Abstract
Pseudomonas protegens strain Pf-5 is a rhizosphere bacterium that suppresses soilborne plant diseases and produces at least seven different secondary metabolites with antifungal properties. We derived mutants of Pf-5 with single and multiple mutations in biosynthesis genes for seven antifungal metabolites: 2,4-diacetylphoroglucinol (DAPG), pyrrolnitrin, pyoluteorin, hydrogen cyanide, rhizoxin, orfamide A, and toxoflavin. These mutants were tested for inhibition of the pathogens Fusarium verticillioides and Fusarium oxysporum f. sp. pisi. Rhizoxin, pyrrolnitrin, and DAPG were found to be primarily responsible for fungal antagonism by Pf-5. Previously, other workers showed that the mycotoxin fusaric acid, which is produced by many Fusarium species, including F. verticillioides, inhibited the production of DAPG by Pseudomonas spp. In this study, amendment of culture media with fusaric acid decreased DAPG production, increased pyoluteorin production, and had no consistent influence on pyrrolnitrin or orfamide A production by Pf-5. Fusaric acid also altered the transcription of biosynthetic genes, indicating that the mycotoxin influenced antibiotic production by Pf-5 at the transcriptional level. Addition of fusaric acid to the culture medium reduced antibiosis of F. verticillioides by Pf-5 and derivative strains that produce DAPG but had no effect on antibiosis by Pf-5 derivatives that suppressed F. verticillioides due to pyrrolnitrin or rhizoxin production. Our results demonstrated the importance of three compounds, rhizoxin, pyrrolnitrin, and DAPG, in suppression of Fusarium spp. by Pf-5 and confirmed that an interspecies signaling system mediated by fusaric acid had parallel effects on antifungal metabolite production and antibiosis by the bacterial biological control organism.
INTRODUCTION
Pseudomonas is a heterogeneous genus of Gammaproteobacteria composed of species with diverse ecological roles; certain strains are members of the plant microbiome contributing to plant growth and health. Our studies focus on the soil bacterium Pseudomonas protegens Pf-5, which is known for its capacity to suppress plant diseases and produce a large spectrum of metabolites with antibiotic activity (1, 2). The antibiotics produced by Pf-5 include pyrrolnitrin (3), pyoluteorin (4), analogs of rhizoxin (5, 6), hydrogen cyanide (7), 2,4-diacetylphloroglucinol (DAPG) (8), monoacetylphloroglucinol (MAPG) (9) (an intermediate in the DAPG biosynthetic pathway [10, 11]), the lipopeptide orfamide A (12), and toxoflavin (13). The antibiotics produced by Pf-5 have distinct but overlapping activity spectra, and it is likely that multiple compounds contribute to the interactions of Pf-5 with target plant pathogens that result in disease suppression. Commonly, the role of an antibiotic in biological control is assessed by comparing the level of disease suppression provided by a wild-type strain versus an antibiotic-deficient mutant (14). For strain Pf-5, however, this approach has not been highly successful in providing evidence for the roles of antibiotics in biological control: mutations in a specific biosynthetic locus, which eliminate the capacity of the bacterium to produce a single antibiotic, can exhibit wild-type levels of biological control activity (7). We hypothesize that the large spectrum of antibiotics produced by Pf-5 provides both enhanced potency and redundancy in the suppression of plant disease. Consequently, biological control may not depend on the production of any single antibiotic but may involve the production of several antibiotics. Here, we developed and characterized a set of mutants needed to test that hypothesis; collectively, the mutants provide the tools for a systematic approach to identify the specific antibiotics contributing to Pf-5's capacity to suppress plant disease.
In Pseudomonas spp., the production of antibiotics and other exoproducts requires the Gac/Rsm signal transduction system, which controls the expression of target genes through a complex signal transduction pathway involving regulatory RNAs and translational repression (15). Due to the preeminent role of the response regulator GacA in this pathway, gacA mutants of Pf-5 do not produce any of the antibiotics produced by wild-type Pf-5 (6, 12, 13, 16–18). Consequently, compared to wild-type strains, gacA mutants can reveal the combined contributions of antibiotics and other exoproducts to a range of biological activities of Pseudomonas spp. In this study, we built upon the well-characterized effects of a gacA mutation on the phenotypes and transcriptome of Pf-5 (16, 17) to construct a mutant set that represents all seven of the known gacA-regulated antibiotics produced by Pf-5 (i.e., pyrrolnitrin, pyoluteorin, analogs of rhizoxin, hydrogen cyanide, DAPG and its intermediate MAPG, orfamide A, and toxoflavin). As proof of principle, we used the mutant set to identify the specific antibiotics responsible for inhibition of Fusarium, a genus of filamentous ascomycete fungi that includes many plant pathogens of global agricultural importance.
Diseases caused by Fusarium spp. include wilts, rots, cankers, and blights of numerous agronomic, horticultural, and forest plants grown in agricultural, landscape, and natural settings (19). F. verticillioides causes ear rot of maize and sorghum (20) and Pokkah Boeng disease of sugarcane (21). Members of the F. oxysporum species complex cause wilt diseases of more than one hundred plant species (19). Diseases caused by these Fusarium spp. are difficult to control with existing fungicides or soil fumigants, and many agricultural plants lack resistance to them (20, 22). To meet the need for alternative disease management strategies, scientists have turned to microorganisms that inhibit Fusarium spp. in search of biological control agents (22). Pseudomonas spp. are prominent members of the suppressive soil microflora, as components of Fusarium-suppressive soils (23–25) or as strains applied to seeds, soil, or plant growth media to achieve biological control (23, 26–29). Among the strains of Pseudomonas spp. effective in biological control are P. protegens Pf-5 and the closely related strain P. protegens CHA0 (30). These strains of P. protegens produce a nearly identical spectrum of antibiotics, with the exception of the rhizoxin analogs, which are produced by Pf-5 but not by CHA0. The production of hydrogen cyanide contributes to the capacity of strain CHA0 to suppress crown and root rot of tomato caused by F. oxysporum f. sp. radicis-lycopersici (31, 32), but the antibiotic(s) responsible for inhibition of Fusarium spp. by strain Pf-5 remains unknown.
Many Fusarium spp. produce toxic secondary metabolites, such as trichothecenes, fumonisins (33), and fusaric acid (FA) (34). These mycotoxins can be produced by the fungus in infected plant tissues, thereby contaminating agricultural products, rendering them unacceptable for food or feed. Beyond their roles as virulence factors in some plant diseases, mycotoxins are thought to enhance the fitness of the producing fungi by other mechanisms, such as protection from oxidative stress (35) and from microbial competitors in natural habitats (36). During the saprotrophic and parasitic phases of their life cycle, Fusarium spp. encounter many environmental stresses, including exposure to antifungal metabolites produced by their microbial coinhabitants (37). An early example of the role of mycotoxins in defense against antibiosis was provided by Duffy and Défago in 1997, who demonstrated that FA produced by isolates of F. oxysporum f. sp. radicis-lycopersici acts as a chemical signal that represses DAPG production by P. protegens CHA0 (28). Since that discovery was reported, others have substantiated that FA represses the production of DAPG (31, 38) and phenazines (39) and alters the production of pyoverdine siderophores (40, 41) by strains of Pseudomonas spp. FA acts, directly or indirectly, by influencing the expression of biosynthesis genes, including genes for the biosynthesis of DAPG (42–44) and phenazine-1-carboxylate (39).
The purpose of this study was 3-fold. Our first goal was to identify the specific antifungal compounds responsible for antibiosis of Fusarium spp. by Pf-5 using a set of mutants with deletions in each of the seven known antifungal compounds produced by Pf-5 under the control of GacA. Second, we set out to evaluate the influence of FA on the production of these antifungal compounds, thereby extending knowledge of the effects of this mycotoxin to a larger set of secondary metabolites produced by this bacterium. Our final objective was to determine the influence of FA on antibiosis against F. verticillioides by Pf-5 and derivative strains.
MATERIALS AND METHODS
Microorganisms and culture conditions.
P. protegens strain Pf-5 was provided by Charles Howell, who isolated it from soil in College Station, TX (3). Pf-5 and derivative strains (Table 1) were stored in Difco nutrient broth (Becton, Dickinson and Company, Sparks, MD) containing 15% glycerol at −80°C. Fresh cultures were started from frozen stocks for each experiment by plating on King's medium B (KBM) (45) and incubating plates at 27°C. Other media used in this study were Difco potato dextrose agar (PDA) (Becton, Dickinson and Company, Sparks, MD), Difco potato dextrose broth (PDB) (Becton, Dickinson and Company), PCG (46), and nutrient yeast broth (8 g Difco nutrient broth and 5 g Bacto yeast extract) supplemented with 1% glycerol (NYBGly) (47).
TABLE 1.
Pseudomonas protegens Pf-5 and derivative strains used in this study
| Strain | Designation | Descriptiona | Reference(s) |
|---|---|---|---|
| Pf-5 | JL4585 | Soil isolate; produces Ofa, Prn, Plt, HCN, MAPG, DAPG, Rzx | 3, 68 |
| Mutants of Pf-5 | |||
| ΔgacA | JL4577 | Insertion of aphI at the site of a 626-bp (nt 1–626) deletion in gacA; altered in the many phenotypes regulated by GacA; Kmr | 16 |
| ΔofaA | JL4807 | 1,143-bp deletion internal to ofaA; has FRT scar; Ofa− | 16 |
| ΔphlA | LK023 | 639-bp deletion of BglII fragment internal to phlA; DAPG−, MAPG− | 9 |
| ΔphlD | JL4804 | 162-bp deletion in phlD; has FRT scar; DAPG−, Plt− | 49, 9 |
| ΔpltA | JL4805 | 275-bp deletion in pltA; has FRT scar; Plt− | 50 |
| ΔprnC | JL4793 | 86-bp insertion of FRT site into prnC; Prn− | 50 |
| ΔrzxB | JL4808 | 1,342-bp deletion in rzxB; has FRT scar; Rzx− | 50 |
| ΔhcnB | JL4809 | 239-bp deletion in hcnB; has FRT scar; HCN− | 53 |
| ΔphlD ΔprnC | JL4830 | DAPG−, MAPG−, Plt−, Prn−; derived by adding a phlD deletion to JL4793 | This study |
| ΔphlA ΔprnC | LK026 | DAPG−, MAPG−, Prn− | 50 |
| ΔphlA ΔrzxB | LK027 | DAPG−, MAPG−, Rzx− | 50 |
| ΔphlD ΔrzxB | JL4901 | DAPG−, MAPG−, Plt−, Rzx−, derived by adding an rzxB deletion to JL4804 | This study |
| ΔprnC ΔrzxB | JL4902 | Prn−, Rzx−; derived by adding an rzxB deletion to JL4793 | This study |
| ΔphlD ΔrzxB ΔprnC | JL4844 | Prn−, DAPG−, MAPG−, Plt−, Rzx−; derived by adding an rzxB deletion to JL4830 | This study |
| ΔphlA ΔrzxB ΔprnC | LK031 | Prn−, DAPG−, MAPG−, Rzx− | 50 |
| ΔphlD ΔrzxB ΔprnC ΔpltA | JL4855 | Prn−, DAPG−, MAPG−, Plt−, Rzx−; derived by adding a pltA deletion to JL4844 | This study |
| ΔphlD ΔrzxB ΔprnC ΔhcnB ΔpltA | JL4865 | Prn−, DAPG−, MAPG−, Plt−, Rzx−, HCN−; derived by adding an hcnB deletion to JL4855 | This study |
| ΔphlD ΔrzxB ΔprnC ΔhcnB ΔpltA ΔofaA | JL4909 | Prn−, DAPG−, MAPG−, Plt−, Rzx−, HCN−, Ofa−; derived by adding an ofaA deletion to JL4865 | This study |
| ΔphlD ΔrzxB ΔprnC ΔhcnB ΔpltA ΔofaA ΔtoxB | JL4932 | Prn−, DAPG−, MAPG−, Plt−, Rzx−, HCN−, Ofa−, Tox−; derived by adding a toxB deletion to JL4909 | This study |
Phenotype abbreviations: DAPG, 2,4-diacetylphloroglucinol; HCN, hydrogen cyanide; MAPG, monoacetylphloroglucinol; Ofa, orfamide A; Plt, pyoluteorin; Prn, pyrrolnitrin; Rzx, rhizoxin derivatives; Tox, toxoflavin. Mutants of Pf-5 containing deletions in ofaA, phlD, pltA, prnC, rzxB, hcnB, and toxB have FRT scars (85- to 86-bp Flp recombinase target sites) in those genes. In-frame deletions were generated in phlA, and the deleted gene does not have an inserted FRT sequence. Primers used to generate and confirm each mutation are provided in the reference to the corresponding single mutant or in Table 2. nt, nucleotides.
Fusarium verticillioides T4-0, which was isolated from corn seed in Oregon, USA, was a gift from Cynthia Ocamb (Oregon State University). Fusarium oxysporum f. sp. pisi R2-238 (race 2) and R5 (race 5), which were isolated from pea near Mount Vernon, WA, USA, were provide by Lyndon Porter (Washington State University). Fusarium spp. were maintained at room temperature in pure culture on PDA immersed in sterilized water. Inoculum for experiments was grown by transferring a PDA plug from these maintenance cultures to fresh PDA and incubating plates at 27°C for 5 days.
Mutant construction.
A set of mutants was assembled to compare to wild-type Pf-5 for the inhibition of Fusarium spp. (Table 1). Mutants were constructed using an overlap extension PCR method (48) followed by a mating and selection process described previously (16, 49, 50). Briefly, the DNAs flanking a target gene were amplified and combined using overlap extension PCR to generate a DNA fragment with a deletion in the target gene. The resulting DNA fragment was cloned into the suicide plasmid pEX18Tc (51) in Escherichia coli S17-1 (52), and the recombinant plasmid was introduced into Pf-5 by conjugation, selecting for tetracycline resistance. The fragment containing the deletion was integrated into the Pf-5 genome by homologous recombination, and resolved merodiploids were selected by tetracycline sensitivity and growth on LB containing 5% sucrose due to the presence of genes for tetracycline resistance and levan sucrase on pEX18Tc. The sequence of each mutated locus was confirmed to be as expected by performing PCR across the deletion site and sequencing the resultant product. Primers used to construct and confirm mutations were published previously (9, 16, 49, 50, 53) or are listed in Table 2. Multiple mutants were created by repetition of the mating, selection, and confirmation process. Certain mutants were constructed by a process involving a gentamicin-resistant intermediate, in which the gentamicin resistance gene was evicted using Flp recombinase, resulting in a target gene deletion flanked by 85- to 86-bp Flp recombinase target (FRT) sites (48). Mutants having these FRT sequences are noted in Table 1, and a detailed description of this method for generating Pf-5 mutants has been published (49).
TABLE 2.
Primers used in this study
| Function and primer | Locus tag | cDNA target | Sequence (5′→3′) |
|---|---|---|---|
| RT-qPCR | |||
| zwfq F | PFL_4610 | zwf | ATCTGGCGCTGCGTAAGCTG |
| zwfq F | TGTCGCTCTGGGTGTTGGAG | ||
| phlDqI F | PFL_5957 | phlD | ACGTACTGATCGTGTCC |
| phlDqI R | CTTGATGTAGTGCTCGC | ||
| pltA2-qF | PFL_2787 | pltA | GACCTCGGCAGATTCC |
| pltA2-qR | GGGTCTTTTCTCCGA | ||
| prnCq F | PFL_3606 | prnC | GACTTCCGCCTATGGG |
| prnCq R | ATGAGGGCGTGAATCC | ||
| hcnAq F | PFL_2577 | hcnA | CAACTTCGATATTCAGCCG |
| hcnAq R | GTGGGCTCCGTTTCAG | ||
| rzxBq F | PFL_2989 | rzxB | CGTTTCGACTCCCAAG |
| rzxBq R | CGTTTCGACTCCCAAG | ||
| pvdAq F | PFL_4079 | pvdA | CCCACGTTCACGATTTG |
| pvdAq R | GCTTGAGGTAGTTGACGA | ||
| pchC2 qF | PFL_3490 | pchC | CCACAACGGTTGATCG |
| pchC2 qR | AGGCATAGGCTTCGTC | ||
| Derivation of toxB mutation | |||
| Gm-F | CGAATTAGCTTCAAAAGCGCTCTGA | ||
| Gm-R | CGAATTGGGGATCTTGAAGTTCCT | ||
| JR Pf5 1033 UpF1 | TCAGACAAGCAAGCTTCGACCAGCACACCGAGATCA | ||
| JR Pf5 1034 UpR1 | TCAGAGCGCTTTTGAAGCTAATTCGGCACAACAGCCACTACCTGCAA | ||
| JR Pf5 1034 DnF1 | AGGAACTTCAAGATCCCCAATTCGGATGCCGTTGTCCTTCAGGG | ||
| JR Pf5 1035 DnR1 | TCAAGCAAGCAAGCTTGACCCGGCATTTCTACTTTTGC | ||
| Confirmation of toxB mutation | |||
| 1034 5′F | CACGGTGTCGGTTTCCACG | ||
| 1034 3′R | CATCGACGACCTGGTGACC | ||
Antagonism assays.
Suppression of Fusarium spp. by Pf-5 and derivative strains was assessed from a dual-culture assay on PDA supplemented with 0.1 mM FeCl3 (PDA-Fe). FeCl3 was added to PDA to avoid the influence of siderophores produced by Pf-5 on growth of Fusarium spp. Each petri plate was inoculated with 10 μl of a bacterial suspension (optical density at 600 nm [OD600] = 0.05, approximately 108 CFU ml−1) from each of three strains: Pf-5 (wild type), a ΔgacA mutant (JL4577), and one other mutant of Pf-5. In some experiments (specified in Results), the medium was supplemented with 0.5 mM FA (Sigma-Aldrich, St. Louis, MO). A PDA plug (2 cm in diameter) from a culture of Fusarium spp. was placed on the agar surface at the center of the plate. Inhibition of the Fusarium spp. was measured after 5 days of incubation at 27°C. The Fusarium growth inhibition index was calculated according to the formula [1 − (x1/x2)] × 100 for Pf-5 and derivative strains, as illustrated in Fig. 1. Each experiment was done at least twice, with four replicate petri dishes for each inhibition assay in all experiments.
FIG 1.
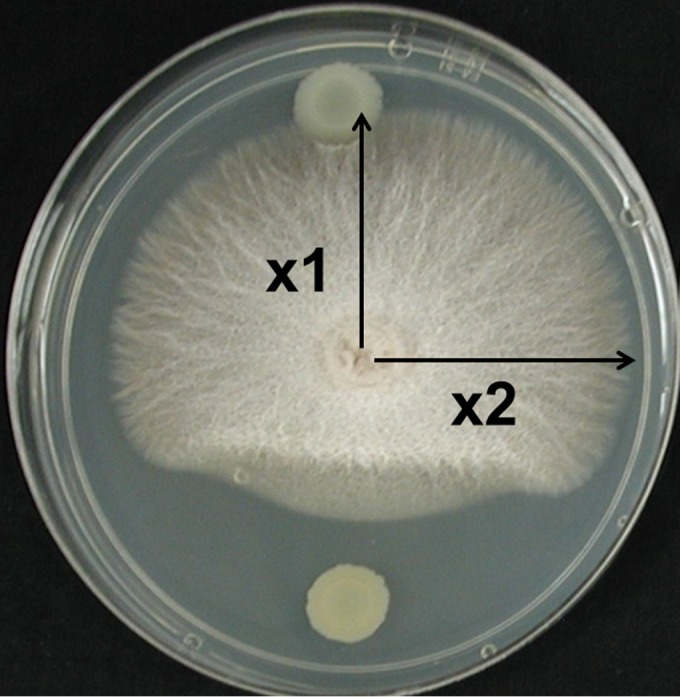
Inhibition of Fusarium verticillioides T4-0 by P. protegens Pf-5 (bottom) and a ΔgacA mutant (top) on PDA amended with 10−4 M FeCl3. Percent inhibition was calculated from measurements of fungal radial growth toward (x1) versus perpendicular to (x2) the bacterial colony according to the formula [1 − (x1/x2)] × 100.
Quantification of secondary metabolite production by P. protegens Pf-5 by HPLC.
Cultures of P. protegens Pf-5 or derivative strains were grown at 20°C or 27°C with shaking (200 rpm) in culture tubes (18-mm diameter) containing 5 ml NYBGly amended with 0.35 mM ZnSO4 (NYBGly-Zn), a medium conducive to the production of DAPG, MAPG, and pyoluteorin by P. protegens strain CHA0 (47). In our preliminary experiments, we found that the medium was also conducive to the production of pyrrolnitrin, orfamide A, and rhizoxin WF-1360F, the primary rhizoxin analog produced by Pf-5. Cultures were harvested after 48 h of growth because the six metabolites most relevant to this study could be detected consistently from cultures of Pf-5 harvested at this time. For experiments comparing mutants to wild-type Pf-5, cultures were grown at 20°C because the production of at least one secondary metabolite, pyoluteorin, is optimized at this temperature (54). Eight cultures were grown for each strain, and cultures from four tubes were pooled prior to centrifugation, resulting in two pooled replicates. For experiments evaluating the influence of FA on secondary metabolite production, cultures were grown at 27°C, which allowed comparison to the antagonism assays done at that temperature. Four replicate cultures were grown for each treatment. Culture supernatants and pellets were extracted separately, as described previously (9, 18), and extracts were combined prior to high-pressure liquid chromatography (HPLC) analysis. In brief, 4 ml of culture was centrifuged to separate the supernatant and pellet. Supernatants were extracted twice with 2 ml ethyl acetate. In one experiment noted in Results, culture supernatants were acidified to pH 2.0 with 1 M HCl prior to extraction, which can enhance extraction of some secondary metabolites such as pyoluteorin but also results in degradation of rhizoxin (6, 55). Pellets were extracted with 4 ml acetone following sonication and centrifugation. Extracts were dried down separately under vacuum. Supernatant and pellet extracts were then resuspended in methanol, combined, and dried down under vacuum. For HPLC analysis, combined extracts were resuspended in 50 μl of 75% aqueous acetonitrile, sonicated, and centrifuged. Analysis of metabolites was performed on 5 μl per sample. Analysis of Pf-5 metabolites was performed on a Shimadzu Prominence HPLC using a Synergi Fusion-RP 80A column (4.6 by 250 mm, 4 mm; Phenomenex). The mobile phase consisted of H2O with 0.1% acetic acid (solvent A) and acetonitrile (solvent B). A linear gradient was applied over 45 min from 20% to 100% solvent B, followed by isocratic elution at 100% solvent B for 10 min. The flow rate was 1.5 ml min−1, and a Shimadzu SPD-M20A photodiode array detector was used for UV detection at 210, 254, 270, and 310 nm. For quantitative analysis, standard curves for each metabolite were constructed from injections of five concentrations, ranging from 5 ng to 1 mg, of standards using a linear-fit model (Shimadzu EZStart software, version 7.3 SP1). Pure orfamide A and rhizoxin WF-1360F were provided by Harald Gross (University of Tübingen, Germany), pure pyoluteorin was provided by Brian Nowak-Thompson (Cornell College, Mt. Vernon, IA, USA), and pure MAPG and DAPG were provided by Christoph Keel (Lausanne University, Switzerland).
Effect of pyrrolnitrin and rhizoxin on the germination of Fusarium microconidia.
Microconidia of Fusarium spp. were produced by inoculating 50 ml of PDB contained in 250-ml flasks with an agar plug from a 5-day culture on PDA. PDB cultures were incubated for 2 weeks at 25°C with shaking (100 rpm) under continuous light. Cultures were passed through 8 layers of sterile cheesecloth. The residue was then placed on a sterile 2-μm filter and washed with sterile water, and spores were suspended in sterile water to a final density of 20,000 spores ml−1. Fifty microliters of the spore suspension was placed in each well of a 96-well tissue culture plate to a final concentration of 103 spores per well. Pyrrolnitrin (Sigma-Aldrich, St. Louis, MO) was suspended in methanol to a concentration of 1 mg ml−1 and diluted with sterile distilled water (dH2O) to yield final concentrations used in the experiments. To each well containing the microconidia, 50 μl of the pyrrolnitrin stock or diluted solutions was added to yield final concentrations of 0, 0.05, 0.1, 0.5, 1.0, 2.5, and 5.0 μg ml−1 pyrrolnitrin. The control was diluted methanol at a concentration equivalent to the highest level of pyrrolnitrin tested. Rhizoxin WF-1360F was suspended in dimethyl sulfoxide (DMSO) to a concentration of 10 mg ml−1 and diluted with sterile dH2O to yield final concentrations used in the experiments. To each well containing the microconidia, 50 μl of rhizoxin WF-1360F stock or dilutions was added to yield a final concentration of 0, 5, or 20 μg ml−1. The control was DMSO at a concentration equivalent to that present in the solution containing highest level of rhizoxin WF-1360F tested. Plates were incubated at 27°C, and microscopic observations were recorded at 20 h. There were four replicates at each concentration, and the experiment was done three times with essentially equivalent results.
Assessing the influence of FA on antibiotic and siderophore production by P. protegens Pf-5.
A stock solution of FA was prepared by dissolving 1 g of FA in 5 ml of methanol, bringing the concentration to 10 mg ml−1 with sterile water, and adjusting the pH to 6.5 with 5 N NaOH. FA was added to the medium immediately prior to inoculation with P. protegens, and controls received a corresponding volume of a methanol-water solution. Cultures were grown for 48 h at 27°C on a rotary shaker (200 rpm) in 5 ml of liquid medium. Bacterial growth was assessed throughout the experiment by measuring the optical density at 600 nm. Secondary metabolites were extracted from four replicate cultures and quantified by HPLC as described above.
In preliminary experiments, we evaluated the influence of FA on Pf-5 in PCG medium, which was used to assess the influence of FA on the related strain P. protegens CHA0 (28, 31). Strain Pf-5 did not grow as well as CHA0 in PCG medium, establishing a final optical density at 600 nm of 2.3, versus 4.0 reported for CHA0. Therefore, we tested the influence of FA on secondary metabolite production by Pf-5 in NYBGly-Zn (28) and PDB supplemented with 0.1 mM FeCl3 (PDB-Fe) (pH 4.3), as these media supported growth and secondary metabolite production by Pf-5. No influence of 0.5 mM FA on the growth of Pf-5 was observed in these two media.
RNA extraction and quantitative reverse transcriptase PCR (RT-qPCR).
Pf-5 was grown in 20 ml of NYBGly-Zn and FA (0 and 0.5 mM) at 27°C with shaking (200 rpm). RNA was extracted from cultures at late exponential growth phase (OD600 = 3.0, 12 h), and stationary growth phase (OD600 = 7.5, 24 h) using an RNeasy kit (Qiagen) with an adapted protocol supplied by the manufacturer. These two time points were selected because in our previous studies, genes for the biosynthesis of many antibiotics by Pf-5 were expressed at late exponential phase and stationary phase, and this expression was correlated with antibiotic production (9, 16, 17, 56). RNA samples were subjected to an on-column DNase treatment (RNeasy minikit with DNase I; Qiagen), and removal of DNA was confirmed by PCR. RNA quality was verified on a BioAnalyser 2100 (Agilent, Palo Alto, CA) by the Center for Genome Research and Biocomputing (CGRB) Core Laboratories, Oregon State University, Corvallis, OR. Total RNA (1 to 10 μg) was reverse transcribed into cDNA using random hexamer primers (Invitrogen, Life Technologies, Grand Island, NY) and 200 U Superscript II RNase H− reverse transcriptase (Invitrogen) according to the procedure supplied with the enzyme.
Quantitative PCR was performed on the cDNA using SYBR green on a Roche LightCycler (Roche Diagnostics Corporation, Indianapolis, IN, USA). Primers (Table 2) were designed through LightCycler probe design software (Roche). Melting curve analysis of products was used to verify amplification of a specific product. The zwf gene (PFL_4610), which encodes glucose-6-phosphate 1-dehydrogenase, was selected as the reference gene because we have observed little variation in zwf expression in several studies of transcriptome of Pf-5 on seed and in culture or in many RT-qPCR experiments (17, 56). The fluorescence-per-cycle data for each reaction were imported into the LinRegPCR program (57, 58). LinRegPCR was used to determine the cycle threshold (CT) value for each reaction and to determine the average amplification efficiency of each primer set. Only primers with amplification efficiencies of 1.6 or greater were used. The Pfaffl method (59) was used to determine a relative quantification of the target genes in comparison to the reference gene. Results were evaluated statistically using the relative expression software tool (60).
RESULTS AND DISCUSSION
Inhibition of Fusarium spp. by P. protegens Pf-5 and derivative strains deficient in antibiotic production.
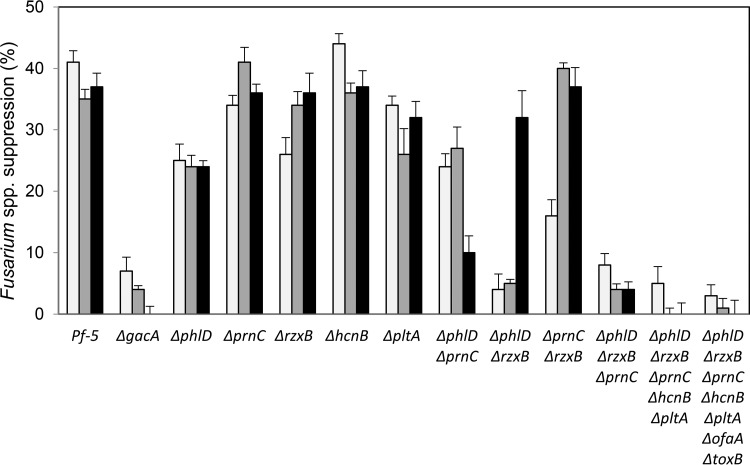
Pf-5 suppressed mycelial growth of three isolates of Fusarium spp. by 30% to 40% on PDA-Fe, whereas a ΔgacA mutant of Pf-5 suppressed mycelial growth by less than 10% on this medium (Fig. 2). These results confirm that a diffusible antifungal compound(s) produced by Pf-5 under the positive control of the Gac/Rsm signal transduction system is largely responsible for antibiosis, as established previously for Pf-5 and many other strains of Pseudomonas spp. (61). To identify the specific compounds contributing to suppression of Fusarium spp., we generated and evaluated a panel of mutants (Table 1) having single or multiple mutations in all known antibiotic biosynthetic gene clusters expressed under the positive control of GacA (16, 17).
FIG 2.
Inhibition of Fusarium spp. by P. protegens Pf-5 and derivative strains on PDA amended with 10−4 M FeCl3. Pf-5 and derivatives having deletions in specified genes are shown on the x axis. The percent inhibition of F. verticillioides isolate T4-0 (white bars), F. oxysporum f. sp. pisi race 2 isolate R2-238 (gray bars), and F. oxysporum f. sp. pisi race 5 isolate R5 (black bars) was determined as shown in Fig. 1. Cultures were grown for 5 days at 27°C before radial growth of Fusarium spp. was measured. Means represent four replicate plates, and error bars represent the standard error of the mean.
To characterize the mutant set, we compared the antibiotic production profiles of representative mutants to that of wild-type Pf-5. Cultures were grown in NYBGly-Zn, a medium conducive to the production of six of Pf-5's known antibiotics, and extracts of culture supernatants were evaluated by HPLC (Table 3). The antibiotic production profiles of Pf-5 varied somewhat between experiments, so Pf-5 was included in each experiment as a reference for comparison to the mutants evaluated. As expected, derivatives of Pf-5 having deletions in biosynthesis genes for five metabolites (JL4865, ΔphlD ΔrzxB ΔprnC ΔhcnB ΔpltA) or seven metabolites (JL4932, ΔphlD ΔrzxB ΔprnC ΔhcnB ΔpltA ΔofaA ΔtoxB) lacked production of the corresponding five or seven compounds (Table 3). At the time this experiment was done, we had not yet established that Pf-5 produces toxoflavin and did not have a method in place to quantify toxoflavin, so toxoflavin production is not reported in Table 3. We have since demonstrated that a ΔtoxB mutant of Pf-5 is deficient in the production of toxoflavin, a compound with broad antimicrobial activity and phytotoxicity (13). Derivatives of Pf-5 with individual deletions lacked production of the expected metabolite, and all but the ΔphlD mutant continued to produce all of the other compounds (Table 3). As described previously, the type III polyketide synthase PhlD is required for the production of both pyoluteorin and DAPG by Pf-5 (9). PhlD synthesizes phloroglucinol, an intermediate in the biosynthesis of DAPG. Phloroglucinol is not an intermediate in pyoluteorin biosynthesis but instead is involved in regulation of the pyoluteorin biosynthesis gene cluster in Pf-5 (56). Our HPLC analysis of the ΔphlD mutant confirmed that PhlD is essential for MAPG, DAPG, and pyoluteorin production (Table 3) and also demonstrated that the ΔphlD mutant produces orfamide A and rhizoxin at levels similar to those produced by wild-type Pf-5. We also observed quantitative differences in the production of several antibiotics by certain mutants with single deletions. For example, the ΔofaA mutant produced less DAPG, MAPG, and rhizoxin WF-1360F than wild-type Pf-5. In contrast, the ΔhcnB mutant produced higher levels of DAPG, MAPG, rhizoxin WF-1360F, and orfamide A than wild-type Pf-5. The mechanism for this is not known, but it could be due in part to the enhanced growth of the ΔhcnB mutant relative to the wild type (data not shown), which in turn could be due to the inherent toxicity of HCN to the producing strain. The ΔpltA mutant produced enhanced levels of DAPG and MAPG, which is likely due to the known reciprocal regulation of pyoluteorin and the phloroglucinol derivatives (9, 42, 44, 62).
TABLE 3.
Concentrations of antibiotics produced in culture by P. protegens Pf-5 and derivative strains having mutations in biosynthetic genesa
| Expt and strain/genotype | Designation | Concn, μg ml−1 (mean ± SEM)b |
|||||
|---|---|---|---|---|---|---|---|
| 2,4-Diacetylphloroglucinol | Monoacetylphloroglucinol | Pyoluteorin | Pyrrolnitrin | Rhizoxin WF-1360F | Orfamide A | ||
| 1 | |||||||
| Pf-5 | JL4585 | 0.10 ± 0.04 | 0.13 ± 0.06 | 8.0 ± 2.1 | 1.2 ± 0.3 | 4.4 ± 0.4 | 100 ± 14 |
| ΔprnC | JL4793 | 0.47c | 0.73c | 7.6 ± 0.4 | BD | 5.6 ± 0.7 | 110 ± 14 |
| ΔpltA | JL4805 | 7.2 ± 0.4 | 5.6 ± 0.6 | BD | 1.0 ± 0.1 | 8.1 ± 0.4 | 150 ± 3 |
| ΔrzxB | JL4808 | 0.04 ± 0.01 | 0.07 ± 0.02 | 8.0 ± 0.7 | 1.1 ± 0.1 | BD | 100 ± 7 |
| ΔhcnB | JL4809 | 9.5 ± 0.9 | 12 ± 1 | 11 ± 7 | 1.6 ± 0.3 | 36 ± 11 | 270 ± 20 |
| ΔphlA | LK023 | BD | BD | 7.2 ± 1.8 | 1.2 ± 0.2 | 3.8 ± 0.5 | 99 ± 11 |
| 2 | |||||||
| Pf-5 | JL4585 | 0.26 ± 0.15 | 0.65 ± 0.36 | 8.0 ± 0.5 | 1.5 ± 0.1 | 4.5 ± 0.1 | 12 ± 1 |
| ΔofaA | JL4807 | 0.03 ± 0.01 | 0.09 ± 0.03 | 7.5 ± 0.6 | 1.6 ± 0.3 | 2.4 ± 0.1 | 0.73 ± 0.01 |
| ΔphlA ΔrzxB ΔprnC | LK031 | BD | BD | 8.0 ± 0.1 | BD | BD | 110 ± 2 |
| 3 | |||||||
| Pf-5 | JL4585 | 0.05 ± 0.02 | 0.14 ± 0.1 | 5.9 ± 0.4 | 1.3 ± 0.1 | 3.8 ± 0.1 | 92 ± 1 |
| ΔgacA | JL4577 | BD | BD | BD | BD | BD | BD |
| ΔphlD | JL4804 | BD | BD | BD | 0.63 ± 0.04 | 4.0 ± 0.2 | 130 ± 7 |
| ΔphlD ΔrzxB ΔprnC ΔhcnB ΔpltA ΔofaA ΔtoxB | JL4932 | BD | BD | BD | BD | BD | 0.54 ± 0.11 |
| 4 | |||||||
| Pf-5 | JL4585 | 1.4 ± 0.4 | 2.4 ± 0.6 | 5.5 ± 0.6 | 1.1 ± 0.1 | 5.0 ± 0.1 | 110 ± 7 |
| ΔrzxB ΔprnC | JL4902 | 0.30 ± 0.21 | 0.9 ± 0.4 | 6.2 ± 0.6 | BD | BD | 90 ± 1 |
| ΔphlA ΔrzxB | LK027 | BD | BD | 5.8 ± 0.1 | 1.2 ± 0.1 | BD | 88 ± 4 |
| 5 | |||||||
| Wild type | JL4585 | 0.16 ± 0.04 | 0.33 ± 0.07 | 4.0 ± 2.9 | 0.70 ± 0.51 | 2.3 ± 1.4 | 56 ± 32 |
| ΔphlD ΔprnC | JL4830 | BD | BD | BD | BD | 7.9 ± 0.8 | 130 ± 14 |
| ΔphlD ΔrzxB ΔprnC | JL4844 | BD | BD | BD | BD | BD | 130 ± 7 |
| ΔphlD ΔrzxB ΔprnC ΔhcnB ΔpltA | JL4865 | BD | BD | BD | BD | BD | 300 ± 1 |
| ΔphlD ΔrzxB | JL4901 | BD | BD | BD | 1.0 ± 0.1 | BD | 120 ± 1 |
P. protegens Pf-5 or derivative strains were grown in 5 ml of NYBGly supplemented with 0.35 mM zinc sulfate (NYBGly-Zn). Cultures were incubated for 48 h at 20°C with shaking at 200 rpm before the concentrations of the antibiotics were quantified by HPLC.
The values represent the mean and standard error of the mean for two replicates, each composed of four broth cultures. Values were rounded to two significant digits. BD, below detection, with detection limits being approximately 0.02 μg ml−1 for each compound.
The compound was detected in only one of the two replicates. The value shown is one half the concentration determined from the replicate in which the compound was detected.
To identify the specific metabolite(s) contributing to inhibition of the Fusarium spp. on PDA-Fe, we first compared the 5-fold mutant (ΔphlD ΔrzxB ΔprnC ΔhcnB ΔpltA) and the 7-fold mutant (ΔphlD ΔrzxB ΔprnC ΔhcnB ΔpltA ΔofaA ΔtoxB) to the ΔgacA mutant and Pf-5. Compared to wild-type Pf-5, both the 5-fold and 7-fold mutants showed reduced inhibition of Fusarium spp., indicating that one or more of the mutagenized genes had a role in inhibition (Fig. 2). The percent inhibition of Fusarium spp. by both mutants was similar to that by the ΔgacA mutant (see Table S1 in the supplemental material). In contrast, all mutants deficient in only one compound suppressed the Fusarium spp., as the percent inhibition exhibited by each single mutant was greater than that by the ΔgacA mutant (Fig. 2; see Table S1 in the supplemental material). These results suggest that more than one compound contributes to fungal inhibition by Pf-5; consequently, antibiosis was not lost when the function of a single biosynthetic gene cluster was lost due to mutation. To determine the mixture of compounds responsible for inhibition, we then tested the 3-fold ΔphlD ΔrzxB ΔprnC mutant, which did not differ significantly from the ΔgacA, 5-fold, or 7-fold mutant in suppression of Fusarium spp. on PDA-Fe (Fig. 2; see Table S1 in the supplemental material).Because phlD is required for production of both DAPG and pyoluteorin, as described above, we repeated the antibiosis tests with a set of mutants having a phlA mutation, which is required for DAPG but not pyoluteorin production by Pf-5 (9). The ΔphlA ΔrzxB ΔprnC mutant, like the ΔphlD ΔrzxB ΔprnC mutant, did not differ significantly from the ΔgacA mutant in antibiosis against the two Fusarium spp. tested (see Fig. S1 in the supplemental material). These results indicate that DAPG, rhizoxin derivatives, and pyrrolnitrin are the primary metabolites responsible for suppression of the isolates of Fusarium spp. This finding is not surprising, as the toxicities of DAPG (63), rhizoxin WF-1360F (5, 64), and pyrrolnitrin (65) against Fusarium spp. are known. Pf-5 mutants deficient in the production of any two of the three compounds varied in inhibition of Fusarium spp., however, suggesting variation in the relative roles of the three compounds in antibiosis. For example, the ΔphlD ΔrzxB double mutant did not suppress mycelial growth of isolate R2-238 or T4-0 but did suppress mycelial growth of isolate R5 by approximately 30%. These results indicate that pyrrolnitrin production contributed to Pf-5's suppression of isolate R5 but did not contribute to Pf-5's suppression of isolate R2-238 or T4-0. One possible explanation for this result is that the isolates of Fusarium spp. differ in sensitivity to the antifungal compounds produced by Pf-5.
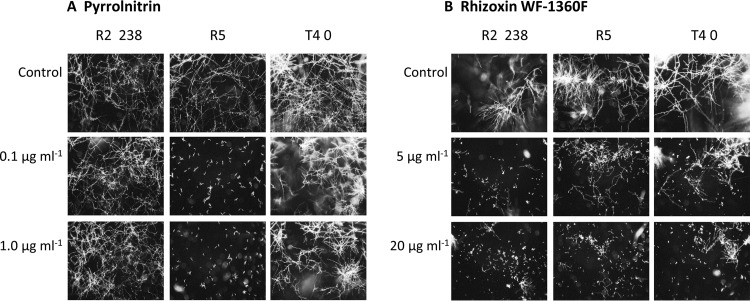
Isolates of Fusarium spp. differ in sensitivity to pyrrolnitrin and rhizoxin.
To determine the relative sensitivities of the Fusarium spp. to the Pf-5 metabolites, the three Fusarium isolates were exposed to purified pyrrolnitrin and WF-1360F, the predominant rhizoxin analog produced by Pf-5, and hyphal growth was assessed. Of the three isolates, F. oxysporum f. sp. pisi R5 was most sensitive to pyrrolnitrin (Fig. 3A). At 0.1 μg ml−1 pyrrolnitrin, hyphal growth of R5 was severely inhibited, whereas isolates R2-238 and T4-0 grew even at a 10-fold-higher concentration of pyrrolnitrin. Of the three isolates, R2-238 was the most sensitive to the rhizoxin analog WF-1360F: 5 μg ml−1 WF-1360F reduced hyphal growth of R2-238, whereas 20 μg ml−1 of the compound was required to cause a similar level of inhibition for isolates R5 and T4-0 (Fig. 3B). Therefore, as expected from the experiments evaluating the antibiosis of mutants of Pf-5, isolate R5 was more sensitive than isolate R2-238 to pyrrolnitrin and was less sensitive to WF-1360F (Fig. 3). The differential sensitivities of the two isolates to these compounds correlates to their inhibition by the ΔphlD ΔrzxB double mutant versus the ΔphlD ΔrzxB ΔprnC triple mutant of Pf-5 (Fig. 2).
FIG 3.
Inhibition of conidial germination and germination tube elongation of F. oxysporum f. sp. pisi R2-238, F. oxysporum f. sp. pisi R5, and F. verticillioides T4-0 by pyrrolnitrin (A) and rhizoxin WF-1360F (B). Twenty hours after conidia of the Fusarium spp. were placed in antibiotic solutions, germination and germination tube elongation were observed under a dissecting microscope and photographed. The blurred regions visible in some photographs are propagules outside the depth of field of the microscope. Four replicate conidial suspensions were assessed for each antibiotic concentration, and the experiment was done three times with nearly identical results.
FA influences antibiotic production by Pf-5.
Having identified the primary metabolites responsible for inhibition of Fusarium spp. by Pf-5, we then set out to determine the influence of the signaling compound FA on antibiotic production by Pf-5. Antibiotic production profiles were obtained from cultures of Pf-5 grown in NYBGly-Zn to correspond to our HPLC profiling of Pf-5 mutants and in PDB-Fe to correspond with assays evaluating inhibition of Fusarium spp. Amendment of the media with 0.5 mM FA did not alter growth rate of Pf-5 (data not shown) but decreased the production of DAPG and MAPG by Pf-5 by 75% to 99% (Table 4), as reported previously for P. protegens strain CHA0 (31) and other Pseudomonas spp. (66). Amendment of the media with 0.5 mM FA increased pyoluteorin production in NYBGly-Zn, but pyoluteorin production in PDB-Fe was near or below the detection limits regardless of FA amendment. In a previous study, 100 μg ml−1 FA decreased pyoluteorin production by P. protegens strain CHA0 grown in PCG medium (28). The different effects of FA on pyoluteorin production in the two studies could be due to different FA concentrations, strains, or media evaluated. Amendment of the media with 0.5 mM FA slightly increased pyrrolnitrin and orfamide A production in PDB-Fe but had no significant influence on the production of either antibiotic in NYBGly-Zn (Table 4). Although we did not assess the influence of FA on siderophore production by Pf-5, FA is known to increase pyoverdine production by Pf-5 (41). These results extend those from previous studies demonstrating that FA reduces the production of DAPG (31, 44), pyoluteorin (28), and phenazines (39, 67) by Pseudomonas spp. Our results show that FA can enhance the production of antibiotics, such as pyoluteorin, and that the effect of FA on secondary metabolite production can vary with the growth medium.
TABLE 4.
Influence of fusaric acid on antibiotic production by Pseudomonas protegens Pf-5a
| Antibiotic | Concn, μg ml−1 (mean ± SE) inb: |
|||
|---|---|---|---|---|
| NYBGly-Zn |
PDB-Fe |
|||
| Without FA | With FA | Without FA | With FA | |
| DAPG | 9.8 ± 0.9 | 0.06 ± 0.02** | 2.2 ± 0.3 | 0.56 ± 0.13** |
| MAPG | 6.6 ± 0.9 | 0.07 ± 0.03** | 15 ± 1 | 0.93 ± 0.05** |
| Orfamide A | 95 ± 9 | 78 ± 11 | 15 ± 1 | 18 ± 1* |
| Pyoluteorin | 0.87 ± 0.04 | 1.4 ± 0.1** | BD | 0.02 ± 0.01 |
| Pyrrolnitrin | 0.36 ± 0.06 | 0.31 ± 0.06 | 1.2 ± 0.1 | 1.4 ± 0.1* |
Strain Pf-5 was grown in 5 ml of NYBGly supplemented with 0.35 mM zinc sulfate (NYBGly-Zn) or in 5 ml of PDB with 0.1 mM FeCl3 (PDB-Fe). Both media were amended with fusaric acid (FA) at 0 or 0.5 mM. Cultures were incubated for 48 h at 27°C with shaking at 200 rpm. Prior to extraction, culture supernatants were acidified to pH 2.0, which enhances extraction of some antibiotics but causes degradation of rhizoxin (6, 55). Consequently, rhizoxin WF-1360F concentrations were not assessed in this experiment. Concentrations of antibiotics listed were quantified by HPLC.
Values represent the mean and standard error for four replicate broth cultures. Values were rounded to two significant digits. BD, below detection, with detection limits being approximately 0.02 μg/ml for all compounds. For each medium, asterisks (* and **) in the same row indicate values that are significantly different from the control value (α = 0.05 and 0.01, respectively) according to Student's t test.
FA influences antibiotic and siderophore gene expression.
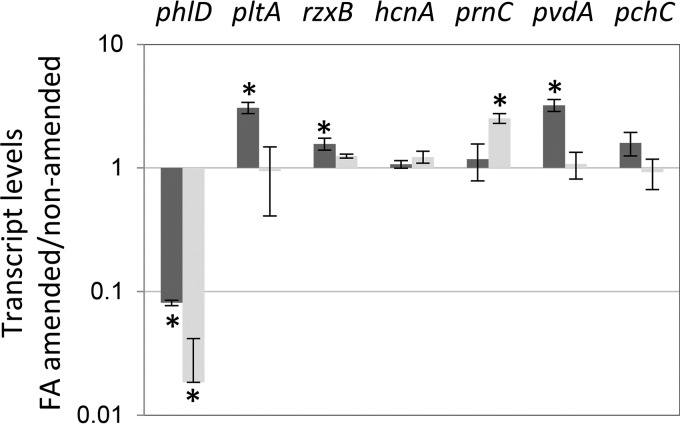
The influence of FA on transcription of secondary metabolite biosynthetic genes was assessed by RT-qPCR of cells harvested from late-exponential- and stationary-phase cultures of Pf-5 in NBGly-Zn. FA decreased the transcript abundance of phlD by 10-fold in late-exponential-phase cells and by approximately 50-fold in stationary-phase cells of Pf-5 (Fig. 4), in agreement with previous studies demonstrating that FA decreased the expression of DAPG biosynthesis genes of P. protegens CHA0 (43, 44). No significant effect of FA on the transcript abundance of hcnA or pchC was detected. The effects of FA on expression of other metabolic biosynthesis genes were smaller in magnitude and dependent on the growth phase of Pf-5. The transcript abundances of pltA, rzxB, and pvdA were significantly higher in late-exponential-phase cultures grown in FA-amended medium than in nonamended medium, but no significant effect was observed in stationary-phase cells. In contrast, the transcript abundance of prnC was enhanced by FA only in stationary-phase cells.
FIG 4.
Influence of fusaric acid on the expression of selected genes for secondary metabolite and siderophore biosynthesis by P. protegens Pf-5. RNA was extracted from late-exponential-phase (OD600 of 3.0) (black bars) and stationary-phase (OD600 of 7.2) (gray bars) cultures of Pf-5 grown in NBGly-Zn amended with 0 or 0.5 mM fusaric acid. Values represent the expression ratio of the target gene in cultures of Pf-5 grown in NBGly-Zn amended with fusaric acid versus nonamended medium. Bars represent the mean for four replicate cultures, and error bars show the standard error of the mean. Asterisks indicate values that differ significantly (P ≤ 0.05) between cultures grown in fusaric acid-amended versus nonamended medium, as determined by the method of Pfaffl et al. (60).
In this study, FA had a parallel influence on the transcription of phlD and production of DAPG. Similarly, the influence of FA on pltA transcription by cells in late-exponential phase (i.e., 24-h cultures) correlated to its influence on pyoluteorin production, assessed from 48-h cultures. These results align with those from previous studies where the expression of genes for DAPG and pyoluteorin biosynthesis by cells from late-exponential or stationary-phase cultures correlated with the levels of these metabolites that subsequently accumulated (16, 17, 56). In contrast, the influence of FA on transcription of biosynthesis genes for pyrrolnitrin did not correlate with the levels of this metabolite quantified from 48-h cultures. Secondary metabolite production is regulated at many levels, and in this example, the transcription of a single biosynthesis gene was not predictive of subsequent levels of the metabolite that accumulated in cultures of Pf-5.
FA influences antibiosis of Pf-5 against F. verticillioides.
We then evaluated the influence of FA on the antibiosis of Pf-5 and derivative strains against F. verticillioides T4-0 on PDA-Fe. FA decreased the inhibition of F. verticillioides T4-0 by Pf-5 and all derivative strains that produced DAPG (i.e., the ΔrzxB, ΔprnC, and ΔrzxB ΔprnC mutants) (Table 5). Accordingly, FA reduced the production of DAPG by Pf-5 in this medium, as determined by HPLC (Table 4). In contrast, FA did not alter the antibiosis exhibited by the ΔphlA, Δphl ΔprnC, and ΔphlA ΔrzxB mutants (Table 5), which relied on pyrrolnitrin and/or rhizoxin to inhibit Fusarium spp. With the exception of rhizoxin, which we were unable to quantify in the experiment described in Table 4, the data show that FA has distinct effects on the production of different antibiotics by Pf-5 that influence the antifungal activity of the bacterium.
TABLE 5.
Influence of fusaric acid on the suppression of Fusarium verticillioides T4-0 by Pf-5 and derivative strains on PDA-Fea
| Strain | Inhibition, %b |
|
|---|---|---|
| Without FA | With FA | |
| Wild type | 30 | 16** |
| ΔgacA | 6 | 1** |
| ΔphlA | 21 | 15 |
| ΔrzxB | 21 | 9** |
| ΔprnC | 28 | 19** |
| ΔprnC ΔrzxB | 13 | 6** |
| ΔphlA ΔprnC | 15 | 17 |
| ΔphlA ΔrzxB | 9 | 7 |
| ΔphlA ΔrzxB ΔprnC | 2 | 1 |
Inhibition of radial growth of F. verticillioides on PDA-Fe was measured after 5 days of incubation at 2 °C.
The results are means for four replicate agar plates. Asterisks (* and **) in the same row indicate values that are significantly different from the control value (α = 0.05 and 0.01, respectively) according to Student's t test.
Conclusions.
P. protegens Pf-5 produces an array of antifungal compounds toxic to phytopathogenic fungi. Here, we assembled a set of mutants of Pf-5 having individual or multiple mutations in gene clusters for each of the known antifungal compounds and used the mutant set to pinpoint the specific compounds contributing to inhibition of F. oxysporum f. sp. pisi and F. verticillioides. Three metabolites—DAPG, rhizoxin derivatives, and pyrrolnitrin—were the primary determinants of antibiosis exhibited by Pf-5 against these Fusarium spp. Isolates of Fusarium spp. varied in their sensitivities to the three compounds, and their sensitivities determined the relative importance of the three compounds in antibiosis by Pf-5. FA, a mycotoxin produced by Fusarium spp., had a differential effect on the production of Pf-5's metabolites, greatly decreasing the production of DAPG and moderately increasing the production of pyoluteorin, pyrrolnitrin, and orfamide A by Pf-5 in one of two media evaluated. We observed parallel influences of FA on the expression of the pyoluteorin biosynthetic gene pltA and pyoluteorin production and on the expression of phlD and DAPG production. In accordance with a recent report showing that FA enhanced pyoverdine production by Pf-5 (41), we observed that FA increased the expression of pvdA, a biosynthesis gene for pyoverdine production. Finally, we assessed the influence of FA on the inhibition of F. verticillioides by Pf-5 and selected derivative strains. FA diminished antibiosis exhibited by Pf-5 and derivative strains that produced DAPG but had no significant influence on antibiosis exhibited by phlA mutants, which do not produce DAPG but suppress F. verticillioides due to rhizoxin and pyrrolnitrin production. In summary, using a systematic approach employing a set of mutants representing the known antifungal metabolites of Pf-5, we demonstrated the roles of three compounds in antibiosis of Fusarium spp. and the parallel influences of the signaling molecule FA on antifungal metabolite production and antibiosis of this phytopathogenic fungus.
Supplementary Material
ACKNOWLEDGMENTS
We thank the following people for their generosity in providing materials for this work: Cynthia Ocamb, Lyndon Porter, and Walter Maccheroni, Jr., for strains of Fusarium spp., Herbert Schweizer for plasmid pEX18Tc, Harald Gross for purified orfamide A and rhizoxin WF-1360F, Brian Nowak-Thompson for purified pyoluteorin, and Christoph Keel for purified MAPG and DAPG. We are grateful to Charles Bacon for valuable advice on assessing the influence of FA on metabolite production by Pf-5.
We dedicate this article to the late Aline A. Pizzirani-Kleiner, without whom this study could not have been done.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02574-15.
REFERENCES
- 1.Gross H, Loper JE. 2009. Genomics of secondary metabolite production by Pseudomonas spp. Nat Prod Rep 26:1408–1446. doi: 10.1039/b817075b. [DOI] [PubMed] [Google Scholar]
- 2.Loper JE, Kobayashi DY, Paulsen IT. 2007. The genomic sequence of Pseudomonas fluorescens Pf-5: insights into biological control. Phytopathology 97:233–238. doi: 10.1094/PHYTO-97-2-0233. [DOI] [PubMed] [Google Scholar]
- 3.Howell CR, Stipanovic RD. 1979. Control of Rhizoctonia solani in cotton seedlings with Pseudomonas fluorescens and with an antibiotic produced by the bacterium. Phytopathology 69:480–482. doi: 10.1094/Phyto-69-480. [DOI] [Google Scholar]
- 4.Howell CR, Stipanovic RD. 1980. Suppression of Pythium ultimum-induced damping-off of cotton seedlings by Pseudomonas fluorescens and its antibiotic, pyoluteorin. Phytopathology 70:712–715. doi: 10.1094/Phyto-70-712. [DOI] [Google Scholar]
- 5.Brendel N, Partida-Martinez LP, Scherlach K, Hertweck C. 2007. A cryptic PKS-NRPS gene locus in the plant commensal Pseudomonas fluorescens Pf-5 codes for the biosynthesis of an antimitotic rhizoxin complex. Org Biomol Chem 5:2211–2213. doi: 10.1039/b707762a. [DOI] [PubMed] [Google Scholar]
- 6.Loper JE, Henkels MD, Shaffer BT, Valeriote FA, Gross H. 2008. Isolation and identification of rhizoxin analogs from Pseudomonas fluorescens Pf-5 by using a genomic mining strategy. Appl Environ Microbiol 74:3085–3093. doi: 10.1128/AEM.02848-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraus J, Loper JE. 1992. Lack of evidence for a role of antifungal metabolite production by Pseudomonas fluorescens Pf-5 in biological control of Pythium damping-off of cucumber. Phytopathology 82:264–271. doi: 10.1094/Phyto-82-264. [DOI] [Google Scholar]
- 8.Nowak-Thompson B, Gould SJ, Kraus J, Loper JE. 1994. Production of 2,4-diacetylphloroglucinol by the biocontrol agent Pseudomonas fluorescens Pf-5. Can J Microbiol 40:1064–1066. doi: 10.1139/m94-168. [DOI] [Google Scholar]
- 9.Kidarsa TA, Goebel NC, Zabriskie TM, Loper JE. 2011. Phloroglucinol mediates crosstalk between the pyoluteorin and 2,4-diacetylphloroglucinol biosynthetic pathways in Pseudomonas fluorescens Pf-5. Mol Microbiol 81:395–414. doi: 10.1111/j.1365-2958.2011.07697.x. [DOI] [PubMed] [Google Scholar]
- 10.Bangera MG, Thomashow LS. 1999. Identification and characterization of a gene cluster for synthesis of the polyketide antibiotic 2,4-diacetylphloroglucinol from Pseudomonas fluorescens Q2-87. J Bacteriol 181:3155–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanahan P, Glennon JD, Crowley J, Donnelly D, O'Gara F. 1993. Liquid chromatographic assay of microbially derived phloroglucinol antibiotics for establishing the biosynthetic route to production, and the factors affecting their regulation. Anal Chim Acta 272:271–277. doi: 10.1016/0003-2670(93)80579-A. [DOI] [Google Scholar]
- 12.Gross H, Stockwell VO, Henkels MD, Nowak-Thompson B, Loper JE, Gerwick WH. 2007. The genomisotopic approach: a systematic method to isolate products of orphan biosynthetic gene clusters. Chem Biol 14:53–63. doi: 10.1016/j.chembiol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Philmus BJ, Shaffer BT, Kidarsa TA, Yan Q, Raaijmakers JM, Begley TP, Loper JE. 2015. Investigations into the biosynthesis, regulation and self-resistance of toxoflavin in Pseudomonas protegens Pf-5. Chembiochem 16:1782–1790. doi: 10.1002/cbic.201500247. [DOI] [PubMed] [Google Scholar]
- 14.Raaijmakers JM, Mazzola M. 2012. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu Rev Phytopathol 50:403–424. doi: 10.1146/annurev-phyto-081211-172908. [DOI] [PubMed] [Google Scholar]
- 15.Lapouge K, Schubert M, Allain FH, Haas D. 2008. Gac/Rsm signal transduction pathway of gamma-proteobacteria: from RNA recognition to regulation of social behaviour. Mol Microbiol 67:241–253. [DOI] [PubMed] [Google Scholar]
- 16.Hassan KA, Johnson A, Shaffer BT, Ren Q, Kidarsa TA, Elbourne LDH, Hartney S, Duboy R, Goebel NC, Zabriskie TM, Paulsen IT, Loper JE. 2010. Inactivation of the GacA response regulator in Pseudomonas fluorescens Pf-5 has far-reaching transcriptomic consequences. Environ Microbiol 12:899–915. doi: 10.1111/j.1462-2920.2009.02134.x. [DOI] [PubMed] [Google Scholar]
- 17.Kidarsa TA, Shaffer BT, Goebel NC, Roberts DP, Buyer JS, Johnson A, Kobayashi DY, Zabriskie TM, Paulsen I, Loper JE. 2013. Genes expressed by the biological control bacterium Pseudomonas protegens Pf-5 on seed surfaces under the control of the global regulators GacA and RpoS. Environ Microbiol 15:716–735. doi: 10.1111/1462-2920.12066. [DOI] [PubMed] [Google Scholar]
- 18.Whistler CA, Corbell NA, Sarniguet A, Ream W, Loper JE. 1998. The two-component regulators GacS and GacA influence accumulation of the stationary-phase sigma factor and the stress response in Pseudomonas fluorescens Pf-5. J Bacteriol 180:6635–6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma L-J, Geiser DM, Proctor RH, Rooney AP, O'Donnell K, Trail F, Gardiner DM, Manners JM, Kazan K. 2013. Fusarium pathogenomics. Annu Rev Microbiol 67:399–416. doi: 10.1146/annurev-micro-092412-155650. [DOI] [PubMed] [Google Scholar]
- 20.Yates IE, Arnold JW, Hinton DM, Basinger W, Walcott RR. 2003. Fusarium verticillioides induction of maize seed rot and its control. Can J Bot 81:422–428. doi: 10.1139/b03-034. [DOI] [Google Scholar]
- 21.Mohammadi A, Nejad RF, Mofrad NN. 2012. Fusarium verticillioides from sugarcane, vegetative compatibility groups and pathogenicity. Plant Protect Sci 48:80–84. [Google Scholar]
- 22.Fravel D, Olivain C, Alabouvette C. 2003. Fusarium oxysporum and its biocontrol. New Phytol 157:493–502. doi: 10.1046/j.1469-8137.2003.00700.x. [DOI] [PubMed] [Google Scholar]
- 23.Lemanceau P, Alabouvette C. 1993. Suppression of Fusarium wilt by fluorescent pseudomonads—mechanisms and applications. Biocontrol Sci Technol 3:219–234. doi: 10.1080/09583159309355278. [DOI] [Google Scholar]
- 24.Mazurier S, Corberand T, Lemanceau P, Raaijmakers JM. 2009. Phenazine antibiotics produced by fluorescent pseudomonads contribute to natural soil suppressiveness to Fusarium wilt. ISME J 3:977–991. doi: 10.1038/ismej.2009.33. [DOI] [PubMed] [Google Scholar]
- 25.Weller DM, Raaijmakers JM, McSpadden Gardener BB, Thomashow LS. 2002. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40:309–348. doi: 10.1146/annurev.phyto.40.030402.110010. [DOI] [PubMed] [Google Scholar]
- 26.Anjaiah V, Koedam N, Nowak-Thompson B, Loper JE, Hofte M, Tambong JT, Cornelis P. 1998. Involvement of phenazines and anthranilate in the antagonism of Pseudomonas aeruginosa PNA1 and Tn5 derivatives toward Fusarium spp. and Pythium spp. Mol Plant Microbe Interact 11:847–854. doi: 10.1094/MPMI.1998.11.9.847. [DOI] [Google Scholar]
- 27.Chin-A-Woeng TFC, Bloemberg GV, van der Bij AJ, van der Drift KMGM, Schripsema J, Kroon B, Scheffer RJ, Keel C, Bakker PAHM, Tichy H-V, de Bruijn FJ, Thomas-Oates JE, Lugtenberg BJJ. 1998. Biocontrol by phenazine-1-carboxamide-producing Pseudomonas chlororaphis PCL1391 of tomato root rot caused by Fusarium oxysporum f.sp. radicis-lycopersici. Mol Plant Microbe Interact 11:1069–1077. doi: 10.1094/MPMI.1998.11.11.1069. [DOI] [Google Scholar]
- 28.Duffy BK, Défago G. 1997. Zinc improves biocontrol of Fusarium crown and root rot of tomato by Pseudomonas fluorescens and represses the production of pathogen metabolites inhibitory to bacterial antibiotic biosynthesis. Phytopathology 87:1250–1257. doi: 10.1094/PHYTO.1997.87.12.1250. [DOI] [PubMed] [Google Scholar]
- 29.Raaijmakers JM, Leeman M, van Oorschot MMP, van der Sluis I, Schippers B, Bakker PAHM. 1995. Dose-response relationships in biological control of fusarium wilt of radish by Pseudomonas spp. Phytopathology 85:1075–1081. doi: 10.1094/Phyto-85-1075. [DOI] [Google Scholar]
- 30.Sharifi-Tehrani A, Zala M, Natsch A, Moenne-Loccoz Y, Defago G. 1998. Biocontrol of soil-borne fungal plant diseases by 2,4-diacetylphloroglucinol-producing fluorescent pseudomonads with different restriction profiles of amplified 16S rDNA. Eur J Plant Pathol 104:631–643. doi: 10.1023/A:1008672104562. [DOI] [Google Scholar]
- 31.Duffy B, Keel C, Defago G. 2004. Potential role of pathogen signaling in multitrophic plant-microbe interactions involved in disease protection. Appl Environ Microbiol 70:1836–1842. doi: 10.1128/AEM.70.3.1836-1842.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezzonico F, Zala M, Keel C, Duffy B, Moënne-Loccoz Y, Défago G. 2007. Is the ability of biocontrol fluorescent pseudomonads to produce the antifungal metabolite 2,4-diacetylphloroglucinol really synonymous with higher plant protection? New Phytol 173:861–872. doi: 10.1111/j.1469-8137.2006.01955.x. [DOI] [PubMed] [Google Scholar]
- 33.Woloshuk CP, Shim W-B. 2013. Aflatoxins, fumonisins, and trichothecenes: a convergence of knowledge. FEMS Microbiol Rev 37:94–109. doi: 10.1111/1574-6976.12009. [DOI] [PubMed] [Google Scholar]
- 34.Brown DW, Lee S-H, Kim L-H, Ryu J-G, Lee S, Seo Y, Kim YH, Busman M, Yun S-H, Proctor RH, Lee T. 2015. Identification of a 12-gene fusaric acid biosynthetic gene cluster in Fusarium through comparative and functional genomics. Mol Plant Microbe Interact 28:319–332. doi: 10.1094/MPMI-09-14-0264-R. [DOI] [PubMed] [Google Scholar]
- 35.Reverberi M, Ricelli A, Zjalic S, Fabbri AA, Fanelli C. 2010. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl Microbiol Biotechnol 87:899–911. doi: 10.1007/s00253-010-2657-5. [DOI] [PubMed] [Google Scholar]
- 36.Palumbo J, O'Keeffe T, Abbas H. 2008. Microbial interactions with mycotoxigenic fungi and mycotoxins. Toxin Rev 27:261–285. doi: 10.1080/15569540802416301. [DOI] [Google Scholar]
- 37.Duffy B, Schouten A, Raaijmakers JM. 2003. Pathogen self-defense: mechanisms to counteract microbial antagonism. Annu Rev Phytopathol. 41:501–538. doi: 10.1146/annurev.phyto.41.052002.095606. [DOI] [PubMed] [Google Scholar]
- 38.Saikia R, Varghese S, Singh BP, Arora DK. 2009. Influence of mineral amendment on disease suppressive activity of Pseudomonas fluorescens to Fusarium wilt of chickpea. Microbiol Res 164:365–373. doi: 10.1016/j.micres.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 39.van Rij ET, Girard G, Lugtenberg BJJ, Bloemberg GV. 2005. Influence of fusaric acid on phenazine-1-carboxamide synthesis and gene expression of Pseudomonas chlororaphis strain PCL1391. Microbiology 151:2805–2814. doi: 10.1099/mic.0.28063-0. [DOI] [PubMed] [Google Scholar]
- 40.Landa BB, Cachinero-Díaz JM, Lemanceau P, Jimínez-Díaz RM, Alabouvette C. 2002. Effect of fusaric acid and phytoanticipins on growth of rhizobacteria and Fusarium oxysporum. Can J Microbiol 48:971–985. doi: 10.1139/w02-094. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz JA, Bernar EM, Jung K. 2015. Production of siderophores increases resistance to fusaric acid in Pseudomonas protegens Pf-5. PLoS One 10:e0117040. doi: 10.1371/journal.pone.0117040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baehler E, Bottiglieri M, Péchy-Tarr M, Maurhofer M, Keel C. 2005. Use of green fluorescent protein-based reporters to monitor balanced production of antifungal compounds in the biocontrol agent Pseudomonas fluorescens CHA0. J Appl Microbiol 99:24–38. doi: 10.1111/j.1365-2672.2005.02597.x. [DOI] [PubMed] [Google Scholar]
- 43.Notz R, Maurhofer M, Dubach H, Haas D, Défago G. 2002. Fusaric acid-producing strains of Fusarium oxysporum alter 2,4-diacetylphloroglucinol biosynthetic gene expression in Pseudomonas fluorescens CHA0 in vitro and in the rhizosphere of wheat. Appl Environ Microbiol 68:2229–2235. doi: 10.1128/AEM.68.5.2229-2235.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnider-Keel U, Seematter A, Maurhofer M, Blumer C, Duffy B, Gigot-Bonnefoy C, Reimmann C, Notz R, Défago G, Haas D, Keel C. 2000. Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J Bacteriol 182:1215–1225. doi: 10.1128/JB.182.5.1215-1225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44:301–307. [PubMed] [Google Scholar]
- 46.Toyoda H, Hashimoto H, Utsumi R, Kobayashi H, Ouchi S. 1988. Detoxification of fusaric acid by a fusaric acid-resistant mutant of Pseudomonas solanacearum and its application to biological control of Fusarium wilt of tomato. Phytopathology 78:1307–1311. doi: 10.1094/Phyto-78-1307. [DOI] [Google Scholar]
- 47.Duffy BK, Defago G. 1999. Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas. Appl Environ Microbiol 65:2429–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi K-H, Schweizer HP. 2005. An improved method for rapid generation of unmarked Pseudomonas aeruginosa deletion mutants. BMC Microbiol 5:30–30. doi: 10.1186/1471-2180-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brazelton JN, Pfeufer EE, Sweat TA, McSpadden Gardener BB, Coenen C. 2008. 2,4-diacetylphloroglucinol alters plant root development. Mol Plant Microbe Interact 21:1349–1358. doi: 10.1094/MPMI-21-10-1349. [DOI] [PubMed] [Google Scholar]
- 50.Henkels MD, Kidarsa TA, Shaffer BT, Goebel NC, Burlinson P, Mavrodi DV, Bentley MA, Rangel LI, Davis EW II, Thomashow LS, Zabriskie TM, Preston GM, Loper JE. 2014. Pseudomonas protegens Pf-5 causes discoloration and pitting of mushroom caps due to the production of antifungal metabolites. Mol Plant Microbe Interact 27:733–746. doi: 10.1094/MPMI-10-13-0311-R. [DOI] [PubMed] [Google Scholar]
- 51.Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86. doi: 10.1016/S0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 52.Simon R, Priefer U, Puhler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 53.Loper JE, Hassan KA, Mavrodi DV, Davis EW II, Lim CK, Shaffer BT, Elbourne LDH, Stockwell VO, Hartney SL, Breakwell K, Henkels MD, Tetu SG, Rangel LI, Kidarsa TA, Wilson NL, van de Mortel JE, Song C, Blumhagen R, Radune D, Hostetler JB, Brinkac LM, Durkin AS, Kluepfel DA, Wechter WP, Anderson AJ, Kim YC, Pierson LS III, Pierson EA, Lindow SE, Kobayashi DY, Raaijmakers JM, Weller DM, Thomashow LS, Allen AE, Paulsen IT. 2012. Comparative genomics of plant-associated Pseudomonas spp.: insights into diversity and inheritance of traits involved in multitrophic interactions. PLoS Genet 8:e1002784. doi: 10.1371/journal.pgen.1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nowak-Thompson B. 1997. An integrative approach to understanding pyoluteorin biosynthesis in the biological control bacterium Pseudomonas fluorescens Pf-5. Oregon State University, Corvallis, OR. [Google Scholar]
- 55.Nowak-Thompson B, Chaney N, Wing JS, Gould SJ, Loper JE. 1999. Characterization of the pyoluteorin biosynthetic gene cluster of Pseudomonas fluorescens Pf-5. J Bacteriol 181:2166–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clifford JC, Buchanan A, Vining O, Kidarsa TA, Chang JH, McPhail KL, Loper JE. 3 September 2015. Phloroglucinol functions as an intracellular and intercellular chemical messenger influencing gene expression in Pseudomonas protegens. Environ Microbiol doi: 10.1111/1462-2920.13043. [DOI] [PubMed] [Google Scholar]
- 57.Ramakers C, Ruijter JM, Deprez RH, Moorman AF. 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339:62–66. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 58.Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJB, Moorman AFM. 2009. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kay E, Dubuis C, Haas D. 2005. Three small RNAs jointly ensure secondary metabolism and biocontrol in Pseudomonas fluorescens CHA0. Proc Natl Acad Sci U S A 22:17136–17141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brodhagen M, Henkels MD, Loper JE. 2004. Positive autoregulation and signaling properties of pyoluteorin, an antibiotic produced by the biological control organism Pseudomonas fluorescens Pf-5. Appl Environ Microbiol 70:1758–1766. doi: 10.1128/AEM.70.3.1758-1766.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keel C, Schnider U, Maurhofer M, Voisard C, Laville J, Burger U, Wirthner P, Haas D, Défago G. 1992. Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Mol Plant Microbe Interact 5:4–13. doi: 10.1094/MPMI-5-004. [DOI] [Google Scholar]
- 64.Takeuchi K, Noda N, Katayose Y, Mukai Y, Numa H, Yamada K, Someya N. 2015. Rhizoxin analogs contribute to the biocontrol activity of newly isolated Pseudomonas strain. Mol Plant Microbe Interact 28:333–342. doi: 10.1094/MPMI-09-14-0294-FI. [DOI] [PubMed] [Google Scholar]
- 65.Burkhead KD, Schisler DA, Slininger PJ. 1994. Pyrrolnitrin production by biological control agent Pseudomonas cepacia B37w in culture and in colonized wounds of potatoes. Appl Environ Microbiol 60:2031–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saikia R, Sarma RK, Yadav A, Bora TC. 2011. Genetic and functional diversity among the antagonistic potential fluorescent pseudomonads isolated from tea rhizosphere. Curr Microbiol 62:434–444. doi: 10.1007/s00284-010-9726-y. [DOI] [PubMed] [Google Scholar]
- 67.van Rij ET, Wesselink M, Chin-A-Woeng TF, Bloemberg GV, Lugtenberg BJJ. 2004. Influence of environmental conditions on the production of phenazine-1-carboxamide by Pseudomonas chlororaphis PCL1391. Mol Plant Microbe Interact 17:557–566. doi: 10.1094/MPMI.2004.17.5.557. [DOI] [PubMed] [Google Scholar]
- 68.Paulsen IT, Press CM, Ravel J, Kobayashi DY, Myers GSA, Mavrodi DV, DeBoy RT, Seshadri R, Ren Q, Madupu R, Dodson RJ, Durkin AS, Brinkac LM, Daugherty SC, Sullivan SA, Rosovitz MJ, Gwinn ML, Zhou L, Schneider DJ, Cartinhour SW, Nelson WC, Weidman J, Watkins K, Tran K, Khouri H, Pierson EA, Pierson LS III, Thomashow LS, Loper JE. 2005. Complete genome sequence of the plant commensal Pseudomonas fluorescens Pf-5. Nat Biotechnol 23:873–878. doi: 10.1038/nbt1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.