Abstract
The use of additional radiotherapy for resected stage IIIA N2 non-small-cell lung cancer in the setting of standard adjuvant chemotherapy remains controversial. A comprehensive search (last search updated in March 2015) for relevant studies comparing patients with stage IIIA N2 non-small-cell lung cancer undergoing resection after treatment with adjuvant postoperative chemotherapy alone or adjuvant postoperative chemoradiotherapy (POCRT) was conducted. Hazard ratios (HRs) were extracted from these studies to give pooled estimates of the effects of POCRT on overall survival (OS) and disease-free survival (DFS). Six studies were included. The meta-analysis demonstrated that POCRT had a greater OS benefit than postoperative chemotherapy (HR =0.87, 95% confidence interval [CI]: 0.79–0.96, P=0.006). Unfortunately, there was no significant difference in DFS between the two groups: the combined HR for DFS was 0.91 (95% CI: 0.57–1.46, P=0.706). In a subgroup analysis of two randomized controlled trials (n=172 patients), adding radiation was of no benefit to either OS (HR =0.72, 95% CI: 0.49–1.06, P=0.094) or DFS (HR =1.45, 95% CI: 1.00–2.09, P=0.047). In summary, compared with postoperative chemotherapy, POCRT was beneficial to OS but not DFS in patients with stage IIIA N2 non-small-cell lung cancer.
Keywords: NSCLC, N2-stage, therapy, surgery
Introduction
Despite treatment advances, lung cancer remains the leading cause of cancer-associated mortality both worldwide and in the People’s Republic of China.1 Approximately 25%–30% of non-small-cell lung cancer (NSCLC) patients are diagnosed at a locally advanced stage (IIIA or IIIB), and postoperative 5-year survival rates range from 13% to 42.8%.2–5 Based on several prospective clinical trials that have validated the survival benefit of concurrent chemoradiotherapy over radiotherapy alone6 or chemotherapy followed by sequential radiotherapy for stage IIIA N2 NSCLC,7,8 concurrent chemoradiotherapy has become the standard care for these patients. However, stage IIIA N2 NSCLC patients have heterogeneous disease presentation; thus, the optimal treatment strategy for patients with operable stage IIIA N2 NSCLC remains controversial.9
Many retrospective analyses have reported a possible survival benefit associated with surgery followed by adjuvant treatment in selected patients with stage IIIA N2 NSCLC.10–15 Postoperative chemotherapy (POCT) has been shown to improve patient survival in several randomized trials. However, up to 40% locoregional recurrence rate has been reported even after complete resection followed by chemotherapy.16,17 Postoperative radiation therapy (PORT) can reduce the risk of local recurrence in N2 disease following surgical treatment.11,12 Whether the addition of radiotherapy to adjuvant chemotherapy after surgical resection improves survival compared with adjuvant chemotherapy alone remains in dispute.
Recently, a large-scale retrospective study13 demonstrated that patients with N2 NSCLC who underwent PORT after radical resection and adjuvant chemotherapy exhibited improved overall survival (OS) compared to patients who underwent adjuvant chemotherapy alone. Thus, we conducted the current meta-analysis to investigate the role of PORT in N2 NSCLC patients who received adjuvant chemotherapy after radical resection.
Methods
Search strategy
We searched the PubMed, Embase, and Medline databases (last search updated in March 2015) for relevant studies using the following key words or MeSH terms: (Chemoradiotherapy OR Chemotherapy OR Radiotherapy OR Chemoradiation) AND NSCLC AND N2 AND surgery. We also searched meeting abstracts from several of the most important international meetings on lung cancer (American Society of Clinical Oncology, European Society of Medical Oncology, European Cancer Conference, and World Conference on Lung Cancer) from January 2005 to June 2015. When duplicate publications were identified, we included the most complete and recent data.
Two authors (X-LX and WC) independently conducted the literature search. All relevant studies were reviewed to identify potentially eligible articles. We also manually reviewed the bibliographies of the identified articles. Corresponding authors were contacted for further information when necessary. Notably, this meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.18
Eligibility criteria
Studies meeting the following eligibility criteria were included: 1) studies involving patients with locally advanced N2 stage cancer; 2) studies comparing surgery followed by chemoradiotherapy to surgery followed by chemotherapy alone; 3) studies providing data (eg, survival curves, hazard ratios [HRs] and 95% confidence interval [CIs]) on OS and/or disease-free survival (DFS); and 4) studies published in English. Studies were excluded if they were published as reviews or case reports. Studies were also excluded if they used only cell or animal models.
Data extraction and quality assessment
Two reviewers (X-LX and WC) independently abstracted data with a predefined information sheet. The following items were abstracted from the published articles: first author name, publication year, patient source, study design, sample size, treatment groups, number of patients in treatment groups, and DFS and OS HRs for treatment groups. Two other authors (Y-PX and W-MM) discussed and resolved all discrepancies in the extracted data. For each study, HRs and associated 95% CIs for DFS and OS were either directly extracted from the research article or calculated by two independent reviewers (WC and Y-PX) using available statistical information and a HR-calculation spreadsheet provided by Tierney et al.19
The quality of the included randomized controlled trials (RCTs) was evaluated according to the PEDro scale.20 The quality of the included studies was evaluated according to the Methodological Index for Non-Randomized Studies.21 The Methodological Index for Non-Randomized Studies includes 12 items that are each scored as 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate).
Statistical analysis
Depending on study heterogeneity, either a random effects model or a fixed effects model was used to calculate pooled HRs, 95% CIs, and P-values. Two-sided P-values less than 0.05 were considered statistically significant. χ2 and I2 statistics were used to assess statistical heterogeneity.22 To identify sources of heterogeneity, subgroup analyses were performed. The results of our meta-analysis are presented in forest plots with point estimates and 95% CIs for each trial as well as overall. We also used Begg’s funnel plot23 together with Egger’s test24 for asymmetry to assess publication bias among the included studies. We used Stata v.11.0 (StataCorp LP, College Station, TX, USA) for all statistical analysis.
Results
Literature search and summary of studies
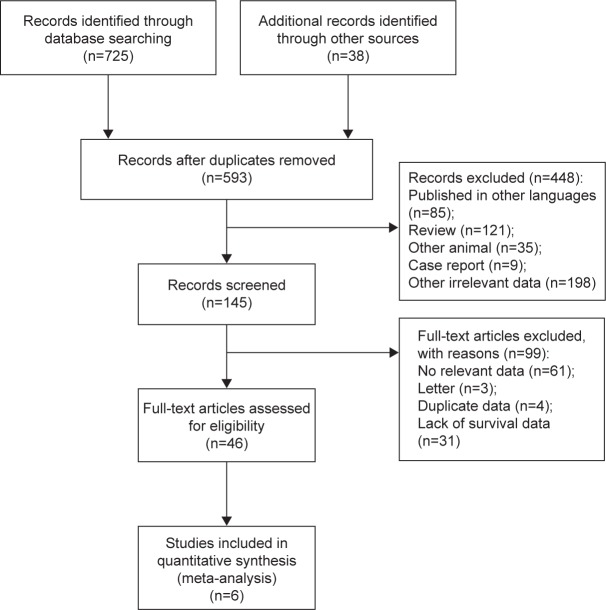
Our article selection process is shown in Figure 1. Our initial database search identified 763 potential full-text trials. Of these, 675 articles were excluded because they did not meet the inclusion criteria, and six were judged to be eligible for inclusion13,14,25–28 (Figure 1). The research in the included studies was performed by Robinson et al13 in the US, Shen et al14 in the People’s Republic of China, Kim et al25 in Korea, Zou et al28 in the People’s Republic of China, Perry et al26 in France, and Douillard et al27 in the US. All studies were conducted between 2007 and 2015.
Figure 1.
Flow chart of study design.
A total of six studies, including two RCTs14,26 and four retrospective studies,13,25,27,28 were enrolled in this meta-analysis for a total of 5,172 cases of N2 NSCLC. This cohort included 2,125 cases of postoperative chemoradiotherapy (POCRT) and 3,047 cases of POCT. Only two studies13,14 directly provided HRs and 95% CIs for OS or DFS; the other four studies25–28 provided survival curves. Thus, we obtained HRs and 95% CIs for OS and DFS using an HR-calculation spreadsheet provided by Tierney et al.19 Only one study14 evaluated the role of concurrent PORT in N2 NSCLC patients who received adjuvant chemotherapy after curative resection. The other five studies13,25–28 compared sequential POCRT with POCT and curative surgery. The minimum follow-ups for all studies ranged from 22.0 to 48.1 months. All patients received two to six cycles of adjuvant chemotherapy. The patients in the POCRT group received an additional dose of over 45 Gy of radiotherapy. Among the six studies included in the analysis, only three studies14,25,26 reported toxicities related to chemoradiotherapy or chemotherapy. Because these data were insufficient, a formal analysis could not be performed.
Meta-analysis and evaluation of heterogeneity
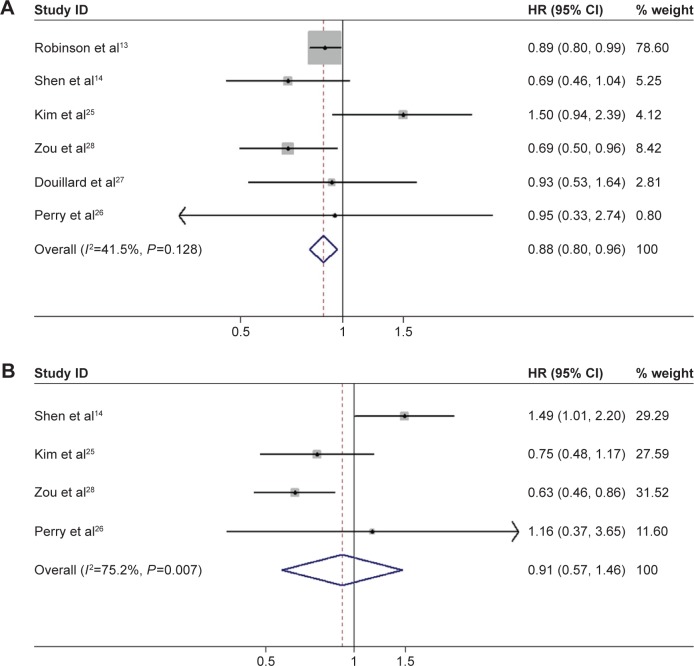
Six studies met our criteria for analysis: two RCTs14,26 and four retrospective reviews.13,25,27,28 The meta-analysis demonstrated a greater OS benefit associated with POCRT versus POCT (HR =0.87, 95% CI: 0.79–0.96, P=0.006) (Figure 2A). Modest homogeneity was detected between the six studies (χ2=8.55, P=0.128, I2=41.5%). DFS was investigated in four studies, including two RCTs14,26 and two retrospective reviews.25,28 Unfortunately, there was no significant difference in DFS between the two groups, as the combined HR for DFS was 0.91 (95% CI: 0.57–1.46, P=0.706) (Figure 2B). Significant heterogeneity (χ2=12.08, P=0.007, I2=75.2%) was observed between these four studies.
Figure 2.
Forest plot for overall survival (A) and disease-free survival (B) associated with adjuvant chemotherapy plus radiotherapy compared to chemotherapy alone in surgically treated N2 non-small-cell lung cancer patients.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Subgroup analyses
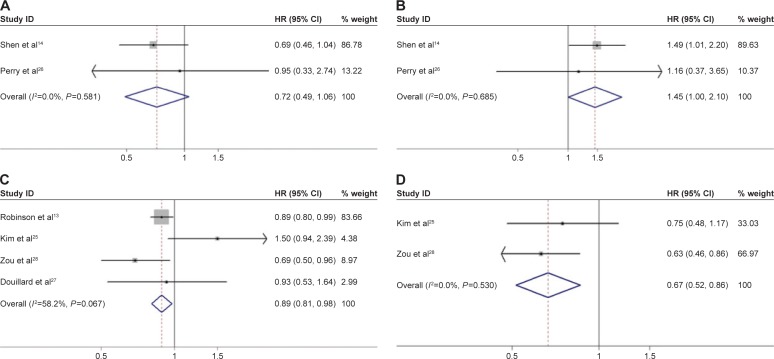
Subgroup analysis was carried out based on the study design. The subgroup analysis was performed on two RCTs14,26 (n=172 patients), which demonstrated that adding radiation did not benefit either OS (HR =0.72, 95% CI: 0.49–1.06, P=0.094) (Figure 3A) or DFS (HR =1.45, 95% CI: 1.00–2.09, P=0.047) (Figure 3B) with adequate homogeneity. In the four retrospective reviews,13,25,27,28 additional PORT significantly improved OS (HR =0.89, 95% CI: 0.81–0.98) (Figure 3C) with modest homogeneity (χ2=7.18, P=0.067, I2=58.2%). These four reviews did not report on how additional PORT affected DFS. The results of this pooled analysis demonstrated that the application of PORT in an adjuvant setting significantly improves DFS (HR =0.67, 95% CI: 0.52–0.86) (Figure 3D) with adequate homogeneity (I2=0.0%).
Figure 3.
Forest plot for overall survival and disease-free survival in the randomized controlled trials (A and B) and retrospective studies (C and D) associated with adjuvant chemotherapy plus radiotherapy compared to chemotherapy alone in surgically treated N2 non-small cell lung cancer patients.
Note: Overall survival (A and C), and disease-free survival (B and D).
Abbreviations: CI, confidence interval; HR, hazard ratio.
Publication bias and sensitivity analysis
Funnel plots and Egger’s regression test were performed for all meta-analyses and revealed no significant publication bias (P>0.05). Sensitivity analysis was performed by excluding studies one by one. When excluding the study conducted by Robinson et al,13 the result of the total meta-analysis on OS only marginally significantly improved (HR =0.84, 95% CI: 0.69–1.04, P=0.102) in the POCRT group. When excluding the other studies, the results were consistent.
Quality assessment
The study scores are shown in Table 1. Overall, the methodological quality of the two RCTs14,26 was good. Points were lost because the blinding of patients and investigators was not reported. The Methodological Index for Non-Randomized Studies scores of the other four non-RCTs13,25,27,28 ranged from 18 to 20 points and were deemed acceptable.
Table 1.
Characteristics of the included studies
| First author | Year | Study years | Country | Study design | Stage | Number of patients
|
DFS (HRs and 95% CIs) | OS (HRs and 95% CIs) | Sequencing of CT and RT | Quality assessment
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Undergoing POCRT | Undergoing POCT | PEDro scale | MINORS | |||||||||
| Robinson et al13 | 2015 | 2006–2010 | USA | Retrospective | IIIA (N2) | 1,850 | 2,633 | NA | 0.89 (0.80–0.99) | Sequential | / | 20 |
| Shen et al14 | 2014 | 2004–2009 | People’s Republic of China | RCT | IIIA (N2) | 66 | 69 | 1.49 (1.01–2.20) | 0.69 (0.46–1.04) | Concurrent | 9 | / |
| Kim et al25 | 2014 | 2000–2011 | Korea | Retrospective | N2 | 38 | 178 | 0.75 (0.48–1.17) | 1.50 (0.94–2.39) | Sequential | / | 18 |
| Zou et al28 | 2010 | 1998–2005 | People’s Republic of China | Retrospective | III (N2) | 104 | 79 | 0.63 (0.46–0.86) | 0.69 (0.50–0.96) | Sequential | / | 19 |
| Douillard et al27 | 2008 | 1998–2000 | USA | Retrospective | N2 | 48 | 70 | NA | 0.93 (0.53–1.64) | Sequential | / | 19 |
| Perry et al26 | 2007 | NA | France | RCT | IIIA (N2) | 19 | 18 | 1.16 (0.37–3.65) | 0.95 (0.33–2.74) | Sequential | 8 | / |
Abbreviations: CI, confidence interval; CT, chemotherapy; DFS, disease-free survival; HR, hazard ratio; MINORS, Methodological Index for Non-Randomized Studies; NA, non-available; OS, overall survival; POCRT, postoperative chemoradiotherapy; POCT, postoperative chemotherapy; RCT, randomized controlled trial; RT, radiation therapy.
Discussion
Patients undergoing complete resection of N2 stage NSCLC are at risk of locoregional and distant recurrence. Adjuvant chemotherapy, the standard postoperative treatment for high-risk, completely resected N2 stage NSCLC, can eliminate residual micrometastases in both the local tumor bed and at distant sites. PORT has long been proposed as a means of reducing risk of death and locoregional recurrence in N2 disease following surgical treatment.11,29,30 Because only a small number of studies have focused on N2 NSCLC, the use of combination POCT and radiotherapy for N2 disease remains controversial. To the best of our knowledge, this is the first meta-analysis to assess the role of PORT in the setting of adjuvant chemotherapy in N2 NSCLC patients who underwent complete resection. Thus, it was necessary to clarify the benefits of POCRT compared with POCT in a cohort of N2 NSCLC patients. Therefore, we reviewed the results of all relevant studies that have assessed the use of POCRT following surgery in patients with stage III N2 NSCLC.
In the present study, we showed that POCRT is superior to POCT with respect to OS, but not with respect to DFS. Among the six included studies, Robinson et al13 used the largest population-based registry of patients with completely resected stage IIIA N2 NSCLC; they identified a total of 4,483 patients (POCRT, n=1,850; POCT, n=2,633). Because of the large number of cases in the study by Robinson et al, it accounted for a large weight in our meta-analysis. Thus, it contributed a major role to the positive OS result. All patients in our current study received treatment in 2006 or later. Therefore, the majority of patients were treated with modern radiation techniques and received a median dose of 54 Gy over 43 days. Based on the large number of cases and the consistency of radiation techniques, it is reasonable to presume that the results of the current study are reliable. Notably, the toxicities reported in the included studies were generally tolerable for both groups. However, the impact of additional PORT on DFS in stage III N2 NSCLC produced conflicting results in our subgroup analysis: it produced favorable effects in the retrospective studies while remaining a risk in the RCTs. Overall, the combined HR for DFS was 0.91 in the total analysis. This value suggests that additional PORT did not impact DFS.
When combining the results of the two included RCTs,14,26 the patients in the POCRT group exhibited a higher DFS risk than those in the POCT group; however, OS was unaffected. This failure to demonstrate the superiority of adjuvant POCRT might be due to the wide variety of criteria used to diagnose patients with N2 disease. Compared to patients with N0 or N1 disease, N2 lymph node involvement results in higher rates of local recurrence and poorer OS.31,32 The included N2 NSCLC patients had heterogeneous disease presentation that could be roughly divided into three groups: patients with minimal N2 involvement found during or after surgery, patients with postoperatively diagnosed N2 disease, and patients with multisite bulky-N2 involvement.33,34 A clearly accepted subgroup classification has not yet been defined. This ambiguity might be responsible for the noted discrepancies in treatment. Additionally, the small sample sizes of the two included RCTs may have led to conflicting results.
One of the most significant strengths of the current study is that it is the first meta-analysis to compare adjuvant chemotherapy plus radiotherapy to chemotherapy alone in a large cohort of surgically treated stage IIIA N2 patients. To accomplish this, a comprehensive review was conducted using the most up-to-date published data. In addition, we contacted corresponding authors by email to obtain relevant unpublished data. Furthermore, subgroup and sensitivity analyses were performed to reduce heterogeneity. However, our meta-analysis also had several limitations. First, retrospective studies were included; therefore, biases (eg, recall bias and selection bias) might have existed. Second, no individual patient data were used. Additionally, except for one study,13 the sample sizes of the included studies were small. There was also modest heterogeneity in dose regimens between studies; for example, different cycles of chemotherapy, varied chemotherapy regimens, different follow-up durations, and varied radiation doses and radiation technologies were used. Finally, potential publication bias and language bias were unavoidable.
Conclusion
In summary, compared to adjuvant chemotherapy alone, adjuvant chemotherapy plus radiotherapy significantly improves OS but not DFS in N2 NSCLC patients. Due to the lack of studies and especially of RCTs on the use of POCRT in N2 NSCLC, the therapeutic benefit of this strategy remains unclear. Thus, large-scale, multicenter clinical trials are urgently needed.
Acknowledgments
This work was supported by the Medical Science and Technology Project of Zhejiang province (No. 2015KYB054-01, 2011KYA032) and the High-Level Backbone Talent Project of Zhejiang province medical platform (No. 2011RCA014).
Footnotes
Author contributions
W-MM, TL, and X-LX designed the study, collected, analyzed, and interpreted the data, and wrote the article. WC and Y-PX participated in the study, collected, analyzed, and interpreted data, and critically revised the article.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 3.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111(6):1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 4.Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer. 2005;50(2):227–234. doi: 10.1016/j.lungcan.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Sawabata N, Miyaoka E, Asamura H, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol. 2011;6(7):1229–1235. doi: 10.1097/JTO.0b013e318219aae2. [DOI] [PubMed] [Google Scholar]
- 6.Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990;323(14):940–945. doi: 10.1056/NEJM199010043231403. [DOI] [PubMed] [Google Scholar]
- 7.Dillman RO, Herndon J, Seagren SL, Eaton WL, Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88(17):1210–1215. doi: 10.1093/jnci/88.17.1210. [DOI] [PubMed] [Google Scholar]
- 8.Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23(25):5883–5891. doi: 10.1200/JCO.2005.55.405. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8(7):740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 10.Corso CD, Rutter CE, Wilson LD, Kim AW, Decker RH, Husain ZA. Re-evaluation of the role of postoperative radiotherapy and the impact of radiation dose for non-small-cell lung cancer using the National Cancer Database. J Thorac Oncol. 2015;10(1):148–155. doi: 10.1097/JTO.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 11.Patel SH, Ma Y, Wernicke AG, Nori D, Chao KS, Parashar B. Evidence supporting contemporary post-operative radiation therapy (PORT) using linear accelerators in N2 lung cancer. Lung Cancer. 2014;84(2):156–160. doi: 10.1016/j.lungcan.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Wisnivesky JP, Halm EA, Bonomi M, Smith C, Mhango G, Bagiella E. Postoperative radiotherapy for elderly patients with stage III lung cancer. Cancer. 2012;118(18):4478–4485. doi: 10.1002/cncr.26585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson CG, Patel AP, Bradley JD, et al. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data Base. J Clin Oncol. 2015;33(8):870–876. doi: 10.1200/JCO.2014.58.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen WY, Ji J, Zuo YS, et al. Comparison of efficacy for postoperative chemotherapy and concurrent radiochemotherapy in patients with IIIA-pN2 non-small cell lung cancer: an early closed randomized controlled trial. Radiother Oncol. 2014;110(1):120–125. doi: 10.1016/j.radonc.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Pisters KM, Kris MG, Gralla RJ, et al. Randomized trial comparing postoperative chemotherapy with vindesine and cisplatin plus thoracic irradiation with irradiation alone in stage III (N2) non-small cell lung cancer. J Surg Oncol. 1994;56(4):236–241. doi: 10.1002/jso.2930560407. [DOI] [PubMed] [Google Scholar]
- 16.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 17.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 19.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PEDro scale. 1999. [Accessed September 1, 2015]. Available from: http://www.pedro.org.au/english/downloads/pedro-scale/
- 21.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim BH, Kim HJ, Wu HG, et al. Role of postoperative radiotherapy after curative resection and adjuvant chemotherapy for patients with pathological stage N2 non-small-cell lung cancer: a propensity score matching analysis. Clin Lung Cancer. 2014;15(5):356–364. doi: 10.1016/j.cllc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Perry MC, Kohman LJ, Bonner JA, et al. A phase III study of surgical resection and paclitaxel/carboplatin chemotherapy with or without adjuvant radiation therapy for resected stage III non-small-cell lung cancer: Cancer and Leukemia Group B 9734. Clin Lung Cancer. 2007;8(4):268–272. doi: 10.3816/CLC.2007.n.005. [DOI] [PubMed] [Google Scholar]
- 27.Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72(3):695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 28.Zou B, Xu Y, Li T, et al. A multicenter retrospective analysis of survival outcome following postoperative chemoradiotherapy in non-small-cell lung cancer patients with N2 nodal disease. Int J Radiat Oncol Biol Phys. 2010;77(2):321–328. doi: 10.1016/j.ijrobp.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 29.Hsu HC, Wang CJ, Huang EY, Sun LM. Post-operative adjuvant thoracic radiotherapy for patients with completely resected non-small cell lung cancer with nodal involvement: outcome and prognostic factors. Br J Radiol. 2004;77(913):43–48. doi: 10.1259/bjr/21845347. [DOI] [PubMed] [Google Scholar]
- 30.Dai H, Hui Z, Ji W, et al. Postoperative radiotherapy for resected pathological stage IIIA-N2 non-small cell lung cancer: a retrospective study of 221 cases from a single institution. Oncologist. 2011;16(5):641–650. doi: 10.1634/theoncologist.2010-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andre F, Grunenwald D, Pignon JP, et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol. 2000;18(16):2981–2989. doi: 10.1200/JCO.2000.18.16.2981. [DOI] [PubMed] [Google Scholar]
- 32.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 33.Ruckdeschel JC. Combined modality therapy of non-small cell lung cancer. Semin Oncol. 1997;24(4):429–439. [PubMed] [Google Scholar]
- 34.Eberhardt WE, Albain KS, Pass H, et al. Induction treatment before surgery for non-small cell lung cancer. Lung Cancer. 2003;42(Suppl 1):S9–S14. doi: 10.1016/s0169-5002(03)00300-3. [DOI] [PubMed] [Google Scholar]