Abstract
Objective
To investigate the cognitive impairment in patients with age-related macular degeneration (AMD).
Methods
Relevant articles were identified through a search of the following electronic databases through October 2015, without language restriction: 1) PubMed; 2) the Cochrane Library; 3) EMBASE; 4) ScienceDirect. Meta-analysis was conducted using STATA 12.0 software. Standardized mean differences with corresponding 95% confidence intervals were calculated. All of the included studies met the following four criteria: 1) the study design was a case–control or randomized controlled trial (RCT) study; 2) the study investigated cognitive function in the patient with AMD; 3) the diagnoses of AMD must be provided; 4) there were sufficient scores data to extract for evaluating cognitive function between cases and controls. The Newcastle–Ottawa Scale criteria were used to assess the methodological quality of the studies.
Results
Of the initial 278 literatures, only six case–control and one RCT studies met all of the inclusion criteria. A total of 794 AMD patients and 1,227 controls were included in this study. Five studies were performed with mini-mental state examination (MMSE), two studies with animal fluency, two studies with trail making test (TMT)-A and -B, one study with Mini-Cog. Results of the meta-analysis revealed lower cognitive function test scores in patients with AMD, especially with MMSE and Mini-Cog test (P≤0.001 for all). The results also showed that differences in the TMT-A (except AMD [total] vs controls) and TMT-B test had no statistical significance (P>0.01). The Newcastle–Ottawa Scale score was ≥5 for all of the included studies. Based on the sensitivity analysis, no single study influenced the overall pooled estimates.
Conclusion
This meta-analysis suggests lower cognitive function test scores in patients with AMD, especially with MMSE and Mini-Cog test. The other cognitive impairment screening tests, such as animal fluency test and TMT, need more studies to assess.
Keywords: age-related macular degeneration, cognitive impairment, meta-analysis, mini mental state examination
Introduction
As the aging population grows, an increasing number of people would be affected by age-related diseases, such as age-related macular degeneration (AMD) and Alzheimer’s disease. AMDs are increasingly affecting both the society and family. Although they are degenerative diseases of different tissues, as the retina is an integral part of the central nervous system, there may be an association between the two diseases. However, the pathogenesis and etiology of the two diseases are still not very clear.
Recently, several studies have shown amyloid β, the main constituent of senile amyloid plaques in the brains of Alzheimer’s disease patients, is also deposited in the drusen of eyes with AMD.1,2 Several factors, such as complement factor H3 and angiogenesis-related factors,4 that may reveal this link have been proposed. Not only the pathology, the epidemiology survey also found positive links between two age-related diseases. A cross-sectional study performed by Lindekleiv et al5 found that large drusen were associated with decreased performance in cognitive function test. The hypothesis of the correlation between the two diseases originated from molecular research in cognitive function tests.6
Many studies have shown a significant difference in investigating the cognitive function between AMD and controls.7,8 However, a few studies reported conflicting results.9 In view of the fact that the sample size of the study was not large enough and there was some contradiction between studies, we performed a meta-analysis of case–control and randomized controlled trial studies to assess the association between cognitive function and AMD disease. To our knowledge, this is the first meta-analysis to assess cognitive impairment in patients with AMD.
Methods
Search and identification strategy
We searched PubMed, EMBASE, Cochrane and ScienceDirect databases on the last day of October for the published literatures through October 2015. The following search terms were used: (“Mild Cognitive” OR “Mild cognitive impairments” OR “Cognitive impairment” OR “Cognitive impairments” OR “Cognitive deficit” OR “CI”) AND (“Macular Degeneration” OR “Wet Macular Degenerations” OR “Wet Macular” OR “Macular Degenerations” OR “Dry Macular Degeneration” OR “Dry Macular Degenerations” OR “Geographic Atrophies” OR “Dry Macular” OR “Age-related macular degeneration” OR “AMD”).
Inclusion and exclusion criteria
The study was included without language restriction and sample size limited if it met the following criteria: 1) the study design was a case–control or randomized controlled trial study; 2) the study investigated cognitive function in the patient with AMD; 3) the diagnosis of AMD must be provided; 4) there were sufficient scores data to extract for evaluating cognitive function between cases and controls.
Two investigators, Sun and Lv, independently evaluated the eligibility of all studies retrieved from the database on the basis of the predetermined selection criteria. Studies not designed as case–control, systematic reviews were excluded from this meta-analysis. Disagreements were resolved by discussion or in consultation with the third investigator.
Data extraction and study quality assessment
Two reviewers, Sun and Wei, independently extracted the following data for each eligible study using a standard form including: first author’s last name, year of publication, area, design of study, education years, control group selection, sex, age, sample size, measure of cognitive function, mean ± standard deviation (SD) of cognitive function test scores and assessed the methodological quality of the included studies with the Newcastle–Ottawa Scale.10 Discrepancies were addressed in consultation with the third reviewer.
Statistical analysis
All the data analyses were performed using STATA 12.0 (StataCorp LP, College Station, TX, USA). Standard mean difference (SMD) was used to evaluate the specified relationship. The Z-test was used to estimate the statistical significance of the pooled data. The Cochrane’s Q-statistic and I2 test were used to evaluate interstudy heterogeneity. If the Q-test showed a P<0.05 or I2 test exhibited >50%, indicating the presence of significant heterogeneity, the random effects model was used; otherwise, the fixed-effects model was used. Subgroup analyses were performed to investigate potential sources of heterogeneity. Sensitivity analysis was performed to evaluate the influence of a single study on the overall estimate. A P<0.05 was identified as statistically significant except for the heterogeneity tests where a level of 0.10 was used.
Results
Identification of included studies
Two hundred and seventy-eight studies were identified by our search strategy through PubMed (n=50), EMBASE (n=185), ScienceDirect (n=24), and Cochrane (n=19) library. After duplicate literatures (n=79) were removed, 199 studies were screened with the title or abstract. From the remaining studies (n=50), we excluded cohort study (n=32) and cross-sectional study (n=6). By further screening, five studies were excluded for the following reasons: two meeting abstracts with no detail data, one study on patients with visual impairment, one study investigating depression, and one investigating visual acuity and driving performance among drivers. Finally, seven studies were eligible for inclusion in this meta-analysis. The literature search, selection process, and reasons for exclusion are shown in Figure 1.
Figure 1.
Flow chart of the studies selection process.
Characteristics and quality of included studies
The age, a most critical factor in cognitive impairment, was described in all the included studies. Other related factors, sex, and educational years were investigated by some but not all studies. Due to the different measurement methods, we did a horizontal comparison between different studies to view if they had the same measurement. If the preceding conditions existed, we performed pool analysis of the related studies. On assessing the quality of included studies, we found that no studies had described non-response rate in AMD or control group. All of the included studies gave the age data, while not every study gave the education data. The Newcastle–Ottawa Scale scores were ≥5 for all the included studies. Details of each included study are described in Tables 1 and 2.
Table 1.
Baseline characteristic and quality of the included studies
| Author | Year | Area | Design of study | Cases
|
Control selection | Sex (female %)
|
Age (years)
|
Education (years)
|
NOS score | Measure of cognitive | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMD | CON | AMD | CON | AMD | CON | AMD | CON | |||||||
| Woo12 | 2012 | Korea | Case-control | 170 | 190 | Community dwelling without AMD | 47.1 | 41.6 | 72.5±7.6 | 76.8±6.1 | 12.0±7.3 | 9.8±5.2 | 7 | MMSE, DST, TMT, WLT, etc |
| Rozzini21 | 2014 | Italy | Case-control | 51 | 24 | Spouses, family member | 53 | 83 | 73.7±7.2 | 71.7±6.2 | 8.4±4.3 | 7.5±2.8 | 8 | MMSE, MOCA TMT, etc |
| Peiretti22 | 2014 | Italy | Case-control | 136 | 38 | Normal individuals | 44.9 | 55.3 | 76.5±9.7 | 75.3±5.5 | N | 7 | MMSE | |
| Mandas23 | 2014 | Italy | Case-control | 119 | 730 | No vision problem | N | 78.4±6.9 | 78.5±6.7 | N | 7 | MMSE, ADL, CIRS | ||
| Al-Salem6 | 2014 | US | Case-control | 138 | 91 | No retinal disease | 52.2 | 54.9 | 76.6±8.6 | 75±8 | N | 5 | Mini-Cog test | |
| Demirci7 | 2015 | Turk | Case-control | 59 | 49 | Healthy subjects | 47 | 48 | 74.3±7.3 | 73.9±5.5 | 3.5±2.6 | 3.6±2.8 | 7 | Mini-MMSE, animals, FAB, etc |
| Kelly24 | 2015 | Irish | RCT | 121 | 105 | Low MP | 66.9 | 50.5 | 65±9 | 47±12.1 | P<0.00l | 7 | FAS, animals, PAL, etc | |
Abbreviations: NOS, Newcastle-Ottawa Scale; ADL, activities of daily living; AMD, age-related macular degeneration; CIRS, cumulative illness rating scale; CON, control; DST, digit span test; FAB, frontal assessment battery; FAS, a phonemic fluency score; MMSE, mini-mental state examination; MOCA, Montreal Cognitive Assessment; MP, macular pigment; N, information not provided in this study; PAL, paired associates learning; RCT, randomized controlled clinical trials; TMT, trail making test; WLT, word list memory test.
Table 2.
Cognitive function test scores in each AMD subtype and control subject of the included studies
| Study | AMD (total)
|
AMD (wet)
|
AMD (dry)
|
Control
|
Measure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | P-value | n | Mean | SD | P-value | n | Mean | SD | P-value | n | Mean | SD | ||
| Woo12 | 170 | 24.97 | 3.30 | <0.001 | 107 | 24.5 | 0.26 | <0.001 | 17 | 23.42 | 0.62 | <0.001 | 190 | 25.99 | 2.79 | MMSE |
| Rozzini21 | 51 | 26.8 | 3.2 | 0.050 | 31 | 27.2 | 2.8 | 0.081 | 20 | 26.2 | 3.6 | 0.011 | 24 | 28.3 | 1.3 | MMSE |
| Peiretti22 | 136 | 27.8 | 1.5 | 0.721 | 38 | 27.7 | 1.6 | MMSE | ||||||||
| Mandas23 | 119 | 20.9 | 6.3 | 0.008 | 730 | 22.5 | 6.0 | MMSE | ||||||||
| Al-Salem6 | 138 | 3.68 | 1.61 | <0.001 | 56 | 3.95 | 1.65 | 0.001 | 82 | 3.5 | 1.59 | <0.001 | 91 | 4.63 | 0.85 | Mini-Cog test |
| Demirci7 | 59 | 24.3 | 3.88 | 0.002 | 45 | 25.46 | 2.80 | 0.071 | 14 | 20.71 | 4.71 | <0.001 | 49 | 26.59 | 3.16 | MMSE |
| Demirci7 | 59 | 13.7 | 5.50 | 0.21 | 45 | 14.84 | 5.20 | 0.845 | 14 | 10.14 | 5.03 | 0.005 | 49 | 15.06 | 5.66 | Animal fluency |
| Kelly24 | 121 | 15.5 | 4.0 | <0.001 | 105 | 21.6 | 5.8 | Animal fluency | ||||||||
| Woo12 | 170 | 83.49 | 78.10 | 0.001 | 107 | 96.34 | 5.35 | <0.001 | 17 | 135.03 | 13.09 | <0.001 | 190 | 64.83 | 37.61 | TMT-A |
| Rozzini21 | 51 | 78.4 | 57.4 | 0.09 | 31 | 80.4 | 66.4 | 0.154 | 20 | 74.8 | 37.2 | 0.104 | 24 | 60 | 20.9 | TMT-A |
| Woo12 | 170 | 201.22 | 86.09 | 0.002 | 107 | 222.59 | 7.39 | <0.001 | 17 | 213.98 | 18.04 | 0.291 | 190 | 192.84 | 81.93 | TMT-B |
| Rozzini21 | 51 | 131.9 | 72.5 | 0.92 | 31 | 130.9 | 88.4 | 0.856 | 20 | 133.7 | 32.9 | 0.600 | 24 | 127.3 | 44.9 | TMT-B |
Abbreviations: AMD, age-related macular degeneration; MMSE, mini-mental state examination; SD, standard deviation; TMT, trail making test.
Analysis of cognitive test scores between AMD patients and control subjects
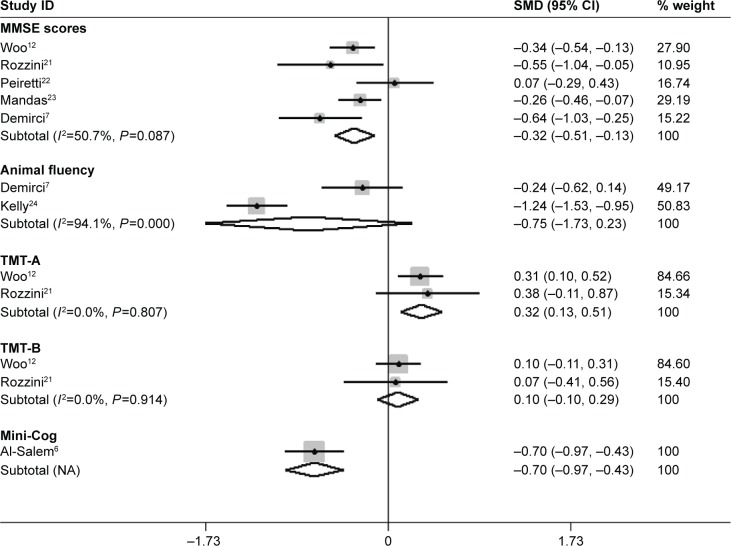
Heterogeneity between the results of different studies was conducted by STATA 12.0 and random-effects models were used. The forest plot (Figure 2) showed heterogeneity test results of included studies on cognitive function in AMD patients and controls. It showed no statistically significant difference among included studies of mini-mental state examination (MMSE) scores, trail making test (TMT)-A and -B. The I2 and P-value were 50.7%, 0.087; 0.0%, 0.807; and 0.0%, 0.904; respectively. The difference among included studies of animal fluency test (AFT) was statistically significant. The I2 and P-value were 94.1%, 0.000.
Figure 2.
Meta-analysis of the cognitive function in AMD patients and controls by MMSE, Animal fluency, Mini-Cog, TMT-A and -B.
Note: Weights are from random-effects analysis.
Abbreviations: AMD, age-related macular degeneration; CI, confidence interval; MMSE, mini-mental state examination; NA, not applicable; SMD, standard mean difference; TMT, trail making test.
The results of meta-analysis are showed in Table 3. The results of meta-analysis showed: AMD (total) patients had lower MMSE scores (SMD=−0.32, 95% confidence interval (CI) −0.51 to −0.13, Z=3.28, P=0.001), lower Mini-Cog scores (SMD=−0.70, 95% CI −0.97 to −0.43, Z=5.03, P<0.001), and higher TMT-A (SMD=0.32, 95% CI 0.13–0.51, Z=3.27, P=0.001) scores than controls, while differences in the animal fluency (SMD=−0.75, 95% CI −1.73 to 0.23, Z=1.51, P=0.132) and TMT-B (SMD=0.10, 95% CI −0.10 to 0.29, Z=0.98, P=0.326) test were not statistically significant.
Table 3.
Stratified analyses according to AMD subtype and different tests
| Subgroups | Number of studies | SMD (95% CI) | Meta-analyses
|
Heterogeneity
|
||
|---|---|---|---|---|---|---|
| Z | P-value | I2 (%) | P-value | |||
| AMD (total) | ||||||
| MMSE | 5 | −0.32 (−0.51, −0.13) | 3.28 | 0.001 | 50.7 | 0.087 |
| Animal fluency | 2 | −0.75 (−1.73, 0.23) | 1.51 | 0.132 | 94.1 | <0.001 |
| TMT-A | 2 | 0.32 (0.13, 0.51) | 3.27 | 0.001 | 0 | 0.807 |
| TMT-B | 2 | 0.10 (−0.10, 0.29) | 0.98 | 0.326 | 0 | 0.914 |
| Mini-Cog | 1 | −0.70 (−0.97, −0.43) | 5.03 | <0.001 | 0 | 1 |
| AMD (wet) | ||||||
| MMSE | 3 | −0.58 (−0.77, −0.38) | 5.80 | <0.001 | 0 | 0.463 |
| Animal fluency | 1 | −0.04 (−0.45, 0.36) | 0.20 | 0.845 | 0 | 1 |
| TMT-A | 2 | 0.76 (0.13, 1.39) | 2.38 | 0.017 | 78.1 | 0.033 |
| TMT-B | 2 | 0.32 (−0.04, 0.69) | 1.73 | 0.084 | 45.3 | 0.176 |
| Mini-Cog | 1 | −0.56 (−0.90, −0.22) | 3.23 | 0.001 | 0 | 1 |
| AMD (dry) | ||||||
| MMSE | 3 | −1.12 (−1.59, −0.64) | 4.59 | <0.001 | 48.6 | 0.143 |
| Animal fluency | 1 | −0.89 (−1.50, −0.27) | 2.84 | 0.005 | 0 | 1 |
| TMT-A | 2 | 1.23 (−0.18, 2.63) | 1.71 | 0.087 | 91.8 | <0.001 |
| TMT-B | 2 | 0.22 (−0.16, 0.61) | 1.15 | 0.250 | 0 | 0.785 |
| Mini-Cog | 1 | −0.90 (−1.21, −0.59) | 5.63 | <0.001 | 0 | 1 |
Abbreviations: AMD, age-related macular degeneration; CI, confidence interval; MMSE, mini-mental state examination; SMD, standard mean difference; TMT, trail making test.
Analysis of cognitive test scores of dry and wet AMD patients with control subjects
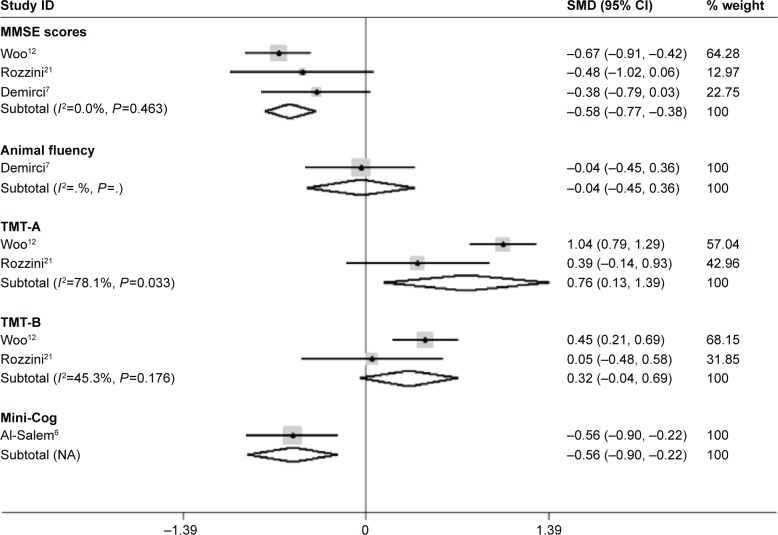
The forest plot (Figure 3) shows heterogeneity test results of included studies of wet AMD patients and controls. It showed no statistically significant difference among included studies of MMSE scores and TMT-B test. The I2 and P-value were 0%, 0.463 and 45.3%, 0.176, respectively. The difference among included studies of TMT-A test is statistically significant. The I2 and P-value were 78.1%, 0.033. The results of meta-analysis are shown in Table 3. It showed AMD (wet) patients had lower MMSE scores (SMD=−0.58, 95% CI −0.77 to −0.38, Z=5.80, P<0.001), lower Mini-Cog scores (SMD=−0.56, 95% CI −0.90 to −0.22, Z=3.23, P=0.001), and higher TMT-A scores (SMD=0.76, 95% CI 0.13 to 1.39, Z=2.38, P=0.017) than controls, while differences in the animal fluency (SMD=−0.04, 95% CI −0.45 to 0.36, Z=0.20, P=0.845) and TMT-B test (SMD=0.32, 95% CI −0.04 to 0.69, Z=1.73, P=0.084) were not statistically significant.
Figure 3.
Meta-analysis of the cognitive function in wet-AMD patients and controls by MMSE, Animal fluency, Mini-Cog, TMT-A and -B.
Note: Weights are from random-effects analysis.
Abbreviations: AMD, age-related macular degeneration; CI, confidence interval; MMSE, mini-mental state examination; NA, not applicable; SMD, standard mean difference; TMT, trail making test.
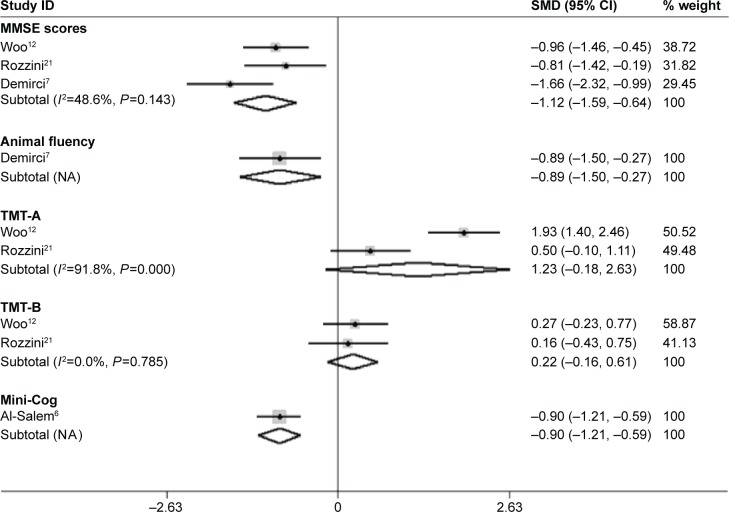
The forest plot (Figure 4) shows heterogeneity test results of included studies of dry AMD patients and controls. It showed no statistically significant difference among included studies of MMSE scores and TMT-B test. The I2 and P-value were 48.6%, 0.143 and 0%, 0.785, respectively. The difference among included studies of TMT-A test was statistically significant. The I2 and P-value were 91.8%, <0.001. The results of meta-analysis are shown in Table 3. It shows AMD (dry) patients had lower MMSE scores (SMD=−1.12, 95% CI −1.59 to −0.64, Z=4.59, P<0.001), lower Mini-Cog scores (SMD=−0.90, 95% CI −1.21 to −0.59, Z=5.63, P<0.001), and lower animal fluency scores (SMD=−0.89, 95% CI −1.50 to −0.27, Z=2.84, P=0.005) than controls, while differences in the TMT-A (SMD=1.23, 95% CI −0.18 to 2.63, Z=1.71, P=0.087)and TMT-B (SMD=0.22, 95% CI −0.16 to 0.61, Z=1.15, P=0.250) test were not statistically significant.
Figure 4.
Meta-analysis of the cognitive function in dry-AMD patients and controls by MMSE, Animal fluency, Mini-Cog, TMT-A and -B.
Note: Weights are from random-effects analysis.
Abbreviations: AMD, age-related macular degeneration; CI, confidence interval; MMSE, mini-mental state examination; NA, not applicable; SMD, standard mean difference; TMT, trail making test.
Sensitivity analyses of the included studies for the cognitive function in patients with AMD
The sensitivity analysis results suggest that no single study influenced the overall pooled estimates (Figure 5).
Figure 5.
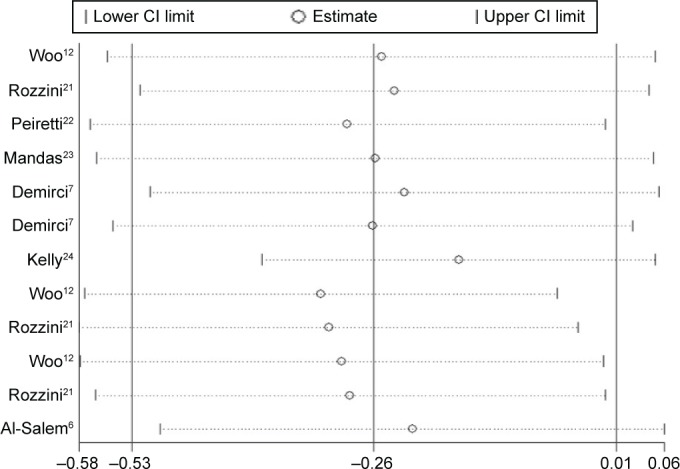
Sensitivity analysis of the included studies for the cognitive function in patients with AMD.
Abbreviations: AMD, age-related macular degeneration; CI, confidence interval.
Discussion
In the present analysis, we confirmed that patients with AMD have lower cognitive function test scores and showed that MMSE and TMT test have positive significance in cognitive function assessment. Consistent with the hypothesis that patients with sensory dysfunction, vision11 or hearing impairment, are more likely to have a cognitive impairment and dementia than age-matched people with normal vision and hearing, we showed AMD may be a significant factor for cognitive impairment.
Although the sensitivity of the MMSE is approximately 49%–63%, it is widely used to screen for dementia because of its high specificity. It is also the common examination used in most of the included studies. According to the realistic diversity of different countries, there are different versions. Therefore, Woo et al12 from Korea used a Korean version for global cognition examination. The boundary value of MMSE screening has been highly controversial, for example, 17,13 21,14 and 24.13 The differences of age and education year may be the main source of heterogeneity. Of course, the controls’ selection and ethnicity may also contribute to the heterogeneity. In spite of the difference of baseline characters listed, the heterogeneity is acceptable (I2=50.7% in total AMD, 0% in wet-AMD and 48.6% in dry-AMD). Irrespective of the sample size, AMD patients, either wet or dry subtypes, have lower MMSE scores compared with controls, taking into account its high specificity.
Compared with global cognition that MMSE indicated, the AFT mainly represents semantic long-term memory15 and may suggests neurodegeneration in the frontotemporal lobe.16 It has a statistically significant correlation with education level, primordial intelligence, current global cognitive and memory function, while having a weak association with age and sex of subjects. In this meta-analysis, only the patients with dry AMD showed a statistically significant difference with the controls (one study).7 More research is needed to explore the significance of AFT in patients with AMD.
TMT is one of the most popular neuropsychological tests. It provides information on visual search, scanning, speed of processing, mental flexibility, and executive functions.17 TMT-A and -B reflect the function of the right and left brain hemispheres, respectively. The present meta-analysis shows the different outcomes between TMT-A and -B. Whether poor performance on the TMT-A is caused by longstanding visual deterioration is unclear and should be determined in the future.
Compared with other dementia screening tests, the Mini-Cog test is unique with its acceptable sensitivity of 53.7% and a high specificity of 95.5%.18 As a replacement test tool of MMSE, the Mini-Cog test has similar sensitivity and specificity, whereas its biggest advantage is that it is a simple test.19 In addition, the Mini-Cog test is not affected by language or education years.20 The study performed by Al-Salem and Schaal6 also showed the same conclusion compared with MMSE scores in our meta-analysis.
As the first meta-analysis on cognitive impairment in patients with AMD, our study has some limitations. First, this meta-analysis included only seven studies. In addition, the meta-analysis is a retrospective study that may lead to subject selection bias. Importantly, the inclusion criteria of cases and controls were not always well defined in the included studies. Diagnostic criteria of AMD are not clear at present, especially the early diagnosis. Considering that not all cognitive function tests are influenced by age factor, we did not take age as an inclusion criterion even though most included studies with subjects over the age of 65 years.
In summary, this meta-analysis suggests lower cognitive function test scores in patients with AMD, especially with MMSE and Mini-Cog test. Other cognitive impairment screening tests, such as AFT and TMT, need more studies to assess. However, due to limitations mentioned above, more studies are still necessary to confirm these findings.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dentchev T, Milam AH, Lee VM, Trojanowski JQ, Dunaief JL. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol Vis. 2003;9:184–190. [PubMed] [Google Scholar]
- 2.Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH. The Alzheimer’s A beta-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99(18):11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukiw WJ, Surjyadipta B, Dua P, Alexandrov PN. Common micro RNAs (miRNAs) target complement factor H (CFH) regulation in Alzheimer’s disease (AD) and in age-related macular degeneration (AMD) Int J Biochem Mol Biol. 2012;3(1):105–116. [PMC free article] [PubMed] [Google Scholar]
- 4.Ding JD, Lin J, Mace BE, Herrmann R, Sullivan P, Bowes Rickman C. Targeting age-related macular degeneration with Alzheimer’s disease based immunotherapies: anti-amyloid-beta antibody attenuates pathologies in an age-related macular degeneration mouse model. Vision Res. 2008;48(3):339–345. doi: 10.1016/j.visres.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindekleiv H, Erke MG, Bertelsen G, et al. Cognitive function, drusen, and age-related macular degeneration: a cross-sectional study. Eye (Lond) 2013;27(11):1281–1287. doi: 10.1038/eye.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Salem KM, Schaal S. Mini-cognitive testing in patients with age-related macular degeneration. Retina. 2014;34(5):868–873. doi: 10.1097/IAE.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 7.Demırcı S, Guneş A, Demırcı K, Demircİ S, Tök L, Tök Ö. Is Alzheimer disease related to age-related macular degeneration? Turk J Med Sci. 2015;45(5):1115–1121. doi: 10.3906/sag-1406-135. [DOI] [PubMed] [Google Scholar]
- 8.Harrabi H, Kergoat MJ, Rousseau J, et al. Age-related eye disease and cognitive function. Invest Ophthalmol Vis Sci. 2015;56(2):1217–1221. doi: 10.1167/iovs.14-15370. [DOI] [PubMed] [Google Scholar]
- 9.Bertone A, Wittich W, Watanabe D, Overbury O, Faubert J. The effect of age-related macular degeneration on non-verbal neuropsychological test performance. Int Congr Ser. 2005:26–30. [Google Scholar]
- 10.Wells GA, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Accessed February 19, 2016]. Available from: http://www.medicine.mcgill.ca/rtamblyn/Readings/The%20Newcastle%20-%20Scale%20for%20assessing%20the%20quality%20of%20nonrandomised%20studies%20in%20meta-analyses.pdf.
- 11.Jefferis JM, Collerton J, Taylor JP, et al. The impact of visual impairment on Mini-Mental State Examination Scores in the Newcastle 85+ study. Age and ageing. 2012;41(4):565–568. doi: 10.1093/ageing/afs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo SJ, Park KH, Ahn J, et al. Cognitive impairment in age-related macular degeneration and geographic atrophy. Ophthalmology. 2012;119(10):2094–2101. doi: 10.1016/j.ophtha.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Pham TQ, Kifley A, Mitchell P, Wang JJ. Relation of age-related macular degeneration and cognitive impairment in an older population. Gerontology. 2006;52(6):353–358. doi: 10.1159/000094984. [DOI] [PubMed] [Google Scholar]
- 14.Dag E, Ornek N, Ornek K, Gunay F, Turkel Y. Mini mental state exam versus Montreal cognitive assessment in patients with age-related macular degeneration. Eur Rev Med Pharmacol Sci. 2014;18(20):3025–3028. [PubMed] [Google Scholar]
- 15.Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia. 2004;42(9):1212–1222. doi: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Libon DJ, McMillan C, Gunawardena D, et al. Neurocognitive contributions to verbal fluency deficits in frontotemporal lobar degeneration. Neurology. 2009;73(7):535–542. doi: 10.1212/WNL.0b013e3181b2a4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 18.Chen C-Y, Leung K-K, Chen C-Y. A quick dementia screening tool for primary care physicians. Archives of Gerontology and Geriatrics. 2011;53(1):100–103. doi: 10.1016/j.archger.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Improving identification of cognitive impairment in primary care. International Journal of Geriatric Psychiatry. 2006;21(4):349–355. doi: 10.1002/gps.1470. [DOI] [PubMed] [Google Scholar]
- 20.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Simplifying detection of cognitive impairment: comparison of the Mini-Cog and Mini-Mental State Examination in a multiethnic sample. Journal of the American Geriatrics Society. 2005;53(5):871–874. doi: 10.1111/j.1532-5415.2005.53269.x. [DOI] [PubMed] [Google Scholar]
- 21.Rozzini L, Riva M, Ghilardi N, et al. Cognitive dysfunction and age-related macular degeneration. American journal of Alzheimer’s disease and other dementias. 2014;29(3):256–262. doi: 10.1177/1533317513517032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peiretti E, Mandas A, Abete C, et al. Age-related macular degeneration and cognitive impairment show similarities in changes of neutral lipids in peripheral blood mononuclear cells. Experimental Eye Research. 2014;124:11–16. doi: 10.1016/j.exer.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Mandas A, Mereu RM, Catte O, et al. Cognitive impairment and age-related vision disorders: Their possible relationship and the evaluation of the use of aspirin and statins in a 65 years-and-over Sardinian population. Frontiers in Aging Neuroscience. 2014:6. doi: 10.3389/fnagi.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly D, Coen RF, Owusu Akuffo K, et al. Cognitive function and its relationship with macular pigment optical density and serum concentrations of its constituent carotenoids. Journal of Alzheimer’s Disease. 2015;48(1):261–277. doi: 10.3233/JAD-150199. [DOI] [PMC free article] [PubMed] [Google Scholar]