Abstract
Background
Greater public awareness of venous thromboembolism may be an important next step for optimizing venous thromboembolism prevention and treatment. “Lifetime risk” is an easily interpretable way of presenting risk information. Therefore, we sought to calculate the lifetime risk of venous thromboembolism (deep vein thrombosis and/or pulmonary embolism) using data from two large, prospective cohort studies: the Cardiovascular Health Study (CHS) and the Atherosclerosis Risk in Communities (ARIC) study.
Methods
We followed participants aged 45–64 years in ARIC (n=14,185) and ≥65 in CHS (n=5,414) at baseline visits (1987-89 in ARIC, 1989-90 and 1992–93 in CHS) for incident venous thromboembolism (n=728 in ARIC through 2011 and n=172 in CHS through 2001). We estimated lifetime risks and 95% confidence intervals of incident venous thromboembolism using a modified Kaplan-Meier method, accounting for the competing risk of death from other causes.
Results
At age 45, the remaining lifetime risk of venous thromboembolism in ARIC was 8.1% (95% confidence interval: 7.1-8.7). High-risk groups were African Americans (11.5% lifetime risk), those with obesity (10.9%), heterozygous for the factor V Leiden (17.1%), or with sickle cell trait or disease (18.2%). Lifetime risk estimates differed by cohort; these differences were explained by differences in time period of venous thromboembolism ascertainment.
Conclusions
At least 1 in 12 middle-aged adults will develop venous thromboembolism in their remaining lifetime. This estimate of lifetime risk may be useful to promote awareness of venous thromboembolism and guide decisions at both clinical and policy levels.
Keywords: epidemiology, thrombosis, embolism, risk factors
A recent Surgeon General's Call to Action1 aimed to “raise consumer awareness about DVT/PE [deep vein thrombosis/pulmonary embolism] and the magnitude of the burden caused by these conditions.” Indeed, there is a case for raising awareness of deep vein thrombosis and pulmonary embolism, collectively termed venous thromboembolism, as awareness in the general population is very low. One telephone survey found that only a quarter of respondents had heard of deep vein thrombosis, fewer than 1 in 10 had any knowledge of its symptoms or risk factors, and only 1 in 17 knew that deep vein thrombosis could be prevented2. In other words, only about 6% of Americans know what deep vein thrombosis is and that it can be prevented. Compare this to the 93% who had heard of diabetes and allergies, and 91% who had heard of stroke. Even colitis was better known than deep vein thrombosis, with 42% of the survey population having heard of it. The Surgeon General's Call to Action also aimed to raise policymakers’ awareness of deep vein thrombosis/pulmonary embolism, in hopes that they will in turn support public awareness campaigns; venous thromboembolism education for health care professionals, including the dissemination of evidence-based guidelines; and the development of tools that facilitate implementation of knowledge (such as hospital-based prevention of venous thromboembolism) into clinical practice. Venous thromboembolism is a substantial source of morbidity and mortality, so prevention is imperative3. Promoting awareness of venous thromboembolism may be an important next step for venous thromboembolism prevention and treatment.
Lifetime risk estimates of venous thromboembolism - defined as the cumulative incidence of venous thromboembolism between an index age and death - could help in promoting awareness of venous thromboembolism. Studies show that the general public finds lifetime risk of disease more easily interpretable than some other formats of risk information (e.g., an annual incidence rate)4,5. Interpretability is essential for a public awareness campaign. The most widely cited lifetime risk is for breast cancer – one in eight – and was effective at promoting awareness, prevention, and treatment of breast cancer. In addition, lifetime risk estimates allow for easy comparison of burden between diseases, and hence can be an important tool to guide decisions at both clinical and policy levels.
To our knowledge, no estimates of the lifetime risk of venous thromboembolism exist. Therefore, we calculated the lifetime risk of venous thromboembolism using data from two large, prospective community-based cohort studies: the Cardiovascular Health Study (CHS)6 and the Atherosclerosis Risk in Communities (ARIC)7 study, which have similar protocols.
Methods
Study population
Between 1987–89, ARIC recruited and examined 15,792 participants aged 45–64 years living in four US communities: Forsyth County, NC; Jackson, MS (African Americans only); suburban Minneapolis, MN; and Washington County, MD7. Response rates for the baseline exam were 46% in Jackson and 65 to 67% at other clinic sites; differences between respondents and non-respondents have been described8.
CHS sampled from Medicare lists, recruited, and examined 5,888 community-dwelling participants aged ≥ 65 years living in four US communities (Pittsburgh (Allegheny County), PA; Forsyth County, NC; Sacramento County, CA; and Washington County, MD) between 1989-90 and 1992–93 (African Americans only)6. The response rate for the baseline exam was 61% of those eligible; differences between respondents and non-respondents have been described6.
We excluded individuals from all analyses if they had a history of venous thromboembolism or anticoagulant use at baseline (n = 347 in ARIC, 419 in CHS); were of a race other than African American or white (due to small numbers) (n = 48 in ARIC, 38 in CHS); in ARIC, were African American from Washington County or Minneapolis suburbs (due to small numbers) (n = 55); or had missing data on any variable included in the main analysis (n excluded in ARIC = 1,157: 25 missing body mass index (BMI) measurements, 547 missing factor V Leiden status, 340 missing prothrombin G20210A status, 238 missing blood group measurements, and 7 missing sickle cell trait measurements; n excluded in CHS = 17 missing BMI measurements). Our final sample size for statistical analyses was 14,185 in ARIC and 5,414 in CHS. The Institutional Review Boards of the collaborating institutions approved both studies. The investigators obtained informed consents from all participants before inclusion in the studies.
Venous thromboembolism ascertainment
Incident venous thromboembolism was defined as the first occurrence of a validated deep vein thrombosis or pulmonary embolism from baseline through the end of follow-up: December 31, 2011 for the ARIC Study and December 31, 2001 for CHS.
Hospitalizations were identified through participant report in both studies, through Medicare records in CHS, and though surveillance of community hospitals’ discharge lists in ARIC9. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) discharge codes from all identified hospitalizations were recorded to identify possible cases of venous thromboembolism. Records with ICD-9-CM discharge codes (where x can take any value) 38.7 (procedure code), 415.1, 415.1X, 451, 451.1, 451.1X, 451.2, 451.8, 451.8X, 451.9, 453, 453.X, 453.XX, 996.7, 996.7X, 997.2, or 999.2 were considered to indicate possible venous thromboembolism. The records were copied and independently reviewed by two physicians using standardized criteria, with differences resolved by discussion9.
Definite deep vein thrombosis was defined as a positive venogram, duplex ultrasound, or autopsy. Probable deep vein thrombosis was defined as a positive Doppler examination or impedance plethysmography. Definite pulmonary embolism was defined as a positive pulmonary angiogram, ventilation/perfusion scans indicating “high probability” of pulmonary embolism or at least two segmental perfusion defects without ventilation defects, computed tomography, or autopsy9. Repeat classification has shown excellent repeatability.
Baseline measurements
Age, race, sex, and history of venous thromboembolism were self-reported in both studies. BMI was calculated as weight (kg) divided by height (m)2; obesity was defined as BMI ≥ 30 kg/m2. In ARIC only, factor V Leiden10, prothrombin G20210A11, ABO blood group12, and hemoglobin S genotype13 were measured as previously described.
Statistical analyses
Analyses were performed using SAS (version 9.2, SAS Institute, Cary, North Carolina). Because age-specific incidence rates (IRs) differed by cohort, all analyses were stratified by cohort. We computed person-years of follow-up from the date of the baseline examination to whichever came first: venous thromboembolism event, death, loss to follow-up, or the end of follow-up. Person-years of follow-up were allocated to 5-year age-specific groups. Age-specific venous thromboembolism IRs per 1,000 person-years were calculated by dividing the age-specific number of incident venous thromboembolism events by age-specific person-years of follow-up, and multiplying this number by 1,000.
Lifetime risk was approximated as the cumulative incidence14 of venous thromboembolism through age 85, because many ARIC participants were not older than 85 at the end of follow-up. We used the Practical Incidence Estimators SAS Macro15 to estimate the remaining lifetime risk of incident venous thromboembolism conditional on survival (alive and free of venous thromboembolism) to selected index ages, and then repeated the estimation stratified by subgroups: sex, race, obesity status at baseline, factor V Leiden, prothrombin G20210A, blood group, and sickle cell trait. These variables were chosen because they are common risk factors for venous thromboembolism in the general population, and most are static across time. As tends to be the case in prospective cohort studies with a long period of follow-up, not all individuals in ARIC and CHS were followed for the entire duration. To account for this, we used a modified Kaplan-Meier approach to approximate cumulative incidence15 (i.e., lifetime risk and its confidence interval). We adjusted lifetime risk estimates for the competing risk of death15,16. Thus, each participant was classified as either a venous thromboembolism event, censored (loss to follow-up or the end of follow-up), or a non-venous thromboembolism-related death, whichever came first.
Because age-specific venous thromboembolism IRs varied by cohort, we conducted sensitivity analyses to explore these differences. We restricted ARIC's follow-up to 2001 to match CHS’ and calculated IRs. We also investigated the effect of cohort and time period of venous thromboembolism ascertainment on IRs by plotting cohort- and age-specific IRs by time period.
Results
At baseline, the mean age of ARIC participants was 54 years, approximately 50% were female, one-quarter were African American, and one-quarter were obese. In comparison, the CHS cohort at baseline was older (mean age of 73), had a similar sex distribution, a lower proportion of African Americans, and a lower proportion of participants who were obese (Table 1).
Table 1.
Baseline characteristics of ARIC and CHS participants.
| Characteristics (means or prevalences) | ARIC (N = 14,185) | CHS (N = 5,414) |
|---|---|---|
| Age, years (SD) | 54.1 (5.8) | 72.8 (5.6) |
| Male, % | 44.7 | 42.8 |
| African American, % | 26.1 | 15.7 |
| Obese*, % | 27.2 | 19.3 |
| Factor V Leiden, % AA or AG† | 4.5 | N/A |
| Prothrombin G20210A, % AA or AG† | 2.2 | N/A |
| Non-O group blood type, % | 57.5 | N/A |
| HbS genotype among African Americans, % AS (sickle cell trait) or SS (sickle cell disease)‡ | 6.9 | N/A |
Baseline ARIC Study: 1987-89.
Baseline CHS: 1989-90 and 1992-93.
ARIC, Atherosclerosis Risk in Communities study; CHS, Cardiovascular Health Study; HbS, hemoglobin S; SD, standard deviation.
Obesity is defined as a body mass index of ≥ 30.
AA: homozygous mutant, AG: heterozygous mutant.
AS: heterozygosity for hemoglobin S, SS: homozygosity for hemoglobin S. HbS genotype was measured in ARIC African Americans only (N = 3,704).
After the index age of 45 years, ARIC participants developed 728 venous thromboembolism events over 288,535 person-years of follow-up. After the index age of 65 years, CHS participants developed 172 venous thromboembolism events over 54,207 person-years of follow-up. As evidenced by sparse person-years (Table 2), few ARIC participants were followed past age 85, and few CHS participants survived past age 95. Incidence rates of venous thromboembolism increased exponentially across increasing age groups. Notably, the age-specific rates in ARIC were higher than for CHS.
Table 2.
Age-specific incidence rates of venous thromboembolism per 1,000 person-years, ARIC and CHS.
| ARIC (N = 14,185) | CHS (N= 5,414) | |||||
|---|---|---|---|---|---|---|
| Age group | # of venous thromboembolisms | Person-years | Incidence rates of venous thromboembolism | # of venous thromboembolisms | Person-years | Incidence rates of venous thromboembolism |
| 45-49 | 6 | 11,517 | 0.5 | - | - | - |
| 50-54 | 15 | 30,127 | 0.5 | - | - | - |
| 55-59 | 45 | 46,562 | 1.0 | - | - | - |
| 60-64 | 106 | 60,075 | 1.8 | - | - | - |
| 65-69 | 168 | 60,664 | 2.8 | 4 | 4,819 | 0.8 |
| 70-74 | 165 | 43,301 | 3.8 | 38 | 14,145 | 2.7 |
| 75-79 | 132 | 24,622 | 5.4 | 47 | 17,353 | 2.7 |
| 80-84 | 80 | 10,121 | 7.9 | 43 | 11,320 | 3.8 |
| 85-89 | 11 | 1,548 | 7.1 | 31 | 4,986 | 6.2 |
| 90-94 | 0 | 1 | 0.0 | 8 | 1,358 | 5.9 |
| 95-99 | - | - | - | 1 | 205 | 4.9 |
| 100-104 | - | - | - | 0 | 22 | 0.0 |
Baseline through end of follow-up in the ARIC Study = 1987-89 through 2011.
Baseline through end of follow-up in CHS = 1989-90 through 2001.
ARIC, Atherosclerosis Risk in Communities study; CHS, Cardiovascular Health Study; HbS, hemoglobin S.
Conditional on survival (alive and free of venous thromboembolism) to age 45, the remaining lifetime risk of venous thromboembolism in ARIC was 8.1% (95% confidence interval: 7.1-8.7) (Table 3). There was little difference in lifetime risk of venous thromboembolism by sex. Particularly high-risk groups were African Americans, with a lifetime risk (95% confidence interval) of 11.5% (8.8-13.1), participants who were obese at baseline (10.9% (8.7-12.3)), participants with factor V Leiden (17.1% (11.4-21.4)), and participants with sickle cell trait or disease (18.2% (3.8-25.1)). As expected, remaining lifetime risk of venous thromboembolism decreased across increasing index ages, reflecting the shorter life expectancy and period at risk of older participants (Supplemental Figure 1, Panels A-E).
Table 3.
Lifetime risk (95% CI) of venous thromboembolism, ARIC.
| Lifetime risk of venous thromboembolism (95% CI), % |
|
|---|---|
| Total | 8.1 (7.1, 8.7) |
| Sex | |
| Male | 7.7 (6.3, 8.6) |
| Female | 8.4 (7.0, 9.3) |
| Race | |
| White | 6.9 (5.9, 7.7) |
| African American | 11.5 (8.8, 13.1) |
| Obesity status at baseline* | |
| Obese | 10.9 (8.7, 12.3) |
| Not obese | 7.0 (5.9, 7.7) |
| Factor V Leiden† | |
| AA or AG | 17.1 (11.4, 21.4) |
| GG | 7.6 (6.6, 8.3) |
| Prothrombin G20210A† | |
| AA or AG | 6.3 (1.6, 9.7) |
| GG | 8.1 (7.1, 8.8) |
| Blood type | |
| Non-O group blood type | 8.9 (7.5, 9.8) |
| O group blood type | 7.0 (5.5, 7.9) |
| HbS Genotype among African Americans‡ | |
| AS (sickle cell trait) or SS (sickle cell disease) | 18.2 (3.8, 25.1) |
| No S | 11.0 (8.3, 12.5) |
Lifetime risk is to age 85, conditional on survival free of venous thromboembolism to age 45, with death free of venous thromboembolism considered a competing event.
Baseline through end of follow-up in the ARIC Study = 1987-89 through 2011.
Obesity is defined as a body mass index ≥ 30.
AA: homozygous mutant, AG: heterozygous mutant, GG: wild type.
HbS genotype was measured in ARIC African Americans only (N = 3,704). AS: heterozygosity for hemoglobin S, SS: homozygosity for hemoglobin S, No S: wild type.
ARIC, Atherosclerosis Risk in Communities study; CI, confidence interval; HbS, hemoglobin S.
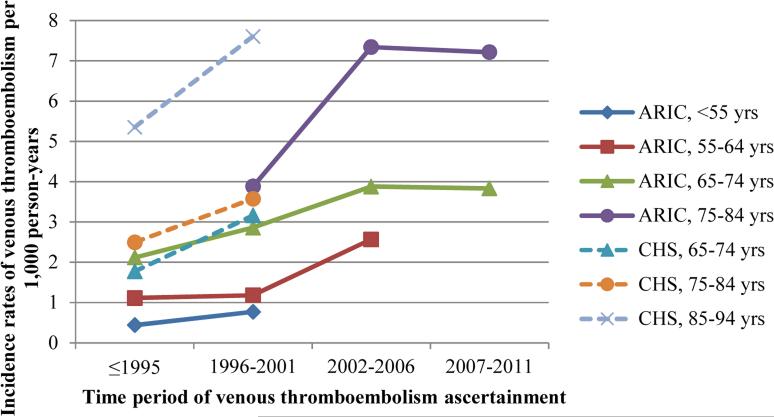
When comparing lifetime risk estimates between the cohorts, those for CHS were about half those for ARIC (Lifetime risk at index age 65: ARIC – 7.1%, CHS – 3.9%; lifetime risk at index age 75: ARIC – 5.2%, CHS – 2.6%) (Supplemental Figure 1, Panel A). At least part of this difference by cohort may relate to ARIC, but not CHS, having follow-up for 2002-2011. When the follow-up for ARIC was restricted to 2001 to match that for CHS, the IRs were more similar (Supplemental Table 1). Additionally, when we compared cohort- and age-specific IRs by time period of venous thromboembolism ascertainment, the IRs per 1,000 person years were similar by study (≤1995, 65-74 yrs: ARIC = 2.1, CHS = 1.8; 1996-2001, 65-74 yrs: ARIC = 2.9, CHS = 3.2; 1996-2001, 75-84 yrs: ARIC = 3.9, CHS = 3.6), and age-specific IRs of venous thromboembolism increased across increasing time periods of venous thromboembolism ascertainment (Figure 1).
Figure 1.
Cohort- and age- specific venous thromboembolism incidence rates by time period of venous thromboembolism ascertainment.
Discussion
The lifetime risk of venous thromboembolism in ARIC was one in 12. High-risk groups were African Americans (1 in 9), those with obesity (1 in 9), those with factor V Leiden (1 in 6), and those with sickle cell trait or disease (1 in 5). The lifetime risk of venous thromboembolism decreased with age, as expected, but still remained relatively high at older ages. IRs and lifetime risk estimates differed between ARIC and CHS; these differences were largely explained by differences in time period of venous thromboembolism ascertainment, with IRs of venous thromboembolism being higher in recent years.
The lifetime risk of venous thromboembolism is substantial, although lower than for other major cardiovascular diseases. At age 40 years, the lifetime risk estimates of coronary heart disease are 1 in 2 for males and 1 in 3 for females17; atrial fibrillation, 1 in 418; and congestive heart failure, at least 1 in 519,20. At age 55, the lifetime risk estimates of stroke are 1 in 6 for males and 1 in 5 for females21. At age 40 years, the lifetime risk of breast cancer is 1 in 8 for females22.
To reiterate, these estimates of lifetime risk may be useful to promote awareness of venous thromboembolism and guide decisions at policy levels. In addition, the lifetime risk estimates could plausibly be used to counsel patients. For instance, screening for sickle cell disease in newborns is now mandated in the U.S., so the lifetime risk estimate of venous thromboembolism in those with sickle trait may be useful in counseling families of identified sickle cell trait newborns. Additionally, factor V Leiden was associated with a high lifetime risk; this estimate might be useful in counseling individuals with factor V Leiden. However, testing for the factor V Leiden mutation in clinical practice is controversial, as it is unknown whether testing improves outcomes in those with venous thromboembolism or in family members of those with a mutation. If testing was paired with education, it could result in awareness of symptoms and risk factors for venous thromboembolism23.
Our estimates of lifetime risk of venous thromboembolism are only generalizable to US populations similar to ARIC. Participants in this study were exclusively African American or white, so results may not generalize to other race groups. Asians and Hispanics in the U.S. have lower incidence rates of venous thromboembolism than whites. African Americans may have higher rates24,25 than whites, although a recent paper calls this into question26. It is important to remember the lifetime risk estimates represent population averages; an individual's estimated risk of venous thromboembolism will vary depending on the presence or absence of various factors.
Differences in IRs and lifetime risk estimates between ARIC and CHS may be explained by changes over time in how venous thromboembolism was ascertained. Computed tomographic pulmonary angiography (CTPA) - a highly sensitive imaging technique to detect pulmonary embolism - was introduced in 1998; CTPA largely and rapidly replaced other tests for pulmonary embolism27. Thought to be in large part a result of the introduction of CTPA, pulmonary embolism incidence in the US increased substantially after 199828. In concordance, our data shows a sharp increase in IRs during the time period that CTPA was adopted (Figure 1). Of course, if venous thromboembolism IRs truly increase over time or the detection of venous thromboembolism becomes more sensitive, estimates of lifetime risk will correspondingly increase.
Other possible explanations for the observed differences between ARIC and CHS exist. CHS participants were aged 65 or older at recruitment. Baseline exclusions and self-selection could have made the CHS cohort healthier in old age than ARIC, for whom exclusions at recruitment occurred when participants were 20 years younger (recruited at ages 45-64) and thus included more unhealthy people by age 65 plus. Also, ARIC had a higher proportion of African Americans (26 versus 15%) and participants with obesity (27 versus 19%) compared to CHS. However, race- and obesity- specific IRs did not explain the discrepancies between cohorts (data not shown).
It is likely that we underestimated the incidence of venous thromboembolism, and thus lifetime risk, in these cohorts. As in most studies, fatal and undiagnosed venous thromboembolisms would have been under-ascertained. Further, cases of venous thromboembolism treated solely in outpatient settings were not captured. An ARIC pilot study estimated that 40% of venous thromboembolisms (based just on ICD code) were outpatient for 1991-2009. However, this is an overestimate, because these potential venous thromboembolism events are not validated. Data from REasons for Geographic and Racial Differences in Stroke (REGARDS), a nationally representative cohort study, estimates a lower proportion of outpatient venous thromboembolisms: between 2003-07, only 10.3% of validated venous thromboembolisms (39 out of 379) were treated in an outpatient setting. And although most venous thromboembolisms occur later in life, we missed venous thromboembolisms before the age of 45, including most venous thromboembolisms related to pregnancy and oral contraceptive use. And finally, approximating lifetime risk estimates as the cumulative incidence of venous thromboembolism through age 85 assumed that nobody developed venous thromboembolism after age 85, further supporting that our estimates are underestimates.
Strengths of this study include a prospective design; two large, population-based biracial samples with a wide geographic distribution in the United States; venous thromboembolism validation; and a long period of follow-up. Drawbacks of this study warrant discussion as well. Participants were exclusively African American or white, so results may not generalize to other race groups. IRs and lifetime risk estimates were unadjusted for other variables, raising the possibility that differences in these metrics between risk subgroups were due to differences in the distribution of other variables. We potentially misclassified obesity, as BMI is not necessarily static across time. However, the lifetime risk estimates in the ‘obese at baseline’ strata are interpretable: In ARIC, it is the lifetime risk of venous thromboembolism if obese in middle-age; in CHS, it is the lifetime risk of venous thromboembolism if obese in older age. And, as discussed above, we likely underestimated lifetime risk.
In conclusion, at least 1 in 12 middle-aged adults develop venous thromboembolism in their lifetime. The fact that 8% of individuals will develop venous thromboembolism during their life highlights the high morbidity of this condition. Considering that fewer than 1 in 10 Americans have any knowledge of deep vein thrombosis2, these findings reiterate the need for venous thromboembolism awareness. This estimate of lifetime risk may be useful to promote awareness of venous thromboembolism and guide decisions at both clinical and policy levels.
Supplementary Material
Clinical significance.
At least 1 in 12 middle-aged adults will develop VTE in their remaining lifetime.
This estimate of lifetime risk may be useful to promote awareness of VTE and guide decisions at both clinical and policy levels.
Acknowledgments
The authors thank the staff and participants of the ARIC and CHS studies for their important contributions.
Funding Sources
The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). CHS was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the NHLBI, with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). Investigator E.J.B. was supported by NHLBI training grant T32HL007779. This content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
REFERENCES
- 1.Office of the Surgeon General (US) The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: 2008. [PubMed] [Google Scholar]
- 2.American Public Health Association Deep-Vein Thrombosis: Advancing Awareness to Protect Patient Lives. Public Health Leadership Conference on Deep-Vein Thrombosis. White Paper. [Google Scholar]
- 3.Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis : A major contributor to global disease burden #2729 ISTH Steering Committee for World Thrombosis Day The members of the ISTH Steering Committee for World Thrombosis Day : Thromb Res. 2014;134(5):931–938. doi: 10.1016/j.thromres.2014.08.014. doi:10.1016/j.thromres.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Sasieni PD, Adams J. Standardized lifetime risk. Am J Epidemiol. 1999;149(9):869–875. doi: 10.1093/oxfordjournals.aje.a009903. [DOI] [PubMed] [Google Scholar]
- 5.Fortin JM, Hirota LK, Bond BE, O'Connor AM, Col NF. Identifying patient preferences for communicating risk estimates: a descriptive pilot study. BMC Med Inform Decis Mak. 2001;1:2. doi: 10.1186/1472-6947-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(4):358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 7.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 8.Jackson R, Chambless LE, Yang K, et al. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol. 1996;49(12):1441–1446. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 9.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19–25. doi: 10.1016/j.amjmed.2004.01.018. doi:10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 10.De Ronde H, Bertina RM. Laboratory diagnosis of APC-resistance: a critical evaluation of the test and the development of diagnostic criteria. Thromb Haemost. 1994;72(6):880–886. [PubMed] [Google Scholar]
- 11.Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88(10):3698–3703. [PubMed] [Google Scholar]
- 12.Ohira T, Cushman M, Tsai MY, et al. ABO blood group, other risk factors and incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE). J Thromb Haemost. 2007;5(7):1455–1461. doi: 10.1111/j.1538-7836.2007.02579.x. doi:10.1111/j.1538-7836.2007.02579.x. [DOI] [PubMed] [Google Scholar]
- 13.Folsom AR, Tang W, Roetker NS, et al. Prospective study of sickle cell trait and venous thromboembolism incidence. J Thromb Haemost. 2014 doi: 10.1111/jth.12787. doi:10.1111/jth.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rychetnik L, Hawe P, Waters E, Barratt A, Frommer M. A glossary for evidence based public health. J Epidemiol Community Health. 2004;58(7):538–545. doi: 10.1136/jech.2003.011585. doi:10.1136/jech.2003.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beiser A, D'Agostino RB, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer's disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19(11-12):1495–1522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Pintilie M. Analysing and interpreting competing risk data. Stat Med. 2007;26(6):1360–1367. doi: 10.1002/sim.2655. doi:10.1002/sim.2655. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353(9147):89–92. doi: 10.1016/S0140-6736(98)10279-9. doi:10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. doi:10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. doi:10.1016/S0140-6736(05)75030-3. [DOI] [PubMed] [Google Scholar]
- 20.Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013;61(14):1510–1517. doi: 10.1016/j.jacc.2013.01.022. doi:10.1016/j.jacc.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seshadri S, Beiser A, Kelly-Hayes M, et al. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37(2):345–350. doi: 10.1161/01.STR.0000199613.38911.b2. doi:10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 22.Feuer EJ, Wun LM, Boring CC, Flanders WD, Timmel MJ, Tong T. The lifetime risk of developing breast cancer. J Natl Cancer Inst. 1993;85(11):892–897. doi: 10.1093/jnci/85.11.892. [DOI] [PubMed] [Google Scholar]
- 23.Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301(23):2472–2485. doi: 10.1001/jama.2009.853. doi:10.1001/jama.2009.853. [DOI] [PubMed] [Google Scholar]
- 24.White RH, Zhou H, Murin S, Harvey D. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost. 2005;93(2):298–305. doi: 10.1160/TH04-08-0506. doi:10.1267/THRO05020298. [DOI] [PubMed] [Google Scholar]
- 25.White RH, Zhou H, Romano PS. Incidence of idiopathic deep venous thrombosis and secondary thromboembolism among ethnic groups in California. Ann Intern Med. 1998;128(9):737–740. doi: 10.7326/0003-4819-128-9-199805010-00006. [DOI] [PubMed] [Google Scholar]
- 26.Zakai NA, McClure LA, Judd SE, et al. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation. 2014;129(14):1502–1509. doi: 10.1161/CIRCULATIONAHA.113.006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittram C, Meehan MJ, Halpern EF, Shepard JO, McLoud TC, Thrall JH. Trends in thoracic radiology over a decade at a large academic medical center. J Thorac Imaging. 2004;19(3):164–170. doi: 10.1097/01.rti.0000117623.02841.e6. [DOI] [PubMed] [Google Scholar]
- 28.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831–837. doi: 10.1001/archinternmed.2011.178. doi:10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.