Abstract
In the current study, we examined the effects of depletion of phagocytes on the progression of Plasmodium yoelii 17XNL infection in mice. Strikingly, the depletion of phagocytic cells, including macrophages, with clodronate in the acute phase of infection significantly reduced peripheral parasitemia but increased mortality. Moribund mice displayed severe pathological damage, including coagulative necrosis in liver and thrombi in the glomeruli, fibrin deposition, and tubular necrosis in kidney. The severity of infection was coincident with the increased sequestration of parasitized erythrocytes, the systematic upregulation of inflammation and coagulation, and the disruption of endothelial integrity in the liver and kidney. Aspirin was administered to the mice to minimize the risk of excessive activation of the coagulation response and fibrin deposition in the renal tissue. Interestingly, treatment with aspirin reduced the parasite burden and pathological lesions in the renal tissue and improved survival of phagocyte-depleted mice. Our data imply that the depletion of phagocytic cells, including macrophages, in the acute phase of infection increases the severity of malarial infection, typified by multiorgan failure and high mortality.
INTRODUCTION
Malaria remains the most devastating protozoan infection in the world, with a heavy disease burden estimated to affect 300 to 500 million individuals, with 0.6 million deaths annually. Plasmodium falciparum is the most important etiological agent of malaria, causing high levels of morbidity and mortality worldwide. The severity of infection is broad-ranging, from a slight febrile illness to severe complications, which can include cerebral malaria, acidosis, acute respiratory distress syndrome, and acute renal and hepatic failure (1–3). Transmission occurs via the bite of an infected female Anopheles mosquito, which releases sporozoites into the bloodstream. The inoculated motile sporozoites invade the hepatocytes and replicate intracellularly. Following the clinically silent liver stage, the merozoite progeny move into the bloodstream and initiate the blood stage, which is associated with the clinical manifestations of malaria (1, 4).
The intensive replication of the parasite within the erythrocytes (red blood cells [RBCs]) and the exportation of parasite proteins to the RBC surface alter the RBC membrane characteristics, allowing RBCs to adhere to the endothelium and causing their sequestration in the blood microvessels of various organs. Parasitized erythrocytes (pRBCs) can also adhere to other RBCs, leukocytes, and platelets, forming intravascular rosettes and clumps, which can impede the microcirculation and disrupt the microvascular blood flow, leading to hypoxia and multiorgan failure (4). In fact, disruption of the microvascular blood flow is commonly observed in experimental and human clinical studies of severe malaria. Together with this sequestration, the overproduction of inflammatory cytokines and soluble mediators by the host immune cells at the sites of infection promotes endothelial cell injury (5–7). The loss of endothelial barrier integrity results in microvascular leakage and organ injury and dysfunction. Thrombosis is triggered by the exposure of tissue factor (TF) expressed on the surfaces of endothelial cells and monocytes to thrombin, leading to fibrin deposition in the blood vessels and obstruction of blood flow (6, 7).
Efficient control of Plasmodium infection by the host is dependent on the function of spleen via filtering pRBCs and regulating the consequent immune response to the infection (8). In the spleen, the blood leaves the terminal arterioles and flows into sinuses of marginal zone, where marginal zone macrophages and marginal metallophilic macrophages are positioned at a ring form surrounding the sinus. This strategic position of macrophages allows them to sufficiently trap and phagocytize blood pathogens (9, 10). However, it is still unclear whether splenic macrophages are crucial for the clearance of the pRBCs. In the present study, clodronate liposome (CLL), which is known to deplete splenic phagocytes (mainly macrophages) in marginal zones and red pulp (10, 11), was used to investigate the contribution of macrophages to host protection against the blood stage of P. yoelii infection in C57BL/6 mice. Our data show that resistance to nonlethal P. yoelii infection is crucially dependent on the function of phagocytic cells, including macrophages, in mice.
MATERIALS AND METHODS
Mice and infection.
Specific-pathogen-free 6-to-8-week-old female C57BL/6 mice purchased from Clea (Tokyo, Japan) were used in this study. Plasmodium yoelii 17XNL was recovered from frozen pRBC stock by passage in mice after intraperitoneal (i.p.) inoculation, and the challenge infections were induced with i.p. inoculations of 1 × 107 fresh pRBCs from donor mice. Parasitemia and survival rates were monitored daily thereafter. Parasitemia was determined by microscope in methanol-fixed thin blood smears stained with 10% Giemsa solution. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Ministry of Education, Culture, Sports, Science and Technology, Japan. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Obihiro University of Agriculture and Veterinary Medicine (permit number 24-17, 25-66).
Depletion of phagocytes and treatment with aspirin.
Phagocytes were depleted by the i.p. administration of 300 μl of CLL once 2 days before infection (−2 postinfection [p.i.]) for the early phase of infection, on day 6 p.i. for the acute phase of infection, on day 14 p.i. for the late acute phase of infection, and on day 30 p.i. for the resolved phase of infection. Mice in another group were injected in parallel with phosphate-buffered saline (PBS) plus liposomes (PL). Uninfected mice received either CLL or PL. CCL and PL were purchased from www.clodronateliposomes.org (Haarlem, the Netherlands). In a separate experiment, mice infected with P. yoelii were injected i.p. with either 25 mg/kg aspirin (acetylsalicylic acid; Sigma-Aldrich, St. Louis, MO, USA) or PBS from day 5 to day 8 p.i. (12). On day 6 p.i., the mice received either CLL or PL (300 μl) by i.p. injection.
qRT-PCR.
The mice were killed, and their organs (including spleen, liver, kidney, lungs, heart, and brain) were harvested and placed in TRI reagent (Sigma). Total RNA was then isolated and reverse transcribed to first-strand cDNA (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. The expression of P. yoelii 18S rRNA (Py18S), cytokines, and adhesion molecules was analyzed with quantitative real-time PCR (qRT-PCR) using an ABI Prism genetic analyzer (Applied Biosystems, Carlsbad, CA, USA) with SYBR green (Applied Biosystems) and the specific primers (see Table S1 in the supplemental material). The primers for qRT-PCR were designed with Primer Express software (Applied Biosystems). Specific gene expression was normalized to the expression of ubiquitin, using β-actin, GAPDH (glyceraldehyde 3-phosphate dehydrogenase), and 18S rRNA genes as the housekeeping genes. The optimal reference gene was selected based on results from the Cotton EST database (http://archive.is/www.leonxie.com). Relative levels of gene expression were calculated with the threshold cycle (ΔΔCT) method (user bulletin no. 2; Perkin-Elmer, Boston, MA, USA [http://www3.appliedbiosystems.com/cms/groups/mcb_support/documents/generaldocuments/cms_040980.pdf]).
Detection of serum cytokines and coagulation factors.
Each mouse serum sample was assayed with an enzyme-linked immunosorbent assay (ELISA) for gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-10 (Pierce Biotechnology, Rockford, IL, USA), prothrombin (Ptn; USCN Life Science, Wuhan, China), fibrinogen (Fbg), and d-dimer (DD; BMASSAY, Beijing, China), according to each manufacturer's instruction.
Hematology and serum biochemistry.
Blood cell counts were performed with an automatic cell counter (Celltac α; Nihon Kohden, Tokyo, Japan). Serum samples for biochemical analysis were examined with a clinical chemistry automated analyzer (Toshiba Medical Systems Co., Tochigi, Japan) to measure the concentrations of total protein (TP), aspartate transaminase (AST), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GTP), creatinine (CRE), and blood urea nitrogen (BUN) with specific detection reagents (Denka Seiken, Tokyo, Japan).
Histopathology, immunohistopathology, immunofluorescence, and electron microscopy.
Organs were fixed in 4% (wt/vol) buffered paraformaldehyde, embedded in paraffin, cut into 4-μm-thick sections, and then stained with hematoxylin and eosin (HE) and phosphotungstic acid hematoxylin (PTAH). The immunohistochemical staining assays were performed with specific antibodies directed against intercellular adhesion molecule 1 (ICAM1; Sino Biological Inc., Beijing, China) (diluted 1:500), von Willebrand factor (vWF; Dako, Copenhagen, Denmark) (diluted 1:1,000), CD41 (Abcam, Cambridge, MA, USA) (diluted 1:500), and P. yoelii merozoite surface protein-1 (PyMSP-1/19 rabbit antiserum; Malaria Research and Reference Reagent Resource Center) (diluted 1:3,000) as the primary antibodies. The sections were exposed to each primary antibody at 4°C overnight and were then incubated with the secondary antibody conjugated to horseradish-peroxidase-labeled polymer (EnVision+ kit; Dako) for 40 min at 37°C. The signals were detected with diaminobenzidine (ImmPACT DAB; Vector Laboratories Inc., Burlingame, CA, USA), followed by counterstaining with Mayer's hematoxylin. For immunofluorescence assays targeting CD41, vWF, and P. yoelii, fresh frozen tissues were cut into 5-μm-thick sections and fixed with cold acetone. Sections were exposed to each primary antibody at 4°C overnight and then incubated with Alexa 568-labeled/Alexa 488-labeled goat anti-rabbit IgG antibody for 40 min at 37°C. For the ultrastructural studies with electron microscopy, kidneys fixed in 4% buffered paraformaldehyde were immersed in 2.66% glutaraldehyde, postfixed in 1% osmium tetroxide, embedded in resin, and processed routinely for semithin and ultrathin sectioning. The sections were observed with a transmission electron microscope (HT7700; Hitachi, Tokyo, Japan).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA, USA). The statistical significance of differences between the groups of mice in the levels of parasitemia was analyzed by two-way analysis of variance (ANOVA). Results are presented as means ± standard errors of the means (SEM). The significance of the differences was evaluated by a one-way ANOVA followed by Tukey's multiple-comparison procedure. Student's t test was used to compare the differences in the results from two independent groups. The significance of the differences in survival was analyzed with a Kaplan-Meier nonparametric model, and the curves were compared using the log-rank test. Results were considered statistically significant when P was <0.05.
RESULTS
Depletion of phagocytes in the acute phase of P. yoelii 17XNL infection causes severe pathogenesis in C57BL/6 mice.
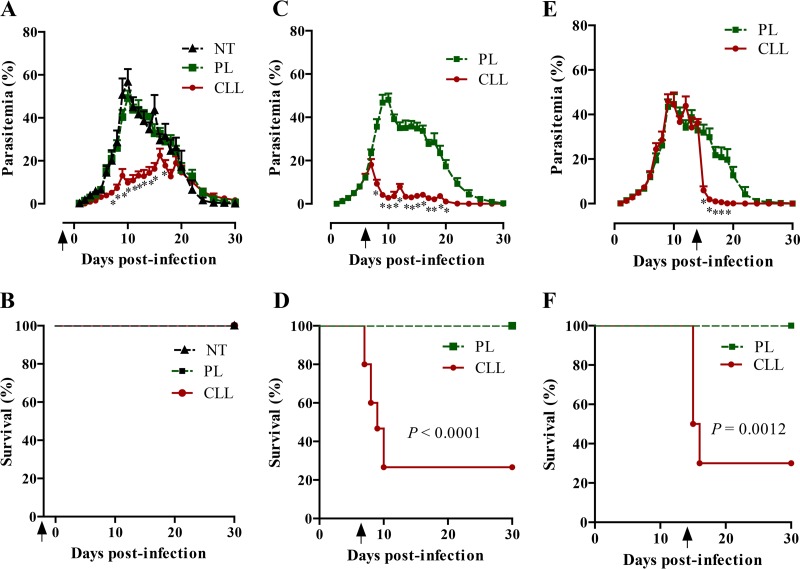
To examine the contribution of phagocytic cells, including macrophages, to the resistance to P. yoelii 17XNL infection, mice were treated with CLL at different times during the course of infection and were monitored for their parasitemia and survival. The infection of mice with P. yoelii 17XNL led to patent parasitemia by day 2 p.i. which increased slowly, peaked by days 11 to 14 p.i., and resolved within week 4 of infection in mice treated with PL or left untreated (Fig. 1A). Mice that received a single injection of CLL 2 days before infection (−2 p.i.) displayed a reduction in parasitemia compared with mice treated with PL but displayed no mortality (Fig. 1A and B). In contrast, mice that received a single injection of CLL on either day 6 or day 14 p.i. showed sharp reductions in parasitemia with coincident 70 to 73% mortality (Fig. 1C to F). Mice that received a single injection of CLL or PL on day 30 p.i. did not show any increase of their parasitemia and showed no mortality (data not shown). To gain further insight into the cellular changes in the spleen of infected mice at the acute stage after treatment with CLL, splenocytes of these mice were assayed by flow cytometry for populations of macrophages, dendritic cells (DCs), natural killer (NK) cells, natural killer T (NKT) cells, and T (CD4+ and CD8+) cells. The percentages of CD11b+ F4/80+, CD11c+, and CD3+ CD8+ cells were reduced in both noninfected and infected mice after treatment with CLL (see Fig. S1A and B in the supplemental material). In addition, P. yoelii-infected mice showed significant reductions in the percentages of Gr.1+ and CD3− NK.1+ cells and elevations in the percentages of CD3+ CD4+ cells after treatment with CLL compared with those in PL-injected mice (see Fig. S1B). Taking the data together, the depletion of phagocytes by using CLL resulted in an alteration of cellular populations in spleen in P. yoelii-infected mice. These results demonstrated the absolute need for phagocytes, including macrophages, to control acute infection of P. yoelii in mice. To identify the mechanism underlying the pathogenesis seen after phagocyte depletion in the acute phase of infection (day 6 p.i.), hematological and biochemical analyses of blood samples from the mice were performed. Ongoing infection caused a gradual decline in the numbers of peripheral RBCs and platelets and in hematocrit (HCT) values in PL-injected mice (see Table S2 in the supplemental material). Strikingly, phagocyte-depleted mice displayed significantly greater numbers of RBCs and platelets and higher HCT values on day 8 p.i. than mice injected with PL (see Table S2). These results demonstrated that the depletion of phagocyte minimized the progression of anemia and thrombocytopenia caused by P. yoelii infection. Moreover, blood levels of ALP, γ-GTP, AST, BUN, and CRE were significantly elevated in the infected mice on day 7 p.i. after treatment with CLL (Fig. 2A). Consistently, urine test results revealed increased levels of TP and CRE, but not BUN, in the phagocyte-depleted mice compared with the PL-treated mice on day 7 p.i. (see Fig. S2 in the supplemental material). Thus, the depletion of phagocytes resulted in severe pathogenesis of a nonlethal P. yoelii infection, typified by significant elevation of biochemical markers for liver and kidney dysfunction in the blood and urine.
FIG 1.
Parasitemia and survival of P. yoelii-infected mice. Mice were injected with clodronate liposomes (CLL) or PBS plus liposomes (PL) on day −2 p.i. (A and B), day 6 p.i. (C and D), or day 14 p.i. (E and F) and then monitored for 30 days for parasitemia (A, C, and E) and survival (B, D, and F). NT, infected mice with no treatment. The results represent the average levels of parasitemia and survival of mice (n = 10 for A, B, E, and F; n = 15 for C and D; n = 5 for NT mice). Data are combined from the results of two or three independent experiments. *, significant difference in the levels of parasitemia of CLL-treated mice compared with control mice as analyzed by two-way ANOVA. Arrowheads indicate the times of administration of CLL or PL.
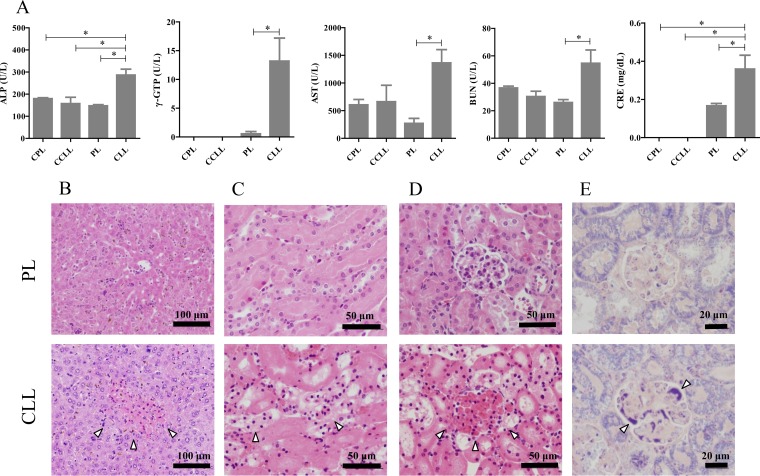
FIG 2.
Hepatic and renal failure in P. yoelii-infected mice. (A) Biochemical analysis of serum markers of liver and kidney function. Samples were collected on day 7 p.i. from P. yoelii-infected mice treated with either PBS plus liposomes (PL) or clodronate liposomes (CLL) on day 6 p.i. Uninfected mice were treated with PBS plus liposomes (CPL) or clodronate liposomes (CCLL) and sampled 1 day after treatment. The results represent the average values (± SEM) for each experimental group (uninfected, n = 5; infected, n = 15). *, significant difference between the groups as determined by one-way ANOVA, followed by Tukey's multiple-comparison test. U/L, units/liter; ALP, alkaline phosphatase; γ-GTP, γ-glutamyl transpeptidase; AST, aspartate transaminase; BUN, blood urea nitrogen; CRE, creatinine. (B to E) Histopathological observations of hepatic and renal tissues of P. yoelii-infected mice. Tissues were sampled for histopathological studies on day 7 p.i. after treatment with clodronate liposomes (CLL) or PBS plus liposomes (PL) on day 6 p.i. Hepatic sections (B) and renal sections (C to E) are shown. Infected macrophage-depleted mice (CLL) showed focal hepatic necrosis (B), tubular necrosis (C), blood stasis (D), and fibrin deposition in the glomeruli (E). Results of HE staining (B to D) and PTAH staining (E) are presented. Arrowheads indicate lesions. Scale bars are indicated.
Lethal infection is associated with severe pathological lesions in the liver and kidney.
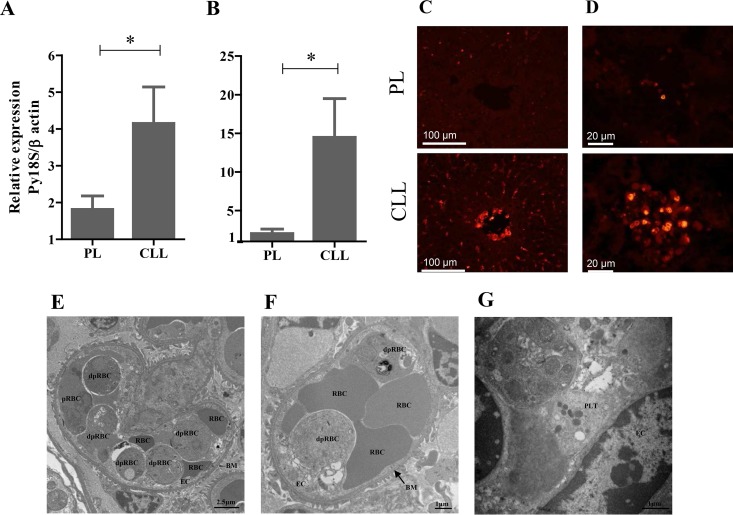
To gain better insight into the pathogenesis of the infection after the depletion of phagocytes, including macrophages, in mice, histopathological examinations of their vital organs were made. The organs were sampled from mice 1 day after the administration of CLL on day 7 p.i., when the mice started to die. Although no marked specific pathological changes were observed in the brains, hearts, lungs, or spleens of the infected mice, phagocyte-depleted mice showed severe lesions in their livers and kidneys (Fig. 2B to E). It is noteworthy that the histopathological lesions observed in the CLL-injected mice on day 7 p.i. included focal hepatic necrosis (Fig. 2B), tubular necrosis, blood stasis, thrombi, and mild fibrin deposition in the glomeruli and arterioles of the kidney (Fig. 2C to E). Concurrent hepatic and renal lesions were observed in 53.3% (8/15) of the infected phagocyte-depleted mice. Because histopathological hepatic and renal tissues were not seen in uninfected mice treated with CLL (see Fig. S3 in the supplemental material), the observed phenotypes were due to the parasite infection but not to the effect of CLL treatment. To assess the correlation between the pathological lesions and the parasite burden in the organs, we examined the parasite burdens and their localization in various tissues with qRT-PCR and immunofluorescence tests, respectively. Consistent with our histopathological observations, the expression of Py18S gene was significantly higher in the livers and kidneys of the phagocyte-depleted mice than in those of the PL-treated mice (Fig. 3A and B). An immunofluorescence study using antiserum to PyMSP-1 demonstrated that pRBCs had accumulated in the blood vessels of the liver and the glomerular capillaries of the kidney (Fig. 3C and D). To obtain direct evidence that the accumulation of pRBCs in the blood vessels of the glomeruli in moribund mice led to pathological lesions, the renal tissues were examined with transmission electron microscopy. Notably, the glomerular capillaries appeared to be dilated and filled with RBCs, pRBCs, and degenerate pRBCs (dpRBCs) (Fig. 3E). The pRBCs and dpRBCs were directly attached to activated or degenerate endothelium (Fig. 3F). Furthermore, platelets were found between the pRBCs and endothelium (Fig. 3G). From these data, we inferred that the depletion of phagocytes resulted in an increase in the levels of pRBCs attached to the endothelia of capillaries, disrupting the microvascular blood flow and causing pathological lesions in the liver and kidney.
FIG 3.
Parasite burden in the livers and kidneys of P. yoelii-infected mice. Tissues were sampled on day 7 p.i. after mice were treated with either clodronate liposomes (CLL) or PBS plus liposomes (PL) on day 6 p.i. (A and B) Analysis of the relative levels of expression of P. yoelii 18S rRNA by qRT-PCR in hepatic (A) and renal (B) tissues. Results represent the average 2−ΔΔCT value of the expression (± SEM) of the parasite gene in untreated mice (n = 15) relative to the levels in PL-treated mice (n = 15). Data are combined from three independent experiments. *, significant difference between the groups as analyzed by Student's t test. (C and D) Specific immunofluorescent staining of the parasite using antiserum directed against PyMSP-1 in the liver (C) and kidney (D). (E to G) Ultrastructural examination of renal sections from phagocyte-depleted infected mice. (E and F) Electron micrograph of a glomerular capillary lumen filled with RBCs, parasitized RBCs (pRBCs), and degenerate pRBCs (dpRBCs) (E), apparently attached to the degenerate endothelium (F). (G) Platelets were found between pRBCs and the endothelium. EC, endothelial cells; BM, basement membrane; PLT, platelets. Scale bars are indicated.
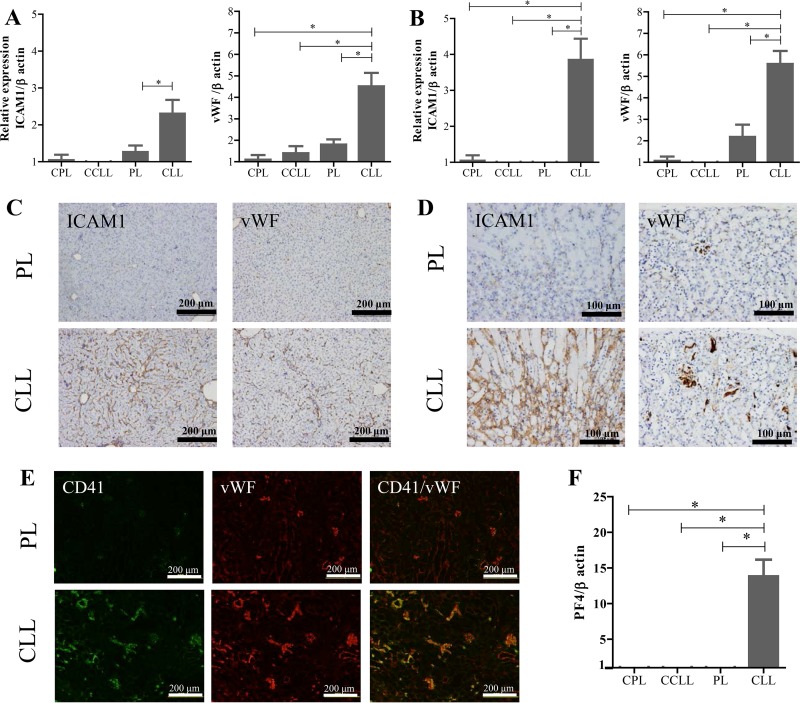
Increased parasite burden in the liver and kidney alters vascular endothelial function.
The expression of adhesion molecules by vascular endothelial cells was examined with qRT-PCR to assess their correlation with parasite burden and clinical complications. Strikingly, the expression of several endothelial biomarkers, including ICAM1, vascular cell adhesion molecule 1 (VCAM1), platelet endothelial cell adhesion molecule (PECAM), P-selectin, E-selectin, and Von Willebrand factor (vWF), was significantly upregulated in the hepatic and renal tissues of the phagocyte-depleted infected mice sampled on day 7 p.i. (Fig. 4A and B; see also Fig. S4 in the supplemental material). In contrast, uninfected mice treated with CLL showed no upregulation of the targeted genes (Fig. 4A and B; see also Fig. S4), indicating that treatment with CLL did not activate the endothelial cells. The levels of expression of ICAM1 and vWF, known to be predictors of severe malarial infection, were then examined with immunohistochemistry. Consistent with the qRT-PCR results, the staining patterns for ICAM1 and vWF in the vessels were markedly more intense in livers and kidneys of the phagocyte-depleted infected mice than in those of the PL-treated mice, which showed fewer and less intensely stained vessels (Fig. 4C and D). Because vWF-expressing platelets mediate the adhesion of pRBCs to the endothelium and the adhesion and aggregation of platelets at sites of vascular injury, leading to thrombosis, we performed immunofluorescence staining of the platelets with both anti-CD41 antibody and anti-vWF antibody. Importantly, the renal sections from the phagocyte-depleted infected mice, but not those from the PL-treated mice, displayed stronger expression of both molecules at overlapping locations, indicating the accumulation of activated platelets in the capillaries (Fig. 4E). Consistent with this, the phagocyte-depleted infected mice showed significantly upregulated expression of platelet factor 4 (PF4), a marker of platelet activation, in the renal tissues (Fig. 4F). Thus, the activation of the endothelium was coincident with increases in both the adhesion/accumulation of pRBCs and the population of activated platelets in the renal vessels.
FIG 4.
mRNA expression and immunohistochemical analyses of endothelial markers in hepatic and renal tissues. (A and B) Gene expression of endothelial-cell adhesion molecules in hepatic (A) and renal tissues (B). Samples from P. yoelii-infected mice treated with either PBS plus liposomes (PL) or clodronate liposomes (CLL) on day 6 p.i. were collected on day 7 p.i. Uninfected mice were treated with PBS plus liposomes (CPL) or clodronate liposomes (CCLL) and sampled 1 day after treatment. The results represent the average 2−ΔΔCT value of the expression (± SEM) of each targeted gene in the mice (uninfected, n = 5; infected, n = 15) relative to that of the corresponding gene in uninfected mice treated with PBS plus liposomes (CPL). *, significant difference between the groups as determined by one-way ANOVA. (C to E) Immunohistochemical observations. Sections of liver (C) and kidney (D) were stained with specific antibody directed against ICAM1 or vWF. (E) Specific immunofluorescent staining for activated endothelial cells/platelets and platelets in renal tissues using antibodies directed against vWF and CD41. Scale bars are indicated. (F) Expression of PF4, an platelet activation marker in renal tissues. The results represent the average 2−ΔΔCT value of the expression (± SEM) of each targeted gene in the mice (uninfected, n = 5; infected, n = 15) relative to that of the corresponding gene in uninfected mice treated with PBS plus liposomes (CPL). *, significant difference between the groups as determined by one-way ANOVA, followed by Tukey's multiple-comparison test.
Levels of coagulation and inflammatory responses are elevated in phagocyte-depleted infected mice.
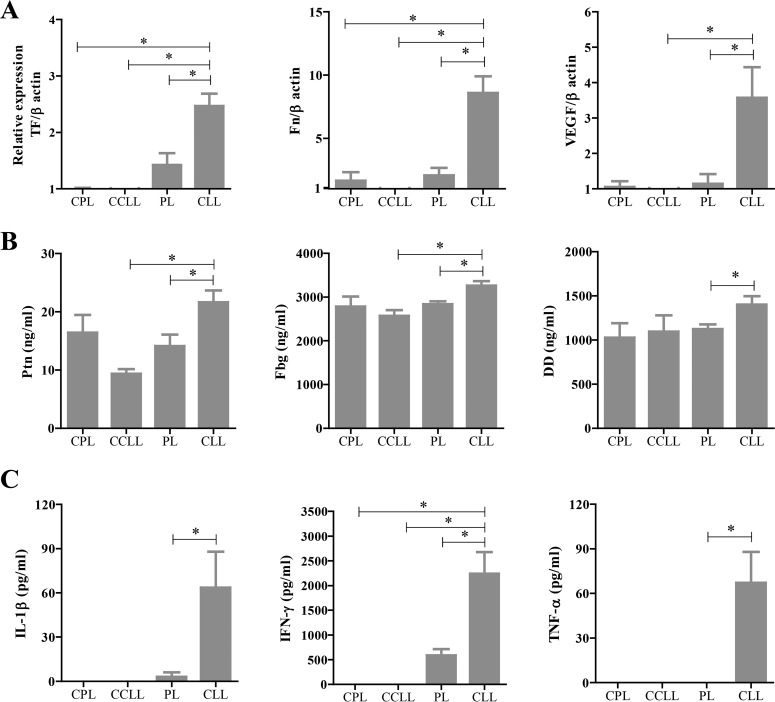
Given the importance of activated platelets in initiating the coagulation cascade and inflammatory response, several biomarker molecules were examined in the tissues and blood of the mice. Importantly, the expression of the genes encoding TF (the initiator of the extrinsic coagulation cascade), fibronectin (Fn), and vascular endothelial growth factor (VEGF), markers of fibrosis, was upregulated in the renal tissues of the phagocyte-depleted mice compared with the levels seen in the PL-treated mice on day 7 p.i. with P. yoelii (Fig. 5A). Consistently, blood levels of coagulation factors, including Ptn, Fbg, and DD, were significantly increased in the phagocyte-depleted mice on day 7 p.i. (Fig. 5B). We next examined the blood levels of IL-1β, IFN-γ, TNF-α, and IL-10, which are known to play essential roles in the pathogenesis of malaria infection, as well as their levels of expression in spleen tissues. Notably, the phagocyte-depleted mice exhibited significantly increased levels of serum IL-1β, IFN-γ, and TNF-α (Fig. 5C) but not of IL-10 (data not shown) on day 7 p.i. compared with PL-treated mice. Similarly, the expression of the genes encoding IFN-γ and TNF-α, but not IL-10, was upregulated in the spleens of the phagocyte-depleted mice on day 7 p.i. (see Fig. S5 in the supplemental material). The gene expression of TGF-β was not detected in any of the samples of spleens (data not shown). Collectively, these results demonstrated a correlation between glomerular fibrin deposition and the activation of the coagulation and inflammatory responses in phagocyte-depleted infected mice.
FIG 5.
Expression and serum levels of coagulation factors and serum inflammatory cytokine in P. yoeli-infected mice. (A) Expression of coagulation and damage markers in renal tissues. The results represent the average 2−ΔΔCT value of the expression (± SEM) of each targeted gene in the mice (uninfected, n = 5; infected, n = 15) relative to that of the corresponding gene in uninfected mice treated with PBS plus liposomes (CPL). (B and C) Serological detection of coagulation factors (B) and inflammatory cytokines (C). P. yoelii-infected mice treated with either PBS plus liposomes (PL) or clodronate liposomes (CLL) on day 6 p.i. were collected on day 7 p.i. Uninfected mice were treated with PBS plus liposomes (CPL) or clodronate liposomes (CCLL) and sampled 1 day after treatment. *, significant difference between the groups as determined by one-way ANOVA, followed by Tukey's multiple-comparison test.
Aspirin therapy attenuates the severity of P. yoelii infection in phagocyte-depleted mice.
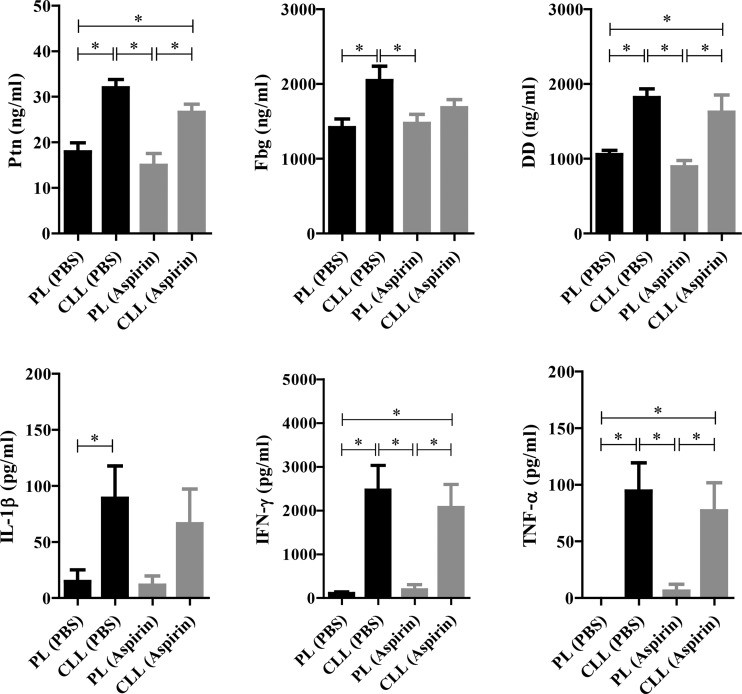
To clarify the mechanism of pathogenesis observed in our study, aspirin, an antiplatelet drug, was used to reduce the serious vascular events attributable to severe infection. Interestingly, the treatment of phagocyte-depleted mice with aspirin caused a slight increase in their peripheral blood parasitemia that was coincident with their improved survival (Fig. 6A and B). In parallel, the parasite burden in the kidney, but not that in the liver, was significantly reduced in the phagocyte-depleted mice treated with aspirin compared with the phagocyte-depleted mice treated with PBS (Fig. 6C and D). Renal lesions, including tubular necrosis and thrombi in the glomeruli, were also observed in the infected phagocyte-depleted mice treated with either aspirin or PBS (see Fig. S6 in the supplemental material). However, the frequency of these lesions in the renal tissues was lower in the aspirin-treated mice (30%, 3/10) than in the PBS-treated mice (60%, 6/10) after infection with P. yoelii. Hepatic necrosis was also observed in the infected phagocyte-depleted mice that were injected with aspirin (20%) or PBS (30%). Consistent with the pathological observations, the serum levels of biochemical markers of liver and kidney dysfunction were significantly elevated in the macrophage-depleted mice compared with the PL-treated mice on day 7 p.i. However, the levels of ALP, γ-GTP, AST, and BUN but not CRE were significantly lower in phagocyte-depleted mice treated with aspirin than in those treated with PBS (Fig. 6E). Next, we examined the coagulation and inflammatory responses, the activation and integrity of the endothelial cells, and the platelet activation in the renal tissues of the infected mice after treatment with aspirin. The phagocyte-depleted infected mice displayed significantly higher blood levels of Ptn, Fbg, DD, IFN-γ, TNF-α, and IL-1β (Fig. 7) and greater expression of the genes for the activation of endothelial cells and platelets than did the PL-treated mice on day 7 p.i. (see Fig. S7). Importantly, the elevation of these markers tended to be reduced in the infected phagocyte-depleted mice after aspirin treatment, although there was no statistically significant difference (Fig. 7; see also Fig. S7). These results showed that improved survival correlated strongly with a reduction in the parasite burden in the renal tissues and was partially associated with the coagulation/inflammatory responses and the activation of endothelial cells and platelets.
FIG 6.
Effects of aspirin treatment on the outcomes of infection with P. yoelii. (A and B) Parasitemia (A) and survival (B) of mice in the 30 days after infection. The results represent the average levels of parasitemia ± SEM in the mice (n = 10). (C and D) Parasite burden in hepatic (C) and renal (D) tissues as examined by qRT-PCR. The relative levels of expression of P. yoelii 18S rRNA in the livers (C) and kidneys (D) of infected mice sampled on day 7 p.i. are indicated. The results represent the average 2−ΔΔCT values of expression (± SEM) in the infected mice (n = 10) relative to that in mice treated with PBS plus liposomes and injected with PBS [PL(PBS)]. (E) Biochemical analysis of serum markers of liver and kidney function in infected mice. Mice were injected with either clodronate liposomes (CLL) or PBS plus liposomes (PL) on day 6 p.i. and were also treated with either aspirin or PBS from day 5 to day 8 p.i. *, significant difference between the groups as determined by one-way ANOVA, followed by Tukey's multiple-comparison test.
FIG 7.
Effects of aspirin treatment on serum levels of coagulation factors and inflammatory cytokines. Mice were injected with either clodronate liposomes (CLL) or PBS plus liposomes (PL) on day 6 p.i. and were also treated with either aspirin (gray column) or PBS (black column) on days 5 and 6 p.i. and were sampled for analyses on day 7 p.i. The results represent the average values (± SEM) for the analyzed mice (n = 10). *, significant difference between the groups as determined by one-way ANOVA, followed by Tukey's multiple-comparison test.
DISCUSSION
We have investigated the contribution of phagocytic cells, including macrophages, to protection against nonlethal infection of P. yoelii in mice. The depletion of phagocytes in the acute phase of P. yoelii infection, but not in the early phase or resolved phase, impaired the resistance of C57BL/6 mice to the infection. The elevated parasite burden in the liver and kidney of phagocyte-depleted mice was accompanied by several pathophysiological features, including microvascular obstruction, increased levels of coagulation factors and proinflammatory cytokines, and the activation of the endothelium. An earlier study had shown that depletion of macrophages using two injections of CLL in the early phase of infection (days −2 and 3 postinfection) resulted in increased parasitemia in P. yoelii 17XNL-infected mice and rapid mortality that occurred a day earlier than that seen with control mice infected with lethal P. yoelii 17XL (13). The differences in these findings are most likely due to differences in the times of drug administration, numbers of injections, and doses of challenge infection. Likewise, Stevenson and colleagues noted that depletion of macrophages on day 6 postinfection with P. chabaudi AS was more effective than depletion of macrophages in the early phase of infection (day −1 postinfection) (14). Here, single administration of CLL to P. yoelii-infected mice at day 6 or day 14 but not at day −2 or day 30 postinfection resulted in severe pathogenesis in mice typified by hepatic and renal failure. The severe pathogenesis after depletion of phagocytes at the acute phase of infection is most probably due to the high parasitemia and to the presence of abundant pRBCs with mature-stage parasites that may sequestrate in the microvascular and cause sudden disruption in the blood flow of organs (4).
Treatment with a single injection of CLL in the acute phase of P. yoelii infection resulted in a reduction in the splenic populations of macrophages, DCs, granulocytes, and NK and CD8+ T cells and an increase in the population of CD4+ T cells. Likewise, naive mice treated with CLL had reduced splenic populations of macrophages, DCs, and CD8+ T cells. This treatment is known to mainly affect macrophages and DCs in the marginal zone of spleen as well as Kupffer cells in the liver (10). Indeed, clodronate is specifically delivered into phagocytic cells by the use of liposomes as vehicles. Within these cells, phospholipid bilayers of liposome are disrupted by lysosomal phospholipases and clodronate can be intracellularly released, causing irreversibly damaged cells and cell death by initiating apoptosis (10). Therefore, we believe that the impairment of the clearance of pRBCs and their particles in spleen by resident splenic phagocytic cells, including macrophages, abrogated the resistance to nonlethal malarial infection. Nonetheless, the decrease in levels of splenic granulocytes and NK cells in infected mice is probably due to the loss of macrophages and DCs, which act as antigen-presenting cells regulating the immune response to the infection. The increase of the population of CD4+ T cells might be caused by the elevation of the burden of parasites and their antigens after depletion of phagocytic cells. The CD4+ T cells are known to be key players in protective immunity and in the pathogenesis of blood-stage malaria infection. Moreover, CD4+ CD25+ Foxp3+ regulatory T cells are activated and their populations expanded in response to malarial infection in mice and humans to control proinflammatory immune responses upon Plasmodium infection (15, 16). However, the contradictory findings in studies of murine malaria suggested diverse (protective or pathological) roles of regulatory T cells during infection (15, 16). Therefore, further study is needed to address the correlation between the regulatory T cells and severe pathogenesis in phagocyte-depleted mice.
Furthermore, phagocyte-depleted mice exhibited higher levels of IFN-γ, TNF-α, and IL-1β, which are known to play important roles in protection and in the pathogenesis of malaria (17). Therefore, the depletion of phagocytes not only impairs the clearance of pRBCs but also alters the consequent immune responses to the infection. Enhanced sequestration of parasitized RBC may then trigger inflammation and cause concomitant enhancement of proinflammatory cytokine levels. Elevated plasma levels of IFN-γ, TNF-α, and IL-1β cytokines in severe malaria are associated with systemic complications and pathological abnormalities in several organs. A report of a previous clinical study noted that renal failure in severe malaria is positively correlated with elevated blood concentrations of TNF-α (18). In support of this concept, mice deficient in TNF-α/β or IFN-γ are completely resistant to experimental cerebral malaria (19, 20). Therefore, further study is required to investigate the contribution of elevation of levels of cytokines to increased pathogenesis of malaria.
Phagocyte-depleted mice suffered acute renal failure, characterized by tubular necrosis, glomerular thrombosis, mild fibrin deposition, and proteinuria, with elevated levels of creatinine in the blood. The pathological lesions observed in moribund mice may have been due to the increased attachment of pRBCs in the glomerular capillaries, triggering regional intravascular coagulation, blood hyperviscosity, and blood stasis. These results are consistent with the concept that the outcome of severe malarial infection depends on the degree of sequestration and accumulation of pRBCs in the microvasculature of vital organs (21, 22). The absence of electron-dense deposits in the basement membranes of the glomerular capillaries and monocyte infiltration into the glomerular capillaries indicates that the underlying mechanism of acute renal failure in our mouse model is not immune-complex-mediated glomerulonephritis. In an analogous fashion, acute renal failure has frequently been reported to be a serious complication of P. falciparum and P. vivax malaria in nonimmune adults and children in Africa and Southeast Asia (23, 24). Renal ischemia arising from the sequestration of pRBCs in the glomerular and tubulointerstitial capillaries is believed to be the major key pathophysiology related to acute renal failure in humans and experimental animals (25, 26). Moreover, patients suffering from acute renal failure arising from malarial infection may display oliguria, proteinuria, hyperbilirubinemia, and high levels of plasma creatinine (27, 28). Renal biopsy specimens of patients show acute tubular necrosis, tubulointerstitial nephritis, and glomerular changes with mesangial proliferation, with or without immune complex deposition. Glomerular hypercellularity, typified by the sequestration of pRBCs with or without the accumulation of monocytes in the glomerular capillaries, is a common pathological feature in these patients (29, 30). Fibrin deposition in the glomeruli has also been observed postmortem in some malarial patients (29). Taking the data together, the high degree of pathophysiological similarity between our findings and those from human malaria patients suffering from renal failure highlights the importance of our model for investigating novel strategies for therapeutic interventions.
The presence of fibrin and thrombi in the glomerular capillaries is the most common pathological feature of many renal diseases characterized by the interruption of blood flow, irreversible ischemia, and necrosis. The mechanism of fibrin deposition in the small arteries and glomerular capillaries has been shown to depend on the interaction between endothelial cells and the macrophages or platelets that initiate thrombosis and fibrosis (29, 30). However, phagocyte-depleted mice exhibited an increase in the number of activated platelets, which is most probably due to the elevation in parasite burden and in production of cytokines as well as the endothelial damage in liver and kidney. Generally, the direct interaction or communication between activated platelets and the inflamed endothelium results in a coagulation response via the amplification of thrombin from TF, leading to fibrin deposition in the glomerular capillaries and arterioles (6, 30). In our study, therefore, the aggregation of pRBCs, dpRBCs, and activated platelets in the inflamed microvasculature of the renal tissues that coincided with increased coagulation and inflammatory responses in the CLL-treated mice may explain the observation of thrombi and fibrin deposition in the capillaries. Consistent with this notion, platelets are known to play important roles in cerebral malaria by facilitating the cytoadhesion of P. falciparum pRBCs to the activated endothelium (31). Platelets respond rapidly to pRBCs, forming aggregations of pRBCs and platelets via the activity of vWF which then attach firmly to the inflamed endothelia by their surface adhesion molecules (32, 33). The upregulated expression of adhesive ligands of platelets stabilizes plug formation and recruits other cells, resulting in microvascular obstruction with or without fibrin deposition (6, 31). Concurrently, malarial patients display thrombocytopenia resulting from the sequestration/aggregation of activated platelets to the vascular endothelium and the removal of platelet-bound pRBCs from the blood by phagocytosis (5). Taking the data together, these findings suggest that inadequate removal of aggregated platelets and pRBCs by phagocytes results in microvascular obstruction in the vital organs, such as the kidney, thus causing severe malaria.
Because platelets are crucial in initiating the coagulation cascade, we presumed that the blockage of platelet activation by aspirin may reduce the coagulation-inflammation responses. Our results demonstrated that treatment with aspirin attenuated the severity of P. yoelii infection in phagocyte-depleted mice, which was evidenced by their improved survival. The phagocyte-depleted mice treated with aspirin displayed a reduced parasite burden in the kidney, with fewer renal lesions than the phagocyte-depleted mice treated with PBS. A possible interpretation of the improved survival of the phagocyte-depleted mice after treatment with aspirin is that fewer pRBCs were sequestered to the glomerular capillaries because platelet aggregation and the binding of pRBCs and platelets to the endothelium were disrupted by administration of aspirin. However, these results emphasize that phagocytic cells, including macrophages, are the central determinants of the infection outcome, probably as the result of mediating the clearance of pRBCs and aggregated platelets.
In conclusion, our data imply that severe malarial infection in patients and experimental animals may be attributable to the inappropriate function of phagocytic cells, including macrophages, in the removal of pRBCs, leading to their excessive sequestration and impairment of the microvascular blood flow.
Supplementary Material
ACKNOWLEDGMENTS
We thank Motomi Torii (Department of Molecular Parasitology, Ehime University School of Medicine, Japan) for providing P. yoelii 17XNL. We thank Youko Matsushita, Megumi Noda, and Yoshie Imura (National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine) for their excellent technical assistance. We sincerely thank Satoru Kawai (Center for Tropical Medicine and Parasitology, Dokkyo Medical University) for his helpful advice and discussion of the electron microscopy study.
Funding Statement
This research was supported by the Japan Society for the Promotion of Science (JSPS) through the Funding Program for Next-Generation World-Leading Researchers (NEXT Program), initiated by the Council for Science and Technology Policy. JSPS provided funding to Yoshifumi Nishikawa under grant number 2011/LS003.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01005-15.
REFERENCES
- 1.Miller LH, Baruch DI, Marsh K, Doumbo OK. 2002. The pathogenic basis of malaria. Nature 415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 2.Miller LH, Ackerman HC, Su XZ, Wellems TE. 2013. Malaria biology and disease pathogenesis: insights for new treatments. Nat Med 19:156–167. doi: 10.1038/nm.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schofield L, Grau GE. 2005. Immunological processes in malaria pathogenesis. Nat Rev Immunol 5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 4.Dondorp AM, Ince C, Charunwatthana P, Hanson J, van Kuijen A, Faiz MA, Rahman MR, Hasan M, Bin Yunus E, Ghose A, Ruangveerayut R, Limmathurotsakul D, Mathura K, White NJ, Day NP. 2008. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis 197:79–84. doi: 10.1086/523762. [DOI] [PubMed] [Google Scholar]
- 5.Faille D, El-Assaad F, Alessi MC, Fusai T, Combes V, Grau GE. 2009. Platelet-endothelial cell interactions in cerebral malaria: the end of a cordial understanding. Thromb Haemost 102:1093–1102. [DOI] [PubMed] [Google Scholar]
- 6.Francischetti IM, Seydel KB, Monteiro RQ. 2008. Blood coagulation, inflammation, and malaria. Microcirculation 15:81–107. doi: 10.1080/10739680701451516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogetseder A, Ospelt C, Reindl M, Schober M, Schmutzhard E. 2004. Time course of coagulation parameters, cytokines and adhesion molecules in Plasmodium falciparum malaria. Trop Med Int Health 9:767–773. doi: 10.1111/j.1365-3156.2004.01265.x. [DOI] [PubMed] [Google Scholar]
- 8.Engwerda CR, Beattie L, Amante FH. 2005. The importance of the spleen in malaria. Trends Parasitol 21:75–80. doi: 10.1016/j.pt.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Buffet PA, Safeukui I, Deplaine G, Brousse V, Prendki V, Thellier M, Turner GD, Mercereau-Puijalon O. 2011. The pathogenesis of Plasmodium falciparum malaria in humans: insights from splenic physiology. Blood 117:381–392. doi: 10.1182/blood-2010-04-202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aichele P, Zinke J, Grode L, Schwendener RA, Kaufmann SH, Seiler P. 2003. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J Immunol 171:1148–1155. doi: 10.4049/jimmunol.171.3.1148. [DOI] [PubMed] [Google Scholar]
- 11.van Rooijen N, Hendrikx E. 2010. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol 605:189–203. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]
- 12.McMorran BJ, Marshall VM, de Graaf C, Drysdale KE, Shabbar M, Smyth GK, Corbin JE, Alexander WS, Foote SJ. 2009. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science 323:797–800. doi: 10.1126/science.1166296. [DOI] [PubMed] [Google Scholar]
- 13.Couper KN, Blount DG, Hafalla JC, van Rooijen N, de Souza JB, Riley EM. 2007. Macrophage-mediated but gamma interferon-independent innate immune responses control the primary wave of Plasmodium yoelii parasitemia. Infect Immun 75:5806–5818. doi: 10.1128/IAI.01005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevenson MM, Ghadirian E, Phillips NC, Rae D, Podoba JE. 1989. Role of mononuclear phagocytes in elimination of Plasmodium chabaudi AS infection. Parasite Immunol 11:529–544. doi: 10.1111/j.1365-3024.1989.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 15.Scholzen A, Minigo G, Plebanski M. 2010. Heroes or villains? T regulatory cells in malaria infection. Trends Parasitol 26:16–25. [DOI] [PubMed] [Google Scholar]
- 16.Hansen DS, Schofield L. 2010. Natural regulatory T cells in malaria: host or parasite allies? PLoS Pathog 29:e1000771. doi: 10.1371/journal.ppat.1000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angulo I, Fresno M. 2002. Cytokines in the pathogenesis of and protection against malaria. Clin Diagn Lab Immunol 9:1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day NP, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TT, Mai NT, Phu NH, Sinh DX, White NJ, Ho M. 1999. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis 180:1288–1297. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 19.Rudin W, Eugster HP, Bordmann G, Bonato J, Müller M, Yamage M, Ryffel B. 1997. Resistance to cerebral malaria in tumor necrosis factor-alpha/beta-deficient mice is associated with a reduction of intercellular adhesion molecule-1 up-regulation and T helper type 1 response. Am J Pathol 150:257–266. [PMC free article] [PubMed] [Google Scholar]
- 20.Rudin W, Favre N, Bordmann G, Ryffel B. 1997. Interferon-gamma is essential for the development of cerebral malaria. Eur J Immunol 27:810–815. doi: 10.1002/eji.1830270403. [DOI] [PubMed] [Google Scholar]
- 21.Baptista FG, Pamplona A, Pena AC, Mota MM, Pied S, Vigário AM. 2010. Accumulation of Plasmodium berghei-infected red blood cells in the brain is crucial for the development of cerebral malaria in mice. Infect Immun 78:4033–4039. doi: 10.1128/IAI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haque A, Best SE, Amante FH, Ammerdorffer A, de Labastida F, Pereira T, Ramm GA, Engwerda CR. 2011. High parasite burdens cause liver damage in mice following Plasmodium berghei ANKA infection independently of CD8(+) T cell-mediated immune pathology. Infect Immun 79:1882–1888. doi: 10.1128/IAI.01210-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naqvi R, Ahmad E, Akhtar F, Naqvi A, Rizvi A. 2003. Outcome in severe acute renal failure associated with malaria. Nephrol Dial Transplant 18:1820–1823. doi: 10.1093/ndt/gfg260. [DOI] [PubMed] [Google Scholar]
- 24.Elsheikha HM, Sheashaa HA. 2007. Epidemiology, pathophysiology, management and outcome of renal dysfunction associated with plasmodia infection. Parasitol Res 101:1183–1190. doi: 10.1007/s00436-007-0650-4. [DOI] [PubMed] [Google Scholar]
- 25.Houba V. 1979. Immunologic aspects of renal lesions associated with malaria. Kidney Int 16:3–8. doi: 10.1038/ki.1979.96. [DOI] [PubMed] [Google Scholar]
- 26.Ehrich JH, Eke FU. 2007. Malaria-induced renal damage: facts and myths. Pediatr Nephrol 22:626–637. doi: 10.1007/s00467-006-0332-y. [DOI] [PubMed] [Google Scholar]
- 27.Sowunmi A. 1996. Renal function in acute falciparum malaria. Arch Dis Child 74:293–298. doi: 10.1136/adc.74.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguansangiam S, Day NP, Hien TT, Mai NT, Chaisri U, Riganti M, Dondorp AM, Lee SJ, Phu NH, Turner GD, White NJ, Ferguson DJ, Pongponratn E. 2007. A quantitative ultrastructural study of renal pathology in fatal Plasmodium falciparum malaria. Trop Med Int Health 12:1037–1050. doi: 10.1111/j.1365-3156.2007.01881.x. [DOI] [PubMed] [Google Scholar]
- 29.Boonpucknavig V, Sitprija V. 1979. Renal disease in acute Plasmodium falciparum infection in man. Kidney Int 16:44–52. doi: 10.1038/ki.1979.101. [DOI] [PubMed] [Google Scholar]
- 30.Hertig A, Rondeau E. 2004. Role of the coagulation/fibrinolysis system in fibrin-associated glomerular injury. J Am Soc Nephrol 15:844–853. doi: 10.1097/01.ASN.0000115400.52705.83. [DOI] [PubMed] [Google Scholar]
- 31.Cox D, McConkey S. 2010. The role of platelets in the pathogenesis of cerebral malaria. Cell Mol Life Sci 67:557–568. doi: 10.1007/s00018-009-0211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larkin D, de Laat B, Jenkins PV, Bunn J, Craig AG, Terraube V, Preston RJ, Donkor C, Grau GE, van Mourik JA, O'Donnell JS. 2009. Severe Plasmodium falciparum malaria is associated with circulating ultra-large von Willebrand multimers and ADAMTS13 inhibition. PLoS Pathog 5:e1000349. doi: 10.1371/journal.ppat.1000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francischetti IM, Seydel KB, Monteiro RQ, Whitten RO, Erexson CR, Noronha AL, Ostera GR, Kamiza SB, Molyneux ME, Ward JM, Taylor TE. 2007. Plasmodium falciparum-infected erythrocytes induce tissue factor expression in endothelial cells and support the assembly of multimolecular coagulation complexes. J Thromb Haemost 5:155–165. doi: 10.1111/j.1538-7836.2006.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.