Abstract
Background: Micro-focused ultrasound with visualization has been cleared by the United States Food and Drug Administration to noninvasively lift the eyebrow, lift submental and neck tissue, and improve lines and wrinkles of the décolleté. Objective: The objective of this prospective, open-label pilot study was to evaluate the efficacy and safety of patient-specific, customized micro-focused ultrasound with visualization treatment with vertical vectoring to lift and tighten facial and neck tissue. Methods and materials: Subjects 25 to 60 years of age (N=20) with areas of skin laxity on the face and neck were enrolled and treated. A dual depth treatment was administered using a vectored pattern. Subjects were evaluated after 90 days, 180 days, and one year. Results: Overall improvements in Subject Global Aesthetic Improvement Scale and Physician Global Aesthetic Improvement Scale scores were reported by 90 and 100 percent of subjects at 90 and 180 days, respectively, and 95 percent for both measures at one year. Six of 14 evaluable subjects were rated as improved by blinded assessment at one year. Self-reported improvements maintained for up to one year included less sagging (79%), fewer lines and wrinkles (58%), and smoother skin texture (47%). Conclusion: Based on these results, treatment with micro-focused ultrasound with visualization with vertical vectoring demonstrated appreciable lifting and tightening of facial and neck tissue resulting in improved Global Aesthetic Improvement Scale scores and a high degree of patient satisfaction for up to one year. ClinicalTrials.gov Identifier: NCT01708512.
To meet the continuing demand for noninvasive facial rejuvenation, a device has been developed that integrates micro-focused ultrasound with high-resolution ultrasound visualization (MFU-V). When applied to tissue of the superficial musculoaponeurotic system and dermis, MFU-V causes collagen to become denatured and contract and leads to additional de novo collagen synthesis and remodeling.1,2 This device can be used to produce significant skin lifting and tightening when applied to areas of lax skin on the face and neck.3-7 MFU-V has been demonstrated to be safe and effective in numerous clinical trials as a noninvasive aesthetic treatment and has been cleared by the United States Food and Drug Administration (FDA) to noninvasively lift tissues in the eyebrow, neck, and submentum, and improve lines and wrinkles of the décolleté (Ulthera® System; Merz Device Innovation Center, Mesa, Arizona).8,9
The MVU-V device uses six transducers that emit ultrasound energy at a range of frequencies and multiple, discrete focal depths, which includes the varying depths of the superficial muscular aponeurotic system.8 Thermal coagulation points are formed when ultrasound energy is focused to a point less than 1mm3 in size below the skin’s surface. There, 95 percent of the delivered ultrasound energy is converted to heat, briefly raising the local temperature to ~65°C, which is optimal for collagen denaturation and subsequent stimulation of neocollagenesis while the superficial layer of skin and other intervening tissue remains un-affected.2,10 This results in immediate collagen contraction and initiates collagen synthesis. Each line of treatment consists of a row of thermal coagulation points at a Single focal depth and each line is typically spaced 2 to 3mm apart during a treatment. Each ultrasound trans-ducer can also provide tissue imaging to a depth of 8mm. The treatment zone for Standard transducers is 25mm in length while the treatment zone for narrow transducers (designated with an N) are 14mm in length.
MFU-V has proven to be effective for lifting and tightening lax skin on the face and neck when a Single pass of focused ultrasound is delivered at varying focal depths3,5,7; however, it has been shown that some subjects receiving MFU-V at two different focal depths in the submental and submandibular areas may obtain greater aesthetic improvement and greater satisfaction with the results they achieve.11 It has also been shown that vectoring treatment by applying vertical treatment lines over hor-izontal treatment lines at different treatment depths and applying a greater number of treatment lines produced significantly greater lifting in the brows and marionette lines.11
Since the clinical presentation of each patient is unique, MFU-V treatment can be customized for each patient. The MFU-V device utilizes a computer-driven platform that enables the clinician to visualize the proposed treatment area with ultrasound-imaging transducers and form a treatment plan prior to applying micro-focused ultrasound energy. As vertical vectoring, additional treatment lines, and dualplane delivery of MFU-V are all important aspects of an individualized treatment plan,11 the objective of this prospective, open-label pilot study was to evaluate the efficacy and safety of patient-specific, customized MFU-V treatment with vertical vectoring to lift and tighten facial and neck tissue.
METHODS
Subjects. Healthy men and women between 25 and 60 years of age with areas of skin laxity on their face and neck were eligible for enrollment. Each subject provided informed consent and expressed their willingness to comply with all protocol requirements. Women of childbearing potential were required to have a negative urine pregnancy test prior to treatment and agreed to use an acceptable method of birth control during the study.
Reasons for exclusion from the study included the presence of a disease or condition that might affect wound healing; body mass index (BMI) ≥30; concomitant drug therapy that might place the subject at risk or interfere with the objectives of the study, such as anticoagulants or antipsychotic medications; a history of drug or alcohol abuse or smoking during the last five years; autoimmune disease; concurrent enrollment in a study involving the use of an investigational drug or device; use of systemic retinoids within the past six months or topical retinoids within the past two weeks; or any of the following conditions or treatments affecting the planned treatment area: severe solar elastosis; excessive subcutaneous fat or skin laxity; significant scarring, wounds or lesions, severe or cystic acne, marked facial asymmetry, ptosis, excessive dermatochalasis, or thick sebaceous skin; presence of a metal Stent or implant; microdermabrasion or prescription level glycolic acid treatments within two weeks; a skin tightening procedure within the past year; use of an injectable hyaluronic acid or hydroxyapatite fillers within 12 months, a poly-L-lactic acid filier within 24 months, or a permanent filier at any time; neurotoxin, nonablative rejuvenative laser or light treatment within the past six months; ablative resurfacing laser treatment, surgical dermabrasion, or deep facial peels; facelifts, blepharoplasty, or brow lift within the past year; or any use of contour threads.
Treatment device. The MFU-V device employs transducers capable of emitting focused ultrasound at frequencies of 4.0, 7.0, and 10.0MHz at focal depths of 1.5, 3.0, and 4.5mm, respectively. Prior to delivering ultrasound energy, these transducers were also used for ultrasound imaging to visualize underlying tissue prior to treatment and to ensure adequate acoustic coupling of the transducer with the skin surface.
Procedures. Standardized two-dimensional photographs of each subject were obtained using fixed camera and lighting conditions prior to the MFU-V procedure. Pre-treatment analgesic medications could be administered at the discretion of the physician and subject. Prior to initiating treatment, the investigator assessed the subject’s skin tissue quality based on age and gender, BMI, and the amount of subcutaneous soft tissue in the region to be treated. Based on this assessment, a customized treatment plan was developed, which included 600 to 700 dual plane treatment lines with vertical vectoring. Prior to treatment, ultrasound gel was applied to the target area and an ultrasound image was obtained to ensure acoustic coupling with the skin.
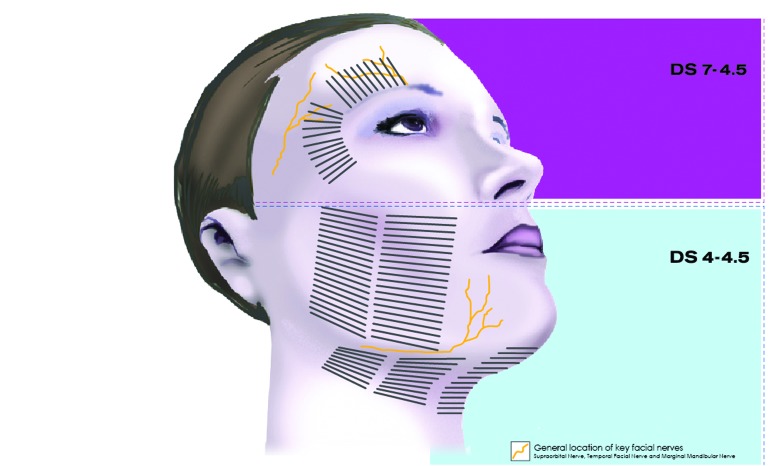
The anatomical depth of focal tissue heating is fixed and determined by the set focus depth of a given transducer and by the ultrasound power and exposure duration. The length of each treatment line was a maximum of 25mm and were spaced 2 to 3mm apart. The anatomical regions treated with MFU-V and the individual zones of thermal coagulation for the upper and lower face are shown in Figures 1 and 2, respectively. These figures also indicate which transducers were used to treat each area.
Figure 1.
Treatment patterns in targeted anatomical regions of the upper face
Figure 2.
Treatment patterns in targeted anatomical regions of the lower face
Treatments were delivered using a vectored pattern beginning with the 4MHz/4.5mm transducer. Based on each subject’s response, the investigator could either continue the treatment at the existing treatment parameters or reduce the treatment energy. The investigator then continued the treatment with a second pass using the 7MHz/3.0mm transducer.
A blinded qualitative assessment compared 90-day post-treatment photos with baseline photos and quantitative improvement in tissue lift was measured on post-treatment Days 90, 180, and one year. The Subject Global Aesthetic Improvement Scale (SGAIS) and the Physician Global Aesthetic Improvement Scale (PGAIS) and Patient Satisfaction Questionnaires (PSQ) were also completed on post-treatment Days 90, 180, and one year.
Efficacy endpoints. The primary efficacy endpoint was the overall improvement in skin lifting and tightening in the treated areas as determined by a blinded qualitative assessment of baseline photographs compared with photos obtained 90 days post-treatment. Secondary efficacy endpoints were overall aesthetic improvements at all follow-up Visits as determined with PGAIS and SGAIS and the responses to the PSQ.
Quantitative assessments of brow and lower face tissue lift were completed using 2D photographs from all follow-up Visits. Baseline and post-treatment photos were matched to ensure proper alignment. For the upper face, a lift measurement was considered improved if the eyebrow was raised ≥0.5mm. For the lower face, an improved lift measurement was defined as a submental lift ≥ 1.0mm. An improved measurement area was defined as a noticeably improved submental area ≥20mm2 in size
Safety endpoints. During the treatment procedure, subjects were asked to rate the mean level of pain they experienced using a validated 11-point numerical rating Scale with 0 denoting no pain and 10 denoting the worst possible pain. Pain scores were recorded for each anatomical treatment area and for each transducer used. Following all treatments, each subject was examined for evidence of an acute response, such as edema or erythema approximately 30 minutes after the exposure. During each follow-up evaluation, subjects were queried about adverse events and changes in concomitant medications and the treatment site was visually examined.
Ethics. The protocol and informed consent form used in this study were approved by a commercial Institution-al Review Board (Asentral, Inc. IRB, Newburyport, Massachusetts). Informed consent and HIPAA auth-orization was obtained from each subject before participating in any study-related activity. Clinical Trials.gov Identifier: NCT01708512.
RESULTS
Subject demographics. Twenty-one subjects were enrolled and 20 subjects (3 male, 17 female) having a mean age of 47 years (range, 34-60 years) were treated. Additional demographic data is provided in Table 1. All 20 subjects returned for the 90-day follow-up (100%) and 19 returned for the 180-day and one-year follow-up Visits (95%).
TABLE 1.
Demographic characteristics
| Mean age, years (min, max) | 47 (34, 60) |
| Mean BMI, kg/m2 (min, max) | 25.2 (20.9, 28.5) |
| Gender, N (%) | |
| Female | 17 (85) |
| Male | 3(15) |
| Race/Ethnicity, N (%) | |
| Caucasian | 19 (95%) |
| Hispanic/Latino | 1 (5%) |
| Fitzpatrick Skin Type, N (%) | |
| Type II | 16 (80%) |
| Type III | 4 (20%) |
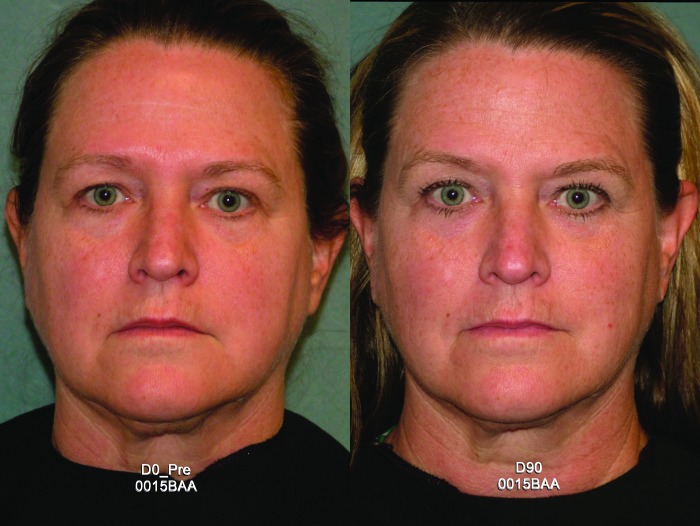
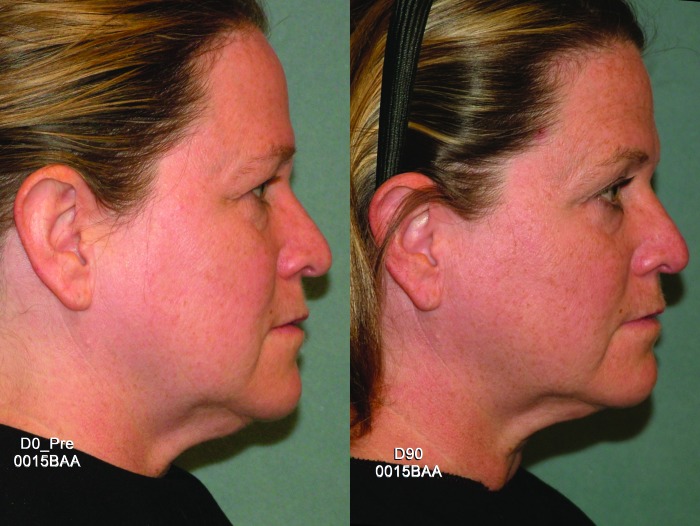
Efficacy endpoints. Subjects received a mean of 683 treatment lines (range 609-700) on the cheeks, submentum, submandular, periorbital, and brow regions. Despite attention to Photographie quality, lighting, and positioning, photos were excluded from blinded assessment for six subjects. Among the 14 evaluable subjects, the blinded assessment results were positive for six (43%). Substantial 90-day improvements can be seen in frontal and lateral views of a treated subject in Figures 3 and 4, respectively.
Figure 3.
Frontal view of a representative subject at baseline and post-treatment Day 90
Figure 4.
Lateral view of a representative subject at baseline and post-treatment Day 90
Results of the PGAIS show 100 percent of subjects were rated as having aesthetic improvement at both 90 and 180 days and 95 percent at one year. The SGAIS results indicated 90 percent of subjects pereeived aesthetic improvement at 90 days and 95 percent at 180 days and one year. Detailed PGAIS and SGAIS data are provided in Table 2. The results of the PSQ indicated that 95 to 100 percent of subjects were Satisfied or Very Satisfied with the aesthetic results they achieved at 90 and 180 days and one year post-treatment (Table 3). All 20 subjects (100%) indicated that they noticed improvement in face and neck characteristics at 90 days, and 17 subjects (90%) noticed improvement at 180 days and one year.
TABLE 2.
Global aesthetic improvement scale scores
| PHYSICIAN SCORES, N (%) | 90 DAYS (N=20) | 180 DAYS (N=19) | 1 YEAR (N=19) |
|---|---|---|---|
| Very much improved | 1 (5) | 1 (5) | 2(11) |
| Much improved | 11 (55) | 10 (53) | 9(47) |
| Improved | 8 (40) | 8 (42) | 7(37) |
| No change | 0(0) | 0(0) | 1 (5) |
| Worse | 0(0) | 0(0) | 0(0) |
| All improved | 20 (100) | 19 (100) | 18 (95) |
| SUBJECT SCORES, N (%) | 90 DAYS (N=20) | 180 DAYS (N=19) | 1 YEAR (N=19) |
| Very much improved | 5(25) | 3(16) | 2(11) |
| Much improved | 5(25) | 7(37) | 6 (32) |
| Improved | 8 (40) | 8 (42) | 10 (53) |
| No change | 2(10) | 1 (5) | 1 (5) |
| Worse | 0(0) | 0(0) | 0(0) |
| All improved | 18 (90) | 18 (95) | 18 (95) |
TABLE 3.
Patient satisfaction questionnaire
| 90 DAYS (N=20) | 180 DAYS (N=19) | 1 YEAR (N=19) | |
|---|---|---|---|
| Patient satisfaction, N (%) | |||
| Very Satisfied | 9 (45) | 9(47) | 7(37) |
| Satisfied | 10 (50) | 10 (53) | 11 (58) |
| Dissatisfied | 1 (5) | 0(0) | 1 (5) |
| Very dissatisfied | 0(0) | 0(0) | 0(0) |
| Very Satisfied + Satisfied | 19 (95) | 19 (100) | 18 (95) |
| Improvement noticed, N (%) | |||
| Lines/wrinkles | 10 (50) | 7(37) | 11 (58) |
| Less Sagging | 18 (90) | 16 (84) | 15 (79) |
| More Even Skin Tone/Color | 2(10) | 4(21) | 0(0) |
| Smoother Skin Texture | 9 (45) | 9(47) | 9(47) |
| Other | 2(10) | 0(0) | 0(0) |
| No Improvement | 0(0) | 1 (5) | 1 (5) |
| Would recommend treatment, N (%) | |||
| Yes | 19 (95) | 19 (100) | 19 (100) |
| No | 1 (5) | 0(0) | 0(0) |
Among 10 subjects with evaluable lower face measurements, the best quantitative data was observed on Day 90 with 30 to 40 percent reporting ≥1mm lift on the right and left sides, respectively, decreasing to 22 to 33 percent at one year. Similarly, 40 to 50 percent reported improvement over ≥20mm2 at Day 90 on the right and left sides, respectively, decreasing to 33 percent for both sides at one year. The proportion of subjects with ≥0.5mm eyebrow lift was 31 to 38 percent on the right and left sides, respectively, 55 to 35 percent at Day 180, and 44 to 33 percent at one year.
Safety endpoints. Among the 20 treated subjects, 19 were pretreated with oral tramadol with acetaminophen and one received hydrocodone with acetaminophen. The mean pain scores for the different transducers used were 4.0 (range, 1-9) for 4.0MHz/4.5mm, 3.2 (range, 0-10) for 7.0MHz/3.0mm, and 5.5 (range, 0-10) for the 7.0MHz/4.5mm. Among the eight reported adverse events, only swelling under right eye (N=1) was considered to be possibly treatment-related and it resolved within four days. There were no serious adverse events.
DISCUSSION
During initial studies with MFU-V, a Single pass of focused ultrasound was delivered at varying focal depths to the face and neck.3,5,7 Using this method, clinically significant improvements were demonstrated by blinded assessment in 86 percent of treated subjects (N=36) after 90 days3 and by 81 and 78 percent of patients after 90 (N=16) and 180 days (N=45), respectively.7 These improvements were enhanced by treating the face and neck areas with two passes of MFU-V at different depths. When subjects were treated with MFU-V treatment at two depths, the percentage of subjects rated by the investigator as having any improvement on the face and neck was 53 percent at Day 60 (N=30), increasing to 79 percent at Day 90 (N=29), and 93 percent at Day 180 (N=29) (Baumann L, Zelickson B. Clinical Trial ULT-110: Evaluation of Micro-Focused Ultrasound for Lifting and Tightening Neck. ClinicalTrials.gov Identifier: NCTO1368874, unpublished). A pilot study showed that the addition of vertical or superolateral vectoring as well as dual-depth treatments resulted in statistically significant increases in brow lifting and marionette line tightening.11
Based on these results, the current study was designed to follow this treatment progression and evaluate the efficacy and safety of patient-specific customization of MFU-V treatment using high treatment density treatment with vertical vectoring for facial and neck tissue tightening and lift. Among the 20 treated subjects, 14 were evaluable for the primary endpoint (blinded assessment) due to challenges with matching fighting and positioning in the photos. Overall improvement in skin lifting and tightening were observed in six subjects 43%); however, overall improvements in SGAIS and PGAIS scores were reported by 90 and 100 percent of subjects at 90 days while 95 percent of subjects were satisfied with the results they achieved. At 180 days, overall improvements in SGAIS and PGAIS scores were again 95 and 100 percent, respectively, and remained 95 percent for both measures at one year following treatment, demonstrating the durable nature of MFU-V treatment. Self-reported improvements that persisted for up to one year included less sagging (79%), less lines and wrinkles (58%), and smoother skin texture (47%). Previous studies have documented clinical improvements that persisted for up to 18 months.6,7 This is the first study to document MFU-V-related improvements persisting for up to one year after treatment.
Safety endpoints were consistent with the known safety profile of MFU-V.12 Similar to previous studies, MFU-V was well-tolerated with oral analgesic pretreatment. Only one transient adverse event was possibly treatment-related. There were no serious or treatment-related adverse events.
A study limitation was that subjects with modest wrinkles and skin laxity were enrolled, which limited the extent of improvement in the quantitative assessments. Future studies should place greater emphasis on subject enrollment and use of more quantitative assessments.
CONCLUSION
This study is the first to prospectively follow subjects out to one year after a Single MFU-V treatment. Based on these results, treatment with high-density MFU-V with vertical vectoring demonstrated appreciable lifting and tightening of facial and neck tissue resulting in improved GAIS scores and a high degree of patient satisfaction for at least one year. MFU-V continues to demonstrate a high degree of safety. Additional studies with patient-specific customization of MFU-V treatment using higher treatment density treatment with vertical vectoring for treating the face and neck are warranted.
ACKNOWLEDGMENTS
This study was sponsored by Merz Device Innovation Center, Mesa, Arizona. The author acknowledges the editorial assistance of Dr. Carl Hornfeldt during the preparation of this manuscript.
Footnotes
DISCLOSURE:This study was sponsored by Merz Device Innovation Center, Mesa, Arizona.
REFERENCES
- 1.Laubach HJ, Makin IR, Barthe PG, et al. Intense focused ultrasound: evaluation of a new treatment modality for precise microcoagulation within the skin. Dermatol Surg. 2008;34:727–734. doi: 10.1111/j.1524-4725.2008.34196.x. [DOI] [PubMed] [Google Scholar]
- 2.White WM, Makin IR, Barthe PG, et al. Selective creation of thermal injury zones in the superficial musculoaponeurotic system using intense ultrasound therapy: a new target for noninvasive facial rejuvenation. Arch Facial Plast Surg. 2007;9:22–29. doi: 10.1001/archfaci.9.1.22. [DOI] [PubMed] [Google Scholar]
- 3.Alam M, White LE, Martin N, et al. Ultrasound tightening of facial and neck skin: a rater-blinded prospective cohort study. J Am Acad Dermatol. 2010;62:262–269. doi: 10.1016/j.jaad.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 4.Chan NP, Shek SY, Yu CS, et al. Safety study of transcutaneous focused ultrasound for non-invasive skin tightening in Asians. Lasers Surg Med. 2011;43:366–375. doi: 10.1002/lsm.21070. [DOI] [PubMed] [Google Scholar]
- 5.Suh DH, Shin MK, Lee SJ, et al. Intense focused ultrasound tightening in Asian skin: clinical and pathologic results. Dermatol Surg. 2011;37:1595–1602. doi: 10.1111/j.1524-4725.2011.02094.x. [DOI] [PubMed] [Google Scholar]
- 6.Suh DH, Oh YJ, Lee SJ, et al. A intense-focused ultrasound tightening for the treatment of infraorbital laxity. J Gosmet Laser Ther. 2012;14:290–295. doi: 10.3109/14764172.2012.738912. [DOI] [PubMed] [Google Scholar]
- 7.Fabi SG, Goldman MP. Retrospective evaluation of micro-focused ultrasound for lifting and tightening the face and neck. Dermatol Surg. 2014;40:569–575. doi: 10.1111/dsu.12471. [DOI] [PubMed] [Google Scholar]
- 8. ULTHERA® Operation & Maintenance Manual. Ulthera, Inc., Mesa, Arizona.
- 9.Fabi SG, Goldman MP, Dayan SH, et al. A prospective multicenter pilot study of the safety and efficacy of microfocused ultrasound with visualization for improving lines and wrinkles of the decollete. Dermatol Surg. 2015;41:327–335. doi: 10.1097/DSS.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 10.Day D. Microfocused ultrasound for facial rejuvenation: current perspectives. Research and Reports in Focused Ultrasound. 2014;2:13–17. [Google Scholar]
- 11.Sasaki GH, Tevez A. Clinical efficacy and safety of focused-image ultrasonography: a 2-year experience. Aesthet Surg J. 2012;32:601–612. doi: 10.1177/1090820X12445576. [DOI] [PubMed] [Google Scholar]
- 12.Hitchcock TM, Dobke MK. Review of the safety profile for microfocused ultrasound with visualization. J Gosmet Dermatol. 2014;13:329–335. doi: 10.1111/jocd.12111. [DOI] [PubMed] [Google Scholar]