Abstract
Purpose
To examine associations between corneal mechanical thresholds and metrics of dry eye.
Methods
This was a cross-sectional study of individuals seen in the Miami Veterans Affairs eye clinic. The evaluation consisted of questionnaires regarding dry eye symptoms and ocular pain, corneal mechanical detection and pain thresholds, and a comprehensive ocular surface examination. The main outcome measures were correlations between corneal thresholds and signs and symptoms of dry eye and ocular pain.
Results
A total of 129 subjects participated in the study (mean age 64 ± 10 years). Mechanical detection and pain thresholds on the cornea correlated with age (Spearman's ρ = 0.26, 0.23, respectively; both P < 0.05), implying decreased corneal sensitivity with age. Dry eye symptom severity scores and Neuropathic Pain Symptom Inventory (modified for the eye) scores negatively correlated with corneal detection and pain thresholds (range, r = −0.13 to −0.27, P < 0.05 for values between −0.18 and −0.27), suggesting increased corneal sensitivity in those with more severe ocular complaints. Ocular signs, on the other hand, correlated poorly and nonsignificantly with mechanical detection and pain thresholds on the cornea. A multivariable linear regression model found that both posttraumatic stress disorder (PTSD) score (β = 0.21, SE = 0.03) and corneal pain threshold (β = −0.03, SE = 0.01) were significantly associated with self-reported evoked eye pain (pain to wind, light, temperature) and explained approximately 32% of measurement variability (R = 0.57).
Conclusions
Mechanical detection and pain thresholds measured on the cornea are correlated with dry eye symptoms and ocular pain. This suggests hypersensitivity within the corneal somatosensory pathways in patients with greater dry eye and ocular pain complaints.
Keywords: dry eye, aesthesiometer, mechanical thresholds, ocular pain
Chronic dry eye affects between 5% and 30% of the population aged 50 and above and is one of the most common presenting complaints to eye care professionals.1 Its primary symptoms include ocular pain, irritation, and blurred vision, and its presence can have a significant impact on the ability to perform common activities such as reading, watching television, using a computer, driving, and professional work.1,2 Clinicians use a combination of symptoms and signs to arrive at a diagnosis, as there is currently not an accepted clinical test that can definitively diagnose the disease; therefore, the population of patients with dry eye is made up of heterogeneous subgroups, with a potential mixture of pathophysiological elements. For many patients, standard treatments aimed at correcting aqueous tear deficiency or evaporative deficiency do not eliminate symptoms of dry eye,3 and studies have shown that measurable tear film parameters do not correlate well with the report and severity of symptoms measured across dry eye patients.4–6
Recent data suggest that there may be another underlying mechanism, not directly related to tear dysfunction, that contributes to symptoms of dry eye for at least a subgroup of patients, that is, the presence of corneal somatosensory dysfunction.7,8 However, typical testing methods used in routine clinical practice are inadequate to evaluate corneal somatosensory status and to identify subgroups of dry eye patients based on this parameter. Confocal microscopy has been used to image the corneal subbasal plexus; and alterations in corneal nerve morphology, including nerve sprouting and thickening,9 low density,10–12 tortuosity, and beading,10 have been described in some dry eye patients as compared to controls. This evidence suggests that nerve dysfunction may contribute to dry eye status for some patients. Other groups, however, have not found differences in corneal nerve densities in subjects with and without dry eye.9
Another emerging method to evaluate neurologic dysfunction within the corneal somatosensory system is quantitative sensory testing (QST). Quantitative sensory testing can be used to quantitatively measure somatosensory function in response to different stimuli.13 Quantitative sensory testing has been used in research studies for many decades to help understand the physiology of the somatosensory system, and has recently been gaining popularity as a tool for the evaluation and diagnosis of neuropathic pain.14,15 Quantitative sensory testing can identify and quantify somatosensory dysfunction, including both hypoesthesias (decreased sensitivity to a stimulus) and hyperesthesias (increased sensitivity to a stimulus).16 Thus, QST, when specifically applied to the cornea, may aid in identifying neuronal dysfunction contributing to the pathophysiology of the painful manifestations of dry eye.
The Cochet-Bonnet and Belmonte aesthesiometry devices are QST devices that have been used to characterize somatosensory disturbances within the eye. The Cochet-Bonnet aesthesiometer has been applied to patients with dry eye, with some patients displaying reduced sensitivity to mechanical stimuli.11,17,18 In these patients, dry eye signs, such as corneal staining, correlated negatively with corneal mechanical sensitivity, suggesting that patients with more severe signs of ocular dryness had decreased mechanical sensitivity at the cornea.17 The Cochet-Bonnet device, however, is not ideal for assessing mechanical sensitivity at the cornea because it requires contact with the eye and has a limited range of testing values.
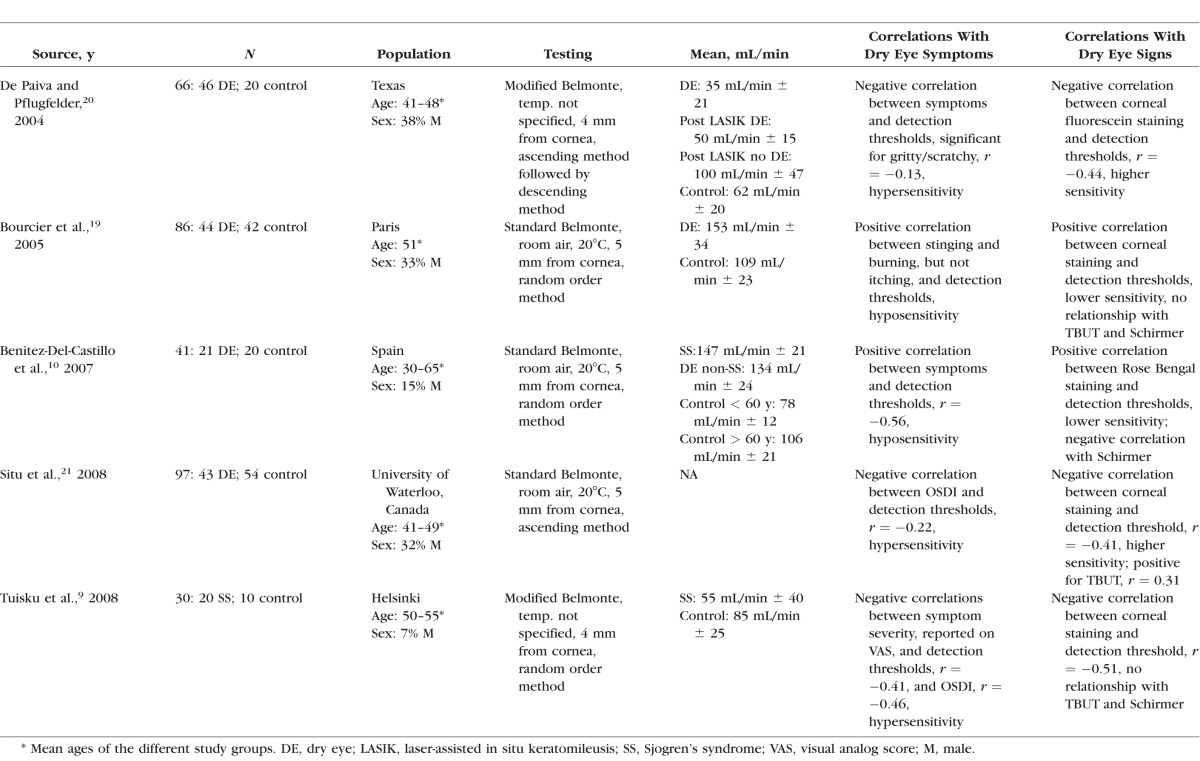
The Belmonte aesthesiometer has also been used to evaluate corneal detection thresholds to mechanical and thermal stimuli, and allows for greater precision than the Cochet-Bonnet device as it has a wider range of testing values and can assess three different modalities (mechanical sensitivity by adjusting air flow, chemical sensitivity using carbon dioxide, and thermal sensitivity by adjusting temperature of stimuli). Of the five studies of patients with dry eye, two found reduced corneal sensitivity to mechanical, chemical, and thermal stimuli compared to controls10,19 while three found increased sensitivity to mechanical stimuli (Table 1).9,20,21 The reason for these equivocal results is unclear, although the heterogeneous nature of dry eye, combined with differences in diagnostic criteria among the studies, may be partly responsible.
Table 1.
Review of Studies That Have Tested for Mechanical Detection Thresholds by Belmonte Aesthesiometry in Dry Eye
In the present study, we had three primary objectives: (1) to evaluate mechanical detection thresholds in our unique population of patients (US veterans with a high frequency of posttraumatic stress disorder [PTSD] and depression) in order to compare this metric with prior dry eye studies; (2) to expand the role of the Belmonte aesthesiometer as a QST instrument for measuring corneal mechanical pain thresholds, a metric that has been used to investigate the functional integrity of the nociceptive system in other chronic pain populations,7 but not in dry eye; and (3) to determine the utility of pain threshold measures as potential indicators of corneal somatosensory system dysfunction by examining relationships between these thresholds and self-reported symptoms of dry eye and of ocular pain specifically. We hypothesized that mechanical detection and pain thresholds measured on the corneal surface would be significantly related to the severity of self-reported eye pain and dry eye symptoms.
Methods
Study Population
Patients with normal eyelid and corneal anatomy were prospectively recruited from the Miami Veterans Affairs (VA) Healthcare System eye clinic between October 2013 and January 2015 and underwent a complete ocular surface examination. Patients were excluded from participation if they wore contact lenses; had undergone refractive surgery; used ocular medications with the exception of artificial tears; had human immunodeficiency virus, sarcoidosis, graft versus host disease, or a collagen vascular disease; had an active external ocular process; had had cataract surgery within the last 6 months or undergone any glaucoma or retinal surgery in the past. Informed consent was obtained from all subjects. Miami VA Institution Review Board approval was obtained to allow the prospective evaluation of subjects. The study was conducted in accordance with the principles of the Declaration of Helsinki and complied with the requirements of the United States Health Insurance Portability and Accountability Act.
Measures
Questionnaires.
For each individual, demographic information (age, sex, race, ethnicity), past ocular and medical history, and medication information were collected. Medications were categorized into anxiolytics, antidepressants, analgesics, antihistamines, and the gabapentinoids.
Patients filled out standardized questionnaires regarding dry eye symptoms, including the Dry Eye Questionnaire 5 (DEQ5)22 and the Ocular Surface Disease Index (OSDI).23 The DEQ5 is a validated, five-item questionnaire that combines patient responses regarding “eye discomfort” (frequency and intensity), “eye dryness” (frequency and intensity), and “watery eyes” (frequency) during the past month. Scores on the DEQ5 can range from 0 to 22, with higher scores indicating greater severity of symptoms. The OSDI is a 12-item questionnaire that assesses the frequency of dry eye symptoms and their impact on function on a 0 to 100 scale, with higher scores indicating greater severity of disease.23 It has good psychometric properties23 and has been used in a number of studies of dry eye and quality of life.24,25
Pain questionnaires were used to assess for the presence and quality of ocular pain. A numerical rating scale (NRS; score 0–10) was used to assess the “average intensity of eye pain during the past week.” The Neuropathic Pain Symptom Inventory (NPSI),26 modified for the eye, was used to quantify the severity of clinically relevant dimensions of neuropathic pain. This questionnaire was chosen as it has been validated and used in a number of patient populations with various neuropathic pain conditions,26–30 though it has not been specifically validated for eye pain. The NPSI consists of 10 scored items that help identify and assess the severity of spontaneous and paroxysmal pain, paresthesias, allodynia, and hyperalgesia. In order to modify the NPSI so that it was relevant to neuropathic ocular pain (NOP), we replaced three of the original questions regarding the severity of allodynia or hyperalgesia caused by (1) light touch, (2) pressure, or (3) contact with something cold on the skin, with questions specific to ocular allodynia or hyperalgesia (eye pain caused or increased by [1] wind, [2] light, and [3] heat or cold). A total NPSI eye score was calculated, along with five subscores (burning spontaneous pain, pressing spontaneous pain, paroxysmal pain, evoked pain, and paresthesia/dysesthesia), as an indication of the severity of neuropathic-like ocular pain.
Regarding mental health indices, symptoms of PTSD were assessed via the PTSD Checklist–Military Version (PCL-M) (score 17–85)31,32 and symptoms of depression via the Patient Health Questionnaire 9 (PHQ9) (score 0–27).33
Ocular QST/Belmonte Aesthesiometry Testing.
Mechanical detection and pain thresholds of the central cornea were assessed with a modified Belmonte noncontact aesthesiometer, which was developed based on the original Belmonte instrument (Figs. 1, 2).34 The tip of the aesthesiometer (0.5 mm in diameter) was placed perpendicular to, and 4 mm from, the surface of the cornea of the right eye. Stimulation consisted of pulses of air at room temperature (approximately 23–26°C)35 applied to the corneal surface. The method of limits, using ascending series only, was used to measure threshold. We opted to test only one eye (right), as dry eye symptoms are largely present bilaterally, previous testing with the Belmonte aesthesiometer has shown insignificant differences in detection thresholds between the eyes,36 and we wanted to optimize testing time and minimize patient fatigue. Further justification for testing only one side comes from other studies showing that right-to-left differences in QST measures at other body sites, including mechanical detection and pain threshold, are very small and insignificant.37
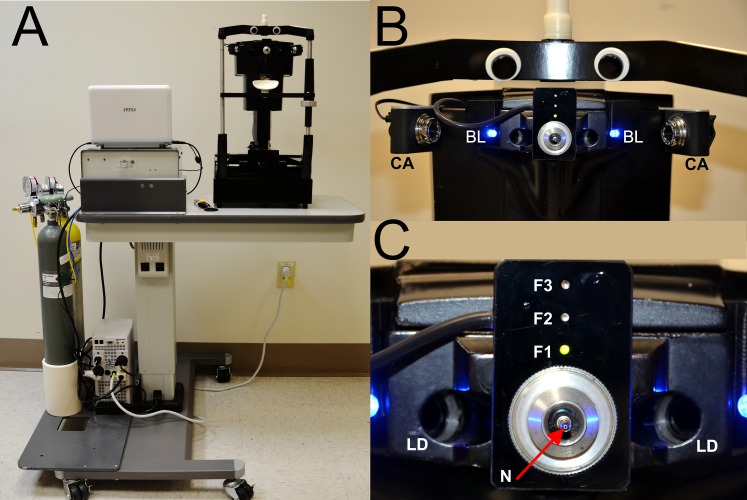
Figure 1.
Modified Belmonte aesthesiometer, patient side. (A) The instrument is offset on the motorized table for patients in wheelchairs. (B) Two low-power red laser diodes (LD) focus on the cornea apex, and two charge-coupled device (CCD) cameras (CA) and two blue light-emitting diodes (LEDs) (BL) assess the distance between the cornea and the nozzle. (C) Air–gas nozzle (N) and patient fixation LEDs that can target the midcornea periphery (F1), the limbal area (F2), and the anterior conjunctiva (F3).
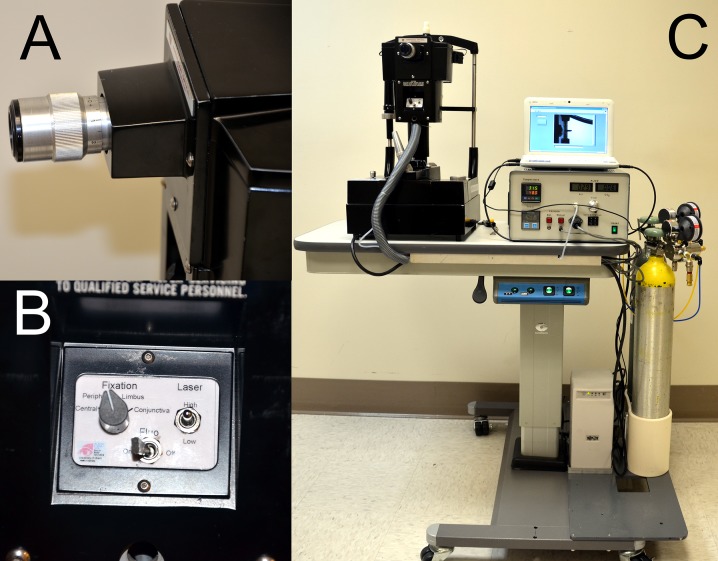
Figure 2.
Modified Belmonte aesthesiometer, operator side. (A) Adjustable ocular with crosshair; the corneal reflex becomes visible only when both laser diodes coincide with the cornea. (B) Controls for laser diode intensity, fixation target, and blue light illumination. (C) Air–gas generator with adjustment and indicators for flow and temperature. Medical air and CO2 tanks are equipped with on/off valves and precision low-pressure two-stage regulators for safety.
For corneal detection threshold measurements, subjects were presented with a stimulus immediately following a blink, and asked to indicate whether they felt the stimulus by pressing a button. The initial flow rate was set at a level below threshold (50 mL/min for most individuals) and increased by 10 mL/min (with 15-second intervals between stimuli) until the subject stated that he or she felt the stimulus or the maximum allowable flow rate (200 mL/min) was reached. Two ascending series were conducted, and detection threshold was defined as the arithmetic mean of the value at which the subject pressed the button across the two series. To estimate ocular pain threshold, the flow rate was further increased beyond the detection threshold in 10 mL/min increments until the subject reported the stimulus as painful or the maximum allowable flow rate (200 mL/min) was reached. Two ascending series were conducted in this way, and pain threshold was defined as a mean of the two series. All threshold measures were performed during the morning hours by the same operator, with room temperature varying between 73°F and 83°F and humidity ranging between 38% and 53%.
Ocular Surface Evaluation.
All patients underwent a standard tear film assessment,38 including measurement of (1) tear osmolarity (TearLAB, San Diego, CA, USA) (once in each eye)—higher measures indicate greater abnormality; (2) tear evaporation measured via tear breakup time (TBUT; 5 μL fluorescein placed, three measurements taken in each eye and averaged)—lower levels indicate more rapid tear evaporation from the surface of the eye; (3) corneal epithelial cell disruption measured via corneal staining (National Eye Institute [NEI] scale,38 five areas of cornea assessed; score 0–3 in each, total 15)—higher levels indicate greater abnormality; (4) tear production measured via Schirmer's strips with anesthesia—lower levels indicate decreased tear production; and (5) meibomian gland assessment. Eyelid vascularity was graded on a scale of 0 to 3 (0 none; 1 mild engorgement; 2 moderate engorgement; 3 severe engorgement) and meibum quality on a scale of 0 to 4 (0 = clear; 1 = cloudy; 2 = granular; 3 = toothpaste; 4 = no meibum extracted). Higher values in both categories indicate more abnormal tear parameters.
Statistical Analysis
Statistical analyses were performed using the SPSS 22.0 (SPSS, Inc., Chicago, IL, USA) statistical package. Descriptive statistics were used to summarize patient demographic and clinical information. Normality of distributions was assessed using the Kolmogorov-Smirnov (K-S) test statistic, and nonnormally distributed data were log transformed and/or analyzed using nonparametric statistical tests. Correlations (Pearson and Spearman) were used to evaluate the strength of association between evoked sensory responses on the cornea and severity of dry eye signs and symptoms. Student's t-tests and Mann-Whitney U tests were used (as appropriate) to evaluate for differences in means between groups. Analysis of covariance was used to evaluate the effect of age on variables of interest. Linear regression analyses with forward selection were used to evaluate the contribution of variables on the variability of dry eye symptoms and ocular pain. We inspected residuals from the linear regression analysis for departures from normality and heterogeneity. In this paper, we opted to give information on all variables being compared as opposed to correcting the P value (e.g., Bonferroni) since the latter methodology has its own limitations.39
Results
Study Sample
One hundred twenty-nine veterans with varying dry eye symptoms (none to severe) participated in the study (mean age 64 ± 10 years, 93% men). Full demographic characteristics of the study sample are presented in Table 2.
Table 2.
Demographics of the Patient Population (n = 129)
Distribution of Corneal Detection and Pain Thresholds in Our Population
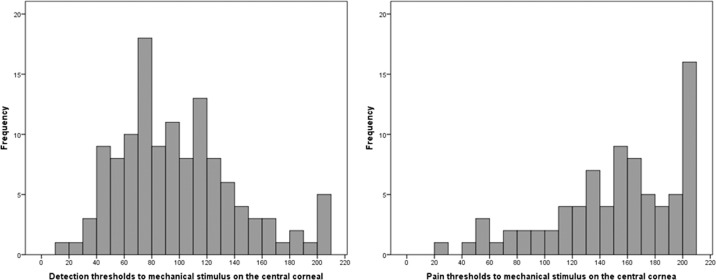
As measured by the modified Belmonte aesthesiometer, the mean mechanical detection threshold of the central cornea was 101 ± 47 mL/min (range, 15–210; median = 90), and the mean pain detection threshold was 171 ± 47 mL/min (range, 25–210; median = 200) (Fig. 3). Of note, 5 individuals did not detect the stimulus at the maximum flow of 200 mL/min, and 49 individuals did not report pain at the maximum value and were assigned a value of 210 to differentiate them from individuals for whom detection and/or pain threshold was reached at the maximum value. Kolmogorov-Smirnov tests of normality revealed that the distribution of detection and pain thresholds was significantly different from the normal distribution, and, as such, both were log transformed. This transformation normalized detection thresholds (based on K-S test) but not pain thresholds; so parametric analyses were used for log-transformed detection thresholds, and both parametric and nonparametric tests were used for log-transformed ocular pain thresholds. Intertrial reliability (agreement between the first and second measurement within a session), as measured by intraclass correlation coefficients (ICC; 1-way random effects), was “substantial” (0.81–1.0)35 for both detection thresholds (ICC = 0.87) and pain thresholds (ICC = 0.88). Measures of detection and pain threshold on the eye were significantly correlated (Pearson r = 0.56, Spearman's ρ = 0.57, both P < 0.0005).
Figure 3.
Distribution of corneal detection (left) and pain (right) thresholds on the central cornea in the study sample.
Relationship Between Corneal Thresholds and Demographics, Medications, and Psychologic Status
Age was positively correlated with log-transformed detection (Pearson r = 0.20, P = 0.01) and pain thresholds (r = 0.14, P = 0.12; Spearman's ρ = 0.23, P = 0.01). Sex, race, and ethnicity did not significantly affect ocular detection and pain thresholds (Mann-Whitney U test, all P > 0.05). Patients on anxiolytics had lower detection thresholds than those subjects not currently taking anxiolytics (median 80 vs. 100 mL/min, P = 0.02, Mann-Whitney U test), but this effect did not remain significant when adjusting for age (by analysis of covariance). None of the other medications significantly affected threshold measures at the cornea. Self-reported symptoms of depression and PTSD were negatively correlated with log-transformed detection thresholds (r = −0.15, P = 0.09 and r = −0.19, P = 0.03, respectively), and nonsignificantly with log-transformed pain thresholds (r = −0.13, P = 0.15 and r = −0.15, P = 0.18, respectively) after controlling for age.
Relationships Among Symptoms of Dry Eye, Ocular Pain Report, and Corneal Thresholds
The mean DEQ5 score for all patients was 11 ± 5 (range, 0–21), and the mean OSDI score was 36 ± 28 (range, 0–98). These two measures of dry eye symptom severity were highly correlated (r = 0.71; ρ = 0.74, both P < 0.0005). Estimates of ocular pain severity, obtained using NRS for average ocular pain over a 1-week recall period (mean = 3.5 ± 2.8 [range, 0–9]) and total score from the NPSI eye (22 ± 23 [range, 0–95]), were highly correlated (r = 0.79; ρ = 0.85, both P < 0.0005). Both measures of dry eye symptoms were significantly correlated with both measures of ocular pain severity (DEQ5 and [1] NRS r = 0.74, P < 0.0005; [2] NPSI eye r = 0.72, P < 0.0005; OSDI and [1] NRS r = 0.73, P < 0.0005; [2] NPSI eye r = 0.73, P < 0.0005).
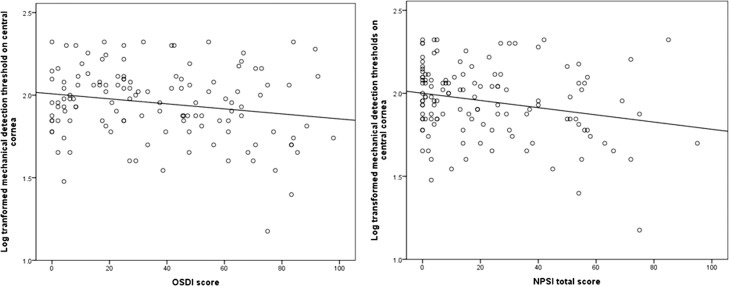
Correlations between self-report measures of ocular symptoms and QST measures, after controlling for age, are presented in Table 3. Log-transformed corneal detection and pain thresholds were significantly and negatively correlated with measures of dry eye symptom severity (r between −0.17 and −0.20, P ≤ 0.05 for all), and with most scores (and subscores) of ocular pain severity. An example of the negative correlation between log-transformed detection thresholds and dry eye symptoms and ocular pain is presented in Figure 4.
Table 3.
Correlations Between Evoked Sensory Responses and Ocular Symptoms (Controlling for Age)
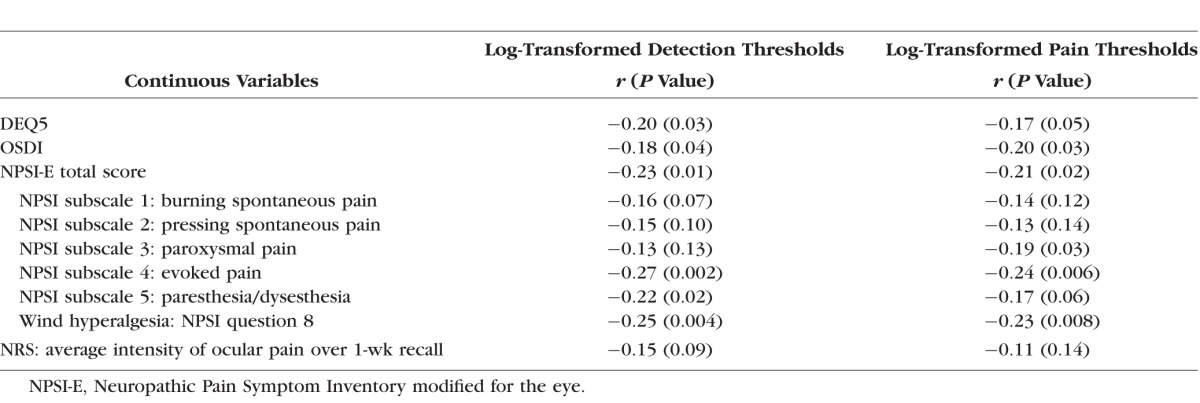
Figure 4.
Negative correlations between the Ocular Surface Disease Index (OSDI; left) and NPSI eye total score (right) and mechanical detection thresholds (log transformed) on central cornea.
Correlations Between Corneal Thresholds and Ocular Signs
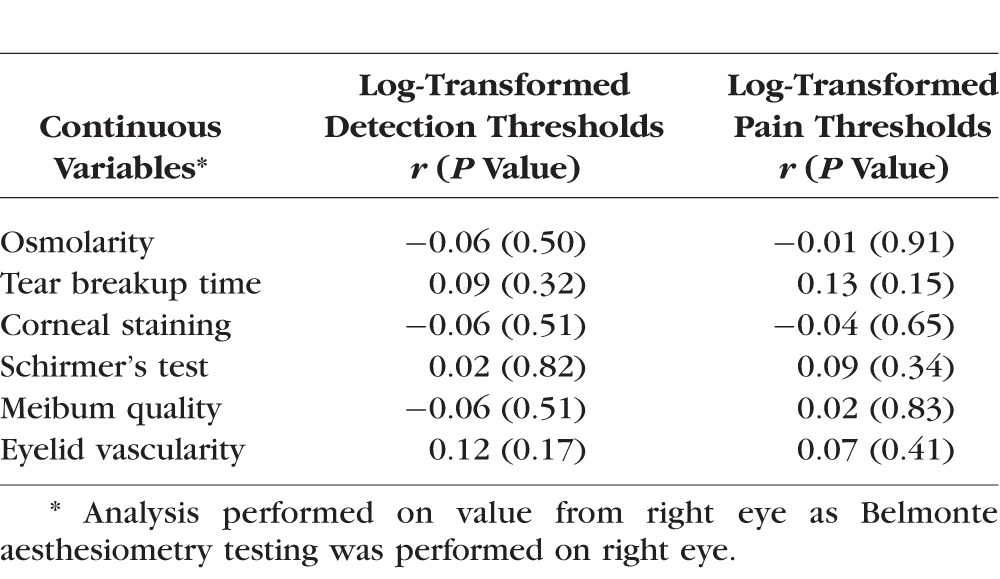
None of the measured signs of dry eye were significantly correlated with log-transformed corneal detection or pain thresholds when controlling for age (Table 4).
Table 4.
Correlations Between Evoked Sensory Responses and Ocular Signs (Controlling for Age)
Linear Regression Analysis Models
In order to provide an omnibus evaluation, forward multivariable linear regression models considering age, PTSD, depression, use of anxiolytics and antidepressants, detection and pain thresholds, and ocular signs were performed to evaluate which factors significantly contributed to the severity of ocular pain and dry eye symptom report. Detection and pain thresholds did not remain significantly associated with ocular pain severity assessed by the NRS and NPSI eye total score regression models, when accounting for other factors entered in the model. In both of these models, PTSD score was most strongly associated with ocular pain (R = 0.44 for NRS, R = 0.48 for NPSI eye). When examining the NPSI subscore for evoked eye pain (sensitivity to light/wind/change in temperature), both PTSD score (β = 0.21, SE = 0.03) and corneal pain threshold (β = −0.03, SE = 0.01) remained significantly associated with evoked eye pain and explained approximately 32% of measurement variability (R = 0.57). Evaluation of residuals revealed that there was no substantial deviation from normality or heterogeneity of variance. Detection and pain thresholds likewise did not remain significantly associated with dry eye severity as measured via the DEQ5 and OSDI when accounting for variability contributed by the above-mentioned factors. Again, PTSD score was the only variable to associate with DEQ5 symptoms (R = 0.45), and both PTSD and depression scores were significantly associated with OSDI scores (R = 0.59).
Discussion
This is the first study to measure corneal pain thresholds using the Belmonte aesthesiometer and to assess the relationship between pain thresholds and primary (non-Sjogren's) dry eye. In this study, we evaluated corneal sensory detection and pain thresholds to mechanical stimuli (air puff) as metrics of somatosensory function and examined relationships between these metrics and signs and symptoms of dry eye and ocular pain. In our unique population, consisting primarily of elderly male veterans, we found that corneal thresholds were negatively associated with the severity of ocular pain and dry eye symptoms (i.e., those with more severe eye pain and dry eye symptoms displayed greater sensitivity to innocuous and to noxious mechanical stimuli on the cornea) but were not significantly correlated with ocular surface parameters traditionally thought to be signs of dry eye. Thus, our results support our hypothesis that corneal somatosensory dysfunction is related to dry eye symptoms for at least a subgroup of patients.8
There are few instruments available to assess the integrity of the corneal somatosensory system in humans. Cotton-tipped applicators enable simple and fast qualitative assessment. The Cochet-Bonnet aesthesiometer enables quantitative measurement of corneal sensitivity and is widely used. Other methods such as chemical stimulation using capsaicin and thermal stimulation with a carbon dioxide laser are not in wide use. Belmonte et al.34 developed a noncontact gas aesthesiometer that can be used to quantitatively measure corneal thresholds to a variety of stimuli (mechanical to air flow, chemical to CO2, and thermal). Threshold measurements obtained with the Belmonte aesthesiometer have been shown to be reliable across test sessions on different days (ICC for mechanical detection = 0.77).39 Results from the present study further support the reliability of measurements of mechanical detection threshold (ICC = 0.87 across two measurements collected within the same study session), and provide new information concerning the reliability of mechanical pain thresholds as well (ICC = 0.88).
While not commercially available, standard and modified versions of this instrument have been used by several groups to evaluate detection thresholds to mechanical stimuli in healthy subjects and in dry eye patients. A wide range of mean detection thresholds has been reported in both populations: healthy (59–109 mL/min)9,10,19,20,34,36,40,41 and dry eye (35–153 mL/min).9,10,19,20 This variability likely arises from many factors including demographic differences between populations (i.e., age) and different models and implementations of the aesthesiometer (temperature of air flow, distance from cornea, methodology), among other parameters.
Three previous studies have reported decreased detection thresholds (increased sensitivity) to nonnoxious mechanical stimuli in patients with various dry eye signs and symptoms.9,20,21 Two other studies, on the other hand, reported increased thresholds (decreased sensitivity) to nonnoxious mechanical stimuli in patients with dry eye.10,19 Other studies have reported differences by type of stimulus used. For example, fibromyalgia patients (who as a group had higher dry eye symptoms and signs than controls, n = 20) were found to have decreased corneal sensitivity to heat and chemical stimuli but no difference in corneal mechanical sensitivity compared to controls (n = 18).41 On the other hand, mechanical thresholds significantly differed between dry eye patients with Sjogren's syndrome versus controls while chemical thresholds did not.9 Similar discrepancies have been found with regard to signs of dry eye as well.9,10,19,20,35 For example, corneal staining was negatively correlated with detection thresholds in one study,20 but positively correlated in another.19 It is unclear what biologic differences may account for the observed clinical variability.
A potential unifying hypothesis for our findings and that of others is that dry eye represents a heterogeneous condition that includes variable dysfunction of the ocular surface and/or corneal somatosensory system, including neural mechanisms underlying nociception. Different dry eye subpopulations may have different profiles with regard to frequencies and magnitudes of dysfunction in these locations, and this may result in the observed differences in corneal sensitivity testing. Subcategorizing dry eye patients based on the specific mechanisms (e.g., peripheral and/or central dysfunction) has important implications for the diagnosis and treatment of dry eye. For example, patients found to have peripheral abnormalities such as an adverse ocular surface environment (e.g., rapid tear evaporation or decreased production) would likely benefit from therapies primarily targeting ocular surface inflammation and hyperosmolarity, while patients with neuropathic-like ocular pain symptoms, in the absence of ocular surface abnormalities, may benefit from more centrally acting neuromodulators.42
There is biologic plausibility as to why nociceptive dysfunction (i.e., NOP) may develop in some dry eye patients. Peripheral nerve injury is the typical initiating event, and virtually any ocular surface perturbation (surgery, air pollution, an arid environment) can create ocular surface inflammation and corneal primary afferent neuronal damage.43 This can lead to a temporary (or prolonged) change in the structure and function of peripheral nociceptors, with altered neuronal nuclear, cytosolic, and membrane signaling mechanisms and increased responsiveness to stimuli. These changes may eventually lead to central nervous system changes (central sensitization) in susceptible patients, at which point pain complaints may no longer display a relationship with ocular surface pathology, but may be attributed to centralized mechanisms. Based on this idea, it is interesting that the evoked-pain subscore of the modified NPSI (i.e., allodynia and hyperalgesia) was the subscore most strongly associated with corneal detection and pain thresholds.
Recently, a large twin study suggested that chronic widespread pain, chronic pelvic pain, migraine, irritable bowel syndrome, and dry eye were heritable common chronic pain disorders with shared genetic factors that influence environmental responses.44 This finding suggests that nociceptive system dysfunction may be at least partially responsible for the dry eye symptoms reported by some patients. Furthermore, as in our patients, many patients with overlapping chronic pain disorders also have psychiatric comorbidities.45,46
As with all studies, our study has limitations, which must be considered when interpreting the study results. First, the study sample consisted of US veterans and may not be generalized to other populations. Specifically, veterans have a higher frequency of mental health disorders such as depression and PTSD compared to the general population. It is well established that depression, PTSD, and pain often coexist.47 For example, the Medical Outcomes Study reported that patients with depression reported greater bodily pain compared to those without depression.48 Individuals with PTSD may also have amplified emotional reactions and increased attention when faced with pain-related stimuli, which may lead to an increased sensitivity to painful stimuli.49 Given these potential confounders, future studies are needed to compare relationships among corneal thresholds, pain intensity ratings, and dry eye symptoms at both ocular and nonocular sites in different populations of patients. Second, all measurements were taken on one day, in the right eye only. Repeated measurements in both eyes and on different days may have given different results. Third, using other emerging techniques to evaluate tear film parameters, such as meniscometry, optical coherence tomography,50 and interferometry,51 may have led to more robust relationships between these signs and dry eye symptom report. Fourth, we used only the mechanical portion of the Belmonte aesthesiometer and did not test for chemical and thermal corneal thresholds. Therefore, we cannot generalize our findings to other submodalities of nociceptive system processing outside of mechanical pain.
Despite these limitations, this study is important as it is the first to report relationships between evoked corneal pain sensitivity and measures of ocular pain and dry eye symptom severity. Our findings therefore have potential implications for dry eye diagnosis and treatment. This suggests that, while important, the status of the ocular surface alone is not sufficient to understand dry eye and that corneal somatosensory function and psychologic status must be considered when evaluating a patient with dry eye. More work needs to be done to determine which noninvasive tests can differentiate dry eye patients based on ocular pain sensitivity and which treatment algorithms will be most appropriate in different dry eye patient subgroups.
Acknowledgments
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Denver, Colorado, United States, May 2015.
We thank Cornelis J. Rowaan, BSME; William G. Lee; Mariela C. Aguilar, MSBME; and Karam Alawa, BSBME, of the Ophthalmic Biophysics Center for providing engineering support and further improving the modified Belmonte aesthesiometer.
Supported financially and materially by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development's Career Development Award CDA-2-024-10S (AG), National Institutes of Health (NIH) Center Core Grant P30EY014801, a Research to Prevent Blindness Unrestricted Grant (institutional), the Henri and Flore Lesieur Foundation (JMP); and NIH NIDCR RO1 DE022903 (RCL). The sponsors or funding organizations had no role in the design or conduct of this research. The contents of this study do not represent the views of the Department of Veterans Affairs or the United States government.
Disclosure: O. Spierer, None; E.R. Felix, None; A.L. McClellan, None; J.M. Parel, None; A. Gonzalez, None; W.J. Feuer, None; C.D. Sarantopoulos, None; R.C. Levitt, None; K. Ehrmann, None; A. Galor, None
References
- 1. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5: 93–107. [DOI] [PubMed] [Google Scholar]
- 2. Pouyeh B,, Viteri E,, Feuer W,, et al. Impact of ocular surface symptoms on quality of life in a United States veterans affairs population. Am J Ophthalmol. 2012; 153: 1061–1066, e1063. [DOI] [PubMed] [Google Scholar]
- 3. Stonecipher K,, Perry HD,, Gross RH,, Kerney DL. The impact of topical cyclosporine A emulsion 0.05% on the outcomes of patients with keratoconjunctivitis sicca. Curr Med Res Opin. 2005; 21: 1057–1063. [DOI] [PubMed] [Google Scholar]
- 4. Galor A,, Feuer W,, Lee DJ,, Florez H,, Venincasa VD,, Perez VL. Ocular surface parameters in older male veterans. Invest Ophthalmol Vis Sci. 2013; 54: 1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galor A,, Felix ER,, Feuer W,, et al. Dry eye symptoms align more closely to non-ocular conditions than to tear film parameters. Br J Ophthalmol. 2015; 99: 1126–1129. [DOI] [PubMed] [Google Scholar]
- 6. Schein OD,, Tielsch JM,, Munoz B,, Bandeen-Roche K,, West S. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology. 1997; 104: 1395–1401. [DOI] [PubMed] [Google Scholar]
- 7. Vehof J,, Kozareva D,, Hysi PG,, et al. Relationship between dry eye symptoms and pain sensitivity. JAMA Ophthalmol. 2013; 131: 1304–1308. [DOI] [PubMed] [Google Scholar]
- 8. Galor A,, Levitt RC,, Felix ER,, Martin ER,, Sarantopoulos CD. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye. 2015; 29: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tuisku IS,, Konttinen YT,, Konttinen LM,, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjogren's syndrome. Exp Eye Res. 2008; 86: 879–885. [DOI] [PubMed] [Google Scholar]
- 10. Benitez-Del-Castillo JM,, Acosta MC,, Wassfi MA,, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007; 48: 173–181. [DOI] [PubMed] [Google Scholar]
- 11. Labbe A,, Alalwani H,, Van Went C,, Brasnu E,, Georgescu D,, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012; 53: 4926–4931. [DOI] [PubMed] [Google Scholar]
- 12. Labbe A,, Liang Q,, Wang Z,, et al. Corneal nerve structure and function in patients with non-Sjögren dry eye: clinical correlations. Invest Ophthalmol Vis Sci. 2013; 54: 5144–5150. [DOI] [PubMed] [Google Scholar]
- 13. Backonja MM,, Attal N,, Baron R,, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013; 154: 1807–1819. [DOI] [PubMed] [Google Scholar]
- 14. Hansson P. Neuropathic pain: clinical characteristics and diagnostic workup. Eur J Pain. 2002; 6 (suppl A): 47–50. [DOI] [PubMed] [Google Scholar]
- 15. Backonja MM,, Walk D,, Edwards RR,, et al. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009; 25: 641–647. [DOI] [PubMed] [Google Scholar]
- 16. Walk D,, Sehgal N,, Moeller-Bertram T,, et al. Quantitative sensory testing and mapping: a review of nonautomated quantitative methods for examination of the patient with neuropathic pain. Clin J Pain. 2009; 25: 632–640. [DOI] [PubMed] [Google Scholar]
- 17. Adatia FA,, Michaeli-Cohen A,, Naor J,, Caffery B,, Bookman A,, Slomovic A. Correlation between corneal sensitivity, subjective dry eye symptoms and corneal staining in Sjogren's syndrome. Can J Ophthalmol. 2004; 39: 767–771. [DOI] [PubMed] [Google Scholar]
- 18. Xu KP,, Yagi Y,, Tsubota K. Decrease in corneal sensitivity and change in tear function in dry eye. Cornea. 1996; 15: 235–239. [DOI] [PubMed] [Google Scholar]
- 19. Bourcier T,, Acosta MC,, Borderie V,, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005; 46: 2341–2345. [DOI] [PubMed] [Google Scholar]
- 20. De Paiva CS,, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004; 137: 109–115. [DOI] [PubMed] [Google Scholar]
- 21. Situ P,, Simpson TL,, Fonn D,, Jones LW. Conjunctival and corneal pneumatic sensitivity is associated with signs and symptoms of ocular dryness. Invest Ophthalmol Vis Sci. 2008; 49: 2971–2976. [DOI] [PubMed] [Google Scholar]
- 22. Chalmers RL,, Begley CG,, Caffery B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010; 33: 55–60. [DOI] [PubMed] [Google Scholar]
- 23. Schiffman RM,, Christianson MD,, Jacobsen G,, Hirsch JD,, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000; 118: 615–621. [DOI] [PubMed] [Google Scholar]
- 24. Grubbs JR,, Jr, Tolleson-Rinehart S,, Huynh K,, Davis RM. A review of quality of life measures in dry eye questionnaires. Cornea. 2014; 33: 215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010; 21: 310–316. [DOI] [PubMed] [Google Scholar]
- 26. Bouhassira D,, Attal N,, Fermanian J,, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004; 108: 248–257. [DOI] [PubMed] [Google Scholar]
- 27. Attal N,, Fermanian C,, Fermanian J,, Lanteri-Minet M,, Alchaar H,, Bouhassira D. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain. 2008; 138: 343–353. [DOI] [PubMed] [Google Scholar]
- 28. Crawford B,, Bouhassira D,, Wong A,, Dukes E. Conceptual adequacy of the neuropathic pain symptom inventory in six countries. Health Qual Life Outcomes. 2008; 6: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Freeman R,, Baron R,, Bouhassira D,, Cabrera J,, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain. 2014; 155: 367–376. [DOI] [PubMed] [Google Scholar]
- 30. Sommer C,, Richter H,, Rogausch JP,, Frettloh J,, Lungenhausen M,, Maier C. A modified score to identify and discriminate neuropathic pain: a study on the German version of the Neuropathic Pain Symptom Inventory (NPSI). BMC Neurol. 2011; 11: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gaylord KM,, Cooper DB,, Mercado JM,, Kennedy JE,, Yoder LH,, Holcomb JB. Incidence of posttraumatic stress disorder and mild traumatic brain injury in burned service members: preliminary report. J Trauma. 2008; 64 (2 suppl): S200–S205, discussion S205–S206. [DOI] [PubMed] [Google Scholar]
- 32. Bjornestad AG,, Schweinle A,, Elhai JD. Measuring secondary traumatic stress symptoms in military spouses with the posttraumatic stress disorder checklist military version. J Nerv Ment Dis. 2014; 202: 864–869. [DOI] [PubMed] [Google Scholar]
- 33. Sidebottom AC,, Harrison PA,, Godecker A,, Kim H. Validation of the Patient Health Questionnaire (PHQ)-9 for prenatal depression screening. Arch Womens Ment Health. 2012; 15: 367–374. [DOI] [PubMed] [Google Scholar]
- 34. Belmonte C,, Acosta MC,, Schmelz M,, Gallar J. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci. 1999; 40: 513–519. [PubMed] [Google Scholar]
- 35. Situ P,, Simpson TL,, Fonn D. Eccentric variation of corneal sensitivity to pneumatic stimulation at different temperatures and with CO2. Exp Eye Res. 2007; 85: 400–405. [DOI] [PubMed] [Google Scholar]
- 36. Teson M,, Calonge M,, Fernandez I,, Stern ME,, Gonzalez-Garcia MJ. Characterization by Belmonte's gas esthesiometer of mechanical chemical, and thermal corneal sensitivity thresholds in a normal population. Invest Ophthalmol Vis Sci. 2012; 53: 3154–3160. [DOI] [PubMed] [Google Scholar]
- 37. Rolke R,, Baron R,, Maier C,, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006; 123: 231–243. [DOI] [PubMed] [Google Scholar]
- 38. Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5: 108–152. [DOI] [PubMed] [Google Scholar]
- 39. Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998; 316: 1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Acosta MC,, Tan ME,, Belmonte C,, Gallar J. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 2001; 42: 2063–2067. [PubMed] [Google Scholar]
- 41. Gallar J,, Morales C,, Freire V,, Acosta MC,, Belmonte C,, Duran JA. Decreased corneal sensitivity and tear production in fibromyalgia. Invest Ophthalmol Vis Sci. 2009; 50: 4129–4134. [DOI] [PubMed] [Google Scholar]
- 42. Belmonte C. Eye dryness sensations after refractive surgery: impaired tear secretion or “phantom” cornea? J Refract Surg. 2007; 23: 598–602. [DOI] [PubMed] [Google Scholar]
- 43. Rosenthal P,, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012; 10: 2–14. [DOI] [PubMed] [Google Scholar]
- 44. Vehof J,, Zavos HM,, Lachance G,, Hammond CJ,, Williams FM. Shared genetic factors underlie chronic pain syndromes. Pain. 2014; 155: 1562–1568. [DOI] [PubMed] [Google Scholar]
- 45. Argoff CE. The coexistence of neuropathic pain sleep, and psychiatric disorders: a novel treatment approach. Clin J Pain. 2007; 23: 15–22. [DOI] [PubMed] [Google Scholar]
- 46. Gore M,, Brandenburg NA,, Dukes E,, Hoffman DL,, Tai KS,, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005; 30: 374–385. [DOI] [PubMed] [Google Scholar]
- 47. Bair MJ,, Robinson RL,, Katon W,, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003; 163: 2433–2445. [DOI] [PubMed] [Google Scholar]
- 48. Wells KB,, Stewart A,, Hays RD,, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. JAMA. 1989; 262: 914–919. [PubMed] [Google Scholar]
- 49. Bryant RA,, Marosszeky JE,, Crooks J,, Baguley IJ,, Gurka JA. Interaction of posttraumatic stress disorder and chronic pain following traumatic brain injury. J Head Trauma Rehabil. 1999; 14: 588–594. [DOI] [PubMed] [Google Scholar]
- 50. Wang J,, Cui L,, Shen M,, Perez VL,, Wang MR. Ultra-high resolution optical coherence tomography for monitoring tear meniscus volume in dry eye after topical cyclosporine treatment. Clin Ophthalmol. 2012; 6: 933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ban Y,, Ogawa Y,, Ibrahim OM,, et al. Morphologic evaluation of meibomian glands in chronic graft-versus-host disease using in vivo laser confocal microscopy. Mol Vis. 2011; 17: 2533–2543. [PMC free article] [PubMed] [Google Scholar]