/or their toxic components, including the venoms of three snakes (the burrowing asp [or Israeli mole viper], western diamondback rattlesnake, and southern copperhead) (Metz et al., 2006), honey bee (Metz et al., 2006), Gila monster (Akahoshi et al., 2011), and two species of scorpions (the Death stalker and Arizona bark scorpions) (Akahoshi et al., 2011) as well as to sarafotoxin 6b, a major toxin in Israeli mole viper snake venom (Metz et al., 2006) and helodermin, a toxin in Gila monster venom (Akahoshi et al., 2011). Consistent with our findings in c-kit mutant MC-deficient mice (Metz et al., 2006), MC-deficient C57BL/6-Cpa3-Cre+;Mcl-1fl/fl mice also are substantially more susceptible to challenge with lethal doses of honey bee venom than are their Cpa3-Cre+;Mcl-1+/+ controls (Marichal et al., 2013).
Data from shRNA knockdown of CPA3 in MCs adoptively transferred to MC-deficient mice, as well as pharmacological evidence, indicated that the key MC-associated enzyme responsible for the effect of MCs on enhanced survival of mice challenged with Israeli mole viper venom or its major toxin, sarafotoxin 6b, is CPA3. Pharmacological evidence also implicated CPA3 in the MC-dependent enhanced resistance of mice to venom from the western diamondback rattlesnake and southern copperhead (Metz et al., 2006). Experiments by Schneider et al. (2007) using CpaY356L, E378A mice (which have a catalytically inactive CPA3) showed that the molecular mechanism by which CPA3 confers enhanced resistance to the toxic effects of sarafotoxin 6b in vivo is by cleaving the terminal tryptophan from the toxic peptide, markedly reducing its toxicity. By contrast, experiments using Cpa3Y356L, E378A mice and Mcpt4−/− mice identified mMCP-4, rather than CPA3, as the key MC-associated protease that contributes to MC-associated enhanced resistance to Gila monster venom (and to helodermin, the VIP-like toxin in such venom), as well as to the tested scorpion venoms (Akahoshi et al., 2011). Notably, CPA3 and mMCP-4 appeared to account for much or all of the protective effects against various venoms that were attributable to MCs, suggesting that these proteases constitute major MC-expressed effector molecules under such conditions.
It will be of interest to assess whether and to what extent other MC-associated proteases (or any of the non-protease mediators derived from MCs) can contribute to enhanced resistance to additional types of animal venoms, or to toxic products derived from other sources such a microorganisms. As reviewed elsewhere (Akahoshi et al., 2011; Marichal et al., 2013; Metz et al., 2006), the observation that two of the MC-derived proteases, CPA3 and mMCP-4, can degrade both endogenous biologically active peptides (endothelin-1 [ET-1] and VIP, respectively) and similar peptides present in animal venoms (sarafotoxin 6b and helodermin, respectively), which are thought to act in mammals via the same receptors which bind the similar endogenous peptides, is of interest. These findings suggest that the MC can help to protect the host in two different settings: (1) situations associated with excessive, and potentially harmful, concentrations of the endogenous peptides, such as in sepsis (in which levels of ET-1 are markedly elevated) or in subjects with VIP-producing tumors and (2) during envenomation by animals whose venoms contain similar peptides. Recent work in MC-deficient C57BL/6-KitW−sh/W−sh and Cpa3-Cre;Mcl-1fl/fl mice suggests that MCs also can contribute to the IgE antibody- and FcεRI-dependent enhanced survival after challenge with potentially lethal amounts of honeybee venom that is observed in animals which develop a TH2 response after an initial subcutaneous exposure to a sublethal amount of that venom (Marichal et al., 2013).
5.1.4 Effects on inflammation during innate and adaptive immune responses
Previous work in KitW/W−v and KitW−sh/W−sh mice (which at baseline have reduced or elevated levels of blood neutrophils, respectively) has provided evidence that MCs can contribute to orchestrating neutrophil recruitment in various inflammatory responses (Biedermann et al., 2000; Echtenacher, Mannel, & Hultner, 1996; Malaviya & Abraham, 2000; Malaviya, Ikeda, Ross, & Abraham, 1996; Piliponsky et al., 2010; Wershil, Murakami, & Galli, 1988; Wershil, Wang, Gordon, & Galli, 1991; Yu et al., 2006). Evidence for a positive role for MCs in the early stages of neutrophil recruitment during LPS-induced inflammation also has been provided by work in Mcpt5-Cre;iDTR mice and Mcpt5-Cre;R-DTA mice (De Filippo et al., 2013). Dudeck et al. (2011) evaluated immunoregulatory functions of MCs using MC-depleted Mcpt5-Cre;iDTR mice and found significant reductions in DNFB-induced-lymph node hypertrophy and DC emigration from the skin after epicutaneous application of DNFB or FITC in the absence of MCs. These findings are consistent with previous observations in experiments performed in c-kit mutant mice (Bryce et al., 2004; Jawdat, Rowden, & Marshall, 2006; McLachlan et al., 2003; Suto et al., 2006). A recent study employing both c-kit mutant and c-kit normal MC-deficient mice provides evidence that MCs can promote leukocyte recruitment and activation, and exacerbate tissue inflammation and pathology, in a mouse model of experimental stroke (Arac et al., 2014). We also recently used the MC knockin approach in both c-kit mutant and c-kit-independent MC-deficient mice, as well as DT-induced local ablation of MCs in the ankle joint of Cpa3-Cre;iDTR mice, to provide evidence that MCs and MC-derived IL-1β can contribute to the early stages of monosodium urate (MSU) crystal-induced acute arthritis (Reber, Marichal, et al., 2014) (Fig. 2).
Figure 2. Evidence from constitutively mast cell (MC)-deficient mice, MC knockin mice, and mice locally depleted of MCs, indicates that MC-derived IL-1β contributes to MSU crystal-induced acute arthritis in mice.
(A) c-kit mutant MC-deficient WBB6F1-KitW/W−v and C57BL/6-KitW−sh/W−sh mice were engrafted intra-articularly (i.a.) with 2 × 106 wild-type (WT) BMCMCs in one ankle and 2 × 106IL-1β−/− BMCMCs or vehicle (PBS) in the contra-lateral ankle joint. Six weeks after MC engraftment or vehicle injection, these mice and their respective WT control mice (i.e., WBB6F1-Kit+/+ and C57BL/6J-Kit+/+ mice, respectively) were injected i.a. with PBS or 0.5 mg MSU crystals (as indicated) and ankle swelling was measured over 24 h. (B) c-kit-independent MC-deficient Cpa3-Cre+;Mcl-1fl/fl mice were engrafted with WT BMCMCs in one ankle and vehicle (PBS) in the contra-lateral ankle joint. Six weeks after engraftment, these mice and their respective Cpa3-Cre+;Mcl-1+/+ littermate controls were injected i.a. with PBS or 0.5 mg MSU crystals (as indicated) and ankle swelling was measured over 24 h. (C) Cpa3-Cre+;DTRfl/+ mice and their Cpa3-Cre−;DTRfl/+ littermate controls were injected i.a. with diphtheria toxin (DT) (two successive weekly injections of 50 ng) in one ankle and vehicle (PBS) in the contra-lateral ankle. Mice were injected i.a. with 0.5 mg MSU crystals into both ankles 1 week after the last DT injection and ankle swelling was measured over 24 h. Data are shown as means ± SEM from n = 6–20 mice pooled from two or three independent experiments. *, **, or *** = P < 0.05, 0.01 or 0.001 versus indicated groups by ANOVA. NS, not significant (P > 0.05). Adapted with permission from Reber, Marichal, et al. (2014).
5.1.5 Mouse models of bacterial infection
Several groups have assessed the role of MCs in the cecal ligation and puncture (CLP) model of sepsis using the MC knockin system in kit mutant mice. Most of these studies were performed using MC-deficient KitW/W−v mice, and most of the results obtained support the conclusion that MCs can improve survival after CLP in KitW/W−v mice (Echtenacher et al., 1996; Mallen-St Clair, Pham, Villalta, Caughey, & Wolters, 2004; Maurer et al., 1998, 2004; Piliponsky et al., 2010, 2008, 2012; Supajatura et al., 2002; Sutherland, Olsen, McKinstry, Villalta, & Wolters, 2008). Given that KitW/W−v mice have reduced numbers of neutrophils (Chervenick & Boggs, 1969; Nigrovic et al., 2008; Piliponsky et al., 2010; Zhou et al., 2007) and other abnormalities in hematopoietic cells besides MCs, it is possible that these findings in part reflect a more important role for MCs in host defense during CLP in KitW/W−v mice than in the corresponding WT mice. Notably, treatment of WT mice with SCF increased MC numbers and also improved survival in the CLP model (Maurer et al., 1998). These effects of SCF treatment appeared to reflect actions of SCF on MCs, at least in KitW/W−v mice, since SCF was able to improve survival of KitW/W−v mice in the CLP model only when such mice were engrafted with WT BMCMCs (Maurer et al., 1998).
Many studies have used engraftment of KitW/W−v mice with WT BMCMCs or with various mutant BMCMCs to try to dissect the pathways leading to MC activation in this setting, as well as to define which mediators released by MCs can contribute to enhanced survival in CLP. Studies in KitW/W−v mice indicated that MC expression of TLR4 but not TLR2 is required for MCs to enhance survival in CLP in KitW/W−v mice (Supajatura et al., 2002). MC also can be activated by ET-1, an endogenous peptide with potent vasoconstrictor properties, primarily through the ETA receptor. Activation by ET-1 promotes MC degranulation and the release of proteases which in turn can degrade ET-1, thus enhancing survival by limiting the toxic effects of ET-1 (Maurer et al., 2004). While initial results from pharmacological studies implicated chymase (mMCP-4) in the degradation of ET-1 in CLP (Maurer et al., 2004), later work employing shRNA knockdown of CPA3 in BMCMCs before their transfer into c-kit mutant MC-deficient mice (Metz et al., 2006), or utilizing mice genetically engineered to have a catalytically inactive CPA3 (Schneider et al., 2007), showed that CPA3 was the critical MC-associated protease that degraded ET-1. Other reports have presented evidence that MC-derived IL-12 (Nakano et al., 2007), and MC expression of the cysteinyl protease dipeptidyl peptidase I (DPPI) (Mallen-St Clair et al., 2004) and of the transcription factor Smad3 (Kanamaru et al., 2005) also can contribute to survival in the tested CLP models.
The initial report describing protective effects of MCs in CLP by Echtenacher et al. (1996) showed that injection of low doses of recombinant TNF can increase survival of KitW/W−v mice, while high doses of TNF worsened survival. Furthermore, injection of an anti-TNF blocking antibody interfered with the protection conferred by selective engraftment of KitW/W−v mice with WT BMCMCs (Echtenacher et al., 1996). Although MCs were known to produce TNF (Gordon & Galli, 1990a), it was not clear from this initial study to what extent the results reflected MC production of TNF during CLP versus effects of MCs on the production of TNF by other cells, including neutrophils which might be recruited to the site of inflammation.
Piliponsky et al. confirmed that MCs can enhance survival after moderately severe CLP (i.e., in a model that induced 20–50% death of the wild-type mice within 4 days after CLP) in three types of c-kit mutant MC-deficient mice (WBB6F1-KitW/W−v, C57BL/6-KitW−sh/W−sh, and WBB6F1-KitW−sh/W−sh mice). However, in KitW/W−v MC knockin mice, MC-derived TNF was not required for the protective role of MCs in moderate CLP. By contrast, while KitW/W−v mice exhibited increased mortality versus the WT mice in a more severe CLP model (in which > 50% of the WT mice die within 4 days), C57BL/6-KitW−sh/W−sh mice (and to a lesser extent WBB6F1-KitW−sh/W−sh mice) show improved survival compared to the corresponding WT mice. Moreover, engraftment experiments showed that MCs, and MC-derived TNF, can increase mortality in C57BL/6-KitW−sh/W−sh mice (Piliponsky et al., 2010). These results highlight two points: (1) that the type of mutation affecting Kit expression can influence aspects of the phenotype analyzed in the CLP model in such mice and (2) that in mice on the same strain background, the role(s) of MCs in CLP may differ in moderately severe versus severe models.
Studies in Mcpt4−/− mice indicate that mMCP-4 has effects that can enhance survival in a moderately severe model of CLP. This may have reflected, in part, the ability of mMCP-4 to degrade TNF and thus limit the potentially toxic effects of high levels of this cytokine (Piliponsky et al., 2012). These findings highlight what may be a complex relationship between the production and potential degradation of TNF by MCs in CLP and perhaps other settings. One may speculate that, in some innate or acquired immune responses, MCs can (1) contribute to the early production of small amounts of TNF (derived from MCs and/or produced by other sources in response to MC activation) that can enhance certain aspects of the responses (Echtenacher et al., 1996; Malaviya et al., 1996; Zhang, Ramos, & Jakschik, 1992), and also (2) release proteases which can limit the toxicity of TNF (which can be degraded by mMCP-4) and other toxic agents, such as ET-1 (which can be degraded by CPA3), particularly in settings in which severe inflammation itself contributes to pathology. MCs also have the potential to limit levels of potentially toxic endogenous mediators in the setting of CLP via the actions of intracellular enzymes, such as in MC-neurolysin-dependent degradation of neurotensin (Piliponsky et al., 2008). In a study which indicated that the activity of a MC-derived chymase can be constrained by an effect of a MC-derived cytokine, Orinska et al. (2007) reported evidence that intracellular IL-15 expression in MCs can transcriptionally limit the amount of mMCP-2 in the cells, resulting in decreased MC-associated chymase activity in vitro, decreased MC antibacterial properties, and reduced survival of the mice after CLP. These interesting findings were unexpected, since prior work by had detected little catalytic activity of mMCP-2 under the conditions that were tested (Andersson, Pemberton, Miller, & Hellman, 2008; Pemberton et al., 2003).
Some groups have also used MC-deficient or MC-associated protease-deficient mice to assess the role of MCs or their proteases in models of experimental infection with different types of bacteria. After i.p. injection of Klebsiella pneumonia, MCs can mediate neutrophil recruitment through the release of TNF (Malaviya et al., 1996) and IL-6 (Sutherland et al., 2008). Two reports suggest a central role for the tryptase mMCP-6 in defense against K. pneumonia (Huang et al., 2001; Thakurdas et al., 2007). In the first report, the authors showed that injection of recombinant mMCP-6 or its human ortholog human hTryptase β1 24 h before inoculation with K. pneumonia significantly improved the ability of KitW/W−v mice to control the bacterial infection (Huang et al., 2001). In the second study, the group demonstrated that mice deficient for mMCP-6 are less efficient than the corresponding WT mice at clearing K. pneumonia following i.p. injection of the bacteria (Thakurdas et al., 2007). There is evidence that MCs also can play protective roles in mouse models of infection with Mycoplasma pneumonia (Xu et al., 2006) or E. coli (Malaviya et al., 1996). Malaviya, Navara, and Uckun (2001) reported that, during infection with E. coli, neutrophil recruitment and bacterial clearance is controlled by JAK3 activation in MCs; this effect was attributed to the diminished ability of Jak3−/− MCs to produce TNF. Subsequent studies using the MC knockin system have provided evidence that MC-derived TNF also can contribute to host defense against bacteria by promoting both DC recruitment to the site of infection and the migration of DCs into draining lymph nodes (DLNs) and by inducing hypertrophy of DLNs and enhancing the development of an acquired immune response against the organisms (McLachlan et al., 2003; Shelburne et al., 2009). By contrast, in MC-engrafted KitW−sh/W−sh mice, we found that MC-derived TNF can enhance bacterial growth and hasten death after i.p. inoculation of Salmonella typhimurium (Piliponsky et al., 2010).
Recently, Chan et al. analyzed the potential roles of MCs in a mouse model of bladder infection with uropathogenic E. coli. Specifically, they assessed the role of MC-derived IL-10 in this model using both the MC knockin approach in KitW−sh/W−sh mice engrafted with WT or Il-10−/− BMCMCs and by employing c-kit-independent mice in which only MCs were unable to produce IL-10 (i.e., Mcpt5-Cre+;Il-10fl/fl mice). They demonstrated a key role for MC-derived IL-10 in limiting production of E. coli-specific antibodies and promoting persistence of bacteria in the bladder (Chan, St John, & Abraham, 2013). Therefore, beside potential roles in promoting immediate innate immune responses to bacteria, and in some settings enhancing the development of acquired immune responses to bacteria, MCs also appear to be able to suppress adaptive immune responses during infections, at least in a model of bladder infection with uropathogenic E. coli.
It will be of great interest to continue to evaluate the roles of MCs in CLP and other models of infection using some of the newer, c-kit-independent, models of MC deficiency, as well as in additional types of MC-associated protease-deficient mice. For example, it was recently reported that MC-deficient Mcpt5-Cre;DTA mice and their MC-sufficient littermate controls are equally susceptible to i.p. infection with Staphylococcus aureus (Ronnberg et al., 2014). Such work will help to clarify which roles of MCs in such settings are variably redundant with the roles of other cell types (such as neutrophils or macrophages) and which MC roles—whether to enhance or suppress aspects of these innate or acquired immune responses—may be nonredundant.
5.1.6 Tissue remodeling and pathology in disease settings
MC-deficient mice and to a lesser extent MC knockin mice have been used by many groups to study various models of tissue remodeling and disease. We will not attempt to review here all of that work, but will note that in many cases the work was conducted using a single type of MC-deficient mouse. As discussed in more detail below, in general, it is wise to examine the biological response of interest in two (or more) models of MC deficiency, particularly when studying complex biological responses in which multiple cell types may have partially overlapping roles. However, there are examples of disease models in which elimination of MCs or individual MC-associated proteases have been reported to have similar effects. For instance, mMCP-4 appears to account for much of the protective role of MCs in a model of kidney fibrosis (Beghdadi et al., 2013) and in a model of brain/spinal cord inflammation (Hendrix et al., 2013; Nelissen et al., 2013); in the latter model, mMCP-4 is thought to function to reduce levels of potentially pathogenic cytokines (Nelissen et al., 2013), a role reminiscent of that of mMCP-4 in degrading TNF in the setting of CLP (Piliponsky et al., 2012).
In settings in which MCs have been shown to have a detrimental impact, the effects of individual MC protease deficiencies are in several cases in line with the effect of a global MC deficiency. One example is the reported detrimental roles of the chymase mMCP-4 (Sun et al., 2009) and tryptase (i.e., mMCP-6) (Zhang et al., 2011) in the formation of experimental abdominal aortic aneurysms (AAAs), where there is evidence that these proteases represent major effector molecules produced by MCs. In further agreement with a role of MC-associated proteases in AAA pathology, chymase inhibitors have been shown to ameliorate the development of AAAs (Inoue et al., 2009; Tsunemi et al., 2004). It also has been reported that mMCP-4 can account for most of the detrimental impact of MCs in a model of bullous pemphigoid (Lin et al., 2011).
5.2. Settings in which divergent results have been obtained using multiple models of MC deficiency or deficiencies in MC-associated proteases
5.2.1 Wound healing and tissue remodeling
There has been considerable speculation that MCs may play important roles in multiple aspects of wound healing (Douaiher et al., 2014; Ng, 2010), either by beneficially promoting wound healing or by detrimentally enhancing the formation of keloids or hypertrophic scars when MCs are over-activated (Douaiher et al., 2014). Using a mouse model of splinted cutaneous excisional wounds (this approach minimizes wound contraction—that is prominent in mice—and therefore is thought to more closely resemble physiological repair of cutaneous wounds in humans), Nauta et al. (2013) found no differences in the kinetics of wound closure nor scar formation in three types of MC-deficient mice (WBB6F1-KitW/W−v mice, C57BL/6-KitW−sh/W−sh mice, or Cpa3-Cre;Mcl-1fl/fl mice) and the respective MC-sufficient control mice. In an excisional skin injury model (Willenborg et al., 2014), Mcpt5-Cre;iDTR mice conditionally deleted of MCs also did not reveal any differences in the kinetics of re-epithelialization or in the formation of vascularized granulation tissue or scar compared to their controls.
Activin is a growth and differentiation factor that is strongly induced upon skin injury (Hubner, Hu, Smola, & Werner, 1996). To investigate whether MCs are involved in activin’s healing-promoting activity, Antsiferova et al. (2013) crossed transgenic mice overexpressing Activin A (under the control of keratin 14 promoter) with Cpa3Cre/+ mice and induced excisional wounds in these mice. The lack of MCs did not influence neutrophil infiltration (as assessed by MPO activity or numbers of Ly6G-positive cells), granulation tissue formation, re-epithelialization, or density of the late granulation tissue/early scar tissue in mice of either the Activin + or Activin − transgenic background. Time to wound closure was not decreased in Cpa3Cre/+ mice, but these mice had slightly smaller wounds than the corresponding controls at the early stages of wound healing. These recent findings in c-kit normal MC-deficient mice are consistent with those of previous studies which analyzed the healing of cutaneous excisional wounds in adult mice and found the rates of wound closure were not different in KitW/W−v mice and the corresponding WT mice (Egozi, Ferreira, Burns, Gamelli, & Dipietro, 2003; Iba, Shibata, Kato, & Masukawa, 2004). In contrast, in an earlier study, the closure of unsplinted cutaneous excisional wounds, which in part reflects contraction of the wounds, was found to be delayed in MC-deficient WBB6F1-KitW/W−v mice versus WT mice during the first 6 days after wounding, but the wounds eventually closed at the same time as those in WT mice (Weller, Foitzik, Paus, Syska, & Maurer, 2006). In addition, Wulff et al. (2012) reported that the scarring associated with full thickness cutaneous wounds examined 7 or 10 days after wounding on fetal day 18 was less in KitW/W−v mice than in WT mice. It would be of interest to determine whether, and to what extent, similar findings might be observed in other types of MC-deficient mice.
Wound healing after skin scald injury has been examined in MC-deficient KitW/W−v mice and in various strains of protease-deficient mice (Bankova et al., 2014; Shiota et al., 2010; Younan et al., 2010). The kinetics of wound closure and re-epithelialization after a 100 °C scald injury were not different between KitW/W−v mice and +/+ mice, but there was a slight reduction in fibrosis at the edge of the wound and slightly less wound vascularization in KitW/W−v mice (Shiota et al., 2010). In another study, KitW/W−v mice exhibited less erythema and ulceration after scald injuries induced at 54 °C, but not at 56 °C or 58 °C (Younan et al., 2010). Mcpt4−/− and Mcpt5−/− mice exhibited decreased injury after scald burns at 54 °C, whereas Mcpt6−/−, Mcpt7−/−, and Cpa3Y356A, E378A mice were not protected from burn-induced pathology (Bankova et al., 2014; Younan et al., 2010). Taken together, these finding indicate that the chymases mMCP-4 and mMCP-5 can contribute to MC-mediated skin damage during burn injury under certain conditions [e.g., in scald injuries induced at 54 °C (Bankova et al., 2014; Younan et al., 2010)], but that MCs are not required for the injuries elicited by higher temperature burns (> 54 °C).
Discordant results regarding the potential roles of MCs also have been obtained when the pathology induced by the same agent is studied in different anatomical sites. For example, MC-deficient Mcpt5-Cre;iDTR mice were not protected from the development of bleomycin-induced skin fibrosis (Willenborg et al., 2014) and the amount of collagen deposition was comparable in MC-depleted Mcpt5-Cre;iDTR and control mice (Willenborg et al., 2014). However, in a model of bleomycin-induced lung fibrosis, there is evidence that MCs, and the chymase mMCP-4, can contribute to the pathology (Reber, Daubeuf, Pejler, Abrink, & Frossard, 2014). The latter finding is thus in agreement with the reported beneficial effects of chymase inhibitors in bleomycin-induced pulmonary fibrosis (see Section 2.1.4). Thus, the roles of MCs in bleomycin-induced pathology may differ based on the protocol of bleomycin administration, the anatomic site examined, and/or the strain background of the mice analyzed.
5.2.2 Mouse models of autoimmune arthritis
Studies in mouse models of autoimmune arthritis have provided partly contradictory data about the effects of a global MC deficiency versus deficiencies in MC proteases. Evidence for an important role of MCs in autoimmune arthritis was provided by Lee et al. (2002), who reported that MC-deficient KitW/W−v and KitlSl/Sl−d mice developed substantially reduced joint inflammation and destruction in a mouse model of human inflammatory arthritis elicited by injection of K/BxN serum, and that local engraftment of wild type, but not Il-1−/− MCs, restored disease susceptibility in KitW/W−v mice (Lee et al., 2002; Nigrovic et al., 2007). Mancardi and Feyerabend subsequently confirmed Lee’s findings in KitW/W−v mice (Feyerabend et al., 2011; Mancardi et al., 2011). However, both Lee’s group (unpublished data) (Nigrovic et al., 2007) and Mancardi et al. (2011) reported that KitW−sh/W−sh mice developed robust arthritis in the K/BxN arthritis model (Mancardi et al., 2011). KitW−sh/W−sh mice also strongly developed a model of proliferative arthritis induced by injection of anti-type II collagen antibody (Zhou et al., 2007), whereas KitW/W−v mice were protected in this arthritis model (Zhou et al., 2007).
Given the discrepant observations in mouse models of autoimmune arthritis in different c-kit mutant mice, Feyerabend et al. (2011) tested Cpa3Cre/+ mice in the K/BxN serum transfer model and found that these mice, like KitW−sh/W−sh mice, were fully susceptible to developing the joint pathology. However, findings in Cpa3Cre/+ and KitW−sh/W−sh mice appear to be inconsistent with those derived from studies in heparin-deficient Ndst2−/− mice (that have deficiencies in multiple MC-associated proteases (see Fig. 1)) and in tryptase- (mMCP-6-) deficient C57BL/6 mice (which also lack mMCP-7), which developed diminished levels of pathology after injections of K/BxN serum (Shin et al., 2009). mMCP-4 has been reported to contribute to disease progression in the collagen-induced-model of arthritis (Magnusson, Pejler, Kleinau, & Abrink, 2009; Shin et al., 2009), although it should be noted that mMCP-4 was only partially responsible for disease progression in this setting.
Together, the discrepant observations in different strains of MC-deficient or MC-protease-deficient mice suggest that MCs or their individual proteases can have redundant or partially nonredundant roles in mouse models of autoimmune arthritis, depending on the strains of mice examined, the amount (and/or specificity) of the autoantibodies, or the protocols used to induce the disease. Because of the important contribution of neutrophils in autoimmune arthritis models, it is possible that partially “redundant” contributions of MCs may be masked in presence of neutrophilia (in KitW−sh/W−sh mice) or even with normal levels of neutrophils (in Cpa3Cre/+ “Cre-Master” mice), but can be revealed in presence of neutropenia (in KitW/W−v mice). Differences in genetic backgrounds of WBB6F1-KitW/W−v mice versus that (e.g., C57BL/6) in other types of MC-deficient mice also may contribute to different experimental outcomes.
As noted above, the choice of experimental protocol also may influence outcomes in such experiments: the studies of the effects of a global MC deficiency were all performed using various models of passively induced, antibody-dependent disease, whereas the study on the role of mMCP-4 was performed after active immunization of mice with collagen (Magnusson et al., 2009). It is possible that passive induction of arthritis may bypass steps in disease progression that are dependent on MCs. In agreement with a detrimental role of MCs in actively induced arthritis, a recent study showed that overactivation of MCs by MC-specific ablation of the NF-κB negative feedback regulator A20 caused exacerbated collagen-induced arthritis (Heger, Fierens, et al., 2014).
By contrast, the study that identified a detrimental role for tryptase in arthritis (Shin et al., 2009) was performed using the passive K/BxN model, i.e., the model that was used in the study where no global impact of MCs was seen in tests in c-kit-independent MC-deficient Cpa3Cre/+ mice (Feyerabend et al., 2011). Although it is not possible at present to explain fully the apparent contradiction between these two studies, one potential explanation is that a global and constitutive absence of MCs may result in compensatory mechanisms that fully or partially mask the contribution of individual MC-derived mediators, in this case tryptase. Another explanation might be that MC proteases, although being essentially restricted to MCs under baseline conditions, under certain circumstances (including the disease setting) may be expressed by other cell types. Finally, it should be noted that the experiments in the two labs employed different amounts of K/BxN serum to elicit the model, with 150 µl being used for studies in the Cpa3Cre/+ mice (Feyerabend et al., 2011) versus 50 µl in the protease-deficient mice (Shin et al., 2009).
5.2.3 Experimental autoimmune encephalomyelitis
Findings in experimental autoimmune encephalomyelitis (EAE) elicited in MC-deficient mice have not been consistent. Compared to the corresponding WT mice, WBB6F1-KitW/W−v mice immunized with myelin oligodendrocyte glycoprotein (MOG)35–55 peptide developed disease with delayed onset and milder severity (Secor, Secor, Gutekunst, & Brown, 2000). Similarly, SJL-KitW/W−v mice exhibited significantly reduced disease severity when immunized with proteolipid protein (PLP)131–159 peptide (Sayed, Walker, & Brown, 2011). Disease susceptibility in these MC-deficient KitW/W−v mice was restored to WT levels by MC engraftment, supporting the conclusion that MCs contribute to the EAE-associated pathology in these models. While evidence for a detrimental role of MCs in EAE was independently confirmed in KitW−sh/W−sh mice in one study (Stelekati et al., 2009), Bennett et al. (2009) reported that both KitW/W−v and KitW−sh/W−sh mice were fully susceptible to EAE, whereas Li et al. (2011) reported earlier and more severe EAE in KitW−sh/W−sh mice in comparison to the corresponding Kit+/+ mice.
To evaluate the possible effects of different experimental protocols on different disease outcomes, Piconese et al. (2011) used three different conditions of immunization to induce EAE and found disease exacerbation in MC-deficient KitW−sh/W−sh mice versus the corresponding WT mice regardless of which protocol was used. It recently has been reported that there are abnormalities in populations of myeloid-derived suppressor cells (MDSCs) in KitW−sh/W−sh mice (Michel et al., 2013). However, it is unclear whether any problems with MDSCs contribute to disease exacerbation in KitW−sh/W−sh mice in the EAE models tested. Furthermore, Piconese et al. showed that KitW/W−v mice immunized with the lower doses of MOG and adjuvants exhibited worse disease than WT mice, resembling the disease phenotype observed in the KitW−sh/W−sh strain. By contrast, they did confirm that EAE was milder in KitW/W−v mice compared with Kit+/+ mice when the disorder was induced using a strong immunization protocol similar to that used by Secor et al. (2000). Feyerabend et al. (2011) found no difference in EAE disease severity in Cpa3Cre/+ or KitW/W−v mice and the respective MC-containing control mice and concluded that MCs are dispensable for EAE development, at least in the models tested.
We think that the simplest conclusion from all of this work is that the roles of MCs in EAE may be highly overlapping with those of other cell types, and that these roles are apparent only under certain conditions of experimental testing. It is likely that a large number of factors can influence the outcome in such studies, including the strain backgrounds and ages of the mice, the strength of the immunization protocols used to induce the disease, and perhaps the composition of the animals’ microbiota. However, as in the studies of models of autoimmune arthritis discussed above, the goal of clarifying what sort of contributions MCs and their products make (or do not make) to disease development in EAE models certainly has benefitted from efforts to study the models in multiple types of MC-deficient mice and using multiple protocols of disease induction.
5.2.4 Mouse models of asthma
A few studies have investigated mouse models of asthma using MC knockin approaches in c-kit mutant KitW/W−v and KitW−sh/W−sh mice. Such studies have provided evidence that a key role of MCs in the examined models of allergic airway inflammation is to amplify the expression of multiple features of the pathology that can be elicited by antigen challenge of the actively immunized animals. For example, studies in mouse models of allergic airway inflammation that either omitted artificial adjuvants at the time of antigen (Ag) sensitization (Nakae et al., 2007; Reuter et al., 2008; Taube et al., 2004; Williams & Galli, 2000; Yu et al., 2006, 2011) or employed relatively low doses of Ag for sensitization or challenge (Kobayashi et al., 2000; Kung et al., 1995) have revealed that MCs can directly or indirectly enhance the magnitude of multiple features of the responses, including AHR to cholinergic stimulation (Kobayashi et al., 2000; Kung et al., 1995; Nakae et al., 2007; Taube et al., 2004; Williams & Galli, 2000; Yu et al., 2006, 2011), infiltration of eosinophils and other leukocytes into the airways and/or bronchoalveolar lavage fluid (Kung et al., 1995; Nakae et al., 2007; Williams & Galli, 2000; Yu et al., 2006, 2011), increased numbers of airway goblet cells (Reuter et al., 2008; Yu et al., 2006, 2011), increased lung collagen deposition (Yu et al., 2006, 2011), and increased numbers of MCs in the airways, including some within the epithelium (Yu et al., 2006, 2011). MC-associated TNF has been identified as one of the key mediators that contribute to AHR and airway inflammation (Kim et al., 2007; Nakae et al., 2007; Reuter et al., 2008), goblet cell metaplasia (Reuter et al., 2008), and lymphocyte recruitment and TH2 cytokine production in such settings (Nakae et al., 2007). In one model of chronic allergic airway inflammation, activation of MCs through the FcR γ chain and the IFNγ receptor 1 is required for the full development of many features of allergic airway responsiveness, inflammation, and tissue remodeling (Yu et al., 2006, 2011).
Recently, the essential role of MCs in OVA-induced AHR has been confirmed in experiments using Mas-TRECK mice (Sawaguchi et al., 2012). Heger, Fierens, et al. (2014) investigated the effect of MC-specific A20 deficiency in mouse asthma models. Loss of MC-specific A20 in c-kit normal Mcpt5-Cre;A20fl/fl mice significantly enhanced airway inflammation, Ag-specific serum IgE, DC recruitment in the lung, and vascular leakage upon antigen challenge when the mice were immunized by intranasal administration of HDM extracts or i.p. injections of OVA without, but not with, alum (Heger, Fierens, et al., 2014). These findings are in agreement with the notion that MCs can contribute to pathology in asthma models in mice.
On the other hand, it has long been known that the direct contribution of MCs to various features of allergic asthma can be masked (or, rendered redundant) in some models of allergic airway inflammation, including very commonly used models that employ strong artificial adjuvants and relatively high doses of Ag for sensitization and challenge (Brusselle et al., 1994; Nogami et al., 1990; Okudaira et al., 1991; Takeda et al., 1997; Williams & Galli, 2000). In our opinion, a reasonable generalization from studies in various models of allergic airway inflammation in mice is that the importance of MCs in the expression of airway inflammation and other features of asthma in mice is greatest when relatively weak stimulants/inducers are used to induce the response. Moreover, it is clear that strain background also can influence the importance of the MCs’ roles in such asthma models (Becker et al., 2011). These general points also may apply to other acquired immune responses that are associated with the production of antigen-specific IgE.
Given the widely held view that MCs can have detrimental effects in human asthma, and in light of the potential to develop agents that might inhibit the actions of MC-associated proteases in this setting, there is great interest in the possible contributions of MC-associated proteases to the pathology of asthma. However, there have been relatively few studies of asthma models in MC protease-deficient mice. A recent report indicates that MC-expressed γ-tryptase has a detrimental impact on lung inflammation, albeit of a nonallergic type (Hansbro et al., 2014). By contrast, studies in Mcpt4−/− mice indicate that mMCP-4 can have protective effects in two different models of allergic lung inflammation (Waern et al., 2009; Waern, Lundequist, Pejler, & Wernersson, 2013), and that such protective effects might reflect, at least in part, degradation of IL-33 by the chymase (Fig. 3).
Figure 3. Roles of mast cells (MCs) and mMCP-4 in allergen-induced airway inflammation, airway hyper-responsiveness (AHR), and tissue remodeling.
In the depicted model of allergic airway inflammation (see Nakae et al., 2007; Waern et al., 2009, 2013; Williams & Galli, 2000; Yu et al., 2006, 2011), challenge of sensitized C57BL/6 and WBB6F1 mice with allergen via the airways i.n. produces different tissue responses in c-kit mutant mice lacking MCs or mMCPT-4 than in the corresponding wild-type mice. In mice lacking MCs (A), allergen challenge induces lower levels of AHR to methacholine challenge, airway inflammation, and tissue changes compared to those observed when MCs are present (B and C). (B) In the presence of wild-type MCs, the binding of allergen by IgE molecules bound to adjacent FcεRI molecules induces FcεRI aggregation, activating MCs to secrete preformed mediators (e.g., mMCP-4 and some TNF), lipid mediators, and many cytokines, chemokines, and growth factors. Some aeroallergens (e.g., HDM) can directly induce MC degranulation and secretion of mMCP-4. The secreted mediators can induce migration, maturation, and activation of DCs, amplify inflammatory responses and TH2 cytokine production, enhance AHR, and promote tissue changes, such as goblet cell metaplasia and overproduction of mucus, collagen deposition, and hyperplasia of airway smooth muscle cells. The activation of airway MCs can potentially be modulated by tissue factors, e.g., IFNγ, S1P, adenosine, and IL-33, or by cells, e.g., TH2 cells and Treg cells, which may be present in these sites. Studies in MC knockin mice indicate that some actions of MCs (such as increasing the numbers of epithelial goblet cells) can occur in a model of chronic asthma by MC-dependent mechanisms that do not require MC signaling via the FcεRIγ chain, whereas MCs must express both the FcεRIγ chain and the INFγR to mediate robust increases in lung eosinophils, neutrophils, and collagen (Yu et al., 2006, 2011). (C) In Mcpt4−/− mice which have MCs but lack MC-associated mMCP-4, there are higher levels of serum IgE after sensitization, which may result in increased IgE levels in the airway tissues, as is depicted in the figure. The increased levels of IgE can favor the expression of increased numbers of FcεRI on MCs and basophils. Moreover, compared to the airway changes in wild-type mice (B), in mMCP-4-deficient mice (C), allergen challenge induces exacerbated AHR and enhanced thickening of airway smooth muscle, increased levels of inflammatory cell infiltration (with increases in eosinophils, lymphocytes, and neutrophils), and elevated levels of IL-33 in the airway. In wild-type mice (B), the degradation of IL-33 by MC-derived mMCP-4 can potentially dampen IL-33-mediated eosinophil recruitment, TH2 responses, and IgE production. AHR, airway hyper-responsiveness; TNF, tumor necrosis factor; HDM, house dust mite; DCs, dendritic cells; IFNγ, interferon γ; S1P, sphingosine-1-phosphate.
5.2.5 Cutaneous contact hypersensitivity
Several reports have assessed the role of MCs during cutaneous contact hypersensitivity (CHS) responses elicited by sensitization and challenge with various haptens (including oxazolone, 2,4,6-trinitrochlorobenzene [TNCB], or 1-fluoro-2,4-dinitrobenzene [DNFB], or urushiol). Some of these studies suggested that MCs can promote multiple features of these CHS reactions (including tissue swelling and leukocyte infiltration) (Askenase et al., 1983; Biedermann et al., 2000; Bryce et al., 2004; Dudeck et al., 2011; Norman et al., 2008), others showed that MCs play no significant (or a redundant) role (Galli & Hammel, 1984) and some reports indicated that MCs can play a protective role during severe CHS reactions (Grimbaldeston et al., 2007; Hershko et al., 2011; Norman et al., 2008). We think that it is likely that multiple factors may account for the diversity of these findings, including the choice of MC-deficient mice and their strain background, the choice and amounts of hapten, the protocols for sensitizing and challenging the mice, the severity of the reactions analyzed, and even the sex or microbiomes of the animals.
For example, using the MC knockin approach in both KitW/W−v and KitW−sh/W−sh MC-deficient mice, we found that MCs substantially limited the skin pathology associated with sensitization and challenge with urushiol (the active substance found in poison ivy and poison oak, and which is responsible for the development of allergic contact dermatitis in humans) or the hapten DNFB (Grimbaldeston et al., 2007). MCs limited multiple features of these severe models of CHS, including tissue swelling, infiltrates of leukocytes, epidermal hyperplasia, and epidermal necrosis. Using engraftment of c-kit mutant mice with WT or Il-10−/− BMCMCs, we provided evidence that production of IL-10 by MCs can significantly contribute to the anti-inflammatory or immunosuppressive effects of MCs in these CHS models (Grimbaldeston et al., 2007). Finally, we showed that MCs and MC-derived IL-10 also can limit the tissue swelling, epidermal thickening, and leukocyte recruitment associated with chronic low dose ultraviolet (UV)B irradiation of the c-kit mutant mice (Grimbaldeston et al., 2007).
These findings were recently challenged by those of Dudeck et al. (2011), who showed that while c-kit mutant mice have slightly increased DNFB-induced CHS reactions, c-kit-independent inducible (Mcpt5-Cre;iDTR) or constitutive (Mcpt5-Cre;R-DTA) MC-deficient mice have diminished CHS responses in these reactions. These authors also found similar responses in Mcpt5-Cre+;Il-10fl/fl mice (in which only MCs cannot make IL-10) and their littermate controls (Dudeck et al., 2011). Otsuka et al. (2011) also reported that DT-inducible MC depletion in Mas-TRECK mice can diminish tissue swelling and leukocyte recruitment in a model of DNFB-induced CHS.
We think that these apparently contradictory results might either be explained by differences in details of CHS protocols or the effects of other factors that might influence these responses in the different models of MC deficiency used (or both). It is also possible that MCs and MC-derived IL-10 may suppress certain CHS responses in KitW−sh/W−sh mice but have redundant or no roles in the corresponding responses in WT mice. However, it is important to note that the CHS protocol employed by Dudeck et al. (2011) induced substantially lower levels of tissue swelling compared to the protocol we reported (Grimbaldeston et al., 2007) and it is possible that MCs can have a proinflammatory role in settings of moderate inflammation and a protective role during severe reactions. This hypothesis was discussed by Norman et al. (2008), who observed diminished tissue swelling in MC-deficient KitW/W−v mice versus the corresponding WT mice in a CHS model elicited using sensitization and challenge with low doses of the hapten oxazolone (Ox; specifically, 50 µl of 2% Ox on one ear, with challenge on the same ear 5–7 later with 10 µl of 0.8% Ox), while they found increased responses in KitW/W−v mice when using higher doses of the same hapten for both sensitization and challenge (i.e., 50 µl of 5% Ox on one ear, with challenge on the same ear 5–7 later with 10 µl of 1.0% Ox). Consistent with a potential role of MCs in suppressing severe CHS responses, Hershko et al. (2011) used the MC knockin approach in c-kit mutant mice to provide evidence that MC-derived IL-2 can contribute to suppression of severe chronic CHS reactions induced by sensitization and repeated challenges with Ox.
5.2.6 Experimental glomerulonephritis
Studies in KitW/W−v and MC knockin mice indicate that MCs can have either protective (Hochegger et al., 2005; Kanamaru et al., 2006) or detrimental (Timoshanko, Kitching, Semple, Tipping, & Holdsworth, 2006) roles in mouse models of immune complex-mediated glomerulonephritis. Hochegger et al. and Kanamaru et al. (Hochegger et al., 2005; Kanamaru et al., 2006) used “accelerated” models of anti-glomerular basement membrane (GBM) disease in mice that were pre-immunized with rabbit IgG with adjuvant, followed by i.v. administration of anti-mouse GBM, and showed that MCs can exert protective effects by modulating the recruitment of effector T cells and macrophages and promoting repair and remodeling processes. By contrast, Timoshanko et al. (2006), using a different model of the disease, found evidence for a detrimental role for MCs in promoting inflammatory cell infiltrates in the kidney.
Notably, work with mMCP-4-deficient mice indicates that mMCP-4 can have detrimental effects in a model of kidney inflammation (Scandiuzzi et al., 2010), even though studies in KitW/W−v mice (Kanamaru et al., 2006) suggested that MCs themselves might have an overall protective effect. Intriguingly, these findings suggest that in this setting, as in certain models of allergic airway inflammation (discussed above), MCs and their individual mediators can orchestrate effects that can either promote or dampen features of pathological processes, perhaps related to the evolution and stage of that process. According to this hypothesis, the global impact of MCs in a given setting is thus the result of the balance between, and timing of, such enhancing or suppressing activities.
5.3. Potential effects of strain background, the host microbiome, and/or differences in animal husbandry
When studying the function of the various MC-associated proteases, it is important to take into account that some of the commonly used mouse strains show important differences in their expression of such proteases. The best known example is the tryptase, mMCP-7. Due to a point mutation in the exon 2/intron 2 splice site of the Mcpt7 gene, which causes a premature stop codon, C57BL/6 mice lack expression of Mcpt7 (Hunt et al., 1996). By contrast, many of the commonly used lab strains such as BALB/c, C3H, and DBA-1 express Mcpt7, and Mcpt7 expression is also intact in 129/Sv mice, i.e., the genetic background that is usually used for generation of knockout mice. As a consequence of the latter, and the fact that the Mcpt6 and Mcpt7 genes are closely located on chromosome 17, the generation of a Mcpt6 knockout using 129/Sv ES cells, and subsequent backcrossing to C57BL/6 background, resulted in mice in which MCs are Mcpt6−/Mcpt7+ but the WT counterpart mice were Mcpt6+/Mcpt7− (Shin et al., 2008). By employing a slightly different approach, using (129/SvJ X C57BL/6) F1 ES cells, Thakurdas et al. (2007) generated a mouse line of C57BL/6 background that was Mcpt6−/Mcpt7−. Another example of genetic background influencing MC protease expression is the expression of γ-tryptase. When studying the expression of this protease, it was found that MCs from C57BL/6 mice express γ-tryptase, whereas MCs from BALB/c and 129/Sv mice lack γ-tryptase expression (Wong et al., 1999).
In the case of MC-deficient mice, we already have noted above that different conclusions are supported about the importance of MCs in features of allergic airway inflammation based on studies performed with KitW−sh/W−sh mice on the C57BL/6 background (which exhibit substantially weaker responses than do the corresponding WT mice) or the BALB/c background (in which responses are similar to those in the corresponding WT mice). Given the many potential genetic contributions to multiple features of any biological response which might be investigated (including those affecting signaling pathways and other aspects of MC biology), there no doubt will be many more examples of strain background influencing the nature and importance of MC contributions to biological responses in vivo. Moreover, given the fact that strain background differences can influence MC functions and/or other features of biological responses, it becomes critical to ensure that MC- or MC-associated-protease- deficient mice are on the same genetic background as the corresponding WT mice to which they are being compared. In the case of KitW−sh/W−sh mice, we have reviewed elsewhere (Piliponsky et al., 2010) that the extent to which the KitW−sh/HNihrJaeBsmJ mice (JAX stock number 005051) are on the C57BL/6 background was not readily discernable from the literature, so we extensively backcrossed these mice to C57BL/6J mice. These C57BL/6J-KitW−sh/W−sh (B6.Cg-KitW−sh/HNihrJaeBsmGlliJ) mice are available from Jackson Laboratories (stock number 012861).
The major potential effects of the host microbiome on diverse immune responses in mice have been extensively reviewed (Gagliani, Hu, Huber, Elinav, & Flavell, 2014; Hansen, Metzdorff, & Hansen, 2013; Honda & Littman, 2012). While the extent to which host microbiome-related differences might contribute to the divergent results obtained by different groups studying the roles of MCs in individual models of disease or host defense is not clear, this possibility needs to be considered. Similarly, differences in multiple aspects of animal husbandry, as well as differences in the time of day when experiments are conducted, all have the potential to influence experimental outcomes and ideally should be carefully controlled.
5.4. Importance of experimental design in studying the roles of mast cells and mast cell-associated proteases in vivo
As reviewed above, individual mouse models of MC deficiency, or models for altering the expression of MC-associated products, differ in their features and may vary in their advantages and limitations for studies of MC function. The newer models of MC deficiency are particularly attractive because they lack the Kit-related phenotypic alterations associated with c-kit mutant MC-deficient mice. However, because the newer models only recently have been described, it is likely that there is still more to be learned about their phenotype, and especially about the effects of disease models on their phenotype, including features that may influence the interpretation of experiments designed to investigate MC functions. In addition, one often can select from among a wide variety of experimental protocols and conditions to investigate particular hypotheses about MC functions in examples of host defense or disease.
Both factors, i.e., (1) the choice of MC-deficient mouse model(s) and (2) the selection of particular experimental conditions for investigating the roles of MCs in various types of biological responses, can influence the results of such work. Moreover, the choice of experimental protocol may be particularly important in biological responses in which the MC is more likely to have a redundant rather than unique (nonredundant) role. This rather obvious point was evident even before the introduction of the new models of MC deficiency, as is illustrated by the results of efforts to employ c-kit mutant MC-deficient mice to investigate the roles of MCs in CHS responses or in allergic inflammation of the airways.
There are at least two ways to view the fact that work by different groups (or even different experiments performed by the same group; Bryce et al., 2004; Galli & Hammel, 1984) support “different conclusions” about the importance of MCs in various biological responses. The first is that this constitutes a “controversy” regarding the roles of MCs in that type of biological response (Brown & Hatfield, 2012; Rodewald & Feyerabend, 2012). The second (which is not necessarily incompatible with the first) is that such discrepancies identify interesting opportunities for understanding the basis for the differences, thereby to gain additional insights into the regulation of these biological responses. For example, in the case of both CHS and allergic airway inflammation, current findings are compatible with the conclusion that the ability of the MC to enhance particular features of the models is most readily detected when one attempts to elicit “relatively weak” responses, particularly in mice of suitable genetic background. In the case of allergic inflammation of the airways, inducing reactions with relatively low doses of antigen for sensitization and challenge may more closely mimic clinical settings than do protocols which use strong adjuvants for sensitization and large amounts of antigen for airway challenge. However, even when employing a relatively weak model of OVA-induced allergic airway inflammation, the strong effects of MCs on multiple features of the airway pathology that were revealed when the experiments were performed using MC-deficient mice on the C57BL/6 or WBB6F1 backgrounds (that are relatively “Th1 biased”) (Kobayashi et al., 2000; Kung et al., 1995; Nakae et al., 2007; Reuter et al., 2008; Taube et al., 2004; Williams & Galli, 2000; Yu et al., 2006, 2011) were not observed in studies of MC-deficient KitW−sh/W−sh mice on the “TH2-biased” BALB/c background (Becker et al., 2011). With respect to models of antibody-dependent arthritis, it seems plausible, although not yet formally proven, that the relative neutrophil deficiency of WBB6F1-KitW/W−v mice (as well as perhaps other abnormalities affecting hematopoietic cells in addition to MCs and neutrophils) contributes to the inability of this type of MC-deficient mouse fully to develop the features of the pathology.
In more general terms, it seems reasonable both to think that evolution has engineered redundancy into the mechanisms needed to sustain many critical biological processes in order to ensure that they remain robust, as well as to propose that such mechanistic redundancy also applies to many pathological processes. Indeed, we speculate that there may be only a small number of biological responses in which MCs are uniquely critical (e.g., as an important source of proteases that may be highly expressed in MCs), as defined by the finding that little or no response would be detectable in the absence of MCs under any conditions of testing. Accordingly, in many types of complex biological responses in which the roles of MCs may be partially overlapping with those of other effector elements, we expect that the choice of experimental model, including the intensity (i.e., type, amount, and/or frequency of administration) of the stimulus used to elicit the response as well as the selection of which specific features of the response to analyze, may be critical in determining whether the contributions of MCs will be of sufficient importance that their absence will be reflected in a significant impairment of the response. Similarly, it seems reasonable to propose that the more critical the MC’s contributions to a particular biological response, the more likely one will find abnormalities in that response when it is tested in each of the different types of MC-deficient mice.
6. GENERAL RECOMMENDATIONS REGARDING THE USE OF MAST CELL-DEFICIENT OR MAST CELL-ASSOCIATED PROTEASE-DEFICIENT MICE TO ANALYZE BIOLOGICAL RESPONSES IN VIVO
For these reasons, we recommend attempting to test hypotheses about MC function using more than one model of MC deficiency. In our lab, we used to perform pilot experiments for any new project in both WBB6F1-KitW/W−v and WCB6F1-KitlSl/Sl−d mice. We then switched to testing WBB6F1-KitW/W−v and C57BL6-KitW−sh/W−sh mice and we now do pilot experiments in C57BL6-KitW−sh/W−sh and Cpa3-Cre;Mcl-1fl/fl mice. If we obtain concordant results in both types of MC-deficient mice, we then proceed to further studies, which can include using mice genetically deficient in particular MC products of interest. Examples are our recent study of the roles of MCs and the MC-associated chymase, mMCP-4, in enhancing resistance to the venoms of the Gila monster and two scorpions, and to the endogenous peptide, VIP (Akahoshi et al., 2011) and our demonstration that MCs can contribute importantly to the early inflammation elicited in a mouse model of acute gout (Reber, Marichal, et al., 2014).
By contrast, if tests in two different types of MC-deficient mice yield discordant findings, we generally do not continue. While pursuing such a project might reveal interesting information about why a MC role may be “revealed” in one type of MC-deficient mouse and not in the other, there is also the risk that we might not succeed in explaining the discrepancy and thereby consume in an ultimately futile effort resources which could be used for more promising lines of inquiry. While in many settings it may not be practical also to test multiple protocols to elicit the type of host defense or disease of interest, one certainly should keep in mind, as discussed above, that the roles of MCs in influencing various features of particular biological responses clearly can differ based on the selection of experimental conditions to examine.
Although we still are in early days with respect to some of the newer models that can be employed for MC research, there already are examples where work in older and newer models have provided either very similar or rather discordant evidence for particular MC functions. Some of the possible reasons for why discrepant results have been obtained regarding the roles of MCs in particular biological responses are noted above and in Table 3 of our recent review (Reber et al., 2012). We have summarized in Table 3 some conclusions about the nature and importance of various roles of MCs and their proteases in different types of biological responses that we think are compatible with current evidence derived from work in multiple older and newer models of MC-deficient mice and/or in mice deficient in certain MC-associated proteases or other products that can be derived from MCs.
Table 3.
A hierarchy of mast cell and/or mast cell-derived protease role(s) and some examples of concordant and discordant findings
| Rolea,b | Definition | Examples |
|---|---|---|
| Unique or nonredundant | Only MCs can perform that function | Findings in multiple model systems (including kit mutant MC-deficient mice and MC knockin mice, and in the more recent MC-deficient mice with normal Kit) support the conclusion that MCs have important or even in some cases nonredundant/unique roles in many acute, IgE-dependent responses (Heger, Seidler, et al., 2014; Lilla et al., 2011; Miyajima et al., 1997; Sawaguchi et al., 2012; Takeishi et al., 1991; Wershil et al., 1987; Zhou et al., 2007) Studies in both various MC-deficient mice and in MC-associated protease-deficient mice are consistent in supporting an important role for MCs (Akahoshi et al., 2011; Marichal et al., 2013; Metz et al., 2006) and their proteases [namely, CPA3 (Schneider et al., 2007) and mMCP-4 (Akahoshi et al., 2011)] in reducing the pathology and mortality induced by the venoms (Akahoshi et al., 2011; Marichal et al., 2013; Metz et al., 2006) or toxic components of venoms (Metz et al., 2006; Schneider et al., 2007) of certain reptiles (Akahoshi et al., 2011; Metz et al., 2006; Schneider et al., 2007) or arthropods (Akahoshi et al., 2011; Marichal et al., 2013; Metz et al., 2006) |
| Important | That aspect of the response would be substantially different (e.g., by 50% or more) in the absence of MCs | |
| Redundant or overlapping | MCs can contribute to the assessed feature of the response along with other effector or regulatory elements, but their potential contribution may only be revealed if one or more of the other partially redundant or overlapping elements is impaired | Different models of MC deficiency have yielded different results when the possible contributions of MCs have been examined in certain complex biological responses. In each of those responses, it is likely that multiples types of immune cells may have redundant or overlapping roles. Such responses include antibody-dependent arthritis (Elliott et al., 2011; Lee et al., 2002; Mancardi et al., 2011; Nigrovic & Lee, 2007; Zhou et al., 2007), EAE (Bennett et al., 2009; Brown & Hatfield, 2012; Feyerabend et al., 2011; Li et al., 2011; Piconese et al., 2011; Secor et al., 2000), and cutaneous contact hypersensitivity (Askenase et al., 1983; Biedermann et al., 2000; Bryce et al., 2004; Dudeck et al., 2011; Galli & Hammel, 1984; Grimbaldeston et al., 2007; Mekori, Chang, Wershil, & Galli, 1987; Mekori & Galli, 1985; Norman et al., 2008; Otsuka et al., 2011). In models of airway inflammation and airway hypersensitivity, it has been reported that both the details of the model system (Kobayashi et al., 2000; Kung et al., 1995; Nakae et al., 2007; Sawaguchi et al., 2012; Takeda et al., 1997; Williams & Galli, 2000; Yu et al., 2006, 2011), and mouse strain background (Becker et al., 2011), can influence the extent to which MCs are important for the development of various features of the responses; this is also likely to be true regarding the ability to discern the importance of the MC’s contribution to many other biological responses |
| Non-contributory | MCs play no role in that feature of the response | As an example, MCs are not required (or are “non-contributory”) for development of a chronic IgE-dependent cutaneous response that is dependent on basophils (Lilla et al., 2011; Mukai et al., 2005) |
In particular biological responses, such as models of host defense or disease, MCs may influence some features of the response more importantly than others. Indeed, in some biological responses, MCs might have any combination of the four roles listed, depending on which features of the response one measures. The number of biological responses in which essentially all features of the response are fully and uniquely dependent on MCs may be small.
In certain biological responses, the roles of individual MC-associated proteases may be to limit some of the “global” effects of MCs in that setting. For example, evidence from studies in MC knockin mice and other models indicates that MCs can contribute to airway allergic inflammation, airway hyper-reactivity, and tissue remodeling in certain models of allergic inflammation of the airways (Kobayashi et al., 2000; Kung et al., 1995; Nakae et al., 2007; Reuter et al., 2008; Taube et al., 2004; Williams & Galli, 2000; Yu et al., 2006, 2011). However, in a model of ovalbumin-induced allergic airway inflammation (Waern et al., 2009) similar to one that was shown to be enhanced by MCs (Williams & Galli, 2000), and in another model of allergic airway inflammation elicited by HDM (Waern et al., 2013), MC-associated mMCP-4 was reported to have effects that limit airway inflammation (Waern et al., 2009, 2013), airway hyper-reactivity to methacholine (Waern et al., 2009, 2013), and airway smooth muscle thickening (Waern et al., 2009, 2013).
This is a modified and updated version of the information in Boxes 2 and 4 from Reber et al. (2012).
7. PERSPECTIVE
This is an exciting time in MC research. The continued availability of “old” models (including “MC knockin c-kit mutant mice” and various MC protease-deficient mice), combined with the introduction of several promising “new” models of MC deficiency or for MC-targeted systemic or local deletion of MC-associated proteases or other MC-associated products, offers a wealth of opportunities to enhance progress in solving the longstanding “riddle of the mast cell,” at least in mice. Some of this work may even suggest new approaches for the treatment of diseases in which MCs or their individual products have been implicated. However, based on the results obtained so far with both the older and newer models for MC research, we think that the most robust conclusions about what MCs and their proteases can do (or do not do) in various biological responses in vivo, and regarding the importance of such MC contributions, are likely to be derived from investigations that employ multiple informative model systems. This approach increases the cost of such work, but permits one to exploit the attractive features of the various models while keeping in mind the known and potential limitations in each of them.
Acknowledgments
We thank the members of the Galli lab and our collaborators and colleagues for their contributions to some of the work reviewed herein, and we apologize to the many contributors to this field whose work was not cited because of space limitations. G. P. is supported by grants from The Swedish Research Council, The Swedish Cancer Foundation, Formas and the Swedish Heart and Lung Foundation. L. L. R. acknowledges support from the Arthritis National Research Foundation (ANRF) and National Institutes of Health grant K99AI110645; T. M. is supported by a Marie Curie International outgoing Fellowship for Career Development: FP7-PEOPLE-2011-IOF, grant 299954; S. J. G. acknowledges support from National Institutes of Health grants U19 AI104209, NS 080062 and from Tobacco-Related Disease Research Program at University of California.
REFERENCES
- Abe T, Nawa Y. Localization of mucosal mast cells in W/Wv mice after reconstitution with bone marrow cells or cultured mast cells, and its relation to the protective capacity to Strongyloides ratti infection. Parasite Immunology. 1987;9:477. doi: 10.1111/j.1365-3024.1987.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Abonia JP, Friend DS, Austen WG, Jr, Moore FD, Jr, Carroll MC, Chan R, et al. Mast cell protease 5 mediates ischemia-reperfusion injury of mouse skeletal muscle. Journal of Immunology. 2005;174:7285. doi: 10.4049/jimmunol.174.11.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nature Reviews Immunology. 2010;10:440. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åbrink M, Grujic M, Pejler G. Serglycin is essential for maturation of mast cell secretory granule. The Journal of Biological Chemistry. 2004;279:40897. doi: 10.1074/jbc.M405856200. [DOI] [PubMed] [Google Scholar]
- Akahoshi M, Song CH, Piliponsky AM, Metz M, Guzzetta A, Abrink M, et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. The Journal of Clinical Investigation. 2011;121:4180. doi: 10.1172/JCI46139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson MK, Pemberton AD, Miller HR, Hellman L. Extended cleavage specificity of mMCP-1, the major mucosal mast cell protease in mouse-high specificity indicates high substrate selectivity. Molecular Immunology. 2008;45:2548. doi: 10.1016/j.molimm.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Ando A, Martin TR, Galli SJ. Effects of chronic treatment with the c-kit ligand, stem cell factor, on immunoglobulin E-dependent anaphylaxis in mice. Genetically mast cell-deficient Sl/Sld mice acquire anaphylactic responsiveness, but the congenic normal mice do not exhibit augmented responses. The Journal of Clinical Investigation. 1993;92:1639. doi: 10.1172/JCI116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T, Xiao W, Gao P, Namiranian S, Matsumoto K, Tomimori Y, et al. Critical role for mast cell Stat5 activity in skin inflammation. Cell Reports. 2014;6:366. doi: 10.1016/j.celrep.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antsiferova M, Martin C, Huber M, Feyerabend TB, Forster A, Hartmann K, et al. Mast cells are dispensable for normal and activin-promoted wound healing and skin carcinogenesis. Journal of Immunology. 2013;191:6147. doi: 10.4049/jimmunol.1301350. [DOI] [PubMed] [Google Scholar]
- Arac A, Grimbaldeston M, Nepomuceno ARB, Olayiwola O, Pereira MP, Nishiyama Y, et al. Evidence that meningeal mast cells can worsen stroke pathology in mice. The American Journal of Pathology. 2014;184:2493–2504. doi: 10.1016/j.ajpath.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias K, Chu DK, Flader K, Botelho F, Walker T, Arias N, et al. Distinct immune effector pathways contribute to the full expression of peanut-induced anaphylactic reactions in mice. The Journal of Allergy and Clinical Immunology. 2011;127:1552. doi: 10.1016/j.jaci.2011.03.044. [DOI] [PubMed] [Google Scholar]
- Aridor M, Rajmilevich G, Beaven MA, Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric G protein Gi3 . Science. 1993;262:1569. doi: 10.1126/science.7504324. [DOI] [PubMed] [Google Scholar]
- Arizono N, Kasugai T, Yamada M, Okada M, Morimoto M, Tei H, et al. Infection of Nippostrongylus brasiliensis induces development of mucosal-type but not connective tissue-type mast cells in genetically mast cell-deficient Ws/Ws rats. Blood. 1993;81:2572. [PubMed] [Google Scholar]
- Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. Journal of the National Cancer Institute. 2006;98:1806. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askenase PW, Van Loveren H, Kraeuter-Kops S, Ron Y, Meade R, Theoharides TC, et al. Defective elicitation of delayed-type hypersensitivity in W/Wv and SI/SId mast cell-deficient mice. Journal of Immunology. 1983;131:2687. [PubMed] [Google Scholar]
- Bankova LG, Lezcano C, Pejler G, Stevens RL, Murphy GF, Austen KF, et al. Mouse mast cell proteases 4 and 5 mediate epidermal injury through disruption of tight junctions. Journal of Immunology. 2014;192:2812. doi: 10.4049/jimmunol.1301794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M, Reuter S, Friedrich P, Doener F, Michel A, Bopp T, et al. Genetic variation determines mast cell functions in experimental asthma. Journal of Immunology. 2011;186:7225. doi: 10.4049/jimmunol.1100676. [DOI] [PubMed] [Google Scholar]
- Beghdadi W, Madjene LC, Claver J, Pejler G, Beaudoin L, Lehuen A, et al. Mast cell chymase protects against renal fibrosis in murine unilateral ureteral obstruction. Kidney International. 2013;84:317. doi: 10.1038/ki.2013.98. [DOI] [PubMed] [Google Scholar]
- Bennett JL, Blanchet MR, Zhao L, Zbytnuik L, Antignano F, Gold M, et al. Bone marrow-derived mast cells accumulate in the central nervous system during inflammation but are dispensable for experimental autoimmune encephalomyelitis pathogenesis. Journal of Immunology. 2009;182:5507. doi: 10.4049/jimmunol.0801485. [DOI] [PubMed] [Google Scholar]
- Berlin AA, Hogaboam CM, Lukacs NW. Inhibition of SCF attenuates peribronchial remodeling in chronic cockroach allergen-induced asthma. Laboratory Investigation. 2006;86:557. doi: 10.1038/labinvest.3700419. [DOI] [PubMed] [Google Scholar]
- Berlin AA, Lincoln P, Tomkinson A, Lukacs NW. Inhibition of stem cell factor reduces pulmonary cytokine levels during allergic airway responses. Clinical and Experimental Immunology. 2004;136:15. doi: 10.1111/j.1365-2249.2004.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann T, Kneilling M, Mailhammer R, Maier K, Sander CA, Kollias G, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. The Journal of Experimental Medicine. 2000;192:1441. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends in Immunology. 2004;25:266. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Blankenhaus B, Reitz M, Brenz Y, Eschbach ML, Hartmann W, Haben I, et al. Foxp3+ regulatory T cells delay expulsion of intestinal nematodes by suppression of IL-9-driven mast cell activation in BALB/c but not in C57BL/6 mice. PLoS Pathogens. 2014;10:e1003913. doi: 10.1371/journal.ppat.1003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot I, Bot M, van Heiningen SH, van Santbrink PJ, Lankhuizen IM, Hartman P, et al. Mast cell chymase inhibition reduces atherosclerotic plaque progression and improves plaque stability in ApoE−/− mice. Cardiovascular Research. 2011;89:244. doi: 10.1093/cvr/cvq260. [DOI] [PubMed] [Google Scholar]
- Bowie MB, Kent DG, Copley MR, Eaves CJ. Steel factor responsiveness regulates the high self-renewal phenotype of fetal hematopoietic stem cells. Blood. 2007;109:5043. doi: 10.1182/blood-2006-08-037770. [DOI] [PubMed] [Google Scholar]
- Boyce JA. Mast cells and eicosanoid mediators: A system of reciprocal paracrine and autocrine regulation. Immunological Reviews. 2007;217:168. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- Braga T, Grujic M, Lukinius A, Hellman L, Abrink M, Pejler G. Serglycin proteoglycan is required for secretory granule integrity in mucosal mast cells. The Biochemical Journal. 2007;403:49. doi: 10.1042/BJ20061257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. The Journal of Clinical Investigation. 2003;112:1666. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA, Hatfield JK. Mast cells are important modifiers of autoimmune disease: With somuch evidence, why is there still controversy? Frontiers in Immunology. 2012;3:147. doi: 10.3389/fimmu.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, et al. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clinical and Experimental Allergy. 1994;24:73. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nature Methods. 2005;2:419. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- Caughey GH. Tryptase genetics and anaphylaxis. The Journal of Allergy and Clinical Immunology. 2006;117:1411. doi: 10.1016/j.jaci.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey GH. Mast cell proteases as protective and inflammatory mediators. Advances in Experimental Medicine and Biology. 2011;716:212. doi: 10.1007/978-1-4419-9533-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CY, St John AL, Abraham SN. Mast cell IL-10 drives localized tolerance in chronic bladder infection. Immunity. 2013;38:349. doi: 10.1016/j.immuni.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervenick PA, Boggs DR. Decreased neutrophils and megakaryocytes in anemic mice of genotype W/Wv. Journal of Cellular Physiology. 1969;73:25. doi: 10.1002/jcp.1040730104. [DOI] [PubMed] [Google Scholar]
- Christy AL, Walker ME, Hessner MJ, Brown MA. Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. Journal of Autoimmunity. 2013;42:50. doi: 10.1016/j.jaut.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Clark JM, Abraham WM, Fishman CE, Forteza R, Ahmed A, Cortes A, et al. Tryptase inhibitors block allergen-induced airway and inflammatory responses in allergic sheep. American Journal of Respiratory and Critical Care Medicine. 1995;152:2076. doi: 10.1164/ajrccm.152.6.8520778. [DOI] [PubMed] [Google Scholar]
- Costanzo MJ, Yabut SC, Zhang HC, White KB, de Garavilla L, Wang Y, et al. Potent, nonpeptide inhibitors of human mast cell tryptase. Synthesis and biological evaluation of novel spirocyclic piperidine amide derivatives. Bioorganic & Medicinal Chemistry Letters. 2008;18:2114. doi: 10.1016/j.bmcl.2008.01.093. [DOI] [PubMed] [Google Scholar]
- Cozzi E, Ackerman KG, Lundequist A, Drazen JM, Boyce JA, Beier DR. The naïve airway hyperresponsiveness of the A/J mouse is Kit-mediated. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12787. doi: 10.1073/pnas.1106582108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig SS, Schwartz LB. Tryptase and chymase, markers of distinct types of human mast cells. Immunologic Research. 1989;8:130. doi: 10.1007/BF02919075. [DOI] [PubMed] [Google Scholar]
- Crowle PK. Mucosal mast cell reconstitution and Nippostrongylus brasiliensis rejection by W/Wv mice. The Journal of Parasitology. 1983;69:66. [PubMed] [Google Scholar]
- Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Current Opinion in Immunology. 2007;19:31. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- Dombrowicz D, Flamand V, Miyajima I, Ravetch JV, Galli SJ, Kinet JP. Absence of Fc€RI α chain results in upregulation of FcγRIII-dependent mast cell degranulation and anaphylaxis. Evidence of competition between Fc€RI and FcγRIII for limiting amounts of FcR α and γ chains. The Journal of Clinical Investigation. 1997;99:915. doi: 10.1172/JCI119256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson LE, Schmitt E, Huntley JF, Newlands GF, Grencis RK. A critical role for stem cell factor and c-kit in host protective immunity to an intestinal helminth. International Immunology. 1996;8:559. doi: 10.1093/intimm/8.4.559. [DOI] [PubMed] [Google Scholar]
- Douaiher J, Succar J, Lancerotto L, Gurish MF, Orgill DP, Hamilton MJ, et al. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Advances in Immunology. 2014;122:211. doi: 10.1016/B978-0-12-800267-4.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Casteran N, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4:e7258. doi: 10.1371/journal.pone.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- Egozi EI, Ferreira AM, Burns AL, Gamelli RL, Dipietro LA. Mast cells modulate the inflammatory but not the proliferative response in healing wounds. Wound Repair and Regeneration. 2003;11:46. doi: 10.1046/j.1524-475x.2003.11108.x. [DOI] [PubMed] [Google Scholar]
- Elliott ER, Van Ziffle JA, Scapini P, Sullivan BM, Locksley RM, Lowell CA. Deletion of Syk in neutrophils prevents immune complex arthritis. Journal of Immunology. 2011;187:4319. doi: 10.4049/jimmunol.1100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoksson M, Lyberg K, Moller-Westerberg C, Fallon PG, Nilsson G, Lunderius-Andersson C. Mast cells as sensors of cell injury through IL-33 recognition. Journal of Immunology. 2011;186:2523. doi: 10.4049/jimmunol.1003383. [DOI] [PubMed] [Google Scholar]
- Erin EM, Leaker BR, Zacharasiewicz A, Higgins LA, Nicholson GC, Boyce MJ, et al. Effects of a reversible β-tryptase and trypsin inhibitor (RWJ-58643) on nasal allergic responses. Clinical and Experimental Allergy. 2006;36:458. doi: 10.1111/j.1365-2222.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- Fantozzi R, Brunelleschi S, Giuliattini L, Blandina P, Masini E, Cavallo G, et al. Mast cell and neutrophil interactions: A role for superoxide anion and histamine. Agents and Actions. 1985;16:260. doi: 10.1007/BF01983155. [DOI] [PubMed] [Google Scholar]
- Fawcett DW. Cytological and pharmacological observations on the release of histamine by mast cells. The Journal of Experimental Medicine. 1954;100:217. doi: 10.1084/jem.100.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry X, Brehin S, Kamel R, Landry Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides. 2002;23:1507. doi: 10.1016/s0196-9781(02)00090-6. [DOI] [PubMed] [Google Scholar]
- Feyerabend TB, Hausser H, Tietz A, Blum C, Hellman L, Straus AH, et al. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Molecular and Cellular Biology. 2005;25:6199. doi: 10.1128/MCB.25.14.6199-6210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyerabend TB, Terszowski G, Tietz A, Blum C, Luche H, Gossler A, et al. Deletion of Notch1 converts pro-T cells to dendritic cells and promotes thymic B cells by cell-extrinsic and cell-intrinsic mechanisms. Immunity. 2009;30:67. doi: 10.1016/j.immuni.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Feyerabend TB, Weiser A, Tietz A, Stassen M, Harris N, Kopf M, et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Finkelman FD. Anaphylaxis: Lessons from mouse models. The Journal of Allergy and Clinical Immunology. 2007;120:506. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, et al. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400:773. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- Franco CB, Chen CC, Drukker M, Weissman IL, Galli SJ. Distinguishing mast cell and granulocyte differentiation at the single-cell level. Cell Stem Cell. 2010;6:361. doi: 10.1016/j.stem.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. The Journal of Cell Biology. 1996;135:279. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumoto Y, Charles N, Olivera A, Leung WH, Dillahunt S, Sargent JL, et al. PTEN deficiency in mast cells causes a mastocytosis-like proliferative disease that heightens allergic responses and vascular permeability. Blood. 2011;118:5466. doi: 10.1182/blood-2010-09-309955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Hu B, Huber S, Elinav E, Flavell RA. The fire within: Microbes inflame tumors. Cell. 2014;157:776. doi: 10.1016/j.cell.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nature Immunology. 2011;12:1035. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: Negative, as well as positive, regulators of immunity. Nature Reviews Immunology. 2008;8:478. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Hammel I. Unequivocal delayed hypersensitivity in mast cell-deficient and beige mice. Science. 1984;226:710. doi: 10.1126/science.6494907. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annual Review of Immunology. 2005;23:749. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nature Immunology. 2005;6:135. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nature Medicine. 2012;18:693. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Wershil BK, Tam SY, Costa JJ. Regulation of mouse and human mast cell development, survival and function by stem cell factor, the ligand for the c-kit receptor. International Archives of Allergy and Immunology. 1995;107:51. doi: 10.1159/000236928. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Zsebo KM, Geissler EN. The kit ligand, stem cell factor. Advances in Immunology. 1994;55:1. doi: 10.1016/s0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- Gaudenzio N, Espagnolle N, Mars LT, Liblau R, Valitutti S, Espinosa E. Cell-cell cooperation at the T helper cell/mast cell immunological synapse. Blood. 2009;114:4979. doi: 10.1182/blood-2009-02-202648. [DOI] [PubMed] [Google Scholar]
- Gekara NO, Weiss S. Mast cells initiate early anti-Listeria host defences. Cellular Microbiology. 2008;10:225. doi: 10.1111/j.1462-5822.2007.01033.x. [DOI] [PubMed] [Google Scholar]
- Gerbaulet A, Wickenhauser C, Scholten J, Peschke K, Drube S, Horny HP, et al. Mast cell hyperplasia, B-cell malignancy, and intestinal inflammation in mice with conditional expression of a constitutively active kit. Blood. 2011;117:2012. doi: 10.1182/blood-2008-11-189605. [DOI] [PubMed] [Google Scholar]
- Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Critical Reviews in Immunology. 2011;31:475. doi: 10.1615/critrevimmunol.v31.i6.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind C, Louahed J, Faulkner H, Vink A, Warnier G, Grencis R, et al. Intraepithelial infiltration by mast cells with both connective tissue-type and mucosal-type characteristics in gut, trachea, and kidneys of IL-9 transgenic mice. Journal of Immunology. 1998;160:3989. [PubMed] [Google Scholar]
- Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature. 1990a;346:274. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Gordon JR, Galli SJ. Phorbol 12-myristate 13-acetate-induced development of functionally active mast cells in W/Wv but not Sl/Sld genetically mast cell-deficient mice. Blood. 1990b;75:1637. [PubMed] [Google Scholar]
- Grayson MH, Cheung D, Rohlfing MM, Kitchens R, Spiegel DE, Tucker J, et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. The Journal of Experimental Medicine. 2007;204:2759. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant KitW−sh/W−sh mice as a model for investigating mast cell biology in vivo. The American Journal of Pathology. 2005;167:835. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ. Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses. Current Opinion in Immunology. 2006;18:751. doi: 10.1016/j.coi.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nature Immunology. 2007;8:1095. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- Grujic M, Calounova G, Eriksson I, Feyerabend T, Rodewald HR, Tchougounova E, et al. Distorted secretory granule composition in mast cells with multiple protease deficiency. Journal of Immunology. 2013;191:3931. doi: 10.4049/jimmunol.1301441. [DOI] [PubMed] [Google Scholar]
- Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37:25. doi: 10.1016/j.immuni.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Gushchin IS, Miroshnikov AI, Martynov VI, Sviridov VV. Histamine releasing and anti-inflammatory activities of MCD-peptide and its modified forms. Agents and Actions. 1981;11:69. doi: 10.1007/BF01991459. [DOI] [PubMed] [Google Scholar]
- Ha TY, Reed ND, Crowle PK. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infection and Immunity. 1983;41:445. doi: 10.1128/iai.41.1.445-447.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haff LA. Improved quantitative PCR using nested primers. PCR Methods and Applications. 1994;3:332. doi: 10.1101/gr.3.6.332. [DOI] [PubMed] [Google Scholar]
- Hallgren J, Karlson U, Poorafshar M, Hellman L, Pejler G. Mechanism for activation of mouse mast cell tryptase: Dependence on heparin and acidic pH for formation of active tetramers of mouse mast cell protease 6. Biochemistry. 2000;39:13068. doi: 10.1021/bi000973b. [DOI] [PubMed] [Google Scholar]
- Hansbro PM, Hamilton MJ, Fricker M, Gellatly SL, Jarnicki AG, Zheng D, et al. Importance of mast cell Prss31/transmembrane tryptase/tryptase-γ in lung function and experimental chronic obstructive pulmonary disease and colitis. The Journal of Biological Chemistry. 2014;289:18214–18227. doi: 10.1074/jbc.M114.548594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CH, Metzdorff SB, Hansen AK. Customizing laboratory mice by modifying gut microbiota and host immunity in an early “window of opportunity”. Gut Microbes. 2013;4:241. doi: 10.4161/gmic.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Kunisada T, Ogawa M, Yamaguchi K, Nishikawa S. Exon skipping by mutation of an authentic splice site of c-kit gene in W/W mouse. Nucleic Acids Research. 1991;19:1267. doi: 10.1093/nar/19.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Peng Q, Walls AF. Potent induction of a neutrophil and eosinophil-rich infiltrate in vivo by human mast cell tryptase: Selective enhancement of eosinophil recruitment by histamine. Journal of Immunology. 1997;159:6216. [PubMed] [Google Scholar]
- He S, Walls AF. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. British Journal of Pharmacology. 1998a;125:1491. doi: 10.1038/sj.bjp.0702223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Walls AF. The induction of a prolonged increase in microvascular permeability by human mast cell chymase. European Journal of Pharmacology. 1998b;352:91. doi: 10.1016/s0014-2999(98)00343-4. [DOI] [PubMed] [Google Scholar]
- Heger K, Fierens K, Vahl JC, Aszodi A, Peschke K, Schenten D, et al. A20-deficient mast cells exacerbate inflammatory responses in vivo. PLoS Biology. 2014;12:e1001762. doi: 10.1371/journal.pbio.1001762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heger K, Seidler B, Vahl JC, Schwartz C, Kober M, Klein S, et al. CreERT2 expression from within the c-Kit gene locus allows efficient inducible gene targeting in and ablation of mast cells. European Journal of Immunology. 2014;44:296. doi: 10.1002/eji.201343731. [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925. [PubMed] [Google Scholar]
- Hellman L, Thorpe M. Granule proteases of hematopoietic cells, a family of versatile inflammatory mediators—An update on their cleavage specificity, in vivo substrates, and evolution. Biological Chemistry. 2014;395:15. doi: 10.1515/hsz-2013-0211. [DOI] [PubMed] [Google Scholar]
- Hendrix S, Kramer P, Pehl D, Warnke K, Boato F, Nelissen S, et al. Mast cells protect from post-traumatic brain inflammation by the mast cell-specific chymase mouse mast cell protease-4. The FASEB Journal. 2013;27:920. doi: 10.1096/fj.12-204800. [DOI] [PubMed] [Google Scholar]
- Hepworth MR, Danilowicz-Luebert E, Rausch S, Metz M, Klotz C, Maurer M, et al. Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6644. doi: 10.1073/pnas.1112268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko AY, Suzuki R, Charles N, Alvarez-Errico D, Sargent JL, Laurence A, et al. Mast cell interleukin-2 production contributes to suppression of chronic allergic dermatitis. Immunity. 2011;35:562. doi: 10.1016/j.immuni.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill PB, MacDonald AJ, Thornton EM, Newlands GF, Galli SJ, Miller HR. Stem cell factor enhances immunoglobulin E-dependent mediator release from cultured rat bone marrow-derived mast cells: Activation of previously unresponsive cells demonstrated by a novel ELISPOT assay. Immunology. 1996;87:326. doi: 10.1046/j.1365-2567.1996.455545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y, Yasuhara T, Yoshida H, Nakajima T, Fujino M, Kitada C. A new mast cell degranulating peptide “mastoparan” in the venom of Vespula lewisii. Chemical & Pharmaceutical Bulletin (Tokyo) 1979;27:1942. doi: 10.1248/cpb.27.1942. [DOI] [PubMed] [Google Scholar]
- Hochegger K, Siebenhaar F, Vielhauer V, Heininger D, Mayadas TN, Mayer G, et al. Role of mast cells in experimental anti-glomerular basement membrane glomerulonephritis. European Journal of Immunology. 2005;35:3074. doi: 10.1002/eji.200526250. [DOI] [PubMed] [Google Scholar]
- Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annual Review of Immunology. 2012;30:759. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell JB, Altounyan RE. A double-blind trial of disodium cromoglycate in the treatment of allergic bronchial asthma. Lancet. 1967;2:539. doi: 10.1016/s0140-6736(67)90499-0. [DOI] [PubMed] [Google Scholar]
- Huang R, Abrink M, Gobl AE, Nilsson G, Aveskogh M, Larsson LG, et al. Expression of a mast cell tryptase in the human monocytic cell lines U-937 and Mono Mac 6. Scandinavian Journal of Immunology. 1993;38:359. doi: 10.1111/j.1365-3083.1993.tb01738.x. [DOI] [PubMed] [Google Scholar]
- Huang C, De Sanctis GT, O’Brien PJ, Mizgerd JP, Friend DS, Drazen JM, et al. Evaluation of the substrate specificity of human mast cell tryptase βI and demonstration of its importance in bacterial infections of the lung. The Journal of Biological Chemistry. 2001;276:26276. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- Huang C, Friend DS, Qiu WT, Wong GW, Morales G, Hunt J, et al. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. Journal of Immunology. 1998;160:1910. [PubMed] [Google Scholar]
- Hubner G, Hu Q, Smola H, Werner S. Strong induction of activin expression after injury suggests an important role of activin in wound repair. Developmental Biology. 1996;173:490. doi: 10.1006/dbio.1996.0042. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Humphries DE, Wong GW, Friend DS, Gurish MF, Qiu WT, Huang C, et al. Heparin is essential for the storage of specific granule proteases in mast cells [see comments] Nature. 1999;400:769. doi: 10.1038/23481. [DOI] [PubMed] [Google Scholar]
- Hunt JE, Stevens RL, Austen KF, Zhang J, Xia Z, Ghildyal N. Natural disruption of the mouse mast cell protease 7 gene in the C57BL/6 mouse. The Journal of Biological Chemistry. 1996;271:2851. doi: 10.1074/jbc.271.5.2851. [DOI] [PubMed] [Google Scholar]
- Iba Y, Shibata A, Kato M, Masukawa T. Possible involvement of mast cells in collagen remodeling in the late phase of cutaneous wound healing in mice. International Immunopharmacology. 2004;4:1873. doi: 10.1016/j.intimp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:1502. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N, Muramatsu M, Jin D, Takai S, Hayashi T, Katayama H, et al. Effects of chymase inhibitor on angiotensin II-induced abdominal aortic aneurysm development in apolipoprotein E-deficient mice. Atherosclerosis. 2009;204:359. doi: 10.1016/j.atherosclerosis.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Ito T, Smrz D, Jung MY, Bandara G, Desai A, Smrzova S, et al. Stem cell factor programs the mast cell activation phenotype. Journal of Immunology. 2012;188:5428. doi: 10.4049/jimmunol.1103366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawdat DM, Rowden G, Marshall JS. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. Journal of Immunology. 2006;177:1755. doi: 10.4049/jimmunol.177.3.1755. [DOI] [PubMed] [Google Scholar]
- Jippo T, Lee YM, Ge Y, Kim DK, Okabe M, Kitamura Y. Tissue-dependent alteration of protease expression phenotype in murine peritoneal mast cells that were genetically labeled with green fluorescent protein. The American Journal of Pathology. 2001;158:1695. doi: 10.1016/S0002-9440(10)64125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jippo T, Tsujino K, Kim HM, Kim DK, Lee YM, Nawa Y, et al. Expression of mast-cell-specific proteases in tissues of mice studied by in situ hybridization. The American Journal of Pathology. 1997;150:1373. [PMC free article] [PubMed] [Google Scholar]
- Jogie-Brahim S, Min HK, Fukuoka Y, Xia HZ, Schwartz LB. Expression of α-tryptase and β-tryptase by human basophils. The Journal of Allergy and Clinical Immunology. 2004;113:1086. doi: 10.1016/j.jaci.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Juurikivi A, Sandler C, Lindstedt KA, Kovanen PT, Juutilainen T, Leskinen MJ, et al. Inhibition of c-kit tyrosine kinase by imatinib mesylate induces apoptosis in mast cells in rheumatoid synovia: A potential approach to the treatment of arthritis. Annals of the Rheumatic Diseases. 2005;64:1126. doi: 10.1136/ard.2004.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakura Y, Thompson H, Nakano T, Yamamura T, Asai H, Kitamura Y, et al. Multiple bidirectional alterations of phenotype and changes in proliferative potential during the in vitro and in vivo passage of clonal mast cell populations derived from mouse peritoneal mast cells. Blood. 1988;72:877. [PubMed] [Google Scholar]
- Kanamaru Y, Scandiuzzi L, Essig M, Brochetta C, Guerin-Marchand C, Tomino Y, et al. Mast cell-mediated remodeling and fibrinolytic activity protect against fatal glomerulonephritis. Journal of Immunology. 2006;176:5607. doi: 10.4049/jimmunol.176.9.5607. [DOI] [PubMed] [Google Scholar]
- Kanamaru Y, Sumiyoshi K, Ushio H, Ogawa H, Okumura K, Nakao A. Smad3 deficiency in mast cells provides efficient host protection against acute septic peritonitis. Journal of Immunology. 2005;174:4193. doi: 10.4049/jimmunol.174.7.4193. [DOI] [PubMed] [Google Scholar]
- Karlson U, Pejler G, Tomasini-Johansson B, Hellman L. Extended substrate specificity of rat mast cell protease 5, a rodent α-chymase with elastase-like primary specificity. The Journal of Biological Chemistry. 2003;278:39625. doi: 10.1074/jbc.M301512200. [DOI] [PubMed] [Google Scholar]
- Kennelly R, Conneely JB, Bouchier-Hayes D, Winter DC. Mast cells in tissue healing: From skin to the gastrointestinal tract. Current Pharmaceutical Design. 2011;17:3772. doi: 10.2174/138161211798357854. [DOI] [PubMed] [Google Scholar]
- Khan AI, Horii Y, Tiuria R, Sato Y, Nawa Y. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. International Journal for Parasitology. 1993;23:551. doi: 10.1016/0020-7519(93)90159-v. [DOI] [PubMed] [Google Scholar]
- Kim YS, Ko HM, Kang NI, Song CH, Zhang X, Chung WC, et al. Mast cells play a key role in the development of late airway hyperresponsiveness through TNF-α in a murine model of asthma. European Journal of Immunology. 2007;37:1107. doi: 10.1002/eji.200636612. [DOI] [PubMed] [Google Scholar]
- Kim CE, Lim SK, Kim JS. In vivo antitumor effect of cromolyn in PEGylated liposomes for pancreatic cancer. Journal of Controlled Release. 2012;157:190. doi: 10.1016/j.jconrel.2011.09.066. [DOI] [PubMed] [Google Scholar]
- Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annual Review of Immunology. 1989;7:59. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- Klein S, Seidler B, Kettenberger A, Sibaev A, Rohn M, Feil R, et al. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nature Communications. 2013;4:1630. doi: 10.1038/ncomms2626. [DOI] [PubMed] [Google Scholar]
- Kneilling M, Hultner L, Pichler BJ, Mailhammer R, Morawietz L, Solomon S, et al. Targeted mast cell silencing protects against joint destruction and angiogenesis in experimental arthritis in mice. Arthritis and Rheumatism. 2007;56:1806. doi: 10.1002/art.22602. [DOI] [PubMed] [Google Scholar]
- Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. The Journal of Experimental Medicine. 2000;192:1849. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Miura T, Haba T, Sato M, Serizawa I, Nagai H, et al. An essential role of mast cells in the development of airway hyperresponsiveness in a murine asthma model. Journal of Immunology. 2000;164:3855. doi: 10.4049/jimmunol.164.7.3855. [DOI] [PubMed] [Google Scholar]
- Komai-Koma M, Brombacher F, Pushparaj PN, Arendse B, McSharry C, Alexander J, et al. Interleukin-33 amplifies IgE synthesis and triggers mast cell degranulation via interleukin-4 in naïve mice. Allergy. 2012;67:1118–1126. doi: 10.1111/j.1398-9995.2012.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama K, Ito Y. Mucosal mast cell responses are not required for protection against infection with the murine nematode parasite Trichuris muris. Parasite Immunology. 2000;22:13. doi: 10.1046/j.1365-3024.2000.00270.x. [DOI] [PubMed] [Google Scholar]
- Krishna MT, Chauhan A, Little L, Sampson K, Hawksworth R, Mant T, et al. Inhibition of mast cell tryptase by inhaled APC 366 attenuates allergen-induced late-phase airway obstruction in asthma. The Journal of Allergy and Clinical Immunology. 2001;107:1039. doi: 10.1067/mai.2001.115631. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy N, Oriss TB, Paglia M, Fei M, Yarlagadda M, Vanhaesebroeck B, et al. Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nature Medicine. 2008;14:565. doi: 10.1038/nm1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung TT, Stelts D, Zurcher JA, Jones H, Umland SP, Kreutner W, et al. Mast cells modulate allergic pulmonary eosinophilia in mice. American Journal of Respiratory Cell and Molecular Biology. 1995;12:404. doi: 10.1165/ajrcmb.12.4.7695919. [DOI] [PubMed] [Google Scholar]
- Lal S, Malhotra S, Gribben D, Hodder D. Nedocromil sodium: A new drug for the management of bronchial asthma. Thorax. 1984;39:809. doi: 10.1136/thx.39.11.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- Latour S, Bonnerot C, Fridman WH, Daeron M. Induction of tumor necrosis factor-α production by mast cells via FcγR. Role of the FcγRIII γ subunit. The Journal of Immunology. 1992;149:2155. [PubMed] [Google Scholar]
- Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: A cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- Lee YM, Jippo T, Kim DK, Katsu Y, Tsujino K, Morii E, et al. Alteration of protease expression phenotype of mouse peritoneal mast cells by changing the microenvironment as demonstrated by in situ hybridization histochemistry. The American Journal of Pathology. 1998;153:931. doi: 10.1016/S0002-9440(10)65634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li Y, Reddel SW, Cherrian M, Friend DS, Stevens RL, et al. Identification of basophilic cells that express mast cell granule proteases in the peripheral blood of asthma, allergy, and drug-reactive patients. Journal of Immunology. 1998;161:5079. [PubMed] [Google Scholar]
- Li H, Nourbakhsh B, Safavi F, Li K, Xu H, Cullimore M, et al. Kit (W−sh) mice develop earlier and more severe experimental autoimmune encephalomyelitis due to absence of immune suppression. Journal of Immunology. 2011;187:274. doi: 10.4049/jimmunol.1003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilla JN, Chen CC, Mukai K, BenBarak MJ, Franco CB, Kalesnikoff J, et al. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Bankaitis E, Heimbach L, Li N, Abrink M, Pejler G, et al. Dual targets for mouse mast cell protease-4 in mediating tissue damage in experimental bullous pemphigoid. The Journal of Biological Chemistry. 2011;286:37358. doi: 10.1074/jbc.M111.272401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nature Medicine. 2009;15:940. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Lincoln PM, Brownell E, Pullen DM, Schock HJ, et al. Stem cell factor (c-kit ligand) influences eosinophil recruitment and histamine levels in allergic airway inflammation. Journal of Immunology. 1996;156:3945. [PubMed] [Google Scholar]
- Lunderius-Andersson C, Enoksson M, Nilsson G. Mast cells respond to cell injury through the recognition of IL-33. Frontiers in Immunology. 2012;3:82. doi: 10.3389/fimmu.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SE, Pejler G, Kleinau S, Abrink M. Mast cell chymase contributes to the antibody response and the severity of autoimmune arthritis. The FASEB Journal. 2009;23:875. doi: 10.1096/fj.08-120394. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Abraham SN. Role of mast cell leukotrienes in neutrophil recruitment and bacterial clearance in infectious peritonitis. Journal of Leukocyte Biology. 2000;67:841. doi: 10.1002/jlb.67.6.841. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Navara C, Uckun FM. Role of Janus kinase 3 in mast cell-mediated innate immunity against gram-negative bacteria. Immunity. 2001;15:313. doi: 10.1016/s1074-7613(01)00184-4. [DOI] [PubMed] [Google Scholar]
- Mallen-St Clair J, Pham CT, Villalta SA, Caughey GH, Wolters PJ. Mast cell dipeptidyl peptidase I mediates survival from sepsis. The Journal of Clinical Investigation. 2004;113:628. doi: 10.1172/JCI19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancardi DA, Jonsson F, Iannascoli B, Khun H, Van Rooijen N, Huerre M, et al. Cutting edge: The murine high-affinity IgG receptor FcγRIV is sufficient for autoantibody-induced arthritis. Journal of Immunology. 2011;186:1899. doi: 10.4049/jimmunol.1003642. [DOI] [PubMed] [Google Scholar]
- Manova K, Bachvarova RF. Expression of c-kit encoded at the Wlocus of mice in developing embryonic germ cells and presumptive melanoblasts. Developmental Biology. 1991;146:312. doi: 10.1016/0012-1606(91)90233-s. [DOI] [PubMed] [Google Scholar]
- Manova K, Nocka K, Besmer P, Bachvarova RF. Gonadal expression of c-kit encoded at the Wlocus of the mouse. Development. 1990;110:1057. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- Marichal T, Starkl P, Reber LL, Kalesnikoff J, Oettgen HC, Tsai M, et al. A beneficial role for immunoglobulin E in host defense against honeybee venom. Immunity. 2013;39:963. doi: 10.1016/j.immuni.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TR, Takeishi T, Katz HR, Austen KF, Drazen JM, Galli SJ. Mast cell activation enhances airway responsiveness to methacholine in the mouse. The Journal of Clinical Investigation. 1993;91:1176. doi: 10.1172/JCI116277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, Echtenacher B, Hultner L, Kollias G, Mannel DN, Langley KE, et al. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. The Journal of Experimental Medicine. 1998;188:2343. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nature Immunology. 2003;4:1199. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, et al. Mast cell activators: A new class of highly effective vaccine adjuvants. Nature Medicine. 2008;14:536. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- Mekori YA, Chang JC, Wershil BK, Galli SJ. Studies of the role of mast cells in contact sensitivity responses. Passive transfer of the reaction into mast cell-deficient mice locally reconstituted with cultured mast cells: Effect of reserpine on transfer of the reaction with DNP-specific cloned T cells. Cellular Immunology. 1987;109:39. doi: 10.1016/0008-8749(87)90290-5. [DOI] [PubMed] [Google Scholar]
- Mekori YA, Galli SJ. Undiminished immunologic tolerance to contact sensitivity in mast cell-deficient W/Wv and Sl/Sld mice. Journal of Immunology. 1985;135:879. [PubMed] [Google Scholar]
- Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. The Journal of Allergy and Clinical Immunology. 2009;124:639. doi: 10.1016/j.jaci.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- Michel A, Schuler A, Friedrich P, Doner F, Bopp T, Radsak M, et al. Mast cell-deficient KitW−sh “Sash” mutant mice display aberrant myelopoiesis leading to the accumulation of splenocytes that act as myeloid-derived suppressor cells. Journal of Immunology. 2013;190:5534. doi: 10.4049/jimmunol.1203355. [DOI] [PubMed] [Google Scholar]
- Milenkovic N, Frahm C, Gassmann M, Griffel C, Erdmann B, Birchmeier C, et al. Nociceptive tuning by stem cell factor/c-Kit signaling. Neuron. 2007;56:893. doi: 10.1016/j.neuron.2007.10.040. [DOI] [PubMed] [Google Scholar]
- Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and FcγRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. The Journal of Clinical Investigation. 1997;99:901. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari JF, Moore WR, Clark J, Tanaka R, Butterfield JH, Abraham WM. Role of tryptase in immediate cutaneous responses in allergic sheep. Journal of Applied Physiology. 1995;79:1966. doi: 10.1152/jappl.1995.79.6.1966. [DOI] [PubMed] [Google Scholar]
- Molinari JF, Scuri M, Moore WR, Clark J, Tanaka R, Abraham WM. Inhaled tryptase causes bronchoconstriction in sheep via histamine release. American Journal of Respiratory and Critical Care Medicine. 1996;154:649. doi: 10.1164/ajrccm.154.3.8810600. [DOI] [PubMed] [Google Scholar]
- Moon TC, St Laurent CD, Morris KE, Marcet C, Yoshimura T, Sekar Y, et al. Advances in mast cell biology: New understanding of heterogeneity and function. Mucosal Immunology. 2010;3:111. doi: 10.1038/mi.2009.136. [DOI] [PubMed] [Google Scholar]
- Motakis E, Guhl S, Ishizu Y, Itoh M, Kawaji H, de Hoon M, et al. Redefinition of the human mast cell transcriptome by deep-CAGE sequencing. Blood. 2014;123:e58. doi: 10.1182/blood-2013-02-483792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23:191. doi: 10.1016/j.immuni.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Musch W, Wege AK, Mannel DN, Hehlgans T. Generation and characterization of alpha-chymase-Cre transgenic mice. Genesis. 2008;46:163. doi: 10.1002/dvg.20378. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nabe T, Kijitani Y, Kitagawa Y, Sakano E, Ueno T, Fujii M, et al. Involvement of chymase in allergic conjunctivitis of guinea pigs. Experimental Eye Research. 2013;113:74. doi: 10.1016/j.exer.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Nagle DL, Kozak CA, Mano H, Chapman VM, Bucan M. Physical mapping of the Tec and Gabrb1 loci reveals that the Wsh mutation on mouse chromosome 5 is associated with an inversion. Human Molecular Genetics. 1995;4:2073. doi: 10.1093/hmg/4.11.2073. [DOI] [PubMed] [Google Scholar]
- Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, et al. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. The Journal of Allergy and Clinical Immunology. 2007;120:48. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Nakano N, Nishiyama C, Kanada S, Niwa Y, Shimokawa N, Ushio H, et al. Involvement of mast cells in IL-12/23 p40 production is essential for survival from polymicrobial infections. Blood. 2007;109:4846. doi: 10.1182/blood-2006-09-045641. [DOI] [PubMed] [Google Scholar]
- Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, et al. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. The Journal of Experimental Medicine. 1985;162:1025. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta AC, Grova M, Montoro DT, Zimmermann A, Tsai M, Gurtner GC, et al. Evidence that mast cells are not required for healing of splinted cutaneous excisional wounds in mice. PLoS One. 2013;8:e59167. doi: 10.1371/journal.pone.0059167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen S, Vangansewinkel T, Geurts N, Geboes L, Lemmens E, Vidal PM, et al. Mast cells protect from post-traumatic spinal cord damage in mice by degrading inflammation-associated cytokines via mouse mast cell protease 4. Neurobiology of Disease. 2013;62C:260. doi: 10.1016/j.nbd.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Newlands GF, Miller HR, MacKellar A, Galli SJ. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N brasiliensis infection. Blood. 1995;86:1968. [PubMed] [Google Scholar]
- Ng MF. The role of mast cells in wound healing. International Wound Journal. 2010;7:55. doi: 10.1111/j.1742-481X.2009.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigrovic PA, Binstadt BA, Monach PA, Johnsen A, Gurish M, Iwakura Y, et al. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2325. doi: 10.1073/pnas.0610852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, et al. Genetic inversion in mast cell-deficient Wsh mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. The American Journal of Pathology. 2008;173:1693. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigrovic PA, Lee DM. Synovial mast cells: Role in acute and chronic arthritis. Immunological Reviews. 2007;217:19. doi: 10.1111/j.1600-065X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, et al. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W 37, W v, W 41 and W. The EMBO Journal. 1990;9:1805. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogami M, Suko M, Okudaira H, Miyamoto T, Shiga J, Ito M, et al. Experimental pulmonary eosinophilia in mice by Ascaris suum extract. The American Review of Respiratory Disease. 1990;141:1289. doi: 10.1164/ajrccm/141.5_Pt_1.1289. [DOI] [PubMed] [Google Scholar]
- Norman MU, Hwang J, Hulliger S, Bonder CS, Yamanouchi J, Santamaria P, et al. Mast cells regulate the magnitude and the cytokine microenvironment of the contact hypersensitivity response. The American Journal of Pathology. 2008;172:1638. doi: 10.2353/ajpath.2008.070559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AA. Pharmacology of sodium cromoglycate. Clinical and Experimental Allergy. 1996;26(Suppl. 4):5. doi: 10.1111/j.1365-2222.1996.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Matsuzaki Y, Nishikawa S, Hayashi S, Kunisada T, Sudo T, et al. Expression and function of c-kit in hemopoietic progenitor cells. The Journal of Experimental Medicine. 1991;174:63. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Pae CI, Lee DK, Jones F, Chiang GK, Kim HO, et al. Tryptase inhibition blocks airway inflammation in a mouse asthma model. Journal of Immunology. 2002;168:1992. doi: 10.4049/jimmunol.168.4.1992. [DOI] [PubMed] [Google Scholar]
- Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. Journal of Immunology. 2010;184:344. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, et al. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Letters. 2001;502:53. doi: 10.1016/s0014-5793(01)02663-1. [DOI] [PubMed] [Google Scholar]
- Oka T, Kalesnikoff J, Starkl P, Tsai M, Galli SJ. Evidence questioning cromolyn’s effectiveness and selectivity as a ‘mast cell stabilizer’ in mice. Laboratory Investigation. 2012;92:1472. doi: 10.1038/labinvest.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunologic Research. 2006;34:97. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama Y, Kirshenbaum AS, Metcalfe DD. Expression of a functional high-affinity IgG receptor, FcγRI, on human mast cells: Up-regulation by IFN-γ. Journal of Immunology. 2000;164:4332. doi: 10.4049/jimmunol.164.8.4332. [DOI] [PubMed] [Google Scholar]
- Oku Y, Itayama H, Kamiya M. Expulsion of Trichinella spiralis from the intestine of W/Wv mice reconstituted with haematopoietic and lymphopoietic cells and origin of mucosal mast cells. Immunology. 1984;53:337. [PMC free article] [PubMed] [Google Scholar]
- Okudaira H, Nogami M, Matsuzaki G, Dohi M, Suko M, Kasuya S, et al. T-cell-dependent accumulation of eosinophils in the lung and its inhibition by monoclonal anti-interleukin-5. International Archives of Allergy and Applied Immunology. 1991;94:171. doi: 10.1159/000235354. [DOI] [PubMed] [Google Scholar]
- Oliveira SH, Lukacs NW. Stem cell factor: A hemopoietic cytokine with important targets in asthma. Current Drug Targets. Inflammation and Allergy. 2003;2:313. doi: 10.2174/1568010033483990. [DOI] [PubMed] [Google Scholar]
- Oliveira SH, Taub DD, Nagel J, Smith R, Hogaboam CM, Berlin A, et al. Stem cell factor induces eosinophil activation and degranulation: Mediator release and gene array analysis. Blood. 2002;100:4291. doi: 10.1182/blood.V100.13.4291. [DOI] [PubMed] [Google Scholar]
- Orinska Z, Maurer M, Mirghomizadeh F, Bulanova E, Metz M, Nashkevich N, et al. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nature Medicine. 2007;13:927. doi: 10.1038/nm1615. [DOI] [PubMed] [Google Scholar]
- Otsu K, Nakano T, Kanakura Y, Asai H, Katz HR, Austen KF, et al. Phenotypic changes of bone marrow-derived mast cells after intraperitoneal transfer into W/W v mice that are genetically deficient in mast cells. The Journal of Experimental Medicine. 1987;165:615. doi: 10.1084/jem.165.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka A, Kubo M, Honda T, Egawa G, Nakajima S, Tanizaki H, et al. Requirement of interaction between mast cells and skin dendritic cells to establish contact hypersensitivity. PLoS One. 2011;6:e25538. doi: 10.1371/journal.pone.0025538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyamada S, Bianchi C, Takai S, Chu LM, Sellke FW. Chymase inhibition reduces infarction and matrix metalloproteinase-9 activation and attenuates inflammation and fibrosis after acute myocardial ischemia/reperfusion. The Journal of Pharmacology and Experimental Therapeutics. 2011;339:143. doi: 10.1124/jpet.111.179697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniagua RT, Sharpe O, Ho PP, Chan SM, Chang A, Higgins JP, et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. The Journal of Clinical Investigation. 2006;116:2633. doi: 10.1172/JCI28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton WD. Compound 48/80: A potent histamine liberator. British Journal of Pharmacology and Chemotherapy. 1951;6:499. doi: 10.1111/j.1476-5381.1951.tb00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejler G, Åbrink M, Ringvall M, Wernersson S. Mast cell proteases. Advances in Immunology. 2007;95:167. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- Pemberton AD, Brown JK, Wright SH, Knight PA, McPhee ML, McEuen AR, et al. Purification and characterization of mouse mast cell proteinase-2 and the differential expression and release of mouse mast cell proteinase-1 and-2 in vivo. Clinical and Experimental Allergy. 2003;33:1005. doi: 10.1046/j.1365-2222.2003.01720.x. [DOI] [PubMed] [Google Scholar]
- Pereira PJ, Bergner A, Macedo-Ribeiro S, Huber R, Matschiner G, Fritz H, et al. Human β-tryptase is a ring-like tetramer with active sites facing a central pore. Nature. 1998;392:306. doi: 10.1038/32703. [DOI] [PubMed] [Google Scholar]
- Peters EM, Maurer M, Botchkarev VA, Jensen K, Welker P, Scott GA, et al. Kit is expressed by epithelial cells in vivo. The Journal of Investigative Dermatology. 2003;121:976. doi: 10.1046/j.1523-1747.2003.12478.x. [DOI] [PubMed] [Google Scholar]
- Piconese S, Costanza M, Musio S, Tripodo C, Poliani PL, Gri G, et al. Exacerbated experimental autoimmune encephalomyelitis in mast-cell-deficient Kit W−sh/W−sh mice. Laboratory Investigation. 2011;91:627. doi: 10.1038/labinvest.2011.3. [DOI] [PubMed] [Google Scholar]
- Piliponsky AM, Chen CC, Grimbaldeston MA, Burns-Guydish SM, Hardy J, Kalesnikoff J, et al. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW−sh/W−sh mice. The American Journal of Pathology. 2010;176:926. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piliponsky AM, Chen CC, Nishimura T, Metz M, Rios EJ, Dobner PR, et al. Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis. Nature Medicine. 2008;14:392. doi: 10.1038/nm1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piliponsky AM, Chen CC, Rios EJ, Treuting PM, Lahiri A, Abrink M, et al. The chymase mouse mast cell protease 4 degrades TNF, limits inflammation, and promotes survival in a model of sepsis. The American Journal of Pathology. 2012;181:875. doi: 10.1016/j.ajpath.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos L, Pena G, Cai B, Deitch EA, Ulloa L. Mast cell stabilization improves survival by preventing apoptosis in sepsis. Journal of Immunology. 2010;185:709. doi: 10.4049/jimmunol.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. European Journal of Pharmacology. 2006;533:327. doi: 10.1016/j.ejphar.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Reber LL, Daubeuf F, Pejler G, Abrink M, Frossard N. Mast cells contribute to bleomycin-induced lung inflammation and injury in mice through a chymase/mast cell protease 4-dependent mechanism. Journal of Immunology. 2014;192:1847. doi: 10.4049/jimmunol.1300875. [DOI] [PubMed] [Google Scholar]
- Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends in Immunology. 2012;33:613. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber LL, Marichal T, Mukai K, Kita Y, Tokuoka SM, Roers A, et al. Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice. The Journal of Allergy and Clinical Immunology. 2013;132:881. doi: 10.1016/j.jaci.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber L, Marichal T, Sokolove J, Starkl P, Gaudenzio N, Iwakura Y, et al. Contribution of mast cell-derived interleukin-1β to uric acid crystal-induced acute arthritis in mice. Arthritis and Rheumatology. 2014;66:2881–2891. doi: 10.1002/art.38747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S, Heinz A, Sieren M, Wiewrodt R, Gelfand EW, Stassen M, et al. Mast cell-derived tumour necrosis factor is essential for allergic airway disease. The European Respiratory Journal. 2008;31:773. doi: 10.1183/09031936.00058907. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Crivellato E. Mast cells, angiogenesis and cancer. Advances in Experimental Medicine and Biology. 2011;716:270. doi: 10.1007/978-1-4419-9533-9_14. [DOI] [PubMed] [Google Scholar]
- Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Advances in Immunology. 2008;98:85. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: Fact or fiction? Immunity. 2012;37:13. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Ronnberg E, Johnzon CF, Calounova G, Faroldi GG, Grujic M, Hartmann K, et al. Mast cells are activated by Staphylococcus aureus in vitro but do not influence the outcome of intraperitoneal Staphylococcus aureus infection in vivo. Immunology. 2014;143:155–163. doi: 10.1111/imm.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild AM. Mechanisms of histamine release by compound 48−80. British Journal of Pharmacology. 1970;38:253. doi: 10.1111/j.1476-5381.1970.tb10354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi M, Takai S, Jin D, Okamoto Y, Muramatsu M, Kim S, et al. A specific chymase inhibitor, NK3201, suppresses bleomycin-induced pulmonary fibrosis in hamsters. European Journal of Pharmacology. 2004;493:173. doi: 10.1016/j.ejphar.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Yoshimoto T, Maruyama H, Tegoshi T, Ohta N, Arizono N, et al. IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast cell-dependent type 2 innate immunity. The Journal of Experimental Medicine. 2005;202:607. doi: 10.1084/jem.20042202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y, et al. Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. Journal of Immunology. 2012;188:1809. doi: 10.4049/jimmunol.1101746. [DOI] [PubMed] [Google Scholar]
- Sayed BA, Walker ME, Brown MA. Cutting edge: Mast cells regulate disease severity in a relapsing-remitting model of multiple sclerosis. Journal of Immunology. 2011;186:3294. doi: 10.4049/jimmunol.1003574. [DOI] [PubMed] [Google Scholar]
- Scandiuzzi L, Beghdadi W, Daugas E, Abrink M, Tiwari N, Brochetta C, et al. Mouse mast cell protease-4 deteriorates renal function by contributing to inflammation and fibrosis in immune complex-mediated glomerulonephritis. Journal of Immunology. 2010;185:624. doi: 10.4049/jimmunol.0902129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer B, Piliponsky AM, Oka T, Song CH, Gerard NP, Gerard C, et al. Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice. The Journal of Allergy and Clinical Immunology. 2012;131:541–548. doi: 10.1016/j.jaci.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemann M, Kugler EM, Buhner S, Eastwood C, Donovan J, Jiang W, et al. The mast cell degranulator compound 48/80 directly activates neurons. PLoS One. 2012;7:e52104. doi: 10.1371/journal.pone.0052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nature Immunology. 2007;8:665. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell-mediated innate defense against endothelin and snake venom sarafotoxin. The Journal of Experimental Medicine. 2007;204:2629. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten J, Hartmann K, Gerbaulet A, Krieg T, Muller W, Testa G, et al. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Research. 2008;17:307. doi: 10.1007/s11248-007-9153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. The Journal of Experimental Medicine. 2000;191:813. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafin WE, Dayton ET, Gravallese PM, Austen KF, Stevens RL. Carboxypeptidase A in mouse mast cells. Identification, characterization, and use as a differentiation marker. Journal of Immunology. 1987;139:3771. [PubMed] [Google Scholar]
- Sharma N, Kumar V, Everingham S, Mali RS, Kapur R, Zeng LF, et al. SH2 domain-containing phosphatase 2 is a critical regulator of connective tissue mast cell survival and homeostasis in mice. Molecular and Cellular Biology. 2012;32(14):2653–2663. doi: 10.1128/MCB.00308-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne CP, Nakano H, St John AL, Chan C, McLachlan JB, Gunn MD, et al. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host & Microbe. 2009;6:331. doi: 10.1016/j.chom.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, et al. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase heparin complexes. Journal of Immunology. 2009;182:647. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, et al. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. Journal of Immunology. 2008;180:4885. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota N, Nishikori Y, Kakizoe E, Shimoura K, Niibayashi T, Shimbori C, et al. Pathophysiological role of skin mast cells in wound healing after scald injury: Study with mast cell-deficient W/WV mice. International Archives of Allergy and Immunology. 2010;151:80. doi: 10.1159/000232573. [DOI] [PubMed] [Google Scholar]
- Smit JJ, Willemsen K, Hassing I, Fiechter D, Storm G, van Bloois L, et al. Contribution of classic and alternative effector pathways in peanut-induced anaphylactic responses. PLoS One. 2011;6:e28917. doi: 10.1371/journal.pone.0028917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nature Medicine. 2007;13:1211. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- Steimer DA, Boyd K, Takeuchi O, Fisher JK, Zambetti GP, Opferman JT. Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood. 2009;113:2805. doi: 10.1182/blood-2008-05-159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelekati E, Bahri R, D’Orlando O, Orinska Z, Mittrucker HW, Langenhaun R, et al. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity. 2009;31:665. doi: 10.1016/j.immuni.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Stevens RL, Friend DS, McNeil HP, Schiller V, Ghildyal N, Austen KF. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:128. doi: 10.1073/pnas.91.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RL, Qui D, McNeil HP, Friend DS, Hunt JE, Austen KF, et al. Transgenic mice that possess a disrupted mast cell protease 5 (mMCP-5) gene can not store carboxypeptidase A (mMC-CPA) protein in their granules. The FASEB Journal. 1996;10:17772. [Google Scholar]
- Stokman G, Stroo I, Claessen N, Teske GJ, Weening JJ, Leemans JC, et al. Stem cell factor expression after renal ischemia promotes tubular epithelial survival. PLoS One. 2010;5:e14386. doi: 10.1371/journal.pone.0014386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. The Journal of Allergy and Clinical Immunology. 2002;109:658. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- Sun J, Arias K, Alvarez D, Fattouh R, Walker T, Goncharova S, et al. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. Journal of Immunology. 2007;179:6696. doi: 10.4049/jimmunol.179.10.6696. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. The Journal of Clinical Investigation. 2002;109:1351. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. Journal of Immunology. 2008;181:5598. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. Journal of Immunology. 2006;176:4102. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Leach S, Liu W, Ralston E, Scheffel J, Zhang W, et al. Molecular editing of cellular responses by the high-affinity receptor for IgE. Science. 2014;343:1021. doi: 10.1126/science.1246976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghon T, Yui MA, Rothenberg EV. Mast cell lineage diversion of T lineage precursors by the essential T cell transcription factor GATA-3. Nature Immunology. 2007;8:845. doi: 10.1038/ni1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi K, Okuda-Ashitaka E, Mabuchi T, Katano T, Ohnishi T, Matsumura S, et al. Involvement of stem cell factor and its receptor tyrosine kinase c-kit in pain regulation. Neuroscience. 2008;153:1278. doi: 10.1016/j.neuroscience.2008.02.073. [DOI] [PubMed] [Google Scholar]
- Takato H, Yasui M, Ichikawa Y, Waseda Y, Inuzuka K, Nishizawa Y, et al. The specific chymase inhibitor TY-51469 suppresses the accumulation of neutrophils in the lung and reduces silica-induced pulmonary fibrosis in mice. Experimental Lung Research. 2011;37:101. doi: 10.3109/01902148.2010.520815. [DOI] [PubMed] [Google Scholar]
- Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, et al. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. The Journal of Experimental Medicine. 1997;186:449. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeishi T, Martin TR, Katona IM, Finkelman FD, Galli SJ. Differences in the expression of the cardiopulmonary alterations associated with anti-immunoglobulin E-induced or active anaphylaxis in mast cell-deficient and normal mice. Mast cells are not required for the cardiopulmonary changes associated with certain fatal anaphylactic responses. The Journal of Clinical Investigation. 1991;88:598. doi: 10.1172/JCI115344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Koike K, Kamijo T, Ishida S, Nakazawa Y, Kurokawa Y, et al. STI571 inhibits growth and adhesion of human mast cells in culture. Journal of Leukocyte Biology. 2003;74:1026. doi: 10.1189/jlb.0602284. [DOI] [PubMed] [Google Scholar]
- Tanzola MB, Robbie-Ryan M, Gutekunst CA, Brown MA. Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course. Journal of Immunology. 2003;171:4385. doi: 10.4049/jimmunol.171.8.4385. [DOI] [PubMed] [Google Scholar]
- Taube C, Wei X, Swasey CH, Joetham A, Zarini S, Lively T, et al. Mast cells, Fc€RI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. Journal of Immunology. 2004;172:6398. doi: 10.4049/jimmunol.172.10.6398. [DOI] [PubMed] [Google Scholar]
- Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. The Journal of Experimental Medicine. 2003;198:423. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, et al. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. The Journal of Biological Chemistry. 2007;282:20809. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- Timoshanko JR, Kitching AR, Semple TJ, Tipping PG, Holdsworth SR. A pathogenetic role for mast cells in experimental crescentic glomerulonephritis. Journal of the American Society of Nephrology. 2006;17:150. doi: 10.1681/ASN.2005080799. [DOI] [PubMed] [Google Scholar]
- Tomimori Y, Muto T, Saito K, Tanaka T, Maruoka H, Sumida M, et al. Involvement of mast cell chymase in bleomycin-induced pulmonary fibrosis in mice. European Journal of Pharmacology. 2003;478:179. doi: 10.1016/j.ejphar.2003.08.050. [DOI] [PubMed] [Google Scholar]
- Tremaine WJ, Brzezinski A, Katz JA, Wolf DC, Fleming TJ, Mordenti J, et al. Treatment of mildly to moderately active ulcerative colitis with a tryptase inhibitor (APC 2059): an open-label pilot study. Alimentary Pharmacology & Therapeutics. 2002;16:407. doi: 10.1046/j.1365-2036.2002.01194.x. [DOI] [PubMed] [Google Scholar]
- Tsai M, Grimbaldeston MA, Yu M, Tam SY, Galli SJ. Using mast cell knock-in mice to analyze the roles of mast cells in allergic responses in vivo. Chemical Immunology and Allergy. 2005;87:179. doi: 10.1159/000087644. [DOI] [PubMed] [Google Scholar]
- Tsai M, Shih LS, Newlands GF, Takeishi T, Langley KE, Zsebo KM, et al. The rat c-kit ligand, stem cell factor, induces the development of connective tissue-type and mucosal mast cells in vivo. Analysis by anatomical distribution, histochemistry, and protease phenotype. The Journal of Experimental Medicine. 1991;174:125. doi: 10.1084/jem.174.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, et al. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:6382. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Tsunemi K, Takai S, Nishimoto M, Jin D, Sakaguchi M, Muramatsu M, et al. A specific chymase inhibitor, 2-(5-formylamino-6-oxo-2-phenyl-1,6-dihydropyrimidine-1-yl)-N-[{3,4-dioxo-1-phenyl-7-(2-pyridyloxy)}-2-heptyl]acetamide (NK3201), suppresses development of abdominal aortic aneurysmin hamsters. The Journal of Pharmacology and Experimental Therapeutics. 2004;309:879. doi: 10.1124/jpet.103.063974. [DOI] [PubMed] [Google Scholar]
- Vaali K, Lappalainen J, Lin AH, Mayranpaa MI, Kovanen PT, Berstad A, et al. Imatinib mesylate alleviates diarrhea in a mouse model of intestinal allergy. Neurogastroenterology and Motility. 2012;24:e325. doi: 10.1111/j.1365-2982.2012.01941.x. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Liang HE, Locksley RM. Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice. Journal of Immunology. 2008;180:4742. doi: 10.4049/jimmunol.180.7.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- Waern I, Jonasson S, Hjoberg J, Bucht A, Abrink M, Pejler G, et al. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. Journal of Immunology. 2009;183:6369. doi: 10.4049/jimmunol.0900180. [DOI] [PubMed] [Google Scholar]
- Waern I, Lundequist A, Pejler G, Wernersson S. Mast cell chymase modulates IL-33 levels and controls allergic sensitization in dust-mite induced airway inflammation. Mucosal Immunology. 2013;6:911. doi: 10.1038/mi.2012.129. [DOI] [PubMed] [Google Scholar]
- Wastling JM, Knight P, Ure J, Wright S, Thornton EM, Scudamore CL, et al. Histochemical and ultrastructural modification of mucosal mast cell granules in parasitized mice lacking the β-chymase, mouse mast cell protease-1. The American Journal of Pathology. 1998;153:491. doi: 10.1016/s0002-9440(10)65592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Tomimori Y, Saito K, Miura K, Wada A, Tsudzuki M, et al. Chymase inhibitor improves dermatitis in NC/Nga mice. International Archives of Allergy and Immunology. 2002;128:229. doi: 10.1159/000064256. [DOI] [PubMed] [Google Scholar]
- Weidner N, Austen KF. Heterogeneity of mast cells at multiple body sites. Fluorescent determination of avidin binding and immunofluorescent determination of chymase, tryptase, and carboxypeptidase content. Pathology Research and Practice. 1993;189:156. doi: 10.1016/S0344-0338(11)80086-5. [DOI] [PubMed] [Google Scholar]
- Welle M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. Journal of Leukocyte Biology. 1997;61:233. doi: 10.1002/jlb.61.3.233. [DOI] [PubMed] [Google Scholar]
- Weller K, Foitzik K, Paus R, Syska W, Maurer M. Mast cells are required for normal healing of skin wounds in mice. The FASEB Journal. 2006;20:2366. doi: 10.1096/fj.06-5837fje. [DOI] [PubMed] [Google Scholar]
- Wernersson S, Pejler G. Mast cell secretory granules: Armed for battle. Nature Reviews Immunology. 2014;14:478–494. doi: 10.1038/nri3690. Published online 06 June. [DOI] [PubMed] [Google Scholar]
- Wershil BK, Mekori YA, Murakami T, Galli SJ. 125I-fibrin deposition in IgE-dependent immediate hypersensitivity reactions in mouse skin. Demonstration of the role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. Journal of Immunology. 1987;139:2605. [PubMed] [Google Scholar]
- Wershil BK, Murakami T, Galli SJ. Mast cell-dependent amplification of an immunologically nonspecific inflammatory response. Mast cells are required for the full expression of cutaneous acute inflammation induced by phorbol 12-myristate 13-acetate. Journal of Immunology. 1988;140:2356. [PubMed] [Google Scholar]
- Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. The Journal of Clinical Investigation. 1991;87:446. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener Z, Buzas E, Kovacs P, Csaba G, Szabo D, Kittel A, et al. Highly reduced peritoneal mast cell number and decreased c-kit expression in histidine decarboxylase knock out mice. Inflammation Research. 2001;50(Suppl. 2):S55. doi: 10.1007/PL00022406. [DOI] [PubMed] [Google Scholar]
- Willenborg S, Eckes B, Brinckmann J, Krieg T, Waisman A, Hartmann K, et al. Genetic ablation of mast cells redefines the role of mast cells in skin wound healing and bleomycin-induced fibrosis. The Journal of Investigative Dermatology. 2014;134(7):2005–2015. doi: 10.1038/jid.2014.12. [DOI] [PubMed] [Google Scholar]
- Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. The Journal of Experimental Medicine. 2000;192:455. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, et al. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient KitW−sh/KitW−sh sash mice. Clinical and Experimental Allergy. 2005;35:82. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GW, Foster PS, Yasuda S, Qi JC, Mahalingam S, Mellor EA, et al. Biochemical and functional characterization of human transmembrane tryptase (TMT)/tryptase γTMT is an exocytosed mast cell protease that induces airway hyperresponsiveness in vivo via an interleukin-13/interleukin-4 receptor α/signal transducer and activator of transcription (STAT) 6-dependent pathway. The Journal of Biological Chemistry. 2002;277:41906. doi: 10.1074/jbc.M205868200. [DOI] [PubMed] [Google Scholar]
- Wong GW, Tang Y, Feyfant E, Sali A, Li L, Li Y, et al. Identification of a new member of the tryptase family of mouse and human mast cell proteases which possesses a novel COOH-terminal hydrophobic extension. The Journal of Biological Chemistry. 1999;274:30784. doi: 10.1074/jbc.274.43.30784. [DOI] [PubMed] [Google Scholar]
- Wright CD, Havill AM, Middleton SC, Kashem MA, Dripps DJ, Abraham WM, et al. Inhibition of allergen-induced pulmonary responses by the selective tryptase inhibitor 1,5-bis-{4-[(3-carbamimidoyl-benzenesulfonylamino)-methyl]-phenoxy}-pentane (AMG-126737) Biochemical Pharmacology. 1999;58:1989. doi: 10.1016/s0006-2952(99)00304-4. [DOI] [PubMed] [Google Scholar]
- Wulff BC, Parent AE, Meleski MA, DiPietro LA, Schrementi ME, Wilgus TA. Mast cells contribute to scar formation during fetal wound healing. The Journal of Investigative Dermatology. 2012;132:458. doi: 10.1038/jid.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss D, Bonneau O, Trifilieff A. Mast cell involvement in the adenosine mediated airway hyperreactivity in a murine model of ovalbumin-induced lung inflammation. British Journal of Pharmacology. 2005;145:845. doi: 10.1038/sj.bjp.0706271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhang D, Lyubynska N, Wolters PJ, Killeen NP, Baluk P, et al. Mast cells protect mice from Mycoplasma pneumonia. American Journal of Respiratory and Critical Care Medicine. 2006;173:219. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi R, Tanaka S, Motomura Y, Kubo M. Regulation of the Il4 gene is independently controlled by proximal and distal 3’ enhancers in mast cells and basophils. Molecular and Cellular Biology. 2007;27:8087. doi: 10.1128/MCB.00631-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younan G, Suber F, Xing W, Shi T, Kunori Y, Abrink M, et al. The inflammatory response after an epidermal burn depends on the activities of mouse mast cell proteases 4 and 5. Journal of Immunology. 2010;185:7681. doi: 10.4049/jimmunol.1002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Eckart MR, Morgan AA, Mukai K, Butte AJ, Tsai M, et al. Identification of an IFN-γ/mast cell axis in a mouse model of chronic asthma. The Journal of Clinical Investigation. 2011;121:3133. doi: 10.1172/JCI43598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. The Journal of Clinical Investigation. 2006;116:1633. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ramos BF, Jakschik BA. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992;258:1957. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]
- Zhang J, Sun J, Lindholt JS, Sukhova GK, Sinnamon M, Stevens RL, et al. Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circulation Research. 2011;108:1316. doi: 10.1161/CIRCRESAHA.111.243758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Qian L, Bieszczad CK, Noelle R, Binder M, Levy NB, et al. Mcl-1 in transgenic mice promotes survival in a spectrum of hematopoietic cell types and immortalization in the myeloid lineage. Blood. 1998;92:3226. [PubMed] [Google Scholar]
- Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in KitW−sh mice does not impair antibody-mediated arthritis. The Journal of Experimental Medicine. 2007;204:2797. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]