SUMMARY
Regular review of the management of bioterrorism is essential for maintaining readiness for these sporadically occurring events. This review provides an overview of the history of biological disasters and bioterrorism. I also discuss the recent recategorization of tier 1 agents by the U.S. Department of Health and Human Services, the Laboratory Response Network (LRN), and specific training and readiness processes and programs, such as the College of American Pathologists (CAP) Laboratory Preparedness Exercise (LPX). LPX examined the management of cultivable bacterial vaccine and attenuated strains of tier 1 agents or close mimics. In the LPX program, participating laboratories showed improvement in the level of diagnosis required and referral of isolates to an appropriate reference laboratory. Agents which proved difficult to manage in sentinel laboratories included the more fastidious Gram-negative organisms, especially Francisella tularensis and Burkholderia spp. The recent Ebola hemorrhagic fever epidemic provided a check on LRN safety processes. Specific guidelines and recommendations for laboratory safety and risk assessment in the clinical microbiology are explored so that sentinel laboratories can better prepare for the next biological disaster.
INTRODUCTION
Biological events that have caused significant mass morbidity, mortality, and fear are well chronicled in human history. Outbreaks of disease were recorded as early as 500 BC, when the Plague of Athens, an unknown disease similar to typhoid fever, may have caused as many as 100,000 deaths. A number of plagues in Europe had significant effects on the development of governance and art. They ranged from the Antonine Plague (similar to smallpox), which caused a death toll of 30% of the population (165 to 180), to the Plague of Justinian (541 to 542) and the Black Death (plague; 1346 to 1350), which resulted in mortality estimates of as high as 70% of the population. The social impact was phenomenal, leaving deserted towns that were never rebuilt and are recorded only through archeology (1, 2).
Biological disasters have also occurred in modern times, ranging from the yellow fever epidemic of 1793 in the United States to plague in the Middle East in the early 1800s, cholera throughout the 1800s, and smallpox in the Americas, as well as measles and mumps. As vaccines were developed, other diseases became prominent, including severe acute respiratory syndrome (SARS) in 2002 and 2003, influenza in the 20th century, and Ebola, most recently in West Africa in 2014. The features that distinguish mass biological outbreaks from seasonal or routine infection cycles include sociological behaviors and population-based fear, especially in earlier generations that did not have scientific knowledge to explain and address the occurrences.
Early in the course of military history, it was recognized that population-based fear could be used as a significant advantage in warfare. This may have been observed independently, with or without intent, on multiple occasions. For example, when Cortez invaded Mexico, he did not deliberately introduce smallpox as a biological warfare agent. However, the rapid decimation of the population was an immediate contributor to the success of his operation. Other independent occurrences include the use of dead bodies infected with Yersinia pestis to contaminate wells in Italy (1155; Battle of Tortona) and the catapulting of the corpses of dead soldiers in Bohemia in 1422. Recognition of the role of primitive smallpox vaccination in the American Revolution allowed for several successful campaigns. Attempted biological warfare by Luke Blackburn, using smallpox-contaminated clothing, was recorded in the Civil War of the United States and was officially banned in 1863 by U.S. Army General Order No. 100, which stated that “The use of poison in any manner, be it to poison wells, or food, or arms is wholly excluded from modern warfare” (1, 2).
As knowledge regarding microbiology developed, the use of such agents, overtly or covertly, returned. In World War I, Bacillus anthracis and Burkholderia mallei (agents of anthrax and glanders, respectively) were considered for infection of horses and mules. In 1925, the United States signed the Geneva Protocol, which prohibited the use of chemical or biological agents. This was not approved by Congress until 50 years later, during the era of the 1972 Biological Weapons Convention (2). In World War II, the United States continued its studies and developed Ft. Detrick, MD, as a site of biological research and development. The Cold War instigated continued investigations in the United States and Russia. Biological warfare was more formally defined as the conscious use of biological and chemical agents deliberately chosen as weapons because of their potentially injurious or lethal effects (1, 2). In current dialogue, the deliberate effort to engage in biological warfare is called bioterrorism. The term applies when chemical and biological terrorism is used as an overt or covert means to cause harm for ideological, political, or financial gain.
LABORATORY RESPONSE NETWORK
The scope of this article is to describe current approaches used by clinical microbiology laboratories to address agents associated with bioterrorism and biological disasters. Clinical microbiology specialists actually developed a coordinated national approach prior to 9/11 and the anthrax bioterrorism attack of 2001. However, the ultimate outcome and development of the network system were also markedly influenced by the anthrax bioterrorism event.
A week following the attacks on the World Trade Center in New York on 11 September 2001, letters laced with anthrax arrived via the U.S. Postal Service at the offices of NBC News, the New York Post, a Florida media outlet, and Senator Tom Daschle in Washington, DC. Other mail was contaminated during the postal service processing and infected other recipients. Twenty-two cases of anthrax were identified (11 inhalational and 11 cutaneous cases); 5 of the inhalational cases were fatal (3).
Preceding this event and recognizing the potential for such an attack, the Centers for Disease Control and Prevention (CDC), the Federal Bureau of Investigation (FBI), and the Association for Public Health Laboratories (APHL) developed the Laboratory Response Network (LRN) in 1999 (http://www.bt.cdc.gov/lrn/biological.asp; http://www.aphl.org/aphlprograms/preparedness-and-response/partnerships-and-outreach/). The mission of the LRN is to “maintain an integrated national and international network of laboratories that are fully equipped to respond quickly to acts of chemical or biological terrorism, emerging infectious diseases, and other public health threats and emergencies.”
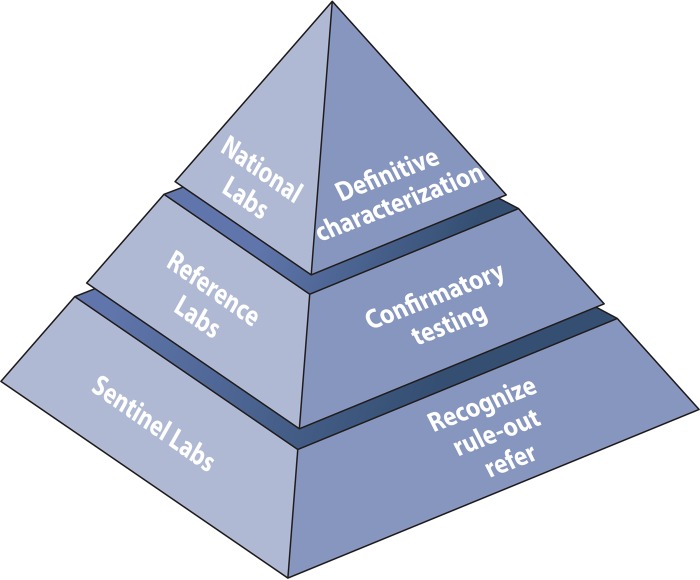
The LRN is typically presented as a pyramid (Fig. 1). At the base of the pyramid are the sentinel laboratories. These are clinical microbiology laboratories, where the primary identification of an infectious agent typically occurs. These laboratories tend to be associated with acute care hospitals or larger reference laboratories. There are thousands of such laboratories in the United States. According to the CDC, a sentinel laboratory is one “capable of analyzing or referring specimens or samples that may contain microbiology agents or biological toxins” (http://www.bt.cdc.gov/lrn/biological.asp; http://www.aphl.org/aphlprograms/preparedness-and-response/partnerships-and-outreach/). A sentinel laboratory is able to perform high-complexity testing in accordance with the Clinical Laboratory Improvement Amendments of 1988 (CLIA; Centers for Medicare & Medicaid Services). Sentinel laboratory services are also available from Department of Defense (DOD) laboratories and veterinary diagnostic laboratories. In-house testing includes Gram stains and at least one of the following: lower respiratory tract, wound, or blood cultures (http://www.bt.cdc.gov/lrn/biological.asp; http://www.aphl.org/aphlprograms/preparedness-and-response/partnerships-and-outreach/).
FIG 1.
CDC Laboratory Response Network (LRN): partners in preparedness. (Adapted from the CDC [http://www.bt.cdc.gov/lrn/pdf/lrn-overview-presentation.pdf].)
The responsibilities of a sentinel clinical laboratory include policies and procedures to refer diagnostic specimens or isolates of public health significance to local or state public health laboratories. Also, laboratory personnel must meet federal regulations for packing and shipping of infectious agents. The laboratory should have policies and procedures that reflect the “Sentinel Level Clinical Laboratory Protocols for Suspected Biological Threat Agents and Emerging Infectious Diseases” of the American Society for Microbiology (ASM) (http://www.asm.org/index.php/issues/sentinel-laboratory-guidelines). In addition, the laboratory should maintain the testing outlined in the ASM guidelines and demonstrate competency by participating in proficiency testing or exercises. From a facility standpoint, the laboratory should have a class II or higher certified biological safety cabinet. Also, the laboratory should comply with biosafety level II (BSL-2) practices and applicable Occupational Safety and Health Administration (OSHA) regulations. Lastly, the laboratory should comply with the rules and regulations of the Select Agent Program. Although sentinel laboratories are not required to register with the Select Agent Rule, they must be familiar with the Rule.
The second level of the pyramid is represented by confirmatory reference laboratories. These are typically public health laboratories, which may represent states, counties, or city services in large metropolitan areas. There are approximately 160 reference laboratories, whose role is to confirm or rule out suspected bioterrorism agents or emerging infectious agents. They have a responsibility to produce high-confidence test results for threat analysis and for interventions by public health authorities. At the apex of the pyramid are the national laboratories that definitively characterize samples and microbial isolates. The CDC and the U.S. Army Medical Research Institute for Infectious Diseases (USAMRIID) laboratory also have special containment areas with biosafety level IV (BSL-4) facilities.
SELECT AGENTS
Since 1997, the United States has defined biological select agents as agents derived from biological sources that can cause significant harm to public health and safety. Select agents are listed by either the U.S. Department of Health and Human Services (HHS) (those affecting humans) or the U.S. Department of Agriculture (USDA) (those affecting agriculture). The complete list of HHS and USDA select agents and toxins can be found at http://www.selectagents.gov/SelectAgentsandToxinsList.html (4). Toxins of various types, bacterial infections known to be spread easily and to have high morbidity and mortality, and a variety of hemorrhagic and encephalitic viruses are highlights of this list. Detailed information regarding the epidemiology of these agents can be found in the work of Elschner et al. (5).
In October 2012, the select agent list was updated, and 13 tier 1 agents were identified (4). Tier 1 agents are those that are at higher risk for causing high-consequence events. The criteria for a tier 1 agent are as follows: (i) the ability to cause a mass casualty event or economic devastation, (ii) communicability or dispersibility, (iii) a low infectious dose, and (iv) a history of interest in weaponization. The 2012 update also added the SARS-associated coronavirus and Chapare and Lujo viruses (Arenaviridae) to the list.
For the purposes of this discussion, I focus on the tier 1 select agents, since these agents have come to the most recent attention either as actual outbreaks or as presumed weaponized agents. The tier 1 select agents are as follows: botulinum neurotoxins, botulinum neurotoxin-producing species of Clostridium, Ebola virus, Francisella tularensis, Marburg virus, Bacillus anthracis, Burkholderia mallei, Burkholderia pseudomallei, variola major virus (smallpox), Yersinia pestis, and foot-and-mouth disease virus (aphthovirus). Botulinum neurotoxins and the Clostridium species that produce them have been known for many years to be risks for biological disasters. Their remarkable toxicity is related to the very low dose required for the neurotoxin effect, which largely causes muscle paralysis. The median lethal dose (MLD) is 0.3 to 1.2 ng/kg of body weight intramuscularly and 10 to 13 ng/kg when delivered via an aerosol route (6). Seven different immunotypes of botulinum toxins have been identified (7). Historically, poisoning was from poorly heated food products; however, sporadic cases still occur, such as the recent cases of wound botulism related to black-tar heroin contamination in southern California (8). Also, within the past 10 years, significant advances have occurred in our understanding of the four-step toxin inhibition of acetylcholine release from the presynaptic nerve terminal, which causes local nerve inactivation. Recently, a large commercial market has made several neurotoxins (three type A toxins and one type B toxin) readily available globally (9). By creating a flaccid paralysis, these toxins, when targeted appropriately, provide treatment for muscle contraction disorders, such as dystonias. Cosmetic applications are also a major use. The low MLD, availability, and easy administration warrant the tier 1 designation.
Another tier 1 select agent of particular note is Ebola virus. In 2014, the Ebola virus outbreak in West Africa caused massive numbers of deaths. Despite global interventions, the outbreak displayed the difficulties in managing an epidemic of this type. As of December 2014, over 18,000 cases had been reported, and nearly 12,000 of those were confirmed by laboratory testing. Over 6,800 deaths have also been confirmed (http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html). Ebola virus causes a form of hemorrhagic fever which begins with systemic clinical findings of fever, nausea, diarrhea, and muscle pain over an incubation period of up to 21 days. Transmission is through body fluids. However, because Ebola virus is an enveloped RNA virus, decontamination methods that disrupt the envelope are effective. Four of the five strains of Ebola virus are known to infect humans, including the Zaire strain represented in the recent outbreaks (10, 11). Preliminary estimates also indicate that very high viral loads are present in infected individuals (12). A quantitative investigation of RNA obtained from outbreaks indicated that mortality is associated with a 2-log increase in viral load. There is much to be learned from the recent Ebola epidemic. Ebola virus has a relatively limited transmission mechanism, with transmission occurring through body fluids. An even larger outbreak would have occurred well before public health agencies implemented effective interventions if Ebola virus were transmittable, for example, by aerosol. As noted by Bill Gates on behalf of the global community, a catastrophic epidemic is one of the few disasters that could derail world development (13). A more comprehensive discussion of preventative measures for laboratory staff and health care workers, based on this recent experience, is provided later in this article. Marburg virus was originally linked taxonomically to Ebola virus because of its filamentous form and other similarities in structure. However, it is antigenically and genomically distinct. A single species, Marburg marburgvirus, is currently recognized. Significantly more research has been completed on Marburg virus than on Ebola virus (14). The first outbreak was noted in Germany and was related to zoonotic transmission from research primates in 1967. Cases have since been observed in Uganda, and although the animal reservoir is uncertain (possibly bats), transmission occurs via body fluids. As more was learned about this virus, it was separated from the Ebola virus taxonomy (in 2001) (5, 11). Infection presents clinically as a hemorrhagic fever. The pathogenesis of Ebola and Marburg filoviruses appears to affect the host immune system by infecting monocytes and macrophages and producing a surplus of proinflammatory cytokines. The cytokines in turn disrupt the vascular system systemically (15).
Variola major virus, or smallpox virus, continues to be listed as a tier 1 select agent. Variola major virus and a less pathogenic related virus, variola minor virus, have caused epidemics throughout recorded history. Typically, the incubation period is 12 days for this large DNA poxvirus; it is easily spread both through contact with fomites from the large macular lesions and through an airborne route. The storied history of smallpox need not be replayed here. However, it was a frightening and disfiguring disease with high mortality and probably has afflicted humans for as many as 10,000 years (16). Vaccination was developed from crude preparations of lesions in the 18th century and became a common practice in the 19th century and well into the 20th century. In 1979, the World Health Organization (WHO) declared the virus eradicated. However, stocks persisted in the United States and Russia as putative bioterrorism agents. The final disposition of the stocks in Russia has never been confirmed. Also, the full DNA sequence is available, and there is some fear concerning possible regeneration of the virus or pathogenic viral components. As such, it persists as a tier 1 select agent (5, 17).
The World Health Organization has published an excellent 10-year review of the scientific research on variola virus (1999 to 2010) (18). In general, numerous molecular assays have been developed for smallpox and poxviruses. A total of 45 smallpox virus strains recovered between 1940 and 1977, with various epidemiologies, have been sequenced (19). The sequences were relatively homologous, allowing for ease in molecular targeting and testing. However, the sequence analysis may have been limited by the strains available historically in the repository. Serological assays are less well developed (18). Serological testing may allow further epidemiological assessment of poxvirus groups. None of the molecular or serological tests have been developed fully and cleared by regulatory agencies for use in general clinical microbiology laboratories.
I briefly mention here the animal virus causing foot-and-mouth disease, typically in cloven-hoofed domestic animals and wild animal populations. The virus is an aphthovirus and an RNA virus of the picornaviruses (20). Its primary risk is to the economy and the agricultural industry (21). Widespread epidemics have decimated domestic animal populations. It was probably first noted as a disease of domestic animals in Europe, in 1514. At the beginning of the 20th century, further work identified the causative agent as a virus. Foot-and-mouth disease virus is an RNA virus of about 8,500 bases and is a species within the Picornaviridae genus (21). As an unenveloped virus, foot-and-mouth disease virus can survive in a variety of zoonotic environments. It mutates readily, and as a consequence, vaccination has been challenging. Using inactivated vaccine preparations, the antibody response is also often delayed, allowing for susceptibility after inoculation (22). The last major outbreaks in the United States occurred in the early 20th century, originating in Michigan and spreading to the Chicago stockyards in 1914, causing a loss of $4.5 million dollars at that time.
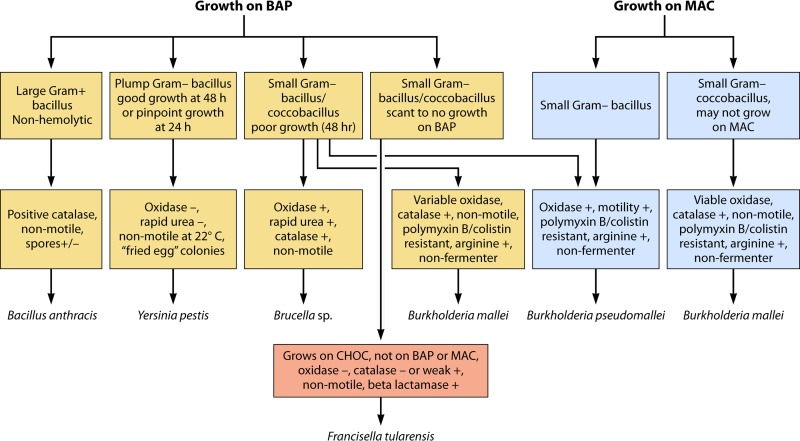
The remaining bacterial members of the tier 1 select agents include a group of four infrequently isolated, fastidiously growing Gram-negative bacteria and Bacillus anthracis. As cultivable agents, these bacteria present special issues to sentinel laboratories because they may first be identified at the “hands-on” level in a hospital or reference clinical microbiology laboratory. Some are fastidious and not immediately identified. Also, the use of automated identification equipment may lead to misidentifications as well as contamination problems. Special consideration of the diagnostic features of the Gram-negative organisms may be valuable for this group, as shown in Fig. 2. The Gram-negative bacteria to be discussed include Francisella tularensis, Burkholderia mallei, Burkholderia pseudomallei, and Yersinia pestis.
FIG 2.
Cultivable bioterrorism agents. BAP, blood agar plate; MAC, MacConkey plate.
Francisella tularensis is the causative agent of the disease tularemia. Humans are typically infected through deer fly or tick vectors as a zoonosis from rabbits, hares, and other wildlife (23, 24). Tularemia has an ulceroglandular and fever presentation, and infection is caused by a very small inoculum (10 to 50 organisms) (5, 25). Francisella tularensis subsp. tularensis is more pathogenic than F. tularensis subsp. holarctica. Classically, there are six different presentations: ulceroglandular (the most common), oculoglandular, pneumonic, oropharyngeal, gastrointestinal, and typhoidal (24). Francisella tularensis is unusual as an intracellular pathogen given that it has a broad animal host range, from mammals to reptiles and invertebrates. Humans tend to become infected most commonly from mammalian hosts, hence the common name “rabbit fever.” It can be transmitted by arthropods as well as environmental sources. It grows on chocolate agar but not on blood agar or MacConkey medium. It is relatively inert, oxidase negative, catalase negative (or weakly positive), and nonmotile. A notable feature is that it is beta-lactamase positive. Molecular diagnostics are complicated by the wide range of related organisms found in the environment. Vaccines have been derived from killed and attenuated sources, usually for veterinary purposes. Weaponization techniques allowing the organism to be aerosol dispersed, with survival times of up to 3 weeks, have been the reason for its serious consideration as a bioterrorism agent (25).
Burkholderia mallei and Burkholderia pseudomallei are similar, nonfermentative, fastidiously growing Gram-negative organisms. These two organisms are the causative agents of glanders and melioidosis, respectively. Melioidosis affects humans and animals and can be acquired from a contaminated environment, usually through percutaneous inoculation but also by inhalation and ingestion (5, 26). It is typically seen in Southeast Asia and Oceania. It can have 40% mortality when presenting as septicemia. Glanders primarily affects animals and can be transmitted from animal to animal and from animal to human. Most cases currently occur in relation to the agricultural or veterinarian work environment. However, it was implicated in the first modern attempt at biological warfare in World War I, when the Germans used it as a biological weapon against horses.
Burkholderia mallei is not an environmental pathogen compared to its close relative, B. pseudomallei (5). Distinguishing these two organisms in the clinical setting can be quite challenging, especially given their infrequency of isolation. B. pseudomallei grows on MacConkey medium and is motile (in contrast to B. mallei, which is nonmotile and does not grow on MacConkey medium). Both organisms show polymyxin B/colistin resistance. An excellent recent review discusses the molecular mechanisms of virulence in these two species (27). The B. mallei genome is smaller than the B. pseudomallei genome. B. pseudomallei also has two circular chromosomes. Most pathogenic mechanisms identified allow the organisms to survive intracellularly and to evade host immune responses.
Yersinia pestis is the etiological agent of bubonic plague. As such, it is another tier 1 select agent with a significant history of repeat plagues over hundreds of years. The plagues probably refashioned the societal changes that occurred in medieval Europe. Y. pestis is a member of the Enterobacteriaceae and is included with two other Yersinia spp.: Yersinia enterocolitica and Yersinia pseudotuberculosis. Yersinia pestis is primarily a rodent pathogen and is usually transmitted by an infected flea but can be transmitted by air, especially during pandemics (5, 28). It is found focally in animal-flea reservoirs in the southwestern United States but occurred as an outbreak in San Francisco as recently as the early 1900s, when plague erupted in Chinatown, and later, during the renovation of the city after the earthquake of 1906 (28). It is a plump Gram-negative bacillus with good growth at 48 h on blood agar media but pinpoint growth at 24 h. It is a relatively inert organism, being oxidase negative, rapid urea negative, and nonmotile at 22°C. The colonies have a “fried egg” appearance. The Y. pestis genome was recently fully sequenced. It appears to be very similar to that of Y. pseudotuberculosis, and some propose that it is a recently derived clone that evolved 1,500 to 20,000 years ago (29–31). More recent phylogenetic analysis indicates that Y. pestis evolved in or near China and spread westward in multiple iterations. In other words, the historically documented plagues actually reflect probable earlier behaviors of this pathogen (32). The particular fears related to Y. pestis as a bioterrorism agent include easy transmission as an airborne agent and its high mortality when epidemic, especially if it is weaponized to enhance organism stability and antibiotic resistance.
Bacillus anthracis is a notorious tier 1 select agent, especially given its close association with the events of 9/11 (33; http://www.cdc.gov/anthrax/news-multimedia/lab-incident/index.html). In the B. anthracis terrorist attack, the bacterium was distributed in a fine particulate form that infected not just the mail recipients but also other individuals whose mail was contaminated and postal workers who handled the mail. B. anthracis is endemic to livestock and survives for long periods as a desiccated spore, and infection can appear clinically in the following three forms: (i) a cutaneous form, (ii) gastrointestinal infection (rare), and (iii) pulmonary edema (very high mortality). Zoonoses caused by anthrax are found globally. The most typical presentation in humans is a skin lesion, which is the result of exposure to animals or animal products containing anthrax spores. A large outbreak occurred in Africa in the 1980s, with 10,000 human cases (34). The outbreak emphasized the impact of exposure to domestic animals as well as the importance of veterinary vaccination. Pathogenesis is caused by elements on two virulence plasmids: pXO1 and pXO2 (34). Both are essential for expressing toxicity (34). pXO1 contains three genes that create toxin virulence, namely, the genes encoding protective antigen (pag), lethal factor (lef), and edema factor (cya). pXO2 contains a five-gene operon responsible for capsule synthesis (34).
Among the cultivable bacteria on the list, B. anthracis is probably identified the most easily and quickly by standard culture methods (5). B. anthracis is a large Gram-positive bacillus that grows within 24 h on standard blood agar media. A distinguishing factor compared to other Gram-positive bacilli with similar morphology is a lack of hemolysis on blood agar plates. It is also positive for catalase, is nonmotile, and is an endospore-forming organism. Multiple molecular methods have also been developed for identification of B. anthracis, given the interest in developing field and general laboratory applications for bioterrorism agents. Amplification methods recently approved for emergency use include a LightCycler PCR assay developed by Roche and film array multiplex PCR technology developed by Idaho Technologies and BioFire. If it is not handled carefully, B. anthracis can create laboratory safety incidents, such as that which occurred in 2014 at the CDC (http://www.cdc.gov/anthrax/news-multimedia/lab-incident/index.html). However, the primary mode of dispersal for bioterrorism incidents is the spore and spore toxin, not the live, non-spore-forming bacillus. The spore is especially durable under conditions of drying and aerosolization (5).
AGENTS WITH HIGH RISK OF OCCUPATIONAL EXPOSURE AND PUBLIC ATTENTION
Simply considering the tier 1 agents does not adequately address other issues related to contamination and exposures in the clinical laboratory setting. Given the need to avoid occupational exposures in the clinical microbiology laboratory, several additional agents should receive high levels of attention among clinical microbiology laboratory directors. These organisms prove challenging to identify, and some have previously been considered select agents.
Brucella spp. are an example. Brucella spp. are a group of small Gram-negative coccobacilli that still are the most frequently reported laboratory-associated bacterial infections (5, 35, 36). They may have poor growth on blood agar media but are usually recognizable as oxidase positive and rapid urease positive (positive in 4 to 24 h, depending on the species). They are also catalase positive, with nonmotile growth. A dose of less than 5 CFU is sufficient for initiation of infection and disease. Serology still plays an important role in exposure analysis for this organism. For diagnosis of systemic infections, molecular methods have been more challenging. However, several recently described approaches can include both recent and relapsed cases (37, 38). Given the delay in early growth on standard culture media and the low infectious inocula, there are opportunities for aerosol transmission before the realization that an isolate is suspected to be Brucella. A recent literature review indicates that most cases are due to aerosolization of organisms during routine identification activities or unknown circumstances compared to a defined laboratory accident (36). A relatively low concentration of organisms (10 to 100 bacteria) can establish infection in humans. The three common species, Brucella abortus, Brucella melitensis, and Brucella suis, cause zoonoses that can be severe and chronic and cause spontaneous abortions and fetal death in pregnant women. Laboratory personnel in the United States are at risk because these are uncommon infections associated with nonspecific signs and symptoms. The incubation period is 8 weeks, and high-risk exposure cases are more likely to develop in cases of laboratory-acquired infection.
Another common bacterial species that can cause laboratory-acquired infection is Mycobacterium tuberculosis. M. tuberculosis is easily transmitted by low-inoculum aerosols and causes over 9 million new cases of human tuberculosis per year (39), with over 2 million deaths per year. The incidence of tuberculosis varies globally (39). It is 10- to 30-fold higher in Asia and Africa than in developed countries. As an easily aerosolized bacterium, M. tuberculosis presents unique containment issues. Although the incidence in the United States is low, at 10 per 100,000 persons, management within clinical laboratories to prevent occupational exposure has always had a high priority (40). Mycobacterium spp. are aerobic, nonmotile bacteria traditionally characterized by their acid-alcohol-fast staining properties and unique culture requirements. As a member of the Actinobacteria, M. tuberculosis is derived from a Gram-positive lineage and an original soil-based habitat (41). Close relatives include Nocardia and Rhodococcus. Molecular investigations of this organism have been extensive (42). Unique genes have been shown to promote infection with this organism, as it causes intracellular infection of macrophages (43). Testing for latent disease still requires the tuberculin skin test or a gamma interferon release assay (44). Sputum analysis and liquid culture are still recommended as standard methods for diagnosing active tuberculosis (44), but new tests are entering the market. An example is the Xpert MTB/RIF assay produced by Cepheid. This assay can detect M. tuberculosis and RIF, its multidrug resistance gene, in 2 h. Preliminary analysis showed a test sensitivity of 77% for smear-negative, culture-positive patients (45). However, most clinical laboratories either refer testing or refer samples for identification. Also, respiratory specimens are frequently received with clinical indications when tuberculosis is being considered. Thus, the overall risk to laboratory personnel is potentially decreased by this awareness of risk from the time of specimen receipt. Despite its designation as a BSL-3 organism, many laboratories engaged in M. tuberculosis testing have BSL-2 facilities that are designated by the CLIA laboratory director for BSL-3 manipulation with the use of enhanced safety practices. In a survey of over 1,000 clinical microbiology laboratories self-defined as sentinel laboratories, only 20% had full BSL-3 capabilities (40).
Finally, a former fungal select agent, Coccidioides immitis, should be mentioned as a risk to laboratory personnel. C. immitis and its close relative, Coccidioides posadasii, are pathogenic fungi found in the dry desert regions of the Southwest United States and Mexico (46). Like other occupational risk agents, it is easily aerosolized from its barrel-shaped arthroconidial form and can survive harsh, dry environments for prolonged periods. Also, the symptoms of infection can be nonspecific and may have a delayed presentation (6 to 8 weeks). Coccidioidomycosis can mimic common respiratory infections, hence the common term for the disease, valley fever. However, in some ethnic populations, the infection becomes systemic, chronic, and difficult to treat (47). Until October 2012, C. immitis was in fact considered a select agent by the CDC, as a BSL-3 agent. It was removed from the select agent list given recent advances in therapy and medical science. Clinical laboratories in regions of endemicity also tend to have significant experience with the likelihood of isolation and use of appropriate exposure restrictions. However, it is responsible for an estimated 150,000 undiagnosed cases per year. When arising as an unrecognized fungal infection acquired during travel, it can lead to laboratory occupational exposure in regions where the agent is not endemic (48; http://www.cdc.gov/fungal).
CLINICAL MICROBIOLOGY RISK ASSESSMENT FOR SELECT AND HIGH-RISK AGENTS
The term “biosurveillance” has become the umbrella term for a more comprehensive approach to bioterrorism and biological events. Over time, the divisions between public health, veterinary medicine, geopolitical events, and bioterrorism have become less distinct (49). Clinical microbiology laboratories may be confused regarding the numerous ways that their data can affect larger events. They should be aware that clinical laboratory records tend to fall within the detection step of data-driven biosurveillance schematics. Laboratory records, whether internal (occupational exposure) or external (epidemics or bioterrorism), are the most frequent records used in evaluating an outbreak (49).
There are several mechanisms by which individual laboratories may wish to develop an individualized risk analysis regarding their role in biosurveillance, including (i) the military and bioterrorism perspective, (ii) the public health standpoint, and (iii) the laboratory safety perspective. No one mechanism of risk management prioritization covers all opportunities for recovery of a biologically dangerous organism in a given laboratory. However, a brief discussion of these three aspects may guide clinical microbiology laboratory directors in evaluating risks for their operation.
Biological warfare threats are described militarily as one of three types of weapons of mass destruction: nuclear, chemical, and biological (50). In assessments of technology requirements, cost, and signature, biological weapons are relatively easy and cheap to manufacture and are considered high risk. Conversely, however, mechanisms such as vaccines may be available for protection of troops and the population, depending on the agent selected. Biological weapons can also be used to purposefully attack animal and plant food sources if introduced into a population. Weaponization refers to a modification of the infectious agent or toxin in a manner that makes it deliverable as an efficient weapon. An example is the case of anthrax. Anthrax is not transmittable from human to human, and its typical reservoir is domestic animals. Humans may become infected, however, through cutaneous, inhalational, or gastrointestinal exposure. Inhalational delivery is by far the most efficient method, causing mortality in 80 to 100% of humans infected by anthrax spores. A massive mediastinal respiratory distress and, often, secondary septicemia and meningitis result in shock and death within 24 to 36 h (51). To become an inhalational weapon, however, anthrax spores must be delivered in a breathable form, which requires processing them in a way that creates particulates of 5 nm or less (static-free). Once this is accomplished, 10,000 spores or fewer are sufficient to cause inhalational disease. A gram of anthrax spores contains 1 × 108 spores. It is not the purpose of this discussion to describe weaponization techniques. However, the possibility of the anthrax spore being aerosolized and its ability to survive in a dry powder, with a low inoculum for fatal disease, make it an intrinsically high risk militarily for weaponization. The following four questions (50) assist in assessing military risk. (i) Is the inoculum size (MLD) low? (ii) Can the agent be delivered easily? (iii) Does it cause high mortality? (iv) Can the agent survive in harsh environments?
The ability to work effectively with outside agencies is critical to the clinical microbiology laboratory's response to a potential bioterrorism agent. Guidance regarding data stream interaction and reporting of bioterrorism agents from a military perspective recently became more fully formed (52, 53). Even before widespread awareness occurred with the anthrax attack, 153 cases of actual or threatened use of bioterrorism agents were reported between 1990 and 1999 (54). That number has continued to increase. The reporting mechanisms from sentinel laboratories to federal, state, regional, and local agencies are important to our vigilance regarding a bioterrorism attack.
Prioritization of risk for presentation of a biologically dangerous agent can also be assessed based on public health reporting, the foundation of which is epidemiology. Public health activities are related to a number of laboratory sciences (microbiology, toxicology, and behavioral and survey research). Reporting is the foundation of the epidemiological sequence. The list of a state's reportable infectious agents is an excellent perspective from which to evaluate the risk of receiving a highly infectious organism. Also, the geography of a state often highlights important local infectious agents. For example, Yersinia pestis is found as sylvatic plague in the southwestern United States (55). As such, public health agencies nationally record a typical number of cases that are higher in the regions where rodent fleas infected with plague come into human contact. Some parts of the country have an abundance of “opportunity” for exposure based on geography. The southwestern United States, for example, has higher prevalences of tularemia, plague, anthrax, and coccidioidomycosis (56–58). As a consequence, the clinical laboratories in these states may have a higher risk for encountering these agents.
Population dynamics can also affect disease presentation and should be part of an individual laboratory's risk assessment. The movement of disease is clearly described by plague contemporaries. Giovanni Villani of Florence reports in 1348 of plague: “Having grown to vigor in Turkey and Greece, the said pestilence leaped to Sicily, and Sardinia and Corsica” (59). Daniel Defoe wrote in 1665 of the movement of the plague through the different regions of London and the exodus of the wealthy from large cities (60). Reading these early accounts is quite fascinating because of their similarities to the human reactions to more recent outbreaks, such as Ebola.
Other types of dynamics are more limited but also predictable. Histoplasma capsulatum, for example, was suddenly common in newly diagnosed HIV-positive patients in the western United States in the 1990s. This yeast is typically encountered as a respiratory pathogen in the Midwest. What happened? A midwestern population had migrated to the cities of the West, in particular Los Angeles and San Francisco, and become immunocompromised by HIV, and activated H. capsulatum infection acquired elsewhere became a prominent disease (61). Similarly, immigration across national boundaries contributes to public health scenarios. An example is the northern travel of Trypanosoma cruzi into the southwestern United States (62). Each clinical microbiology laboratory should build contemporary migrations and local migrations into their assessment for presentation of infectious diseases in their laboratory.
The third area to consider in a risk assessment by the clinical microbiology laboratory director is laboratory safety. Some of the microbiology laboratory safety considerations are very familiar. The precautions based on biosafety level (BSL) are intrinsic to managing risk within laboratory operations. The four BSLs each have their own containment controls that include laboratory practices, safety equipment, and facility requirements (63; http://www.cdc.gov/training/quicklearns/biosafety). Every laboratory should begin with standard microbiological practices. Appropriate equipment, including personal protective equipment (PPE), and facility requirements are added for each higher risk category. Several training modules and texts are available for easy reference (63; http://www.cdc.gov/training/quicklearns/biosafety). However, the risk management in this model is always a baseline expectation and is based on the risk of the agent being managed.
If these practices are presumed to always be in place, why do events still occur? A break in the BSL management process may be only one cause. Considerable effort should be made by clinical microbiology laboratory directors to also become familiar with the other risk priorities, as described above. In addition, they should have procedures in place for working with unknown high-risk etiologic agents or newly discovered agents. What we have learned about these additional procedures is discussed later in this document, with reference to the recent Ebola virus infections in the United States.
CLINICAL MICROBIOLOGY RESOURCES FOR MANAGEMENT OF BIOTERRORISM AND OUTBREAKS
Shortly after the anthrax attack of 2001, professional societies and government agencies came together to enhance the development of resources for front-line sentinel laboratories. The American Society for Microbiology (ASM) was in the forefront of this effort. Using the expertise of its scientific membership, ASM created the template for a bioterrorism readiness plan for sentinel laboratories (64). This document provides a comprehensive outline for each laboratory. It includes a communication plan, a discussion of the Laboratory Response Network (LRN), basic guidelines for bioterrorism agents, packing and shipping instructions, an information checklist, instructions for handling of possible bioterrorism agents, information on therapy for exposure, and a policy sign-off procedure. This comprehensive template now provides the basis for most clinical microbiology laboratory bioterrorism preparedness plans. ASM continues to keep this updated to meet developing scenarios. An overview of this information is also available at http://www.asm.org/index.php/guidelines/sentinel-guidelines.
The College of American Pathologists (CAP), the professional organization for pathologists in the United States and Canada, subsequently incorporated a requirement in its accreditation standards requiring a bioterrorism preparedness plan for each CAP-accredited clinical microbiology laboratory. CAP also created the Laboratory Preparedness Exercise (LPX), based on a model of survey specimen distribution and grading. This exercise was created in collaboration with the CDC and APHL and is discussed later in this article (40).
APHL (http://www.aphl.org) is the membership organization representing public health and governmental laboratories. It works with local, state, national, and international public health laboratories to ensure high-quality public health laboratory systems. APHL was also integral to the initial development of the LRN and worked closely with the CDC to accomplish this effort. It currently has over 800 laboratory members. An important role for APHL is the coordination of various state systems with the federal system of laboratories. Also, APHL is an important partner in LPX development.
The CDC is one of the major operational units of the U.S. Department of Health and Human Services. The CDC identifies and targets health problems and preventative mechanisms for disease. It provides reporting and statistical support and a national surveillance operation, provides laboratory expertise to the public health network, has an epidemiological investigative unit for introduced and newly recognized diseases, and provides regular reports of the latest surveillance and guideline information for all areas of health, from smoking cessation to infectious diseases. The CDC serves as the lead agency for the Public Health Service (PHS). There are many resources on bioterrorism and biological disasters available at the CDC website (http://www.cdc.gov).
In the United States, each state also has a public health network designed for the public health needs of the individual state. These needs can vary considerably, based on the geographic location of the state, the population, adjacencies to other countries, climate, occupational health and safety requirements, and agriculture and manufacturing activities. Some states have local and regional public health laboratories. California is an example of a state with multiple public health laboratories: it has county-based public health laboratories in some highly populated counties. Other states use a centralized state public health laboratory (South Dakota) or may have integral connections to academic or federal activities (Iowa). The reporting mechanisms begin with clinical microbiology sentinel laboratories for each state's reportable infectious agent list. Reporting occurs first to the local/regional level, then the state level, and finally the federal (CDC) level in most common circumstances. Certain exigencies can occur which can cause federal interests to work more closely with public health and clinical laboratories at the local level.
Other resources exist at the federal level, including the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) (http://www.usamriid.army.mil). USAMRIID has BSL-4 laboratories that can work with other federal agencies for serious BSL-4 outbreaks. USAMRIID also develops diagnostic assays. It was founded in 1969 to protect the military from biological risks and has since become a scientific resource for the study of bioterrorism agents. USAMRIID has expertise in testing vaccines and aerosols as well as many BSL-4 agents. It works closely with the U.S. Department of Health and Human Services. The Department of Agriculture also has a research unit, the Agricultural Research Service (ARS) (http://www.ars.usda.gov). This service works with state and academic agricultural cooperative organizations to address agricultural research objectives. It also specifically addresses some of the potential biological disasters that involve agriculturally associated transmissions or targets.
SENTINEL LABORATORY CHECKLISTS, EDUCATION, AND PERFORMANCE
Assessing preparedness for a biological emergency became a major topic of discussion after the anthrax attack of 2001. A key challenge is the level to which preparedness should be assessed (65). In one method, structure (resources and staff) can be incorporated into routine checklists. The College of American Pathologists includes checklist items related to bioterrorism protocols (66). Shortly after the anthrax outbreak, the CAP Commission on Laboratory Accreditation recommended checklist items for bioterrorism that include “proper collection, transporting, handling, testing, and shipping specimens collected from possibly exposed patients” (66). Other approaches include the use of metrics, which will depend somewhat on the frequency of bioterrorism organism encounters and development of an audit system for biosafety procedures. Recently, the roles of the CLIA laboratory director and sentinel laboratories were discussed explicitly from the biosecurity perspective, including the requirement of a CLIA laboratory director to adhere to biosafety guidelines and public health reporting mechanisms (67). Also noted in that recent review is the lack of biosecurity for select agents for international laboratories and shipping companies.
Sentinel Laboratory Performance
State activities.
There are various approaches to developing preparedness through the use of test exercises at the state level. These test exercises are sometimes confusingly referred to as proficiency testing, a term used for federal CLIA purposes. State public health policy may incorporate a test exercise in response to this need (68). The Wisconsin State Laboratory of Hygiene, located at the University of Wisconsin-Madison, has an active program that defines a sentinel laboratory and provides proficiency testing exercises as a free service to clinical laboratories in Wisconsin (http://www.slh.wisc.edu). The Wisconsin program allows participation of clinical, environmental, and international customers. Several states additionally use the LPX surveys (discussed below) to also assess performance at the state level. Examples include newsletters (Maryland State Public Health Laboratory) and summaries provided by the Michigan Department of Community Health, Bureau of Laboratories.
Education.
Education has been an important component directed toward performance. Clinical care physicians acknowledge the importance of sentinel laboratories in the evaluation of potential bioterrorism and emerging infections (69). Clinicians have been advised that education is warranted regarding awareness of overt threats as well as the potential for covert actions. In requesting testing from the sentinel clinical microbiology laboratory, clinicians additionally are advised to obtain optimal specimens. Laboratories are educated to limit culture manipulation and to refrain from viral culture. Also, they are advised to contact their local public health laboratory and to restrict manipulation of certain potential agents to a certified class II biological safety cabinet or a laboratory with BSL-3 conditions (67). Laboratories are receiving education using the continuously available resources of the CDC and APHL. Automated and commercial biochemical identification systems can be a source of contamination, in addition to often providing misleading information (69). Education alone, however, is never a complete answer.
National exercises.
As the LRN became more widely recognized, it also became imperative to develop more “real life” challenges that both educate and provide hands-on experience with techniques. This approach incorporates both the educational and test training and exercise aspects of managing potential bioterrorism agents. CAP, APHL, and the CDC collaborated in 2007 to develop a bioterrorism response educational exercise, incorporating the sending of attenuated or vaccine strains of bioterrorism agents, to achieve the following goals: (i) to provide LRN sentinel laboratories with a realistic bioterrorism agent challenge exercise; (ii) to provide an educational exercise that would test most aspects of a clinical microbiology laboratory response, including (a) ruling out and referral of potential bioterrorism agents by using appropriate LRN sentinel laboratory guidelines, (b) notification by the participating LRN sentinel laboratory to the appropriate local LRN reference laboratory of a potential bioterrorism agent, (c) packaging and shipping of organisms to the appropriate LRN reference laboratory (some laboratories provide actual shipping, and others use checklist approaches), and (d) assessment of the knowledge of appropriate laboratory protocols that address the safe handling of highly pathogenic organisms; and (iii) to provide information to state and local public health LRN reference laboratories about gaps in the LRN sentinel laboratory system (40).
The CAP/APHL/CDC bioterrorism exercises were among the first national programs to examine these capabilities for participating laboratories. Voluntary participant laboratories were sent two mailings (LPXA and LPXB) a year. Subscribers were informed that bioterrorism agents might be part of each challenge and that sterilization or appropriate disposal of the provided agents was required after performance of the exercise. Also, participating laboratories were required to “opt in” to the LPX program by submitting a signed affidavit stating that the laboratory was equipped with a certified class II biosafety cabinet and indicating that it would comply with BSL-2 practices (40).
The organisms presented in the challenges in the first 2 years were as follows: Bacillus anthracis (Sterne strain 34F2), Bacillus megaterium, Burkholderia thailandensis, Yersinia pestis (CDC A1122), Klebsiella pneumoniae, Aggregatibacter (Actinobacillus) actinomycetemcomitans, Francisella tularensis subsp. holarctica (NDBR 101), Aggregatibacter aphrophilus, Escherichia coli, Brucella abortus (strain RB51; vaccine strain), Salmonella enterica serogroup Typhimurium, Shigella sonnei, Yersinia enterocolitica, Corynebacterium diphtheriae (nontoxigenic), Staphylococcus aureus, and Malbranchea species. Between 1,100 and 1,200 laboratories participated in each exercise (two per year). Participating laboratories included public health laboratories as well as sentinel laboratories. Identification options ranged from full identification through a series of acceptable options depending on the laboratory type, including full identification, genus-level identification, determination of Gram-negative/positive morphology, determination of aerobic/anaerobic status, referral to rule out a bioterrorism agent, and detection of a nonbioterrorism agent (full identification not required) (40).
Options that could be selected for notification or reporting of the results included the following: contact the appropriate local LRN reference laboratory and follow its instructions, call the CDC, refer the isolate to the normal commercial reference laboratory, refer the isolate to the CDC, take no further action, and “other.” Data on the time interval between specimen processing and notification to an appropriate LRN reference laboratory about an identified or suspected bioterrorism agent were also collected, as well as identification procedures, including characteristics and tests (40).
Satisfactory responses for challenges meant to alert laboratories to a possible bioterrorism agent (B. anthracis, F. tularensis, and Y. pestis) during the 2 years of the survey tended to show improvement (Table 1) (40). For an agent meant to closely mimic bioterrorism agents, i.e., Burkholderia thailandensis (a mimic of B. pseudomallei and B. mallei), 28.1% of laboratories identified the organism as B. pseudomallei, and 39.2% were not able to rule out B. pseudomallei. The remaining participants identified this challenge as either B. thailandensis or a Gram-negative bacillus. In cases where standard biochemicals are used, the identification may depend on relatively few and esoteric features. For example, B. thailandensis and B. pseudomallei are both motile, and B. mallei is not. Also, B. thailandensis assimilates l-arabinose and adonitol and does not assimilate dulcitol and erythritol, in contrast to B. pseudomallei. What was learned from these exercises is that judging the capacity of a laboratory depends considerably on the expertise and facilities of each laboratory. Referral to a reference laboratory may be the most acceptable response for laboratories with more limited service. Reference laboratories also participated in these surveys. The percentage of reference laboratories reporting themselves as reference laboratories tended to be similar to the percentage performing full identification, although direct linking of these data to demographics was not performed (40).
TABLE 1.
Numbers of laboratories with acceptable identifications of Bacillus anthracis, Yersinia pestis, and Francisella tularensis in the LPX in 2007 and 2008
| Test organism | % satisfactory responses (no. of participants/total no. of participants) |
|
|---|---|---|
| 2007 | 2008 | |
| B. anthracis | 90.1 (1,109/1,231) | 99.9 (1,095/1,096) |
| Y. pestis | 83.8 (1,028/1,227) | 87.6 (1,135/1,296) |
| F. tularensis | 86.6 (959/1,107) | 91.6 (1,184/1,293) |
Some of the challenges included food and water pathogens more familiar to clinical microbiologists (Salmonella and Shigella). The laboratories tended to perform very well on these challenges and showed a familiarity with the appropriate testing mechanisms. Similarly, Corynebacterium diphtheriae was well identified and managed. Clearly, the more difficult organisms to detect were the fastidious Gram-negative organisms, such as F. tularensis and B. thailandensis. As training proceeds for sentinel laboratories, perhaps these types of organisms should be emphasized more thoroughly. Also, it was evident that not all participants used some essential tests for identification. Catalase testing for a Y. pestis isolate was “not performed,” for example, by 19.4% of reporting laboratories. The aspect of appropriate test availability for a sentinel laboratory may require examination by the collaborating organizations.
Another issue that needs to be addressed is the use of automated equipment to identify organisms. Currently, it is recommended for sentinel and reference laboratories that agents suspected of being bioterrorism agents should not be placed on automated instruments. This recommendation may change as new approaches, such as the use of film array technology panels for bioterrorism, become more prevalent. However, in the interim, while manual and automated identification procedures are more traditional, it is recommended that manual identification methods be used. In the 2008 Y. pestis challenge, 69.2% of laboratories applied automated detection on commercial systems, which is a cause for concern.
Over time, sentinel laboratories showed improvement in their understanding of the LRN and appropriate notification procedures. The number of participants indicating that they would notify their reference laboratories increased over time for the period of 3 to 6 days, which may reflect an appropriate interval depending on the organism examined. Also, the number of laboratories taking more than 10 days to report findings decreased over the same 2-year study (40).
Areas for Improvement in Sentinel Laboratories
In reviewing LPX results over time, there is a great deal of variation in the level of service covered under the definition of a sentinel laboratory. It may be relevant to examine the differences in more detail in order to standardize sentinel laboratories. Among the areas to consider for standardization are (i) the availability of required biochemical testing, (ii) the use of automated identification systems, and (iii) an expectation of notification turnaround time. Some of the tests used to fully identify the organisms associated with bioterrorism appear to be increasingly unavailable in clinical microbiology laboratories, as limited service has become a cost-saving trend. An example from the LPX survey is the use of catalase and urease tests, two key tests that need to be available for correct assessment of an unknown organism (40). If a laboratory accepts the designation of sentinel laboratory, criteria should include appropriate testing for the types of isolates anticipated. Also, there is considerable confusion over the use of automated identification methods for these types of agents. Many of the microorganisms in this category are fastidious. They may be overgrown by commensal flora and misidentified, or the algorithms for identification in automated equipment may not provide an accurate response. Also, the use of automated equipment provides the potential for contamination with a bioterrorism pathogen. Sentinel laboratories should carefully consider how they approach fastidious organisms as part of their procedures as automated equipment is implemented and should avoid using automated equipment for potential select agents. This recommendation also applies to reference laboratories. Finally, all sentinel laboratories should determine explicitly, as a part of their procedure, the expected turnaround time for notification of a public health laboratory of a potential bioterrorism agent. There has been improvement in this aspect in the LPX surveys, but there is still room for improvement.
The LPX surveys continue to be available through the CAP/APHL/CDC collaboration and show improvement in the management of potential bioterrorism agents. Refinement of the exercise could also be a goal as part of the standardization of laboratory preparedness methods.
NEW TECHNOLOGY AND BIOTERRORISM PREPAREDNESS
Clinical microbiology laboratories are currently in the midst of dealing with so-called “disruptive” technologies that may markedly advance our ability to detect potential bioterrorism agents in sentinel laboratories. These technologies include mass spectrophotometry, provided as matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry, and film array and similar multiplex PCR technologies. The two commercially available MALDI-TOF systems similarly use a wide-profile mass spectrophotometric method to examine large biomolecules, typically targeting proteins that can be used to identify select bacteria (70). Although an in-depth discussion of the technology is not presented here, it is clear that mass spectrometry methods allow discrimination of bacteria, including Francisella tularensis subspecies and other organisms of bioterrorism interest (71, 72). These methods have also been used to identify Burkholderia species and Bacillus spores (73, 74). A significant issue with MALDI-TOF mass spectrometry is the misidentification of select agents as nonbioterrorism agents because the database does not include select agents. Some laboratories have found it difficult to obtain libraries including select agents (75). Currently, clinical microbiology laboratories are in the process of implementing systems available from bioMérieux/Vitek and Bruker. Depending on the service, most are targeting a more rapid identification of blood culture specimens. Some laboratories are also engaging in laboratory-developed testing using primary positive blood culture bottles with successful CLIA validation to shorten the turnaround time for bacterial identification. The issue of using automated instrumentation for detection of suspected bioterrorism agents looms on the horizon of clinical laboratory decision-making and public health policy. The issue of contamination of automated equipment arose in the management of Ebola cases in the United States. Applying contamination stringencies to MALDI-TOF methods may be the next issue.
In addition to MALDI-TOF mass spectrometry, film array and other multiplex PCR techniques are being applied for the detection of bioterrorism agents. BioFire (formerly Idaho Technology) and Roche have both received emergency use authorization from the FDA for the purpose of identifying Ebola virus and other biological threats. The film array system uses advances in nanotechnology, including microfluidics, microelectronics, and microfabrication, to simplify molecular testing in a sample-to-result automation. The pouch for testing is self-contained, giving the added advantage of reducing the likelihood of cross-contamination of samples. The model of a single specimen per instrument also provides constraints on the number of specimens that can be tested at the same time. Analysis of melting curves for the PCR products allows automated assay interpretation. The first successful approach included a FilmArray respiratory panel that included multiple viruses as well as bacterial representatives, including Bordetella pertussis and Mycoplasma pneumoniae (76). The BioThreat-E FilmArray system includes individually packaged BioThreat-E FilmArray pouches that include detection reagents for 27 targets and 17 pathogens. Multiple targets are provided for Bacillus anthracis, Francisella tularensis, Brucella species, Rickettsia, Coxiella burnetii, Venezuelan equine encephalitis (VEE) virus, Yersinia pestis, and orthopoxviruses. This system's easy-to-use, single-specimen pouch and wide array of detection targets have the potential to bring the routine detection of select agents to the level of the sentinel laboratory, and it was used recently for analysis of Ebola virus infection in the United States. The FDA has approved four molecular tests for bioterrorism testing under emergency circumstances, including the Department of Defense EZ rRT-PCR assay (August 2014), the BioFire FilmArray biothreat panel (October 2014), the Roche LightMix Ebola Zaire rRT-PCR system (December 2014), and the Cepheid Xpert test (March 2015). The BioFire FilmArray panel is currently restricted to use by the Department of Defense. Clearly, this market will continue to develop rapidly and may provide significant assistance to sentinel laboratories in the detection of bioterrorism agents. However, public health agencies at the state and national levels will need to be involved to appropriately standardize reporting and network considerations.
RETHINKING MICROBIOLOGY LABORATORY SAFETY
It is clear that laboratory safety is paramount to the management of all highly infectious agents. In the earlier discussion of risk strategies, it exists as one of the three primary approaches. The current BSL system is the basis for all laboratory safety management in microbiology laboratories. However, we learned new things with the recent Ebola virus presentation in West Africa and in the United States. As a consequence of the Ebola presentation in the United States, many sentinel laboratories and health care institutions identified new safety issues for Ebola. Also, the development of these procedures may provide a template for the implementation of emergency laboratory services in highly infectious settings.
Periodically, bioterrorism and biological disasters present as epidemics. Bioterrorism is a subset of these occurrences. Much can be learned about bioterrorism management from these outbreaks. Ebola virus, a cause of hemorrhagic fever, was the most recent such outbreak. It is suspected that the index case for the Ebola virus outbreak of 2014 occurred in a young boy in Guinea in December 2013 after exposure to bats. Ebola is a viral disease with a fatality rate of 30 to 90%, depending on the virus species within the group of five species associated with Ebola hemorrhagic fever (77). Large outbreaks in sub-Saharan Africa occur for three species: Zaire ebolavirus, Bundibugyo ebolavirus, and Sudan ebolavirus. In October 2014, Baize et al. reported definitive identification of the West Africa Ebola outbreak, through genome sequencing and phylogenetic analysis, as deriving from a clade within Zaire ebolavirus (77).
The first WHO situation report describing the Ebola outbreak was released in August 2014. Guinea, Liberia, and Sierra Leone were already in a state of widespread and intense transmission. The number of cases reported at that time was 3,052, with 1,546 deaths (78). On 2 October 2014, the total number of reported cases had doubled, to 7,157 cases, with 3,330 deaths (78). By 5 November 2014, the WHO situation report described a total of 13,042 cases, with 4,818 deaths (78). Notably, a total of 546 health care workers were known to have been infected at the time of that report, and 310 of them died. Some of the early increase was probably related to improved reporting with the new heightened awareness of the disease. However, the increases were remarkable. The most recent written WHO situation report as of this writing (25 February 2015) indicates that management of the outbreak is working, with a total of 99 new confirmed cases that week and a downward trend for new cases. However, it is clear that all aspects of management have yet to be defined fully.
The CDC was similarly active in direct and supporting roles throughout the Ebola outbreak (79). The CDC issued a level 3 warning for U.S. citizens to avoid travel to Guinea, Liberia, and Sierra Leone. The CDC involvement heightened with the first reported case in the United States, in Dallas, TX. The patient was from Liberia and was visiting family. He died 8 October 2014, after exposing two health care workers who subsequently recovered. During the CDC and Texas public health management of this case, it became apparent that significant gaps existed in current recommendations for the handling of infectious diseases with high communicability from a blood source. The report from the CDC indicates a total of 23,948 cases, with mortality of 9,729 cases (77, 79), as of 2 March 2015.
The current CDC recommendations for clinical laboratory management can be found at the CDC website (http://www.cdc.gov/vhf/ebola/healthcare-us/laboratories/index.html). Care centers are determined to be Ebola assessment hospitals or regular health care facilities. Ebola assessment hospitals are designated facilities that are prepared to receive, isolate, and evaluate a potential Ebola case while the need for Ebola testing is assessed. These institutions also continue to provide care until an Ebola diagnosis is confirmed or ruled out and until a discharge or transfer is completed.
Shortly after wide publicity of the first case in the United States, it became clear that gaps in procedures existed for the safe management of clinical laboratory testing on a patient suspected of infection with this highly virulent blood- and fluid-borne viral pathogen. All laboratories were directed to first comply with the OSHA Bloodborne Pathogens Standard, a valuable specimen management document (80). Performance of site-specific risk assessments was advised for all clinical laboratories, including assessments of all work processes and procedures, to determine potential exposure risks and to mitigate these risks through engineering control, administrative controls, and use of appropriate personal protective equipment (PPE). Additional information regarding these controls and PPE can be obtained from CLSI document M29-A4 (Protection of Laboratory Workers from Occupationally Acquired Infections; Approved Guideline, 4th ed.) (81). Questions occurred regarding the safety of testing specimens on various types of laboratory equipment used for routine testing. The CDC recommended the use of manufacturer-recommended disinfectants or avoidance of use for testing equipment which generates an aerosol (82). The CDC and FDA are working with vendors of laboratory equipment to determine ways that disinfectants can be used and evaluated as part of the instrument review process.
Other specific considerations for safe laboratory testing include the use of closed collection systems and closed testing instrumentation without cap removal. Centrifugation poses a risk for aerosolization and requires review. Use of automated blood culture instruments also requires ensuring that the bottle from a suspected Ebola patient is cleaned with disinfectant or that manual incubation is performed in separate incubators. Point-of-care testing can be considered an option restricted to suspected Ebola patients. However, if the point-of-care instrumentation excludes testing of critically ill patients, this is considered off-label use by the FDA. A validation process for these instruments is necessary for use with critically ill patients in this scenario. If a point-of-care testing station is employed for use on suspected Ebola patients, workers should remember to not overload the flow of the designated biosafety cabinet. Use of a Plexiglas shield can also be considered. Transport of specimens also poses risks. Primary specimens should be handled with proper PPE and placed in a durable, leakproof secondary container; pneumatic transport of specimens should not occur. A specific travel plan for hand-carried specimens to avoid high-traffic areas should be considered in cases where high-risk specimens are to be moved.
As can be seen from the above discussion, several gaps may be identified in a given health care facility. The best advice regarding performing a robust risk assessment for highly virulent biospecimens is available at several resources. These references provide overall detailed information that well serves the development of a biosafety plan for highly virulent infectious agents. The Healthcare Infection Control Practices Advisory Committee (HICPAC), convened by the CDC, has an in-depth guideline for isolation precautions (82). Another excellent resource is a publication from a CDC-convened Biosafety Blue Ribbon Panel, entitled “Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories” (83).
The question still remains, though: how does an individual clinical microbiology laboratory director approach risk assessment and gap analysis for highly virulent biospecimens? We may soon hear more formal recommendations from the CDC. However, a place to begin for an individual clinical laboratory may be the use of prospective failure mode and effects analysis (FMEA) combined with a discrete quality management plan for unique biological disasters, such as the Ebola outbreak.
FMEA is a management tool that prospectively reviews the process map for receiving and testing highly virulent specimens. FMEA is a generic risk assessment tool that can also be used for many other risk questions. It provides a score in a prospective fashion, based on the combined frequency at which a high-risk event might occur, the visibility of a high-risk event, and the severity of the risk outcome (84). A numerical score is applied to each of the 3 factors and multiplied to provide an estimate of high-risk nodes in a process (85).
Assume that a suspected highly virulent specimen (e.g., blood from a suspected Ebola patient) is collected for a complete blood count (CBC) and basic metabolic panel. In an individual laboratory's process, this may involve phlebotomy, transport, specimen processing, testing, residual specimen management, and biological disposal, as a simplification of the process. FMEA would review each of these steps for opportunities for contamination from the specimen. A CBC would have somewhat less risk, for example, than use of some chemistry instruments, because most hematology instruments do not require cap removal to perform the testing. Potential corrective actions are then listed for each step (e.g., PPE for phlebotomy). By working through the process and identifying where risk can occur, each step in a process is accounted for in the analysis rather than taking a scatter approach to biosafety management. Similar processes within the clinical microbiology laboratory can also be performed in this fashion.
The Biosafety Blue Ribbon Panel convened by the CDC in 2012 additionally provides an excellent outline for individual clinical laboratories based on the elements found in a quality management system (83). For each of the 12 quality system essentials (QSE), specific biosafety considerations are provided. For example, in the first QSE, “organization,” a clear reporting structure is established for 24/7 reporting of safety incidents, and an organizational chart for reporting is developed. Similar recommendations are provided for each of these familiar 12 QSEs. Background regarding the quality management plan or system for those who would like to review this activity can also be found in laboratory management references (84). Combining a quality management system approach with FMEA provides an important risk assessment plan while more formal guidelines regarding the importance of laboratory biosafety are being developed by the CDC and other public health agencies.
CONCLUSIONS
A periodic overview of bioterrorism is an important activity for clinical microbiology laboratory directors, as highlighted by recent outbreaks, such as the Ebola outbreak. An earlier review of this topic clearly demarcated the history of bioterrorism and several of the possible agents of bioterrorism (86). Additional activities and knowledge have been acquired since the 2001 anthrax outbreak. These updates were reviewed here, including the recent redefining of the tier 1 agents by federal authorities. I also examined mechanisms that clinical microbiologists can incorporate into their risk assessments for bioterrorism in their laboratories. I reviewed the mechanisms that clinical microbiologists use to stay abreast of managing reporting requirements and specimen management in the face of significant changes in public health monitoring and microbiology testing technology. The performance of the LRN was discussed through an assessment of sentinel laboratory performance, in particular via the LPX. Finally, the importance of internal laboratory safety review was emphasized through a discussion of the recent Ebola epidemic. I cannot underestimate the value of sentinel laboratories to public biological safety, and I look forward to even more discussion with public health agencies regarding safe biological laboratory practices.
ACKNOWLEDGMENTS
I thank Nancy Cornish, Division of Laboratory Programs, Standards and Services, Center for Surveillance, Epidemiology and Laboratory Services, Office of Public Health Scientific Services, Centers for Disease Control and Prevention, for advice and consultation regarding the Ebola outbreak and laboratory biological safety. I also thank Judith Johnson for her excellent administrative assistance.
Biography

Elizabeth Wagar is Professor and Chair of the Department of Laboratory Medicine, University of Texas MD Anderson Cancer Center, Houston, TX, where she is also a Distinguished Professor and holds the Jose M. Trujillo Endowed Chair. She was the Laboratory Medical Director of the University of California, Los Angeles, Clinical Laboratories for over 10 years and served as the Residency Program Director for the Department of Pathology and Laboratory Medicine. Dr. Wagar received her M.D. from Michigan State University, East Lansing, MI, and performed an anatomic pathology and laboratory medicine residency at the University of California, San Francisco. She is board certified in anatomic and clinical pathology and has publications on topics ranging from basic molecular microbiology research to quality and administrative topics, including her 2011 book, Laboratory Administration for Pathologists. Dr. Wagar is on the Board of Governors for CAP and has special interests in quality programs, laboratory management, and clinical microbiology.
REFERENCES
- 1.Eitzen EM Jr, Takafuji ET. 1997. Historical overview of biological warfare, p 415–423. In Sidell FR, Takafuji ET, Franz DR (ed), Medical aspects of chemical and biological warfare. Office of the Surgeon General, Borden Institute, Walter Reed Army Medical Center, Washington, DC. [Google Scholar]
- 2.Stockholm International Peace Research Institute (SIPRI). 1971. The problem of chemical and biological warfare, vol 1. The rise of CB weapons. Humanities Press, New York, NY. [Google Scholar]
- 3.Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, Cetron M, Cohen M, Doyle T, Fischer M, Greene C, Griffith KS, Guarner J, Hadler JL, Hayslett JA, Meyer R, Petersen LR, Phillips M, Pinner R, Popovic T, Quinn CP, Reefhuis J, Reissman D, Rosenstein N, Schuchat A, Shieh WJ, Siegal L, Swerdlow DL, Tenover FC, Traeger M, Ward JW, Weisfuse I, Wiersma S, Yeskey K, Zaki S, Ashford DA, Perkins BA, Ostroff S, Hughes J, Fleming D, Koplan JP, Gerberding JL, National Anthrax Epidemiologic Investigation Team. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis 8:1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Health and Human Services. 2012. Possession, use and transfer of select agents and toxins, biennial review. Fed Regist 77:61084. [PubMed] [Google Scholar]
- 5.Elschner M, Cutler S, Weldmann M, Butaye F (ed). 2012. BSL3 and BSL4 agents: epidemiology, microbiology and practical guidelines. Wiley-Blackwell, Darmstadt, Federal Republic of Germany. [Google Scholar]
- 6.Montecucco C, Molgo J. 2005. Botulinal neurotoxins: revival of an old killer. Curr Opin Pharmacol 5:274–279. doi: 10.1016/j.coph.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Cherington M. 2004. Botulism: update and review. Semin Neurol 24:155–164. doi: 10.1055/s-2004-830901. [DOI] [PubMed] [Google Scholar]
- 8.Los Angeles Times. 17 December 2014. 3 cases of wound botulism in O.C.: here's what it is. http://www.latimes.com/local/lanow/la-me-ln-heroin-wound-botulism-california-20141217-story.html. Accessed 12 March 2015.
- 9.Jankovic J. 2004. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry 75:951–957. doi: 10.1136/jnnp.2003.034702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gire SK, Goba A, Andersen KG, Sealfon RS, Park DJ, Kanneh L, Jalloh S, Momoh M, Fullah M, Dudas G, Wohl S, Moses LM, Yozwiak N, Winnicki SM, Matranga CB, Malboeuf CM, Qu J, Gladden AD, Schaffner S, Yang X, Jiang P, Nekoui M, Colubri A, Coomber MR, Fonnie M, Moigboi A, Gbakie M, Kamara FK, Tucker V, Konuwa E, Saffa S, Sellu J, Jalloh AA, Kovoma A, Koninga J, Mustapha I, Kargbo K, Foday M, Yillah M, Kanneh F, Robert W, Massally JL, Chapman SB, Bochicchio J, Murphy C, Nusbaum C, Young S, Birren BW, Grant D, Scheiffelin JS, Lander ES, Happi CT, Gevao SM, Gnirke A, Rambaut A, Garry RF, Khan SH, Sabeti PC. 2014. Genomic surveillance elucidates Ebola virus origins and transmission during the 2014 outbreak. Science 345:1369–1372. doi: 10.1126/science.1259657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn JH, Becker S, Ebihara H, Geisbert TW, Johnson KM, Kawaoka Y, Lipkin WI, Negredo AI, Netesov SV, Nichol ST, Palacios G, Peters CJ, Tenorio A, Volchkov VE, Jahrling PB. 2010. Proposal for a revised taxonomy of the family Filoviridae: classifications, name of taxa and viruses, and virus abbreviations. Arch Virol 155:2083–2103. doi: 10.1007/s00705-010-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Towner JS, Rollin PE, Bausch DG, Sanchez A, Crary SM, Vincent M, Lee WF, Spiropoulou CF, Ksiazek TG, Lukwiya M, Kaducu F, Downing RR, Nichol ST. 2004. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol 78:4330–4341. doi: 10.1128/JVI.78.8.4330-4341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gates B. 2015. The next epidemic—lessons from Ebola. N Engl J Med 372:1381–1384. doi: 10.1056/NEJMp1502918. [DOI] [PubMed] [Google Scholar]
- 14.Brauburger K, Hume AD, Muhlberger E, Olejnik J. 2012. Forty-five years of Marburg virus research. Viruses 4:1878–1927. doi: 10.3390/v4101878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamadzadeh M, Chen L, Schmaljohn AL. 2007. How Ebola and Marburg viruses battle the immune system. Nature 7:556–567. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins DR. 2002. The greatest killer: smallpox in history. University of Chicago Press, Chicago, IL. [Google Scholar]
- 17.Pennington H. 2003. Smallpox and bioterrorism. Bull World Health Organ 81:762–767. [PMC free article] [PubMed] [Google Scholar]
- 18.Kah AS, Smith GL (ed). 2010. Scientific review of variola virus research, 1999–2010. WHO Document Production Services, Geneva, Switzerland. [Google Scholar]
- 19.Esposito JJ, Sammons SA, Frace AM, Osborne JD, Olsen-Rasmussen M, Zhang M, Govil D, Damon IK, Kline R, Laker M, Li Y, Smith GL, Meyer H, LeDuc JW, Wohlhueter RM. 2006. Genome sequence diversity and clues to the evolution of variola (smallpox) virus. Science 313:807–812. doi: 10.1126/science.1125134. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Salas E, Sobrino F. 2008. Foot and mouth disease virus, p 1–38. In Animal viruses: molecular virology. Caister Academic Press, Norfolk, England. [Google Scholar]
- 21.Grubman MJ, Baxt B. 2004. Foot-and-mouth disease. Clin Microbiol Rev 17:465–493. doi: 10.1128/CMR.17.2.465-493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doel TR. 2003. FMD vaccines. Virus Res 91:81–99. doi: 10.1016/S0168-1702(02)00261-7. [DOI] [PubMed] [Google Scholar]
- 23.Sjostedt A. 2007. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci 1105:1–29. doi: 10.1196/annals.1409.009. [DOI] [PubMed] [Google Scholar]
- 24.Foley JE, Nieto NC. 2010. Tularemia. Vet Microbiol 140:332–338. doi: 10.1016/j.vetmic.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, Lillibridge SR, McDade JE, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Tonat K. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 26.Gilad J, Schwartz D, Amsalem Y. 2007. Clinical features and laboratory diagnosis of infection with the potential bioterrorism agents Burkholderia mallei and Burkholderia pseudomallei. Int J Biomed Sci 3:144–152. [PMC free article] [PubMed] [Google Scholar]
- 27.Galyov EE, Brett PJ, DeShazer D. 2010. Molecular insights into Burkholderia pseudomallei and Burkholderia mallei pathogenesis. Annu Rev Microbiol 64:495–517. doi: 10.1146/annurev.micro.112408.134030. [DOI] [PubMed] [Google Scholar]
- 28.Perry RD, Fetherston JD. 1997. Yersinia pestis: etiologic agent of plague. Clin Microbiol Rev 10:35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkhill J, Wren BW, Thomson NR, Titball RW, Holden MT, Prentice MB, Sebaihia M, James KD, Churcher C, Mungall KL, Baker S, Basham D, Bentley SD, Brooks K, Cerdeño-Tárraga AM, Chillingworth T, Cronin A, Davies RM, Davis P, Dougan G, Feltwell T, Hamlin N, Holroyd S, Jagels K, Karlyshev AV, Leather S, Moule S, Oyston PC, Quail M, Rutherford K, Simmonds M, Skelton J, Stevens K, Whitehead S, Barrell BG. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413:523–527. doi: 10.1038/35097083. [DOI] [PubMed] [Google Scholar]
- 30.Achtman M. 2008. Evolution, population structure and phylogeography of genetically monomorphic bacterial pathogens. Annu Rev Microbiol 62:53–70. doi: 10.1146/annurev.micro.62.081307.162832. [DOI] [PubMed] [Google Scholar]
- 31.Achtman M, Morelli G, Zhu P, Wirth T, Diehl I, Kusecek B, Vogler AJ, Wagner DM, Allender CJ, Easterday WR, Chenal-Francisque V, Worsham P, Thomson NR, Parkhill J, Lindler LE, Carniel E, Keim P. 2004. Microevolution and history of the plague bacillus, Yersinia pestis. Proc Natl Acad Sci U S A 101:17837–17842. doi: 10.1073/pnas.0408026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morelli G, Song Y, Mazzoni CJ, Eppinger M, Roumagnac P, Wagner DM, Feldkamp M, Kusecek B, Vogler AJ, Li Y, Cui Y, Thomson NR, Jombart T, Leblois R, Lichtner P, Rahalison L, Peterson JM, Balloux F, Keim P, Wirth T, Ravel J, Yang R, Carniel E, Achtman M. 2010. Yersinia pestis genome sequencing identifies patterns of global phylogenetic diversity. Nat Genet 42:1140–1143. doi: 10.1038/ng.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer RC. 2003. Bacillus anthracis. J Clin Pathol 56:182–187. doi: 10.1136/jcp.56.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixon TC, Meselson M, Guillem J, Hanna PC. 1999. Anthrax. N Engl J Med 341:815–826. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 35.Robichaud S, Libman M, Behr M, Rubin E. 2004. Prevention of laboratory-acquired brucellosis. Clin Infect Dis 38:e119–e122. doi: 10.1086/421024. [DOI] [PubMed] [Google Scholar]
- 36.Traxler RM, Lehrmen MW, Bosserman EA, Guerra MA, Smith TL. 2013. A literature review of laboratory-acquired brucellosis. J Clin Microbiol 51:3055–3062. doi: 10.1128/JCM.00135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nimri LF. 2003. Diagnosis of recent and relapsed cases of human brucellosis by PCR assay. BMC Infect Dis 3:5–12. doi: 10.1186/1471-2334-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Debeaumont C, Falconnet PA, Maurin M. 2005. Real time PCR for detection of Brucella spp. DNA in human serum samples. Eur J Clin Microbiol Infect Dis 24:842–845. doi: 10.1007/s10096-005-0064-0. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. 2009. WHO report: global tuberculosis control 2009: epidemiology, strategy, financing. WHO/HTM/TB/2009.411. WHO, Geneva, Switzerland. [Google Scholar]
- 40.Wagar EA, Mitchel MJ, Carroll KC, Beavis KG, Petti CA, Schlaberg R, Yasin B. 2010. A review of sentinel laboratory performance: identification and notification of bioterrorism agents. Arch Pathol Lab Med 137:1490–1503. doi: 10.1043/2010-0098-CP.1. [DOI] [PubMed] [Google Scholar]
- 41.Bentley SD, Chuter KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harbper D, Bateman A, Brown S, Chancra G, Chen CW, Collins M, Cromin A, Fraser A, Goble A, Hidalgo J, Horasby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O'Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzurrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147. doi: 10.1038/417141a. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. 2004. Tuberculosis nucleic acid amplification testing performance evaluation program. CDC, Atlanta, GA. [Google Scholar]
- 43.Smith I. 2003. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev 16:463–495. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zumla A, Raviglione M, Hafner R, von Reyn CF. 2013. Tuberculosis. N Engl J Med 368:745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 45.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, Gler MT, Blakemore R, Worodria W, Gray C, Huang L, Caceres T, Mehdiyev R, Raymond L, Whitelaw A, Sagadevan K, Alexander H, Albert H, Cobelens F, Cox H, Alland D, Perkins MD. 2011. Feasibility, diagnostic accuracy and effectiveness of decentralized use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicenter implementation study. Lancet 377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parish JM, Blair JE. 2008. Coccidioidomycosis. Mayo Clin Proc 83:343–348. doi: 10.4065/83.3.343. [DOI] [PubMed] [Google Scholar]
- 47.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, Williams PM. 2005. Coccidioidomycosis. Clin Infect Dis 41:1217–1223. doi: 10.1086/496991. [DOI] [PubMed] [Google Scholar]
- 48.Tsang CA, Anderson SM, Imholte SB, Erhart LM, Chen S, Park BJ, Christ C, Komatsu K, Chiller T, Sunenshine R. 2010. Enhanced surveillance of coccidioidomycosis, Arizona, USA, 2007–2008. Emerg Infect Dis 16:1738–1744. doi: 10.3201/eid1611.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Margevicius KJ, Generous N, Taylor-McCabe KJ, Brown M, Daniel WB, Castro L, Hengartner A, Deshpande A. 2014. Advancing a framework to enable characterization and evaluation of data streams useful for biosurveillance. PLoS One 9:e83730. doi: 10.1371/journal.pone.0083730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eitzen EM. 1997. Use of biological weapons, p 451–466. In Zajtchuk R, Bellamy RF (ed), Textbook of military medicine: warfare, weaponry, and the casualty, part I. Office of the Surgeon General, Department of the Army USA, Washington, DC. [Google Scholar]
- 51.Friedlander AM. 1997. Anthrax, p 467–478. In Zajtchuk R, Bellamy RF (ed), Textbook of military medicine: warfare, weaponry, and the casualty, part I. Office of the Surgeon General, Department of the Army USA, Washington, DC. [Google Scholar]
- 52.Karwa M, Currie B, Kvetan V. 2005. Bioterrorism: preparing for the impossible or the improbable. Crit Care Med 33(Suppl 1):S75–S95. doi: 10.1097/01.CCM.0000151070.56915.22. [DOI] [PubMed] [Google Scholar]
- 53.Cheng KE, Crary DJ, Ra J, Safta C. 2013. Structural models used in real-time biosurveillance outbreak detection and outbreak curve isolation from noisy background morbidity levels. J Am Med Inform Assoc 20:435–440. doi: 10.1136/amiajnl-2012-000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grundmann O. 2014. The current state of bioterrorist attack surveillance and preparedness in the US. Risk Manag Healthc Policy 7:177–187. doi: 10.2147/RMHP.S56047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wimsatt J, Biggins DE. 2009. A review of plague persistence with special emphasis on fleas. J Vector Borne Dis 46:85–99. [PubMed] [Google Scholar]
- 56.Petersen JM, Mean PS, Schriefer ME. 2009. Francisella tularensis: an arthropod-borne pathogen. Vet Res 40:7–16. doi: 10.1051/vetres:2008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blackburn JK, McNyset KM, Curtis A, Hugh-Jones ME. 2007. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecologic niche modeling. Am J Trop Med Hyg 77:1103–1110. [PubMed] [Google Scholar]
- 58.Hector RF, Laniado-Laborin R. 2005. Coccidioidomycosis—a fungal disease of the Americas. PLoS Med 2:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly J. 2006. The great mortality: an intimate history of the black death, the most devastating plague of all time, p 102 Harper Collins, New York, NY. [Google Scholar]
- 60.Defoe D. 2013. A journal of the plague year, 1722: being observations or memorials, of the most remarkable occurences, as well publick as private, which happened in London during the Last Great Visitation in 1665. Forgotten Books, London, United Kingdom: (Original work published 1722.) [Google Scholar]
- 61.Wheat LJ, Chetchotisakd P, Williams B, Connolly P, Shutt K, Haijeh R. 2000. Factors associated with severe manifestations of histoplasmosis in AIDS. Clin Infect Dis 30:877–881. doi: 10.1086/313824. [DOI] [PubMed] [Google Scholar]
- 62.Bern C, Kjos S, Yabsley J, Montcombery SP. 2011. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev 24:655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.US Department of Health and Human Services. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed. HHS publication no. (CDC) 21-1112 HHS, Washington, DC. [Google Scholar]
- 64.American Society for Microbiology. 2006. Sentinel laboratory guidelines for suspected agents of bioterrorism: clinical laboratory bioterrorism readiness plan. http://www.asm.org/ccLibraryFiles/FILENAME/000000002178/BTtemplate3-02-06.pdf ASM, Washington, DC: Accessed 23 February 2015. [Google Scholar]
- 65.Nelson C, Lurie N, Wasserman J. 2007. Assessing public health emergency preparedness: concepts, tools and challenges. Annu Rev Public Health 28:1–18. doi: 10.1146/annurev.publhealth.28.021406.144054. [DOI] [PubMed] [Google Scholar]
- 66.Carlson D. February 2002. Winds of change—training, checklists, pricing. CAP Today 2002:83. [Google Scholar]
- 67.Rao V, Eilers A, Mancini C. 2006. Select agents diagnostic test reporting requirements—exemptions and implications to biosecurity. Appl Biosaf 11:215–221. [Google Scholar]
- 68.Martin W. 2004. Legal and public policy responses of states to bioterrorism. Am J Publ Health 7:1093–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pien BC, Royden J, Saah Miller SE, Woods CW. 2006. Use of sentinel laboratories by clinicians to evaluate potential bioterrorism and emerging infections. Clin Infect Dis 42:1311–1323. doi: 10.1086/503260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seng P, Drancourt M, Gouriet F, La Scola B, Fournier P-E, Rolain JM, Raoult D. 2009. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis 49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 71.Lundquist M, Caspersen MB, Wikstrom P, Forsman M. 2005. Discrimination of Francisella tularensis subspecies using surface enhanced laser desorption ionization mass spectrometry and multivariate data analysis. FEMS Microbiol Lett 243:303–310. doi: 10.1016/j.femsle.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 72.Hsieh SY, Tseng CI, Lee YS, Kuo AJ, Sun CF, Lin YH, Chen JK. 2008. Highly efficient classification and identification of human pathogenic bacteria by MALDI-TOF MS. Mol Cell Proteomics 7:448–456. [DOI] [PubMed] [Google Scholar]
- 73.Vanlaere E, Sergeant K, Dawyndt P, Kallow W, Erhard M, Sutton H, Dare D, Devreese B, Samyn B, Vandamme P. 2008. Matrix-assisted laser desorption ionization–time-of-flight mass spectrometry of intact cells allows rapid identification of Burkholderia cepacia complex. J Microbiol Methods 75:279–286. doi: 10.1016/j.mimet.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 74.Hathout Y, Demirev PA, Ho YP, Bundy J, Ryzhov V, Sapp L, Stutler J, Jackman J, Fenselau C. 1999. Identification of Bacillus spores by matrix-assisted laser desorption ionization–mass spectrometry. Appl Environ Microbiol 65:4313–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cunningham SA, Patel R. 2013. Importance of using Bruker's security-relevant library for biotype identification of Burkholderia pseudomallei, Brucella species, and Francisella tularensis. J Clin Microbiol 51:1639–1640. doi: 10.1128/JCM.00267-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pierce VM, Elkan M, Leet M, McGowan KL, Hodinka RL. 2012. Comparison of the Idaho Technology FilmArray system to real-time PCR for detection of respiratory pathogens in children. J Clin Microbiol 50:364–371. doi: 10.1128/JCM.05996-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, Soropogui B, Sow MS, Keïta S, De Clerck H, Tiffany A, Dominguez G, Loua M, Traoré A, Kolié M, Malano ER, Heleze E, Bocquin A, Mély S, Raoul H, Caro V, Cadar D, Gabriel M, Pahlmann M, Tappe D, Schmidt-Chanasit J, Impouma B, Diallo AK, Formenty P, Van Herp M, Günther S. 2014. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 371:1418–1425. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 78.World Health Organization. 29 August 2014. WHO: Ebola response roadmap: situation report 1. WHO, Geneva, Switzerland: http://www.who.int/csr/disease/ebola/situation-reports/archive/en Accessed 2 March 2015. [Google Scholar]
- 79.Centers for Disease Control and Prevention. 2014. Ebola outbreak in West Africa. CDC, Atlanta, GA: http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html Accessed 2 March 2015. [Google Scholar]
- 80.Occupational Safety and Health Administration. 2011. Bloodborne pathogens standard 29 CFR 1910.1030. OSHA, Washington, DC: https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10051 Accessed 2 March 2015. [Google Scholar]
- 81.Clinical and Laboratory Standards Institute (CLSI). 2014. Protection of laboratory workers from occupationally acquired infections; approved guideline, 4th ed CLSI document M29-A4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 82.Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. 2007. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. CDC, Atlanta, GA: http://www.cdc.gov/ncidod/dhqp/pdf/isolation2007.pdf Accessed 24 February 2015. [Google Scholar]
- 83.Miller JM, Astles R, Baszler T, Chapin K, Carey R, Garcia L, Gray L, Larone D, Pentella M, Pollock A, Shapiro DS, Weirich E, Wiedbrauk D, Biosafety Blue Ribbon Panel, Centers for Disease Control and Prevention (CDC). 2012. Guidelines for safe work practices in human and animal medical diagnostic laboratories. Recommendations of a CDC-convened, Biosafety Blue Ribbon Panel. MMWR Surveill Summ 61(Suppl:1–102. [PubMed] [Google Scholar]
- 84.Wagar EA, Horowitz RE, Siegal G. 2011. Quality management in laboratory medicine, p 130–132. In Laboratory management for pathologists. CAP Press, Northfield, IL. [Google Scholar]
- 85.Mikulak RJ, McDermott R, Beauregard M (ed). 2008. The basics of FMEA, 2nd ed Productivity Press, New York, NY. [Google Scholar]
- 86.Klietmann WF, Ruoff KL. 2001. Bioterrorism: implications for the clinical microbiologist. Clin Microbiol Rev 14:364–381. doi: 10.1128/CMR.14.2.364-381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]