Abstract
Objective:
To determine the diagnostic value of serum white blood cell (WBC) count, fever (>38˚C) and WBC rise (>10%) for bacterial meningitis in patients with severe traumatic brain injury (TBI).
Method:
This cross-sectional study was conducted in Shahid Rajaei hospital affiliated with Shiraz University of Medical Sciences during a 1-year period from 2013 to 2014. We included consecutively all the patients with severe TBI admitted to our center during the study period who were febrile (>38˚C orally) and underwent lumbar puncture (LP) and analysis and culture of cerebrospinal fluid (CSF). Laboratory analysis of CSF and blood were performed within 2 hours of LP. CSF culture was considered the gold standard for diagnosis of bacterial meningitis. The sensitivity, specificity, positive and negative predictive value (PPV, NPV) of peripheral blood WBC count, fever (>38˚C) and WBC rise (>10%) was determined according to the CSF culture.
Results:
Overall we included242 consecutive patients with severe TBI. The mean age of the participants was 32.8 ± 17.4 years. Acinetobacter was the most common organism found in the CSF cultures. The sensitivity and specificity of peripheral WBC count (>10,000)was 48.4% (95% CI: 0.42-0.56) and 47% (95% CI: 0.37-0.58) respectively. The PPV and NPV was 13.1% (95% CI: 0.33-0.52) and 84.8% (95% CI: 0.42-0.61), respectively. The AUC for WBC count was 0.478 (95% CI: 0.37-0.58) indicating low accuracy for the diagnosis of bacterial meningitis. The AUC for WBC rise (>10%) and temperature >38˚C was0.460 (95% CI: 0.351-0.569) and 0.517 (95% CI: 0.410-0.624) respectively, both indicating low accuracy for diagnosis of bacterial meningitis.
Conclusion:
The results of the current study indicates that peripheral blood leukocyte count, fever (>38˚C) and WBC rise (>10%) is a non-reliable marker for diagnosis of bacterial meningitis in patients with severe TBI.
Key Words: Cerebrospical fluid (CSF), Traumatic brain injury (TBI), Leukocyte count, Sensitivity, Specificity; Positive predictive value (PPV), Negative predictive value (NPV)
Introduction
Traumatic brain injury (TBI) is defined as a condition in which the brain function is disrupted via blunt (motor vehicle accidents, falls, and assaults) or penetrating trauma. The severity of TBI is assessed according to the severity of mental status impairment [1,2]. From 1.7 million people who suffer from TBI each year in United States, around 50,000 die and, 90,000 people become disabled. Young men constitute the largest group of patients with TBI [2]. Although infections can complicate TBI both in outpatient and inpatient settings, those hospitalized are at greater risk. The possible TBI related infections are, urinary tract infection (UTI), pneumonia, surgical site infection, catheter associated bacteremia/blood stream infections, and meningitis; with ventilator associated pneumonia (VAP) being the most and meningitis the least common [3].
There is a 10% risk of meningitis among TBI patients [4]. Following brain injury, a cascade of events including the release of proteases, amino acids, reactive oxygen species (ROS) leads to the disruption of blood brain barrier (BBB). The disruption of cerebral homeostasis causes brain edema, increased intracranial pressure (ICP), impaired cerebral perfusion, decreased level of consciousness (LOC) and may make patient vulnerable to meningitis [5,6]. The risk factors of bacterial meningitis in patients with TBI are: having lumbar or ventricular drain, prolonged intensive care unit (ICU) admission (>7 days) and overall hospital stay (>50 days), basilar skull fracture with and without cerebrospinal fluid (CSF) leakage and adjacent facial infections.
Regarding the causative agent, gram positive organisms is the most prevalent organism found in CSF cultures [3].
Meningitis can be diagnosed by patient’s clinical features (neck stiffness, fever, positive brudzinski and kernig sign), CSF culture, gram stain, and its protein, sugar, leukocyte count and differentiation. Although lumbar puncture remains the mainstay in the diagnosis of meningitis, it carries several complications such as brain herniation [7]. In addition, empirical administration of antibiotics in most patients with severe TBI is associated with increased false negative results of CSF analysis [8]. The most important fact is that patients suffering from TBI may have CSF leukocytosis and bloody CSF. Standard CSF tests for the diagnosis of bacterial meningitis in neurosurgical patients have been limitedly studied [8,9]. It has been reported that the examined parameters (total and differential leukocyte counts, gram stains, and values for glucose and total protein) are either not sensitive or not specific enough to reliably distinguish bacterial meningitis from non-bacterial meningitis [8,9]. So, it seems that there is a need to find a less invasive method to detect meningitis in TBI patients. It has also been reported that bacterial meningitis is associated with peripheral blood leukocytosis [10-12]; thus it seems that the white blood cell (WBC) count can be used to predict the possibility of meningitis in TBI patients. This study, thus, aims to determine the diagnostic accuracy of peripheral blood WBC count, fever (>38˚C) and WBC rise (>10%) in predicting bacterial meningitis in patients with severe TBI.
Materials and Methods
Study Population
This was a cross-sectional study being performed in Shahid Rajaei hospital, a Level I trauma center affiliated with Shiraz University of Medical Sciences during a 1 year period from August 2013 to August 2014. The study protocol was approved by the institutional review board (IRB) and medical ethics committee of Shiraz University of Medical Sciences. All the patients’ formal guardians provided their informed written consents before their patient being included in the study. We included consecutively all adult patients (>18 years of age) with severe TBI (Glasgow coma scale (GCS) of less than 8) who were febrile (>38˚C). We excluded all the patients with non-CNS infections such as pneumonia, surgical site infection, UTI, sepsis, peritonitis, pharyngitis, osteomyelitis and wound infections. Patients with malignancies, renal failure, hepatic failure, those with leukemia and those receiving steroids were excluded from the study.
Study Protocol
A data form was designed consisting of two parts. The first part included the demographic information such as age, gender and place of residence and neurological status related parameters such as GCS, brain CT scan findings, and whether the patients undergo intrathecal or systemic antibiotic therapy or any neurosurgical procedure. The second part was a table in which the results of laboratory studies including peripheral WBC count, CSF analysis parameters including protein, sugar, simultaneous blood sugar, gram stain, culture, total cell count, WBC count, differential count of polymorphonuclear (PMN). Lumbar puncture (LP) was performed by inserting a spinal needle in the L4-L5 or L5-S1 sub-arachnoid space of the patient lying on the side in a lateral decubitus position. The procedure was performed by a neurosurgeon. Elevated intracranial pressure (ICP), infection over the puncture site and bleeding diathesis were contraindications to LP. We also recorded the axillary body temperature, and any sudden rise of WBC from the previous test (>10%). Laboratory analysis of CSF and blood were performed within 2 hours of lumbar puncture by the central laboratory for bacteriology and chemistry. Meningitis was diagnosed based on the CSF parameters: CSF PMN>10%+CSF sugar/ Serum Sugar<50%) and a positive CSF culture. The results were considered suggestive of meningitis if PMN>5±CSF Sugar/Serum sugar<50%). Meningitis was excluded if PMN<5 or CSF sugar/ serum sugar>70%. The gold standard for diagnosis of bacterial meningitis was the CSF culture. CSF culture performed in Shahid Rajaei Microbiology laboratory immediately. The standard technique on culture media for 48-hour were used for CSF culture.
Statistical Analysis
All the statistical analyses in the current study were performed by the Statistical Package for Social sciences (SPSS Inc., Chicago, USA) version 16.0. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of peripheral WBC count was assessed according to the results of CSF culture. The receiver operating characteristic (ROC) curve was also drawn and area under curve (AUC) was calculated. AUC of more than 0.9 was considered good diagnostic accuracy while 0.7 to 0.9 was considered moderate and less than 0.7 was considered week accuracy.
Results
Overall we included 242 consecutive febrile patients with severe TBI who full filled the study criteria. The mean age of the patients was 32.8±17.4 (ranging from 15 to 82) years. Among them there were 232 (95.9%) men and 10 (4.1%) women. The baseline characteristics of the patients are summarized in Table 1.
Table 1.
Baseline characteristics of 242 febrile patients with severe traumatic brain injury suspected to have bacterial meningitis
| Variables | Value |
|---|---|
| Age (years) | 32.8 ± 17.4 |
| Gender | |
| Men | 232 (95.9%) |
| Women | 10 (4.1%) |
| Mechanism of injury | |
| Motor vehicle accidents (%) | 193 (79.7%) |
| Falls (%) | 40(16.5%) |
| Assault trauma (%) | 93.7%) |
| Others (%) | 0 (0%) |
| Brain CT-Scan Findings | |
| Fractures (%) | 178 (73.5%) |
| Epideural hematoma (%) | 123 (50.8%) |
| Contusions (%) | 92 (38%) |
| Subdural hematoma (%) | 75 (30.9%) |
| Subarachnoid hemorrhage (%) | 63 (26%) |
| Intracerebral hemorrhage (%) | 39 (16.1%) |
| Others (%) | 10 (4.1%) |
| GCS | 6.73 ± 3.23 |
| Treatment | |
| Surgery (%) | 119 (49.1%) |
| Conservative (%) | 123 (50.8%) |
| Antibiotic therapy | |
| Yes (%) | 191 (78.9%) |
| No (%) | 51 (21.1%) |
| CSF results | |
| Proven bacterial meningitis (%) | 89 (36.8%) |
| Suspected bacterial meningitis (%) | 14 (5.8%) |
| Excluded meningitis (%) | 139 (57.4%) |
| Culture results | |
| Positive (%) | 36 (14.8%) |
| Negative (%) | 206 (85.1%) |
The microorganisms identified in CSF samples of 36 patients with bacterial meningitis consisted of Acinetobacter (n=18), Staphylococcus aureus (n=13), Pseudomonas aeroginosa (n=2), Escherichia coli (n=2) and Klebsiella pneumonia (n=1). The brain CT-Scan findings were brain contusion, epidural, subdural and intracerebral hemtomas, pneumocephalus and subarachnoid hemorrhage (Table 1). The most common mechanism of injury was motor vehicle accidents accounting for 193 (79.7%) of cases followed by falls in 40 (16.5%) and assault trauma in 9 (3.7%) patients. There were 4 (1.65%) patients who were treated with intrathecal antibiotics (Amikacin and Vancomycin).
The Positive and negative predictive values of patients with WBC>10,000 for the diagnosis of post-traumatic meningitis were 13.1% (95% CI: 0.33-0.52) and 84.8% (95% CI: 0.42-0.61), respectively. Its respective sensitivity and specificity were 48.4% (95% CI: 0.42-0.56) and 47% (95% CI: 0.37-0.58). Furthermore, we have assessed the predictive values of temperature of >38oC for the diagnosis of bacterial meningitis. While The PPV was 14.2% (95% CI: 0.25-0.42), the NPV was 84.3% (95% CI: 0.63-0.76). The sensitivity of temperature >38oC for the diagnosis of meningitis was 55.5% (95% CI: 0.44-0.63) and specificity was 41.7% (95% CI: 0.41-0.61).
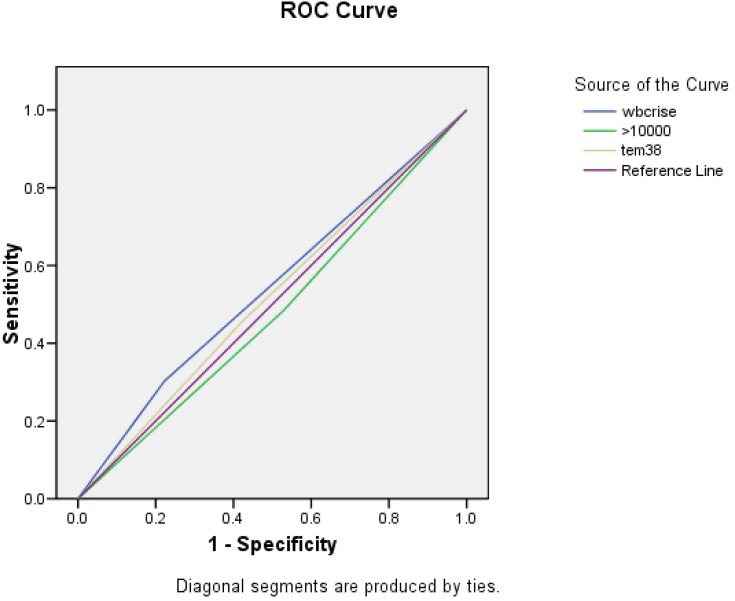
The PPV of 10% WBC rise was 18.1% (95% CI: 0.28-0.41) and its NPV was 86.5% (95% CI: 0.54-0.73). The sensitivity and specificity were 27% (95% CI: 0.33-0.54) and 78.1% (95% CI: 0.42-0.63). The ROC curve for the peripheral WBC >10,000 showed an AUC of 0.478 (95% CI: 0.37-0.58) which indicates low accuracy for the diagnosis of bacterial meningitis. The AUC for WBC rise (>10%) and temperature >38˚C was0.460 (95% CI: 0.351-0.569) and 0.517 (95% CI: 0.410-0.624) respectively, both indicating low accuracy for diagnosis of bacterial meningitis (Figure 1).
Fig. 1.
Receiver-operating characteristic (ROC) Curve for peripheral white blood cell (WBC) count of more than 10,000 (109/l), fever > 38˚C and WBC rise of more than 10% for diagnosis of bacterial meningitis in patients with severe traumatic brain injury (TBI). The area under curve (AUC) was calculated to be 0.478 (95% CI: 0.371-0.584) for peripheral WBC count, 0.517 (95% CI: 0.410-0.624) for fever and 0.460 (95% CI: 0.351-0.569) for WBC rise which demonstrates a low diagnostic accuracy for these parameters
Table 2 compares the inflammatory markers of CSF and peripheral blood between those with proven meningitis and those with excluded one. We found that patients with proved bacterial meningitis based on the CSF results had significantly higher WBC count of WBC ( p<0.001), lower CSF sugar ( p=0.021) and higher PMN count of CSF ( p<0.001) when compared to those with excluded bacterial meningitis. Other markers including the peripheral WBC count were comparable between these two groups (Table 2).
Table 2.
Comparing the inflammatory markers in patients with severe traumatic brain injury with proven and excluded meningitis
| Proven meningitis (n=89) | Excluded meningitis (n=139) | p -value | |
|---|---|---|---|
| Serum Leukocyte count (109/l) | 11,357 ± 4305 | 10,291± 2,161 | 0.701 |
| CSF a leukocyte count (106/l) | 3161 ± 86.35 | 730 ± 57.1 | <0.001 |
| CSF protein (mg/l) | 212.34 ± 191.20 | 151.12 ± 216.15 | 0.832 |
| CSF/Blood glucose ratio | 0.29 ± 0.18 | 0.63 ± 0.31 | 0.021 |
| CSF PMN b count (106/l) | 161 ± 260 | 14 ± 29 | <0.001 |
CSF: Cerebrospinal fluid;
PMN: Polymorphonuclear
Discussion
The occurrence of bacterial meningitis after neurosurgery has been documented in up to 4% of patients [13]. The diagnosis of meningitis has been based on the cytobiochemical characteristics of CSF samples and on positive CSF culture. Unfortunately, CSF culture results are not available early in the course of a diagnostic procedure or may even remain negative in up to 70% of clinically suspected cases [13]. The severe consequences of delayed or untreated bacterial meningitis, in conjunction with the lack of clear diagnostic criteria for meningitis occurring after neurosurgery, explain the prevailing practice of empirically treating all suspected cases with high-dose broad spectrum intravenous antibiotics. The expense and risk of unnecessary treatment of uninfected patients call for a more specific test for the diagnosis of bacterial meningitis after neurosurgery. The results of the current study have shown that serum leukocyte count of more than 10×109/l has a low PPV while it has a NPV of 0.69 to distinguish between those with and without meningitis. It was neither sensitive nor specific for the diagnosis of meningitis. In another study with the same cut-off point for the diagnosis of bacterial meningitis, the results have demonstrated the PPV of 56% and NPV of 80%. However, in addition to the fact that their study focused mainly on bacterial meningitis, the number of patients in the aforementioned study was 20 which limited [14]. Regarding the sensitivity and specificity of serum leukocyte count for the diagnosis of bacterial meningitis, Jereb et al., [14] have shown the sensitivity of 85% and specificity of 45%.
In our study, we have also assessed how fever (auxiliary temperature ≥38˚C) can positively and negatively predict the existence of meningitis in a patient with severe TBI. The results were inconclusive (PPV: 14.2%). However, lack of fever in a patient with TBI can rule out bacterial meningitis with NPV of 84.3%. This is in line with previous studies suggesting that in patients with head trauma, there can be a modest rise in temperature [15]. So, it should not be taken as a diagnostic marker for meningitis. TBI produces focal or multiple brain injuries, blood-brain barrier disruption, ischemia and reperfusion, diffuse axonal injury and development of cerebral microbleeding, intracranial hematomas, or contusion areas [15]. Two studies conducted in sedated patients suffering from severe TBI reported an average brain temperature that was higher by approximately 1°C than the average rectal temperature in the first posttraumatic days [16,17]. In the absence of an infectious cause, one explanation of this phenomenon could be a “resetting” of the hypothalamic thermoregulatory center which has been shown to be disrupted in 42% of patients after severe TBI [18]. The observed elevation in brain temperature could be related to posttraumatic changes in brain metabolism (hyperglycolysis) [19], in cerebral blood flow (hyperemia) [20], or in the local inflammatory response (e.g., increased intracerebral interleukin-1β) [21]. Decoupling of energy metabolism in cases of brain injury could also contribute to the production of heat; in such cases, ATP synthesis can indeed be short-circuited. The rise in WBC had low PPV, NPV and sensitivity while it had 78% specificity. As there is no cut-off value for the WBC in TBI patients, this parameter can be used more specifically to diagnose those suffering from TBI. Although there can be multiple factors responsible for the rise in the WBC such as pneumonia, UTI and other nosocomial infections, meningitis can also be a possibility when those have been ruled out.
We note some limitations to this study. The small number of patients may lead to the lack of sensitivity and specificity observed in this study. Furthermore, as no gold standard was previously determined for the diagnosis of bacterial meningitis in the setting of TBI, we have taken the positive CSF culture results as conclusive for bacterial meningitis.
In conclusion, the results of the current study indicates that peripheral blood leukocyte count, fever (>38˚C) and WBC rise (>10%) is a non-reliable marker for diagnosis of meningitis in patients with severe TBI. So, it seems reasonable to investigate the predictive values of other inflammatory parameters such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) for the diagnosis of meningitis in the setting of head trauma.
Conflict of interest: None declared.
References
- 1.Topolovec-Vranic J, Ennis N, Colantonio A, Cusimano MD, Hwang SW, Kontos P, et al. Traumatic brain injury among people who are homeless: a systematic review. BMC Public Health. 2012;12:1059. doi: 10.1186/1471-2458-12-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronado VG, Faul M, Wald MM, Xu L. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths, 2002-2006. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 3.Kourbeti IS, Vakis AF, Papadakis JA, Karabetsos DA, Bertsias G, Filippou M, et al. Infections in traumatic brain injury patients. Clin Microbiol Infect. 2012;18(4):359–64. doi: 10.1111/j.1469-0691.2011.03625.x. [DOI] [PubMed] [Google Scholar]
- 4.Mazzeo AT, Bullock R. Effect of bacterial meningitis complicating severe head trauma upon brain microdialysis and cerebral perfusion. Neurocrit Care. 2005;2(3):282–7. doi: 10.1385/NCC:2:3:282. [DOI] [PubMed] [Google Scholar]
- 5.Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock. 2001;16(3):165–77. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- 6.Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev. 2011;24(3):557–91. doi: 10.1128/CMR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Crevel H, Hijdra A, de Gans J. Lumbar puncture and the risk of herniation: when should we first perform CT. J Neurol. . 2002;249(2):129–37. doi: 10.1007/pl00007855. [DOI] [PubMed] [Google Scholar]
- 8.Garges HP, Moody MA, Cotten CM, Smith PB, Tiffany KF, Lenfestey R, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters. Pediatrics. 2006;117(4):1094–100. doi: 10.1542/peds.2005-1132. [DOI] [PubMed] [Google Scholar]
- 9.Tavares WM, Machado AG, Matushita H, Plese JP. CSF markers for diagnosis of bacterial meningitis in neurosurgical postoperative patients. Arq Neuropsiquiatr. 2006;64(3A):592–5. doi: 10.1590/s0004-282x2006000400012. [DOI] [PubMed] [Google Scholar]
- 10.Arda B, Sipahi OR, Atalay S, Ulusoy S. Pooled analysis of 2,408 cases of acute adult purulent meningitis from Turkey. Med Princ Pract. 2008;17(1):76–9. doi: 10.1159/000109595. [DOI] [PubMed] [Google Scholar]
- 11.Chen XL, Jiang L. Recurrent bacterial meningitis caused by an occult basilar skull fracture. World J Pediatr. 2011;7(2):179–81. doi: 10.1007/s12519-010-0215-y. [DOI] [PubMed] [Google Scholar]
- 12.Huy NT, Thao NT, Diep DT, Kikuchi M, Zamora J, Hirayama K. Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: a systemic review and meta-analysis. Crit Care. 2010;14(6):R240. doi: 10.1186/cc9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371(9628):1955–69. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- 14.Jereb M, Muzlovic I, Hojker S, Strle F. Predictive value of serum and cerebrospinal fluid procalcitonin levels for the diagnosis of bacterial meningitis. Infection. 2001;29(4):209–12. doi: 10.1007/s15010-001-1165-z. [DOI] [PubMed] [Google Scholar]
- 15.Thompson HJ, Tkacs NC, Saatman KE, Raghupathi R, McIntosh TK. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiol Dis. 2003;12(3):163–73. doi: 10.1016/s0969-9961(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 16.Rossi S, Zanier ER, Mauri I, Columbo A, Stocchetti N. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. J Neurol Neurosurg Psychiatry. 2001;71(4):448–54. doi: 10.1136/jnnp.71.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stocchetti N, Rossi S, Zanier ER, Colombo A, Beretta L, Citerio G. Pyrexia in head-injured patients admitted to intensive care. Intensive Care Med. 2002;28(11):1555–62. doi: 10.1007/s00134-002-1513-1. [DOI] [PubMed] [Google Scholar]
- 18.Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, Stalla GK, Agha A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298(12):1429–38. doi: 10.1001/jama.298.12.1429. [DOI] [PubMed] [Google Scholar]
- 19.Oddo M, Schmidt JM, Carrera E, Badjatia N, Connolly ES, Presciutti M, et al. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med. 2008;36(12):3233–8. doi: 10.1097/CCM.0b013e31818f4026. [DOI] [PubMed] [Google Scholar]
- 20.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita K, Chatzipanteli i K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD. Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery. 2002;51(1):195–203. doi: 10.1097/00006123-200207000-00027. discussion. [DOI] [PubMed] [Google Scholar]