SUMMARY
Herpes simplex virus 2 (HSV-2) is a DNA virus that is efficiently transmitted through intimate genital tract contact and causes persistent infection that cannot be eliminated. HSV-2 may cause frequent, symptomatic self-limited genital ulcers, but in most persons infection is subclinical. However, recent studies have demonstrated that the virus is frequently shed from genital surfaces even in the absence of signs or symptoms of clinical disease and that the virus can be transmitted during these periods of shedding. Furthermore, HSV-2 shedding is detected throughout the genital tract and may be associated with genital tract inflammation, which likely contributes to increased risk of HIV acquisition. This review focuses on HSV diagnostics, as well as what we have learned about the importance of frequent genital HSV shedding for (i) HSV transmission and (ii) genital tract inflammation, as well as (iii) the impact of HSV-2 infection on HIV acquisition and transmission. We conclude with discussion of future areas of research to push the field forward.
INTRODUCTION
Herpes simplex virus 2 (HSV-2) is the leading cause of genital ulcer disease, increases risk of HIV acquisition, and causes neonatal herpes, a rare infection associated with long-term neurologic impairment and high mortality rates. HSV-2 is an alphaherpesvirus in the herpesvirus family of DNA viruses, all of which cause chronic, incurable infection. HSV-2 is transmitted to a sexual partner during sexual contact or during labor and delivery to the neonate through direct mucosal or skin contact. During primary infection, HSV-2 infects epithelial cells and then nerve endings, followed by retrograde axonal transport to establish persistent infection in the sacral ganglia. During reactivation, the virus travels down the axon to skin and mucosal surfaces, where viral shedding may be associated with genital ulcers or, more commonly, is asymptomatic. While HSV-2 infection has in the past been considered to be a “latent” infection with infrequent reactivation, carefully designed natural history studies have shown that there is dynamic interaction between HSV-2, which reactivates frequently and is “shed” on mucosal surfaces, and the mucosal immune response. This review discusses what is known about genital HSV-2 shedding and how HSV-2 shedding may be used to understand genital tract inflammation as well as how it may contribute to increased risk of HIV acquisition.
EPIDEMIOLOGY
As of 2012, HSV-2 was estimated to infect 417 million people worldwide between the ages of 15 to 49 years, giving a global prevalence of 11.3%, with 19.2 million incident infections each year (1). The seroprevalence varies widely depending upon region of the world, from 10% to 70% in women attending antenatal care clinics (2), with the greatest burden borne in Africa (Fig. 1) (1). Worldwide, HSV-2 seroprevalence is nearly 2-fold higher in women than in men (14.8% versus 8% global prevalence, respectively) (1). Sexual exposure is the major risk factor, with higher HSV-2 seroprevalence associated with increasing age and increasing number of lifetime sexual partners (3).
FIG 1.
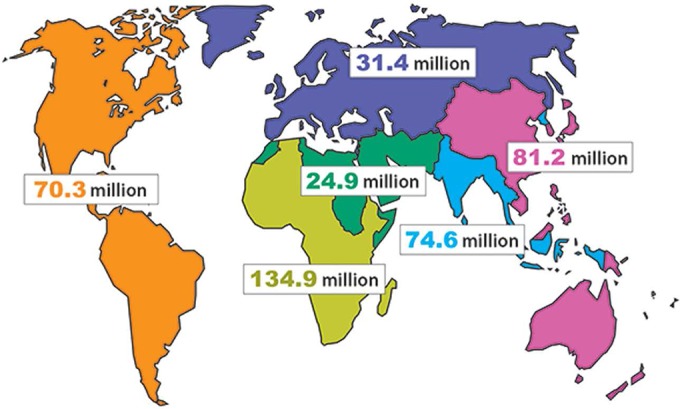
Estimates of the number of people with prevalent HSV-2 infection in 2012, by age, sex, and region. (Adapted from reference 1.)
HSV-2 Epidemiology by Geographic Region
In the most recent global estimates, overall HSV-2 seroprevalence was <10% among persons living in the European, Eastern Mediterranean, Southeast Asian, and Western Pacific regions. The seroprevalence was estimated to be nearly 15% in the Americas and was highest in Africa, at over 30% (1). Another global seroprevalence study estimated HSV-2 seroprevalence in women age 40 to 44; these estimates were 10 to 20% in the East Asian, Pacific, Middle Eastern, North African, and Western European regions, 22.3% in Australia and New Zealand, 29.5% in North America, 39% in South America, and 69% in sub-Saharan Africa (4). In sub-Saharan Africa, HSV-2 is rapidly acquired among HSV-2-seronegative female sex workers, with incidence rates up to 23 per 100-person years (5, 6).
In the United States, seroprevalence has declined in 14- to 49-year-olds in recent years, from a high of 21.2% in 1988 to 1994 to 15.5% in the most recent 2007 to 2010 National Health and Nutrition Examination Survey (NHANES), a population-based estimate of U.S. adults (7). HSV-2 seroprevalence was also found to have declined by 4.8% per year among pregnant women in Seattle for the period 1989 to 2010 (8). The declining seroprevalence has been seen mostly in white persons, with stable rates in black populations, resulting in worsening racial disparities such that for every one white man, four black men are infected, with similar ratios for women (7). Disparities have also been noted in black young men who have sex with men (MSM) (9) and pregnant women (8). In addition, high seroprevalence, up to 50% of persons tested, has been noted in urban populations screened in the emergency department (10).
Clinical Manifestations of Genital HSV Infection
HSV-2 is the leading cause of genital ulcer disease (GUD) in the United States and throughout the world. Multiple studies conducted in the United States, Africa, and Asia using sensitive PCR assays have shown that HSV is found in 60% of genital ulcers (11–15). During primary infection, patients may experience multiple, severe bilateral genital ulcers, in addition to local genital symptoms, including dysuria, cervicitis, and inguinal lymphadenopathy (16). In one study, one-third of cases of proctitis were caused by HSV in MSM, with higher incidence in persons with HIV infection (17). Systemic manifestations such as fever, myalgias, headaches, and symptoms of aseptic meningitis may also accompany primary genital HSV. In the absence of antiviral therapy, lesions from first-episode genital herpes resolve within 3 weeks. During the initial infection, HSV establishes infection in the sacral ganglion, where it is protected from clearance by the host immune response. The sacral ganglion acts as a reservoir for future recurrences and subclinical genital shedding.
Periodic recurrences of GUD are a hallmark of genital HSV infection, with a median of 5 recurrences in the first year after primary infection with genital HSV-2 and a median of 1 recurrence after genital HSV-1 (18). After the first year of infection, the recurrence rate for HSV-2 decreases by a median of 2 recurrences per year. However, recurrence rates may be highly variable, with some persons having no outbreaks and others having ≥9 outbreaks per year (19). Importantly, most people also shed HSV-2 asymptomatically: the frequency of asymptomatic shedding is markedly higher than the symptomatic occurrences described above. Thus, true reactivation and potential exposure of sexual partners to HSV-2 are much more frequent than exposure to clinically apparent genital lesions. Recurrences may be accompanied by a prodrome due to the virus traveling down the nerve, with symptoms including tingling, burning, and itching. Recurrences are less severe than first-episode genital herpes and often are unilateral, are without systemic symptoms, and resolve within 3 to 5 days without antiviral therapy (16). Although GUD is the classic clinical manifestation of genital herpes, patients may have atypical or misdiagnosed symptoms such as itching or fissures, and the infection may be confused with dermatitis or Candida infections. HSV recurrences may occur anywhere in the area of sacral innervation, including the rectum, buttocks, and thigh (20). Recurrences occur at these locations at a frequency similar to that for genital lesions (20).
Genital HSV-1 Infection in High-Income Countries
Genital HSV-1 is now the leading cause of first-episode genital herpes in high-income countries, particularly in women and MSM less than 25 years of age (21). While HSV-1 is acquired nearly universally during childhood in low- and middle-income countries, in high-income countries HSV-1 seroprevalence is declining, particularly in adolescents. For instance, in the United States, the seroprevalence of HSV-1 has dropped 29% among 14- to 19-year-olds, from 42.6% to 30.1%, over the past 30 years (22). As a result of the declining seroprevalence, adolescents and young adults may experience their first exposure to HSV-1 with initiation of sexual activity. Risk factors for genital HSV-1 infection include both oral and vaginal sex (23). Genital HSV-1 recurrences are less frequent than genital HSV-2 recurrences, with a recurrence rate of 1.3/year in the first year after infection and 0.7/year in the second year of infection (24, 25).
DIAGNOSTICS
Viral Diagnostic Assays
For patients presenting with GUD, swabs should be obtained from the genital ulcer for HSV culture or PCR, both of which can be programmed to be type specific. If vesicles or pustules are present, they should be unroofed and the base of the ulcer swabbed to obtain adequate cells for viral detection. Viral culture is widely available and relatively inexpensive; however, sensitivity of viral culture can be 4-fold lower than that of HSV PCR, particularly when low quantities of virus are present (26). Multiple HSV PCR assays are available, several of which detect genes such as that for glycoprotein B (27). Commercially available, FDA-approved PCR assays have been developed for detection of HSV, and it is hoped that these assays will be less expensive and more widely available for patient care (28, 29). Direct fluorescence antigen staining is not recommended due to low sensitivity.
Serology
The ability to define the epidemiology and natural history of genital HSV infection has been made possible by the availability of type-specific IgG antibody assays, which allow for differentiation between HSV-1 and HSV-2 infections. These assays were first developed in the early 1970s and have been refined over time (30). Type-specific immunoblots which detect antibodies to glycoprotein G are still used in the NHANES survey (31), and the University of Washington (UW) Western blot, which detects antibodies to multiple HSV-1 and HSV-2 proteins, is a gold standard assay against which commercial type-specific serologic assays are compared (32). Testing for IgM antibodies is not recommended due to low sensitivity and specificity (33).
Several commercially available type-specific serologic assays that detect IgG antibody to glycoprotein G2 are now available. While these tests perform well against the UW Western blot with high sensitivity and specificity (34), in clinical practice they are limited by false positives, particularly in persons testing positive for HSV-2 and HSV-1 infection and in low-prevalence populations where the positive predictive value is low. For the HerpeSelect test, false-positive results occur most frequently at low index values (1.1 to 3.5), particularly when cross-reactive HSV-1 antibodies are present (35). Specificity at low index values may be improved by following a highly sensitive test, such as the HerpeSelect assay, with a highly specific test, such as the Biokit assay (36). This testing strategy has 99.1% sensitivity and specificity compared to the UW Western blot (36). For HerpeSelect results with low index values (1.1 to 3.5), the CDC now recommends testing with the Biokit or UW Western blot to confirm results (33). Newer point-of-care tests such as the UniGold HSV-2 rapid test have also performed with high sensitivity and specificity in American adults (38).
The Kalon HSV-2 type-specific enzyme-linked immunosorbent assay (ELISA), used in the United Kingdom and Europe (not FDA approved in the United States), has lower sensitivity during first-episode infection but higher specificity than HerpeSelect (39). In low- and high-risk Asian women (40, 41) and African populations (42–44), the Kalon HSV-2 ELISA had greater concordance with UW Western blot results and higher specificity than the HerpeSelect and Biokit assays. Raising the index value cutoff for HerpeSelect improves specificity in international settings as well (43–46). In a systematic review and meta-analysis of HSV testing in sub-Saharan Africa, the specificity of HerpeSelect was found to be significantly lower in persons with HIV infection (44). Other serologic assays, such as the Euroline Western blot, are also limited by low specificity, particularly in African populations (47).
Serologic screening may be helpful both in patients presenting with first-episode genital herpes to determine whether they have recently acquired the virus and in patients presenting without current symptoms of genital herpes, especially given that 80% of persons infected with genital HSV-2 are not aware that they have the infection (3). Due in part to these issues with the specificity of commercially available screening assays, as well as concerns about psychosocial harm and perceived lack of interventions, the U.S. Preventive Services Task Force and the American College of Obstetrics and Gynecology do not recommend routine screening of the general population or pregnant women for genital herpes (48, 49). The 2015 CDC guidelines suggest that testing may be considered in those persons presenting for sexually transmitted infection (STI) screening and/or in those who have high-risk sexual behavior (33), as well as persons with HIV infection and MSM at increased risk for HIV acquisition. Type-specific serologic testing is recommended for recurrent genital symptoms or atypical symptoms without virologic confirmation and for those with a clinical diagnosis of genital herpes who have not had laboratory confirmation, as well as those whose partner has genital herpes (33).
TREATMENT OF GENITAL HERPES
Despite the explosion of new therapeutics for other viral pathogens such as HIV and hepatitis C virus, FDA-approved drugs for treatment of genital herpes have been unchanged for nearly 2 decades. Acyclovir is a guanosine analogue that is monophosphorylated by the HSV-encoded thymidine kinase, with the second and third phosphate groups added by cellular kinases (50, 51). The triphosphorylated nucleotide selectively inhibits the viral DNA polymerase and is incorporated in the growing viral DNA chain, causing chain termination. The drug becomes active only in virus-infected cells, which contributes to its excellent safety profile. Acyclovir is available in both oral and intravenous (i.v.) formulations. Valacyclovir is the valine ester prodrug of acyclovir, which has higher oral bioavailability and requires less frequent dosing (reviewed in reference 52). Famciclovir is a prodrug of penciclovir and similarly is highly bioavailable (reviewed in reference 53).
There are two strategies for treatment of genital herpes infections: episodic treatment or suppressive therapy. Episodic treatment is used only when patients are symptomatic with a genital herpes recurrence and is given for 2 to 10 days, depending on the drug regimen and whether it is being used for a first episode or a recurrent episode of genital herpes (33). There are a number of different dosing regimens recommended for episodic treatment (33). Episodic therapy decreases the length of recurrences and decreases symptoms. For treatment of episodic disease, acyclovir, valacyclovir, and famciclovir are all preferred agents with similar efficacy (33). Suppressive regimens are taken daily, and they decrease the risk of recurrences by ∼50% and improve quality of life for persons with recurrent genital herpes (54, 55). As discussed below, suppressive therapy also reduces genital shedding and reduces the risk of genital herpes transmission among discordant heterosexual couples (56). There are some data that valacyclovir offers better suppression of genital recurrences and subclinical shedding than famciclovir (57).
Two helicase-primase inhibitors, ASP2151 and pritelivir, have entered clinical trials. Testing of these drugs is significant, because they have a novel mechanism of action, different than that of the nucleotide analogues. ASP2151 was tested for episodic therapy in a dose-ranging phase II trial, and several doses tested trended toward a 1-day decrease in time to healing (58). The second agent, pritelivir, was tested for suppression of genital herpesvirus shedding in dose-ranging phase II trials (59). The best-performing dose, 75 mg daily, showed a 87% reduction in genital herpesvirus shedding and also reduced genital lesions by a similar magnitude (59). Pritelivir has an extended half-life (>5 days), providing prolonged concentrations, which is a potential advantage for interrupting viral shedding. This promising agent is, however, currently on an FDA hold due to recently reported toxicity in animal models.
Acyclovir-resistant herpes is very rare in immunocompetent populations but can cause severe infections in immunocompromised hosts. The options for treatment of acyclovir-resistant herpes are limited to the nephrotoxic agent foscarnet (60), continuous-infusion acyclovir (61), or less-validated topical therapies such as 5% imiquimod cream (62) or i.v. or topical cidofovir (63, 64).
In studies of HIV prevention, tenofovir vaginal gel and oral tenofovir-emtricitabine and tenofovir unexpectedly decreased the risk of HSV-2 acquisition by 30 to 50% in African heterosexual populations, although similar protection was not seen in trials conducted with North American MSM (65–67). This finding led to interest in studying tenofovir for reduction in genital lesions and shedding in HSV-2-infected persons. However, in a 3-arm randomized trial, tenofovir gel did not decrease the risk of HSV-2 shedding or lesions in HSV-2-infected persons, and oral tenofovir had only a modest effect on HSV shedding and lesions in the per-protocol analysis (68). Genital HSV shedding is not decreased in persons infected with HIV/HSV-2 who are receiving tenofovir compared to those not receiving tenofovir (69). Therefore, the effect of tenofovir on HSV-2 appears to be isolated to prevention of acquisition through an undefined mechanism, and it does not appear to be virologically active at studied doses against HSV-2 in those already infected.
GENITAL HSV SHEDDING
Historically, HSV has been conceptualized to be in either the “latent” or “lytic” state. Most of the time was thought to be spent in the latent state, characterized by a restricted gene expression/protein expression profile, in the sacral ganglion. Reactivation to lytic replication, when HSV would travel down sensory nerves to genital mucosal or epithelial cells and replicate, resulting in genital ulcer disease, was thought to be a rare event. However, recent studies have shown that the virus reactivates much more frequently than previously understood and is often detected, or “shed” from skin and mucosal surfaces, in the absence of symptoms (70, 72, 74). These data suggest that viral reactivation occurs more often than previously thought. Importantly, transmission to sexual partners and neonates can often occur during these periods of subclinical shedding.
Lesional and Subclinical Shedding
The initial studies of genital HSV shedding used viral culture to detect HSV-2 from genital ulcers. In the absence of antiviral therapy, HSV can be cultured from genital lesions for a mean of 11 to 12 days during primary genital herpes and ∼4 days during genital herpes recurrences (16). The first study that characterized the frequency of and symptoms associated with genital HSV shedding was performed by Wald and colleagues, in which women collected daily viral cultures from the cervix, vulva, and rectum for a median of 105 days and kept a diary detailing daily symptoms (70). This landmark study revealed that shedding occurred in the absence of symptoms on 2% of the days in women with HSV-2 and accounted for 32% of days with shedding and that it occurred more frequently in the 7 days prior to or following a symptomatic episode. In addition, the duration of shedding occurrences was shorter during asymptomatic than during lesional episodes, and shedding rate was associated with infection severity and number of recurrences. Importantly, this study also established that daily cultures collected at home by participants were highly reliable, with 97.4% concordance between participant- and clinician-collected samples, which paved the way for future participant-collected genital sampling studies. The development of a real-time PCR assay for HSV brought 4-fold-greater sensitivity for detection of HSV on mucosal surfaces compared to culture, particularly at low copy numbers of HSV DNA, and this is the method currently used to detect genital shedding (26, 73).
Symptomatic versus Asymptomatic Infection
Over 80% of HSV-2-seropositive persons in the United States are not aware that they are infected with HSV-2 (3). A cohort of participants with asymptomatic HSV-2 infection were enrolled in a genital swabbing study to characterize subclinical genital shedding patterns in this population. Fifty-three participants who were HSV-2 seropositive but did not have a history of symptomatic genital HSV-2 infection collected daily genital swabs for HSV culture and had HSV isolated on 3.8% of days (74). These data have been refined with use of HSV PCR, which showed that among 498 persons with HSV-2 infection, 410 persons who were symptomatic had a mean shedding rate of 20% of days, and persons with asymptomatic infection had a mean shedding rate of 10% of days. Although the subclinical shedding rate was higher in persons with symptomatic disease (13.1% of days, versus 8.8% of days in those with asymptomatic infection), the quantities of virus shed during subclinical shedding episodes were similar (75).
Genital HSV-1 shedding is much less frequent than that for HSV-2, occurring on a mean of 0.5% of days, although this was studied in a small number of women using the less sensitive viral culturing detection method (70). Longitudinal shedding data measured with PCR are lacking for genital HSV-1.
Risk Factors for Genital HSV-2 Shedding
Through the intensive study of large cohorts of HSV-2-seropositive persons, risk factors for both overall shedding and subclinical shedding have emerged (75) (Table 1). Significant risk factors in a multivariate model include a history of symptomatic infection (2-fold-increased risk) and having ≥8 recurrences (1.6-fold-increased risk of shedding compared to having 1 to 7 recurrences) per year. In addition, Caucasians had higher shedding rates than other races (75). These same risk factors were associated with subclinical genital shedding (75). Importantly, several factors were not associated with genital shedding rate, including sex, sexual orientation, HSV-1 serostatus, or time of menstrual cycle (75, 76).
TABLE 1.
Significant risk factors for symptomatic and asymptomatic genital HSV sheddinga
| Risk factor | Overall shedding |
Subclinical Shedding |
||
|---|---|---|---|---|
| aRR | P value | aRR | P value | |
| History of asymptomatic infection | 0.5 | <0.001 | 0.72 | 0.06 |
| Caucasian | 1.74 | <0.001 | 1.45 | 0.03 |
| ≥8 recurrences/yr | 1.61 | <0.001 | 1.46 | 0.02 |
Based on data from reference 75. aRR, adjusted risk ratio.
Importance of Shedding: Transmission
These data demonstrate that HSV-2-seropositive persons both with and without a history of symptomatic genital infection reactivate the virus frequently, which places them at risk for transmitting it to sexual partners. Prospective studies of genital HSV-2 transmission among discordant couples demonstrate the importance of subclinical shedding in transmission events. In one study of 144 discordant couples, transmission occurred in 14 couples; in 9 couples, the source partner was asymptomatic at the time of transmission, and the remaining source partners had prodrome or lesions first noted near the time of sexual activity (77). In another prospective study of HSV-2-seronegative persons who were enrolled in a prophylactic gD2/gB2 subunit HSV-2 vaccine trial, 155 persons acquired HSV-2, and of these, only 57 (37%) were symptomatic (78). The prophylactic vaccine was not effective at preventing HSV-2 disease and therefore did not appear to impact symptomatic acquisition of infection (79). These data demonstrate that HSV-2 is both transmitted and acquired without symptoms in most persons.
Subclinical genital HSV shedding at the time of labor and delivery may also lead to transmission to the neonate, resulting in neonatal herpes. In a large cohort of 40,023 women who had genital HSV cultures collected at the time of labor and delivery, 202 had positive cultures (80). Of these, only 56 (28%) had lesions at the time of delivery; the remainder were shedding subclinically. Of the 10 cases of neonatal herpes seen in this cohort, all were acquired from women who had subclinical shedding. In another study of 15,923 asymptomatic pregnant women, 57 (0.4%) had HSV shedding by genital culture (81).
It is not known whether there is a threshold quantity of HSV DNA present in genital secretions which is associated with transmission. Anecdotal transmission from mother to infant has been reported with HSV culture-negative, PCR-positive genital and cervical secretions, although these collections are often hours from the infant's exposure at delivery (82). Monitoring of genital swabs for HSV DNA at the time of birth in asymptomatic women is not recommended. Mathematical models of transmission suggest that there may be a virologic threshold for sexual transmission, with infrequent transmission predicted at <4 log10 copies/ml HSV DNA from genital swabs and with most transmissions occurring with ≥6 log10 copies of virus (83).
Persistence of Genital HSV Shedding
Genital HSV shedding occurs at the highest rate in the first year after HSV-2 infection and then declines. In one study of 377 persons who had daily self-collected genital swabbing sessions for a median of 64 days, the shedding rate declined significantly between the first year and subsequent years of infection, from 26% to 13% (84). However, even in persons who had been HSV-2 infected for 2 decades, the median shedding rate remained relatively stable, with shedding detected on 11% of days (84).
Kinetics of Genital HSV Shedding
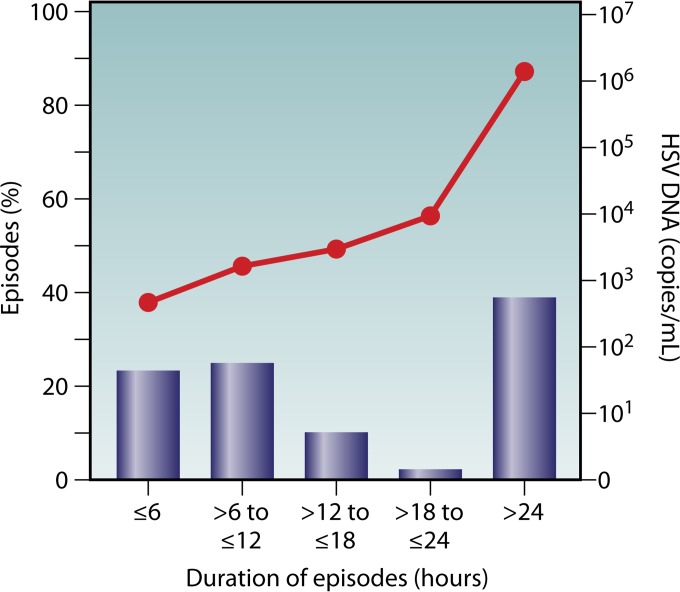
Detailed mathematical modeling has also provided new insight into the kinetics of genital HSV shedding. To obtain shedding rates at the levels seen in natural history studies, mathematical models predict that small quantities of HSV are released from neurons into the genital tract daily, supporting the hypothesis that the neurons act as a reservoir and that the viral infection is dynamic, with frequent reactivation (85). Mathematical modeling also suggested that shedding episodes may be shorter than predicted by once-daily swabbing and that some shedding episodes may be overlapping, reflecting multiple short bursts of reactivation in different anatomic locations (86). This hypothesis led to a swabbing study that used a more intensive sampling frequency, with participants collecting oral and genital swabs four times daily (∼every 6 h while awake) (72). The results revealed that the median shedding episode duration was 13 h, and 60% of shedding episodes were less than 24 h long (72) (Fig. 2). In addition, intensive sampling revealed 3-fold more reactivations of HSV-2 (median, 18 reactivations) per year than with once-daily sampling (median, 6/year). Shedding was not associated with a particular time of day. Modeling studies using data generated from these shedding studies suggest that virus is released from the ganglion in a near-constant stream of small quantities of virus (85). Based on these data, we hypothesize that a robust immune local tissue response must be present and primed to contain these short bursts of genital shedding. These hypotheses are also supported by models showing that an increased quantity of immune cells in the mucosa is associated with decreased shedding duration and severity (88). How much HSV is shed is also an important component of transmission and shedding dynamics. The quantity of virus shed is positively associated with the length of a shedding episode (72).
FIG 2.
The majority of shedding episodes last less than 24 h. HSV DNA was quantified by PCR from self-collected genital swabs taken by a cohort of participants every 6 h for 60 days. Overall, the duration of a shedding episode is associated with viral titer. (Adapted from reference 72 with permission of Oxford University Press.)
Suppression of Genital HSV Shedding
Antiviral therapy, including acyclovir, valacyclovir, and famciclovir, decreases clinical and subclinical genital shedding by 70 to 80% (57, 89, 90), the quantity of virus shed, and the risk of transmission to sexual partners by nearly 50% (91). However, even high-dose antiviral therapy does not eliminate viral shedding, with short episodes of breakthrough shedding occurring despite doses of valacyclovir of 1 g three times daily (92). This suggests that novel interventions that can more potently suppress genital HSV shedding may be more effective for prevention of sexual transmission.
The improved understanding of the frequency of genital HSV shedding has led to its use as a sensitive indicator of disease severity and efficacy for clinical trials, particularly for phase I/II studies of novel antiviral agents and therapeutic vaccines (59, 93).
Anatomic Patterns of Genital HSV Shedding
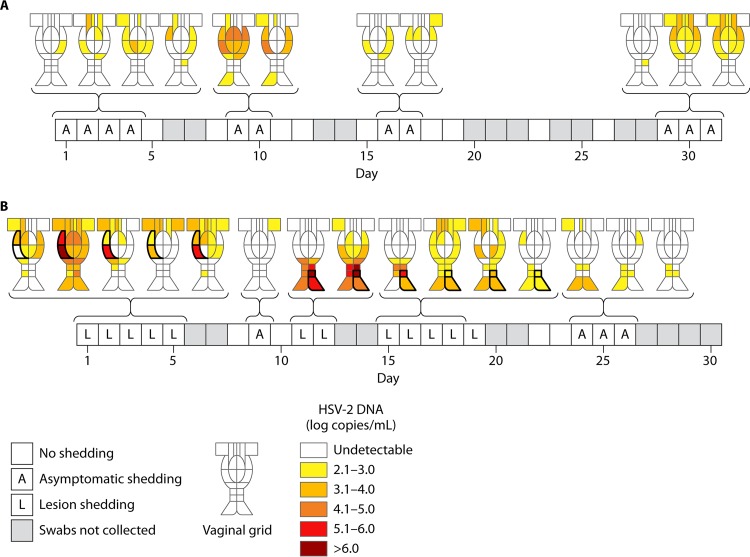
Genital HSV shedding may occur throughout the area innervated by the sacral ganglia. A detailed analysis of anatomic patterns of shedding revealed that shedding may be detected at single small anatomic sites with low quantities of virus or may be detected throughout the genital tract in widespread flashes of shedding, particularly in the setting of genital lesions or with high quantities of virus shed (Fig. 3) (94, 95). These findings suggest that HSV infection involves the entire sacral ganglion and is not limited to only sites where lesions may occur. In addition, these findings provide additional understanding of the limited efficacy of condoms in prevention of genital HSV acquisition (96). Spatially based mathematical models have supported the precise localization of genital HSV shedding (97).
FIG 3.
Anatomic distribution of genital shedding in 2 women who had 22 genital samples collected over a 30-day period. The quantity of virus shed in each region is shown according to the heat map. (A) Widespread genital shedding in a woman with asymptomatic shedding. (B) Diffuse shedding in a woman with a symptomatic genital lesion on days 1 to 5, days 11 and 12, and days 15 to 19. The site of the lesion is highlighted in black. (Adapted from reference 94.)
Summary of Genital HSV Shedding
Taken together, the intensive characterizations of the timing and localization of both symptomatic and subclinical genital HSV shedding have demonstrated that viral reactivation is frequent and widely distributed throughout the genital tract and that subclinical shedding can lead to viral transmission. These studies have refined our longstanding view of the virus as “latent” with infrequent rounds of reactivation to that of a much more dynamic virus for which reactivation occurs frequently and “skirmishes” between the virus and host likely occur daily. Recent data have shown that the host can contain reactivation at the site of viral release in the genital skin (98).
GENITAL HSV-2 INFECTION AND HIV INFECTION
From a public health standpoint, the most important consequence of genital HSV-2 infection is the marked contribution to the spread of HIV infection. Genital HSV infection has long been identified as a risk factor in HIV acquisition (99). Meta-analyses of risk factors for HIV infection have estimated that prevalent HSV-2 infection increases the risk of HIV acquisition 2- to 3-fold (100, 101). Incident HSV-2 infection is associated with a 5- to 7-fold-increased risk of HIV acquisition, with some studies estimating 16-fold-increased risk in men (102–104). The biological basis of increased susceptibility is due to both increased ulceration and increased inflammation present in the skin and mucosa of persons who are HSV-2 infected. Increased numbers of activated CD4+ T cells are seen in cervical secretions from HSV-2-seropositive women (105, 106), and increased inflammation is seen in foreskins of HSV-2-seropositive men, compared to those who are HSV-2 seronegative (107, 108). Importantly, this is seen in the absence of genital lesions, suggesting that HSV-2 infection, likely through the immune response to subclinical shedding, fundamentally alters the inflammatory infiltrate to the genital skin and mucosa. In addition, the immune response to genital lesions is associated with infiltration of CD4+ T cells bearing the HIV coreceptors CCR5 and CXCR4 to the epithelium; these cells are more susceptible to HIV infection ex vivo than control skin biopsy specimens (109). Furthermore, these cells persist in the epithelium for weeks after a lesion, even in the presence of antiviral therapy (109). The impact of genital HSV-1 infection on the risk of HIV acquisition is not known (110).
HSV-2 Infection in HIV-Infected Individuals
In HIV-infected individuals, the presence of GUD is associated with an increased risk of HIV transmission (111). HIV is shed from HSV-2 ulcers in HIV-infected persons (112) and is more frequently shed from the genital tract, at higher quantities, during GUD and in women with more frequent HSV-2 shedding (113), which likely contributes to increased risk of transmission. While some studies have shown that HSV-2 infection is associated with increased plasma HIV RNA levels and that HIV RNA increases during HSV-2 recurrences (114), others have shown no association between HSV-2 shedding (115) or seropositivity (116) and HIV plasma levels and CD4+ T cell trajectories.
The clinical manifestations of HSV-2 in HIV-infected individuals may be similar to those in HIV-seronegative persons. However, HIV-infected persons are also at increased risk of prolonged genital ulcerations (117), acyclovir resistance (118), and atypical presentations, such as hypertrophic lesions (119). The risk of these complications increases with declining CD4 count. HSV-2-seropositive persons with CD4 counts of <250 cells/ml have an increased risk of GUD and HSV-2 shedding in the first 6 months after starting antiretroviral therapy (ART), but the risk returns to baseline after this period (120). Suppressive anti-HSV therapy reduces the risk of both HSV-2 shedding and GUD after initiation of ART (120). Other studies have shown that there is decreased risk of GUD associated with ART, but the overall HSV shedding rate is not significantly different between persons on and off ART (121).
Studies Designed To Disrupt HSV/HIV Interactions
Several randomized controlled clinical trials with HSV-2-seropositive persons have been performed to disrupt the synergy between HIV and HSV-2 through the use of suppressive anti-HSV therapy, such as acyclovir. Suppressive acyclovir has been shown to decrease HIV load and disease progression in HIV-infected persons not on antiretroviral therapy (122–124). High-dose valacyclovir decreases plasma HIV RNA levels by 0.2 to 0.6 log10 copies/ml more than standard-dose acyclovir in HIV-infected persons not on antiretroviral therapy (125, 126). As acyclovir has been found to have activity against HIV (127–129), it is not clear whether this decrease is mediated by a direct antiviral effect on HIV replication or by an indirect effect through HSV suppression leading to decreased inflammation or leading to decreased HSV-mediated HIV replication (130). However, given the increasing availability of antiretroviral therapy and the demonstrated efficacy in preventing disease progression even among persons with CD4 counts of >500 cells/mm3 (131) and in preventing HIV transmission (132), ART is strongly preferred over HSV suppression.
The Partners in Prevention study was designed to study whether suppressive acyclovir could prevent HIV transmission among HIV-discordant couples. In this trial, the HIV-1-seropositive/HSV-2-seropositive partner was randomized to suppressive acyclovir or placebo. Suppressive acyclovir was not effective in preventing HIV transmission (133). Well-executed studies conducted with HSV-2-seropositive, HIV-1-seronegative persons did not demonstrate a benefit of suppressive acyclovir over placebo for reduction of HIV acquisition (134, 135). These data suggest that more potent therapies or HSV-2 prevention strategies, such as a vaccine, will be needed to disrupt the HSV/HIV interaction. The mechanism of this failure of suppressive acyclovir involves the persistence of the inflammatory response to HSV-2 despite current antivirals. Short reactivations of HSV-2 still occur on suppressive HSV-2 therapy, even at much higher doses than used in the clinical practice (92). These reactivations are associated with a persistent inflammatory response of both CD4+ T cells and associated dendritic cells, which increases the likelihood of HIV-1 acquisition (109).
HSV-2 VACCINES
Due to the widespread prevalence of HSV-2, the lack of highly effective prevention strategies, and the influence of HSV-2 infection on the HIV epidemic, HSV-2 vaccines are a global health priority (137). A comprehensive review of HSV-2 vaccines has been recently performed and is beyond the scope of this article (138). Briefly, prophylactic vaccines have been extensively tested, primarily with glycoprotein subunit vaccines (79, 139). While these have been effective in animal models and have induced neutralizing antibodies, they have failed to show efficacy for HSV-2 prevention in phase III clinical trials. The most recent prophylactic vaccine study, “Herpevac,” enrolled over 8,000 HSV-1/HSV-2-seronegative women with a primary endpoint of HSV genital disease (140). The vaccine did not prevent HSV-2 disease or acquisition but did show moderate efficacy in prevention of both HSV-1 infection and disease (141). Protection against HSV-1 acquisition was associated with increasing titers of antibody to gD2, which is the first correlate of protection identified for HSV infection (140). As this vaccine did not prevent HSV-2, it is unlikely to be further pursued. There is currently a phase I study of a live, replication-deficient vaccine, HSV529, which will be tested in HSV-2-naive populations, and there are many other promising prophylactic candidates in preclinical studies.
There is also active research into developing a therapeutic HSV vaccine. These vaccines would be given to persons with HSV-2 infection to reduce HSV-2 genital recurrences, shedding, and potentially transmission. Thus far, two candidates have met their endpoints in recent phase II studies. GEN-003, a subunit vaccine containing gD2 and ICP4 with a novel matrix M adjuvant to stimulate T cell immunity, reduced shedding by 55% (142) in a phase IIB trial. The HerpV vaccine, a polypeptide vaccine comprised of 32 HSV antigens complexed with human heat shock protein 70 and adjuvanted with QS-21, reduced shedding by 15% (143). A DNA vaccine, consisting of gD2 and UL46 and adjuvanted with Vaxfectin, failed to meet its endpoint of reduction in shedding in a phase I/II trial (144). Several other vaccine candidates with diverse platforms are entering phase I/II trials.
MOLECULAR EPIDEMIOLOGY OF HSV-2
One of the emerging concepts in HSV virology is the recognition of widespread genomic heterogeneity, heterogeneity that may play a role in both response to therapy and potential immune protection. To date, few full-length HSV-2 genomes have been sequenced (145, 146, 153, 154). However, with the decreasing cost and increasing efficiency of next-generation sequencing, this number is expected to increase exponentially in the near future. Although a much larger number of HSV-1 genomes have been sequenced from clinical isolates, none of these have originated from genital sites (147, 148). Recent data suggesting that HSV-2 may have evolved from a cross-species transmission event from chimpanzees rather than from HSV-1 have already challenged our assumptions about the evolution of these ancient infections (149).
Limited sequencing of glycoprotein genes from 47 clinical isolates from Tanzania and Scandinavia has suggested greater genetic variability in Tanzania. This is based on finding two divergent genogroups, one which has been identified only in Tanzania and another which is found in both locations (150). In addition, that report suggested frequent homologous recombination events. The best evidence for geographic variability comes from Dudek et al. (151), who demonstrated that dl529, a live attenuated vaccine candidate, required a 5-fold-higher dose for 50% protection against SD90, a strain from South Africa that had undergone minimal passages in the laboratory, than for laboratory strain G in a mouse challenge model (151). They also showed that a vaccine based on a U.S.-derived strain protected significantly better against U.S. strains and that a vaccine based on SD90 protected better against African strains than against U.S. strains, suggesting that there are important differences in epitopes between some genogroups that mediate protection (151). SD90 has subsequently undergone full-length genomic sequencing and has more than 100 nucleotide differences compared to HG52 (an attenuated laboratory strain), although overall conservation was greater than 99% in the unique long and unique short regions of the genome (146). Another variant has been identified in 4 HIV-infected patients from West Africa, characterized by a 27-nucleotide substitution in the UL30 gene (DNA polymerase) and increased sequence diversity of US4 (glycoprotein G) (152). The recent nearly full-length sequencing of 6 strains from the United States and 34 low-passage-number and laboratory strains from around the world has greatly improved our understanding of basic virology of HSV-2, demonstrating the highly conserved nature of HSV-2, with an overall genetic distance of only 0.4% (153, 154). The role of recombination between strains requires additional research, as Kolb et al. described frequent recombination (154) but Newman et al. described a low level of recombination (153). Surprisingly, geographic clustering of strains was not observed (153).
Large-scale strain typing of HSV-2 has not been developed. Restriction fragment length polymorphisms (RFLP) combined with heteroduplex mobility assays (155) and polymorphic short tandem repeats or microsatellites (156) have been studied to differentiate between different strains for epidemiologic purposes, but these are not widely available. The frequency at which HSV-2 superinfection occurs has been an area of previous study, particularly given the potential implications for whether development of a prophylactic vaccine for HSV-2 will be feasible. Superinfection has been demonstrated in several small studies with limited sample sizes (157–160), with a prevalence of up to 25% in HIV-negative persons (157) and up to 100% in HIV-infected persons (160). These data were generated using RFLP (157) or PCR of regions with variable repeats, and novel efforts to use genomically based technologies to define the frequency of and risk factors for superinfection are being explored.
CONCLUSIONS
Genital HSV remains a highly prevalent infectious agent with a significant impact on sexual health and risk of HIV infection. We now understand that HSV-2 infection is not characterized by latency with occasional outbreaks but that it is a dynamic infection with frequent, often subclinical, shedding throughout the genital tract that results in inflammation. Detailed natural history studies that rely on highly motivated participants have contributed to this enhanced understanding of viral shedding and inflammation. We must translate this knowledge into interventions that fully suppress shedding, which will likely be required to prevent transmission and to disrupt HIV/HSV-2 interactions. Novel strategies to prevent or suppress HSV-2 infection such as prophylactic or therapeutic vaccines are urgently needed.
ACKNOWLEDGMENTS
Christine Johnston receives royalty payments from UpToDate, and within the past 24 months, her institution has received funding to conduct clinical trials from the following companies that are developing antivirals or vaccines for HSV-2: Aicuris GmbH, Gilead, Genocea, Vical, and Agenus. Lawrence Corey is a coinventor on several patents involving potential HSV vaccine candidates and is on the scientific advisory board for and holds stock (<1%) in Immune Design Corp.
We thank Mindy Miner for scientific editing assistance.
Biographies

Christine Johnston, M.D., M.P.H., is an Assistant Professor in the Division of Infectious Diseases, Department of Medicine, at the University of Washington. Her clinical research aims to understand the pathogenesis of genital herpesvirus infections through well-designed studies which rely on intensive sampling from human participants, with the ultimate goal of improving available interventions for these infections. She is also interested in the interaction of HSV with other pathogens in the genital tract, particularly HIV, and the vaginal microbiome.

Lawrence Corey, M.D., is president and director emeritus of Fred Hutchinson Cancer Research Center, Seattle, WA. He is also a member of the Vaccine and Infectious Disease Division and principal investigator of the HIV Vaccine Trials Network at Fred Hutchinson Cancer Research Center and Professor of Medicine and Laboratory Medicine at the University of Washington, Seattle, where he holds the Lawrence Corey Endowed Chair in medical virology. Dr. Corey received his medical degree from the University of Michigan and his infectious diseases training at the University of Washington. He has served as faculty at the University of Washington since 1978 and moved his laboratories to the Fred Hutchinson Cancer Research Center in 1998. His areas of research are in medical virology and include a broad range of studies involving herpesviruses (HSV-2 and Kaposi’s sarcoma-associated herpesvirus [KSV] in particular), HIV vaccine development, and infections in immunocompromised hosts, especially those undergoing bone marrow transplant.
REFERENCES
- 1.Looker KJ, Magaret AS, Turner KME, Vickerman P, Gottlieb SL, Newman LM. 2015. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 10:e114989. doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiffer JT, Corey L. 2013. Rapid host immune response and viral dynamics in herpes simplex virus-2 infection. Nat Med 19:280–288. doi: 10.1038/nm.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. 2006. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 4.Kenyon C, Buyze J, Colebunders R. 2014. Classification of incidence and prevalence of certain sexually transmitted infections by world regions. Int J Infect Dis 18:73–80. doi: 10.1016/j.ijid.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Rajagopal S, Magaret A, Mugo N, Wald A. 2014. Incidence of herpes simplex virus type 2 infections in Africa: a systematic review. Open Forum Infect Dis 1:ofu043. doi: 10.1093/ofid/ofu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chohan V, Baeten JM, Benki S, Graham SM, Lavreys L, Mandaliya K, Ndinya-Achola JO, Jaoko W, Overbaugh J, McClelland RS. 2009. A prospective study of risk factors for herpes simplex virus type 2 acquisition among high-risk HIV-1 seronegative women in Kenya. Sex Transm Infect 85:489–492. doi: 10.1136/sti.2009.036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanfair RN, Zaidi A, Taylor LD, Xu F, Gottlieb S, Markowitz L. 2013. Trends in seroprevalence of herpes simplex virus type 2 among non-Hispanic blacks and non-Hispanic whites aged 14 to 49 years—United States, 1988 to 2010. Sex Transm Dis 40:860–864. doi: 10.1097/OLQ.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 8.Delaney S, Gardella C, Saracino M, Magaret A, Wald A. 2014. Seroprevalence of herpes simplex virus type 1 and 2 among pregnant women, 1989-2010. JAMA 312:746–748. doi: 10.1001/jama.2014.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okafor N, Rosenberg ES, Luisi N, Sanchez T, Del Rio C, Sullivan PS, Kelley CF. 2015. Disparities in herpes simplex virus type 2 infection between black and white men who have sex with men in Atlanta, GA. Int J STD AIDS 26:740–745. doi: 10.1177/0956462414552814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel EU, Frank MA, Hsieh YH, Rothman RE, Baker AE, Kraus CK, Shahan J, Gaydos CA, Kelen GD, Quinn TC, Laeyendecker O. 2014. Prevalence and factors associated with herpes simplex virus type 2 infection in patients attending a Baltimore City emergency department. PLoS One 9:e102422. doi: 10.1371/journal.pone.0102422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyrer C, Jitwatcharanan K, Natpratan C, Kaewvichit R, Nelson KE, Chen C-Y, Weiss JB, Morse SA. 1998. Molecular methods for the diagnosis of genital ulcer disease in a sexually transmitted disease clinic population in northern Thailand: predominance of herpes simplex virus infection. J Infect Dis 178:243–246. doi: 10.1086/515603. [DOI] [PubMed] [Google Scholar]
- 12.Makasa M, Buve A, Sandøy IF. 2012. Etiologic pattern of genital ulcers in Lusaka, Zambia: has chancroid been eliminated? Sex Transm Dis 39:787–791. doi: 10.1097/OLQ.0b013e31826ae97d. [DOI] [PubMed] [Google Scholar]
- 13.Mertz KJ, Trees D, Levine WC, Lewis JS, Litchfield B, Pettus KS, Morse SA, St Louis ME, Weiss JB, Schwebke J, Dickes J, Kee R, Reynolds J, Hutcheson D, Green D, Dyer I, Richwald GA, Novotny J, Weisfuse I, Goldberg M, O'Donnell JA, Knaup R. 1998. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J Infect Dis 178:1795–1798. [DOI] [PubMed] [Google Scholar]
- 14.Paz-Bailey G, Rahman M, Chen C, Ballard R, Moffat HJ, Kenyon T, Kilmarx PH, Totten PA, Astete S, Boily MC, Ryan C. 2005. Changes in the etiology of sexually transmitted diseases in Botswana between 1993 and 2002: implications for the clinical management of genital ulcer disease. Clin Infect Dis 41:1304–1312. doi: 10.1086/496979. [DOI] [PubMed] [Google Scholar]
- 15.Brankin AE, Tobian AAR, Laeyendecker O, Suntoke TR, Kizza A, Mpoza B, Kigozi G, Nalugoda F, Iga B, Chen MZ, Gray RH, Wawer MJ, Quinn TC, Reynolds SJ. 2009. Aetiology of genital ulcer disease in female partners of male participants in a circumcision trial in Uganda. Int J STD AIDS 20:650–651. doi: 10.1258/ijsa.2009.009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corey L, Adams HG, Brown ZA, Holmes KK. 1983. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med 98:958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 17.Bissessor M, Fairley CK, Read T, Denham I, Bradshaw C, Chen M. 2013. The etiology of infectious proctitis in men who have sex with men differs according to HIV status. Sex Transm Dis 40:768–770. doi: 10.1097/OLQ.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 18.Benedetti JK, Zeh J, Corey L. 1999. Clinical reactivation of genital herpes simplex virus infection decreases in frequency over time. Ann Intern Med 131:14–20. doi: 10.7326/0003-4819-131-1-199907060-00004. [DOI] [PubMed] [Google Scholar]
- 19.Benedetti J, Corey L, Ashley R. 1994. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med 121:847–854. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- 20.Mindel A, Carney O, Williams P. 1990. Cutaneous herpes simplex infections. Genitourin Med 66:14–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryder N, Jin F, McNulty AM, Grulich AE, Donovan B. 2009. Increasing role of herpes simplex virus type 1 in first-episode anogenital herpes in heterosexual women and younger men who have sex with men, 1992-2006. Sex Transm Infect 85:416–419. doi: 10.1136/sti.2008.033902. [DOI] [PubMed] [Google Scholar]
- 22.Bradley H, Markowitz LE, Gibson T, McQuillan GM. 2014. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999-2010. J Infect Dis 209:325–333. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 23.Cherpes TL, Meyn LA, Hillier SL. 2005. Cunnilingus and vaginal intercourse are risk factors for herpes simplex virus type 1 acquisition in women. Sex Transm Dis 32:84–89. doi: 10.1097/01.olq.0000151414.64297.46. [DOI] [PubMed] [Google Scholar]
- 24.Engelberg R, Carrell D, Krantz E, Corey L, Wald A. 2003. Natural history of genital herpes simplex virus type 1 infection. Sex Transm Dis 30:174–177. doi: 10.1097/00007435-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Lafferty WE, Coombs RW, Benedetti J, Critchlow C, Corey L. 1987. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N Engl J Med 316:1444–1449. [DOI] [PubMed] [Google Scholar]
- 26.Wald A, Huang ML, Carrell D, Selke S, Corey L. 2003. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis 188:1345–1351. doi: 10.1086/379043. [DOI] [PubMed] [Google Scholar]
- 27.Ryncarz AJ, Goddard J, Wald A, Huang ML, Roizman B, Corey L. 1999. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J Clin Microbiol 37:1941–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuypers J, Boughton G, Chung J, Hussey L, Huang ML, Cook L, Jerome KR. 2015. Comparison of the Simplexa HSV1 & 2 Direct kit and laboratory-developed real-time PCR assays for herpes simplex virus detection. J Clin Virol 62:103–105. doi: 10.1016/j.jcv.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Van Der Pol B, Warren T, Taylor SN, Martens M, Jerome KR, Mena L, Lebed J, Ginde S, Fine P, Hook EW III. 2012. Type-specific identification of anogenital herpes simplex virus infections by use of a commercially available nucleic acid amplification test. J Clin Microbiol 50:3466–3471. doi: 10.1128/JCM.01685-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plummer G. 1973. A review of the identification and titration of antibodies to herpes simplex viruses type 1 and type 2 in human sera. Cancer Res 33:1469–1476. [PubMed] [Google Scholar]
- 31.Lee F, Coleman R, Pereira L, Bailey P, Tatsuno M, Nahmias A. 1985. Detection of herpes simplex virus type 2 specific antibody with glycoprotein G J Clin Microbiol 22:641–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. 1988. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 26:662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Workowski KA, Bolan G. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommen Rep 64(RR-3):1–137. [PMC free article] [PubMed] [Google Scholar]
- 34.Wald A, Ashley-Morrow R. 2002. Serological testing for herpes simplex virus (HSV)-1 and HSV-2 infection. Clin Infect Dis 35:S173–182. doi: 10.1086/342104. [DOI] [PubMed] [Google Scholar]
- 35.Golden MR, Ashley-Morrow R, Swenson P, Hogrefe WR, Handsfield HH, Wald A. 2005. Herpes simplex virus type 2 (HSV-2) Western blot confirmatory testing among men testing positive for HSV-2 using the focus enzyme-linked immunosorbent assay in a sexually transmitted disease clinic. Sex Transm Dis 32:771–777. doi: 10.1097/01.olq.0000175377.88358.f3. [DOI] [PubMed] [Google Scholar]
- 36.Morrow RA, Friedrich D, Meier A, Corey L. 2005. Use of “Biokit HSV-2 Rapid Assay” to improve the positive predictive value of Focus HerpeSelect HSV-2 ELISA. BMC Infect Dis 5:84. doi: 10.1186/1471-2334-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reference deleted. [Google Scholar]
- 38.Shevlin E, Morrow RA. 2014. Comparative performance of the Uni-Gold™ HSV-2 Rapid: a point-of-care HSV-2 diagnostic test in unselected sera from a reference laboratory. J Clin Virol 61:378–381. doi: 10.1016/j.jcv.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrow RA, Friedrich D, Krantz E. 2003. Performance of the focus and Kalon enzyme-linked immunosorbent assays for antibodies to herpes simplex virus type 2 glycoprotein G in culture-documented cases of genital herpes. J Clin Microbiol 41:5212–5214. doi: 10.1128/JCM.41.11.5212-5214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ngo TD, Laeyendecker O, Morrow RA, Lai S, Quinn TC. 2008. Comparison of three commercial immunoassays for detection of herpes simplex virus type 2 antibodies in commercial sex workers in Yunnan Province, China. Clin Vaccine Immunol 15:1301–1303. doi: 10.1128/CVI.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ngo TD, Laeyendecker O, La H, Hogrefe W, Morrow RA, Quinn TC. 2008. Use of commercial enzyme immunoassays to detect antibodies to the herpes simplex virus type 2 glycoprotein G in a low-risk population in Hanoi, Vietnam. Clin Vaccine Immunol 15:382–384. doi: 10.1128/CVI.00437-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delany-Moretlwe S, Jentsch U, Weiss H, Moyes J, Ashley-Morrow R, Stevens W, Mayaud P. 2010. Comparison of focus HerpesSelect and Kalon HSV-2 gG2 ELISA serological assays to detect herpes simplex virus type 2 antibodies in a South African population. Sex Transm Infect 86:46–50. doi: 10.1136/sti.2009.036541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng'ayo MO, Friedrich D, Holmes KK, Bukusi E, Morrow RA. 2010. Performance of HSV-2 type specific serological tests in men in Kenya. J Virol Methods 163:276–281. doi: 10.1016/j.jviromet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biraro S, Mayaud P, Morrow RA, Grosskurth H, Weiss HA. 2011. Performance of commercial herpes simplex virus type-2 antibody tests using serum samples from Sub-Saharan Africa: a systematic review and meta-analysis. Sex Transm Dis 38:140–147. doi: 10.1097/OLQ.0b013e3181f0bafb. [DOI] [PubMed] [Google Scholar]
- 45.Lingappa J, Nakku-Joloba E, Magaret A, Friedrich D, Dragavon J, Kambugu F, Joloba M, Whalen C, Coombs R, Celum C, Morrow RA. 2010. Sensitivity and specificity of herpes simplex virus-2 serological assays among HIV-infected and uninfected urban Ugandans. Int J STD AIDS 21:611–616. doi: 10.1258/ijsa.2009.008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mujugira A, Morrow RA, Celum C, Lingappa J, Delany-Moretlwe S, Fife KH, Heffron R, De Bruyn G, Homawoo B, Karita E, Mugo N, Vwalika B, Baeten JM. 2011. Performance of the Focus HerpeSelect-2 enzyme immunoassay for the detection of herpes simplex virus type 2 antibodies in seven African countries. Sex Transm Infect 87:238–241. doi: 10.1136/sti.2010.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neal JD, Tobian AA, Laeyendecker O, Ngo TD, Redd AD, Reynolds SJ, Ashley Morrow R, Manucci JL, Serwadda D, Gray RH, Quinn TC. 2011. Performance of the Euroline Western blot assay in the detection of herpes simplex virus type 2 antibody in Uganda, China and the USA. Int J STD AIDS 22:342–344. doi: 10.1258/ijsa.2009.009327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.US Preventive Services Task Force. July 2015, posting date. Final update summary: genital herpes: screening. US Preventive Services Task Force, Rockville, MD: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/genital-herpes-screening. [Google Scholar]
- 49.ACOG Committee on Practice Bulletins. 2007. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. No. 82 June 2007. Management of herpes in pregnancy. Obstet Gynecol 109:1489–1498. [DOI] [PubMed] [Google Scholar]
- 50.Elion G, Furman P, Fyfe J, deMiranda P, Beauchamp L, Schaeffer H. 1977. The selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A 74:5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaeffer HJ, Beauchamp L, de Miranda P, Elion GB, Bauer DJ, Collins P. 1978. 9-(2-Hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature 272:583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- 52.Perry CM, Faulds D. 1996. Valaciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections. Drugs 52:754–772. [DOI] [PubMed] [Google Scholar]
- 53.Perry CM, Wagstaff AJ. 1995. Famciclovir. A review of its pharmacological properties and therapeutic efficacy in herpesvirus infections. Drugs 50:396–415. [DOI] [PubMed] [Google Scholar]
- 54.Lebrun-Vignes B, Bouzamondo A, Dupuy A, Guillaume J-C, Lechat P, Chosidow O. 2007. A meta-analysis to assess the efficacy of oral antiviral treatment to prevent genital herpes outbreaks. J Am Acad Dermatol 57:238–246. doi: 10.1016/j.jaad.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Fife KH, Almekinder J, Ofner S. 2007. A comparison of one year of episodic or suppressive treatment of recurrent genital herpes with valacyclovir. Sex Transm Dis 34:297–301. [DOI] [PubMed] [Google Scholar]
- 56.Corey L, Ashley R. 2004. Prevention of herpes simplex virus type 2 transmission with antiviral therapy. Herpes 11(Suppl 3):170A–174A. [PubMed] [Google Scholar]
- 57.Wald A, Selke S, Warren T, Aoki FY, Sacks S, Diaz-Mitoma F, Corey L. 2006. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sex Transm Dis 33:529–533. doi: 10.1097/01.olq.0000204723.15765.91. [DOI] [PubMed] [Google Scholar]
- 58.Tyring S, Wald A, Zadeikis N, Dhadda S, Takenouchi K, Rorig R. 2012. ASP2151 for the treatment of genital herpes: a randomized, double-blind, placebo- and valacyclovir-controlled, dose-finding study. J Infect Dis 205:1100–1110. doi: 10.1093/infdis/jis019. [DOI] [PubMed] [Google Scholar]
- 59.Wald A, Corey L, Timmler B, Magaret A, Warren T, Tyring S, Johnston C, Kriesel J, Fife K, Galitz L, Stoelben S, Huang M, Selke S, Stobernack H, Ruebsamen-Schaeff H, Birkmann A. 2014. Helicase-primase inhibitor pritelivir for herpes simplex virus-2 infection. N Engl J Med 370:201–210. doi: 10.1056/NEJMoa1301150. [DOI] [PubMed] [Google Scholar]
- 60.Safrin S, Crumpacker C, Chatis P, Davis R, Hafner R, Rush J, Kessler HA, Landry B, Mills J. 1991. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med 325:551–555. [DOI] [PubMed] [Google Scholar]
- 61.Kim JH, Schaenman JM, Ho DY, Brown JM. 2011. Treatment of acyclovir-resistant herpes simplex virus with continuous infusion of high-dose acyclovir in hematopoietic cell transplant patients. Biol Blood Marrow Transplant 17:259–264. doi: 10.1016/j.bbmt.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 62.Leeyaphan C, Surawan TM, Chirachanakul P, Prasertworonun N, Punyaratabandhu P, Omcharoen V, Jiamton S. 2015. Clinical characteristics of hypertrophic herpes simplex genitalis and treatment outcomes of imiquimod: a retrospective observational study. Int J Infect Dis 33:165–170. doi: 10.1016/j.ijid.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Lalezari JP, Drew WL, Glutzer E, Miner D, Safrin S, Owen WF Jr, Davidson JM, Fisher PE, Jaffe HS. 1994. Treatment with intravenous (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]-cytosine of acyclovir-resistant mucocutaneous infection with herpes simplex virus in a patient with AIDS. J Infect Dis 170:570–572. doi: 10.1093/infdis/170.3.570. [DOI] [PubMed] [Google Scholar]
- 64.Lalezari J, Schacker T, Feinberg J, Gathe J, Lee S, Cheung T, Kramer F, Kessler H, Corey L, Drew WL, Boggs J, McGuire B, Jaffe HS, Safrin S. 1997. A randomized, double-blind, placebo-controlled trial of cidofovir gel for the treatment of acyclovir-unresponsive mucocutaneous herpes simplex virus infection in patients with AIDS. J Infect Dis 176:892–898. doi: 10.1086/516542. [DOI] [PubMed] [Google Scholar]
- 65.Abdool Karim SS, Abdool Karim Q, Kharsany ABM, Baxter C, Grobler AC, Werner L, Kashuba A, Mansoor LE, Samsunder N, Mindel A, Gengiah TN. 2015. Tenofovir gel for the prevention of herpes simplex virus type 2 infection. N Engl J Med 373:530–539. doi: 10.1056/NEJMoa1410649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Celum C, Morrow RA, Donnell D, Hong T, Hendrix CW, Thomas KK, Fife KH, Nakku-Joloba E, Mujugira A, Baeten JM. 2014. Daily oral tenofovir and emtricitabine-tenofovir preexposure prophylaxis reduces herpes simplex virus type 2 acquisition among heterosexual HIV-1-uninfected men and women: a subgroup analysis of a randomized trial of HSV-2 protection with tenofovir-based preexposure prophylaxis. Ann Intern Med 161:11–19. doi: 10.7326/M13-2471. [DOI] [PubMed] [Google Scholar]
- 67.Marcus JL, Glidden DV, McMahan V, Lama JR, Mayer KH, Liu AY, Montoya-Herrera O, Casapia M, Hoagland B, Grant RM. 2014. Daily oral emtricitabine/tenofovir preexposure prophylaxis and herpes simplex virus type 2 among men who have sex with men. PLoS One 9:e91513. doi: 10.1371/journal.pone.0091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bender Ignacio RA, Perti T, Magaret AS, Rajagopal S, Stevens CE, Huang M-L, Selke S, Johnston C, Marrazzo J, Wald A. 4 June 2015. Oral and vaginal tenofovir for genital herpes simplex virus type 2 shedding in immunocompetent women: a double-blind, randomized, cross-over trial. J Infect Dis. doi: 10.1093/infdis/jiv317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan DH, Kaul R, Raboud JM, Walmsley SL. 2011. No impact of oral tenofovir disoproxil fumarate on herpes simplex virus shedding in HIV-infected adults. AIDS 25:207–210. doi: 10.1097/QAD.0b013e328341ddf7. [DOI] [PubMed] [Google Scholar]
- 70.Wald A, Zeh J, Selke S, Ashley RL, Corey L. 1995. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med 333:770–775. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 71.Reference deleted. [Google Scholar]
- 72.Mark K, Wald A, Magaret A, Selke S, Olin L, Huang M, Corey L. 2008. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis 198:1141–1149. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jerome KR, Huang ML, Wald A, Selke S, Corey L. 2002. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol 40:2609–2611. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley R, Krieger JN, Corey L. 2000. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med 342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 75.Tronstein E, Johnston C, Huang ML, Selke S, Magaret A, Warren T, Corey L, Wald A. 2011. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 305:1441–1449. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brock BV, Selke S, Benedetti J, Douglas JM Jr, Corey L. 1990. Frequency of asymptomatic shedding of herpes simplex virus in women with genital herpes. JAMA 263:418–420. [PubMed] [Google Scholar]
- 77.Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. 1992. Risk factors for the sexual transmission of genital herpes. Ann Intern Med 116:197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- 78.Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. 1999. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N Engl J Med 341:1432–1438. [DOI] [PubMed] [Google Scholar]
- 79.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Handsfield HH, Warren T, Marr L, Tyring S. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 282:331–340. [DOI] [PubMed] [Google Scholar]
- 80.Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. 2003. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 289:203–209. doi: 10.1001/jama.289.2.203. [DOI] [PubMed] [Google Scholar]
- 81.Brown ZA, Benedetti J, Selke S, Ashley R, Watts DH, Corey L. 1996. Asymptomatic maternal shedding of herpes simplex virus at the onset of labor: relationship to preterm labor. Obstet Gynecol 87:483–488. doi: 10.1016/0029-7844(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 82.Cone R, Hobson A, Brown Z, Ashley R, Berry S, Ishak L, Winter C, Corey L. 1994. Frequent detection of genital herpes simplex virus DNA by polymerase chain reaction among pregnant women. JAMA 272:792–796. [PubMed] [Google Scholar]
- 83.Schiffer JT, Mayer BT, Fong Y, Swan DA, Wald A. 26 March 2014. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. J R Soc Interface doi: 10.1098/rsif.2014.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Phipps W, Saracino M, Magaret A, Selke S, Remington M, Huang ML, Warren T, Casper C, Corey L, Wald A. 2011. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis 203:180–187. doi: 10.1093/infdis/jiq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schiffer JT, Abu-Raddad L, Mark KE, Zhu J, Selke S, Magaret A, Wald A, Corey L. 2009. Frequent release of low amounts of herpes simplex virus from neurons: results of a mathematical model. Sci Transl Med 1:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crespi CM, Cumberland WG, Wald A, Corey L, Blower S. 2007. Longitudinal study of herpes simplex virus type 2 infection using viral dynamic modelling. Sex Transm Infect 83:359–364. doi: 10.1136/sti.2006.022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reference deleted. [Google Scholar]
- 88.Schiffer JT, Abu-Raddad L, Mark KE, Zhu J, Selke S, Koelle DM, Wald A, Corey L. 2010. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc Natl Acad Sci U S A 107:18973–18978. doi: 10.1073/pnas.1006614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wald A, Zeh JE, Barnum G, Davis LG, Corey L. 1996. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med 124:8–15. doi: 10.7326/0003-4819-124-1_Part_1-199601010-00002. [DOI] [PubMed] [Google Scholar]
- 90.Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. 1997. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest 99:1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Corey L, Wald A, Patel R, Sacks SL, Tyring SK, Warren T, Douglas JM, Paavonen J, Morrow RA, Beutner KR. 2004. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 92.Johnston C, Saracino M, Kuntz S, Magaret A, Selke S, Huang ML, Schiffer JT, Koelle DM, Corey L, Wald A. 2012. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet 379:641–647. doi: 10.1016/S0140-6736(11)61750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wald A, Bernstein D, Fife K, Lee P, Tyring S, Van Wagoner N, Warren T, Magaret A, Flechtner J, Hetherington S. 2013. Program Abstr 53rd Intersci Conf Antimicrob Agents Chemother, Denver, CO. http://www.icaac.org/.
- 94.Johnston C, Zhu J, Jing L, Laing KJ, McClurkan CM, Klock A, Diem K, Jin L, Stanaway J, Tronstein E, Kwok WW, Huang ML, Selke S, Fong Y, Magaret A, Koelle DM, Wald A, Corey L. 2014. Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. J Virol 88:4921–4931. doi: 10.1128/JVI.03285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tata S, Johnston C, Huang M-L, Selke S, Magaret A, Corey L, Wald A. 2010. Overlapping reactivations of herpes simplex virus type 2 in the genital and perianal mucosa. J Infect Dis 201:499–504. doi: 10.1086/650302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin ET, Krantz E, Gottlieb SL, Magaret AS, Langenberg A, Stanberry L, Kamb M, Wald A. 2009. A pooled analysis of the effect of condoms in preventing HSV-2 acquisition. Arch Intern Med 169:1233–1240. doi: 10.1001/archinternmed.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schiffer JT, Swan D, Al Sallaq R, Magaret A, Johnston C, Mark KE, Selke S, Ocbamichael N, Kuntz S, Zhu J, Robinson B, Huang M-L, Jerome KR, Wald A, Corey L. 2013. Rapid localized spread and immunologic containment define herpes simplex virus-2 reactivation in the human genital tract. eLife 2:e00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, Jin L, Diem K, Koelle DM, Wald A, Robins H, Corey L. 2013. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 497:494–497. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stamm WE, Handsfield HH, Rompalo AM, Ashley RL, Roberts PL, Corey L. 1988. The association between genital ulcer disease and acquisition of HIV infection in homosexual men. JAMA 260:1429–1433. [PubMed] [Google Scholar]
- 100.Wald A, Link K. 2002. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis 185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 101.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 102.Kapiga SH, Sam NE, Bang H, Ni Q, Ao TT, Kiwelu I, Chiduo S, Ndibe U, Seage G III, Coplan P, Shao J, Rosenberg ZF, Essex M. 2007. The role of herpes simplex virus type 2 and other genital infections in the acquisition of HIV-1 among high-risk women in northern Tanzania. J Infect Dis 195:1260–1269. doi: 10.1086/513566. [DOI] [PubMed] [Google Scholar]
- 103.Sobngwi-Tambekou J, Taljaard D, Lissouba P, Zarca K, Puren A, Lagarde E, Auvert B. 2009. Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis 199:958–964. doi: 10.1086/597208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.del Mar Pujades Rodriguez M, Obasi A, Mosha F, Todd J, Brown D, Changalucha J, Mabey D, Ross D, Grosskurth H, Hayes R. 2002. Herpes simplex virus type 2 infection increases HIV incidence: a prospective study in rural Tanzania. AIDS 16:451–462. doi: 10.1097/00002030-200202150-00018. [DOI] [PubMed] [Google Scholar]
- 105.Shannon B, Yi TJ, Thomas-Pavanel J, Chieza L, Janakiram P, Saunders M, Tharao W, Huibner S, Remis R, Rebbapragada A, Kaul R. 2014. Impact of asymptomatic herpes simplex virus type 2 infection on mucosal homing and immune cell subsets in the blood and female genital tract. J Immunol 192:5074–5082. doi: 10.4049/jimmunol.1302916. [DOI] [PubMed] [Google Scholar]
- 106.Rebbapragada A, Wachihi C, Pettengell C, Sunderji S, Huibner S, Jaoko W, Ball B, Fowke K, Mazzulli T, Plummer FA, Kaul R. 2007. Negative mucosal synergy between herpes simplex type 2 and HIV in the female genital tract. AIDS 21:589–598. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 107.Johnson KE, Sherman ME, Ssempiija V, Tobian AA, Zenilman JM, Duggan MA, Kigozi G, Serwadda D, Wawer MJ, Quinn TC, Rabkin CS, Gray RH. 2009. Foreskin inflammation is associated with HIV and herpes simplex virus type-2 infections in Rakai, Uganda. AIDS 23:1807–1815. doi: 10.1097/QAD.0b013e32832efdf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnson KE, Redd AD, Quinn TC, Collinson-Streng AN, Cornish T, Kong X, Sharma R, Tobian AAR, Tsai B, Sherman ME, Kigozi G, Serwadda D, Wawer MJ, Gray RH. 2011. Effects of HIV-1 and herpes simplex virus type 2 infection on lymphocyte and dendritic cell density in adult foreskins from Rakai, Uganda. J Infect Dis 203:602–609. doi: 10.1093/infdis/jiq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, Corey L. 2009. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med 15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tan D, Kaul R, Walmsley S. 2009. Left out but not forgotten: should closer attention be paid to coinfection with herpes simplex virus type 1 and HIV? Can J Infect Dis Med Microbiol 20:e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Li X, vanCott T, Quinn TC. 2001. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 112.Schacker TW, Ryncarz AJ, Goddard J, Diem K, Shaughnessy M, Corey L. 1998. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA 280:61–66. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 113.Nagot N, Ouedraogo A, Konate I, Weiss H, Foulongne V, Defer M, Sanon A, Becquart P, Segondy M, Sawadogo A, Van de Perre P, Mayaud P. 2008. Roles of clinical and subclinical reactivated herpes simplex virus type 2 infection and human immunodeficiency virus type 1 (HIV-1)-induced immunosuppression on genital and plasma HIV-1 levels. J Infect Dis 198:241–249. doi: 10.1086/589621. [DOI] [PubMed] [Google Scholar]
- 114.Mole L, Ripich S, Margolis D, Holodniy M. 1997. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis 176:766–770. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 115.Augenbraun M, Corey L, Reichelderfer P, Wright DJ, Burns D, Koelle DM, Robison E, Cohen M. 2001. Herpes simplex virus shedding and plasma human immunodeficiency virus RNA levels in coinfected women. Clin Infect Dis 33:885–890. doi: 10.1086/322654. [DOI] [PubMed] [Google Scholar]
- 116.Hoots BE, Hudgens MG, Cole SR, King CC, Klein RS, Mayer KH, Rompalo AM, Sobel JD, Jamieson DJ, Smith JS. 2011. Lack of association of herpes simplex virus type 2 seropositivity with the progression of HIV infection in the HERS cohort. Am J Epidemiol 173:837–844. doi: 10.1093/aje/kwq432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Siegal F, Lopez C, Hammer GS, Brown AE, Kornfeld SJ, Gold J, Hassett J, Hirschman SZ, Cunningham-Rundles C, Adelsberg BR, Parham DM, Siegal M, Cunningham-Rundles S, Armstrong D. 1981. Severe acquired immunodeficiency in male homosexuals manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med 305:1439–1444. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- 118.Levin MJ, Bacon TH, Leary JJ. 2004. Resistance of herpes simplex virus infections to nucleoside analogues in HIV-infected patients. Clin Infect Dis 39(Suppl 5):S248–S257. doi: 10.1086/422364. [DOI] [PubMed] [Google Scholar]
- 119.Yudin MH, Kaul R. 2008. Progressive hypertrophic genital herpes in an HIV-infected woman despite immune recovery on antiretroviral therapy. Infect Dis Obstet Gynecol 2008:592532. doi: 10.1155/2008/592532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tobian AA, Grabowski MK, Serwadda D, Newell K, Ssebbowa P, Franco V, Nalugoda F, Wawer MJ, Gray RH, Quinn TC, Reynolds SJ. 2013. Reactivation of herpes simplex virus type 2 after initiation of antiretroviral therapy. J Infect Dis 208:839–846. doi: 10.1093/infdis/jit252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Posavad CM, Wald A, Kuntz S, Huang ML, Selke S, Krantz E, Corey L. 2004. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis 190:693–696. doi: 10.1086/422755. [DOI] [PubMed] [Google Scholar]
- 122.Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, Defer MC, Djagbare D, Sanon A, Andonaba JB. 2007. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med 356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 123.Lingappa JR, Baeten JM, Wald A, Hughes JP, Thomas KK, Mujugira A, Mugo N, Bukusi EA, Cohen CR, Katabira E, Ronald A, Kiarie J, Farquhar C, Stewart GJ, Makhema J, Essex M, Were E, Fife KH, de Bruyn G, Gray GE, McIntyre JA, Manongi R, Kapiga S, Coetzee D, Allen S, Inambao M, Kayitenkore K, Karita E, Kanweka W, Delany S, Rees H, Vwalika B, Magaret AS, Wang RS, Kidoguchi L, Barnes L, Ridzon R, Corey L, Celum C. 2010. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet 375:824–833. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reynolds SJ, Makumbi F, Newell K, Kiwanuka N, Ssebbowa P, Mondo G, Boaz I, Wawer MJ, Gray RH, Serwadda D, Quinn TC. 2012. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis 12:441–448. doi: 10.1016/S1473-3099(12)70037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perti T, Saracino M, Baeten JM, Johnston C, Diem K, Ocbamichael N, Huang M-L, Selke S, Magaret A, Corey L, Wald A. 2013. High-dose valacyclovir decreases plasma HIV-1 RNA more than standard-dose acyclovir in persons coinfected with HIV-1 and HSV-2: a randomized crossover trial. J Acquir Immune Defic Syndr 63:201–208. doi: 10.1097/QAI.0b013e3182928eea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mugwanya K, Baeten JM, Mugo NR, Irungu E, Ngure K, Celum C. 2011. High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 coinfected persons: a randomized, crossover trial. J Infect Dis 204:1912–1917. doi: 10.1093/infdis/jir649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lisco A, Vanpouille C, Tchesnokov EP, Grivel JC, Biancotto A, Brichacek B, Elliott J, Fromentin E, Shattock R, Anton P. 2008. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe 4:260–270. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McMahon MA, Siliciano JD, Lai J, Liu JO, Stivers JT, Siliciano RF, Kohli RM. 2008. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem 283:31289–31293. doi: 10.1074/jbc.C800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McMahon MA, Parsons TL, Shen L, Siliciano JD, Siliciano RF. 2011. Consistent inhibition of HIV-1 replication in CD4+ T cells by acyclovir without detection of human herpesviruses. J Virol 85:4618–4622. doi: 10.1128/JVI.02423-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mosca JD, Bednarik DP, Raj NB, Rosen CA, Sodroski JG, Haseltine WA, Pitha PM. 1987. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature 325:67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- 131.INSIGHT START Study Group. 2015. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR. 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, Mujugira A, Baeten JM, Mullins JI, Hughes JP, Bukusi EA, Cohen CR, Katabira E, Ronald A, Kiarie J, Farquhar C, Stewart GJ, Makhema J, Essex M, Were E, Fife KH, de Bruyn G, Gray GE, McIntyre JA, Manongi R, Kapiga S, Coetzee D, Allen S, Inambao M, Kayitenkore K, Karita E, Kanweka W, Delany S, Rees H, Vwalika B, Stevens W, Campbell MS, Thomas KK, Coombs RW, Morrow R, Whittington WL, McElrath MJ, Barnes L, Ridzon R, Corey L. 2010. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med 362:427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany-Moretlwe S, Cowan F, Casapia M, Ortiz A, Fuchs J. 2008. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 371:2109–2119. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Watson-Jones D, Weiss HA, Rusizoka M, Changalucha J, Baisley K, Mugeye K, Tanton C, Ross D, Everett D, Clayton T. 2008. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med 358:1560–1571. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reference deleted. [Google Scholar]
- 137.Gottlieb SL, Low N, Newman LM, Bolan G, Kamb M, Broutet N. 2014. Toward global prevention of sexually transmitted infections (STIs): the need for STI vaccines. Vaccine 32:1527–1535. doi: 10.1016/j.vaccine.2013.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Johnston C, Koelle DM, Wald A. 2014. Current status and prospects for development of an HSV vaccine. Vaccine 32:1553–1560. doi: 10.1016/j.vaccine.2013.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 140.Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R, Deal CD. 2014. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis 209:828–836. doi: 10.1093/infdis/jit651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Genocea. 20 May 2015, posting date http://ir.genocea.com/releasedetail.cfm?ReleaseID=914052. [Google Scholar]
- 143.Agenus. 7 November 2013, posting date. http://www.agenusbio.com/docs/press-releases/2013/herpv-vaccine-for-genital-herpes-meets-primary-endpoint.php. [Google Scholar]
- 144.Vical. 22 June 2015, posting date. http://www.vical.com/investors/news-releases/News-Release-Details/2015/Vical-Reports-Top-Line-Results-From-Phase-12-Trial-of-Therapeutic-Genital-Herpes-Vaccine/default.aspx. [Google Scholar]
- 145.Dolan A, Jamieson FE, Cunningham C, Barnett BC, McGeoch DJ. 1998. The genome sequence of herpes simplex virus type 2. J Virol 72:2010–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Colgrove R, Diaz F, Newman R, Saif S, Shea T, Young S, Henn M, Knipe DM. 2014. Genomic sequences of a low passage herpes simplex virus 2 clinical isolate and its plaque-purified derivative strain. Virology 450-451:140–145. doi: 10.1016/j.virol.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Szpara ML, Gatherer D, Ochoa A, Greenbaum B, Dolan A, Bowden RJ, Enquist LW, Legendre M, Davison AJ. 2014. Evolution and diversity in human herpes simplex virus genomes. J Virol 88:1209–1227. doi: 10.1128/JVI.01987-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Szpara ML, Parsons L, Enquist LW. 2010. Sequence variability in clinical and laboratory isolates of herpes simplex virus 1 reveals new mutations. J Virol 84:5303–5313. doi: 10.1128/JVI.00312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wertheim JO, Smith MD, Smith DM, Scheffler K, Kosakovsky Pond SL. 2014. Evolutionary origins of human herpes simplex viruses 1 and 2. Mol Biol Evol 31:2356–2364. doi: 10.1093/molbev/msu185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Norberg P, Kasubi MJ, Haarr L, Bergstrom T, Liljeqvist J-A. 2007. Divergence and recombination of clinical herpes simplex virus type 2 isolates. J Virol 81:13158–13167. doi: 10.1128/JVI.01310-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dudek TE, Torres-Lopez E, Crumpacker C, Knipe DM. 2011. Evidence for differences in immunologic and pathogenesis properties of herpes simplex virus 2 strains from the United States and South Africa. J Infect Dis 203:1434–1441. doi: 10.1093/infdis/jir047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Burrel S, Abrao EP, Desire N, Seang S, Caumes E, Agut H, Boutolleau D. 2013. Detection of a new variant of herpes simplex virus type 2 among HIV-1-infected individuals. J Clin Virol 57:267–269. doi: 10.1016/j.jcv.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 153.Newman RM, Lamers SL, Weiner B, Ray SC, Colgrove RC, Diaz F, Jing L, Wang K, Saif S, Young S, Henn M, Laeyendecker O, Tobian AAR, Cohen JI, Koelle DM, Quinn TC, Knipe DM. 2015. Genome sequencing and analysis of geographically diverse clinical isolates of herpes simplex virus 2. J Virol 89:8219–8232. doi: 10.1128/JVI.01303-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kolb AW, Larsen IV, Cuellar JA, Brandt CR. 2015. Genomic, phylogenetic, and recombinational characterization of herpes simplex virus 2 strains. J Virol 89:6427–6434. doi: 10.1128/JVI.00416-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martin ET, Koelle DM, Byrd B, Huang ML, Vieira J, Corey L, Wald A. 2006. Sequence-based methods for identifying epidemiologically linked herpes simplex virus type 2 strains. J Clin Microbiol 44:2541–2546. doi: 10.1128/JCM.00054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Burrel S, Ait-Arkoub Z, Voujon D, Deback C, Abrao EP, Agut H, Boutolleau D. 2013. Molecular characterization of herpes simplex virus 2 strains by analysis of microsatellite polymorphism. J Clin Microbiol 51:3616–3623. doi: 10.1128/JCM.01714-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Buchman TG, Roizman B, Nahmias AJ. 1979. Demonstration of exogenous genital reinfection with herpes simplex virus type 2 by restriction endonuclease fingerprinting of viral DNA. J Infect Dis 140:295–304. doi: 10.1093/infdis/140.3.295. [DOI] [PubMed] [Google Scholar]
- 158.Maitland NJ, Smith IW, Peutherer JF, Robertson DH, Jones KW. 1982. Restriction endonuclease analysis of DNA from genital isolates of herpes simplex virus type 2. Infect Immun 38:834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sakaoka H, Aomori T, Gouro T, Kumamoto Y. 1995. Demonstration of either endogenous recurrence or exogenous reinfection by restriction endonuclease cleavage analysis of herpes simplex virus from patients with recrudescent genital herpes. J Med Virol 46:387–396. doi: 10.1002/jmv.1890460416. [DOI] [PubMed] [Google Scholar]
- 160.Roest RW, Maertzdorf J, Kant M, van der Meijden WI, Osterhaus ADME, Verjans GMGM. 2006. High incidence of genotypic variance between sequential herpes simplex virus type 2 isolates from HIV-1-seropositive patients with recurrent genital herpes. J Infect Dis 194:1115–1118. doi: 10.1086/507683. [DOI] [PubMed] [Google Scholar]