Abstract
A levan-producing strain, BD1707, was isolated from Tibetan kefir and identified as Leuconostoc citreum. The effects of carbon sources on the growth of L. citreum BD1707 and levan production in tomato juice were measured. The changes in pH, viable cell count, sugar content, and levan yield in the cultured tomato juice supplemented with 15% (wt/vol) sucrose were also assayed. L. citreum BD1707 could synthesize more than 28 g/liter of levan in the tomato juice-sucrose medium when cultured at 30°C for 96 h. Based on the monosaccharide composition, molecular mass distribution, Fourier transform infrared (FTIR) spectra, and nuclear magnetic resonance (NMR) spectra, the levan synthesized by L. citreum BD1707 was composed of a linear backbone consisting of consecutive β-(2→6) linked d-fructofuranosyl units, with an estimated average molecular mass of 4.3 × 106 Da.
INTRODUCTION
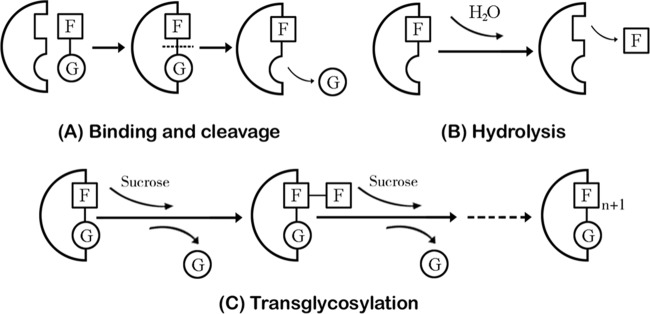
Levan is a natural fructan consisting of d-fructofuranosyl residues linked predominantly by β-(2,6) linkage in the backbone with occasional β-(2,1) branch chains. Sucrose is considered the sole carbon source for microbial levan synthesis. Levansucrase (sucrose:2,6-β-d-fructan 6-β-d-fructosyltransferase; EC 2.4.1.10), belonging to the glycoside hydrolase family 68 (GH68), is responsible for the catalysis of two reactions in the formation of levan: (i) transglycosylation, using the growing fructan chain or sucrose as the acceptor substrate, and (ii) hydrolysis of sucrose, in which water is used as the acceptor (1). First, sucrose is bound to the active center of the levansucrase and cleaved, which results in the release of glucose (Fig. 1A). Then, the remaining fructose moiety is transferred to the lengthening polyfructose chain (Fig. 1C). Alternatively, water reaches the catalytic center of levansucrase after cleavage of donor sucrose, serving as an alternative acceptor, which leads to the release of initially bound fructose and the complete hydrolysis of sucrose (Fig. 1B) (2). In nature, levan exists in a limited number of plant species and a broad range of microbial products. Levans from distinct sources differ in molecular weight and degree of branching. For example, low-molecular-weight levans are found mainly in plants, whereas levans of high molecular weight are primarily synthesized by various microbial species (3).
FIG 1.
Schematic diagram of the levansucrase-catalyzed reactions in levan synthesis (based on work by Ozimek et al. [1]).
Microbial levans are preferred over plant levans for commercial use in the food, pharmaceutical, and cosmetic industries due to their high yield as well as better solubility in water. In the food industry, levan can act as an emulsifier, stabilizer, thickener, encapsulating agent, surface-finishing agent, carrier for flavor and fragrances (4), and prebiotic ingredient (5). For pharmaceutical application, levan has been used as a blood plasma volume extender (6), anti-obesity agent, hypocholesterolemic agent, antitumor agent, hypolipidemic agent, antidiabetic agent, antiviral agent, and antipathogenic agent (7). Owing to its positive properties in terms of moisturizing effect, cell cytotoxicity, cell proliferation effect, and anti-inflammation, levan can also be utilized in cosmetic products (8).
So far, a variety of microorganisms, most of them belonging to Bacillus and Pseudomonas and a few lactic acid bacteria (LAB) belonging to Streptococcus and Lactobacillus as well as Leuconostoc mesenteroides, are well known to synthesize levan (4). However, no study on the synthesis of levan by Leuconostoc citreum has been reported.
In the present work, a levan-producing L. citreum strain, BD1707 (CGMCC 6431), was screened out of Tibetan kefir and its growth characteristics in tomato juice supplemented with sucrose (tomato juice-sucrose medium) were determined, including changes in the viable cell count, pH, and the contents of sucrose, fructose, glucose, and levan during the cultivation. The composition and chemical structure of the levan were determined by analysis of monosaccharide composition, molecular mass distribution, and Fourier transform infrared (FTIR) and nuclear magnetic resonance (NMR) spectra.
MATERIALS AND METHODS
Screening of EPS-producing strain.
Kefir grains collected from Tibet, China, were used to screen for exopolysaccharide (EPS)-producing microorganisms. Five grams of grains was homogenized in 45 ml of sterile saline solution (8.5 g/liter of NaCl) in a stomacher (BagMixer; Interscience, France) for 10 min. Suspensions were serially diluted, and aliquots (100 μl) were spread on M17 agar (Merck KGaA, Darmstadt, Germany) containing 50 g/liter of sucrose as well as 0.08 g/liter of ruthenium red (Sigma-Aldrich Co. LLC, St. Louis, MO) and incubated at 30°C aerobically for 48 h. Nonropy strains form red colonies for the bacterial cell wall being stained by ruthenium red, while ropy strains form white colonies on the same plate for the capsular exopolysaccharide, which would prevent the cells from being stained (9). An isolate designated BD1707, which presented a ropy phenotype in this assay, was chosen for further study.
Strain identification.
Physiological tests were conducted to confirm isolate identity. Gram staining was examined after 24 h of cultivation on M17 agar at 30°C aerobically. Catalase and oxidase activities were determined by the methods of Guo et al. (10). Growth at different temperatures was observed in M17 broth (Merck KGaA, Darmstadt, Germany) at 10 and 45°C for 7 days. Growth at pHs 4.5 and 9.5 was measured in M17 broth at 30°C for 7 days. Salt tolerance of the strain was tested in M17 broth containing 6.5% NaCl. Carbohydrate fermentation tests were carried out with API 50 CH strips (bioMérieux, Marcy l'Etoile, France).
Strain identification was also conducted by 16S rRNA sequence analysis. Briefly, cells grown in M17 broth at 30°C on a rotary shaker at 200 rpm for 12 h were collected to extract genomic DNA using a DNeasy blood and tissue kit (Qiagen, Valencia, CA). The 16S rRNA gene was amplified using universal primers (27F, 5′-AGAGTTTGATCCTGGCTCAG-3′, and 1492R, 5′-GGTTACCTTGTTACGACTT-3′) (11) for bacteria and a PCR thermal cycler (GenAmp PCR System 9700; PE Applied Biosystems, Foster City, CA) with a TaKaRa Taq PCR kit (TaKaRa Shuzo Co. Ltd., Otsu, Japan) by using the following program: 30 cycles consisting of denaturation at 94°C for 1 min, annealing at 52°C for 1 min, and extension at 72°C for 2 min and an additional extension step at 72°C for 10 min. Amplified 16S rRNA genes were purified using a QIAquick gel extraction kit (Qiagen) and sequenced with an automated DNA analyzer (Applied Biosystems). 16S rRNA gene sequence similarity searches were performed in the GenBank data library using the BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi) (12).
Preparation of the carbohydrate-blended tomato juice medium.
The tomato juice medium used in this study was described previously (13). In brief, tomatoes were cut into small cubes, ground in a pulper, and filtered through cotton gauze to remove the majority cake containing peel and seeds. The crude tomato juice was further centrifuged and the supernatant was collected as tomato juice. Glucose, sucrose, arabinose, d-xylose, trehalose, cellobiose, amygdalin, maltose, fructose, mannitose, mannitol, and esculin were added individually as an additional carbon source to the tomato juice at a concentration of 5% (wt/vol); the pH of the mixture was adjusted to 6.5 by adding 5.0 M NaOH drop by drop. After being sterilized at 121°C for 20 min and cooling to ambient temperature, the carbohydrate-blended tomato juice medium was utilized for L. citreum BD1707 cultivation.
Cultivation conditions.
A loop of fresh culture of BD1707 on M17 agar was inoculated into 10 ml of sterile M17 broth and cultivated at 30°C on a rotary shaker at 200 rpm for 24 h. The activation procedure in M17 broth was carried out twice. The cells were collected by centrifugation at 15,000 × g for 5 min at 4°C and washed twice with 20 mM sterile sodium phosphate buffer (pH 6.5). Subsequently, washed cells were resuspended in sodium phosphate buffer (pH 6.5) and adjusted to the initial volume. For analysis of the impact of individual carbon sources on the biomass and exopolysaccharide, 1 ml of the resuspended bacterial cells was inoculated into 100-ml Erlenmeyer flasks containing 50 ml of the sterile tomato juice supplemented with the corresponding carbohydrate and cultivated at 30°C on a shaker at 200 rpm for 72 h. To measure the kinetic change in viable cell count, pH value, and carbohydrate content in the sucrose-supplemented tomato juice medium, the resuspended bacterial cells were inoculated at a 2% (vol/vol) ratio into 250-ml Erlenmeyer flasks containing 150 ml of the broth. The cultivation was carried out at 30°C on a shaker at 200 rpm. During this procedure, 5 ml of sample was withdrawn at 0, 3, 6, 12, 24, 48, 72, and 96 h for further assays.
Preparation and purification of levan.
The cells in the cultivation broth were removed by centrifugation at 15,000 × g for 5 min at 4°C. After 4 volumes of prechilled ethanol were added to the supernatant with gentle stirring, the mixture was stored at 4°C overnight. The precipitate was collected by centrifugation at 15,000 × g at 4°C for 20 min and redissolved in deionized water. This process was repeated twice to isolate the exopolysaccharides. The final aqueous solutions were dialyzed (molecular mass cutoff, 14,000 Da) against distilled water at 4°C for 72 h, then freeze-dried using FreeZone12 (Labconco Corporation, KS), and weighed for the exopolysaccharide yield. Since there was hardly detectable exopolysaccharides in the cultured broth with additional carbon sources such as glucose and arabinose, only the EPS (levan) produced by BD1707 in the tomato juice supplemented with sucrose was furtherly characterized for its composition as well as chemical structure.
Before characterization of the structure of the BD1707 levan, e.g., analysis of the monosaccharide composition and the molecular mass distribution, the residual protein content of the obtained BD1707 EPS solution (5 mg/ml) was determined by the Bradford assay using bovine serum albumin (Sigma-Aldrich Co. LLC, St. Louis, MO) as a standard (14).
Analytical methods.
The viable cell counts of BD1707 in cultured broths were determined by plate counting. Aliquots of cultured broth samples at 0, 3, 6, 12, 24, 48, 72, and 96 h after 10-fold serial dilution in sterilized physiological saline were plated onto M17 agar and cultivated aerobically at 30°C for 48 h. The viable cell counts of BD1707 in the samples were expressed as CFU per milliliter.
The biomass was determined by measuring the dry weight of the cells collected in the 5-ml sample, and the pH value was measured by a pH meter (model PB-10, Sartorius AG, Goettingen, Germany).
Sucrose, glucose, and fructose contents.
The sucrose, glucose, and fructose contents of the samples at various intervals were determined by a high-performance liquid chromatography (HPLC) method described previously (15).
Monosaccharide composition.
The monosaccharide composition of BD1707 EPS was measured according the procedure described by Wood et al. (16). Rhamnose, arabinose, galactose, glucose, xylose, mannose, and fructose (Sigma-Aldrich Co. LLC, St. Louis, MO) were used as references. The percentages of monosaccharides were calculated from the peak areas using a response factor.
Molecular mass distribution.
The molecular mass of the levan produced by BD1707 was determined by high-performance size exclusion chromatography (HPSEC) using a Perkin-Elmer series 200 liquid chromatograph (PerkinElmer, Waltham, MA) equipped with a Perkin-Elmer series 200 refractive index detector. Two TSK-GEL columns (G6000PWXL and G4000PWXL; 7.8 by 300 mm) were maintained in series, utilizing 0.05 M NaNO3 as the eluent at a flow rate of 0.6 ml/min. The columns and guard columns were maintained at 25°C, and 5 mg/ml of BD1707 levan dissolved in the 0.05 M NaNO3 was filtered through a 0.22-μm filter (Sartorius AG) before injection. Commercial pullulans with molecular masses ranging from 6,000 to 2,560,000 Da (Sigma-Aldrich Co. LLC) were used as standards.
FTIR spectroscopic analysis.
The FTIR spectrum of the BD1707 levan was obtained using a Nicolet Nexus 470 spectrometer (Nicolet Co., Waltham, MA). A sample was prepared as a KBr pellet and scanned against a blank KBr pellet background at wavelength range of 4,000 to 500 cm−1 with a resolution of 4.0 cm−1.
1H and 13C NMR spectroscopy analysis.
Ten milligrams of BD1707 levan from the cultured tomato juice-sucrose medium was dissolved in 0.5 ml of pure D2O and then placed into 5-mm NMR tubes. One-dimensional 1H NMR spectra utilizing tetramethoxysilane as an internal standard and 13C NMR spectra were obtained on a Varian Mercury Plus 400 spectrometer (Varian Medical Systems, Inc., CA) at 25°C. The 1H NMR data collection consisted of 256 acquisitions.
Statistical analysis.
All experiments and analysis at every time point from each experiment were performed at least in triplicate. The means, standard errors, and standard deviation were calculated from replicates with the experiments and analyzed using OriginPro 8.0.
Nucleotide sequence accession number.
The full-length 16S rRNA sequence of BD1707 has been deposited in GenBank under accession no. KT626384.
RESULTS
Isolation and identification of an EPS-producing strain.
Strain BD1707 formed mucous, opaque, slightly convex, and irregularly edged colonies on M17 agar with 5% sucrose. When the colony was picked up with a loop, a sticky string could be observed, indicating that strain BD1707 had the capability to synthesize capsular and/or ropy exopolysaccharides (17). Cells of strain BD1707 are Gram-positive and catalase- and oxidase-negative cocci, visible predominantly in chains consisting of 3 to 8 cells under a light microscope. The strain is able to grow at 10°C and at pH 4.0 but unable to grow at 45°C, at pH 9.5, or in 6.5% (wt/vol) NaCl. Carbohydrate fermentation analysis by API 50 CH strips suggested that the new isolate could produce acid from glucose, sucrose, arabinose, d-xylose, trehalose, cellobiose, amygdalin, maltose, fructose, mannitose, mannitol, and esculin, which indicated a 98.6% similarity between this strain and the reported strains of L. citreum. Genetic analysis further confirmed the taxonomic status of strain BD1707. The 16S rRNA gene sequencing analysis showed that BD1707 had over 99% similarity to most of the reported strains belonging to L. citreum (99 to 100% similarity to more than 60 strains). Accordingly, the new isolate was designated L. citreum BD1707.
Effects of carbon sources on growth and EPS production.
To determine the effects of different carbon sources on growth and EPS synthesis, L. citreum BD1707 was inoculated into tomato juice containing 12 individual carbon sources, which were chosen from the carbohydrates known to be metabolizable by this strain. As shown in Table 1, all the carbon sources tested could enhance the growth of L. citreum BD1707, as reflected by the obvious increase in bacterial biomass. The highest EPS production, 15.12 g/liter, was attained by BD1707 cells grown in the presence of 5% (wt/vol) sucrose. When cellobiose was utilized as an additional carbon source besides those already existing in the original tomato juice (13), the EPS synthesized could hardly be detected (although the observed biomass was only slightly less than that with sucrose). The same phenomenon was also observed in the other 10 carbon sources. Consequently, sucrose was supposed to be the optimal carbon source for the growth and EPS synthesis of BD1707, which was in good agreement with the results obtained by other researchers (1).
TABLE 1.
Effects of carbon sources on BD1707 growth and EPS production in tomato juice containing 5% (wt/vol) individual carbon sources
| Carbon source | Biomass (g/liter) | EPS concn (g/liter) |
|---|---|---|
| Amygdalin | 4.85 | 0.37 |
| Arabinose | 3.49 | 0.17 |
| Cellobiose | 5.58 | 0.46 |
| Esculin | 2.81 | 0.14 |
| Fructose | 4.06 | 0.33 |
| Glucose | 3.67 | 0.25 |
| Maltose | 3.98 | 0.26 |
| Mannitol | 4.11 | 0.22 |
| Mannitose | 4.21 | 0.23 |
| Sucrose | 6.12 | 15.12 |
| Trehalose | 3.32 | 0.20 |
| d-Xylose | 2.53 | 0.19 |
Growth characteristics of L. citreum BD1707 in tomato juice-sucrose medium.
The changes in the viable cell counts and the pH during the growth of L. citreum BD1707 in the tomato juice-sucrose medium are shown in Fig. 2A. L. citreum BD1707 grew well in the tomato juice-sucrose medium, with a significant (P < 0.05) increase in the viable cell count, from an initial value of 7.57 log10 to 9.40 log10 CFU/ml, while the pH value of the medium decreased dramatically, from 6.07 to as low as 4.20, during the first 24 h of cultivation. The fast growth of BD1707 cells in the medium might be partially attributed to the initial pH value of 6.07, detected in the inoculated tomato juice-sucrose medium, which was approximate to the optimal pH value reported for the growth of L. citreum (18, 19). However, the viable cell counts were found to decrease slowly after 24 h ofcultivation, which might have resulted from the decrease in the pH value and depletion of nutrients, as well as the accumulation of organic acid in the medium (20).
FIG 2.
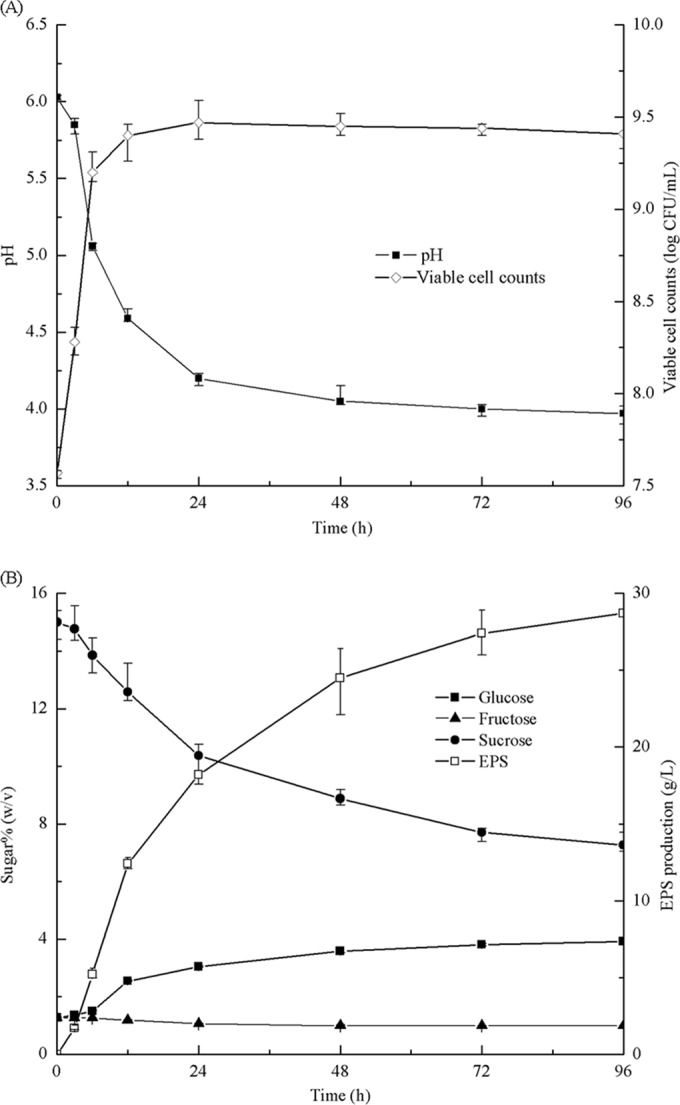
Changes in viable cell counts and pH (A) and sucrose, glucose, and fructose contents and EPS yields (B) during cultivation of L. citreum BD1707 in tomato juice-sucrose medium.
During the 96 h of cultivation, large amount of EPS (levan) accumulated in the cultured broth by L. citreum BD1707, with a climatic yield of 28.70 g/liter. Meanwhile, sucrose decreased from the initial level of 15.00% (wt/vol) to 7.27% (wt/vol) at 96 h, whereas glucose accumulated from the initial level of 1.27% (wt/vol) to a final level of 3.92% (wt/vol), as shown in Fig. 2B. No obvious change in the concentration of fructose, which fluctuated around 1% (wt/vol), was observed.
Molecular mass distribution.
A sharp peak was observed at the retention time of 18.14 min, reflecting the high homogeneity or purity of the prepared EPS. Accordingly, based on the equation of the molecular masses of standards and their corresponding retention times, the average molecular mass of EPS synthesized by L. citreum BD1707 in the tomato juice-sucrose medium was estimated to exceed 4.3 × 106 Da (Fig. 3), which was much higher than those of many other bacterial sources documented before (21, 22).
FIG 3.
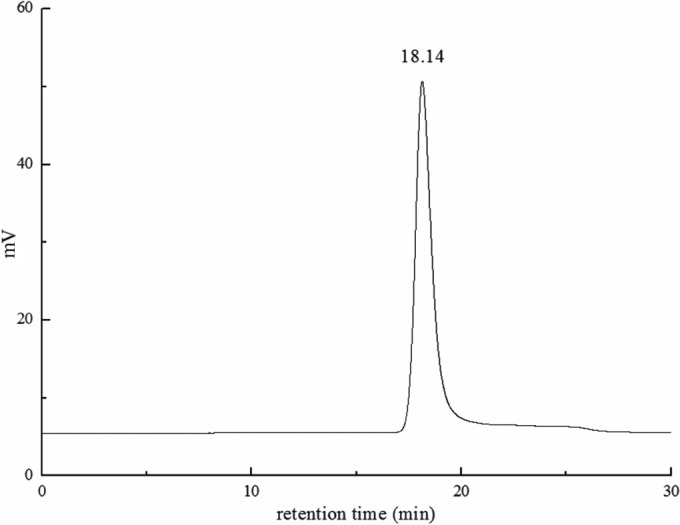
HPSEC profile of EPS produced by L. citreum BD1707 in tomato juice-sucrose medium.
Monosaccharide composition.
To avoid the possible interference of the mono- and disaccharide residues on the monosaccharide analysis of the prepared EPS, a solution of 5 mg/ml of the EPS sample was first subjected to the HPLC method mentioned in Materials and Methods to determine the residual sucrose, fructose, and glucose contents. None of the mentioned mono- or disaccharides was detectable in the prepared EPS by means of ethanol precipitation and washing repeatedly, indicating that the EPS sample was suitable for further monosaccharide analysis.
The retention time of the monosaccharide from the digested sample was 14.50 min, which accurately matched that of fructose, indicating that L. citreum BD1707 synthesized a fructan-type polysaccharide in tomato juice-sucrose medium. Inulin and levan are two basic types of fructan, and only the former had been reported for the L. citreum species until now (23). It was obviously of importance to ascertain whether the L. citreum BD1707 fructan shared a structure similar to that of fructans from other L. citreum strains. Therefore, the structure of L. citreum BD1707 fructan, such as the type of glycosidic linkages, was further characterized by FTIR spectroscopy analysis and nuclear magnetic resonance (NMR).
FTIR spectroscopy analysis.
FTIR spectroscopy analysis was performed to determine the type of the glycosidic linkages and the functional groups of levan produced by L. citreum BD1707. As shown in Fig. 4, the band in the region of 3,306 cm−1 was relevant to the hydroxyl (O—H) stretching vibration, and the two bands at 2,930 and 2,884 cm−1 arose from carbon-hydrogen (C—H) stretching vibration. The band observed at 1,634 cm−1 was due to bound water (24). Vibrations of the C—H plane deformation and aromatic skeleton caused the bands in the region of 1,412 and 1,217 cm−1 (24, 25). The bands between 1,120 and 1,008 cm−1 reflected the stretching vibration of C—O—C and C—O—H glycosidic linkages (26). The characteristic absorption at 923 and 810 cm−1 indicated the presence of the furanoid ring of the sugar units (27). The FTIR spectrum of BD1707 fructan well matched the characteristic peaks in the spectra of levan derived from Halomonas sp. strain AAD6 (28), indicating that the two polysaccharides shared similar structures. The β-(2→6) linkages in BD1707 fructan were further confirmed by 1H and 13C NMR analysis.
FIG 4.
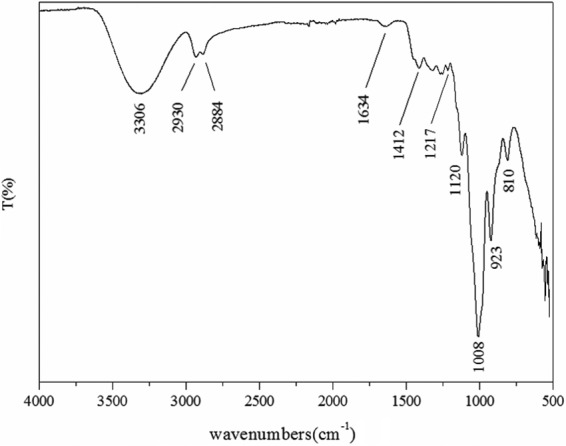
FTIR spectrum for levan synthesized by L. citreum BD1707 in tomato juice-sucrose medium. T, transmission.
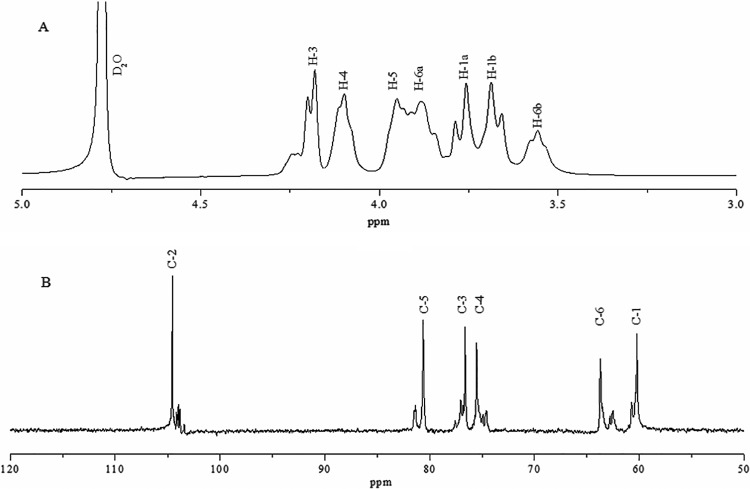
1H and 13C NMR spectroscopy analysis.
The structure of L. citreum BD1707 fructan was revealed by the 1H and 13C NMR spectra shown in Fig. 5. Seven protons corresponding to β-fructofuranosyl units were observed between 3.4 and 4.2 ppm in the 1H NMR spectrum (Fig. 5A), indicating that BD1707 fructan was of the levan type with the linkage of (2→6) fructofuranoside, which is in good agreement with the absorption peak at 923 cm−1 in the FTIR spectrum (Fig. 4). No peaks were found in the anomeric region (5.3 to 4.3 ppm), reflecting the absence of anomeric protons (29). These results provided strong evidence that the polysaccharide from BD1707 is a homopolymer of fructose, which is in accordance with the result of monosaccharide analysis. On the whole, the 1H NMR spectrum of the BD1707 fructan displayed high similarity to that of the levan from Zymomonas mobilis (ZAG-12L) (29).
FIG 5.
1H (A) and 13C (B) NMR (400 MHz, D2O) spectra for levan synthesized by L. citreum BD1707 in tomato juice-sucrose medium.
The six major resonances at 104.537 (C-2), 80.622 (C-5), 76.628 (C-3), 75.532 (C-4), 63.716 (C-6) and 60.234 (C-1) ppm in the 13C-NMR of BD1707 fructan (Fig. 5B) were assigned to the individual carbon atoms associated with the β-configurated fructofuranose units, via comparison with the published data for B. subtilis levan (30). Furthermore, the downfield shifted signal at C-6 confirmed a β-2,6 backbone structure of levan-type fructan (31). The assignments for the individual signals of levan from BD1707 and several published microorganisms (32–34) in D2O are listed in Table 2.
TABLE 2.
Assignments of individual signals in 13C NMR spectra of BD1707 levan and bacterial levan from previous studies as well as inulin
| Carbon atom | Chemical shift (ppm) |
||||
|---|---|---|---|---|---|
| L. citreum BD1707 levan | L. reuteri 121 levana | B. subtilis Takahashi levanb | B. polymyxa levanc | Inulinc | |
| C-1 | 60.234 | 61.7 | 59.9 | 60.7 | 60.9 |
| C-2 | 104.537 | 105.0 | 104.3 | 104.4 | 103.3 |
| C-3 | 76.628 | 78.1 | 76.3 | 77.0 | 77.0 |
| C-4 | 75.532 | 76.6 | 75.2 | 75.7 | 74.3 |
| C-5 | 80.622 | 81.2 | 80.3 | 80.5 | 81.1 |
| C-6 | 63.716 | 64.3 | 63.5 | 63.6 | 62.2 |
As shown in Table 2, the primary carbons (C-1 and C-6) of BD1707 levan were not grouped so closely as that of inulin and the three ring carbons (C-3, C-4, and C-5) were more closely grouped in the case of inulin, a characteristic discrepancy in the 13C-NMR spectra between inulin and levan (35).
DISCUSSION
Kefir is a natural source rich in LAB species, including Leuconostoc spp. (36). In this study, a levan-producing strain, BD1707, was screened out from Tibetan kefir grains on M17 agar containing 5% sucrose by the method of ruthenium red staining. The isolate was identified as L. citreum based on phenotypic and genotyping analysis.
Among the 12 different carbon sources tested, sucrose turned out to be the sole carbon source suitable for levan synthesis, which is in accordance with numerous reports suggesting that microbial levans are extracellularly biosynthesized from sucrose-based medium by transfructosylation of levansucrase (37). A likely explanation for the substrate specificity of levansucrase is that sucrose, because of its small size, easily entered the active site and bound to the levansucrase, resulting in a progressive reaction of polymerization (Fig. 1). Therefore, the disaccharide sucrose was an indispensable fructosyl donor substrate for fructan polymer synthesis (1).
A remarkable increase in the viable cell count and decrease in the pH value were observed during the first 24 h of cultivation. Similar viable cell counts and pH trends were also noted for L. mesenteroides BD1710 growth in tomato juice-sucrose medium for dextran biosynthesis (13), which indicated that tomato juice-sucrose medium could be a potential culture medium for EPS biosynthesis and growth of Leuconostoc species. During the cultivation of strain BD1707, a steady increase in the concentration of levan with a constant decrease in the sucrose concentration was observed, and similar phenomena have also been reported in L. citreum strains able to synthesize other exopolysaccharides. L. citreum had been reported to be capable of synthesizing a variety of homopolysaccharides from sucrose. For example, L. citreum S5 could biosynthesize dextran from sucrose as the sole substrate by dextransucrase (EC 2.4.1.5) (38). Inulosucrase (EC 2.4.1.9) from L. citreum CW28 was capable of hydrolyzing sucrose to synthesize inulin (23). Although a higher concentration of sucrose seemed in favor of the accumulation of levan in the cultured broth of L. citreum BD1707, the sucrose was not completely utilized in the tomato juice supplemented at a 15% (wt/vol) level (Fig. 2). Similar findings were also obtained for other levan-producing strains, such as Z. mobilis (39). The initial amount of glucose in the tomato juice-sucrose medium originated from the tomato juice (13), while the increase in glucose during the fermentation probably derived from two factors: (i) release from sucrose continuously by levansucrase and (ii) insufficient metabolism of the bacterial cells in stationary phase.
A comparatively high yield of levan (28.70 g/liter) was obtained after 96 h of cultivation of L. citreum BD1707, which offered a way to prepare levan efficiently that is different from that utilized by some other LAB, such as Streptococcus salivarius. S. salivarius synthesized only 3 g/liter levan in the medium (40). The efficiency (mass ratio of produced levan to initial sucrose, 19.1%) and conversion yield value (mass ratio of produced levan to consumed sucrose, 37.1%) obtained in present study were much higher than those of other levan-producing strains. Viikari reported that the amount of levan synthesized by Z. mobilis was equivalent to almost 10% of the original sucrose (41). Silbir et al. also found that about 10.3% of initial sucrose and 21.7% of consumed sucrose were utilized by Z. mobilis to biosynthesize levan (39). The high efficiency of L. citreum BD1707 in converting sucrose into levan might be derived from the strong levansucrase activity expressed as well as the fulfillment by the tomato juice of the stringent nutrition requirement by the producer. Tomato juice has been proved to be a suitable medium for many species of lactic acid bacteria, e.g., Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, and Lactobacillus delbrueckii (42) and Leuconostoc mesenteroides (13), to produce probiotic beverages or functional products.
As evidenced by its monosaccharide composition and structural characteristics (via FTIR and 1H and 13C NMR analysis), the levan synthesized by L. citreum BD1707 in the tomato juice-sucrose medium consisted of consecutive β-(2→6)-fructofuranosyl units, highly similar in structure to those produced by other bacterial species, although the former had a much higher molecular mass (the estimated mass of the former exceeded 4.3 × 106 Da). Compared with the levan derived from other bacterial species or plants, the huge molecule of BD1707 levan might endow this polymer with an enhanced ability to perform its functions. It had been reported that the diversity in structure as well as functions of microbial exopolysaccharides was greatly influenced by their molecular masses, monosaccharide compositions, and molecular conformations (degree of branching as well as type of glycosidic or fructosidic linkages) (43). For instance, levans with high molecular masses (>300 kDa) demonstrated antitumor activity (44),while low-molecular-mass ones (<100 kDa) have been employed as blood plasma volume extenders (6) and antiviral agents (45).
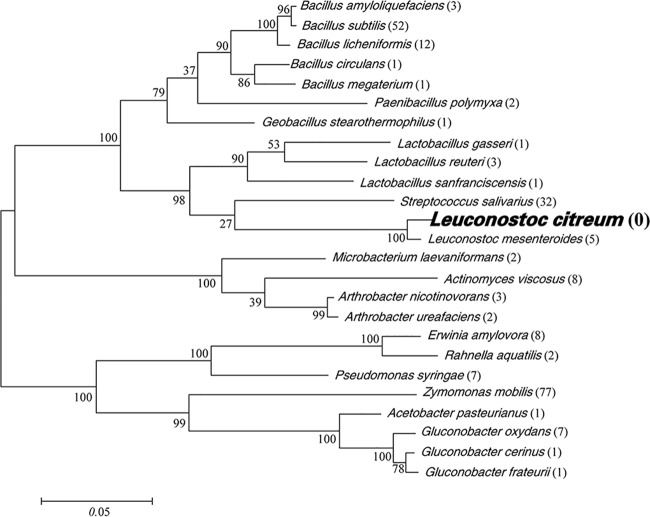
Microbial levans were biosynthesized from sucrose-based substrates by microorganisms secreting levansucrase; most of them were nonfood bacteria, such as Bacillius spp. and Zymomona spp. (Fig. 6). Only a handful LAB species, including Streptococcus spp., L. mesenteroides, and Lactobacillus spp., had been reported to show levan synthesis. Among the published studies regarding LAB levan, most of them (32 of 38) were focused on the physico-chemical and biological characteristics of S. salivarius levan (40, 46).
FIG 6.
Phylogenetic tree of Leuconostoc citreum and reported levan-producing strains calculated on the basis of 16S rRNA gene sequences. Numbers in parentheses are numbers of published studies reporting levan synthesized by members of the species. The scale bar indicates number of changes per nucleotide.
In conclusion, L. citreum strain BD1707 is able to synthesize levan in tomato juice-sucrose medium, which is available worldwide. So far, microbial levans have been primarily derived from nonfood microorganisms, which might confer a safety risk for the biopolymer to be applied commercially. In contrast, L. citreum BD1707 offers an alternative to produce levan in a more safe and low-cost way.
Considering the potential of levan as a functional agent with wide application, further study is necessitated to elucidate the mechanism involved in levan synthesis by BD1707 as well as cultivation parameters affecting the yield.
ACKNOWLEDGMENTS
We gratefully acknowledge the financial support of Key Projects in the National Science and Technology Pillar Program during the 12th Five-Year Plan (no. 2013BAD18B01).
We also greatly appreciate the technical support provided by the Instrumental Analysis Center of Shanghai Jiao Tong University, Shanghai, China, in assisting with NMR analysis.
REFERENCES
- 1.Ozimek LK, Kralj S, Van der Maarel MJ, Dijkhuizen L. 2006. The levansucrase and inulosucrase enzymes of Lactobacillus reuteri 121 catalyse processive and non-processive transglycosylation reactions. Microbiology 152:1187–1196. doi: 10.1099/mic.0.28484-0. [DOI] [PubMed] [Google Scholar]
- 2.Jakob F, Meißner D, Vogel RF. 2012. Comparison of novel GH 68 levansucrases of levan-overproducing Gluconobacter species. Acetic Acid Bacteria 1:e2. [Google Scholar]
- 3.Poli A, Kazak H, Gürleyendağ B, Tommonaro G, Pieretti G, Öner ET, Nicolaus B. 2009. High level synthesis of levan by a novel Halomonas species growing on defined media. Carbohydr Polym 78:651–657. doi: 10.1016/j.carbpol.2009.05.031. [DOI] [Google Scholar]
- 4.Han YW. 1990. Microbial levan. Adv Appl Microbiol 35:171–194. [DOI] [PubMed] [Google Scholar]
- 5.Bello FD, Walter J, Hertel C, Hammes WP. 2001. In vitro study of prebiotic properties of levan-type exopolysaccharides from lactobacilli and non-digestible carbohydrates using denaturing gradient gel electrophoresis. Syst Appl Microbiol 24:232–237. doi: 10.1078/0723-2020-00033. [DOI] [PubMed] [Google Scholar]
- 6.Schechter I, Hestrin S. 1963. Use of levan as an expander of blood-volume. Vox Sang 8:82–85. doi: 10.1111/j.1423-0410.1963.tb04152.x. [DOI] [PubMed] [Google Scholar]
- 7.Byun BY, Lee SJ, Mah JH. 2014. Antipathogenic activity and preservative effect of levan (β-2, 6-fructan), a multifunctional polysaccharide. Int J Food Sci Technol 49:238–245. doi: 10.1111/ijfs.12304. [DOI] [Google Scholar]
- 8.Kim KH, Chung CB, Kim YH, Kim KS, Han CS, Kim CH. 2004. Cosmeceutical properties of levan produced by Zymomonas mobilis. J Cosmet Sci 56:395–406. [PubMed] [Google Scholar]
- 9.Stingele F, Neeser J-R, Mollet B. 1996. Identification and characterization of the eps (exopolysaccharide) gene cluster from Streptococcus thermophilus Sfi6. J Bacteriol 178:1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y, Huang E, Yuan C, Zhang L, Yousef AE. 2012. Isolation of a Paenibacillus sp. strain and structural elucidation of its broad-spectrum lipopeptide antibiotic. Appl Environ Microbiol 78:3156–3165. doi: 10.1128/AEM.07782-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J-H, Ahn H-J, Kim S-G, Chung C-H. 2013. Dextran-like exopolysaccharide-producing Leuconostoc and Weissella from kimchi and its ingredients. Food Sci Biotechnol 22:1047–1053. doi: 10.1007/s10068-013-0182-x. [DOI] [Google Scholar]
- 12.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 13.Han J, Hang F, Guo B, Liu Z, You C, Wu Z. 2014. Dextran synthesized by Leuconostoc mesenteroides BD1710 in tomato juice supplemented with sucrose. Carbohydr Polym 112:556–562. doi: 10.1016/j.carbpol.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 14.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.Villanueva-Suárez MJ, Redondo-Cuenca A, Rodriguez-Sevilla MD, Martinez MD. 2003. Characterization of nonstarch polysaccharides content from different edible organs of some vegetables, determined by GC and HPLC: comparative study. J Agric Food Chem 51:5950–5955. doi: 10.1021/jf021010h. [DOI] [PubMed] [Google Scholar]
- 16.Wood PJ, Weisz J, Blackwell BA. 1994. Structural studies of (1→3),(1→4)-β-d-glucans by 13C-nuclear magnetic resonance spectroscopy and by rapid analysis of cellulose-like regions using high-performance anion-exchange chromatography of oligosaccharides released by lichenase. Cereal Chem 71:301–307. [Google Scholar]
- 17.Ruas-Madiedo P, De Los Reyes-Gavilán C. 2005. Invited review: methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J Dairy Sci 88:843–856. doi: 10.3168/jds.S0022-0302(05)72750-8. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Eom HJ, Lee J, Han J, Han NS. 2004. Statistical optimization of medium composition for growth of Leuconostoc citreum. Biotechnol Bioprocess Eng 9:278–284. doi: 10.1007/BF02942344. [DOI] [Google Scholar]
- 19.Sung I-K, Han NS, Kim BS. 2012. Co-production of biomass and metabolites by cell retention culture of Leuconostoc citreum. Bioprocess Biosyst Eng 35:715–720. doi: 10.1007/s00449-011-0651-7. [DOI] [PubMed] [Google Scholar]
- 20.Shah N, Jelen P. 1990. Survival of lactic acid bacteria and their lactases under acidic conditions. J Food Sci 55:506–509. doi: 10.1111/j.1365-2621.1990.tb06797.x. [DOI] [Google Scholar]
- 21.Dos Santos LF, Bazani Cabral De Melo FC, Martins Paiva WJ, Borsato D, Corradi Custodio Da Silva MDL, Pedrine Colabone Celligoi MA. 2013. Characterization and optimization of levan production by Bacillus subtilis NATTO. Rom Biotechnol Lett 18:8413–8422. [Google Scholar]
- 22.Zhang T, Li R, Qian H, Mu W, Miao M, Jiang B. 2014. Biosynthesis of levan by levansucrase from Bacillus methylotrophicus SK 21.002. Carbohydr Polym 101:975–981. doi: 10.1016/j.carbpol.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz-Soto ME, Olivares-Illana V, Lopez-Munguia A. 2004. Biochemical properties of inulosucrase from Leuconostoc citreum CW28 used for inulin synthesis. Biocatal Biotransformation 22:275–281. doi: 10.1080/10242420400014251. [DOI] [Google Scholar]
- 24.Liu J, Luo J, Ye H, Sun Y, Lu Z, Zeng X. 2010. Medium optimization and structural characterization of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr Polym 79:206–213. doi: 10.1016/j.carbpol.2009.07.055. [DOI] [Google Scholar]
- 25.Schwanninger M, Rodrigues J, Pereira H, Hinterstoisser B. 2004. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib Spectrosc 36:23–40. doi: 10.1016/j.vibspec.2004.02.003. [DOI] [Google Scholar]
- 26.Wu M, Wu Y, Zhou J, Pan Y. 2009. Structural characterisation of a water-soluble polysaccharide with high branches from the leaves of Taxus chinensis var. mairei. Food Chem 113:1020–1024. doi: 10.1016/j.foodchem.2008.08.055. [DOI] [Google Scholar]
- 27.Liu C, Lu J, Lu L, Liu Y, Wang F, Xiao M. 2010. Isolation, structural characterization and immunological activity of an exopolysaccharide produced by Bacillus licheniformis 8-37-0-1. Bioresour Technol 101:5528–5533. doi: 10.1016/j.biortech.2010.01.151. [DOI] [PubMed] [Google Scholar]
- 28.Küçükaşik F, Kazak H, Güney D, Finore I, Poli A, Yenigün O, Nicolaus B, Öner ET. 2011. Molasses as fermentation substrate for levan production by Halomonas sp. Appl Microbiol Biotechnol 89:1729–1740. doi: 10.1007/s00253-010-3055-8. [DOI] [PubMed] [Google Scholar]
- 29.Angeli R, da Paz NV, Maciel JC, Araújo FF, Paiva PM, Calazans GM, Valente AP, Almeida FC, Coelho LC, Carvalho LB. 2009. Ferromagnetic levan composite: an affinity matrix to purify lectin. BioMed Res Int doi: 10.1155/2009/179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bock K, Pedersen C. 1983. Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. Adv Carbohydr Chem Biochem 41:27–66. doi: 10.1016/S0065-2318(08)60055-4. [DOI] [Google Scholar]
- 31.Tomašić J, Jennings HJ, Glaudemans CP. 1978. Evidence for a single type of linkage in a fructofuranan from Lolium perenne. Carbohydr Res 62:127–133. doi: 10.1016/S0008-6215(00)83384-4. [DOI] [Google Scholar]
- 32.Hijum SA, Bonting K, Maarel MJ, Dijkhuizen L. 2001. Purification of a novel fructosyltransferase from Lactobacillus reuteri strain 121 and characterization of the levan produced. FEMS Microbiol Lett 205:323–328. doi: 10.1111/j.1574-6968.2001.tb10967.x. [DOI] [PubMed] [Google Scholar]
- 33.Shih L, Chen L-D, Wang T-C, Wu J-Y, Liaw K-S. 2010. Tandem production of levan and ethanol by microbial fermentation. Green Chem 12:1242–1247. doi: 10.1039/b924765c. [DOI] [Google Scholar]
- 34.Han YW. 1989. Levan production by Bacillus polymyxa. J Ind Microbiol 4:447–451. doi: 10.1007/BF01569641. [DOI] [Google Scholar]
- 35.Han YW, Clarke MA. 1990. Production and characterization of microbial levan. J Agric Food Chem 38:393–396. doi: 10.1021/jf00092a011. [DOI] [Google Scholar]
- 36.Gulitz A, Stadie J, Wenning M, Ehrmann MA, Vogel RF. 2011. The microbial diversity of water kefir. Int J Food Microbiol 151:284–288. doi: 10.1016/j.ijfoodmicro.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Wu F-C, Chou S-Z, Shih I-L. 2013. Factors affecting the production and molecular weight of levan of Bacillus subtilis natto in batch and fed-batch culture in fermenter. J Taiwan Inst Chem Eng 44:846–853. doi: 10.1016/j.jtice.2013.03.009. [DOI] [Google Scholar]
- 38.Son M-J, Jang E-K, Kwon O-S, Seo J-H, Kim I-J, Lee I-S, Park S-C, Lee S-P. 2008. Characterization of dextran produced from Leuconostoc citreum S5 strain isolated from Korean fermented vegetable. Eur Food Res Technol 226:697–706. doi: 10.1007/s00217-007-0579-y. [DOI] [Google Scholar]
- 39.Silbir S, Dagbagli S, Yegin S, Baysal T, Goksungur Y. 2014. Levan production by Zymomonas mobilis in batch and continuous fermentation systems. Carbohydr Polym 99:454–461. doi: 10.1016/j.carbpol.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 40.Newbrun E, Baker S. 1968. Physico-chemical characteristics of the levan produced by Streptococcus salivarius. Carbohydr Res 6:165–170. doi: 10.1016/S0008-6215(00)81506-2. [DOI] [Google Scholar]
- 41.Viikari L. 1984. Formation of levan and sorbitol from sucrose by Zymomonas mobilis. Appl Microbiol Biotechnol 19:252–255. [Google Scholar]
- 42.Yoon KY, Woodams EE, Hang YD. 2004. Probiotication of tomato juice by lactic acid bacteria. J Microbiol 42:315–318. [PubMed] [Google Scholar]
- 43.He X, Niu X, Li J, Xu S, Lu A. 2012. Immunomodulatory activities of five clinically used Chinese herbal polysaccharides. J Exp Integr Med 2:15–27. [Google Scholar]
- 44.Calazans GMT, Lima RC, de França FP, Lopes CE. 2000. Molecular weight and antitumour activity of Zymomonas mobilis levans. Int J Biol Macromol 27:245–247. doi: 10.1016/S0141-8130(00)00125-2. [DOI] [PubMed] [Google Scholar]
- 45.Esawy MA, Ahmed EF, Helmy WA, Mansour NM, El-Senousy WM, El-Safty MM. 2011. Production of levansucrase from novel honey Bacillus subtilis isolates capable of producing antiviral levans. Carbohydrate Polym 86:823–830. doi: 10.1016/j.carbpol.2011.05.035. [DOI] [Google Scholar]
- 46.Gibbons R, Banghart S. 1968. Induction of dental caries in gnotobiotic rats with a levan-forming streptococcus and a streptococcus isolated from subacute bacterial endocarditis. Arch Oral Biol 13:297-IN221. doi: 10.1016/0003-9969(68)90128-3. [DOI] [PubMed] [Google Scholar]