Abstract
Oxygenous terpenoids are active components of many medicinal plants. However, current studies that have focused on enzymatic oxidation reactions cannot comprehensively clarify the mechanisms of oxygenous terpenoid synthesis and diversity. This study shows that an endophytic bacterium can trigger the generation of reactive oxygen species (ROS) that directly increase oxygenous sesquiterpenoid content and diversity in Atractylodes lancea. A. lancea is a famous but endangered Chinese medicinal plant that contains abundant oxygenous sesquiterpenoids. Geo-authentic A. lancea produces a wider range and a greater abundance of oxygenous sesquiterpenoids than the cultivated herb. Our previous studies have shown the mechanisms behind endophytic promotion of the production of sesquiterpenoid hydrocarbon scaffolds; however, how endophytes promote the formation of oxygenous sesquiterpenoids and their diversity is unclear. After colonization by Pseudomonas fluorescens ALEB7B, oxidative burst and oxygenous sesquiterpenoid accumulation in A. lancea occur synchronously. Treatment with exogenous hydrogen peroxide (H2O2) or singlet oxygen induces oxidative burst and promotes oxygenous sesquiterpenoid accumulation in planta. Conversely, pretreatment of plantlets with the ROS scavenger ascorbic acid significantly inhibits the oxidative burst and oxygenous sesquiterpenoid accumulation induced by P. fluorescens ALEB7B. Further in vitro oxidation experiments show that several oxygenous sesquiterpenoids can be obtained from direct oxidation caused by H2O2 or singlet oxygen. In summary, this study demonstrates that endophytic bacterium-triggered ROS can directly oxidize oxygen-free sesquiterpenoids and increase the oxygenous sesquiterpenoid content and diversity in A. lancea, providing a novel explanation of the mechanisms of oxygenous terpenoid synthesis in planta and an essential complementarity to enzymatic oxidation reactions.
INTRODUCTION
Atractylodes lancea is a traditional Chinese medicinal plant and is the main ingredient of many famous Chinese medicines. Oxygenous sesquiterpenoids, such as hinesol, β-eudesmol, atractylone, and caryophyllene oxide, are the main active components in A. lancea and have medicinal efficacy against rheumatic diseases, digestive disorders, night blindness, and influenza (1). The quality of A. lancea strongly depends on the area in which the herb is produced, and A. lancea grown in the Maoshan area of the Jiangsu Province is the geo-authentic herb (2), which is characterized by higher oxygenous sesquiterpenoid content and diversity than herbs grown in other areas (3). In recent years, the geo-authentic A. lancea has become endangered due to habitat destruction and overexploitation. Although cultivation ensures the production of medicinal materials, the oxygenous sesquiterpenoid content and diversity in cultivated A. lancea is considerably decreased compared to that of the wild herb. Currently, knowledge on the biosynthesis and diversity of oxygenous sesquiterpenoids is insufficient. Some studies have explained the mechanisms of oxygenous sesquiterpenoid synthesis by enzymatic reactions (4). However, this cannot explain the diversity seen among oxygenous sesquiterpenoids, as there are no corresponding enzymes to catalyze the synthesis of so many different sesquiterpenoids (5).
Endophytes are microorganisms that asymptomatically colonize the internal tissues of almost all plants (6, 7). Our previous studies have demonstrated that the endophytes inside A. lancea grown in the Maoshan area are more abundant than those inside A. lancea grown in other areas (8–10), and several endophytes, such as Acinetobacter sp. strain ALEB16 and Gilmaniella sp. strain AL12, can efficiently increase oxygenous sesquiterpenoid accumulation in A. lancea (11–13). These endophytes can activate signaling molecules, such as hydrogen peroxide (H2O2), salicylic acid, and jasmonic acid (14, 15), which increase the expression of genes encoding key enzymes involved in sesquiterpenoid biosynthesis pathways (13), promoting the formation of sesquiterpenoid hydrocarbon scaffolds in A. lancea. However, the mechanisms by which endophytes promote the oxidation of sesquiterpenoid hydrocarbon scaffolds and increase oxygenous sesquiterpenoid accumulation are unknown. The endophyte Pseudomonas fluorescens ALEB7B has been isolated from A. lancea grown in the Maoshan area and has been shown to establish a stable mutualistic relationship with A. lancea (10, 16). It is worth noting that P. fluorescens ALEB7B can increase oxygenous sesquiterpenoid accumulation in A. lancea more efficiently than other endophytes, such as Acinetobacter sp. strain ALEB16 (13).
Oxidative burst is one of the earliest plant responses to microbial infection (17). We propose that endophyte-triggered reactive oxygen species (ROS), such as H2O2, not only can act as signaling molecules but also can directly oxidize oxygen-free sesquiterpenoids to generate oxygenous sesquiterpenoids. The diverse array of oxygenous sesquiterpenoids cannot be obtained from enzymatic reactions alone, which oxidize sesquiterpenoids at specific sites. However, the oxidation induced by ROS can lead to the formation of diverse oxygenous sesquiterpenoids because of the random nature of chemical oxidation and differing degrees of oxidation. Therefore, the sesquiterpenoid diversity seen in A. lancea may be a result of direct oxidation caused by ROS.
The aims of this study were to investigate whether the endophytic colonization of A. lancea by P. fluorescens ALEB7B can cause an oxidative burst and to demonstrate the impact of ROS on oxygenous sesquiterpenoid composition in A. lancea. This study confirms that endophytic bacterium-triggered ROS can directly oxidize sesquiterpenoids and increase oxygenous sesquiterpenoid content and diversity in A. lancea.
MATERIALS AND METHODS
A. lancea plantlets and growth conditions.
A. lancea aseptic tissue culture plantlets were established as previously described (13, 18). Buds were collected from cultivated A. lancea and washed under running water, after which all procedures were conducted aseptically. Buds were surface sterilized by immersing in 75% (vol/vol) ethanol for 30 s, soaking in 1% (wt/vol) mercury chloride for 10 min, and thoroughly rinsing 5 times in sterile distilled water. Several buds were randomly selected, homogenized, and inoculated on potato dextrose agar to confirm the absence of endophytes. Intact, surface-sterilized buds then were transferred to 50 ml of Murashige-Skoog medium supplemented with 0.3 mg liter−1 naphthaleneacetic acid (NAA) and 2.0 mg liter−1 6-benzyladenine in sealed 100-ml Erlenmeyer flasks.
When sufficient buds had germinated, they were separated and transplanted into 50 ml of Murashige-Skoog rooting medium supplemented with 0.1 mg liter−1 NAA in sealed 100-ml Erlenmeyer flasks to develop into plantlets. All aseptic tissue culture plantlets were kept in a growth chamber with a photoperiod of 12 h, a light density of 3,400 lm m−2, and a temperature cycle of 25/18°C day/night. Plantlets used in this study were 4 weeks old.
Endophytic bacterium and inoculation.
P. fluorescens ALEB7B was isolated from the geo-authentic A. lancea grown in the Maoshan area (10) and stored at the China Center for Type Culture Collection (CCTCC AB 2013331). The molecular identification of P. fluorescens ALEB7B and its colonization inside A. lancea were confirmed in our previous study (16). Bacterium was grown in Luria-Bertani (LB) broth at 30°C with agitation (220 rpm) for 24 h, and bacterial cells were collected and resuspended in sterile double-distilled water with the concentration adjusted to 106 cells ml−1.
A. lancea aseptic tissue culture plantlets were sprayed with 200 μl of the bacterial suspension. The bacterial suspension flowed from leaf surfaces to the roots. Other plantlets were sprayed with 200 μl of sterile double-distilled water as a control. All bacterium-inoculated and control plantlets were randomized in the growth chamber.
Enumeration of P. fluorescens ALEB7B inside A. lancea.
Plantlets were sampled and surface sterilized (10). The amount of P. fluorescens ALEB7B inside A. lancea was estimated using bacterial cell culture and quantitative PCR (qPCR).
Surface-sterilized plantlets were homogenized, serially diluted in sterile double-distilled water, and inoculated onto LB agar plates. The number of colonies on each plate was counted after incubation at 30°C for 3 days, and the number of CFU per gram of plant tissue was calculated. The 16S rRNA gene sequence of the reisolated bacterial strain had the highest similarity (100%) to the 16S rRNA gene sequence of the original bacterial strain (KF460526), confirming the reisolation of P. fluorescens ALEB7B from A. lancea.
qPCR was performed as described by Wang et al. (19). Primers were designed to amplify a fragment of the gyrB gene in P. fluorescens, which encodes DNA gyrase subunit B (gyrB-qPCR-F, TTGGCGACAGCGAAACCACC; gyrB-qPCR-R, GCCACCCTCGTACTTGAACAGC) (16).
Chemicals and treatments.
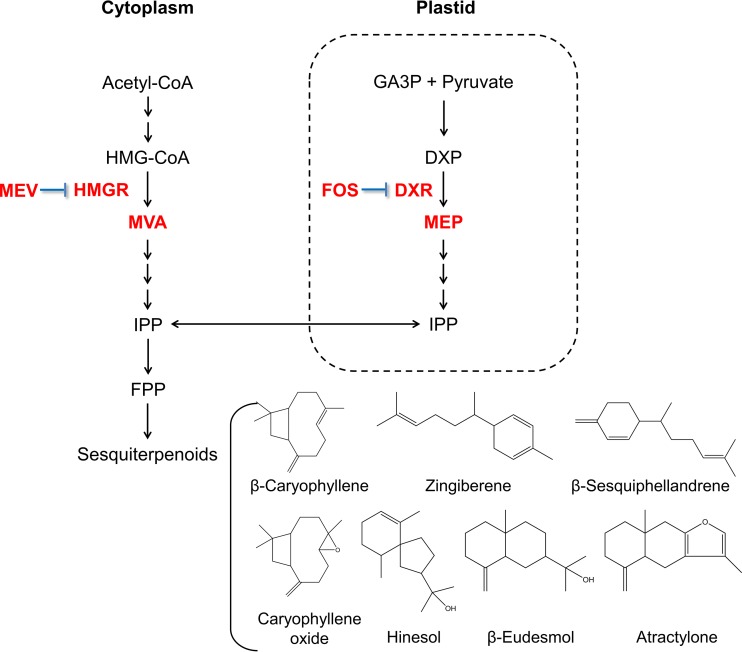
There are two main sesquiterpenoid biosynthesis pathways in A. lancea, including the mevalonate (MVA) pathway and the 2-C-methyl-d-erythritol phosphate (MEP) pathway (Fig. 1). Mevinolin (MEV) and fosmidomycin (FOS) are specific inhibitors of these two pathways. Ascorbic acid (AsA) was chosen as an ROS scavenger, as it can quench four main forms of ROS at the same time (20), including hydrogen peroxide (H2O2), singlet oxygen, superoxide anions, and hydroxyl radicals (21).
FIG 1.
Proposed sesquiterpenoid biosynthesis pathway in Atractylodes lancea adapted from Wang et al. (13). Lines ending with a bar indicate the repression of enzymatic activities by inhibitors (mevinolin and fosmidomycin). Acetyl-CoA, acetyl coenzyme A; DXP, 1-deoxy-d-xylulose 5-phosphate; DXR, 1-deoxy-d-xylulose 5-phosphate reductoisomerase; FOS, fosmidomycin; FPP, farnesyl pyrophosphate; GA-3P, glyceraldehyde 3-phosphate; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; HMGR, 3-hydroxy-3-methylglutaryl coenzyme A reductase gene; IPP, isopentenyl diphosphate; MEP, 2-C-methyl-d-erythritol phosphate; MEV, mevinolin; MVA, 3R-mevalonic acid.
All inhibitors, oxidants, and ROS scavenger, including MEV (30 μM), FOS (200 μM), H2O2 (2, 5, or 10 mM), sodium hypochlorite (NaClO; 0.2, 0.5, or 1 M), and AsA (1,000 ppm), were dissolved or diluted in double-distilled water (13). MEV was previously converted to a water-soluble sodium salt as described by Hagen and Grunewald (22). FOS was purchased from Toronto Research Chemicals Inc. (North York, Canada), and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). All solutions were sterilized by filtration through 0.22-μm sterile filters.
The inhibitors or ROS scavenger were sprayed on plant leaves until dripping wet to allow a 1-day infiltration period before bacterial inoculation or oxidant application (13). The oxidant solutions (200 μl) were sprayed on plantlets, which flowed from leaf surfaces to plant roots. Chemicals and their dosages used here were based on the results of preliminary experiments. An equal volume of sterile double-distilled water was applied to plantlets as controls.
β-Caryophyllene and caryophyllene oxide standards were purchased from Sigma-Aldrich, and zingiberene and β-sesquiphellandrene were synthesized by WuXi AppTec Co., Ltd. (Tianjing, China). Their purities were higher than 99%. Hinesol, β-eudesmol, and atractylone were separated and purified in our laboratory to >95% purity.
Photosynthesis measurement.
Leaf chlorophyll content was assayed using a spectrophotometric method according to Zhu et al. (23). The net photosynthetic rate was monitored using a Li-Cor 6400 portable photosynthesis system (Lincoln, NE) according to Li and Wang (24). Compressed air containing 350 μmol mol−1 CO2 was used as a gas source, and the photosynthetic photon flux density was 200 μmol m−2 s−1. Measurements were conducted from 9:00 a.m. to 10:00 a.m.
Soluble carbohydrate, acetyl coenzyme A, pyruvate, and malondialdehyde measurements in whole A. lancea plantlets.
Soluble carbohydrates were measured according to the method of Wang et al. (18). Acetyl coenzyme A was extracted according to Tumaney et al. (25) and measured using the enzyme-linked immunosorbent assay kit (Shanghai Fankel Biological Technology Co., Ltd., China). Pyruvate content was measured using the 2,4-dinitrophenylhydrazine method, while malondialdehyde content was measured using the thiobarbituric acid method according to Zeng et al. (26).
Measurement and histochemical staining of endogenous reactive oxygen species.
Endogenous H2O2 and hydroxyl radical content were measured with H2O2 and hydroxyl radical assay kits (Nanjing Jiancheng Bio-Engineering Institute, China), respectively (14). Singlet oxygen levels were determined using a spectrophotometric method according to Yang et al. (27). Superoxide anion levels were determined using the nitroblue tetrazolium (NBT) colorimetric method according to Gao et al. (28).
Histochemical staining of H2O2 was conducted using 3,3-diaminobenzidine (DAB) as a substrate according to Wang et al. (29). Staining of superoxide anions was conducted using the NBT staining method according to Montiel et al. (30). Stained leaves and roots were observed and photographed.
Sesquiterpenoid extraction and gas chromatography analysis.
Harvested plantlets were dried at 36°C to a constant weight, and total sesquiterpenoids were extracted according to the method of Wang et al. (13). One gram of dried plantlets was ground and extracted in 4 ml of cyclohexane for 10 h. After sonication and centrifugation, total sesquiterpenoid extracts were dried over anhydrous sodium sulfate and filtered through 0.22-μm sterile filters before gas chromatography (GC) analysis.
GC analysis was performed on an Agilent 7890A GC equipped with a flame ionization detector (Agilent Technologies, Santa Clara, CA) as previously described (13). An Agilent DB-1HT capillary chromatographic column (30 m; 0.32-mm inside diameter, 0.1-μm film) was used with the following temperature program: initial temperature of 100°C for 4 min, increased to 140°C at 10°C min−1 for 10 min, increased to 220°C at 10°C min−1 for 10 min, and increased to 260°C at 10°C min−1 for 2 min. The injector and detector temperatures were 240°C and 350°C, respectively. High-purity nitrogen was used as the carrier gas with a flow rate of 0.8 ml min−1. The injection volume was 1 μl, and the split ratio was 5:1.
Qualitative analyses of seven main sesquiterpenoids (β-caryophyllene, zingiberene, β-sesquiphellandrene, caryophyllene oxide, hinesol, β-eudesmol, and atractylone) were performed according to retention times of authentic standards (15). Quantitative measurements were made by comparing results to standard curves that were constructed according to concentrations and peak areas of the sesquiterpenoid standards in the gas chromatograms.
Sesquiterpenoids oxidized by reactive oxygen species in vitro and identification of oxidized products.
Hydroxyl radicals were generated from the reductive cleavage of H2O2 (21), and singlet oxygen radicals were generated by reacting H2O2 with NaClO (31). Sesquiterpenoids were extracted from 1 g of dried A. lancea plantlets with 4 ml of anhydrous alcohol and were incubated with 4 ml of 5 mM H2O2, 5 mM H2O2 plus 5 U ml−1 peroxidase (Sigma-Aldrich), or 5 mM H2O2 plus 0.2 M NaClO. The reaction was allowed to proceed for 4 h, and oxidized products subsequently were extracted using 4 ml of cyclohexane. Sesquiterpenoids reacted with double-distilled water were used as controls.
Oxidized products were identified using an Agilent 7890A series GC connected to a 5975 mass selective detector (scanned range m/z 50 to 500) fitted with a DB-1ms capillary chromatographic column (30 m; 0.25-mm inside diameter, 0.25-μm film). The electron impact mass spectral data (70 eV) of each peak in the total ion chromatogram was obtained and compared to the National Institute of Standards and Technology (NIST) database to identify the corresponding compound (32).
Statistical analysis.
All experiments were performed in triplicate with three biological replicates in each repeat. As similar results were shown in each repeat, only one typical example was shown for each experiment. The means and standard deviations (SD) were calculated using SPSS Statistics 17.0 software (SPSS Inc., Chicago, USA), and the final experimental data were represented as the means ± SD. When an analysis consisted of only a control and an experimental group, an independent t test was performed using SPSS Statistics 17.0 software, and when three or more groups were compared, a one-way analysis of variance (ANOVA) was performed, followed by Tukey's multiple-comparison test (P < 0.05) (33).
RESULTS
Colonization dynamics of P. fluorescens inside A. lancea.
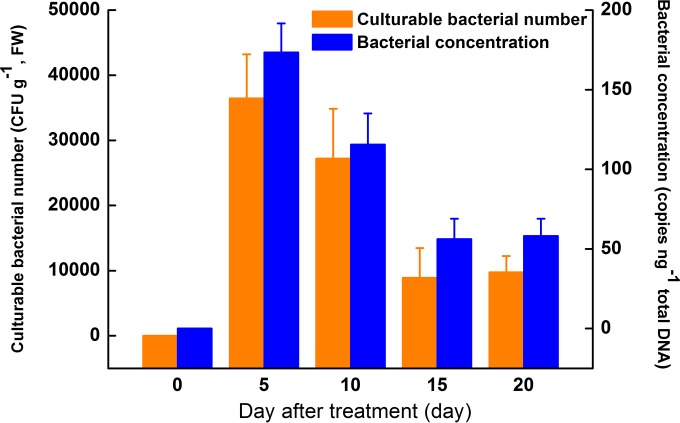
Bacterial cell culture and qPCR were conducted to monitor the colonization dynamics of P. fluorescens ALEB7B inside A. lancea. The number of cultivable bacteria peaked 5 days after inoculation (36,433.3 ± 6,785.4 CFU g−1, fresh weight) and subsequently decreased and became stable after 15 days (Fig. 2). The bacterial DNA concentration also peaked 5 days after inoculation (173.5 ± 18.1 copies ng−1 total DNA) and followed a pattern similar to that of the cultivable bacteria, reaching a stable level after 15 days. Results of both bacterial cell culture and qPCR showed similar bacterial colonization dynamics. The high bacterial load at the early stages of colonization might induce plant defense responses, which might limit the bacterial growth in planta. The stable colonization of P. fluorescens ALEB7B at later stages indicated the adaptation of each symbiotic partner. Therefore, we detected the impact of bacterial colonization on the host plant to further elaborate the symbiotic interaction between A. lancea and P. fluorescens.
FIG 2.
Culturable bacterial number and bacterial DNA concentration of Pseudomonas fluorescens ALEB7B inside Atractylodes lancea. The culturable bacterial number is represented as the number of CFU per gram of plant tissue. The bacterial DNA concentration is represented as the number of gyrB gene copies per nanogram of total DNA extracted from A. lancea inoculated with P. fluorescens ALEB7B. FW, fresh weight.
Endophytic P. fluorescens triggers oxidative burst and increases oxygenous sesquiterpenoid content in A. lancea.
Histochemical staining and quantification of ROS in A. lancea were conducted. To identify any correlation between endophytic bacterium-triggered oxidative burst and the accumulation of sesquiterpenoids, the levels of seven main sesquiterpenoids in A. lancea were measured after inoculation with P. fluorescens ALEB7B.
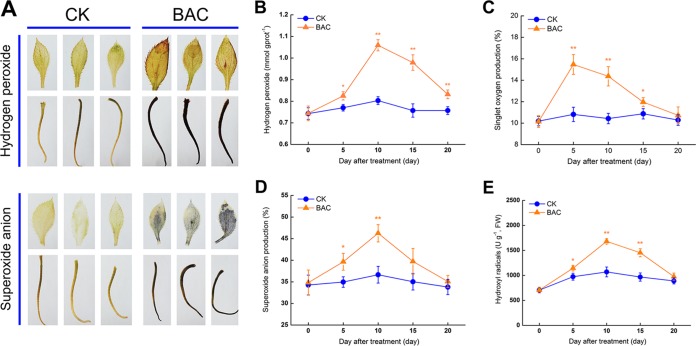
Oxidized DAB (brownish insoluble polymer) was observed in both plant leaves and roots 10 days after bacterial inoculation, allowing us to visualize H2O2 accumulation in situ (Fig. 3A). Superoxide anion production in situ was visualized by the formation of formazan precipitates. Levels of all four forms of ROS in A. lancea increased significantly 5 days after bacterial inoculation. The H2O2 content peaked (1.3-fold higher than the control) 10 days after inoculation and then slowly decreased (Fig. 3B). The singlet oxygen level peaked (1.4-fold greater than the control) after 5 days and returned to the control level after 20 days (Fig. 3C). The level of superoxide anions peaked (1.3-fold greater than the control) after 10 days and then decreased rapidly (Fig. 3D). The hydroxyl radical content remained at a relatively high level (approximately 1.5-fold higher than the control) 10 to 15 days after bacterial inoculation (Fig. 3E).
FIG 3.
Histochemical staining and level of reactive oxygen species in Atractylodes lancea inoculated with Pseudomonas fluorescens ALEB7B. (A) Histochemical staining of hydrogen peroxide (H2O2) and superoxide anions in plant leaves and roots after 10 days. (B to E) Level of H2O2, singlet oxygen, superoxide anions, and hydroxyl radicals in A. lancea over time. Results are means from three biological replicates. Error bars indicate standard deviations. *, significant differences at P < 0.05; **, significant differences at P < 0.01. CK, control; BAC, P. fluorescens ALEB7B.
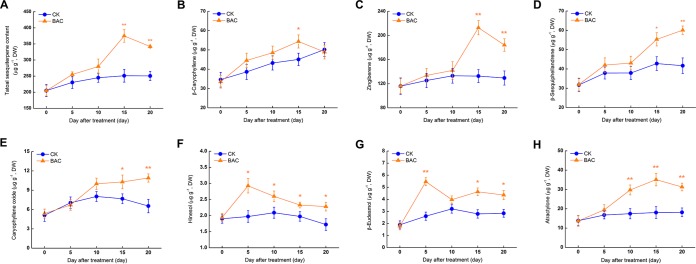
Total sesquiterpenoid accumulation in A. lancea significantly increased 15 days after inoculation with P. fluorescens ALEB7B (Fig. 4A). Accumulation of three oxygen-free sesquiterpenoids (β-caryophyllene, zingiberene, and β-sesquiphellandrene) significantly increased after 15 days (Fig. 4B to D), while caryophyllene oxide content began increasing (1.3-fold higher than the control) after 10 days (Fig. 4E). Hinesol and β-eudesmol content peaked (1.5-fold and 2.1-fold higher than the control, respectively) after 5 days (Fig. 4F and G), and atractylone content significantly increased (1.7-fold above the control) after 10 days (Fig. 4H). The endophytic bacterium-triggered oxidative burst and oxygenous sesquiterpenoid accumulation in A. lancea occurred synchronously, indicating that the endophytic bacterium-triggered ROS directly oxidizes oxygen-free sesquiterpenoids. Therefore, we subsequently investigated the impacts of exogenous hydrogen peroxide and singlet oxygen on oxygenous sesquiterpenoid accumulation in planta. Increased oxygen-free sesquiterpenoid content in planta at later stages might have been the result of increased primary metabolites, as acetyl coenzyme A and pyruvate are precursors of sesquiterpenoids (see Fig. S1 in the supplemental material).
FIG 4.
Accumulation of total sesquiterpenoid and seven main sesquiterpenoids in Atractylodes lancea inoculated with Pseudomonas fluorescens ALEB7B over time. (A) Total sesquiterpenoid. (B) β-Caryophyllene. (C) Zingiberene. (D) β-Sesquiphellandrene. (E) Caryophyllene oxide. (F) Hinesol. (G) β-Eudesmol. (H) Atractylone. Results are means from three biological replicates. Error bars indicate standard deviations. *, significant differences at P < 0.05; **, significant differences at P < 0.01. CK, control; BAC, P. fluorescens ALEB7B.
Exogenous hydrogen peroxide and singlet oxygen increase oxygenous sesquiterpenoid accumulation in A. lancea.
Based on our preliminary experiments, 5 mM H2O2 and 0.2 M NaClO plus 5 mM H2O2 were applied to A. lancea plantlets, as they increased endogenous H2O2 (see Fig. S2A in the supplemental material) and singlet oxygen levels (see Fig. S3A) to those seen when the plants were inoculated with P. fluorescens ALEB7B (Fig. 3B and C). Levels of three oxygen-free sesquiterpenoids (β-caryophyllene, zingiberene, and β-sesquiphellandrene) significantly increased 10 or 15 days after treatment with 5 mM H2O2 and then decreased rapidly (see Fig. S2B to D). Levels of four oxygenous sesquiterpenoids (caryophyllene oxide, hinesol, β-eudesmol, and atractylone) all peaked after 5 days and then reduced to a level that was the same as or lower than that of the control (see Fig. S2E to H).
The β-caryophyllene content significantly decreased 5 days after treatment with 0.2 M NaClO plus 5 mM H2O2 and remained at a level lower than that of the control (see Fig. S3B in the supplemental material). Levels of zingiberene and β-sesquiphellandrene both significantly decreased from 5 to 10 days after the treatment and increased after 15 days (see Fig. S3C and D). Meanwhile, the levels of three oxygenous sesquiterpenoids (caryophyllene oxide, hinesol, and β-eudesmol) all significantly increased from 5 to 15 days after the treatment (see Fig. S3E to G), but atractylone content remained relatively unchanged across the whole experimental period (see Fig. S3H). Oxygenous sesquiterpenoid accumulation in A. lancea plantlets stimulated by exogenous H2O2 or singlet oxygen occurred earlier than the accumulation of oxygen-free sesquiterpenoids. These results indicated that oxygen-free sesquiterpenoids were directly oxidized by H2O2 or singlet oxygen at an early stage posttreatment. Furthermore, increased oxygen-free sesquiterpenoid accumulation at a later stage after the treatment reflected the signaling function of H2O2 or singlet oxygen.
The ROS scavenger AsA reverses oxygenous sesquiterpenoid accumulation caused by endophytic P. fluorescens.
To further confirm the direct oxidation of ROS to oxygen-free sesquiterpenoids, we investigated whether the ROS scavenger AsA could reverse oxygenous sesquiterpenoid accumulation in A. lancea caused by P. fluorescens ALEB7B. Pretreatment of plantlets with 1,000 ppm AsA reduced H2O2 and singlet oxygen levels in A. lancea inoculated with P. fluorescens ALEB7B to control levels (see Fig. S4A and B in the supplemental material). Compared with untreated plantlets, the content of both the oxygen-free (β-caryophyllene, zingiberene, and β-sesquiphellandrene) (see Fig. S4C to E) and oxygenous sesquiterpenoids (caryophyllene oxide, hinesol, β-eudesmol, and atractylone) (see Fig. S4F to I) in pretreated plantlets significantly decreased but still were higher than those in the control plantlets. A single treatment of A. lancea with AsA had no obvious impact on oxidative burst or sesquiterpenoid accumulation. These results indicated that AsA reversed oxygenous sesquiterpenoid accumulation caused by P. fluorescens ALEB7B by reducing ROS levels in planta, further demonstrating the direct oxidation of ROS to oxygen-free sesquiterpenoids. We next conducted in vitro oxidation experiments, which further confirmed the direct impact of ROS on oxygenous sesquiterpenoid formation.
In vitro sesquiterpenoid oxidation by hydrogen peroxide and singlet oxygen.
Total sesquiterpenoids extracted from cultivated A. lancea reacted with 5 mM H2O2 or 0.2 M NaClO plus 5 mM H2O2. After incubation with H2O2 for 4 h, the contents of two compounds in the total extracted sesquiterpenoids were clearly increased compared to those of the control (see Fig. S5A in the supplemental material). Gas chromatography-mass spectrometry (GC-MS) analysis showed that these two compounds were caryophyllene oxide and humulene epoxide II (see Fig. S5B).
When the sesquiterpenoids were reacted with NaClO plus H2O2, the contents of seven oxygenous sesquiterpenoids were much higher than the control levels. These oxygenous sesquiterpenoids included aromadendrene oxide, hinesol, diepicedrene-1-oxide, tricyclo[5.2.2.0 (1, 6)]undecan-3-ol, 2-methylene-6,8,8-trimethyl-, 7-epi-cis-sesquisabinene hydrate, and spathulenol (see Table S1 and Fig. S5C in the supplemental material). Among these, hinesol and spathulenol were previously reported as oxygenous sesquiterpenoids in A. lancea (see Fig. S5D) (34). These results were consistent with the results of in vivo experiments, and hinesol and caryophyllene oxide were found to be derived from direct oxidation both in vivo and in vitro. Many oxygenous sesquiterpenoids were generated from in vitro oxidation, which showed that the sesquiterpenoid diversity in A. lancea was at least partially dependent on the direct oxidation of ROS.
Functions of sesquiterpenoids in the balance of antagonism between A. lancea and P. fluorescens.
We detected the functions of direct oxidation of oxygen-free sesquiterpenoids by ROS in symbiotic interactions between A. lancea and P. fluorescens. When two sesquiterpenoid synthesis pathways in A. lancea (the MVA pathway and MEP pathway) were blocked by MEV and FOS, the impact of endophytic P. fluorescens ALEB7B on A. lancea changed from benefit to harm. It was clear that sesquiterpenoid accumulation in A. lancea plantlets was significantly inhibited by MEV and FOS (Table 1). When sesquiterpenoid synthesis was blocked, the dry weight of plants was significantly decreased and the malondialdehyde content in planta was significantly increased 5 days after bacterial inoculation. Moreover, the aboveground parts of plantlets that had been pretreated with MEV and FOS before bacterial inoculation appeared chlorotic and withered (Fig. 5). A single treatment with MEV and FOS had no obvious adverse effects on any of the indices tested. These results indicated that sesquiterpenoids protected host plants from oxidative damage caused by endophytic bacterial colonization and that oxygen-free sesquiterpenoids that directly consumed ROS played essential roles in maintaining the balance of antagonism between A. lancea and P. fluorescens.
TABLE 1.
Impacts of P. fluorescens ALEB7B on sesquiterpenoid content, plant dry weight, and malondialdehyde content in Atractylodes lancea organisms when two main sesquiterpenoid synthesis pathways are blocked by MEV and FOS
| Parameter and day after treatment | Valuea for treatment with: |
|||
|---|---|---|---|---|
| CK | BAC | FOS+MEV | BAC+FOS+MEV | |
| Total sesquiterpene content (μg g−1, DW) | ||||
| 0 | 206.3 ± 17.8 a | 208.3 ± 17.8 a | 207.3 ± 19.6 a | 202.9 ± 17.2 a |
| 5 | 214.7 ± 9.9 a | 221.4 ± 14.4 a | 223.1 ± 7.9 a | 187.9 ± 14.4 a |
| 10 | 200.2 ± 13.5 b | 245.8 ± 19.2 b | 240.3 ± 16.7 b | 162.1 ± 19.8 a |
| 15 | 227.3 ± 15.9 b | 327.4 ± 15.6 c | 158.4 ± 15.2 a | 151.7 ± 18.6 a |
| 20 | 235.0 ± 10.9 b | 303.6 ± 15.9 c | 156.3 ± 12.4 a | 147.3 ± 19.6 a |
| DW (g) | ||||
| 0 | 0.06 ± 0.01 a | 0.06 ± 0.01 a | 0.06 ± 0.01 a | 0.06 ± 0.01 a |
| 5 | 0.05 ± 0.01 a | 0.07 ± 0.01 a | 0.23 ± 0.05 b | 0.04 ± 0.00 a |
| 10 | 0.05 ± 0.01 b | 0.07 ± 0.01 c | 0.14 ± 0.01 d | 0.03 ± 0.01 a |
| 15 | 0.07 ± 0.01 b | 0.08 ± 0.01 b | 0.11 ± 0.01 c | 0.04 ± 0.01 a |
| 20 | 0.08 ± 0.01 b | 0.09 ± 0.01 b | 0.10 ± 0.01 b | 0.04 ± 0.01 a |
| Malondialdehyde (nmol mg protein−1) | ||||
| 0 | 5.4 ± 0.4 a | 5.5 ± 0.5 a | 5.4 ± 0.4 a | 5.3 ± 0.4 a |
| 5 | 6.2 ± 0.5 a | 5.9 ± 0.6 a | 7.7 ± 0.5 a | 10.3 ± 0.6 b |
| 10 | 6.3 ± 0.5 a | 5.3 ± 0.7 a | 9.3 ± 0.7 b | 11.5 ± 0.9 b |
| 15 | 8.5 ± 0.5 a | 6.9 ± 0.4 a | 9.3 ± 0.5 a | 11.0 ± 0.4 b |
| 20 | 6.1 ± 0.5 a | 4.3 ± 0.5 a | 11.7 ± 0.8 b | 16.6 ± 0.9 c |
Values are means from three biological replicates with the corresponding standard deviations. Values followed by different lowercase letters are significantly different according to Tukey's multiple-comparison test (P < 0.05). CK, control; BAC, P. fluorescens ALEB7B; FOS, fosmidomycin; MEV, mevinolin; DW, dry weight.
FIG 5.
Typical phenotype of Atractylodes lancea whose two main sesquiterpenoid synthesis pathways are blocked by mevinolin and fosmidomycin 20 days after the inoculation of Pseudomonas fluorescens ALEB7B. CK, control; BAC, P. fluorescens ALEB7B; MEV, mevinolin; FOS, fosmidomycin.
DISCUSSION
Oxygenous terpenoids are the active components of many medicinal plants, such as menthol, artemisinin, paclitaxel, and glycyrrhizic acid (35). The oxygen-containing groups are essential for the medicinal activities of oxygenous terpenoids, as in the case of artemisinin, whose activity is dependent upon the presence of a peroxide bridge (36). Enzymatic oxygenous terpenoid synthesis has been well studied (37, 38). Enzymatic reactions can catalyze hydroxylation or ketonization at specific sites of terpenoid hydrocarbon scaffolds. Much work has been done to clarify all of the intermediates and corresponding enzymes involved in the synthesis of artemisinic acid, the direct precursor of artemisinin; however, the enzymes that oxidize artemisinic acid to artemisinin have not yet been identified (35). It is clear, therefore, that enzymatic oxidation cannot explain the synthetic mechanisms of all oxygenous terpenoids. A. lancea contains diverse oxygenous sesquiterpenoids, but their compositions in different individuals vary greatly (3, 39). Directional oxidation catalyzed by enzymes cannot explain the diversity of oxygenous terpenoids that is seen. However, ROS can oxidize sesquiterpenoids at multiple sites, which promotes oxygenous sesquiterpenoid synthesis and gives rise to the diversity of these compounds.
The accumulation of active components in A. lancea strongly depends on its producing area (40). Higher oxygenous sesquiterpenoid diversity and content are characteristics of the geo-authentic A. lancea grown in the Maoshan area (3). Hinesol, β-eudesmol, atractylone, and caryophyllene oxide are characteristic sesquiterpenoid components in the geo-authentic A. lancea (14). The isolation rate of endophytes from the geo-authentic A. lancea is also much higher than that found when A. lancea is grown in other areas (8–10). Oxidative burst is one of the earliest plant responses to microbial infections (17). This study demonstrated that after colonization by P. fluorescens ALEB7B (Fig. 2), the endophytic bacterium-triggered oxidative burst and oxygenous sesquiterpenoid accumulation in A. lancea occurred at the same time (Fig. 3 and 4E to H). Furthermore, we showed that treatment of plants with exogenous H2O2 and singlet oxygen significantly increased the levels of endogenous H2O2 and singlet oxygen, respectively (see Fig. S2A and S3A in the supplemental material). Meanwhile, the oxygenous sesquiterpenoid content significantly increased (see Fig. S2E to H and S3E to H), but oxygen-free sesquiterpenoid content did not change or significantly decrease (see Fig. S2B to S2D and S3B to D). Conversely, when A. lancea plantlets were pretreated with the ROS scavenger AsA, P. fluorescens ALEB7B could not trigger the oxidative burst in planta (see Fig. S4A and B in the supplemental material), and the oxygenous sesquiterpenoid content in pretreated A. lancea plantlets was much lower than that in untreated plants (see Fig. S4F to I). These results indicate that endophytic bacterium-triggered ROS not only can act as signaling molecules (14) but also can directly oxidize oxygen-free sesquiterpenoids, increasing the content and diversity of oxygenous sesquiterpenoids in planta. Moreover, Yan et al. (41) recently reported that the fumitremorgin B endoperoxidase from Aspergillus fumigatus can catalyze the formation of the peroxide bridge in verruculogen, providing a basis for the identification of endophyte-derived oxidases that function in plant oxygenous terpenoid formation. Therefore, we speculate that endophytes promote oxygenous terpenoid formation in planta via three mechanisms. First, ROS induced by endophytic infection may directly oxidize oxygen-free terpenoids. Second, oxidases derived from endophytes may be secreted and function in planta. Third, signaling pathways activated by endophytic colonization increase the amount or activity of relevant oxidases.
In vitro oxidation experiments showed that H2O2 could oxidize total sesquiterpenoids extracted from cultivated A. lancea and generate caryophyllene oxide and humulene epoxide II (see Fig. S5A and B in the supplemental material). Singlet oxygen could oxidize extracted sesquiterpenoids and generate hinesol and spathulenol (see Fig. S5C and D). Caryophyllene oxide, humulene epoxide II, hinesol, and spathulenol all have been reported to be the main oxygenous sesquiterpenoids in wild A. lancea (3, 39). These results further demonstrate that endophyte-triggered ROS can lead directly to the formation of diverse oxygenous sesquiterpenoids in geo-authentic A. lancea. Some studies have suggested that the transformation of artemisinic acid to artemisinin is a nonenzymatic spontaneous oxidation and correlates with singlet oxygen levels in planta (27, 42). This supports our viewpoint, indicating that the ROS-induced direct oxidation of terpenoids have general biological significance. Steenackers et al. (31) have reported that H2O2 oxidizes terpenoids by elongating O—O bonds, and cleaving the OH moiety and singlet oxygen reacts with endocyclic double bonds of terpenoids (43). The process of terpenoids being directly oxidized by endophytic bacterium-triggered ROS is an essential complementarity to enzymatic terpenoid synthesis and modification in planta. As found in this study, peroxidase could protect some sesquiterpenoids, such as atractylone, from being peroxidized by H2O2 (see Fig. S5A). Therefore, we speculate that oxygenous terpenoids can be generated from two sources in planta. On the one hand, oxidases can catalyze oxidations at specific sites of terpenoid hydrocarbon scaffolds. On the other hand, ROS induced by stresses may directly and randomly oxidize terpenoids, and enzymatic reactions and ROS may function synergistically.
Direct oxidation of terpenoids by bacterium-triggered ROS also regulates interactions between endophytes and plants. The interactions between endophytes and plants can vary from mutualism to parasitism (44). The asymptomatic colonization of plants by endophytes is a delicate balance of antagonism between endophytes and host plants, during which plant secondary metabolites play essential roles (45). If host plants cannot effectively control endophyte-triggered ROS, oxidative damage to the plant will occur. Direct oxidation of terpenoids by ROS consumes endophyte-triggered ROS and maintains endogenous ROS levels at those which plants can endure. When two sesquiterpenoid synthesis pathways in A. lancea were blocked (Fig. 1), colonization by P. fluorescens ALEB7B caused chlorosis and withered symptoms in the aboveground parts of plantlets (Fig. 5). Meanwhile, the plant dry weight significantly decreased and the malondialdehyde content in plantlets significantly increased (Table 1). These results indicate that sesquiterpenoids act as antioxidants and participate in protecting plants from oxidative damage caused by environmental stresses. Furthermore, although bacterial colonization can enhance plant photosynthesis (see Fig. S1B and C in the supplemental material), it has little effect on plant biomass (see Fig. S1A). The increased photosynthates induced by endophytic bacterium are preferentially used by A. lancea to synthesize precursors of sesquiterpenoids (see Fig. S1D to F). Furthermore, oxygenous sesquiterpenoids that have been oxidized from oxygen-free sesquiterpenoids have stronger antimicrobial activity (46). These processes restrict the overgrowth of endophytes in planta (47) and are essential strategies used by host plants to modulate the growth of endophytes in vivo and to maintain a mutualistic relationship with endophytes.
In summary, this study shows that endophytic bacterium-triggered ROS can directly oxidize sesquiterpenoids and increase oxygenous sesquiterpenoid content and diversity in A. lancea, which not only provides a novel viewpoint on the mechanisms of oxygenous terpenoid synthesis and diversity in multiple medicinal plants but also is essential to further understanding interactions between endophytes and plants.
Supplementary Material
ACKNOWLEDGMENTS
This work was financially supported by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, Promoting Project for Industrialization of Jiangsu Higher Education Institutions (grant number JHB2012-16), Integration of Production and Research Projects of Nanjing Science and Technology Commission (grant number 201306019), Graduate Education Innovation Project of Jiangsu Province (grant number KYLX15_0734), and National Natural Science Foundation of China (grant number 31070443).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03434-15.
REFERENCES
- 1.Wang HX, Liu CM, Liu Q, Gao K. 2008. Three types of sesquiterpenes from rhizomes of Atractylodes lancea. Phytochemistry 69:2088–2094. doi: 10.1016/j.phytochem.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Ouyang Z, Zhang L, Zhao M, Wang PX, Wei Y, Fang J. 2012. Identification and quantification of sesquiterpenes and polyacetylenes in Atractylodes lancea from various geographical origins using GC-MS analysis. Rev Bras Farmacognosia 22:957–963. doi: 10.1590/S0102-695X2012005000051. [DOI] [Google Scholar]
- 3.Ji L, Ao P, Pan JG, Yang JY, Yang J, Hu SL. 2001. GC-MS analysis of essential oils from rhizomes of Atractylodes lancea (Thunb.) DC. and A. chinensis (DC.) Koidz. China J Chin Mater Med 26:182–185. [PubMed] [Google Scholar]
- 4.Hong GJ, Xue XY, Mao YB, Wang LJ, Chen XY. 2012. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 24:2635–2648. doi: 10.1105/tpc.112.098749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thulasiram HV, Erickson HK, Poulter CD. 2007. Chimeras of two isoprenoid synthases catalyze all four coupling reactions in isoprenoid biosynthesis. Science 316:73–76. doi: 10.1126/science.1137786. [DOI] [PubMed] [Google Scholar]
- 6.Ryan RP, Germaine K, Frank A, Ryan DJ, Dowling DN. 2008. Bacterial endophytes: recent developments and applications. FEMS Microbiology Lett 278:1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 7.Demers JE, Gugino BK, del Mar Jiménez-Gasco M. 2015. Highly diverse endophytic and soil Fusarium oxysporum populations associated with field-grown tomato plants. Appl Environ Microbiol 81:81–90. doi: 10.1128/AEM.02590-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv LX, Wang HW, Liang XF, Hao SJ, Du W, Zhu H, Dai CC. 2014. Effects of different chemotypes and seasonal dynamic variation on species diversity of endophytic fungal communities harbored in Atractylodes lancea. Acta Ecol Sinica 34:7300–7310. [Google Scholar]
- 9.Chen JX, Dai CC, Li X, Tian LS, Xie H. 2008. Endophytic fungi screening from Atracty lancea and inoculating into the host plantlets. Guihaia 28:256–260. [Google Scholar]
- 10.Zhou JY, Jia Y, Wang HW, Dai CC. 2013. Diversity and plant growth-promoting potential of culturable endophytic bacteria isolated from the leaves of Atractylodes lancea. Acta Ecol Sinica 33:1106–1117. doi: 10.5846/stxb201208031103. [DOI] [Google Scholar]
- 11.Ren CG, Dai CC. 2013. Nitric oxide and brassinosteroids mediated fungal endophyte-induced volatile oil production through protein phosphorylation pathways in Atractylodes lancea plantlets. J Integr Plant Biol 55:1136–1146. doi: 10.1111/jipb.12087. [DOI] [PubMed] [Google Scholar]
- 12.Ren CG, Chen Y, Dai CC. 2014. Cross-talk between calcium-calmodulin and brassinolide for fungal endophyte-induced volatile oil accumulation of Atractylodes lancea plantlets. J Plant Growth Reg 33:285–294. doi: 10.1007/s00344-013-9370-4. [DOI] [Google Scholar]
- 13.Wang XM, Yang B, Ren CG, Wang HW, Wang JY, Dai CC. 2015. Involvement of abscisic acid and salicylic acid in signal cascade regulating bacterial endophyte-induced volatile oil biosynthesis in plantlets of Atractylodes lancea. Physiol Plantarum 153:30–42. doi: 10.1111/ppl.12236. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Dai CC, Zhao YW, Peng Y. 2011. Fungal endophyte-induced volatile oil accumulation in Atractylodes lancea plantlets is mediated by nitric oxide, salicylic acid and hydrogen peroxide. Process Biochem 46:730–735. doi: 10.1016/j.procbio.2010.11.020. [DOI] [Google Scholar]
- 15.Ren CG, Dai CC. 2012. Jasmonic acid is involved in the signaling pathway for fungal endophyte-induced volatile oil accumulation of Atractylodes lancea plantlets. BMC Plant Biol 12:128. doi: 10.1186/1471-2229-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou JY, Zhao XY, Dai CC. 2014. Antagonistic mechanisms of endophytic Pseudomonas fluorescens against Athelia rolfii. J Appl Microbiol 117:1144–1158. doi: 10.1111/jam.12586. [DOI] [PubMed] [Google Scholar]
- 17.Nanda AK, Andrio E, Marino D, Pauly N, Dunand C. 2010. Reactive oxygen species during plant-microorganism early interactions. J Integr Plant Biol 52:195–204. doi: 10.1111/j.1744-7909.2010.00933.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Dai CC, Cao JL, Xu DS. 2012. Comparison of the effects of fungal endophyte Gilmaniella sp. and its elicitor on Atractylodes lancea plantlets. World J Microbiol Biotechnol 28:575–584. doi: 10.1007/s11274-011-0850-z. [DOI] [PubMed] [Google Scholar]
- 19.Wang XM, Yang B, Wang HW, Yang T, Ren CG, Zheng HL, Dai CC. 2015. Consequences of antagonistic interactions between endophytic fungus and bacterium on plant growth and defense responses in Atractylodes lancea. J Basic Microbiol 55:659–670. doi: 10.1002/jobm.201300601. [DOI] [PubMed] [Google Scholar]
- 20.Wang SY, Jiao HJ. 2000. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J Agric Food Chem 48:5677–5684. doi: 10.1021/jf000766i. [DOI] [PubMed] [Google Scholar]
- 21.Noctor G, Lelarge-Trouverie C, Mhamdi A. 2015. The metabolomics of oxidative stress. Phytochemistry 112:33–35. doi: 10.1016/j.phytochem.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Hagen C, Grünewald K. 2000. Fosmidomycin as an inhibitor of the non-mevalonate terpenoid pathway depresses synthesis of secondary carotenoids in flagellates of the green alga Haematococcus pluvialis. J Appl Bot 74:137–140. [Google Scholar]
- 23.Zhu X, Song F, Xu H. 2010. Arbuscular mycorrhizae improves low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil 331:129–137. doi: 10.1007/s11104-009-0239-z. [DOI] [Google Scholar]
- 24.Li X, Wang C. 2013. Physiological and metabolic enzymes activity changes in transgenic rice plants with increased phosphoenolpyruvate carboxylase activity during the flowering stage. Acta Physiol Plantarum 35:1503–1512. doi: 10.1007/s11738-012-1191-8. [DOI] [Google Scholar]
- 25.Tumaney AW, Ohlrogge JB, Pollard M. 2004. Acetyl coenzyme A concentrations in plant tissues. J Plant Physiol 161:485–488. doi: 10.1078/0176-1617-01258. [DOI] [PubMed] [Google Scholar]
- 26.Zeng FS, Liu K, Li SD, Zhan YG. 2015. Crosstalk among nitric oxide, calcium and reactive oxygen species during triterpenoid biosynthesis in Betula platyphylla. Funct Plant Biol 42:643–654. doi: 10.1071/FP14352. [DOI] [PubMed] [Google Scholar]
- 27.Yang R, Zeng X, Lu Y, Lu W, Feng L, Yang X, Zeng Q. 2010. Senescent leaves of Artemisia annua are one of the most active organs for overexpression of artemisinin biosynthesis responsible genes upon burst of singlet oxygen. Planta Med 76:734–742. doi: 10.1055/s-0029-1240620. [DOI] [PubMed] [Google Scholar]
- 28.Gao FK, Ren CG, Dai CC. 2012. Signaling effects of nitric oxide, salicylic acid, and reactive oxygen species on isoeuphpekinensin accumulation in Euphorbia pekinensis suspension cells induced by an endophytic fungal elicitor. J Plant Growth Reg 31:490–497. doi: 10.1007/s00344-012-9258-8. [DOI] [Google Scholar]
- 29.Wang HQ, Yang XF, Guo LH, Zeng HM, Qiu DW. 2015. PeBL1, a novel protein elicitor from Brevibacillus laterosporus strain A60, activates defense responses and systemic resistance in Nicotiana benthamiana. Appl Environ Microbiol 81:2706–2716. doi: 10.1128/AEM.03586-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montiel J, Nava N, Cárdenas L, Sánchez-López R, Arthikala M-K, Santana O, Sánchez F, Quinto C. 2012. A Phaseolus vulgaris NADPH oxidase gene is required for root infection by rhizobia. Plant Cell Physiol 53:1751–1767. doi: 10.1093/pcp/pcs120. [DOI] [PubMed] [Google Scholar]
- 31.Steenackers B, Neirinckx A, de Cooman L, Hermans I, de Vos D. 2014. The strained sesquiterpene β-caryophyllene as a probe for the solvent-assisted epoxidation mechanism. ChemPhysChem 15:966–973. doi: 10.1002/cphc.201300981. [DOI] [PubMed] [Google Scholar]
- 32.Hunziker L, Bönisch D, Groenhagen U, Bailly A, Schulz SLW. 2015. Pseudomonas strains naturally associated with potato plants produce volatiles with high potential for inhibition of Phytophthora infestans. Appl Environ Microbiol 81:821–830. doi: 10.1128/AEM.02999-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bianco C, Senatore B, Arbucci S, Pieraccini G, Defez R. 2014. Modulation of endogenous indole-3-acetic acid biosynthesis in bacteroids within Medicago sativa nodules. Appl Environ Microbiol 80:4286–4293. doi: 10.1128/AEM.00597-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan XY. 2008. Analysis of the essential oil compositions in Atractylodes lancea. China Pharm 19:2380–2381. [Google Scholar]
- 35.Kitaoka N, Lu X, Yang B, Peters RJ. 2015. The application of synthetic biology to elucidation of plant mono-, sesqui-, and diterpenoid metabolism. Mol Plant 8:6–16. doi: 10.1016/j.molp.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu YY. 2011. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med 17:1217–1220. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- 37.Yu F, Okamoto S, Harada H, Yamasaki K, Misawa N, Utsumi R. 2011. Zingiber zerumbet CYP71BA1 catalyzes the conversion of α-humulene to 8-hydroxy-α-humulene in zerumbone biosynthesis. Cell Mol Life Sci 68:1033–1040. doi: 10.1007/s00018-010-0506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen DT, Göpfert JC, Ikezawa N, MacNevin G, Kathiresan M, Conrad J, Spring O, Ro D-K. 2010. Biochemical conservation and evolution of germacrene A oxidase in Asteraceae. J Biol Chem 285:16588–16598. doi: 10.1074/jbc.M110.111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dian LH, Liang Y, Song YP. 2004. GC-MS analysis of volatile constituents of Atractylodes lancea (Thunb.) DC. Guangzhou Chem Indust 32:32–34. [Google Scholar]
- 40.Sun YZ, Guo LP, Yang XQ, Huang LQ, Zhu WQ, Pan YZ. 2008. Biomass structure analysis of Atractylodes lancea in different ecological environments. China J Chin Materia Med 33:1516–1518. [PubMed] [Google Scholar]
- 41.Yan W, Song H, Song F, Guo Y, Wu C-H, Her AS, Pu Y, Wang S, Naowarojna N, Weitz A, Hendrich MP, Costello CE, Zhang L, Liu P, Zhang YJ. 2015. Endoperoxide formation by an α-ketoglutarate-dependent mononuclear non-haem iron enzyme. Nature 527:539–543. doi: 10.1038/nature15519. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Brown GD. 2010. The biosynthesis of artemisinin (Qinhaosu) and the phytochemistry of Artemisia annua L. Molecules 15:7603–7698. doi: 10.3390/molecules15117603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sköld M, Karlberg A-T, Matura M, Börje A. 2006. The fragrance chemical β-caryophyllene-air oxidation and skin sensitization. Food Chem Toxicol 44:538–545. doi: 10.1016/j.fct.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Zhao GZ, Varma A, Qin S, Xiong Z, Huang HY, Zhu WY, Zhao LX, Xu LH, Zhang S, Li WJ. 2012. An endophytic Pseudonocardia species induces the production of artemisinin in Artemisia annua. PLoS One 7:e51410. doi: 10.1371/journal.pone.0051410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 46.Duraipandiyan V, Al-Harbi NA, Lgnacimuthu SC. 2012. Antimicrobial activity of sesquiterpene lactones isolated from traditional medicinal plant, Costus speciosus (Koen ex. Retz.) Sm. BMC Complement Altern Med 12:13. doi: 10.1186/1472-6882-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Dai CC, Chen Y. 2009. Antimicrobial activity of volatile oil from Atractylodes lancea against three species of endophytic fungi and seven species of exogenous fungi. Chin J Appl Ecol 20:2778–2784. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.