Abstract
Chlamydia psittaci is an obligate intracellular bacterium responsible for avian chlamydiosis, otherwise known as psittacosis, a zoonotic disease that may lead to severe atypical pneumonia. This study was conducted on seven mule duck flocks harboring asymptomatic birds to explore the circulation and persistence of C. psittaci during the entire breeding process and assess the potential sources of worker exposure. Cloacal swabs and air samples were taken on each occasion requiring humans to handle the birds. In parallel, environmental samples, including dust, water, and soil, were collected. Specific real-time PCR analyses revealed the presence of C. psittaci in all flocks but with three different shedding patterns involving ducks about the age of 4, 8, and 12 weeks with heavy, moderate, and low excretion levels, respectively. Air samples were only positive in flocks harboring heavy shedders. Dust in flocks with heavy or moderate shedders carried chlamydial loads strongly associated with the loads detected in avian and soil samples. Environmental contamination, significantly correlated with shedding dynamics, was considered to be the most probable source of exposure. The high prevalence of bacteriophage Chp1 in all flocks, mostly jointly present with chlamydia, suggests an important factor in C. psittaci persistence, thus creating a greater risk for humans. A survey conducted in these flocks regarding farming practices and activities showed that disinfection seems to be the most promising practice for reducing C. psittaci prevalence in ducks and that the place and the duration of action during operations seem to be potential risk factors. Strict adherence to good practices is strongly recommended.
INTRODUCTION
Chlamydia psittaci, a member of the Chlamydiaceae family, is an obligate intracellular bacterium known worldwide to cause “psittacosis” (also called “ornithosis” or, more generally, “avian chlamydiosis”), which is the most important potentially zoonotic animal chlamydiosis. The disease mainly affects psittacine birds and domestic poultry and is usually systemic with clinical signs that vary greatly in severity depending on the species and age in weeks, as well as the virulence of the chlamydial strain involved (1, 2). Avian strains of Chlamydia psittaci are separated in 13 ompA genotypes named A to F, E/B, 1V, 6N, Matl16, R54, YP84, and CPX308 (3). These avian genotypes present relative host specificity, and most of them have also been identified in cases of zoonotic transmission (4–7).
Transmission to humans occurs through inhalation of contaminated aerosols resulting from avian respiratory tract excretions and droppings and can cause a primarily respiratory disease complex, which may lead to severe atypical pneumonia and death in the most severe cases (8, 9). The risk of psittacosis is highest among individuals in direct contact with birds, e.g., poultry sector workers, veterinarians, pet shop employees, and pet bird owners. A number of recent reports suggest that numerous cases of psittacosis are linked to domestic poultry and that turkeys, ducks, and chickens could be important reservoirs of infection in humans, for example, in poultry farm, hatchery, slaughterhouse, and processing plant workers (6, 10–15). Because of the severity and potentially fatal outcome of human psittacosis and the serious hazard to workers, European Directive 2000/54/EC (16) and the Approved List of Biological Agents of the Advisory Committee on Dangerous Pathogens (17) have classified C. psittaci as a “hazard group 3” human pathogen. This underlines the need for further awareness and efficient risk assessment and management.
Most of the human cases of psittacosis reported in France to the public health authorities are linked to ducks, and especially to mule ducks, a hybrid species related to the production of foie gras (6, 15). More recently, a study conducted in mule duck breeding farms revealed significant shedding of C. psittaci, in mostly asymptomatic birds, revealing a hidden risk for workers (18). The latter study also leads to a hypothesis about how ducks become contaminated: although some of the ducklings could inherit a low level of infection from their parents, the majority seems to arrive perfectly healthy on the breeding farm and become infected later, possibly by the environment.
Because within-flock C. psittaci circulation data are essential to implementing optimal management practices, we performed a field study on seven naturally infected but asymptomatic mule duck flocks. We sought to (i) describe C. psittaci shedding dynamics, (ii) determine environmental contamination, and (iii) identify circulating C. psittaci diversity. In order to assess the potential sources of worker exposure, cloacal swabs and air and environmental samples (dusters, water, and soil) were taken on each occasion on which humans were required to handle birds during the entire breeding process, i.e., from the hatchery to the slaughterhouse. C. psittaci bacteriophage Chp1 and amoebae were also screened for in order to understand their possible involvement in chlamydial persistence in the field, as previously suspected (19, 20). Furthermore, a questionnaire survey was conducted on duck farms to gather information on routine cleaning and disinfection practices and on farming activities in an attempt to explain the maintenance of the microorganism in its environment.
MATERIALS AND METHODS
Sampling.
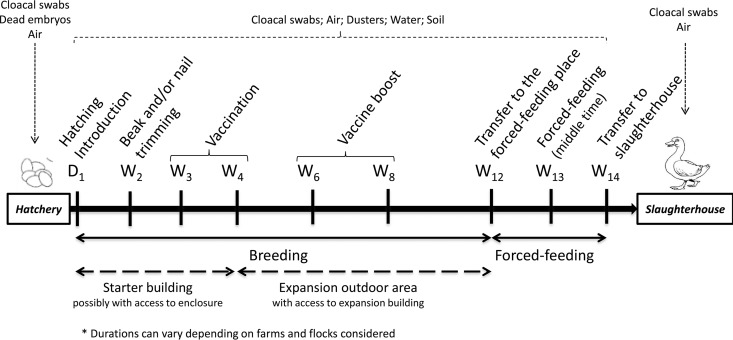
This study was conducted in seven mule duck flocks (A1, A2, B, C, D1, D2, and E) from five breeding farms (A to E) in western France. From January to July 2013, sampling was performed for each flock at each stage of the production process, from the hatchery to the slaughterhouse. A synthesis of the samples collected is presented in Fig. 1.
FIG 1.
Interventions, samples, ages of ducks (in weeks), and living areas.
(i) Avian samples.
In the hatchery, 15 randomly selected 1-day-old ducklings (per flock) were subjected to cloacal sampling and 15 dead embryos (unhatched eggs) were subjected to necropsy. Spleen, liver, lung, and intestine samples were taken during necropsy.
After the introduction of ducklings on farms (at the age of 1 day), 15 randomly selected ducks (per flock) were subjected to cloacal swab sampling whenever birds had to be handled by workers. Samples were taken during introduction, trimming of nails and beaks, vaccination against Pasteurella multocida, booster vaccination, transfer to the force-feeding room, force-feeding, transfer to the slaughterhouse, and slaughtering just after plucking (Fig. 1).
(ii) Air samples.
Air samples (n = 123; Table 1) were collected using a microbial air sampler MAS-100 NT device (MBV, Switzerland). The device was placed as close as possible to the worker. Each sampling period lasted 10 min with the air pumped at a flow of 100 liters per min and impacted a semiliquid agar as previously described (21). Two air samples were taken at each stage, except in the slaughterhouse where four samples were taken: two at the loading dock and two in the hanging area, which are two places where the birds are handled and in an agitated state.
TABLE 1.
Results of chlamydia detection in avian and environmental samples from five duck farmsa
| Flock and sample | Action | Date (day/mo/yr) | Place | Age (days) |
Chlamydiaceae detection |
Bacteriophage detection |
Amoeba detectionc |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cloacal swabs |
Air samples |
Dusters |
Water samples |
Soil samples |
Cloacal swabs |
Air samples |
Dusters |
Water samples |
Soil samples |
Water |
Soil |
||||||||||||||||||||
| 23S-rtPCR |
IncA-rtPCR |
Genotype | |||||||||||||||||||||||||||||
| No. pos/total | Mean Cq | No. pos/totalb | Mean Cq | No. pos/total | Cq | No. pos/total | Mean Cq | No. pos/total | Mean Cq | No. pos/total | Mean Cq | No. pos/total | Mean Cq | No. pos/total | Cq | No. pos/total | Mean Cq | No. pos/total | Mean Cq | No. pos/total | Mean Cq | Amo | Vahl | Amo | Vahl | ||||||
| A1 | |||||||||||||||||||||||||||||||
| 13-0273/1 | Implementation | 24/01/13 | Starter building | 1 | 0/15 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | 2/5 | 37.7 | 0/15 | NA | 0/2 | NA | 1/1 | 35.4 | 0/2 | NA | 3/5 | 34.6 | + | – | + | + | |||
| 13-0458/1 | Nail trimming | 06/02/13 | Starter building | 14 | 1/15 | 35.9 | 0/1 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | ND | 3/15 | 38.5 | 1/2 | 38.2 | 1/1 | 36.8 | 0/2 | NA | ND | + | – | |||||
| 13-0550/1 | Vaccination | 19/02/13 | Starter building + enclosures | 27 | 15/15 | 24.2 | 15/15 | 28.9 | E/B_06-859 | 1/2 | 35.7 | 1/1 | 28.0 | 0/2 | NA | 5/5 | 28.3 | 14/15 | 35.5 | 2/2 | 37.3 | 1/1 | 30.2 | 0/2 | NA | 5/5 | 33.7 | + | + | – | – |
| 13-0904/1 | Vaccination boost | 21/03/13 | Outdoor area | 57 | 13/15 | 34.6 | 13/13 | 34.1 | 0/2 | NA | 2/2 | 36.8 | 0/2 | NA | 1/5 | 40.0 | 14/15 | 35.3 | 0/2 | NA | 2/2 | 35.2 | 0/2 | NA | 1/5 | 37.4 | + | + | + | – | |
| 13-1159/1 | Transfer | 16/04/13 | Outdoor area | 83 | 10/15 | 32.2 | 10/10 | 33.2 | 0/2 | NA | 0/1 | NA | ND | ND | 13/15 | 35.7 | 0/2 | NA | 1/1 | 36.8 | ND | ND | |||||||||
| 13-1159/2 | Start of force-feeding | 16/04/13 | 0/2 | NA | ND | ND | ND | 0/2 | NA | ND | ND | ND | |||||||||||||||||||
| 13-1328/1 | Force-feeding | 23/04/13 | 90 | 6/15 | 37.0 | 5/6 | 36.4 | 0/2 | NA | ND | ND | ND | 6/15 | 35.7 | 0/2 | NA | ND | ND | ND | ||||||||||||
| 13-1328/2 | Transfer | 29/04/13 | 96 | 1/15 | 35.9 | 1/1 | 35.6 | 0/2 | NA | ND | ND | ND | 4/15 | 34.5 | 0/2 | NA | ND | ND | ND | ||||||||||||
| 13-1328/3 | Slaughtering | 29/04/13 | 1/15 | 39.3 | 1/1 | 38.9 | 0/4 | NA | ND | ND | ND | 4/15 | 35.8 | 0/4 | NA | ND | ND | ND | |||||||||||||
| A2 | |||||||||||||||||||||||||||||||
| 13-0749/1 | Implementation | 07/03/13 | Starter building | 1 | 0/15 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | 2/5 | 38.9 | 0/15 | NA | 0/2 | NA | 1/1 | 37.4 | 0/2 | NA | 2/5 | 38.4 | – | – | + | + | |||
| 13-0904/2 | Nail and beak trimming | 18/03/13 | Starter building | 12 | 0/15 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | ND | 2/15 | 38.3 | 1/2 | 39.3 | 0/1 | NA | 0/2 | NA | ND | + | – | |||||||
| 13-1030/1 | Vaccination | 04/04/13 | Starter building + enclosures | 29 | 15/15 | 26.9 | 15/15 | 26.9 | E/B_06-859 | 1/2 | 37.8 | 2/2 | 31.3 | 2/2 | 32.0 | 5/5 | 31.6 | 12/15 | 37.2 | 2/2 | 36.2 | 2/2 | 34.7 | 0/2 | NA | 5/5 | 35.2 | + | + | – | – |
| 13-1376/1 | Vaccination boost | 02/05/13 | Outdoor area | 57 | 7/15 | 33.8 | 6/7 | 32.9 | 0/2 | NA | 0/2 | NA | 0/2 | NA | 2/5 | 38.6 | 10/15 | 35.6 | 1/2 | 37.2 | 0/2 | NA | 0/2 | NA | 2/5 | 38.5 | + | + | + | – | |
| 13-1598/1 | Slaughtering | 03/06/13 | 89 | 3/15 | 37.7 | 3/3 | 37.6 | 0/4 | NA | ND | ND | ND | 4/15 | 36.3 | 1/4 | 39.5 | ND | ND | ND | ||||||||||||
| B | |||||||||||||||||||||||||||||||
| 13-0273/2 | Implementation | 31/01/13 | Starter building | 1 | 0/15 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | 0/5 | NA | 0/15 | NA | 0/2 | NA | 1/1 | 33.2 | 0/2 | NA | 0/5 | NA | + | – | + | + | |||
| 13-0458/2 | Nail trimming | 14/02/13 | Starter building | 15 | 0/15 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | ND | 0/15 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | ND | + | – | |||||||
| 13-0550/2 | Vaccination | 25/02/13 | Starter building + enclosures | 26 | 6/15 | 32.9 | 3/6 | 32.7 | E/B_06-859 | 0/2 | NA | 1/1 | 36.2 | 0/2 | NA | 0/5 | NA | 10/15 | 38.0 | 0/2 | NA | 1/1 | 32.8 | 0/2 | NA | 0/5 | NA | – | – | + | – |
| 13-1030/2 | Vaccination boost | 28/03/13 | Outdoor area | 57 | 11/15 | 35.1 | 10/11 | 34.4 | E/B_06-859 | 0/2 | NA | 0/2 | NA | 0/2 | NA | 3/5 | 37.1 | 15/15 | 33.7 | 0/2 | NA | 2/2 | 33.3 | 0/2 | NA | 5/5 | 36.9 | + | – | + | + |
| 13-1328/4 | Transfer | 23/04/13 | Outdoor area | 83 | 5/15 | 33.0 | 5/5 | 33.0 | 0/2 | NA | 0/1 | NA | ND | ND | 8/15 | 33.7 | 1/2 | 38.0 | 1/1 | 36.7 | ND | ND | |||||||||
| 13-1328/5 | Start of force-feeding | 23/04/13 | 0/2 | NA | ND | ND | ND | 1/2 | 38.1 | ND | ND | ND | |||||||||||||||||||
| 13-1328/6 | Force-feeding | 30/04/13 | 90 | 1/15 | 38.0 | 1/1 | 37.8 | 0/2 | NA | ND | ND | ND | 5/15 | 32.8 | 0/2 | NA | ND | ND | ND | ||||||||||||
| 13-1376/2 | Transfer | 06/05/13 | 96 | 5/15 | 39.1 | 1/5 | 37.2 | 0/2 | NA | ND | ND | ND | 5/15 | 33.7 | 0/2 | NA | ND | ND | ND | ||||||||||||
| 13-1376/3 | Slaughtering | 06/05/13 | 1/15 | 39.7 | 0/1 | NA | 0/4 | NA | ND | ND | ND | 3/15 | 35.7 | 0/4 | NA | ND | ND | ND | |||||||||||||
| C | |||||||||||||||||||||||||||||||
| 13-0458/3 | Implementation | 14/02/13 | Starter building | 1 | 2/15 | 35.8 | 0/2 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | 0/5 | NA | 1/15 | 39.9 | 0/2 | NA | 0/1 | NA | 0/2 | NA | 1/5 | 35.6 | + | – | + | – | |
| 13-0749/2 | Nail trimming | 26/02/13 | Starter building | 13 | 0/15 | NA | 0/1 | NA | 0/1 | NA | 0/2 | NA | ND | 2/15 | 39.1 | 0/1 | NA | 1/1 | 39.1 | 0/2 | NA | ND | + | – | |||||||
| 13-0749/3 | Vaccination | 07/03/13 | Starter building | 22 | 15/15 | 25.4 | 15/15 | 25.5 | E/B_E30 | 1/2 | 36.6 | 1/1 | 28.7 | 2/2 | 27.9 | 5/5 | 28.8 | 15/15 | 28.9 | 2/2 | 34.4 | 1/1 | 26.4 | 2/2 | 35.5 | 5/5 | 31.4 | + | – | – | + |
| 13-1030/3 | Vaccination boost | 28/03/13 | Outdoor area | 43 | 14/15 | 33.7 | 14/14 | 33.4 | 0/2 | NA | 2/2 | 35.0 | 2/2 | 38.2 | 4/5 | 36.0 | 15/15 | 31.3 | 2/2 | 36.1 | 2/2 | 34.9 | 0/2 | NA | 3/5 | 38.4 | + | – | + | – | |
| 13-1376/4 | Transfer | 07/05/13 | Outdoor area | 83 | 5/15 | 36.9 | 4/5 | 37.1 | 0/2 | NA | 0/1 | NA | ND | ND | 11/15 | 34.4 | 0/2 | NA | 1/1 | 28.5 | ND | ND | |||||||||
| 13-1376/5 | Start of force-feeding | 07/05/13 | 0/2 | NA | ND | ND | ND | 1/2 | 38.9 | ND | ND | ND | |||||||||||||||||||
| 13-1458/1 | Force-feeding | 13/05/13 | 89 | 1/15 | 37.6 | 0/1 | NA | 0/2 | NA | ND | ND | ND | 7/15 | 35.5 | 0/2 | NA | ND | ND | ND | ||||||||||||
| 13-1458/2 | Transfer | 21/05/13 | 97 | 3/15 | 37.0 | 2/3 | 37.0 | 0/2 | NA | ND | ND | ND | 7/15 | 34.2 | 0/2 | NA | ND | ND | ND | ||||||||||||
| 13-1458/3 | Slaughtering | 21/05/13 | 1/15 | 39.9 | 0/1 | NA | 0/4 | NA | ND | ND | ND | 6/15 | 37.5 | 0/4 | NA | ND | ND | ND | |||||||||||||
| D1 | |||||||||||||||||||||||||||||||
| 13-0458/4 | Implementation | 14/02/13 | Starter building | 1 | 2/15 | 36.8 | 0/2 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | 0/5 | NA | 0/15 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | 0/5 | NA | + | + | + | + | |
| 13-0749/4 | Vaccination | 07/03/13 | Starter building | 22 | 0/15 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | 0/5 | NA | 0/15 | NA | 1/2 | 38.1 | 1/1 | 36.9 | 0/2 | NA | 1/5 | 36.0 | + | + | + | + | |||
| 13-1030/4 | Vaccination boost | 04/04/13 | Outdoor area | 50 | 0/15 | NA | 0/2 | NA | 0/2 | NA | 0/2 | NA | 0/5 | NA | 3/15 | 39.2 | 0/2 | NA | 1/2 | 35.5 | 0/2 | NA | 0/5 | NA | + | + | + | + | |||
| 13-1376/6 | Transfer | 10/05/13 | Outdoor area | 86 | 7/15 | 37.0 | 5/7 | 37.0 | 0/2 | NA | 0/1 | NA | ND | ND | 11/15 | 32.1 | 2/2 | 34.8 | 1/1 | 30.9 | ND | ND | |||||||||
| 13-1376/7 | Start of force-feeding | 10/05/13 | 0/2 | NA | ND | ND | ND | 1/2 | 33.8 | ND | ND | ND | |||||||||||||||||||
| 13-1458/4 | Force-feeding | 15/05/13 | 91 | 4/15 | 37.4 | 3/4 | 37.5 | 0/2 | NA | ND | ND | ND | 14/15 | 32.6 | 1/2 | 35.6 | ND | ND | ND | ||||||||||||
| 13-1458/5 | Transfer | 22/05/13 | 98 | 5/15 | 37.7 | 4/5 | 36.5 | 0/2 | NA | ND | ND | ND | 13/15 | 34.2 | 2/2 | 38.7 | ND | ND | ND | ||||||||||||
| 13/1458/6 | Slaughtering | 22/05/13 | 1/15 | 39.0 | 1/1 | 39.0 | 0/4 | NA | ND | ND | ND | 14/15 | 34.3 | 2/4 | 39.4 | ND | ND | ND | |||||||||||||
| D2 | |||||||||||||||||||||||||||||||
| 13-1159/3 | Implementation | 11/04/13 | Starter building | 1 | 0/15 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | 0/5 | NA | 0/15 | NA | 0/2 | NA | 1/1 | 39.7 | 0/2 | NA | 0/5 | NA | + | + | – | + | |||
| 13-1376/8 | Vaccination | 06/05/13 | Starter building + enclosures | 26 | 0/15 | NA | 0/1 | NA | 0/1 | NA | 0/2 | NA | 0/5 | NA | 2/15 | 39.6 | 0/1 | NA | 1/1 | 38.8 | 0/2 | NA | 0/5 | NA | + | – | + | + | |||
| 13-1598/2 | Vaccination boost | 03/06/13 | Outdoor area | 55 | 14/15 | 32.8 | 13/14 | 32.1 | E/B_06-859 | 0/2 | NA | 0/2 | NA | 2/2 | 39.4 | 1/5 | 37.9 | 14/15 | 34.5 | 2/2 | 36.8 | 2/2 | 34.8 | 0/2 | NA | 1/5 | 35.1 | + | + | + | + |
| 13-2060/1 | Transfer | 05/07/13 | Outdoor area | 86 | 4/15 | 36.7 | 3/4 | 37.1 | 0/2 | NA | 1/1 | 39.7 | ND | ND | 5/15 | 32.7 | 1/2 | 38.9 | 1/1 | 33.9 | ND | ND | |||||||||
| 13-2060/2 | Start of force-feeding | 05/07/13 | 0/2 | NA | ND | ND | ND | 0/2 | NA | ND | ND | ND | |||||||||||||||||||
| 13-2060/3 | Force-feeding | 08/07/13 | 89 | 2/15 | 37.3 | 2/2 | 37.4 | 0/2 | NA | ND | ND | ND | 4/15 | 35.9 | 0/2 | NA | ND | ND | ND | ||||||||||||
| 13-2060/4 | Transfer | 18/07/13 | 99 | 2/15 | 35.3 | 2/2 | 35.7 | 0/2 | NA | ND | ND | ND | 2/15 | 37.1 | 0/2 | NA | ND | ND | ND | ||||||||||||
| 13-2060/5 | Slaughtering | 18/07/13 | 1/15 | 34.5 | 1/1 | 35.5 | 0/4 | NA | ND | ND | ND | 2/15 | 36.1 | 0/4 | NA | ND | ND | ND | |||||||||||||
| E | |||||||||||||||||||||||||||||||
| 13-0749/5 | Implementation | 28/02/13 | Starter building | 1 | 0/15 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | 0/5 | NA | 0/15 | NA | 0/2 | NA | 1/1 | 37.8 | 0/2 | NA | 1/5 | 35.8 | + | – | – | + | |||
| 13-0904/3 | Nail trimming | 08/03/13 | Starter building + enclosures | 9 | 0/15 | NA | 0/1 | NA | 0/1 | NA | 0/2 | NA | ND | 1/15 | 39.9 | 0/1 | NA | 1/1 | 36.5 | 0/2 | NA | ND | – | – | |||||||
| 13-0904/4 | Vaccination | 21/03/13 | Outdoor area | 22 | 0/15 | NA | 0/2 | NA | 0/1 | NA | 0/2 | NA | 0/5 | NA | 0/15 | NA | 0/2 | NA | 1/1 | 36.0 | 0/2 | NA | 0/5 | NA | + | – | – | – | |||
| 13-1159/4 | Vaccination boost | 11/04/13 | Outdoor area | 43 | 0/15 | NA | 0/2 | NA | 0/2 | NA | 2/2 | 38.7 | 1/5 | 37.6 | 9/15 | 39.1 | 0/2 | NA | 2/2 | 35.9 | 2/2 | 38.3 | 1/5 | 37.1 | + | + | + | – | |||
| 13-1458/7 | Transfer | 17/05/13 | Outdoor area | 79 | 15/15 | 32.3 | 15/15 | 32.7 | E/B_06-859 | ND | ND | ND | ND | 14/15 | 28.5 | ND | ND | ND | ND | ||||||||||||
| 13-1458/8 | Start of force-feeding | 17/05/13 | 0/2 | NA | ND | ND | ND | 2/2 | 33.5 | ND | ND | ND | |||||||||||||||||||
| 13-1598/3 | Force-feeding | 22/05/13 | 84 | 12/15 | 35.6 | 10/12 | 34.7 | E/B_06-859 | 0/2 | NA | ND | ND | ND | 15/15 | 29.5 | 2/2 | 37.2 | ND | ND | ND | |||||||||||
| 13-1598/4 | Transfer | 29/05/13 | 91 | 6/15 | 36.9 | 5/6 | 36.4 | 0/2 | NA | ND | ND | ND | 14/15 | 32.1 | 2/2 | 35.9 | ND | ND | ND | ||||||||||||
| 13-1598/5 | Slaughtering | 29/05/13 | 7/15 | 38.0 | 7/7 | 38.0 | 0/4 | NA | ND | ND | ND | 15/15 | 33.0 | 3/4 | 37.9 | ND | ND | ND | |||||||||||||
ND, not done; NA, not applicable. No. pos/total = number of positive swabs/total number of tested swabs (except as noted in footnote b).
No. pos/total = number of positive swabs/number of Chlamydiaceae PCR-positive swabs.
–, no amplification on electrophoresis; +, amplification on electrophoresis.
(iii) Environmental samples (dusters and water and soil samples).
In order to detect the presence of C. psittaci in the environment of the flocks, 196 environmental samples were obtained, in total, from the stages before transfer for force-feeding (Table 1). First of all, dusters (n = 39; Table 1) were used in farm buildings and runs. Pairs of foot-dusters (n = 34) were worn to cover the entire building area or the areas surrounding bird feeders and watering places in fields and were used for all stages except for the transfer to force-feeding, where a wall-duster (n = 5) was wiped on the building walls. The wall-duster was not used on flocks A2 (because of early slaughtering owing to a herpesvirus infection) and E (because the building had not been occupied for a long time and had already been cleaned). Two water samples from the watering places were obtained at each sampling time from introduction until the booster vaccination stage (n = 52; Table 1). Finally, soil samples (straw, droppings, or soil obtained from five different places frequented by the birds) were taken during the introduction, vaccination, and booster vaccination stages (n = 105; Table 1).
DNA extraction.
Cloacal swabs were subjected to DNA extraction using a QIAamp DNA minikit (Qiagen, Courtaboeuf, France) according to the manufacturer-recommended “buccal swab spin” protocol. DNA was eluted with 150 μl of elution buffer and stored at −20°C before analysis. Air samples were centrifuged at 32,000 × g for 1 h at 10°C, the sediments were suspended in 400 μl of phosphate-buffered saline (PBS). Dusters were placed in plastic bags with 30 ml of PBS and kneaded until the dusts were well mixed, and then 400 μl of supernatant was collected. Both air and dust suspensions were then subjected to the same DNA extraction protocol as the cloacal swabs.
Water and soil samples were subjected to supplementary extraction steps in order to also collect DNA from possibly encysted amoebae. Briefly, for the water samples, 35 ml was centrifuged at 26,500 × g for 1 h, and the sediment was then collected in 500 μl of PBS. For the soil samples, approximately 5 g of sample was mixed with 10 ml of PBS and allowed to sediment, and then 500 μl of supernatant was collected. Subsequently, both water and soil suspensions were subjected to five consecutive thermal shocks (2 min at −80°C in an ethanol bath and 1 min 30 at 99°C in a dry bath) and then ribolyzed, and 200 μl of supernatant was collected and subjected to the same extraction protocol as the other samples.
Tissue samples (spleen, liver, intestine, and lung) collected from the unhatched eggs were submitted to DNA extraction using the QIAamp DNA minikit according to the manufacturer's “tissue protocol.” DNA was eluted with 200 μl of elution buffer and stored at −20°C before analysis.
Direct detection of chlamydiae from DNA samples.
For all DNA samples, a first screening was performed using a Chlamydiaceae-specific real-time PCR targeting the 23S rRNA gene (23S-rtPCR) (22). Each reaction mix contained 2 μl of sample DNA template, 10 μl of 2× Universal Mastermix (Applied Biosystems), 0.5 μl of each primer (25 mM) and 2 μl of the probe (1 mM), and 5 μl of deionized water. DNA samples found to be positive in the 23S-rtPCR were further analyzed by a C. psittaci-specific real-time PCR targeting the incA gene (23). A positive control (C. psittaci Loth strain) was systematically included, and an internal control for potential PCR inhibition (TaqMan exogenous internal positive control; Applied Biosystems) was used for the environmental samples (dusters, water, and soil). All samples with a quantification cycle (Cq) over 40 were considered as negatives. A sample with a 20 < Cq ≤ 30 value was regarded as highly positive, while 30 < Cq ≤ 35 was regarded as moderate, and Cq > 35 was regarded as low positive.
Genotyping.
The most concentrated C. psittaci-positive DNA samples (Cq < 30), obtained from cloacal swabs and environmental DNA samples, were further analyzed to determine the genotypes involved. The ompA gene taken from positive samples was amplified using the 3GPF/5GPB primer set as previously described (24). The PCR products were sequenced at MWG (Biotech France, Roissy, France).
Detection of C. psittaci bacteriophage Chp1.
All DNA samples were screened for the presence of the bacteriophage Chp1. Initially, a real-time PCR assay targeting a 168-pb fragment of the gene encoding the major capsid protein VP1 (ORF 1) was performed with the primers VP1_Chp1_forw (5′-CCGCCTTTTGTTAAGGGTGA-3′) and VP1_Chp1_rev (5′-ATGAACGCCAAAATGACCTTG-3′) and the VP1_Chp1_probe (5′-FAM-GTTTATGTTGATTTAGCGGCTTCA-TAM-3′). Sequencing of both strands of fragments of ORF 1 gene (521 pb), as well as the ORF 2 gene encoding the minor spike protein VP2 (433 pb), was performed on the most Chp1-concentrated DNA samples using the primer pairs VP1_forw (5′-TGGTACGACTGTTGCCCAAA-3′)/VP1_rev (5′-CAGGCTGCTCTCCCAAATGA-3′) and VP2_forw (5′-TTTGGCGGTCTTGCTTCAGG-3′)/VP2_rev (5′-CAGGCAACAACTTGCCAGC-3′), respectively, to confirm the presence of the C. psittaci-specific bacteriophage (Chp1) in the samples. Amplification conditions consisted in an initial denaturation step at 95°C for 10 min followed by 30 cycles of amplification consisting of denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 1 min. This was followed by incubation at 72°C for 10 min.
Detection of amoebae.
All DNA samples from water and soil were also screened for the presence of free-living amoebae. PCR analyses were performed by amplification of the 18S rRNA gene with primers specific for Amoebozoan (Amo_1400_F/Amo-1540-R) and Vahlkampfiidae (Vahl_560_F/Vahl_730_R), as described previously (25).
Survey on farming practices and activities.
Appropriate information regarding the management, the feeding and watering practices, the cleaning and disinfection procedures (in the different buildings and fields and of the equipment used on the breeding farms), the health status of birds, and the infection-limiting precautions and antimicrobial treatments were collected by filling in a questionnaire during farm visits (Table 2). Farmers were also asked about the proximity of other breeding farms and the presence of other avian species (wild or farm birds).
TABLE 2.
Survey on farming practices and activities in farms A, B, C, D, and E
| Item | Observed practices | Farm |
||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| Interactions between two batches | ||||||
| No. of different ages on site | Two different ages present concurrently on site | x | x | x | x | x |
| Only one age | ||||||
| Interval between new batches | One new batch every 7 to 8 wks | x | x | x | x | x |
| Management of enclosure (outdoor area adjacent to starter building)a | ||||||
| Use of lime: depending on the breeder, spreading of lime for each depopulation period or once a year, over the whole enclosure or only in part (immediate surroundings, around hatches, etc.); spreading takes place on a moist surface with about 0.5 to 1 kg of lime per m2 | No | |||||
| Yes, whole enclosure for each batch | x | |||||
| Yes, in part for each batch | x | |||||
| Yes, whole enclosure once a year | x | |||||
| Yes, in part (surrounds and entrances) once a year | x | |||||
| Surface scraping of enclosure | Yes, once a year in spring | x | x | |||
| No | x | x | ||||
| Depopulation | 5 wks | x | x | x | ||
| 3 to 5 wks | x | |||||
| Management of outdoor expansion areab | ||||||
| Use of lime | Yes, whole run once a year | x | x | |||
| No | x | x | x | |||
| Surface scraping of run | Yes, once a year in spring | x | x | x | ||
| No | x | x | ||||
| Depopulation: more than 3 wks on most breeder farms, but highly variable (from 0 days to 8 wks) | 7 to 8 wks | x | ||||
| 6 to 7 wks | x | |||||
| 3 to 6 wks | x | x | ||||
| None | x | |||||
| Cleaning and disinfection of starter building | ||||||
| Cleaning with a high-pressure cold water pump using an alkaline foam detergent, Agromousse, applied to partitions and ceilings | Application of a foam detergent with a contact time of 6 h, then action with high-pressure cold water pump | x | ||||
| Application of a foam detergent with a contact time of 15 min, then action with high-pressure cold water pump | x | x | x | x | ||
| Disinfection using a disinfectant containing quaternary ammonium and glutaraldehyde at a dilution of 1% (a bactericide by contact in 5 min, a virucide in 15 min) | Yes, two disinfections, one after washing and one before arrival of next ducklings | x | ||||
| Yes, one disinfection after washing, followed by one fumigation (Fumagri, orthophenylphenol) | x | |||||
| Yes, 1 disinfection after washing | x | x | x | |||
| Use of lime (all or part, frequency). | Yes, throughout for each batch | x | x | x | x | |
| Not systematically | x | |||||
| Cleaning of starter equipment product recommended by professional agricultural body (0.5% sodium hypochlorite) | Washing with water and disinfection by soaking 24 h with sodium hypochlorite | x | ||||
| Washing with water and disinfection by soaking a few hours with sodium hypochlorite | x | |||||
| Washing with water and disinfection by soaking 24 h with acid descaler-detergent | x | |||||
| Washing with water and disinfection (quaternary ammonium and glutaraldehyde) by spraying with all-round disinfectant | x | |||||
| Washing with water and disinfection by spraying with sodium hypochlorite | x | |||||
| Cleaning of piping | Use of an alkaline (sodium hypochlorite) 30 min, then rinsing, then an acid descaler-detergent for 30 min | x | ||||
| Use of an alkaline (sodium hypochlorite) 30 min to 1 h | x | |||||
| Rinsing with water | x | x | x | |||
| Depopulation of starter building | 1 wk | x | x | x | ||
| 2 wks | x | |||||
| Up to 3 wks | x | |||||
| Cleaning and disinfection of expansion facility | ||||||
| Removal of litter | Yes | x | x | x | ||
| Not systematically after each batch, but regularly (more than twice a year) | x | |||||
| Twice a year | x | |||||
| Washing with high-pressure cold water pump | Yes, all surfaces | x | x | |||
| Not systematically: only all partitions | x | |||||
| Not systematically: only base of partitions | x | |||||
| Carried out once a year in full | x | |||||
| Application of an alkaline foam detergent (Agromousse) applied to partitions and ceiling | Yes, contact time > 15 min | x | x | x | ||
| No | x | x | ||||
| Disinfection using a disinfectant containing quaternary ammonium and glutaraldehyde | Yes, two disinfections, one after washing and one before arrival of ducks | x | ||||
| Yes, one disinfection after washing | x | |||||
| Carried out once a year | x | |||||
| Never | x | x | ||||
| Spreading of lime (all or part, frequency); spreading was carried out on a moist surface with about 0.5 to 1 kg of lime per m2 | Yes, throughout for each batch | x | x | |||
| Yes, throughout for each batch except if depopulation interval is too short (in winter) | x | |||||
| No | x | x | ||||
| Cleaning of expansion facility equipment | Systematic cleaning with cold water without disinfection | x | ||||
| Washing with cold water without disinfection not systematic | x | x | x | |||
| Washing once a year with water, then disinfection with sodium hypochlorite by soaking 1/2 day | x | |||||
| Cleaning of piping | Use of an alkaline (sodium hypochlorite) 30 min, then rinsing, then an acid descaler-detergent 30 min | x | ||||
| Rinsing with water, no use of products | x | x | x | x | ||
| Depopulation of expansion facility | 3 to 4 wks | x | ||||
| 1 wk | x | |||||
| A few days | x | x | ||||
| None | x | |||||
| Interactions between animals of the same batch | ||||||
| Access to building throughout rearing phase (summer) | No, no shelter beyond 3 wks of age | x | x | |||
| No, no shelter beyond 5 wks of age | x | |||||
| Sometimes no | x | |||||
| Always yes | x | |||||
| Access to building throughout rearing phase (winter) | No, no shelter beyond 6 wks of age | x | ||||
| Always yes | x | x | x | x | ||
| Startup density (ducks/m2) | 12 ducks/m2 | x | x | |||
| 14 ducks/m2 | x | |||||
| 19 ducks/m2 | x | x | ||||
| No. of drinkers available at introduction (one drinker available per no. of ducklings) | One drinker for less than 100 ducklings or drinking nipples available | x | ||||
| One drinker for more than 100 but less than 150 ducklings | x | |||||
| One drinker for more than 150 ducklings | x | x | x | |||
| Feeders available at introduction (1 feeder available per no. of ducklings) | One feeder for less than 50 ducklings or chain feeders | x | x | |||
| One feeder for more than 50 but less than 70 ducklings | x | |||||
| One feeder for more than 70 ducklings | x | x | ||||
| Management of straw in starter building | Straw added every day | x | x | x | x | x |
| Mortality over first 4 days (%) | ≤0.1 | x | x | x | ||
| ≤0.2 | x | |||||
| 0.2 to 0.5 | x | |||||
| Density of animals in enclosure adjacent to expansion building (ducks/m2) | 2 ducks/m2 | x | ||||
| 5 ducks/m2 | x | |||||
| 10 ducks/m2 | x | x | ||||
| Time in enclosure adjacent to expansion building (weeks) | 1 week | x | x | |||
| 2 weeks | x | x | ||||
| Surface of outdoor run area available per duck (m2/duck) | 30 m2/duck | x | ||||
| 15 m2/duck | x | |||||
| 5 m2/duck | x | x | x | |||
| Time on outdoor run area (wks) | 7 | x | x | |||
| 8 | x | |||||
| 9 | x | x | ||||
| Drinking troughs available for 2,000 ducks | Three drinking troughs | x | ||||
| Four drinking troughs | x | x | ||||
| Five drinking troughs | x | x | ||||
| Feeding troughs available for 2,000 ducks | Three feeding troughs | x | ||||
| Five feeding troughs | x | x | x | |||
| Eight feeding troughs | x | |||||
| Density in expansion building (ducks/m2) | 7 ducks/m2 | x | x | x | ||
| 14 ducks/m2 | x | |||||
| 15 ducks/m2 | x | |||||
| Management of straw in outdoor run facility | Every day | x | x | x | x | |
| Every second day | x | |||||
| Interactions between humans and animals | ||||||
| Setup | ||||||
| No. of persons present when animals are handled | 1 | x | x | |||
| 2 | x | x | x | |||
| 3 | ||||||
| 4 | ||||||
| 5 | ||||||
| 6 | ||||||
| 7 | ||||||
| 10 | ||||||
| Origin of persons (private company, neighbors, other breeders) | Farm's own personnel | x | x | x | x | x |
| Personnel from neighboring ready-for-gavage farms | ||||||
| Workers from a private company specializing in the handling of poultry and rabbits | ||||||
| Duration of operations | 1 to 2 h | x | x | x | x | x |
| 1/2 day | ||||||
| 1 day or several 1/2 days | ||||||
| More than 1 day | ||||||
| Place of operation (open-air vs indoor facility) | Closed building | x | x | x | x | x |
| Building with wide-open doors | ||||||
| Open-air facility | ||||||
| Debeaking (only for farm C) | ||||||
| No. of persons present when animals are handled | 1 | x | ||||
| 2 | ||||||
| 3 | ||||||
| 4 | ||||||
| 5 | ||||||
| 6 | ||||||
| 7 | ||||||
| 10 | ||||||
| Origin of persons (private company, neighbors, other breeders) | Farm's own personnel | x | ||||
| Personnel from neighboring ready-for-gavage farms | ||||||
| Workers from a private company specializing in the handling of poultry and rabbits | ||||||
| Duration of operations | 1 to 2 h | |||||
| 1/2 day | ||||||
| 1 day or several 1/2 days | ||||||
| More than 1 day | x | |||||
| Place of operation (open air vs indoor facility) | Closed building | x | ||||
| Building with wide-open doors | ||||||
| Open air | ||||||
| Declawing/debeaking | ||||||
| No. of persons present when animals are handled | 1 | |||||
| 2 | x | x | ||||
| 3 | ||||||
| 4 | ||||||
| 5 | ||||||
| 6 | x | x | ||||
| 7 | ||||||
| 10 | ||||||
| Origin of persons (private company, neighbors, other breeders) | Farm's own personnel | x | x | |||
| Personnel from neighboring ready-for-gavage farms | x | |||||
| Workers from a private company specializing in the handling of poultry and rabbits | x | |||||
| Duration of operations | 1 to 2 h | |||||
| 1/2 day | x | x | x | x | ||
| 1 day or several 1/2 days | ||||||
| More than 1 day | ||||||
| Place of operation (open-air vs indoor facility) | Closed building | x | x | x | x | |
| Building with wide-open doors | ||||||
| Open air | ||||||
| Primary vaccination | ||||||
| No. of persons present when animals are handled | 1 | x | x | |||
| 2 | x | |||||
| 3 | ||||||
| 4 | x | |||||
| 5 | ||||||
| 6 | x | |||||
| 7 | ||||||
| 10 | ||||||
| Origin of persons (private company, neighbors, other breeders) | Farm's own personnel | x | x | x | ||
| Personnel from neighboring ready-for-gavage farms | ||||||
| Workers from a private company specializing in the handling of poultry and rabbits | x | x | ||||
| Duration of operations | 1 to 2 h | |||||
| 1/2 day | x | x | x | |||
| 1 day or several 1/2 days | x | |||||
| More than 1 day | x | |||||
| Place of operation (open-air vs indoor facility) | Closed building | x | x | x | x | |
| Building with wide-open doors | x | |||||
| Open air | ||||||
| Booster vaccination | ||||||
| No. of persons present when animals are handled | 1 | x | x | |||
| 2 | x | |||||
| 3 | ||||||
| 4 | x | |||||
| 5 | ||||||
| 6 | x | |||||
| 7 | ||||||
| 10 | ||||||
| Origin of persons (private company, neighbors, other breeders) | Farm's own personnel | x | x | x | ||
| Personnel from neighboring ready-for-gavage farms | ||||||
| Workers from a private company specializing in the handling of poultry and rabbits | x | x | ||||
| Duration of operations | 1 to 2 h | |||||
| 1/2 day | x | x | x | |||
| 1 day or several 1/2 days | x | |||||
| More than 1 day | x | |||||
| Place of operation (open-air vs indoor facility) | Closed building | x | ||||
| Building with wide-open doors | x | x | ||||
| Open air | x | x | ||||
| Transfer to force-feeding | ||||||
| No. of persons present when animals are handled | 1 | |||||
| 2 | ||||||
| 3 | ||||||
| 4 | ||||||
| 5 | ||||||
| 6 | x | x | x | |||
| 7 | ||||||
| 10 | x | x | ||||
| Origin of persons (private company, neighbors, other breeders) | Farm's own personnel | |||||
| Personnel from neighboring ready-for-gavage farms | x | |||||
| Workers from a private company specializing in the handling of poultry and rabbits | x | x | x | x | ||
| Duration of operations | 1 to 2 h | x | x | x | x | x |
| 1/2 day | ||||||
| 1 day or several 1/2 days | ||||||
| More than 1 day | ||||||
| Place of operation (open-air vs indoor facility) | Closed building | |||||
| Building with wide-open doors | x | x | ||||
| Open air | x | x | x | |||
Use for 1 to 3 weeks starting from day 15 or 4 weeks depending on weather conditions.
Use for 8 weeks from age 4 weeks with rotation of two or three runs of 1 Ha each (highly variable depending on available surface area).
Statistical analysis.
The correlation among dependent variables of interest: the prevalence of C. psittaci shedders and the C. psittaci excretion level (shedding), the C. psittaci contamination and load in air and environmental samples, the prevalence and excretion level of bacteriophage Chp1, and the independent variables expressing the ducks' ages, management practices (feeding and watering practices, cleaning and disinfection practices), and worker activities were tested with the use of the nonparametric Spearman's correlation coefficient (Spearman's rho) test. Mann-Whitney and Kruskal-Wallis nonparametric tests were carried out to investigate the effect of the breeding space, as a two-level (indoors and outdoors) or three-level (indoors, enclosure, and outdoor expansion area) factor, respectively, on C. psittaci shedding patterns, as dependent variables of interest. A series of nonparametric Mann-Whitney tests was also performed in order to determine whether the C. psittaci contamination of environmental samples (water and soil) differed based on the occurrence of amoebae in the respective samples. Moreover, the impact of the intervention, as a multilevel factor, on C. psittaci prevalence and shedding was explored using the Kruskal-Wallis nonparametric test accompanied by a post hoc homogeneous subsets stepwise procedure, which is able to find specific significant differences among factor levels. The impact of the month of the year on C. psittaci shedding, also as a multilevel factor, was explored using the Kruskal-Wallis nonparametric test. The significance level of all tests was 0.05. Nonparametric tests were used for all analyses due to the lack of normality in their distribution. Graphical methods such as scatterplots and box plots were used in order to visualize correlation and differences. Statistical analysis was performed using SPSS (Statistical Package for Social Science) software (version 22; IBM Co, New York, NY).
RESULTS
C. psittaci was detected in each of the seven mule duck flocks studied, although none of the birds sampled during this study presented clinical signs of avian chlamydiosis. Flock A2 was slaughtered early, before the force-feeding process, due to a suspicion of a herpesvirus infection causing high mortality in the flock.
Prevalence of C. psittaci shedders and excretion level during the whole breeding process.
In the hatchery, all cloacal swabs obtained from 1-day-old ducklings were negative for chlamydiae when tested with the 23S-rtPCR. Moreover, only five organs taken from five different dead embryos (unhatched eggs) from flocks A1, C, and E were weakly positive (respectively, a lung [Cq 38.8] and an intestine [Cq 39.1], a spleen [Cq 39.5] and a lung [Cq 39.5], and a liver [Cq 38.9]).
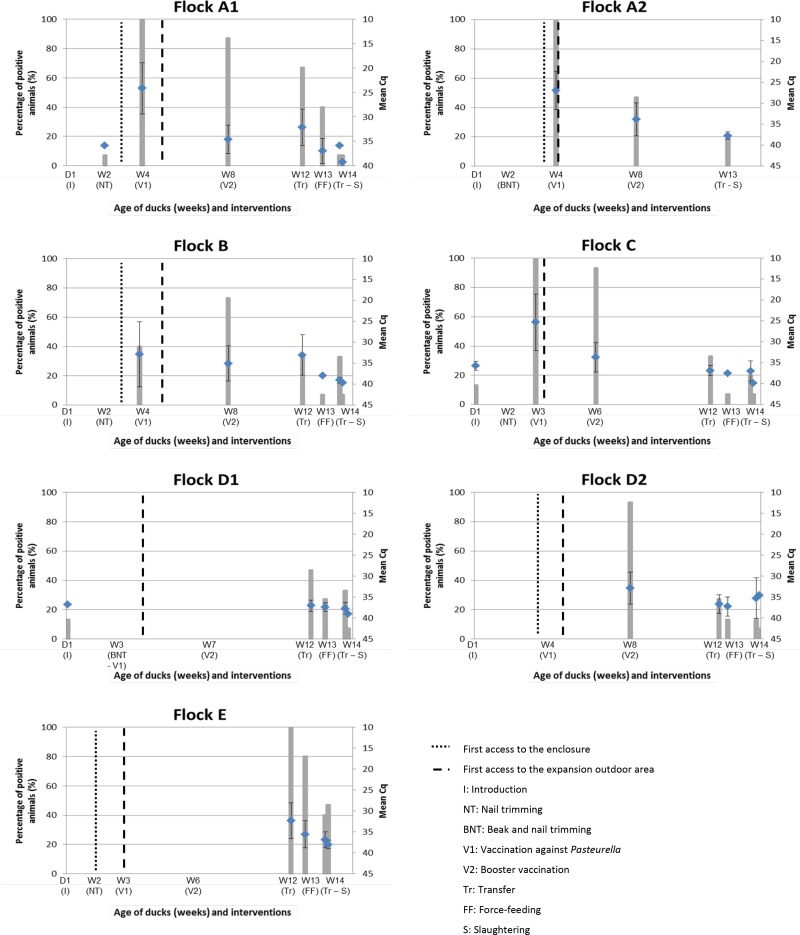
In the farms, all flocks presented C. psittaci-positive birds (shedders) during the breeding process, with the prevalence of shedders reaching up to 100% (15/15) and decreasing to 6.7% (1/15), until reaching the slaughterhouse (Table 1). The higher levels of C. psittaci shedding (lower Cq values) occurred at the peak of shedder prevalence (statistically significant negative correlation between positive birds and mean Cq values; Spearman's rho = −0.636, P < 0.001; see Fig. S1 in the supplemental material). Three different shedding patterns were observed (Fig. 2). The first pattern was observed in flocks A1, A2, and C harboring birds with heavy shedding (mean Cq of 25) about the age of 4 weeks, which for flocks A1 and A2 coincided with the first access of the ducks to a small outdoor enclosure near the livestock building. A second pattern was observed in flocks B and D2 with a moderate shedding (mean Cq values of 32.8 and 35.1) around 8 weeks of age, which coincided with a period when the birds were in the outdoor expansion area. Finally, a third pattern observed in flocks D1 and E was characterized by a low (mean Cq values up to 37) and late shedding detected during the force-feeding process at around 12 weeks of age. A statistically significant positive correlation was observed between mean Cq values and age (Spearman's rho = 0.654, P < 0.001), whereas there was no statistical correlation between C. psittaci prevalence or shedding and months of the year (P > 0.05). Only the E/B genotype of C. psittaci was detected, with the subgenotype E/B_06-859 identified in most of the flocks tested (A1, A2, B, D1, and E) and the subgenotype E/B_E30 in flock C. At the slaughterhouse, in most cases, in addition to the low prevalence of shedders, the level of C. psittaci shedding was particularly low (mean Cq of 39) (Table 1).
FIG 2.
Levels of shedding in flocks A1, A2, B, C, D1, D2, and E. The percentages of positive animals are represented by histograms, and the mean Cq of each sampling time is indicated by a mark with the associated standard deviation.
Air and environmental contamination by C. psittaci.
All air samples collected at the hatchery and the slaughterhouse were found to be negative. Positive air samples with a generally low load of C. psittaci (Cq of 35.7 to 37.7) were detected in three flocks (A1, A2, and C) (Table 1), when the birds were heavy shedders (low mean Cq of 25) (statistically significant negative correlation between Cq values of shedders and positive air samples; Spearman's rho = −0.479, P = 0.003). Dusters were detected positive (Cq values ranging from 28 to 39.7) in flocks A1, A2, and C, as well as in flocks B and D2 harboring the heavy and moderate shedders, respectively (Table 1). The dusters were not taken at the transfer-to-slaughterhouse step for either flock A2 (because of the herpesvirus outbreak) or flock E (because the building was already cleaned and had been empty for 6 weeks). Similarly, the soil samples originating from flocks A1, A2, B, C, and D2 were positive (Cq values ranging from 28.9 to 40). Chlamydia was detected in water samples (Cq values ranging from 27.9 to 39.4) obtained from flocks A2, C, D2, and E, whereas none of the environmental samples obtained from flock D1 were positive (Table 1). A statistically significant positive correlation was observed between the prevalence of shedders and the positive environmental samples: dusters (Spearman's rho = 0.639, P < 0.001), water samples (Spearman's rho = 0.496, P = 0.010), and soil samples (Spearman's rho = 0.818, P < 0.001). As expected, the soil samples were mainly positive when the birds were heavy shedders (statistically significant negative correlation between Cq values of shedders and positive soil samples; Spearman's rho = −0.726 with P = 0.011). Moreover, there was a strong relationship between C. psittaci load in soil samples and those in dusters and water samples; in fact, there was a perfect match between the orderings of the corresponding Cq values, yielding a Spearman's rho equal to 1. Sequencing of the ompA gene confirmed the presence of an E/B genotype of C. psittaci in the environmental samples harboring the same subgenotypes as those identified in the birds (E/B_06-859 or E/B_E30) (Table 1).
Presence of bacteriophage Chp1 and amoebae in avian and environmental samples.
The bacteriophage Chp1 was detected in all flocks, based on the real-time PCR assay targeting the ORF 1 gene. The bacteriophage was mostly detected (with Cq values ranging from 30 to 40) when birds were heavy chlamydial shedders. Specifically, the higher levels of C. psittaci shedding (lower Cq values) occurred at the peak of prevalence of Chp1 shedders (statistically significant negative correlation between Cq values of C. psittaci shedders and cloacal swabs positive for Chp1; Spearman's rho = −0.329 with P = 0.047). Also, there was statistically significant positive correlation between the prevalence of C. psittaci and Chp1 shedders (Spearman's rho = 0.846 with P < 0.001). However, though some Chlamydiaceae-positive DNA samples were negative for Chp1, this phage was also detected on Chlamydiaceae-negative samples. Sequencing of the ORF 1 and ORF 2 genes was performed on DNA collected on swabs from six of the seven flocks (A1, B, C, D1, D2, and E). Sequences were aligned and compared to the corresponding Chp1 sequence (accession number NC_001741). All ORF 1 fragments were identical to the ORF 1 sequence of Chp1, but three different sequences with three point mutations were obtained for the ORF 2 gene (detected in flock B for the first, in flock C for the second, and in flocks A1, D1, D2, and E for the third) compared to the ORF 2 sequence of Chp1 (see Fig. S2 in the supplemental material). The three new sequences of ORF 2 were submitted to the European Nucleotide Archive (accession numbers LN906837 to LN906839).
Free-living amoebae were detected in DNA samples from both water and soil, with a higher prevalence for Amoebozoan (38/52 and 41/105 water and soil samples tested, respectively) than for Vahlkampfiidae (16/52 and 27/105). However, no significant difference was found between the values of C. psittaci contamination in the environmental samples and the occurrence of amoebae (Amoebozoan and Vahlkampfiidae) in the corresponding samples (P > 0.05 in all nonparametric Mann-Whitney tests performed).
Evaluation of farming practices and activities.
Questions concerning the management practices routinely applied in each breeding farm or flock were grouped according to the interactions between two batches (cleaning and disinfection practices) or between birds of the same batch (feeding and watering, as well as bird density), while the interactions between humans and birds were additionally categorized according to the farming activities (interventions) (Table 2). In order to conduct statistical analysis, numerical scores were given for each practice or activity, which were correlated with the prevalence and level of C. psittaci shedding (Table 1) and were evaluated for all spaces separately (outdoors or indoors) or overall (outdoors and indoors). In each farm, none of the outdoor areas were adjacent to the outdoor areas of another farm. Only farm D raised another avian species in parallel (pheasants), whereas measures against wild animals or birds were taken in all cases but without real efficiency. Water came from public networks and occasionally from wells, except for farm C, where it came only from a well. The water network was acid-treated regularly, except in farm B. All farmers used the same cleaning and/or disinfection products supplied by the same company but applied them in different ways and at different frequencies. The analysis showed that the prevalence of C. psittaci shedders was inversely related to the level of disinfection. Lower percentages of C. psittaci shedders were generally associated with high levels of disinfection applied to starter-building facilities; a negative correlation (Spearman's rho = −0.352, P = 0.048), significant with respect to the significance level of 0.05, was detected (see Fig. S3 in the supplemental material). The practice of using an alkaline detergent for cleaning in the expansion building was not followed by farms A and C harboring the heavy C. psittaci shedders (Table 2). Expansion buildings were regularly limed, again with the exception of farms A and C. The manure was removed from the building after each flock except in farm C, where it was removed only twice a year. Furthermore, every farm except farm C underwent a depopulation period of varying durations. Finally, regarding worker activities, a significant correlation (Spearman's rho = −0.629, P < 0.001) was found between the place of interventions (open air versus closed building) and the prevalence of C. psittaci shedders; the prevalence was higher when the corresponding operations took place in the open air (see Fig. S4 in the supplemental material). Moreover, a negative correlation was observed between the duration of operations and Cq values of C. psittaci shedding (Spearman's rho = −0.498, P = 0.035); the load of shedding was higher (low Cq values) when the operation lasted for one or more than 1 day (see Fig. S5 in the supplemental material).
DISCUSSION
This study was carried out on seven mule duck flocks to explore C. psittaci circulation from the hatchery to the slaughterhouse and to identify and evaluate possible zoonotic pathways and means of C. psittaci persistence in these environments. All flocks explored in this study harbored positive birds at different moments during the breeding process, confirming the high prevalence of C. psittaci in mule ducks in France. We chose to analyze cloacal swabs in order to describe C. psittaci shedding dynamics in infected birds since chlamydiae may survive as commensals in the gastrointestinal tracts of virtually all natural hosts and persist for long periods without any overt inflammation or pathology (26). It is worth mentioning that quite similar shedding levels were obtained from cloacal and pharyngeal swabs collected and analyzed in parallel in a previous survey conducted on ducks (6). Only the E/B genotype was detected in these flocks. This genotype has been found to be common in duck farms and has already been implicated in duck-associated human cases of psittacosis in France (6).
In this study, three different patterns of C. psittaci shedding were highlighted involving birds about the age of 4, 8, and 12 weeks with heavy, moderate, and low excretion levels, respectively. The pattern corresponding to the heavy and early shedding detected in 4-week-old birds was novel compared to those identified in a previous study conducted on other duck breeding farms from the same geographical area (18). The latter study raised many questions as to whether the environment could play an important role in maintaining infection in duck farms. In most of the cases in the present study, the peak of C. psittaci shedding was detected in ducks that concomitantly had access to an outdoor run (from 1 to 4 weeks, depending on flocks). In birds of flocks A1 and A2, the heavy shedding coincided with their first access to a small outdoor field (enclosure) near the livestock building, whereas the moderate shedding presented by the birds of flocks B and D2 coincided with the period when they were on the outdoor run (outdoor expansion area). The exception to this rule was flock C, which harbored birds with heavy shedding before access to an outdoor run, but applied the most incomplete cleaning and disinfection practices (further discussed below). Other exceptions were flocks D1 and E, where the peak of shedding occurred later. In farm E, a doxycycline-based treatment had been administered in the birds' drinking water due to severe dyspnea at 4 weeks without any chlamydia being detected in bird samples collected before this age. This antibiotic is active against chlamydiae, and this probably delayed the bacterial replication and the shedding once the birds were exposed to chlamydiae. Concerning flock D1, it is noteworthy that, in contrast to all other flocks, including flock D2, C. psittaci was not detected in any environmental sample collected outdoors (Table 1). The different environmental exposure of flocks D1 and D2, since they were on separate outside fields, could explain their different shedding patterns (middle and late). In any case, variables, i.e., the prevalence and level of C. psittaci shedding, analyzed and evaluated for all breeding spaces, showed significant differences between indoors and outdoors (P = 0.018 for the prevalence and P < 0.001 for the Cq values) (see Fig. S6A and B in the supplemental material). Specifically, significant differences were found between indoors, enclosures, and outdoor expansion areas concerning the level of C. psittaci shedding (P < 0.001) with the higher levels (lower Cq values) corresponding to enclosures (see Fig. S6C in the supplemental material). Moreover, a significant effect of the “intervention” factor was also found on the prevalence (P = 0.008) and level (P = 0.001) of C. psittaci shedding (see Fig. S7 in the supplemental material). It is probable that some interventions may induce more stress for birds and lead to heavier shedding, but this cannot explain all the differences in the prevalence or the excretion level in birds and other factors, such as the “outdoor” factor, that seem to critically impact shedding (Table 1). As has been previously reported, the presence of coinfections could exacerbate chlamydial shedding (27–30). Unlike the cases described in these latter studies, it is interesting that the herpesvirus infection that emerged in flock A2 (resulting in premature slaughtering) did not increase the level of C. psittaci shedding from ducks, which therefore had the same shedding pattern as those of flock A1.
Vertical transmission of C. psittaci was already reported in chickens and turkeys (9, 31, 32). In our study, only five samples from dead embryos (unhatched eggs) were found positive, whereas all cloacal swabs (n = 105) obtained from 1-day-old ducklings at the hatchery were negative for chlamydiae. Moreover, only a few (5 of 105; Table 1) cloacal swabs recovered at introduction and at the nail-trimming stages were positive, suggesting a limited role of both vertical and horizontal (possibly by inhalation) transmission pathways between duck flocks. Taking into account all the above-mentioned findings and keeping in mind that chlamydiae are able to persist within the gastrointestinal tract in a commensal relationship (26), environmental contamination and transmission seemed to constitute the most probable main pathway in duck flocks. In this study, environmental samples such as soil and water obtained from different places frequented by the birds during the first four breeding stages were mostly positively correlated with the occurrence and prevalence of C. psittaci shedders at the respective stages. The strong association between positive soil samples and high shedding level, as well as the perfect relationship shown between the chlamydial load in soil samples and the loads in dusters and water samples, indicate that the contaminated soil may be the most significant source of transmission, even after the shedder birds have been removed from the breeding facilities. Clearly, we can assume that infected birds, possibly infected ducklings from heavily shedding laying flocks (18), introduce chlamydiae into a farm, contaminating its environment. In the hypothesis where chlamydiae are able to survive long enough in the farm environment, uninfected flocks arriving at the same places (indoors and especially outdoors) could then become infected.
In our survey, none of the C. psittaci-infected ducks presented symptoms. The absence of clinical signs coupled with the existence of various C. psittaci shedding patterns in birds could make the exposure to C. psittaci for workers unpredictable, increasing the risk since ducks are frequently handled during the breeding process. Therefore, in order to assess the exposure risks and identify the sources of contamination, it was interesting to analyze environmental samples from flocks at each occasion requiring workers to handle the birds. Among other sources, air samples and dusters from these specific stages were analyzed, since zoonotic transfer mostly occurs through inhalation of contaminated aerosols or contaminated dust particles created from avian excretion (33). Air samples were positive in three flocks (A1, A2, and C) harboring early and heavy shedders. Sampling was carried out during the primary vaccination conducted in closed buildings. Consequently, C. psittaci exposure via aerosols for workers at these farms is real, and the wearing of personal protective equipment appears to be essential. The exposure seems to be higher in breeding farms than at hatcheries and slaughterhouses, since no positive air samples were found at the latter. However, human cases have already been reported in duck hatcheries and slaughterhouses (34, 35). In our study, only a few birds were positive with a low level of shedding at the slaughterhouse, which concurs with findings obtained in this same slaughterhouse in 2013 (36). Confirmed or regularly suspected cases of psittacosis among workers in this slaughterhouse could be explained by the occurrence of heavily shedding ducks not detected in these two independent surveys, even if two duck flocks highly positive for Chlamydiaceae but not for C. psittaci were identified in a previous study (36), or by the fact that exposure to a small number of chlamydiae is enough for active infection in workers. Regarding the hatchery, the differences could possibly be explained by the different prevalences of vertical contamination from parental flocks. Meanwhile, contaminated dust is known to play an important role in worker exposure. A lot of dust is raised during several activities (handling birds during vaccinations, bird transfers, etc.) which could be a source of aerial contamination. In our study, dusters detected positive in the flocks with the heavy (A1, A2, and C) and also the moderate (B and D2) shedders (Table 1) were carrying a chlamydial load strongly associated with the loads detected in avian and soil samples. It is worth mentioning that, in a retrospective investigation conducted on a duck farm, 6 months after the clinical onset in birds and in a duck farmer who presented with a particularly severe pneumonia due to C. psittaci, dust was found to be the only positive among other environmental and avian samples (15).
Since chlamydiae are obligate intracellular bacteria, they could either survive in the cellular structures in which they have been excreted (8) or with the assistance of a host, for example, amoebae. It has indeed been observed that some Chlamydia species are able to survive and even multiply in free-living amoebae (37–39). It is thus possible that C. psittaci could also survive in this host. Environmental samples taken for this study (water and soil samples; Table 1) were also tested for the presence of amoebae, and higher prevalence rates were found for Amoebozoan than for Vahlkampfiidae. However, the link between chlamydial contamination and the presence of amoebae was less evident, obviously owing to the ubiquitous nature of the amoebae. Further in vitro studies on chlamydial survival in the presence of amoebae could validate or refute the hypothesis concerning the role of amoebae as reservoirs for C. psittaci in the environment. Furthermore, it has also been suggested that naturally occurring bacteriophage infection may be yet another persistence induction mechanism (20). In this context, chlamydial infection might be kept at a very low level for prolonged periods (e.g., in the gut) and could be triggered once the phage population was greatly reduced and after the immune response in the local site has abated. Such a scenario would be consistent with long-suspected latent or inapparent in vivo chlamydial infections (40). In our study, the C. psittaci bacteriophage Chp1 was identified in all flocks, mostly in the joint presence of chlamydia. Interestingly, the higher levels of C. psittaci shedding occurred at the peak of prevalence of bacteriophage shedders, implying that Chp1 infection may be a nonnegligible factor in C. psittaci biology and pathogenesis in ducks. However, the phage was not detected in duck C. psittaci strain suspensions available in our laboratory (data not shown), in line with previous observations under which the presence of phage may go undetected in in vitro cultures for long periods of time (41). Chp1 was also detected in 82% of DNA taken from dusters, 29% of DNA from air, 7% from water and 34% from soil recovered from farm environments (Table 1). Chp1 infection, although often “cryptic,” is probably an integral part of duck chlamydiosis in some countries, e.g., the United Kingdom (42). Remarkably, infection with C. psittaci bacteriophage Chp1 was reported for the first time in 1982 in the context of a severe outbreak of human psittacosis at a duck-processing plant in East Anglia (United Kingdom), with the Chp1 being suspected of amplifying the virulence of C. psittaci strains involved (43). The high prevalence of this bacteriophage in the mule duck breeding process could thus generate a greater risk of human contamination.
Intraflock contamination could occur at any time, thus explaining the different shedding patterns observed. This probably depends, in addition to the birds' immune status, partly on antimicrobials periodically administered, as seems to have happened at farm E, where late shedding occurred after a doxycycline-based treatment, but mainly on the cleaning and disinfection procedures implemented. The survey conducted at these farms regarding the management practices routinely applied showed that farms that apply the most incomplete cleaning and disinfection protocols were usually those with the heavy shedders, such as farms A and C. Interestingly, whereas the use of alkaline detergent and lime for cleaning could affect environmental contamination, the application of disinfectant containing quaternary ammonium and glutaraldehyde seems to be the main practice that could reduce the prevalence of C. psittaci in ducks. These findings were expected, since chlamydiae, although remaining infectious in organic material for many months, seem to be susceptible to disinfectants (8). However, it would have been interesting to further examine environmental samples recovered from farm facilities after the removal of birds and before the arrival of new flocks in order to evaluate the efficiency of different disinfection protocols. Finally, regarding worker activities, the place (open air versus closed building) and the duration of operations seem to be potential risk factors. The need to handle birds in closed buildings and the risk associated with this practice should dictate the obligatory use of personal protective equipment (e.g., clothing that does not retain dust, gloves, and face shields). Besides, the knowledge of the risk arising from prolonged exposure emphasizes the need for modification and adaptation of such work protocols and the strict observance of good practices proposed (44). Future research should focus on these topics.
Conclusion.
This study allowed a precise monitoring of mule duck flocks with a closeup on activities implying the interaction of humans with birds in order to assess the potential sources of worker exposure. It confirms the high prevalence of C. psittaci in mule duck flocks in France. The absence of clinical signs, along with the existence of various C. psittaci shedding patterns in ducks, together mean that exposure to C. psittaci for workers is unpredictable and yet considerable. Environmental contamination significantly correlated with shedding dynamics seems to be the most probable main pathway transmission. The high prevalence of Chp1 bacteriophage in mule ducks could be an important factor in C. psittaci persistence, thus creating a greater risk of human contamination. Cleaning and disinfection procedures could have an important impact on persistence and on environmental contamination. The obligatory use of personal protective equipment and the strict observance of good practices are essential to protect workers from exposure during the mule duck breeding process.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by AFSSET (Airchlam-EST-11-110).
We thank the hatchery, the breeders, and the slaughterhouse that opened their doors to us and P. Fortomaris (Laboratory of Animal Husbandry, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece) for sharing views related to husbandry issues. We also thank John Kerr for help in editing the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03179-15.
REFERENCES
- 1.Andersen AA, Vanrompay D. 2000. Avian chlamydiosis. Rev Sci Tech 19:396–404. [PubMed] [Google Scholar]
- 2.Kaleta EF, Taday EM. 2003. Avian host range of Chlamydophila spp. based on isolation, antigen detection, and serology. Avian Pathol 32:435–461. doi: 10.1080/03079450310001593613. [DOI] [PubMed] [Google Scholar]
- 3.Sachse K, Laroucau K, Vorimore F, Magnino S, Feige J, Muller W, Kube S, Hotzel H, Schubert E, Slickers P, Ehricht R. 2009. DNA microarray-based genotyping of Chlamydophila psittaci strains from culture and clinical samples. Vet Microbiol 135:22–30. doi: 10.1016/j.vetmic.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Heddema ER, van Hannen EJ, Duim B, Vandenbroucke-Grauls CM, Pannekoek Y. 2006. Genotyping of Chlamydophila psittaci in human samples. Emerg Infect Dis 12:1989–1990. doi: 10.3201/eid1212.051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanrompay D, Harkinezhad T, van de Walle M, Beeckman D, van Droogenbroeck C, Verminnen K, Leten R, Martel A, Cauwerts K. 2007. Chlamydophila psittaci transmission from pet birds to humans. Emerg Infect Dis 13:1108–1110. doi: 10.3201/eid1307.070074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laroucau K, de Barbeyrac B, Vorimore F, Clerc M, Bertin C, Harkinezhad T, Verminnen K, Obeniche F, Capek I, Bebear C, Durand B, Zanella G, Vanrompay D, Garin-Bastuji B, Sachse K. 2009. Chlamydial infections in duck farms associated with human cases of psittacosis in France. Vet Microbiol 135:82–89. doi: 10.1016/j.vetmic.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 7.Yin L, Kalmar ID, Lagae S, Vandendriessche S, Vanderhaeghen W, Butaye P, Cox E, Vanrompay D. 2013. Emerging Chlamydia psittaci infections in the chicken industry and pathology of Chlamydia psittaci genotype B and D strains in specific pathogen free chickens. Vet Microbiol 162:740–749. doi: 10.1016/j.vetmic.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Longbottom D, Coulter LJ. 2003. Animal chlamydioses and zoonotic implications. J Comp Pathol 128:217–244. doi: 10.1053/jcpa.2002.0629. [DOI] [PubMed] [Google Scholar]
- 9.Harkinezhad T, Geens T, Vanrompay D. 2009. Chlamydophila psittaci infections in birds: a review with emphasis on zoonotic consequences. Vet Microbiol 135:68–77. doi: 10.1016/j.vetmic.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 10.Lagae S, Kalmar I, Laroucau K, Vorimore F, Vanrompay D. 2014. Emerging Chlamydia psittaci infections in chickens and examination of transmission to humans. J Med Microbiol 63:399–407. doi: 10.1099/jmm.0.064675-0. [DOI] [PubMed] [Google Scholar]
- 11.Williams CJ, Sillis M, Fearne V, Pezzoli L, Beasley G, Bracebridge S, Reacher M, Nair P. 2013. Risk exposures for human ornithosis in a poultry processing plant modified by use of personal protective equipment: an analytical outbreak study. Epidemiol Infect 141:1965–1974. doi: 10.1017/S0950268812002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrovay F, Balla E. 2008. Two fatal cases of psittacosis caused by Chlamydophila psittaci. J Med Microbiol 57:1296–1298. doi: 10.1099/jmm.0.2008/001578-0. [DOI] [PubMed] [Google Scholar]
- 13.Tiong A, Vu T, Counahan M, Leydon J, Tallis G, Lambert S. 2007. Multiple sites of exposure in an outbreak of ornithosis in workers at a poultry abattoir and farm. Epidemiol Infect 135:1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaede W, Reckling KF, Dresenkamp B, Kenklies S, Schubert E, Noack U, Irmscher HM, Ludwig C, Hotzel H, Sachse K. 2008. Chlamydophila psittaci infections in humans during an outbreak of psittacosis from poultry in Germany. Zoonoses Public Health 55:184–188. doi: 10.1111/j.1863-2378.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- 15.Carlier L, Kempf M, Aaziz R, Jolivet-Gougeon A, Laroucau K. 2014. A severe case of pneumopathy in a duck breeder due to Chlamydia psittaci diagnosed by 16S rDNA sequencing. JMM Case Rep doi: 10.1099/jmmcr.0.001537. [DOI] [Google Scholar]
- 16.EU. 2000. Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the protection of workers from risks related to exposure to biological agents to work. Official Journal of the European Communities L262/21. EU-OSHA, Bilbao, Spain: https://osha.europa.eu/en/legislation/directives/exposure-to-biological-agents/77. [Google Scholar]
- 17.ACDP. 2013. Approved list of biological agents of the Advisory Committee on Dangerous Pathogens. ACDP/HSE, London, United Kingdom: http://www.hse.gov.uk/pubns/misc208.pdf. [Google Scholar]
- 18.Vorimore F, Thébault A, Poisson S, Cléva D, Robineau J, de Barbeyrac B, Durand B, Laroucau K. 2015. Chlamydia psittaci in ducks a hidden health risk for poultry workers. Pathog Dis 73:1–9. doi: 10.1093/femspd/ftv007. [DOI] [PubMed] [Google Scholar]
- 19.Amann R, Springer N, Schonhuber W, Ludwig W, Schmid EN, Muller KD, Michel R. 1997. Obligate intracellular bacterial parasites of acanthamoebae related to Chlamydia spp. Appl Environ Microbiol 63:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan RJ, Mathews SA, Mukhopadhyay S, Summersgill JT, Timms P. 2004. Chlamydial persistence: beyond the biphasic paradigm. Infect Immun 72:1843–1855. doi: 10.1128/IAI.72.4.1843-1855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Droogenbroeck C, Van Risseghem M, Braeckman L, Vanrompay D. 2009. Evaluation of bioaerosol sampling techniques for the detection of Chlamydophila psittaci in contaminated air. Vet Microbiol 135:31–37. doi: 10.1016/j.vetmic.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 22.Ehricht R, Slickers P, Goellner S, Hotzel H, Sachse K. 2006. Optimized DNA microarray assay allows detection and genotyping of single PCR-amplifiable target copies. Mol Cell Probes 20:60–63. doi: 10.1016/j.mcp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Menard A, Clerc M, Subtil A, Megraud F, Bebear C, de Barbeyrac B. 2006. Development of a real-time PCR for the detection of Chlamydia psittaci. J Med Microbiol 55:471–473. doi: 10.1099/jmm.0.46335-0. [DOI] [PubMed] [Google Scholar]
- 24.Kaltenboeck B, Kousoulas KG, Storz J. 1993. Structures of and allelic diversity and relationships among the major outer membrane protein (ompA) genes of the four chlamydial species. J Bacteriol 175:487–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Calvez T, Trouilhe MC, Humeau P, Moletta-Denat M, Frere J, Hechard Y. 2012. Detection of free-living amoebae by using multiplex quantitative PCR. Mol Cell Probes 26:116–120. doi: 10.1016/j.mcp.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Rank RG, Yeruva L. 2014. Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect Immun 82:1362–1371. doi: 10.1128/IAI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karpinska TA, Kozaczynski W, Niemczuk K, Jasik A, Kycko A, Reichert M. 2013. Mixed infection by fowlpox virus and Chlamydophila psittaci in a commercial laying hen flock. Acta Vet Hung 16:1–10. [DOI] [PubMed] [Google Scholar]
- 28.Van Loock M, Loots K, Van Heerden M, Vanrompay D, Goddeeris BM. 2006. Exacerbation of Chlamydophila psittaci pathogenicity in turkeys superinfected by Escherichia coli. Vet Res 37:745–755. doi: 10.1051/vetres:2006033. [DOI] [PubMed] [Google Scholar]
- 29.Van Loock M, Geens T, De Smit L, Nauwynck H, Van Empel P, Naylor C, Hafez HM, Goddeeris BM, Vanrompay D. 2005. Key role of Chlamydophila psittaci on Belgian turkey farms in association with other respiratory pathogens. Vet Microbiol 107:91–101. doi: 10.1016/j.vetmic.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 30.To KK, Tse H, Chan WM, Choi GK, Zhang AJ, Sridhar S, Wong SC, Chan JF, Chan AS, Woo PC, Lau SK, Lo JY, Chan KH, Cheng VC, Yuen KY. 2014. A novel psittacine adenovirus identified during an outbreak of avian chlamydiosis and human psittacosis: zoonosis associated with virus-bacterium coinfection in birds. PLoS Negl Trop Dis 8:e3318. doi: 10.1371/journal.pntd.0003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickx V, Vanrompay D. 2011. Zoonotic transmission of Chlamydia psittaci in a chicken and turkey hatchery. J Med Microbiol 60:775–779. doi: 10.1099/jmm.0.030528-0. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed B, De Boeck C, Dumont A, Cox E, De Reu K, Vanrompay D. 2015. First experimental evidence for the transmission of Chlamydia psittaci in poultry through eggshell penetration. Transbound Emerg Dis doi: 10.1111/tbed.12358. [DOI] [PubMed] [Google Scholar]
- 33.Beeckman DS, Vanrompay DC. 2009. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect 15:11–17. doi: 10.1111/j.1469-0691.2008.02669.x. [DOI] [PubMed] [Google Scholar]
- 34.Abadia G, Capek I, André-Fontaine G, Laurens E. 2006. Etude de séroprévalence de la chlamydiose aviaire chez certains professionnels avicoles en Bretagne et Pays de la Loire, 2001-2002. BEH 27–28:204–205. [Google Scholar]
- 35.Belchior E, Bradane G, Mercier A-F, Fortin N, Ollivier R, Hubert B. 2010. Investigations de cas humains de psittacose dans deux abattoirs de volailles, Pays de la Loire, Mars-Avril 2009. Epidemiol Santé Anim 57:5–11. [Google Scholar]
- 36.Hulin V, Oger S, Vorimore F, Aaziz R, de Barbeyrac B, Berruchon J, Sachse K, Laroucau K. 2015. Host preference and zoonotic potential of Chlamydia psittaci and C. gallinacea in poultry. Pathog Dis 73:1–11. doi: 10.1093/femspd/ftv007. [DOI] [PubMed] [Google Scholar]
- 37.Essig A, Heinemann M, Simnacher U, Marre R. 1997. Infection of Acanthamoeba castellanii by Chlamydia pneumoniae. Appl Environ Microbiol 63:1396–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coulon C, Eterpi M, Greub G, Collignon A, McDonnell G, Thomas V. 2012. Amoebal host range, host-free survival, and disinfection susceptibility of environmental Chlamydiae as compared to Chlamydia trachomatis. FEMS Immunol Med Microbiol 64:364–373. doi: 10.1111/j.1574-695X.2011.00919.x. [DOI] [PubMed] [Google Scholar]
- 39.Wirz M, Polkinghorne A, Dumrese C, Ziegler U, Greub G, Pospischil A, Vaughan L. 2008. Predator or prey? Chlamydophila abortus infections of a free-living amoebae, Acanthamoeba castellanii 9GU. Microbes Infect 10:591–597. [DOI] [PubMed] [Google Scholar]
- 40.Beatty WL, Morrison RP, Byrne GI. 1994. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev 58:686–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Z, Shou H-Z, Hsia R-C, Bavoil P. 2006. The Chlamydiaphages, p 79–101. In Bavoil PM, Wyrick PB (ed), Obligate intracellular parasitism. Horizon Bioscience, Oxford, United Kingdom. [Google Scholar]
- 42.Bacon EJ, Richmond SJ, Wood DJ, Stirling P, Bevan BJ, Chalmers WS. 1986. Serological detection of phage infection in Chlamydia psittaci recovered from ducks. Vet Rec 119:618–620. [PubMed] [Google Scholar]
- 43.Richmond SJ, Stirling P, Ashley CR. 1982. Virus infecting the reticulate bodies of an avian strain of Chlamydia psittaci. FEMS Microbiol Lett 14:31–36. [Google Scholar]
- 44.Deschuyffeleer TP, Tyberghien LF, Dickx VL, Geens T, Saelen JM, Vanrompay DC, Braeckman LA. 2012. Risk assessment and management of Chlamydia psittaci in poultry processing plants. Ann Occup Hyg 56:340–349. doi: 10.1093/annhyg/mer102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.