Abstract
Objective:
To determine the effects of local administration of platelet rich plasma (PRP) on peripheral nerve regeneration in rat sciatic nerve transection model.
Methods:
Forty-five male white Wistar rats were randomized into three experimental groups (n=15): Normal control group (NC), silicon group (SIL), PRP treated group (SIL/PRP). In NC group left sciatic nerve was exposed through a gluteal muscle incision and after homeostasis muscle was sutured. In SIL group left sciatic nerve was exposed the same way and transected proximal to tibio-peroneal bifurcation leaving a 10-mm gap. Proximal and distal stumps were each inserted into a silicone conduit and filled with 10 µL phosphate buffered solution. In SIL/PRP group silicon conduit was filled with 20 µL PRP. Each group was subdivided into three subgroups of five animals each and were studied 4, 8, 12 weeks after surgery.
Results:
The animals were comparable regarding the baseline characteristics. Behavioral testing, sciatic nerve functional study and gastrocnemius muscle mass showed earlier regeneration of axons in SIL/PRP than in SIL group.
Conclusion:
Local administration of PRP combined with silicon grafting could accelerate functional recovery of peripheral nerve. Easily available growth factors and bioactive proteins present in PRP may have clinical implications for the surgical management of patients after nerve transection.
Key Words: Peripheral nerve repair, Sciatic, Platelet rich plasma (PRP), Local administration, Functional recovery
Introduction
The purpose of regenerative medicine is the augmentation or substitution of damaged cells [1]. Damage to the peripheral nerves in the head and neck, including the inferior alveolar, lingual, facial and hypoglossal nerves, can occur through a number of causes [2,3]. Various nonsurgical and pharmacologic treatments are available for patients with nerve injury. Under normal conditions, spontaneous healing of nerve fibers is expected within a few weeks or months and some cases may necessitate surgical intervention [4-6]. The need to achieve a tension- free repair in peripheral nerve surgery has led to the accepted standard of nerve grafting. However, the associated morbidities and suboptimal clinical results provide a compelling reason to search for alternatives. Inert conduits avoid both the problems inherent in harvesting a nerve for autograft and the potential nerve compression and irritation found with some resorbable synthetic grafts. Allowing to grow through conduits, and thus to be subject to the neurotrophic guidance cues that collect there, may ultimately allow more precise alignment of fascicles and thus better functional recovery than be possible with primary nerve repair or nerve autograft. These conduits appear to offer significant advantages including improved regeneration when compared with traditional repair and are currently available for use clinically. Incorporating neurotropic and neurotrophic factors into conduits are encouraging and likely playing a prominent role in peripheral nerve repair [7].
Regenerating axons react to a host of molecular cues in the local environment. Several attempts have been made to overcome the length restrictions inherent in other synthetic tubes using tissue engineering to combine the advantages of neurotroghic and neurotropic substances that are known to support or enhance axonal growth with the benefits of tubulization. Combining synthetic tubes with biological factors known to encourage and hasten nerve growth an active area of research [8].
Platelet rich plasma (PRP) has been observed to enhance healing of the donor and recipient sites of soft tissue flaps and grafts including skin, mucosa, muscles and dermal fat [9,10]. The addition of PRP to the regenerating nerve fibers immediately after their reanastomosis has improved the process of early regeneration of the nerve fibers [11]. The enhancing effect of PRP is associated with the large number of platelets in PRP that release significant quantities of growth factors that aid the healing process [10, 2-14]. These growth factors act locally to recruit undifferentiated cells to the site of injury, trigger mitosis in these cells and induce angiogenesis [10,15,16].
The platelets that constitute PRP are able to release various growth factors that are essential for the healing of lesions, such as the three platelet growth factor isomers (PRP αα, ββ and αβ), VEGF, transforming growth factor (TGF-β1 and β2) and epithelial growth factor (EGF) [12,17]. The platelets are also responsible for the synthesis and storage of BDNF [18]. The application of PRP increases the number of nerve fibers after peripheral nerve reanastomosis, and can produce a neurotrophic effect, stimulating the proliferation of Schwann cells and myelination, important components during peripheral nerve repair [11].
Data in literature on the local effect of PRP on functional recovery of peripheral nerve regeneration are scarce, encouraging the search for more precise information about its performance. The scarceness of basic research that endorses PRP’s ability to promote functional recovery of peripheral nerve fibers has encouraged the authors to study local effects of PRP on nerve regeneration. Therefore, the present study was designed to attempt to determine if PRP do in fact locally reduce dysfunction after nerve injury in the rat sciatic nerve transection model. Assessment of the nerve regeneration was based on behavioral, functional (Walking Track Analysis) and muscle mass measurement criteria within 12 weeks after surgery.
Materials and Methods
Experimental Design
Forty-five male white Wistar rats weighing approximately 280 g were divided into three experimental groups (n=15), randomly: Normal control group (NC), silicone control group (SIL) and PRP treated group (SIL/PRP). Each group was again subdivided into three subgroups of five animals each and surveyed in four time points of 4, 8 and 12 weeks. Two rats were served as PRP donors. Two weeks before and during the entire experiments, the animals were housed in individual plastic cages with an ambient temperature of 23±3ºC, stable air humidity, and a natural day/night cycle. The rats had free access to standard rodent laboratory food and tap water.
Preparation of the Platelet-Rich Plasma
The platelet-rich plasma was prepared based on a method described by others [19]. In brief, the fresh blood sample was obtained by heart puncture with a tube containing sodium citrate. The collected blood was firstly centrifuged at 160 g, for 20 minutes, at environmental temperature (22ºC). Then, a red lower fraction (red cell component) and an upper straw- yellow turbid fraction (serum component) were observed. A point was marked at 1.4 mm below the line dividing the two fractions. All the content above this point was pipetted and transferred to other 5 ml vacuum tube in which a line corresponding to 0.35 ml was drawn from the tube’s bottom. The sample was then submitted to a new centrifugation at 400 g, for 15 minutes, resulting in two components: one above was platelet-poor plasma and other below the line drawn on the tube was PRP.
Grafting Procedure
Animals were anesthetized by intraperitoneal administration of ketamine-xylazine (ketamine 5%, 90 mg/kg and xylazine 2%, 5mg/kg). All procedures followed a standard microsurgery technique under magnifying lenses (BIO-ART EQUIPMENTOS ODONTOLOGICOS LTDA, Sao Carlos/SP- Brasil). The procedures were carried out based on the guidelines of the Ethics Committee of the International Association for the Study of pain [20]. The University Research Council approved all experiments. Following surgical preparation in the normal control group (NC) the left sciatic nerve was exposed through a gluteal muscle incision and after careful homeostasis the muscle was sutured with resorbable 4/0 sutures, and the skin with 3/0 nylon. In the SIL group the left sciatic nerve was exposed through a gluteal muscle incision and transected proximal to the tibio-peroneal bifurcation where a 7 mm segment was excised, leaving a gap about 10 mm due to retraction of nerve ends. Proximal and distal stumps were each inserted 2 mm into a silicone tube and two 10/0 nylon sutures were placed at each end of the cuff to fix the tube in place and to leave a 10- mm gap between the stumps. The conduit was filled with 20 μL the phosphate buffered saline and sterile Vaseline was used to seal the ends of the tubes to avoid leakage. In the SIL/PRP group the conduit was filled with 20 μL PRP. The animals were anesthetized and euthanized with transcardial perfusion of a fixative containing 2% paraformaldehyde and 1% glutaraldehyde buffer (pH: 7.4) 4, 8 and 12 weeks after surgery.
Behavioral Testing
Functional recovery of the nerve was assessed using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale for rat hind limb motor function [21]. Although BBB is widely used to assess functional recovery in spinal cord injured animals, however, it has been demonstrated that it could be most useful in assessment of never repair processes in peripheral nerve injuries [22].
Scores of 0 and 21 were given when there were no spontaneous movement and normal movement, respectively. BBB recordings were performed by a trained observer who was blinded to the experimental design. The testing was performed in a serene environment. The animals were observed and assessed within a course of a 4-minute exposure to an open area of a mental circular enclosure. BBB scores were recorded once before surgery in order to establish a baseline control and again weekly thereafter to assess functional recovery during 12 weeks.
Functional Assessment of Reinnervation
Sciatic Functional Index (SFI)
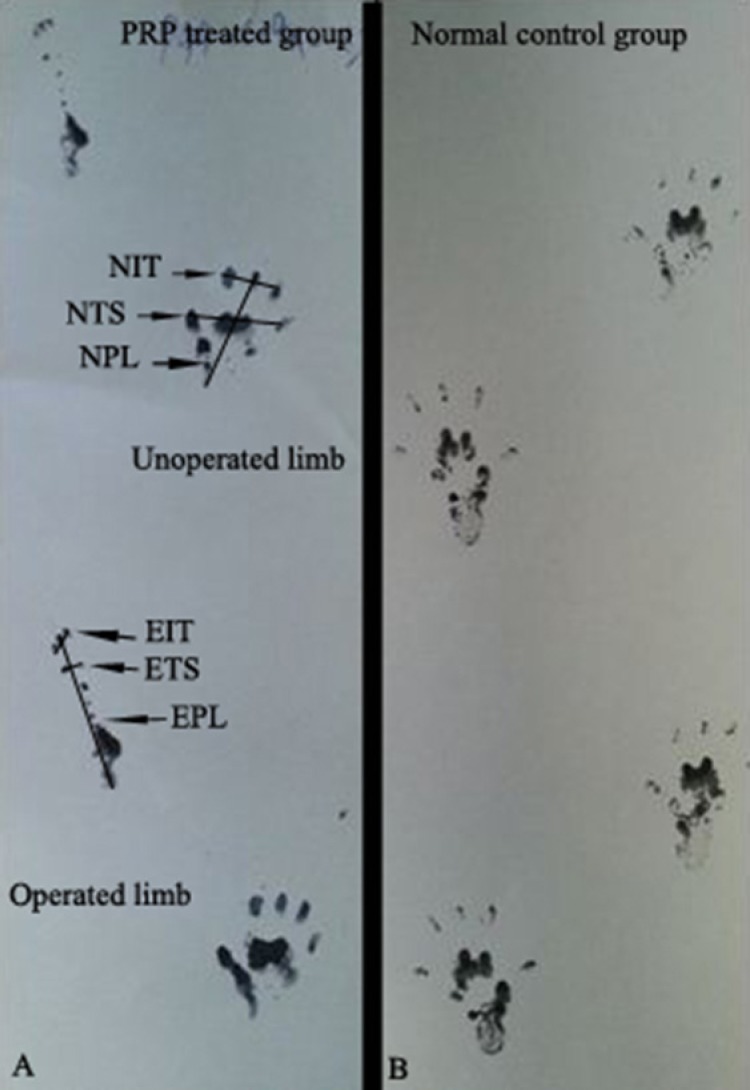
Walking track analysis was performed 4, 8 and 12 weeks after surgery based on the method of others [23]. The lengths of the third toe to its heel (PL), the first to the fifth toe (TS), and the second toe to the fourth toe (IT) were measured on the experimental side (E) and the contralateral normal side (N) in each rat (Figure 1). The sciatic function index (SFI) of each animal was calculated by the following formula: SFI=-38.3×(EPL-NPL)/NPL+109.5×(ETS-NTS)/ NTS+13.3×(EIT-NIT)/NIT-8.8 In general, SFI oscillates around 0 for normal nerve function, whereas around -100 SFI represents total dysfunction. SFI was assessed in the NC group and the normal level was considered as 0. SFI was a negative value and a higher SFI meant the better function of the sciatic nerve.
Fig. 1.
Pawprints in rats 2 weeks after surgery with for designated measurments: PRP-treated (A) and normal rats (B), toe spread (TS), print length (PL), intermediate toe spresd (IT). E: experimental, N: Normal. Note the elongation of the PL and narrowing of TS and IT in operated limb
Measurement of Gastrocnemius Muscles Mass
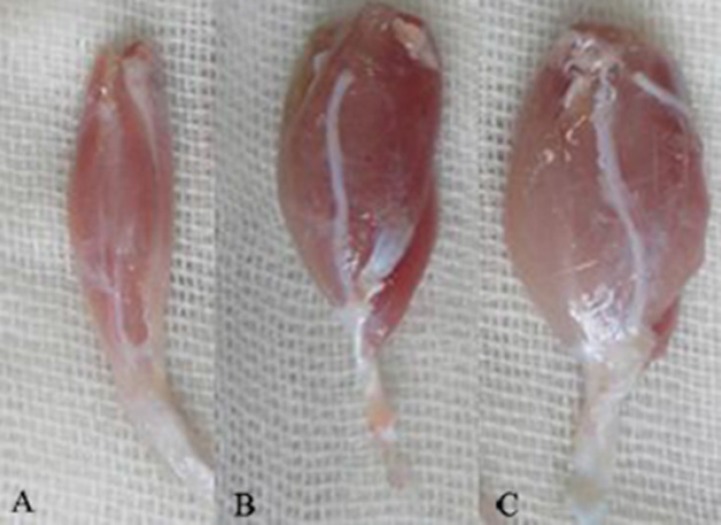
Recovery assessment was also indexed using the weight ratio of the gastrocnemius muscles 12 weeks after surgery. Immediately after sacrificing of animals, gastrocnemius muscles were dissected and harvested carefully from intact and injured sides and weighed while still wet, using an electronic balance (Figure 2). Two independent observers unaware of the analyzed group made all measurements.
Fig. 2.
Gastrocnemius muscles dissected and harvested from injured sides. Note to the lesser mass of gastrocnemius muscle of SIL (A) group compared to those of SIL/PRP (B) and NC group (C) 12 weeks after surgery
Statistical Analysis
Experimental results were expressed as means±SD. Statistical analyses were performed using PASW 18.0 (SPSS Inc., Chicago, IL, USA). In BBB and SFI assessments each rat was assessed 12 weeks on a weekly basis. In muscle mass measurements at the end of the study period each rat was euthanized and the muscle was measured. Therefore, in BBB and SFI assessments repeated measures and in muscle mass measurements two- way ANOVA were used. The differences were considered significant when p<0.05.
Results
BBB Recovery
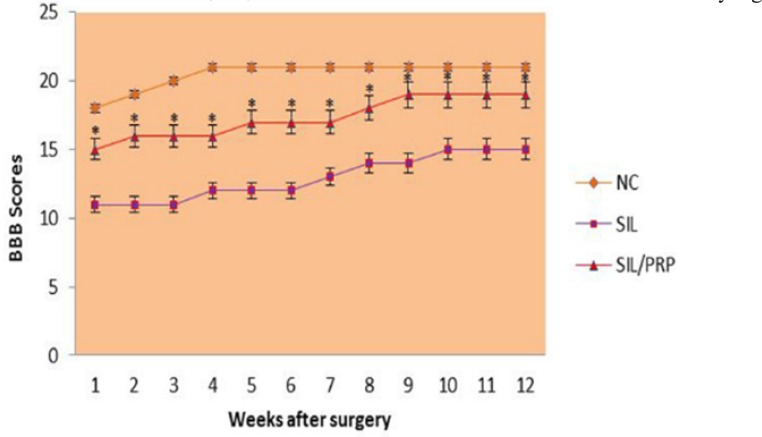
In order to assess hind limb recovery the open field locomotor was used. Figure 3 shows BBB scores compared to the baseline. All experimental groups, except for NC, showed the greatest degree of functional deficit one week after surgery. The PRP treated group showed significant improvement in locomotion of the operated limb compared to the control group during the study period ( p=0.001).
Fig. 3.
BBB score for all experimental groups. Local administration of PRP with silicon grafting gave better scores than in SIL group. Standard error at each data point is shown with bars. *P=0.001 vs SIL group. Data are presented as mean ± SD
Recovery of Sciatic Nerve Function and Reinnervation SFI Outcome
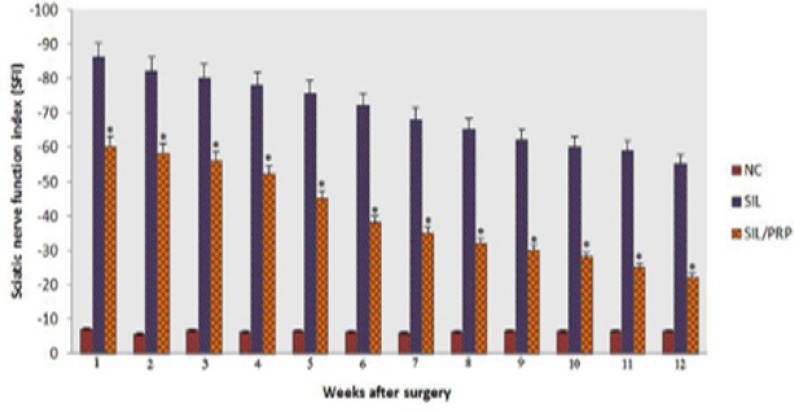
Figure 4 shows sciatic function index (SFI) values in experimental groups. Prior to surgery, SFI values in both groups were near zero. After the nerve transection, the mean SFI decreased to -100 due to the complete loss of sciatic nerve function in all animals. At the end of the study period, animals of SIL/PRP achieved a mean value for SFI of -22.6±-3.15 whereas in control group a mean value of -56.2±-3.5 was found. The statistical analyses revealed that the recovery of nerve function was significantly ( p=0.001) different between SIL and SIL/PRP groups and administration of PRP improved functional recovery in the course of time.
Fig. 4.
Effects on the sciatic nerve function index (SFI) in each experimental group during the study period. Statistically significant improvement (P=0.001) was observed in functional recovery of sciatic nerve in PRP treated animals at the end of the study period. *P=0.001 vs SIL group. Data are presented as mean ± SD
Gastrocnemius Muscles Mass Measurement
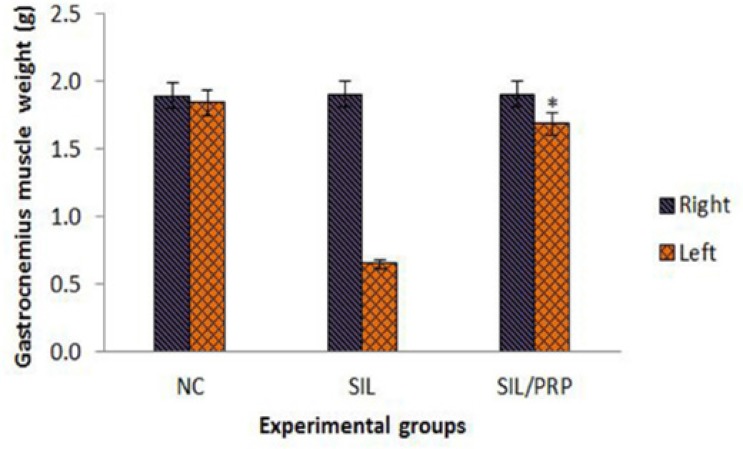
The mean ratios of gastrocnemius muscles weight were measured. There was statistically significant difference between the muscle weight ratios of SIL/ PRP and SIL groups ( p=0.001). The results showed that in SIL/PRP group muscle weight ratio was bigger than SIL group and the gastrocnemius muscle weight loss was improved by local administration of PRP (Figure 5).
Fig. 5.
Measurement of gastrocnemius muscle mass. The gastrocnemius muscles of both sides (operated left and unoperated right) are excised and weighed in the experimental groups at 12 weeks after surgery, *P=0.001 vs SIL group. Data are presented as mean ± SD
Discussion
Complete disruption of the perineurium results in third, fourth and fifth degrees of nerve injuries. With these types of injuries prognosis for recovery, without surgical and biological aids, is slow and poor. In such cases, all of the reparative processes fail, and there may be neuroma formation [24,25]. The results of the present study showed that administration of PRP resulted in faster functional recovery of the sciatic nerve during the study period. We used silicone tube as a scaffold for keeping the delivered drug in situ. Selection of an appropriate method to evaluate functional recovery of nerve regeneration is extremely influential. Although both morphological and functional data have been used to assess neural regeneration after induced crush injuries, the correlation between these two types of assessment is usually poor [26-28]. We did not perform nerve conduction tests because electrophysiological studies have poor correlation with functional indices [29]. Nerve conduction velocity and peak action potential amplitude do not evaluate total nerve function but a fraction of nerve fibers population. Compound action potential is derived from extrinsic direct nerve excitation and does not correlate with proper central or peripheral connections [27]. Classical and newly developed methods of assessing nerve recovery, including histomorphometry, retrograde transport of horseradish peroxidase and retrograde fluorescent labeling do not necessarily predict the reestablishment of motor and sensory functions [27,28,30-32]. Although such techniques are useful in studying the nerve regeneration process, they generally fail in assessing functional recovery [28]. Therefore, research on peripheral nerve injury needs to focus on functional assessment. Castaneda et al., [30] suggested that arrival of sprouts from the proximal stump at the distal nerve stump does not necessarily imply recovery of nerve function. Information taken from BBB scale may be invaluable in evaluation of peripheral nerve process. Results of the present study showed that the PRP treated animals had been improved in locomotion of the operated limb compared to the SIL group during the study period. Walking track analysis has frequently been used to reliably determine functional recovery following nerve repair in rat models [21,23]. Left gastrocnemius muscle weight was significantly greater in the SIL/PRP group than in the SIL group, indicating indirect evidence of successful end organ reinnervation in the PRP treated animals.
Schwann cells, the glial cells of the peripheral nervous system, surround all peripheral nerve axons, and in the case of myelinated nerve fibers, elaborate the myelin sheaths necessary for fast impulse conduction. There are many important developmental and functional interactions between Schwann cells and axons [33]. A major rate-limiting step in the induction of nerve regeneration across a gap is the proliferation, and migration of Schwann cells between the nerve stumps. Therefore, formation of a properly aligned extra cellular matrix scaffold is essential to enhance Schwann cell proliferation in a conduit, through which blood vessels and other cell types migrate and form primordial assembly for the formation of a new nerve structure [34].
We used a silicone rubber chamber as a conduit to provide a scaffold for PRP and to facilitate Schwann cells migration. The silicone as a conduit has been utilized to repair segmental nerve tissue loss which proved to be supportive conduit for peripheral nerve axonal regeneration and maturity [8]. If the nerve is injured, through mechanical trauma, neurotoxins or demyelinating diseases for example, Schwann cells again proliferate to restore the integrity of the Schwann cell sheath and aid regeneration of functional nerve fibers [35].
The role of PRP in tissue repair and regeneration has already been studied [10,12,14,16,36]. PRP is a concentration of fundamental growth factors known to be actively secreted by platelets to initiate wound healing. These growth factors include the isomeres of platelet-derived growth factor (PDGFαα, PDGFββ, and PDGFαβ) [37] the numerous transforming growth factors- β (TGFβ1 and TGFβ2), vascular endothelial growth factor, and epithelial growth factor. Because these concentrated platelets are suspended in a small volume of plasma, PRP is more than just a platelet concentrate. It also contains fibrin, fibronectin and vitronectin, known to act as cell adhesion molecules for osteoconduction and as a matrix for bone, connective tissue and epithelial migration [10,16].
A natural blood clot contains 95% red blood cells, 5% platelets, less than 1% white blood cells and numerous fibrin strands. A PRP clot contains 4% red blood cells, 95% platelets and 1% white blood cells. The healing cascade of the nerve fibers is initiated and controlled by bioactive proteins found in platelets, plasma and white blood cells. Increasing the concentration of these bioactive proteins, may accelerate the healing process of the regenerating nerve fibers [11].
Successful regeneration of peripheral nerve after nerve injury depends on activated Schwann cells and their supportive role in the production of neurotrophic and neurotropic factors (such as nerve growth factors) that enhance neural recovery [25]. The results of the present study showed a statistically significant difference in the outcome of nerve regeneration between the PRP-treated and non-treated groups. This may be partially due to the activation of Schwann cells to a greater extent in the PRP-treated compared with the non-treated groups. Even though our preliminary study shows the neuroprotective action of local PRP in peripheral nerve injuries, determining the molecular mechanisms leading to the neuroprotective action needs to be investigated. We have not given the histological and molecular evidence for neuroprotective action of PRP. This may be considered as a limitation to our study. Therefore, the authors stress that the aim of the current investigation was to evaluate clinical treatment potential of PRP on nerve regeneration including functional assessments of the nerve repair, a case not considered in previous studies. Preparation of PRP is cost saving as a result of simplicity of laboratory procedures, and potential for a bioactive mixture of growth factors and proteins. The results of the present study indicated that local administration of PRP combined with silicone entubulization could be of benefit after sciatic nerve transection. Detailed mechanism of neuroprotective action remains to be investigated. Further clinical and immunohistochemical studies are recommended to support the present results and to explore the possible beneficial effects (e.g. angiogenic effect) of PRP on regeneration of injured peripheral nerves. The experimental model presented here is reproducible and the behavioral and functional methods could be effectively used for the study of local effects of neurotrophics combined with guidance conduits.
In conclusion, the present study demonstrated that local administration of PRP combined with silicon grafting could accelerate functional recovery after nerve transection in sciatic nerve. Growth factors and bioactive proteins present in PRP that is easily available may have clinical implications for the surgical management of patients after nerve transection.
Conflict of interest: None declared.
References
- 1.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 2.Messora MR, Nagata MJH, Furlaneto FAC, Dornelles RCM, Bomfim SRM, Deliberador TM, et al. A standardized research protocol for platelet-rich plasma (PRP) preparation in rats. RSBO(Online) 2011;8(3):299–304. [Google Scholar]
- 3.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–96. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Meek MF, Coert JH. Clinical use of nerve conduits in peripheral nerve repair: review of the literature. J Reconstr Microsurg. . 2002;18(2):97–109. doi: 10.1055/s-2002-19889. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 6.Sammartino G, Tia M, Marenzi G, Lauro AE, D’Agostino E, Claudio PP. Use of autologous platelet-rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofac Surg. 2009;67(11):2369–73. doi: 10.1016/j.joms.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto H, Gurney ME. Human platelets contain brain-derived neurotrophic factor. J Neurosci. 1990;10(11):3469–78. doi: 10.1523/JNEUROSCI.10-11-03469.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis JB, Stroobant P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat schwalm cells. J Cell Biol. 1990;110(4):1353–60. doi: 10.1083/jcb.110.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varejão AS1, Melo-Pinto P, Meek MF, Filipe VM, Bulas-Cruz J. Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurol Res. 2004;26(2):186–94. doi: 10.1179/016164104225013833. [DOI] [PubMed] [Google Scholar]
- 10.Shen N, Zhu J. Application of sciatic functional index in nerve functional assessment. Microsurgery. 1995;16(8):552–5. doi: 10.1002/micr.1920160809. [DOI] [PubMed] [Google Scholar]
- 11.Piñeros-Fernández A1, Rodeheaver PF, Rodeheaver GT. Octyl 2-cyanoacrylate for repair of peripheral nerve. Ann Plast Surg. . 2005;55(2):188–95. doi: 10.1097/01.sap.0000164386.72523.3c. [DOI] [PubMed] [Google Scholar]
- 12.Dellon AL, Mackinnon SE. Sciatic nerve regeneration in the rat. Validity of walking track assessment in the presence of chronic contractures. Microsurgery. 1989;10(3):220–5. doi: 10.1002/micr.1920100316. [DOI] [PubMed] [Google Scholar]
- 13.Elgazzar R F, Mutabagani MA, Abdelaal SE, Sadakah AA. Platelet rich plasma may enhance peripheral nerve regeneration after cyanoacrylate reanastomosis: a controlled blind study on rats. Int J Oral Maxillofac Surg. 2008;37(8):748–55. doi: 10.1016/j.ijom.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Farrag TY, Lehar M, Cerhaegen P, Carson KA, Byrne PJ. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. . 2007;117(1):157–65. doi: 10.1097/01.mlg.0000249726.98801.77. [DOI] [PubMed] [Google Scholar]
- 15.Pogrel MA, McDonald AR, Kaban LB. Gore-Tex tubing as conduit for repair of lingual and inferior alveolar nerve continuity defects: A preliminary report. J Oral Maxillofac Surg. 1998 Mar;56(3):319–21. doi: 10.1016/s0278-2391(98)90107-0. discussion 321-2. [DOI] [PubMed] [Google Scholar]
- 16.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83(1):129–38. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotraum. . 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 18.Kanaya F, Firrell JC, Breidenbach WC. Sciatic function index, nerve conduction tests, muscle contraction, and axon morphometry as indicators of regeneration. Plast Reconstr Surg. 1996;98(7):1264–71. doi: 10.1097/00006534-199612000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Munro CA, Szalai JP, Mackinnon SE, Midha R. Lack of association between outcome measures of nerve regeneration. Muscle Nerve. 1998;21(8):1095–7. doi: 10.1002/(sici)1097-4598(199808)21:8<1095::aid-mus20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Kraut RA, Chahal O. Management of patients with trigeminal nerve injuries after mandibular implant placement. J Am Dent Assoc. 2002;133(10):1351–4. doi: 10.14219/jada.archive.2002.0050. [DOI] [PubMed] [Google Scholar]
- 21.de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77(3):634–43. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- 22.Castaneda F, Kinne RK. Omental graft improves functional recovery of transected peripheral nerve. Muscle Nerve. 2002;26(4):527–32. doi: 10.1002/mus.10229. [DOI] [PubMed] [Google Scholar]
- 23.Mark S, Greenberg MD. Handbook of Neurosurgery. New York: Theime Publishers; 2010. [Google Scholar]
- 24.Salzer JL, Bunge RP Studies of Schwann cell proliferation. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol. 1980;84(3):739–52. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oyama T, Nishimoto S, Tsugawa T, Shimizu F. Efficacy of platlet-rich plasma in alveolar bone grafting. J Oral Maxillofac Surg. 2004;62(5):555–8. doi: 10.1016/j.joms.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 26.Bervar M. Video analysis of standing-an alternative footprint analysis to assess functional loss following injury to the rat sciatic nerve. J Neurosci Methods. 2000;102(2):109–16. doi: 10.1016/s0165-0270(00)00281-8. [DOI] [PubMed] [Google Scholar]
- 27.Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26(2):151–60. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 28.Bolin LB, Iismaa TP, Shooter EM. Isolation of activated adult Schwann cells and a spontaneously immortal Schwann cell clone. J Neurosci Res. . 1992;33(2):231–8. doi: 10.1002/jnr.490330206. [DOI] [PubMed] [Google Scholar]
- 29.Williams LR, Longo FM, Powell HC, Lundborg G, Varon S. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay. J Comp Neurol. 1983;218(4):460–70. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]
- 30.Freymiller EG, Agbaloo TL. Platelet rich plasma: ready or not? Clinical controversies in oral and maxillofacial surgery: part one. J Oral Maxillofac Surg. . 2004;62(7):484–8. doi: 10.1016/j.joms.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Robinson PP, Loescher AR, Smith KG. A prospective, quantitative study on the clinical outcome of lingual nerve repair. Br J Oral Maxillofac Surg. . 2000;38(4):255–63. doi: 10.1054/bjom.2000.0463. [DOI] [PubMed] [Google Scholar]
- 32.Lanigan DT, Hohn FI. Facial nerve injuries after sagittal split mandibular ramus osteotomies for advancement: a report of 2 cases and review of the literature. J Oral Maxillofac Surg. 2004;62(4):503–7. doi: 10.1016/j.joms.2003.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Thorwarth M, Wehrhan F, Schultze-Mosgau S, Wiltfang J, Schlegel KA. PRP modulates expression of bone matrix proteins in vivo without long-term effects on bone formation. Bone. 2006;38(1):30–40. doi: 10.1016/j.bone.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Butterfield K, Bennett J, Gronowicz G, Adams D. Effect of platlet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg. 2005;63(3):370–6. doi: 10.1016/j.joms.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Wieken K, Angioi-Duprez K, Lim A, Marchal L, Merle M. Nerve anastomosis with glue: comparative histologic study of fibrin and cyanoacrylate glue. J Reconstr Microsurg. 2003;19(1):17–20. doi: 10.1055/s-2003-37186. [DOI] [PubMed] [Google Scholar]
- 36.Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Vento AM, et al. Platelet-drived growth factors enhance proliferation of human stromal stem cells. Biomaterails. . 2003;24(18):3095–100. doi: 10.1016/s0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- 37.Marxs RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.1998. 85(6):638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]