Abstract
Objective:
To explore the pros and cons of early versus delayed intervention when dealing with severe blunt liver injury with significant hemoperitoneum and hemodynamic instability.
Methods:
This retrospective cross-sectional study was performed at the Nemazi hospital, Shiraz, Southern Iran, level I trauma Center affiliated with Shiraz University of Medical Sciences. The study population comprised of all patients who were operated with the impression of blunt abdominal trauma and confirmed diagnosis of liver trauma during an 8-year period. All data were extracted from patients’ hospital medical records during the study period. The patients’ outcome was compared between those who underwent perihepatic packing or primary surgical repair.
Results:
Medical records of 76 patients with blunt abdominal liver trauma who underwent surgical intervention were evaluated. Perihepatic packing was performed more in patients who have been transferred to operation room due to unstable hemodynamics (p<0.001) as well as in patients with more than 1000 milliliters of hemoperitoneum based on pre-operative imaging studies (e.g. CT/US) (p=0.002).
Conclusion:
We recommend that trauma surgeons should approach perihepatic packing earlier in patients who have been developed at least two of these three criteria; unstable hemodynamics, more than 1000 milliliters hemoperitoneum and more than 1600 milliliters of intra-operative estimated blood loss. We believe that considering these criteria will help trauma surgeons to diagnose and treat high risk patients in time so significant hemorrhage (e.g. caused by dilatational coagulopathy, hypothermia and acidosis, etc.) can ultimately be prevented and more lives can be saved.
Key Words: Perihepatic, Packing; Repair, Blunt, Liver, Trauma
Introduction
The liver is the most susceptible injured organ despite the supposed protected position in the abdominal cavity [1,2]. Even though mortality from blunt liver trauma has declined in past decades, there are challenges regarding the management of blunt liver trauma. Since 1897 when primary surgical repair (Hepatorrhaphy) has been introduced [3], this method has undergone different supports and oppositions. However against all the advantages and disadvantages, nowadays this method is used in some circumstances like severely ill patients where other techniques cannot be performed [4,5].
The improvements in imaging techniques and designated trauma intensive care unit (ICU) care have contributed to a fallen in morbidity / mortality rate and an enhanced non-operative management of stable patients with low grade liver injuries [6]. In unstable patients the packing may be used to defer the definitive operation [7]. Packing of liver injuries was suspended after observation of serious complications in World War II and the Vietnam War. Though, during the past decade, perihepatic packing has been re-established as an acceptable method of handling liver injuries in some situations [8,9]. Packing should highly be considered seriously when other surgical methods of arresting haemorrhage fail in a haemodynamically unstable patient. Uncontrollable coagulopathy is an absolute indication for perihepatic packing; others include bilobar liver injury, large non-expanding haematoma and capsular avulsion. Recently, packing has been used successfully when dealing with suspected injury to the vena cava or hepatic veins [10]. It is also a useful adjunct to the inexperienced surgeon’s armamentarium for patients who need to be temporarily stabilized for transfer to a specialist center [7,11,12].
The technique of perihepatic packing involves manual closure or approximation of the parenchyma, followed by the consecutive placing of dry abdominal packs around the liver and straight over the injury in an attempt to provide tamponade to a bleeding site. The minimal number of dry laparotomy pads necessary to stop bleeding should be applied for avoiding vena caval compression. The use of plastic sheets minimizes the risk of recurrent bleeding after pack removal. Subhepatic packing may be used to compress the liver against the diaphragm, but there is a risk of infrahepatic caval and renal vein compression [13-15].
In the present study, we evaluated medical records of confirmed patients with blunt liver trauma for perihepatic packing or primary surgical repair was performed during a 8 years period at level I truma center hospital affiliated with the Shiraz University of Medical Sciences, Shiraz, Iran. By comparing different characteristics in patients underwent packing and primary surgical repair, we aim to explore and define indications for deciding which patients and when early perihepatic packing have to be performed instead of late perihepatic packing (after failure of primary repair) in our study population. Also, we want to evaluate clinical features of patients with blunt liver trauma for whom surgery were inevitable as well as their outcome.
Materials and Methods
Study Design and Population
This retrospective cross-sectional study was performed at the Nemazee hospital, Shiraz, Fars province, Southern Iran, level I trauma Center affiliated with Shiraz University of Medical Sciences. The study population comprised of all patients who were operated with an impression of blunt abdominal trauma and a final diagnosis liver trauma between January 2003 until the end of 2010. Complete checking the operation reports, we only included those patients for whom perihepatic packing and/or surgical repair was executed in the operation room. Those who had penetrating trauma or gunshot alone or simultaneous penetrating and blunt liver trauma were excluded. We also excluded those liver trauma patients who were discharged or expired during observation in hospital (Emergency Room (ER), General Ward or ICU) for whom no surgical intervention was performed afterwards. In addition to reviewing the patients’ medical records and in order to prevent procedural data collection errors, we also used The International Classification of Diseases, Tenth Revision, Clinical Modification 10 (ICD-10-CM) code S36.1 (Injury of liver or gallbladder) to identify hospitalizations from the electronic archives of these two hospitals. Finally, a total number of 76 patients fulfilled our criteria. In our Trauma Center, the common guideline in our entire patients with blunt abdominal trauma is evaluating the hemodynamics at the first line. If the patient is hemodynamically stable but we have clinical suspicious for internal bleeding, an abdominal CT scan with intravenous (IV) and oral contrast will be done. If liver laceration (grade IV or V) was confirmed by radiology or extravasation of the contrast dye was detected, the patient will be transferred to operating room for further laparotomic evaluations. Otherwise (i.e. Grade of liver laceration is less than IV and no extravasation of contrast dye was detected), the patient would be observed in the ICU. But if the hemodynamic of the patient is unstable (at the first visit in the ER or next visit when under observation), the patient will be transferred to operation room with the impression of ongoing intra-peritoneal hemorrhage.
The surgical goal approach in all the patients who were taken to operating room, was primarily to control the bleeding from liver by perihepatic packing (damage control approach/laparotomy). If the patient became hemodnamically stable, we would try primary repair (suturing /ligation). If the repair was unsuccessful (persistent hemorrhage), an attempt was make to perform perihepatic packing followed by trauma ICU transfer for further resuscitation. On the other hand, if, after 30-60 minutes, the hemodynamic of the patient does not stabilize (despite intensive transfusion and volume support), the surgeons would check for active bleeding again. If there was continuous bleeding from the liver, other aggressive techniques (other than packing or suturing like clumping the aorta, etc.) would be applied. Hence if the bleeding was controlled and stopped, the surgeon would close the abdomen afterwards and the patient will be observed in the ICU. The final goal in the management of all patients is to definite control of bleeding. An effective packing is defined when the hemorrhage from the liver is ceased completely.
Study Protocol
A case of blunt abdominal liver trauma was defined as a patient with the true history of blunt trauma to the abdomen which was documented by computerized tomographic scan (CT scan) of the abdomen and/ or diagnosis of surgeon in operating room. We searched the databank for patients’ records of our trauma center, based on this definition and retained the available data of the patients operated with a final diagnosis of liver trauma (and followed by surgical interventions), through a data gathering form. Data collection forms included demographics, month of hospitalization, type of accident, laboratory data, physical exam, history and final outcome (complete recovery and death). We compared all the variables between fully-recovered and expired patients, in order to find out probable prognostic factors.
All wards and emergency rooms (including pediatrics, adults, ICUs, post ICUs, infectious, etc.) were evaluated. Reviewing of the patients’ medical records was performed by two well-qualified surgeons. In order to increase the accuracy of results, we only include patients whose medical records were complete in all aspects. The study protocol was approved by the Institutional Review Board (IRB) and the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran. As the study was retrospective, no informed written consents were required for the study.
Statistical Analysis
All data analyses were performed using Statistical Package for the Social Sciences (SPSS Inc., Chicago, Illinois, USA) version 17. Chi-squared or Fisher’s exact tests test were used to compare the qualitative variables between final survived and non-survived patients as well as those operated by perihepatic packing method versus surgical repair method. Also, all quantitative variables were analyzed through independent t-test except the volume of intra- operative bleeding, for which Mann-Whitney test was applied. Data are reported as mean ± Standard deviation (SD) or proportions, as appropriate. A two-tailed P value less than 0.05 was considered Statistically significant.
We calculated sensitivity and specificity of volume of intra-operative bleeding in order to find the best cut-off points for predicting performance of the perihepatic packing method in these patients. A receiver-operating characteristic (ROC) curve was issued (plot of sensitivity vs. 1-specificity) and the areas under the curves (AUCs) estimated. An AUC=1 indicated a perfect predictor, AUC>0.8 indicated high accuracy and AUC between 0.6 and 0.8 indicated moderate accuracy.
Results
Medical records of 76 patients with blunt abdominal liver trauma who underwent surgery were evaluated. Surgical repair was done for 40 patients whereas 36 subjects underwent perihepatic packing. The majority of patients were male (n=59, 77.6%). The mean age of the patients was found to be 24.5±11.8 (range 2-70) years. Table 1 indicates the mechanism of injury on these subjects. Grade of liver injury based on Liver Injury Scale (Revision 1994) [16] was shown in Table 2.
Table 1.
Mechanism of injury in 76 patients with blunt abdominal trauma
| Type of accident | Number |
|---|---|
| Car-car | 27 (35.5%) |
| Car turn over | 5 (6.6%) |
| Car-pedestrian | 22 (28.9%) |
| Falling down | 4 (5.3%) |
| Motor | 15 (19.7%) |
| Assault trauma | 3 (3.9%) |
Table 2.
Grade of liver injuries in patients with blunt liver trauma for whom perihepatic packing or surgical repair was performed
| Perihepatic packing (n=36) | Surgical repair (n=40) | Total (n=76) | |
|---|---|---|---|
| I | 2 (40%) | 3 (60%) | 5 (6.6%) |
| II | 1 (4.5%) | 21 (95.5%) | 22 (28.9%) |
| III | 20 (57.1%) | 15 (42.9%) | 35 (46.1%) |
| IV | 13 (92.9%) | 1 (7.1%) | 14 (18.4%) |
| V | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Total number | 36 (47.9%) | 40 (52.1%) | 76 (100%) |
Twenty eight (36.8%) patients were expired out of all 76 subjects. Those who had primary repair had significantly lower mortality (p=0.024, n=10, 25% vs. n=18, 50%). On the other hand, grade of liver injury was also had a statistical significant association with type of surgery (p≤0.001) i.e. perihepatic packing was more performed for those patients with higher grades of liver injury (II=1, 4.5%, III=20, 57.1%, IV=13, 92.9%).
Table 3 indicates indications for transferring the patient to the operating room. These indications could be presented at the time of admission or during the observation time in the emergency room. We consider developing peritonitis in those who had progressive abdominal pain and/or intractable abdominal pain (severity). Those who had SBP blow 90mmHg and/ or pulse rate over even after 2 liters crystalloid hydration and applying intravenous analgesics were labeled as unstable hemodynamics and were transferred to operating room. If the hemoglobin of the patients decreased, packed cells were transfused. However, there were some individuals who had a hemoglobin drop (2 mg/dL decrease in hemoglobin in less than 6 hours) after transfusing 2 bags of packed cell. These patients were also transferred to OR for further evaluations.
Table 3.
Indications for surgery in 76 patients with blunt liver trauma
| Perihepatic packing (n=36) | Surgical repair (n=40) | Total (n=76) | |
|---|---|---|---|
| Developing peritonitis | 6 (20.7%) | 23 (79.3%) | 29 (38.2%) |
| Unstable hemodynamics | 29 (69%) | 13 (31%) | 42 (55.3%) |
| Decreased haemoglobin | 2 (66.7%) | 1 (33.3%) | 3 (3.9%) |
| Positive radiologic findings | 4 (36.4%) | 7 (63.6%) | 11 (14.5%) |
| Decreased LOC a | 6 (60%) | 4 (40%) | 10 (13.2%) |
LOC: level of consciousness
Perihepatic packing was performed statistically more in patients who have been transferred to OR due to disturbances in hemodynamics (n=29, 69% vs. n=13, 31%, p<0.001).
Positive radiologic findings are presence of severe fluid in abdominal ultrasonography and/or ascites and/or extravasation of contrast agent in the abdominal cavity. Glasgow coma scale (GCS) scoring system was applied for evaluation of the level of consciousness of all patients. That patient who had GCS below 8 on the time of admission or developed low GCS during the period of observation was also transferred to operating room for surgery. All 10 patients who had surgery due to decreased level of consciousness were expired. Of these 10 patients, 9 ones had head trauma and the remained one without head trauma, had more than 2000 milliliters free blood in abdominal cavity which explained the probable cause of death (fatal shock due to massive liver hemorrhage).
Free blood in abdominal cavity at the beginning of operation was evaluated through joint collaboration of surgeons and anesthesiologists. The final amount was calculated by summing these three items: volume and numbers of clots in abdominal cavity, volume of blood in suction equipment and number of long gauze. We divided the amount of free blood in abdomen in five categories (blow 500, 500-100, 1000- 1500, 1500-2000 and more than 2000 milliliters). Perihepatic packing was performed for those patients with higher free blood in abdomen (p=0.029). If we change our dividing system to two groups (less than 1000 and more than 1000 milliliters free blood in abdomen),We will observe that perihepatic packing was performed significantly more in patients with more than 1000 milliliters free blood in abdominal cavity (n=24, 68.6% vs. n=11, 31.4%, p=0.002).
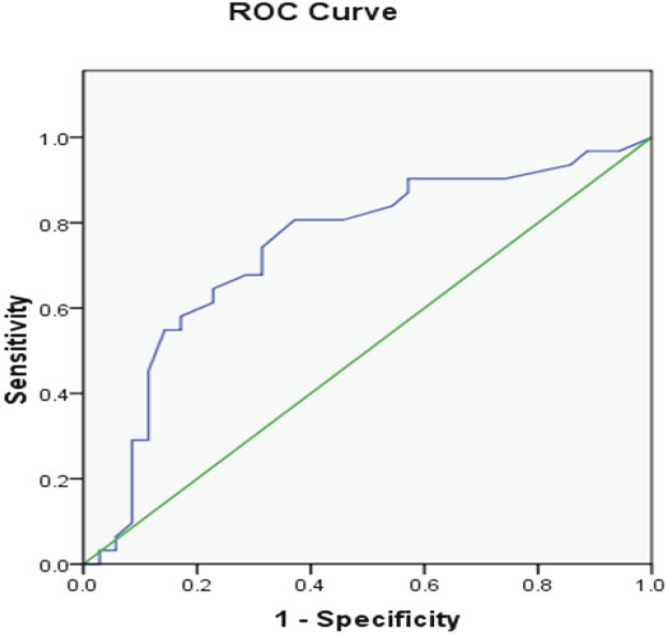
Seventeen patients had more than 2000 milliliters free blood in abdomen. 4 ones underwent surgical repair and 14 ones had perihepatic packing. We observed 100% mortality (n=4) in surgical repair group in comparison to 53.8% mortality (n=7) in perihepatic packing group. Duration of operation was not significantly different in two groups of patient for whom Perihepatic packing and surgical repair was executed (p=0.705, 2.44±1.6 vs. 2.6±1.3 hours respectively). Volume of intra-operative bleeding was significantly more in perihepatic packing group in comparison to surgical repair group (p=0.001, 2996.8±2213.6 vs. 1761.4±2451.5 milliliters respectively). We found that our patients with blunt liver trauma, intra-operative bleeding more than 1600 millilitres is an appropriate cut-off value for predicting performance of perihepatic packing (Sensitivity=74%, Specificity=69%, AUC=0.74, p=0.001, CI=0.62-0.86) (Figure 1). In another words, the surgeon finally have been forced to perform hepatic packing in majority of patients who had intra-operative bleeding over than 1600 milliliters.
Fig. 1.
Receiver-operating characteristics (ROC) curve (plot of sensitivity vs. 1-specificity) of volume of perioperative bleeding in predicting performance of perihepatic packing in patients with blunt liver trauma
None of the patients in both groups developed fever or sepsis during post-operation follow up. Three patients (2 belongs to perihepatic packing and 1 belongs to surgical repair group) developed post- operation fluid collection around the liver. All were cured completely through percutaneous drainage of fluid. The Pringle maneuver was performed in 19 (27.5%) patients (n=14, 73.7% in perihepatic packing group vs. n=5, 26.3% in surgical repair group). Unstable hemodynamics at first visit or the next visits when under observation, more than 1000 milliliters free blood in abdominal cavity at the initiation of surgery and more than 1600 milliliters of intra-operative bleeding are the three statistically significant findings which made surgeons to perform perihepatic packing at last. Eighteen patient had only one criterion (n=5, 27.8% in perihepatic packing group vs. n=13, 72.2% in surgical repair group), fourteen patients had two criteria (n=9, 64.3% in the perihepatic packing group vs. n=5, 35.7% in the surgical repair group) and seventeen subjects had all three criteria (n=14, 82.4% in perihepatic packing group vs. n=3, 17.6% in surgical repair group).
Comparing the presence of these three criteria among all patients indicated that when more than one criteria were present in our population, the surgeons were finally made to perform perihepatic packing (p<0.001).
Discussion
Even with new advances in the non-operative management of liver trauma, there are still some categories of patients who require immediate surgical interventions to control exsanguination from the liver. Delayed inhibition of bleeding will result in developing coagulopathy, acidosis and hyperthermia which are three fatal complications that can’t be reversed by interventions or conservatively leading to death. Perihepatic packing can decrease morbidity and mortality associated with uncontrollable hemorrhage and allows best planning in treating this category of patients. [17,18].
As it was elucidated in Table 3, indications for transferring to the operating room could be presented at the time of admission or during the observation time in the emergency room. We consider developing peritonitis in those who had progressive abdominal pain and/or intractable abdominal pain (severity). Those who had SBP blow 90mmHg and/or pulse rate over even after 2 liters crystalloid hydration and applying IV analgesics were labeled as unstable hemodynamics and were transferred to OR. If the hemoglobin of the patients decreased, packed cells were transfused. However, there were some individuals who had a hemoglobin drop (2 mg/ dl decrease in hemoglobin in less than 6 hours) after transfusing 2 bags of packed cell. These patients were also transferred to OR for further evaluations. We observed that perihepatic packing was performed statistically more in patients who had been transferred to OR with the indication of “unstable hemodynamics” (p<0.001). This indicates that surgeons were made to do perihepatic packing after losing some golden time (i.e. after trying to control the bleeding through suturing but failed at last). Hence, they have to perform perihepatic packing sooner in this specific category of patients. Furthermore, we have determined that patients who were hemodynamically unstable as well as having had greater than 1000 milliliters of hemoperitoneum on initial exploratory laparotomy, forced trauma surgeons to stabilize the situation by doing perihepatic packing rather than locating a time consuming source of bleeding in unstable patient. The same story is applicable for those patients who had more than 1600 milliliters of intra-operative bleeding. The late findings states that if, during the operation, the surgeons noticed that the volume of intra-operative bleeding is going to exceeds 1600 milliliters, the have to shift to the perihepatic packing method as soon as possible in order to prevent more blood loss. We observed that all 10 patients who had surgery due to decreased level of consciousness when associated with liver injuries were not survived. This indicates the synergistic effect of head trauma in patients with liver trauma which deteriorates their situation and outcome. It has been well proved that in patients with liver trauma, simultaneous injuries (polytraumatic patients) will increase mortality and morbidity significantly [19-21]. Most of the surveys focused on hollow viscous injuries. Our findings suggest that presence of head trauma and progressing decreased level of consciousness can be an alarming sign of deterioration the condition of the patient. There was not statistically significant difference between the duration of operation in two groups (p=0.705). On the other hand, the volume of intra- operative bleeding was significantly higher in perihepatic packing group (p=0.001). All these finding strongly suggests that our trauma surgeons timing to perform perihepatic packing were not optimal. In another word, they have postponed perihepatic packing in patients with liver trauma and the delayed-action perihepatic packing was useless in improving final outcome. However, the proper timing for packing remains controversial, although many surgeons recommended that it should be applied when coagulopathy is suspected and long before irreversible shock from blood loss, hypothermia and acidemia arises. Appropriate timing for initiation of definite perihepatic packing will result in less duration of operation as well as less intra-operative bleeding [22-24]. Perihepatic packs should be removed as soon as the patient is hemodynamically stable, adequately resuscitated, and coagulopathy; hypothermia and acidosis all have been corrected. Broad-spectrum antibiotics should be administered intravenously when packs are placed in order to lessen the risk of sepsis (which has been reported to occur in 10-30 percent of patients) [25-27].
We also found out that for 17 patients Pringle maneuver was not performed possibly due to hemodynamic instability as seen in four of these patients. But in 13 patients, it seems that the Pringle maneuver should have been performed at that time. We observed that applying Pringle maneuver (pedicle occlusion) could be useful either concomitant with primary surgical repair or perihepatic packing. In this diagnostic and therapeutic technique, hepatoduodenal ligament (just posterior to duodenum in which hepatic artery, common bile duct and portal vein pass through) is clamped by applying atraumatic vascular clamp or non-crushing bowel clamp. Although there are controversies along with occlusion time, most surgeons agreed that one hour is the optimum time. In addition to control the bleeding temporarily, it will guide the surgeon to identify whether major vascular injury happened or not and if more packing is required [8,28].
Although previous studies had reported some complications like subphrenic or hepatic abscess, bilomas, bile fistulas or sepsis [29], none of them have been occured in our patients. This could be due the appropriate timing for packing removal as well as proper antibacterial coverage of administering intravenous drugs. Another finding which empowers the use of perihepatic packing in severely-ill patients is our outcome in 17 patients who had more than 2000 milliliters free blood in abdominal cavity at the beginning of operation. Although the number of these patients for whom surgical repair was done was few (4 patients) in comparison to perihepatic packing (13 patients), the overall mortality was better in perihepatic packing group. This is in accordance with previous reports [30,31].
We concluded that delay in performing perihepatice packing may contribute to higher mortality and the trauma surgeon should consider the option of perihepatic packing as a primary therapeutic early to decrease morbidity and mortality. It is vivid that prompt assessment of the patient in addition to serial abdominal CT scan has the major role in proper decision making peri-operatively.
We believe that more supportive approach for stabilizing the hemodynamics in addition to early perihepatic packing can lead to more preservation of the liver as well as the brain function. We also have to keep in mind that patient selection is essential in order to prevent overuse or underuse of damage control approach and perihepatic packing in this setting. Hence recognizing alarming clinical and paraclinical findings would help surgeons to decide in which group of patients perform perihepatic packing and in which one to avoid [32].
We suggest that perihepatic packing can prevent mortality in certain group of patients who need early laparotomy due to severe hemorrhage from liver damage. We recommend that perihepatic packing have to be performed earlier in patients who have been developed at least two of these three criteria; unstable hemodynamics at first visit or the next visits when under observation, more than 1000 milliliters free blood in the abdominal cavity at the initiation of surgery and more than 1600 milliliters of intra- operative bleeding. We believe that considering these criteria will help surgeons to diagnose high risk patients so early before the time that the patient has initiated a bloody vicious cycle (dilatational coagulopathy, hypothermia and acidosis). To the best of our knowledge, there have been no randomized clinical trials designed to compare the short-term and long-term squeal as well as total prognosis of early and late perihepatic packing in patients with blunt abdominal liver trauma.
Randomized, prospective, and multi-institutional clinical trials with larger number of participants are needed to sufficiently compare the different aspects of early first-line perihepatic packing to standard-of- care combination of surgical repair and perihepatic packing. Till such surveys are available, primary first-line perihepatic packing should be done as an off-label surgical method for patients who fulfilled mentioned criteria.
Acknowledgements
The authors wish to thank all the staff of the central archive office of Nemazi hospital, Shiraz University of Medical Sciences, Shiraz, Iran, for their kind cooperation.
Conflict of Interest: None declared.
Financial Support
This project was financially supported by the Trauma Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
References
- 1.Matthes G, Stengel D, Seifert J, Rademacher G, Mutze S, Ekkernkamp A. Blunt liver injuries in polytrauma: results from a cohort study with the regular use of whole-body helical computed tomography. World J Surg. 2003;27(10):1124–30. doi: 10.1007/s00268-003-6981-0. [DOI] [PubMed] [Google Scholar]
- 2.Miller PR, Croce MA, Bee TK, Malhotra AK, Fabian TC. Associated injuries in blunt solid organ trauma: implications for missed injury in nonoperative management. J Trauma. 2002;53(2):238–42. doi: 10.1097/00005373-200208000-00008. discussion 42-4. [DOI] [PubMed] [Google Scholar]
- 3.Kousnetzoff M, Pensky J. Sur la resection partielle du foie chez l'homme et chez les animaux. Rev Chir. 1897;17:319–31. [Google Scholar]
- 4.Krige JE. Liver fracture and bleeding. Br J Surg. 2000;87(12):1615–6. doi: 10.1046/j.1365-2168.2000.01660.x. [DOI] [PubMed] [Google Scholar]
- 5.Krige JE, Bornman PC, Terblanche J. Liver trauma in 446 patients. S Afr J Surg. 1997;35(1):10–5. [PubMed] [Google Scholar]
- 6.Lee SK, Carrillo EH. Advances and changes in the management of liver injuries. Am Surg. 2007;73(3):201–6. [PubMed] [Google Scholar]
- 7.Cirocchi R, Contine A, Mazieri M, Bisacci R, Fabbri C, Bisacci C, et al. Perihepatic packing combined with wrapping in the treatment of major bi-lobar hepatic trauma. Chir Ital. 1999;51(3):259–64. [PubMed] [Google Scholar]
- 8.Nicol AJ, Hommes M, Primrose R, Navsaria PH, Krige JE. Packing for control of hemorrhage in major liver trauma. World J Surg. 2007;31(3):569–74. doi: 10.1007/s00268-006-0070-0. [DOI] [PubMed] [Google Scholar]
- 9.Tugnoli G, Casali M, Villani S, Biscardi A, Borrello A, Baldoni E. The "damage control" in severe hepatic injuries: our experience. Ann Ital Chir. 2003;74(5):529–33. discussion 34. [PubMed] [Google Scholar]
- 10.David Richardson J, Franklin GA, Lukan JK, Carrillo EH, Spain DA, Miller FB, et al. Evolution in the management of hepatic trauma: a 25-year perspective. Ann Surg. 2000;232(3):324–30. doi: 10.1097/00000658-200009000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollands MJ, Little JM. Perihepatic packing: its role in the management of liver trauma. Aust N Z J Surg. 1989;59(1):21–4. doi: 10.1111/j.1445-2197.1989.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 12.Sheridan R, Driscoll D, Felsen R. Packing and temporary closure in a liver injury. Injury. 1997;28(9-10):711–2. doi: 10.1016/s0020-1383(97)00128-9. [DOI] [PubMed] [Google Scholar]
- 13.Girgin S, Gedik E, Tacyildiz IH. Evaluation of surgical methods in patients with blunt liver trauma. Ulus Travma Acil Cerrahi Derg. 2006;12(1):35–42. [PubMed] [Google Scholar]
- 14.Zani B, Fiamingo P, Valduga P, Fama R, Prezzi C, Eccher C. Blunt liver trauma: therapeutic options. Chir Ital. 2005;57(1):71–5. [PubMed] [Google Scholar]
- 15.Ebrahimi S, Tahmasebi S, Rouhezamin MR, Mousavi SM, Abbasi HR, Bolandparvaz S, et al. Modified Perihepatic Packing; A Creative and Beneficial Method for Management of High Grade Liver Injury. Bull Emerge Trauma. 2013;1(1):22–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Fabian TC, Bee TK. Liver and Biliary Tract. In: Mattox KL, Moore EE, Feliciano DV, editors. Trauma. 7th ed. NewYork: McGrawHill; 2013. p. 542. [Google Scholar]
- 17.Clemente N, Di Saverio S, Giorgini E, Biscardi A, Villani S, Senatore G, et al. Management and outcome of 308 cases of liver trauma in Bologna Trauma Center in 10 years. Ann Ital Chir. 2011;82(5):351–9. [PubMed] [Google Scholar]
- 18.Colombo F, Sansonna F, Baticci F, Corso R, Scandroglio I, Maggioni D, et al. Liver trauma: experience in the management of 252 cases. Chir Ital. 2005;57(6):695–702. [PubMed] [Google Scholar]
- 19.Beal SL. Fatal hepatic hemorrhage: an unresolved problem in the management of complex liver injuries. J Trauma. 1990;30(2):163–9. [PubMed] [Google Scholar]
- 20.Sikhondze WL, Madiba TE, Naidoo NM, Muckart DJ. Predictors of outcome in patients requiring surgery for liver trauma. Injury. 2007;38(1):65–70. doi: 10.1016/j.injury.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 21.Di Saverio S, Catena F, Filicori F, Ansaloni L, Coccolini F, Keutgen XM, et al. Predictive factors of morbidity and mortality in grade IV and V liver trauma undergoing perihepatic packing: single institution 14 years experience at European trauma centre. Injury. 2012;43(9):1347–54. doi: 10.1016/j.injury.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Croce MA, Fabian TC, Menke PG, Waddle-Smith L, Minard G, Kudsk KA, et al. Nonoperative management of blunt hepatic trauma is the treatment of choice for hemodynamically stable patients. Results of a prospective trial. Ann Surg. 1995;221(6):744–53. doi: 10.1097/00000658-199506000-00013. discussion 53-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Errico E, Goffre B, Mazza D. Blunt abdominal trauma: current management. Chir Ital. 2009;61(5-6):601–6. [PubMed] [Google Scholar]
- 24.Ochiai T, Igari K, Yagi M, Ito H, Kumagai Y, Iida M, et al. Treatment strategy for blunt hepatic trauma: analysis of 183 consecutive cases. Hepatogastroenterology. 2011;58(109):1312–5. doi: 10.5754/hge11042. [DOI] [PubMed] [Google Scholar]
- 25.Cogbill TH, Moore EE, Jurkovich GJ, Feliciano DV, Morris JA, Mucha P. Severe hepatic trauma: a multi-center experience with 1,335 liver injuries. J Trauma. 1988;28(10):1433–8. [PubMed] [Google Scholar]
- 26.Cue JI, Cryer HG, Miller FB, Richardson JD, Polk HC Jr. Packing and planned reexploration for hepatic and retroperitoneal hemorrhage: critical refinements of a useful technique. J Trauma. 1990;30(8):1007–11. doi: 10.1097/00005373-199008000-00010. discussion 11-3. [DOI] [PubMed] [Google Scholar]
- 27.Feliciano DV, Mattox KL, Burch JM, Bitondo CG, Jordan GL Jr. Packing for control of hepatic hemorrhage. J Trauma. 1986;26(8):738–43. doi: 10.1097/00005373-198608000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Badger SA, Barclay R, Campbell P, Mole DJ, Diamond T. Management of liver trauma. World J Surg. 2009;33(12):2522–37. doi: 10.1007/s00268-009-0215-z. [DOI] [PubMed] [Google Scholar]
- 29.Caruso DM, Battistella FD, Owings JT, Lee SL, Samaco RC. Perihepatic packing of major liver injuries: complications and mortality. Arch Surg. 1999;134(9):958–62. doi: 10.1001/archsurg.134.9.958. discussion 62-3. [DOI] [PubMed] [Google Scholar]
- 30.Vyhnanek F, Denemark L, Duchac V. Current diagnostic and therapeutic approaches in liver injuries. Acta Chir Orthop Traumatol Cech. 2003;70(4):219–25. [PubMed] [Google Scholar]
- 31.Markogiannakis H, Sanidas E, Michalakis I, Manouras A, Melissas J, Tsiftsis D. Predictive factors of operative or nonoperative management of blunt hepatic trauma. Minerva Chir. 2008;63(3):223–8. [PubMed] [Google Scholar]
- 32.Chovanes J, Cannon JW, Nunez TC. The evolution of damage control surgery. Surg Clin North Am. 2012;92(4):859–75. doi: 10.1016/j.suc.2012.04.002. vii-viii. [DOI] [PubMed] [Google Scholar]