Abstract
During aerobic respiration, microorganisms consume oxygen (O2) through the use of different types of terminal oxidases which have a wide range of affinities for O2. The Km values for O2 of these enzymes have been determined to be in the range of 3 to 200 nmol liter−1. In this study, we examined the time course of development of aerobic respiratory kinetics of four marine bacterial species (Dinoroseobacter shibae, Roseobacter denitrificans, Idiomarina loihiensis, and Marinobacter daepoensis) during exposure to decreasing O2 concentrations. The genomes of all four species have genes for both high-affinity and low-affinity terminal oxidases. The respiration rate of the bacteria was measured by the use of extremely sensitive optical trace O2 sensors (range, 1 to 1,000 nmol liter−1). Three of the four isolates exhibited apparent Km values of 30 to 60 nmol liter−1 when exposed to submicromolar O2 concentrations, but a decrease to values below 10 nmol liter−1 was observed when the respiration rate per cell was lowered and the cell size was decreased due to starvation. The fourth isolate did not reach a low respiration rate per cell during starvation and exhibited apparent Km values of about 20 nmol liter−1 throughout the experiment. The results clearly demonstrate not only that enzyme kinetics may limit O2 uptake but also that even individual cells may be diffusion limited and that this diffusion limitation is the most pronounced at high respiration rates. A decrease in cell size by starvation, due to limiting organic carbon, and thereby more efficient diffusion uptake may also contribute to lower apparent Km values.
INTRODUCTION
Oxygen (O2), the second most abundant gas in the atmosphere on Earth, plays a critical role in nature, including aquatic environments. As the most favorable electron acceptor, O2 drives the degradation of organic matter and influences the cycling of other elements (e.g., nitrogen, sulfur, phosphorus) (1). For decades, aerobic respiration has been studied by different methods in various environments (2–4). Due to the limit of detection by traditional methods (Winkler methods and methods that use electrochemical sensors and optodes), the measurement of respiratory activity in low-O2 environments, such as oceanic oxygen-minimum zones (OMZs), is rare (5), and even in fully oxygenated waters, the direct measurement of oxygen dynamics is difficult and the more indirect method of measurement of formazan formation has therefore been applied (6). The lack of detailed data on aerobic respiration in low-O2 environments restricts the understanding of carbon budgets (7) and the prediction of the development of O2 within such environments (8). Recently, STOX oxygen sensors have been applied to quantify O2 respiration rates in OMZs (9), and by this technique, it was possible to measure rates down to about 1 nmol−1 liter−1 h−1, which is a level of resolution that is a factor of 10 higher than that obtained by traditional methods (10). Half-saturation constants (apparent Km values) for microbial communities were estimated from the data obtained with STOX oxygen sensors, but highly variable values ranging from 30 to 200 nmol liter−1 were obtained. Another study indicated high half-saturation O2 concentrations of several micromolar, supposedly caused by diffusion limitation around and within aggregates (11).
The final step of aerobic respiration is conducted by a terminal cytochrome oxidase, a membrane-associated protein which transfers electrons to O2 (12). Two main families of terminal oxidases have been classified on the basis of structural and functional differences (13–16): the heme-copper oxidases (HCOs) and the cytochrome bd-type oxidases. Furthermore, three classes have been identified within HCOs according to the combination of different heme subunits (classes A, B, and C). The kinetic parameters maximum respiration rate (Vmax) and Km can be used to describe respiratory activity as a function of the O2 concentration, although it should be kept in mind that the Michaelis-Menten equation strictly applies only to the kinetics of single enzymes. According to the affinity of O2, these terminal oxidases can be classified as high-affinity terminal oxidases (with Km values of about 3 to 8 nmol liter−1) (17) and low-affinity terminal oxidases (with Km values of about 200 nmol liter−1) (18). Respiration rates in aquatic environments have been estimated by different methods, and the kinetics have been estimated using several models. Different equations have been proposed to estimate Vmax and Km values, such as fitting of the data directly to the Michaelis-Menten equation by computer-aided iterative regression (19); the method of Dixon and Webb, as described by Kita et al. (20); and the method described by Appleby and Bergersen (21). Tiano et al. (22) reported that a modified Jassby and Platt equation results in better fits to the measured decrease in the O2 concentration than the Michaelis-Menten model under low O2 concentrations.
The microbial aerobic respiration rate is affected by different factors, such as growth phase, nutrient availability, and the O2 concentration. On the basis of genomic data analysis, some bacteria have both high- and low-affinity terminal oxidase genes, and the high-affinity terminal oxidases may enable these bacteria to maintain high levels of respiratory activity down to low-nanomolar levels of O2 (17, 18). It has, however, been difficult to measure the O2 concentration when it is present at such low concentrations, and the few investigations of O2 at the nanomolar level that have been performed have been done by spectroscopy of oxygenated/deoxygenated leghemoglobins or myoglobins, yielding information about changes in O2 levels only over a very limited range of O2 concentrations without the possibility of investigating the change in kinetics after exposure to conditions with low O2 concentrations (23). Little attention has thus been paid to the kinetics of microbial respiration in marine environments, where low-oxygen conditions prevail in many areas, such as in OMZs. The respiratory activities in north and south eastern tropical Pacific OMZs were found to change with depth, and even the same water sample showed changes in activity under different initial conditions of O2 incubation, which indicates a fast adaptation of the microbial community to changing environmental conditions (9).
In this study, we investigated the respiratory kinetics of pure cultures of marine bacteria to get insight into the basic mechanisms of respiration under low-O2 conditions. The use of pure cultures of marine bacteria provides information on respiration under low-O2 conditions better than that which we can obtain by analyzing heterogeneous marine communities. The change in bacterial respiratory activity was thus examined by incubation of four pure cultures of marine bacteria known to have both high- and low-affinity terminal oxidases in the presence of low O2 concentrations. The incubations were performed both with an ample supply of electron donors and under starvation conditions. The respiration rates were determined in all-glass setups to avoid contamination with O2, and the concentrations of O2 were determined with novel highly sensitive optodes that can resolve nanomolar concentrations over the range of 1 to 1,000 nmol liter−1 (24). The setup enabled us to take samples over the period of incubation to evaluate cell numbers by flow cytometry and cell size by fluorescence microscopy.
MATERIALS AND METHODS
Bacterial culture preparation.
Four strains of bacteria (Dinoroseobacter shibae DSM-16493, Roseobacter denitrificans DSM-7001, Idiomarina loihiensis DSM-15497, and Marinobacter daepoensis DSM-16072) were purchased from the Leibniz Institute, DSMZ, Germany. Bacteria were grown at 25°C on autoclaved marine broth medium containing 5.00 g peptone (BD), 1.00 g yeast extract (Difco), 0.10 g FeC6H5O7, 12.60 g MgCl2·6H2O, 3.24 g Na2SO4, 19.45 g NaCl, 2.38 g CaCl2·2H2O, 0.55 g KCl, 0.16 g NaHCO3, and 0.01 g Na2HPO4·2H2O per liter. Cultures were incubated on a rotary shaker (180 rpm) to ensure fully oxic conditions and to obtain cell suspensions in exponential growth phase.
Setup and incubation.
The Lumos optode optoelectronics device (24) was held by a polyvinyl chloride holder, which was glued on the outside of the bottle. The photodetector was aligned with the preglued glass dot, which was coated with a thin layer of the fluorophore [palladium(II) 5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorphenyl)-porphyrin] in Hyflon AD 60 perfluoropolymer (25) (coated glass dots are referred to here as optode dots). The combination of the specific fluorophore and Hyflon AD 60 results in a highly sensitive optode that can measure concentrations ranging from 1 to 1,000 nmol liter−1. The Lumos device was connected to a computer and was controlled by FireSting Logger software (Pyroscience). Data were recorded every 15 s during the incubations.
Artificial seawater was utilized for bacterial incubation and was prepared as described previously (26). It consisted of 24.99 g NaCl, 4.16 g Na2SO4, 0.79 g KCl, 0.18 g NaHCO3, and individually autoclaved 11.13 g MgCl2·6H2O and 1.58 g CaCl2·2H2O per liter. The pH was adjusted to 7.5 after the different components of the seawater were mixed.
The details of the setup (Fig. 1) and the procedure conducted have been described by Tiano et al. (22). Briefly, seawater was bubbled with N2 for 1 h to remove the dissolved O2 without dramatically changing the pH. Modified bottles with preglued optode dots inside and a glass-coated magnet in the bottom were filled by use of a Tygon and glass tube siphon connected to the reservoir. The reservoir and the gas phase of the bottles were continuously flushed with N2. All bottles, glass-coated magnets, and tubes had previously been cleaned with 0.1 M HCl and subsequently washed with autoclaved water to minimize bacterial contamination. The bottles (volume, 1,160 ml) had a thin (internal diameter, 0.25 cm) and long (length, 25 cm) glass tube, hereafter named the pressure compensation tube, penetrating the glass wall at the periphery of the bottle to allow a change in volume caused by temperature variations and to enable addition and removal of liquid during the incubations.
FIG 1.
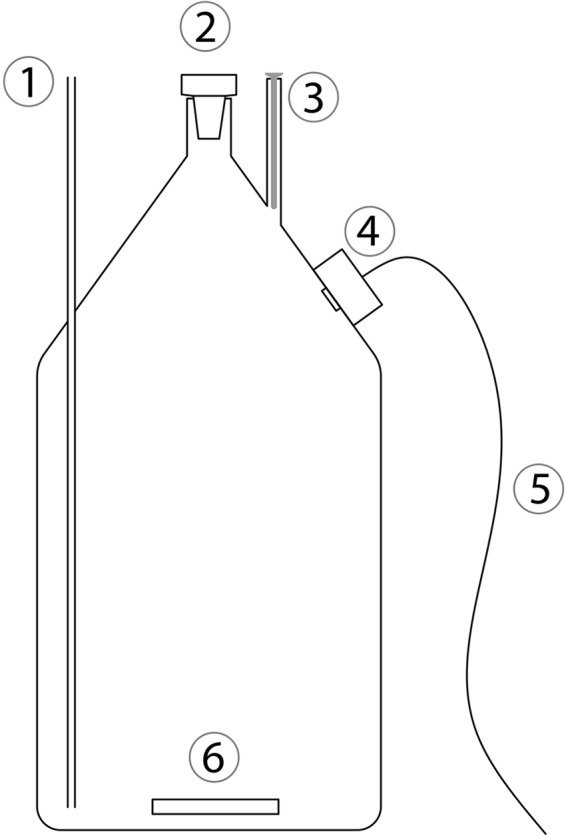
Schematic drawing of the bottle used for incubation. 1, pressure compensation tube; 2, ground-glass stopper; 3, glass tube with an internal diameter of 8.1 mm for insertion of an 8-mm-diameter STOX sensor (for these experiments, a STOX dummy was inserted); 4, optode with optoelectronics; 5, wire for connection to a personal computer for data collection; 6, glass-coated magnet.
During the incubations, the bottles were kept in a water bath kept at a constant temperature of 25°C. Before injection of the bacteria, the bottles with seawater were kept in the water bath for 30 min to reach temperature equilibrium. The incubations lasted for 30 to 40 h.
In order to estimate the growth phase of the bacteria in the inoculum, the optical density at 600 nm was measured with a spectrophotometer (Pharmacia Biotech). The culture was adjusted to the proper concentration with marine broth medium, and a small amount of culture was injected into the incubation bottles to ensure a suitable respiration rate. Data for dilutions and injection volumes are shown in Table 1. One milliliter of air-saturated water was repeatedly injected into the bottles (corresponding to an increase in the O2 concentration of approximately 300 nmol liter−1) when the optode reading indicated anoxia. A long syringe needle that could penetrate the whole pressure compensation tube was used for the injections (and also for the removal of samples). The injection of air-saturated water also served for calibration of the sensor. Samples of 1 ml liquid for microscopic and flow cytometry analysis were taken through the pressure compensation tube every 5 h during the incubation, while 1 ml of oxic water was simultaneously injected. The water sample was fixed with 1% glutaraldehyde (Sigma) at room temperature for 10 min, shock frozen in liquid nitrogen, and stored in a −80°C freezer until further analysis.
TABLE 1.
OD600, dilution before injection, and volume of injection into the reactors for M. daepoensis, I. loihiensis, D. shibae, and R. denitrificans
| Species | Limitationa | OD600b | Dilution (fold) | Injection vol (ml) |
|---|---|---|---|---|
| M. daepoensis | No | 0.090 | 6 | 1 |
| Yes | 0.566 | 5 | 0.4 | |
| I. loihiensis | No | 0.137 | 9 | 1 |
| Yes | 1.104 | 5 | 0.4 | |
| R. denitrificans | No | 1.049 | 7 | 0.4 |
| Yes | 1.244 | 2 | 0.4 | |
| D. shibae | No | 0.235 | 5 | 1 |
| Yes | 0.397 | 1.2 | 0.4 |
One of the incubations for each culture was designed to cause severe electron donor limitation and is indicated by “Yes.”
Data represent the mean values from three experiments.
Determination of cell numbers by flow cytometry.
Bacterial numbers were determined using a NovoCyte flow cytometer (ACEA Biosciences) equipped with a 488-nm laser and a 530-nm filter. Subsamples (100 μl) were stained with 1 μl of an 8×-diluted solution of SYTO 13 (Molecular Probes) for 15 min prior to the counting of the number of cells. The abundance of bacteria was determined by counting the bacteria contained in a fixed sample volume of 25 μl. The number of bacteria in a region in a scatterplot of the SYTO 13 fluorescence signal versus the side scatter that was used for counting was determined by comparison with the number of bacteria in a sample that was not stained.
Measurement of cell size by fluorescence microscopy.
One hundred microliters of fixed sample was filtered onto 0.2-μm-pore-size polycarbonate filters (Maine Manufacturing LLC). The dried samples were stained with 1 drop of prepared SYBR green (1 μl of SYBR green I nucleic acid gel stain [Thermo Fisher Scientific] mixed with 800 μl of glycerol–phosphate-buffered saline [AF1; CitiFluor Ltd.] and 200 μl of Vectashield mounting medium [Vector Laboratories]). Cell sizes were measured using an Axiovert 200M epifluorescence microscope with standard SYBR green and fluorescein filter sets (Zeiss). At least 400 cells per sample were measured. It is difficult to measure an accurate size by fluorescence, but it is possible to observe relative changes in size.
Data analysis and modeling of kinetic parameters and population size.
Two kinetic parameters, the respiration rate of the population and the half-saturation constant (apparent Km), were estimated by use of a modified Jassby and Platt equation as described by Tiano et al. (22). The cell numbers between the two measurement time points were estimated by linear interpolation. The respiration rate per cell was calculated by dividing the respiration rate of the population by the cell numbers.
Statistical analysis and graphing.
To determine if there was a significant difference in the apparent Km values determined during the incubation, the apparent Km values obtained over time were tested by the Tukey test in a one-way repeated-measures analysis of variance (ANOVA). If the normality test (Shapiro-Wilk) or equal variance test failed, the data were transformed by use of a log(x + 1) transformation. The Friedman test was used if the transformed data still failed the normality test or the equal variance test. Differences in doubling times and cell sizes during the incubations were tested by t test. The significance value was set at 0.05 for multiple comparisons in post hoc tests. All the data were plotted by the SigmaPlot (version 12.0) program to construct the figures.
RESULTS
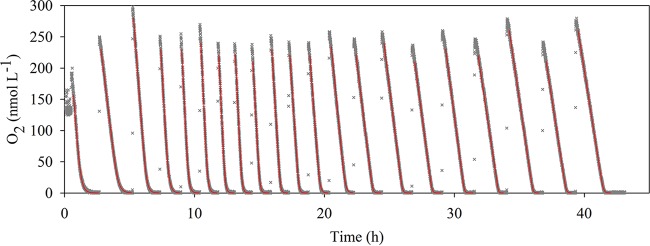
As the setup applied in this study allowed us to measure O2 concentrations over time, it was possible to monitor and analyze the respiratory activity with a high degree of temporal resolution (Fig. 2). The initial O2 concentration during the initial incubation was about 200 nmol liter−1. Subsequent injections of aerated water increased the O2 concentration to about 300 nmol liter−1 right after the injections (Fig. 2).
FIG 2.
Time course of the oxygen concentration during 42 h of incubation of R. denitrificans with artificial seawater under energy-limited conditions. During the incubation, 1 ml of air-saturated water was injected into the bottle through the pressure compensation tube when oxygen was completely consumed. Gray crosses, the data points measured every 15 s (most are hidden by the red curves); red curves, the modeled curves used for extraction of the kinetic parameters.
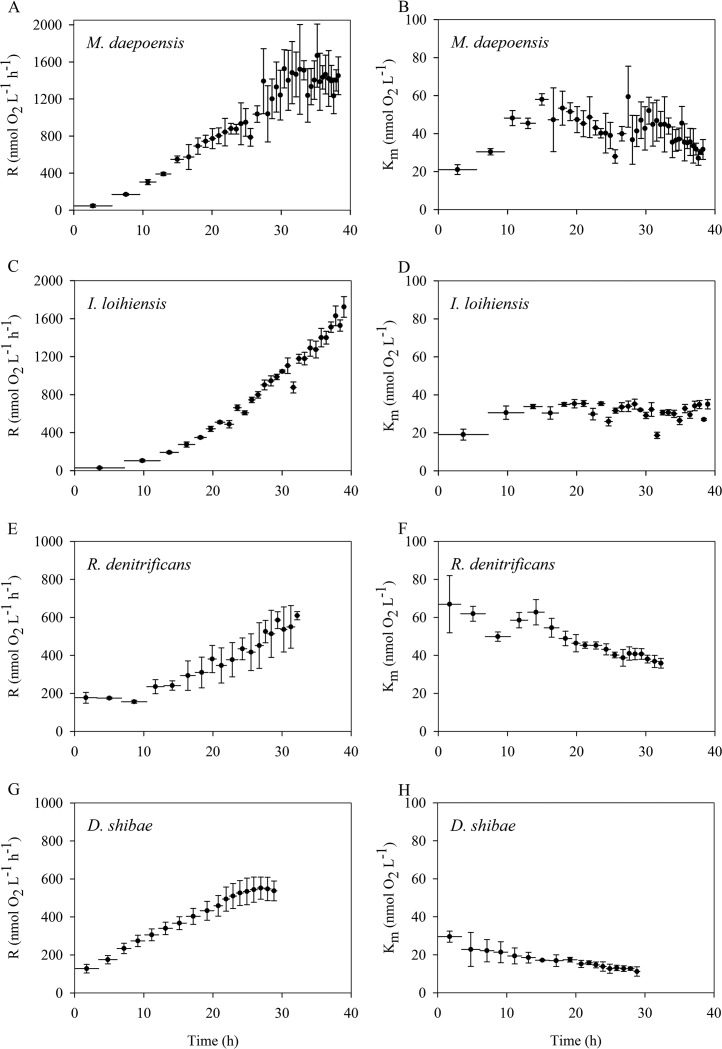
The estimated apparent Km values and respiration rates of the population during the incubations are plotted in Fig. 3 and Fig. 4. The respiration rate of the population during the first type of incubation progressively increased during the whole period of measurement, and therefore, that type of incubation could be considered to have taken place under non-energy-limited (i.e., non-electron-donor-limited) conditions (Fig. 3). The initial respiration rates of the M. daepoensis and I. loihiensis populations (46 ± 15 and 29 ± 8 nmol O2 liter−1 h−1, respectively) were lower than those of the R. denitrificans and D. shibae populations (177 ± 28 and 128 ± 23 nmol O2 liter−1 h−1, respectively). However, the final respiration rates of the populations during the incubation were 1,450 ± 203, 1,724 ± 108, 609 ± 22, and 537 ± 52 nmol O2 liter−1 h−1 for M. daepoensis, I. loihiensis, R. denitrificans, and D. shibae, respectively. These increased respiration rates for the populations indicate growth of the bacteria, and M. daepoensis and I. loihiensis especially exhibited high growth rates. The apparent Km value for I. loihiensis was about 30 nmol liter−1. The apparent Km value decreased from 66 ± 15 to 35 ± 2 nmol liter−1 and from 29 ± 3 to 11 ± 2 nmol liter−1 for R. denitrificans, and D. shibae, respectively. The apparent Km value for M. daepoensis increased from about 27 ± 3 to 45 ± 3 mol liter−1 within the first 10 h and was subsequently stable for the next 20 h and then decreased to about 30 ± 1 nmol liter−1 after a total of 38 h of incubation. It is evident that after approximately 2 to 3 h the first data points for Km values deviated somewhat from the other data points, indicating adaptation to new environmental conditions.
FIG 3.
Time course of the respiration rates (R) of the population (A, C, E, and G) and apparent Km values (B, D, F, and H) for four species, M. daepoensis (A and B), I. loihiensis (C and D), R. denitrificans (E and F), and D. shibae (G and H), during incubation under non-energy-limited conditions. Values are means ± SDs (n = 3 or 4). Horizontal bars represent time periods. Note the differences in the y-axis scales in the left panels (A, C, E, and G).
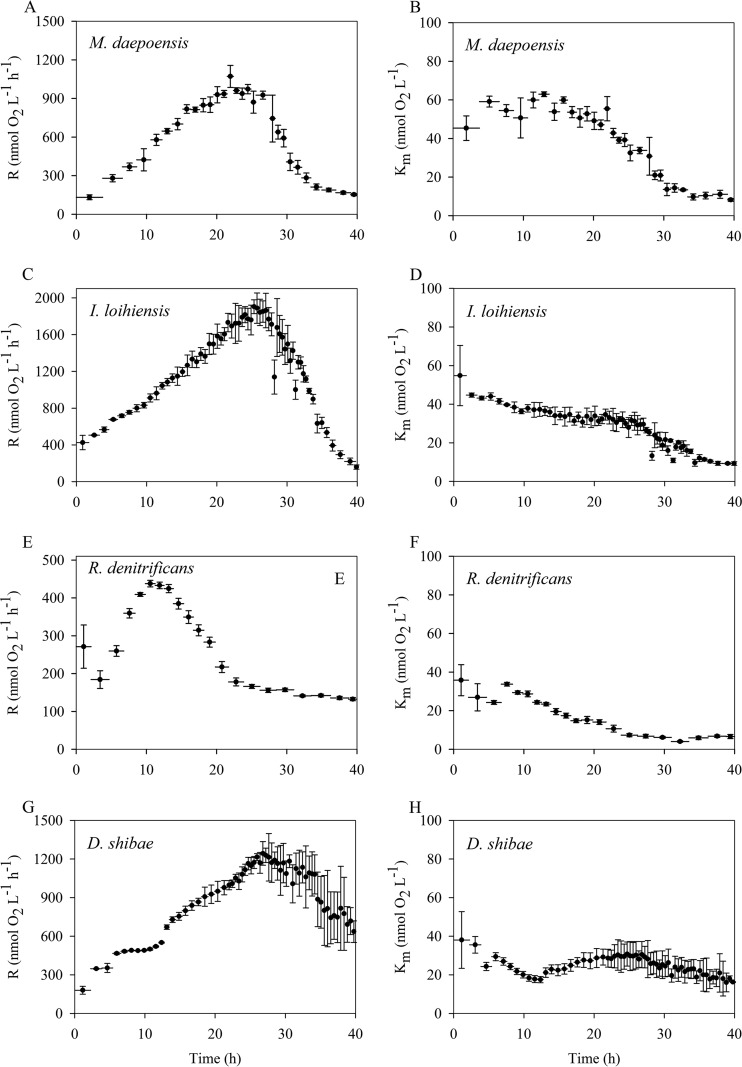
FIG 4.
Time course of the respiration rates (R) of the population (A, C, E, and G) and apparent Km values (B, D, F, and H) for four species, M. daepoensis (A and B), I. loihiensis (C and D), R. denitrificans (E and F), and D. shibae (G and H), during incubation over a period characterized by energy-limited conditions. Values are means ± SDs (n = 3 to 4). The horizontal bars represent time periods. Note the differences in the y-axis scales in the left panels (A, C, E, and G).
The second type of incubation was with injections of larger amounts of bacteria and smaller amounts of medium. With increases in the incubation period and the growth of the population, the respiration rate of the population increased for a period of time and then decreased dramatically (Fig. 4). This incubation was thus considered to have taken place under energy-limited conditions. The maximum values of the respiration rates of the populations during the incubation were 1,071 ± 85, 1,903 ± 75, 438 ± 9, and 1,243 ± 92 O2 nmol liter−1 h−1 for M. daepoensis, I. loihiensis, R. denitrificans, and D. shibae, respectively. The initial apparent Km values ranged from 35 ± 8 to 55 ± 16 nmol liter−1, and the Km values for all four species became stable when the respiration rates of the population reached the maximum. The apparent Km values were thus 49 ± 4 nmol liter−1 at 20 h for M. daepoensis, 32 ± 4 nmol liter−1 at 24 h for I. loihiensis, 29 ± 1 nmol liter−1 at 10 h for R. denitrificans, and 29 ± 7 nmol liter−1 at 25 h for D. shibae. The apparent Km values subsequently decreased and the respiration rates of the populations decreased during starvation, and the Km values gradually reached final low levels that were slightly different for the different cultures. The final stable Km values were 8 ± 1, 9 ± 1, 7 ± 1, and 16 ± 1 nmol liter−1 for M. daepoensis, I. loihiensis, R. denitrificans, and D. shibae, respectively. The D. shibae culture did not, however, exhibit the same large decrease in apparent Km value that the other cultures did, probably because the incubation time was insufficient for it to reach low rates of respiration per cell.
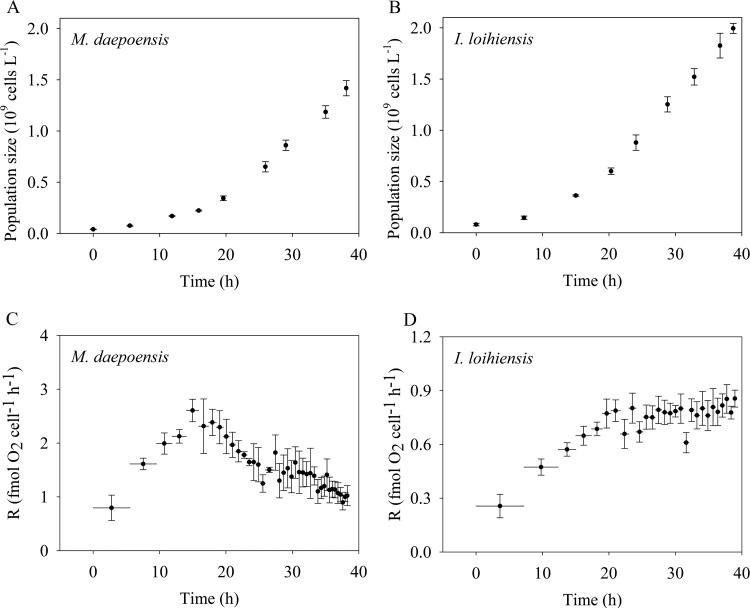
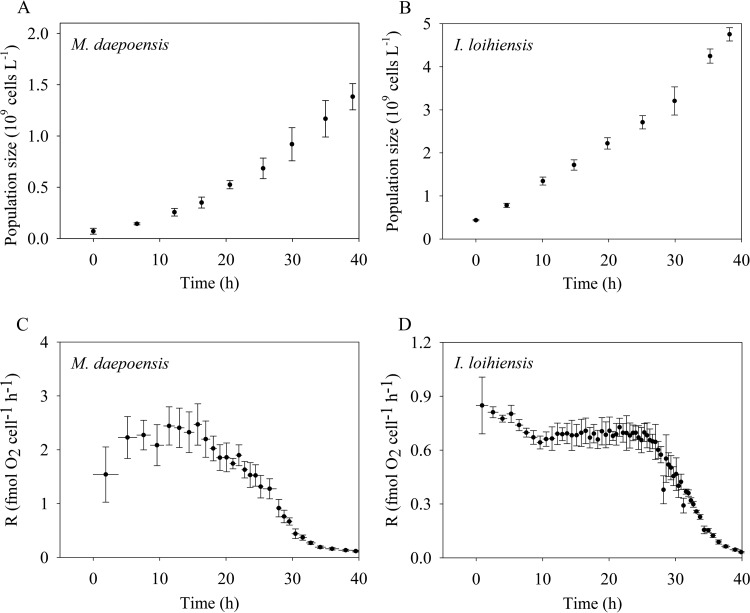
The population sizes and respiration rate per cell of M. daepoensis and I. loihiensis were tracked during incubation under non-energy-limited conditions (Fig. 5) and under energy-limited conditions (Fig. 6). The population sizes increased for all four incubations, and the cultures grew in terms of cell number (but not total biovolume; see below) only slightly faster under non-energy-limited conditions than under energy-limited conditions. During the incubation under non-energy-limited condition (Fig. 5), the respiration rate of M. daepoensis increased from 0.80 ± 0.24 to 2.61 ± 0.21 fmol O2 cell−1 h−1 at about 15 h and then decreased to 1.00 ± 0.09 fmol O2 cell−1 h−1 at the end of the incubation; the respiration rate of I. loihiensis increased from 0.26 ± 0.07 to 0.80 ± 0.08 fmol O2 cell−1 h−1 at about 23 h, and the respiration rate stayed at this level during the rest of the incubation. The low respiration rates obtained from the first determinations indicated a lag phase before maximum growth under the new culture conditions. During the incubation under energy-limited condition (Fig. 6), the respiration rate of M. daepoensis increased from 1.54 ± 0.51 to 2.44 ± 0.35 fmol O2 cell−1 h−1 at about 11 h and then decreased to a very low rate (0.12 fmol O2 cell−1 h−1) at the end of the incubation; the respiration rate of I. loihiensis decreased from 0.85 ± 0.16 to 0.70 ± 0.02 fmol O2 cell−1 h−1 at about 7 h and then decreased to a very low rate (0.03 ± 0.004 fmol O2 cell−1 h−1) at the end of the incubation.
FIG 5.
Time courses of population size (A and B) and the respiration rate per cell (R) (C and D) of M. daepoensis and I. loihiensis during incubation under non-energy-limited conditions. Values are means ± SDs (n = 3 to 4). The horizontal bars (C and D) represent time periods. Note the differences in the y-axis scales in the bottom panels (C and D).
FIG 6.
Time courses of population size (A and B) and the respiration rate per cell (R) (C and D) of M. daepoensis and I. loihiensis during incubation under energy-limited conditions. Values are means ± SDs (n = 3 to 4). The horizontal bars (C and D) represent time periods. Note the differences in the y-axis scales in the bottom panels (C and D).
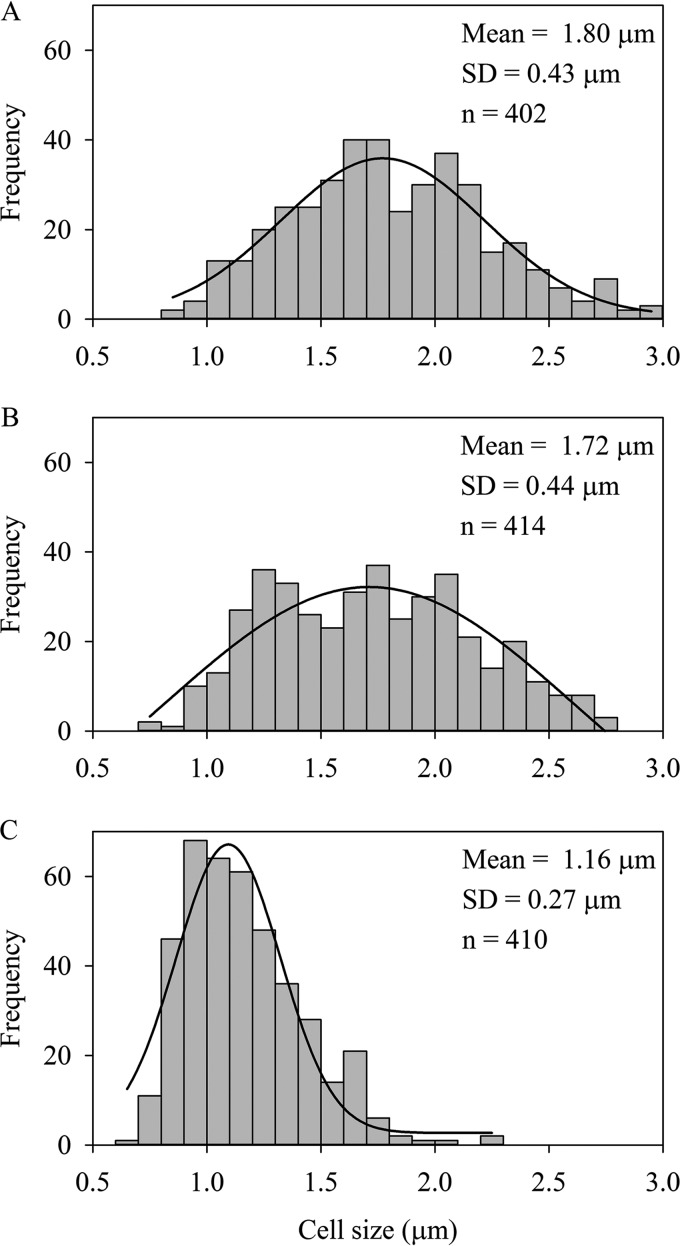
The cell sizes of M. daepoensis during the incubation under energy-limited condition were measured under a fluorescence microscope with SYBR green staining. The lengths of the cells (Fig. 7) decreased significantly (P < 0.05, t test), due to starvation, from 1.80 ± 0.43 μm at the beginning of the incubation to 1.72 ± 0.44 μm at 12.6 h during the incubation and further decreased to 1.16 ± 0.27 μm at the end of the incubation. Although the cell number increased during incubation under energy-limited conditions, this did not correspond to a proportional increase in the total biovolume.
FIG 7.
Cell size distribution of M. daepoensis at 0 h (A), 12.6 h (B), and 39.2 h (C) during incubation under energy-limited conditions.
DISCUSSION
The study of O2 kinetics at low O2 concentrations requires sensors with high degrees of accuracy at low O2 concentrations, an incubation vessel that does not leak O2 into the system, and a homogeneous culture without aggregate formation. The setup applied in this experiment was tested in a previous study (22). The long pressure compensation tube is the only entry for O2 exchange in this glass-sealed system. It was proven both by mathematical calculation and with experimental data (22) that the O2 exchange between the incubation vessel and the environment is negligible. The glass-coated magnet in the bottle ensures homogeneity after injections of bacteria or O2-saturated water. During this experiment, two bottles into which bacteria were not injected were used as controls in each run. The results from these noninoculated bottles also confirmed that there was no external O2 contamination (data not shown).
During our incubations, the cells were exposed to multiple oxic/anoxic shifts. The effects of a change from anoxic to oxic conditions have been investigated in detail for D. shibae, and it was concluded that the energy charge of the cell was back to normal oxic values within less than a minute (27). We assume that the same also held true for the other three organisms and that our measurements of energy metabolism, expressed by the rates of O2 consumption during about 10-min periods of oxic conditions, represent short-term steady-state values (not taking the O2 concentration dependence into account). The prolonged exposure of cultures to anoxia may lead to significant physiological changes (28), and we thus avoided long anoxic periods.
On the basis of an analysis of genomics data, the genomes of all four investigated species have the genes for both high-affinity terminal oxidases and low-affinity terminal oxidases. Thus, they all have the potential to have low apparent Km values by expressing the high-affinity terminal oxidases. During the incubation under non-energy-limited, low-oxygen conditions, the slight decrease in the apparent Km values for R. denitrificans and D. shibae may indicate the expression of a high-affinity terminal oxidase. Considering that the Km value of low-affinity terminal oxidases is about 200 nmol liter−1 (18) and that of high-affinity terminal oxidases is 3 to 8 nmol liter−1 (17), the estimated apparent Km values for M. daepoensis (about 43 nmol liter−1) and I. loihiensis (about 31 nmol liter−1) demonstrate that large portions of the terminal oxidases were the high-affinity ones. A gene fusion study with Escherichia coli (29) showed that genes for high-affinity terminal oxidases are expressed under high-O2 conditions, and even cultures incubated under high-O2 conditions may thus exhibit relatively low apparent Km values. M. daepoensis and I. loihiensis could have lowered their relatively high apparent Km values during the incubation under non-energy-limited condition by decreasing their cell size, but cell size may be governed more by nutrient availability than by O2 concentration. The only source of organic electron donors in our incubations was the organic matter remaining in the culture medium from the injection and internal storage, for example, as polyhydroxybutyric acid. Thus, the electron donors became limiting during the incubations with large amounts of bacteria and small amounts of medium. The population size continued to increase during the periods when the respiration rate of the populations decreased due to starvation. The respiration rate per cell thus decreased dramatically. Meanwhile, it was accompanied by decreasing apparent Km values, suggesting that the apparent Km value was correlated with the availability of an electron donor.
In the following, the integrated effect of O2 gradients around the cell, the difference in cell size, and the difference in respiration rate on the observed apparent Km value are illustrated by modeling. For the convenience of modeling, the cells were assumed to be spherical but to have the same volume as the actual rod-shaped cells. We modeled the following three parameters. (i) The maximum O2 flux to a cell with zero concentration at the cell surface (Jmax) is given by (28)
| (1) |
where rc is the radius of the cell; D is the diffusion coefficient in water, which has a value of 2.40 × 10−5 cm2 s−1 at 25°C; and C∞ is the surrounding bulk O2concentration.
(ii) The theoretical maximum respiration rate without diffusion limitation (Rmax) can be described by the Michaelis-Menten equation:
| (2) |
where Km is the enzyme half-saturation concentration and Vmax is the maximum respiration rate per cell at nonlimiting high O2 concentrations.
(iii) The real respiration rate per cell (Rc) when diffusion limitation is taken into account is given by
| (3) |
where C0 is the O2 concentration at the cell surface, where the terminal oxidase is located.
The flux of O2 to the cell (J) is defined by (30)
| (4) |
When the cell is in a steady state, the processes are balanced and the O2 flux to the cell and the respiration rate per cell are equal:
| (5) |
Substitution from equations 3 and 4 gives
| (6) |
C0 can then be calculated with known values of Vmax, Km, rc, and C∞. Thus, Rc can be calculated in return as a function of C∞.
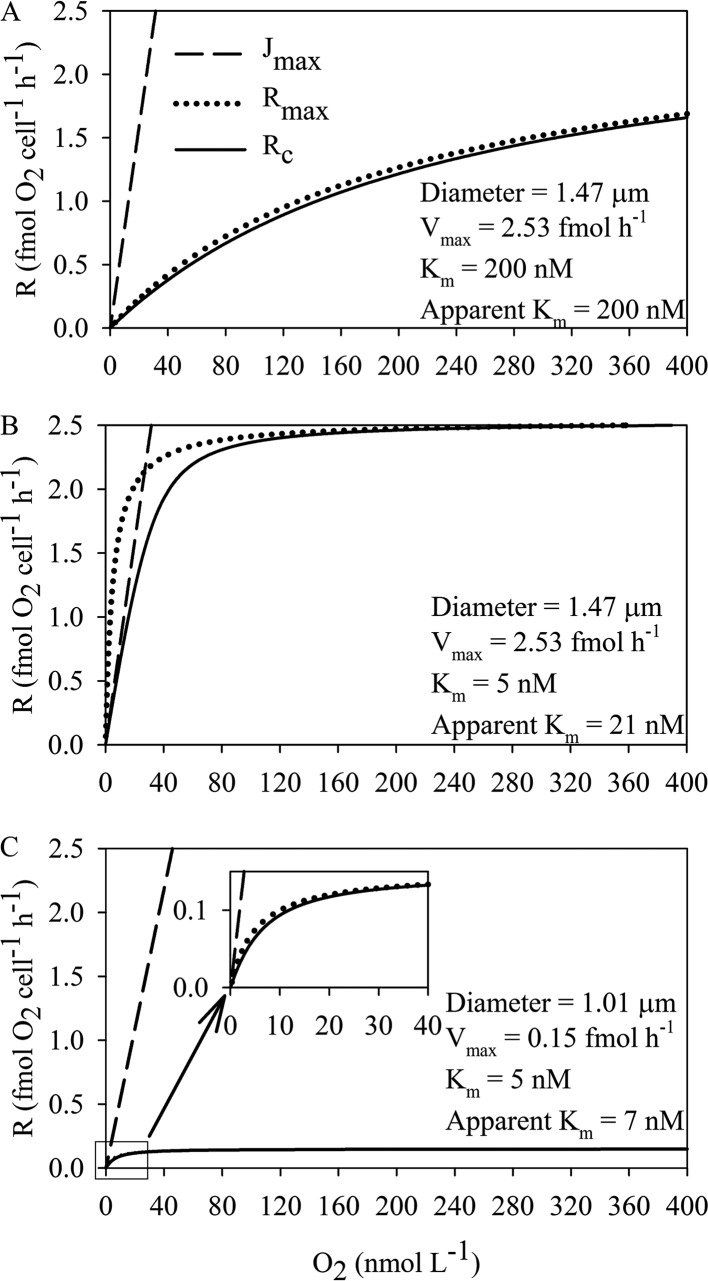
In order to explain the model, we discuss here the data for M. daepoensis assuming two values for respiration under saturating O2 concentrations (Vmax), two different cell sizes, and two different Km values. The large cell representative of the cell size under nonlimited conditions has an equivalent diameter of 1.47 μm, and the small cell representative of the cell size under conditions of starvation has an equivalent diameter of 1.01 μm. The diameters of cells of both sizes were recalculated from values in the literature (31) equating the diameters of cells with a rod shape to the diameters of spherical cells of the same volume. In the example, the large cell is assumed to be from the nonlimited condition with a high Vmax (2.53 fmol O2 cell−1 h−1) and the small cell has a low Vmax (0.15 fmol O2 cell−1 h−1). The actual respiration rate per cell by consideration of both diffusion limitation and Michaelis-Menten enzymatic kinetics (Rc), the maximum O2 flux to the cell under the assumption that the O2 concentration at the cell surface is 0 (Jmax), and the theoretical maximum respiration rate per cell under the assumption that there is no diffusion limitation (Rmax) were compared under three scenarios. (i) When the energy and O2 supplies are sufficient, the cell size of M. daepoensis is assumed to be large, the respiration rate per cell is high, and only low-affinity terminal oxidases are expressed (Km value, 200 nmol liter−1) (Fig. 8A). In that case, the actual respiration rate is close to the theoretical maximum respiration rate, if no diffusion limitation at all O2 concentrations is assumed, and the apparent Km is identical to the enzyme Km. Due to the high Km value, both calculated respiration rates are much lower than Jmax. (ii) In the second example, we consider large M. daepoensis cells having the same Vmax of 2.53 fmol O2 cell−1 h−1, but now we assume that only high-affinity terminal oxidases are present (Km value, 5 nmol liter−1) (Fig. 8B). In this case, the theoretical maximum respiration rate of the cell is limited by diffusion. When both diffusion limitation and the enzyme Km (21 nmol liter−1) are taken into account, the apparent Km value obtained from the curve is much higher than the enzymatic Km value (5 nmol liter−1). We observed an even higher apparent Km value of 43 nmol liter−1 under nonlimited conditions, indicating that our geometric assumptions were oversimplified. Under these conditions, Rc values are close to Jmax values at the lowest O2 concentrations. (iii) When the environment is low in electron donors, the decrease in cell size and low respiration rates enabled the actual respiration rate to get very close to the theoretical non-diffusion-limited respiration rate (Fig. 8C). The apparent Km value (7 nmol liter−1) was similar to the values measured experimentally and approached the enzymatic Km value, and Vmax was thus approached at very low O2 concentrations. It is thus an advantage for the bacterium to decrease its cell size under conditions of limitation of O2 (or any other type of element) (32). Marine bacteria thus decrease their cell volume when nutrients are present at low concentrations (33, 34).
FIG 8.
Modeled curves of O2 consumption (R) of hypothetical spherical cells otherwise having characteristics similar to those of the M. daepoensis cells investigated under the following assumptions. (i) The cell consumes all oxygen diffusing to the cell so that the O2 concentration at the cell surface is zero (Jmax). (ii) The cell terminal oxidases exhibit Michaelis-Menten kinetics, but there is no diffusion limitation (Rmax). (iii) The O2 consumption by the cell is limited by both enzyme kinetics and diffusion to the cell (Rc). The modeling was performed under three scenarios: big cells (diameter, 1.47 μm) with a high respiration rate (2.53 fmol O2 cell−1 h−1) under nonlimiting O2 concentrations (Vmax) and only a low-affinity terminal oxidase (Km value, 200 nmol liter−1) (A), big cells with a high respiration rate and only a high-affinity terminal oxidase (Km value, 5 nmol liter−1) (B), and small cells (diameter, 1.01 μm) with a low respiration rate (0.20 fmol O2 cell−1 h−1) and only a high-affinity terminal oxidase (C).
Km values measured by different methods were summarized in a previous study (23). Until 1994, studies showed that the Km values measured by an O2 electrode are higher than the Km values estimated from the deoxygenation kinetics of myoglobin or leghemoglobin. That might probably indicate that the latter method has a resolution higher than that achieved with standard Clark-type O2 electrodes. Furthermore, the apparent Km values for E. coli in exponential phase determined with a sensitive O2 electrode were found to be 1.80 μmol liter−1 (20) and 0.19 μmol liter−1 (18) in two studies. This 1-order-of-magnitude difference in these two apparent Km values suggests that the sensitivity of the electrode affects the determination of the apparent Km value and that the estimates are nonrealistic. The apparent Km values determined even by our extremely sensitive O2 microsensors with a fast response were at least twice the values estimated by optodes for the four species investigated in this experiment (data not shown). The technique relying on the spectrophotometric differences between the oxygenated and deoxygenated state of myoglobin or leghemoglobin was applied more often than the technique with O2 electrodes after the 1980s to determine Km values for different types of terminal oxidases or whole cells of E. coli (17, 35), pathogens (36), and soil bacteria (23, 37). Previously reported Km values obtained by this technique were also in the nanomolar range. However, the reported Km values determined by the use of leghemoglobin and myoglobin can have a 1-order-of-magnitude difference in the same study (35, 36). Furthermore, it has been observed that the apparent Km values measured with intact cells (Azotobacter vinelandii) which contain a cytochrome bd-type oxidase (a typical high-affinity terminal oxidase) are 4-fold higher than those measured in membranes (23). High apparent Km values indicate a diffusion limitation of the O2 supply. In addition, a previous study showed that the level of expression of the gene encoding the low-affinity terminal oxidase is repressed over 140-fold under anaerobic conditions in E. coli but is still detectable (29). Thus, some low-affinity terminal oxidases may still be functioning even in environments with extremely low O2 concentration, which could contribute to relatively high apparent Km values. The values estimated in our study even surpass the bottom of the range of apparent Km values for intact cells reported previously, and we thus assume that the set of techniques used is more sensitive than previously used methods.
In comparison with previous studies related to respiration kinetics, the advantages of this study are as follows. (i) The setup used in this study enables the continuous recording of the O2 concentration. Thus, it is able to resolve temporal change in kinetics. Previous studies focused on the respiration of bacteria and the apparent Km value of the bacteria during some kind of supposed steady state. It has been recorded that apparent Km values for E. coli in early exponential phase and stationary phase have almost a 1-order-of-magnitude difference (1.80 and 0.27 μmol liter−1, respectively) in the same study (20). We know, however, that the apparent Km value of bacteria must be related to the range of O2 concentrations during the incubation, as a certain transition concentration triggers the expression of high-affinity terminal oxidases (38). However, the change of kinetics for the same types of cells over time is apparently neglected. The myoglobin- or leghemoglobin-based method resolves only the apparent Km values for one state of the cells rather than a temporal change. This study reveals that the apparent Km values decrease over time under conditions with low O2 concentrations and a limited electron donor supply. (ii) The system is completely sealed by glass, except for the pressure compensation tube, which is used for the injection of bacteria and water, and this creates a system that is more isolated from the environment than previous setups. The myoglobin- or leghemoglobin-based method requires a stopper with a fine hole to seal the cuvette (23). Ambient O2 can easily pass through the hole and the gap between the stopper and the cuvette to contaminate the system. Furthermore, our setup can be applied to all types of aquatic media.
The optical trace O2 sensor allows the measurement of respiration rates under conditions with low O2 levels with a high sensitivity and an extremely low detection limit. The apparent Km values estimated from this study are lower than those previously reported in studies of isolated marine bacteria in culture (39) and seawater (22). The conditions of our incubations with energy limitation are closer to in situ ocean conditions than traditional incubations in rich media. As a consequence of starvation, the reduced respiration rate per cell and the reduced cell size decreased the diffusion limitation and resulted in lower apparent Km values, illustrating that even individual fast-respiring bacteria are diffusion limited at very low O2 concentrations. The same holds true for all other substances present in low concentrations.
ACKNOWLEDGMENTS
We gratefully acknowledge Preben G. Sørensen and Lars B. Pedersen for their technical assistance. We thank glass blower J. C. Kondrup at Aarhus University for providing the incubation reactors. We acknowledge Yao Chen for helping with illustrations. The FACS Core facility at Aarhus University provided access to flow cytometer equipment.
This study was supported by the European Research Council (grant no. 2672333).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Canfield DE, Kristensen E, Thamdrup B. 2005. Aquatic geomicrobiology. Advances in marine biology, vol 48 Elsevier Academic Press, San Diego, CA. [DOI] [PubMed] [Google Scholar]
- 2.Kühl M. 2005. Optical microsensors for analysis of microbial communities. Methods Enzymol 397:166–199. doi: 10.1016/S0076-6879(05)97010-9. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen LP, Christensen PB, Revsbech NP, Sørensen J. 1990. Denitrification and oxygen respiration in biofilms studied with a microsensor for nitrous oxide and oxygen. Microb Ecol 19:63–72. doi: 10.1007/BF02015054. [DOI] [PubMed] [Google Scholar]
- 4.Allee WC, Oesting R. 1934. A critical examination of Winkler's method for determining dissolved oxygen in respiration studies with aquatic animals. Physiol Zool 7:509–541. [Google Scholar]
- 5.Williams PJL. 1984. A review of measurements of respiration rates of marine plankton populations, p 357–389. In Hobbie JE, Williams PJL (ed), Heterotrophic activity in the sea. Springer USA, Boston, MA. [Google Scholar]
- 6.Martínez-García S, Karl DM. 2015. Microbial respiration in the euphotic zone at Station ALOHA. Limnol Oceanogr 60:1039–1050. doi: 10.1002/lno.10072. [DOI] [Google Scholar]
- 7.Kirchman DL. 1997. Carbon cycle: microbial breathing lessons. Nature 385:121–122. doi: 10.1038/385121a0. [DOI] [Google Scholar]
- 8.Falkowski PG, Algeo T, Codispoti L, Deutsch C, Emerson S, Hales B, Huey RB, Jenkins WJ, Kump LR, Levin LA, Lyons TW, Nelson NB, Schofield OS, Summons R, Talley LD, Thomas E, Whitney F, Pilcher CB. 2011. Ocean deoxygenation: past, present, and future. Eos Trans Am Geophys Union 92:409. doi: 10.1029/2011EO460001. [DOI] [Google Scholar]
- 9.Tiano L, Garcia-Robledo E, Dalsgaard T, Devol A, Ward BB, Ulloa O, Canfield DE, Revsbech NP. 2014. Oxygen distribution and aerobic respiration in the north and south eastern tropical Pacific oxygen minimum zones. Deep Sea Res Part I Oceanogr Res Pap 94:173–183. doi: 10.1016/j.dsr.2014.10.001. [DOI] [Google Scholar]
- 10.Wikner J, Panigrahi S, Nydahl A, Lundberg E, Bamstedt U, Tengberg A. 2013. Precise continuous measurements of pelagic respiration in coastal waters with oxygen optodes. Limnol Oceanogr Methods 11:1–15. doi: 10.4319/lom.2013.11.1. [DOI] [Google Scholar]
- 11.Kalvelage T, Lavik G, Jensen MM, Revsbech NP, Löscher C, Schunck H, Desai DK, Hauss H, Kiko R, Holtappels M, LaRoche J, Schmitz RA, Graco MI, Kuypers MMM. 2015. Aerobic microbial respiration in oceanic oxygen minimum zones. PLoS One 10:e0133526. doi: 10.1371/journal.pone.0133526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poole RK. 1983. Bacterial cytochrome oxidases: a structurally and functionally diverse group of electron-transfer protein. Biochim Biophys Acta 726:205–243. doi: 10.1016/0304-4173(83)90006-X. [DOI] [PubMed] [Google Scholar]
- 13.Hemp J, Robinson DE, Ganesan KB, Martinez TJ, Kelleher NL, Gennis RB. 2006. Evolutionary migration of a post-translationally modified active-site residue in the proton-pumping heme-copper oxygen reductases. Biochemistry 45:15405–15410. doi: 10.1021/bi062026u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calhoun MW, Thomas JW, Gennis RB. 1994. The cytochrome oxidase superfamily of redox-driven proton pumps. Trends Biochem Sci 19:325–330. doi: 10.1016/0968-0004(94)90071-X. [DOI] [PubMed] [Google Scholar]
- 15.Pitcher RS, Watmough NJ. 2004. The bacterial cytochrome cbb3 oxidases. Biochim Biophys Acta 1655:388–399. doi: 10.1016/j.bbabio.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Brändén G, Gennis RB, Brzezinski P. 2006. Transmembrane proton translocation by cytochrome c oxidase. Biochim Biophys Acta 1757:1052–1063. doi: 10.1016/j.bbabio.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 17.D'Mello R, Hill S, Poole RK. 1996. The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two oxygen-binding haems: implications for regulation of activity in vivo by oxygen inhibition. Microbiology 142:755–763. doi: 10.1099/00221287-142-4-755. [DOI] [PubMed] [Google Scholar]
- 18.Rice CW, Hempfling WP. 1978. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol 134:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith A, Hill S, Anthony C. 1990. The purification, characterization and role of the d-type cytochrome oxidase of Klebsiella pneumoniae during nitrogen fixation. J Gen Microbiol 136:171–180. doi: 10.1099/00221287-136-1-171. [DOI] [PubMed] [Google Scholar]
- 20.Kita K, Konishi K, Anraku Y. 1984. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J Biol Chem 259:3375–3381. [PubMed] [Google Scholar]
- 21.Appleby CA, Bergersen FJ. 1980. Preparation and experimental use of leghaemoglobin, p 315–335. In Bergersen FJ. (ed), Methods for evaluating biological nitrogen fixation. John Wiley & Sons, Chichester, United Kingdom. [Google Scholar]
- 22.Tiano L, Garcia-Robledo E, Revsbech NP. 2014. A new highly sensitive method to assess respiration rates and kinetics of natural planktonic communities by use of the switchable trace oxygen sensor and reduced oxygen concentrations. PLoS One 9:e105399. doi: 10.1371/journal.pone.0105399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Mello R, Hill S, Poole RK. 1994. Determination of the oxygen affinities of terminal oxidases in Azotobacter vinelandii using the deoxygenation of oxyleghaemoglobin and oxymyoglobin: cytochrome bd is a low-affinity oxidase. Microbiology 140:1395–1402. doi: 10.1099/00221287-140-6-1395. [DOI] [Google Scholar]
- 24.Lehner P, Larndorfer C, Garcia-Robledo E, Larsen M, Borisov SM, Revsbech N-P, Glud RN, Canfield DE, Klimant I. 2015. Lumos—a sensitive and reliable optode system for measuring dissolved oxygen in the nanomolar range. PLoS One 10:e0128125. doi: 10.1371/journal.pone.0128125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borisov SM, Lehner P, Klimant I. 2011. Novel optical trace oxygen sensors based on platinum(II) and palladium(II) complexes with 5,10,15,20-meso-tetrakis-(2,3,4,5,6-pentafluorphenyl)-porphyrin covalently immobilized on silica-gel particles. Anal Chim Acta 690:108–115. doi: 10.1016/j.aca.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 26.Hansen H, Koroleff F. 1999. Determination of nutrients, p 159–224. In Grasshoff K, Kremling K, Ehrhardt M (ed), Methods of seawater analysis. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 27.Holert J, Hahnke S, Cypionka H. 2011. Influence of light and anoxia on chemiosmotic energy conservation in Dinoroseobacter shibae. Environ Microbiol Rep 3:136–141. doi: 10.1111/j.1758-2229.2010.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laass S, Kleist S, Bill N, Drüppel K, Kossmehl S, Wöhlbrand L, Rabus R, Klein J, Rohde M, Bartsch A, Wittmann C, Schmidt-Hohagen K, Tielen P, Jahn D, Schomburg D. 2014. Gene regulatory and metabolic adaptation processes of Dinoroseobacter shibae DFL12T during oxygen depletion. J Biol Chem 289:13219–13231. doi: 10.1074/jbc.M113.545004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cotter PA, Chepuri V, Gennis RB, Gunsalus RP. 1990. Cytochrome o (cyoABCDE) and d (cydAB) oxidase gene expression in Escherichia coli is regulated by oxygen, pH, and the fnr gene product. J Bacteriol 172:6333–6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jørgensen BB. 2001. Life in the diffusive boundary layer, p 348–373. In Boudreau BP, Barker JB (ed), The benthic boundary layer. Oxford University Press, New York, NY. [Google Scholar]
- 31.Yoon J-H, Yeo S-H, Kim I-G, Oh T-K. 2004. Marinobacter flavimaris sp. nov. and Marinobacter daepoensis sp. nov., slightly halophilic organisms isolated from sea water of the Yellow Sea in Korea. Int J Syst Evol Microbiol 54:1799–1803. doi: 10.1099/ijs.0.63151-0. [DOI] [PubMed] [Google Scholar]
- 32.Koch AL. 1971. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol 6:147–217. doi: 10.1016/S0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- 33.Kjelleberg S, Humphrey BA, Marshall KC. 1982. Effect of interfaces on small, starved marine bacteria. Appl Environ Microbiol 43:1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjelleberg S, Hermansson M. 1984. Starvation-induced effects on bacterial surface characteristics. Appl Environ Microbiol 48:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Mello R, Hill S, Poole R. 1995. The oxygen affinity of cytochrome bo′ in Escherichia coli determined by the deoxygenation of oxyleghemoglobin and oxymyoglobin: Km values for oxygen are in the submicromolar range. J Bacteriol 177:867–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wainwright LM, Elvers KT, Park SF, Poole RK. 2005. A truncated haemoglobin implicated in oxygen metabolism by the microaerophilic food-borne pathogen Campylobacter jejuni. Microbiology 151:4079–4091. doi: 10.1099/mic.0.28266-0. [DOI] [PubMed] [Google Scholar]
- 37.Contreras ML, Escamilla JE, Del Arenal IP, Davila JR, D'Mello R, Poole RK. 1999. An unusual cytochrome o′-type cytochrome c oxidase in a Bacillus cereus cytochrome a3 mutant has a very high affinity for oxygen. Microbiology 145:1563–1573. doi: 10.1099/13500872-145-7-1563. [DOI] [PubMed] [Google Scholar]
- 38.Bergersen FJ, Turner GL. 1980. Properties of terminal oxidase systems of bacteroids from root nodules of soybean and cowpea and of N2-fixing bacteria grown in continuous culture. Microbiology 118:235–252. doi: 10.1099/00221287-118-1-235. [DOI] [Google Scholar]
- 39.Devol AH. 1978. Bacterial oxygen uptake kinetics as related to biological processes in oxygen deficient zones of the oceans. Deep Sea Res 25:137–146. doi: 10.1016/0146-6291(78)90001-2. [DOI] [Google Scholar]