Abstract
Cardiac disease causes morbidity in several lysosomal storage diseases, which are the result of deficient activity of lysosomal enzymes. Mucopolysaccharidosis (MPS) causes aortic and valvular disease, Pompe disease causes cardiac muscle weakness, and Fabry disease causes left ventricular hypertrophy. Enzyme replacement therapy involves intravenous injection of enzyme modified with mannose 6-phosphate, which can be taken up by cells, and is currently approved for some lysosomal storage diseases. Gene therapy can result in secretion of mannose 6-phosphate-modified enzyme into blood, from where it can; similarly, be taken up by cells. Gene therapy has been effective in animal models of lysosomal storage disease, and holds great promise.
Keywords: Animal models, gene therapy, lysosomal storage disease, mucopolysaccharidosis, valve
Introduction
More than 50 different forms of inherited lysosomal storage diseases are known to occur in humans; most result in high morbidity and mortality. The aggregate incidence is approximately 1 in 7000 live births [1]. Most lysosomal storage diseases are caused by loss of normal function of a specific lysosomal acid hydrolase, resulting in inability of the lysosomes to degrade large complex substrates that have been targeted for degradation after endocytosis or autophagy. Lysosomal accumulation of the substrate affects the architecture and function of cells, tissues, and organs; lysosomal storage diseases that affect the heart include mucopolysaccharidosis (MPS), Pompe disease, and Fabry disease. MPS results from deficiency in any of several enzymes involved in degradation of glycosaminoglycans, and results in mitral and aortic valve thickening and regurgitation, and aortic dilatation. Pompe disease is caused by deficiency of acid-α-glucosidase (AAG), and results in weakness of cardiac muscle because of the accumulation of glycogen [2]. Fabry disease is attributable to deficiency of α-galactosidase A and results in accumulation of globotriaosylceramide, a glycosphingolipid, and results in vascular disease, left ventricular hypertrophy, and cardiac conduction defects [3].
Enzyme replacement therapy has been developed recently for some types of MPS and for Pompe and Fabry diseases. It involves intravenous injection of mannose-6-phosphate-modified enzyme, which can diffuse to cells and be taken up via the mannose-6-phosphate receptor. The active enzyme can be delivered to the lysosome, where it catabolizes stored substrate. Other treatment alternatives include hematopoietic stem cell transplantation for MPS [4,5], and substrate reduction therapy, which is being tested for Fabry disease [3].
Therapeutic gene transfer holds great therapeutic potential, and has proven successful in several animal models of lysosomal storage disease. The most common form of gene therapy involves the transfer into an animal of a cDNA that encodes a functional protein, resulting in long-term expression of the protein that was deficient. As transduced cells in a single organ can secrete enzyme that enters blood or diffuses to adjacent cells, a small percentage of transduced cells can be therapeutic for the entire animal. In-vivo gene therapy involves systemic injection of vector into the circulation, or localized injection into a specific organ. Ex-vivo gene therapy is comprised of removal of cells (such as hematopoietic stem cells), modification in vitro, and infusion of the modified cells back into the animal.
Mucopolysaccharidosis
MPS is caused by deficiency of enzymes that degrade glycosaminoglycans, and results in thickening and elastin fiber fragmentation in the aorta and cardiac valves. This is associated with upregulation of elastin-degrading proteins such as matrix metalloproteinases and cathepsins [6], and the changes can result in valvular stenosis or insufficiency, or both. Intravenous injection of plasmid DNA or viral vectors has been found to result in high expression in the liver, and high concentrations of enzyme in the serum [7]. Neonatal administration of a retroviral vector was shown to reduce the cardiac and aortic manifestations in mice with MPS I, which are deficient in activity of α-l-iduronidase [8]. Good hepatic transgene expression has also been observed with adenoviral vectors [9], SV40 vectors, and a transposon-based plasmid [10], and in-vivo treatment with adeno-associated virus or lentiviral vectors has reduced storage of glycosaminoglycans in the heart, although functional analyses were not performed [11]. Ex vivo hematopoietic-stem-cell-directed gene therapy improved left ventricular function in mice with MPS I [4].
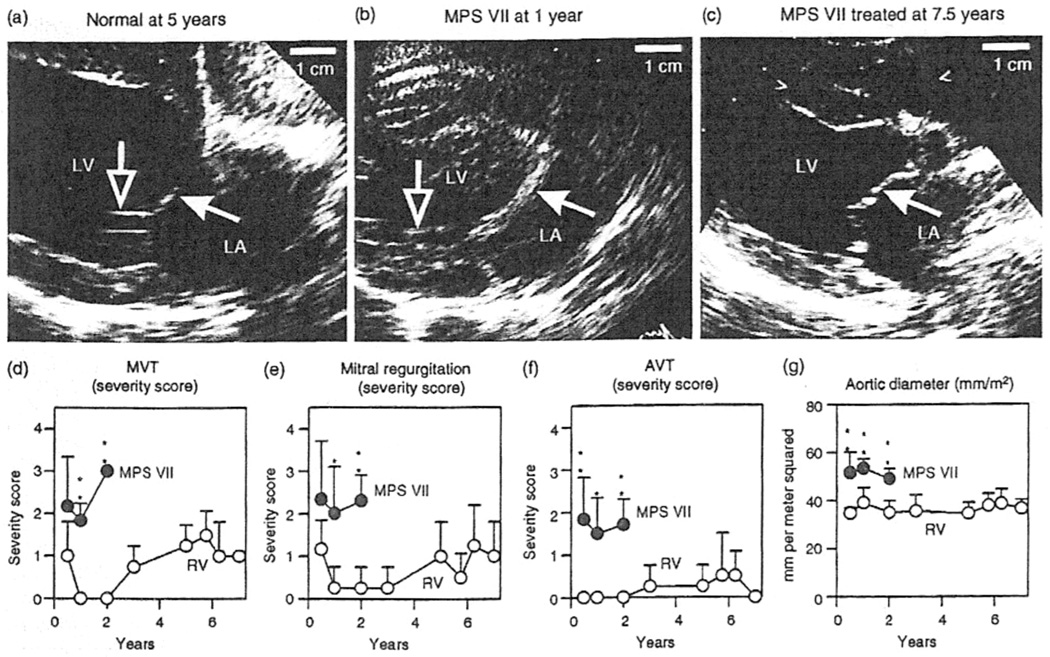
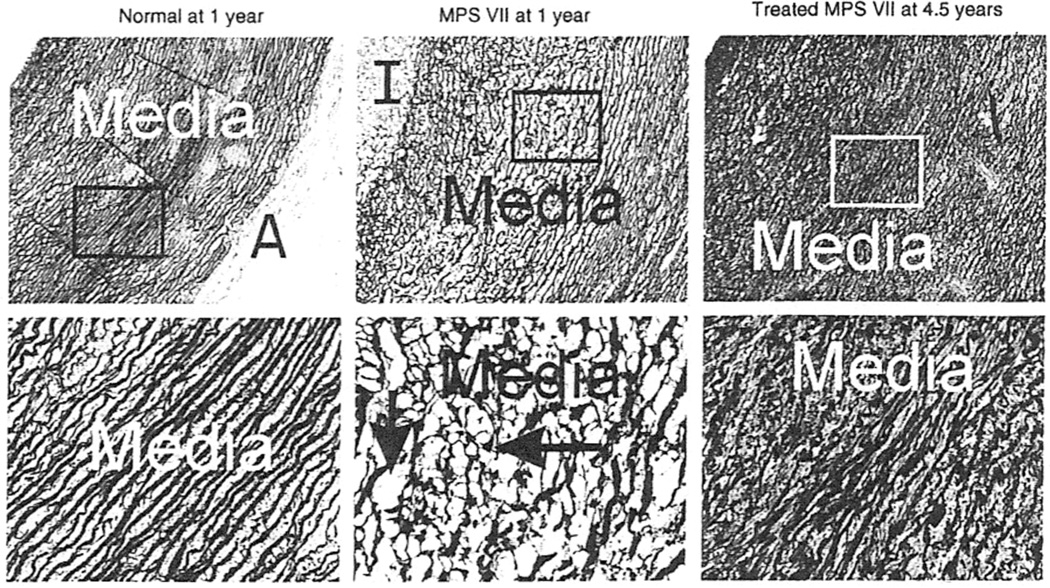
Studies involving large animals are more likely to be predictive of results in humans, because their larger size requires efficient scaling and their longer lifespan allows late evaluation of efficacy and toxicity [11]. Retroviral vector gene therapy has yielded impressive results for dogs with MPS VII or MPS I. Dogs affected with MPS VII due to β-glucuronidase deficiency that were treated at 2–3 days of age using a retroviral vector achieved very high serum β-giucuronidase activity [12] that has been maintained stably for up to 7years (M. Sleeper, M. Haskins, K. Ponder, unpublished data). There was a marked reduction in mitral and aortic valve thickening and insufficiency [12, 13], which has been sustained at 7.5 years (Figure 1). In addition, dogs with untreated MPS VII exhibit aortic dilatation and elastin fragmentation, and neonatal gene therapy with a retrovirai vector has been found to result in a marked reduction in both of these parameters [13] that was maintained in the long term (Figure 1, Figure 2). Similar experiments in dogs affected with MPS I using a retroviral vector containing the canine α-l-iduronidase cDNA also resulted in stable enzyme activity and normal aortic diameters with mild aortic valve thickening and aortic regurgitation at 1 year [14], and improvements have been maintained at 2 years, although moderate mitral regurgitation was present (M. Sleeper, M. Haskins, K. Ponder, unpublished data). Marked improvement in the heart was also noted in MPS I affected cats that were treated as newborns with intravenous injection of a retroviral vector expressing the deficient enzyme (M. Sleeper, M. Haskins, K. Ponder, unpublished data). The improvement lasted for at least 2 years, when transient immune suppression was given at the time of gene therapy [11].
Figure 1.
Echocardiograms in dogs with mucopolysaccharidosis (MPS) VII. Normal dogs, dogs with untreated MPS VII, or dogs with MPS VII treated with retroviral vector underwent echocardiography at the ages indicated above the panels. (a–e) Echocardiographic images of the mitral valve obtained in the long axis from the right parasternum. The cranial (anterior) mitral valve leaflets are indicated by filled white arrows, and the chordae tendinae by open arrows. Note the marked thickening of the valve in the untreated MPS VII dog compared with that in the normal and treated MPS VII dogs. (d–f) Subjective severity score for echocardiogram parameters. Mitral valve thickening (MVT) (d), mitral valve regurgitation (e), and aortic valve thickening (AVT) (f) were scored from 0 (normal) to 4 (severely abnormal) for six or seven dogs with untreated MPS VII and for four dogs with MPS VII treated with retroviral vector, at the indicated ages. Values in the groups at a given age were compared statistically using Student’s t-test (*P = 0.005–0.05; **P = < 0.005). Untreated dogs with MPS VII do not survive beyond 2 years, so it was not possible to compare values in treated dogs with MPS VII with those in age-matched, untreated dogs at older ages. (g) Aortic diameter. The aortic diameter determined in the long axis was normalized to the body surface area (m2), and statistical comparisons were performed as in (d–f). LA, left atrium; LV, left ventricle.
Figure 2.
Pathology of the aorta. Aortae were collected from dogs at the ages indicated above the panels and then stained for elastin with Verhoeff’s van Giesen stain, which results in a dark color in the fibers. All images have the intima (I) at the upper left, and the adventitia (A) in the lower right. Lysosomal storage is indicated by the short vertical arrow, and elastin fiber fragmentation is identified with the long horizontal arrow. Low-power images (original magnification ×5) are shown in the top panels, in which the box indicates the region that is shown at high power (original magnification ×20) in the lower panels.
Pompe disease
Pompe disease results in the accumulation of glycogen in cardiac and skeletal muscle, and is associated with reduced contractility, the causative mechanisms of which remain unclear. In mice with Pompe disease, intravenous administration of an adeno-associated virus vector expressing AAG resulted in efficient transduction of liver, high concentrations of AAG in blood, and reduced lysosomal storage [15]. Cardiac function and mass normalization were sustained with gene therapy for at least 1 year in this model [16]. Therapy was more effective in clearing cardiac glycogen storage in heart muscle of young AAG-knockbut mice than in that of older counterparts [2,15]. These results emphasize that screening of newborns to allow early diagnosis and treatment is an important initiative.
Fabry disease
Fabry disease results in kidney and heart disease, at least in part as a result of the accumulation of storage material in the vascular system. Intravenous administration of adeno-associated virus serotype 8 encoding for human α-galactosidase resulted in normalization of tissue (including cardiac) substrate storage concentrations [17], although the mildness of cardiac disease in this model made it difficult to determine if there was clinical improvement.
Summary
Systemic gene therapy with viral vectors has reduced the clinical manifestations of cardiovascular disease in MPS and in Pompe disease, and will probably have a therapeutic effect for Fabry disease. As early treatment was more beneficial in some studies, identification of patients with lysosomal storage disease by means of screening of newborns [18], which is currently being introduced in some states in the USA, will be important in the future. Studies are currently underway to define the risk of insertional mutagenests and to modify vectors in order to reduce the associated carcinogenic risks. If these concerns can be addressed, it is possible that, in the near future, gene therapy will be used to treat patients with these diseases.
Acknowledgments
This work was supported by National Institutes of Health Grants DK06648 awarded to K. P. P. and DK54481 and RR02512 awarded to M. E. H.
REFERENCES
- 1.Ellinwood NM, Vite CH, Haskins ME. Gene therapy for lysosomal storage diseases: the lessons and promise of animal models. Gene Med. 2004;6:481–506. doi: 10.1002/jgm.581. [DOI] [PubMed] [Google Scholar]
- 2.Geel TM, McLaughlin PM, de Leij LF, Ruiters MH, Niezen-Koning KE. Pompe disease: current state of treatment modalities and animal models. Mol Genet Metab. 2007;92:299–307. doi: 10.1016/j.ymgme.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Seino Y, Takahashi H, Fukumoto H, Utsumi K, Hirai V. Cardiovascular manifestations of Fabry disease and the novel therapeutic strategies. J Nippon Med Sch. 2005;72:254–261. doi: 10.1272/jnms.72.254. [DOI] [PubMed] [Google Scholar]
- 4.Jordan MC, Zheng Y, Ryazantsev S, Rozengurt N, Roos KP, Neufeld EF. Cardiac manifestations in the mouse mode! of mucopolysaccharidosis. I. Mol Genet Metab. 2005;86:233–243. doi: 10.1016/j.ymgme.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y, Ryazantsev S, Ohmi K, et al. Retrovirally transduced bone marrow has a therapeutic effect on brain in the mouse model of mucopolysaccnaridosis IIIB. Mol Genet Metab. 2004;82:286–295. doi: 10.1016/j.ymgme.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Ma X, Tittiger M, Knutsen RH, Schaller L, Mecham RP, Ponder KP. Upregulation of elastase proteins results in aortic dilatation in mucopolysaccharidosis I mice. Mol Genet Metab. 2008;94:298–304. doi: 10.1016/j.ymgme.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camassola M, Braga LM, Delgado-Canedo A, et al. Nonviral in vivo gene transfer in the mucopolysaccharidosis I murine model. J Inherit Metab Dis. 2005;28:1035, 1043–304. doi: 10.1007/s10545-005-0070-5. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Xu L, Hennig AK, et al. Liver-directed neonatal gene therapy prevents cardiac, bone, ear, and eye disease in mucopolysaccharidosis I mice. Mot Ther. 2005;11:35–47. doi: 10.1016/j.ymthe.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Xu F, Ding E, Migone F, et al. Glycogen storage in multiple muscles of old GSD-II mice can be rapidly cleared after a single intravenous injection with a modified adenoviral vector expressing hGAA. J Gene Med. 2005;7:171–178. doi: 10.1002/jgm.660. [DOI] [PubMed] [Google Scholar]
- 10.Aronovich EL, Bell JB, Belur LR, et al. Prolonged expression of a lysosomal enzyme in mouse liver after sleeping beauty transposon-mediated gene delivery: implications for non-viral gene therapy of mucopolysaccharidoses. J Gene Med. 2007;9:403–415. doi: 10.1002/jgm.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponder KP, Haskins ME. Gene therapy for mucopolysaccharidosis. Expert Opin Biol Ther. 2007;7:1333–1345. doi: 10.1517/14712598.7.9.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponder KP, Melniczek R, Xu L, et al. Therapeutic neonatal hepatic gene therapy in mucopolysaccharidosis VII dogs. Proc Natl Acad Sci U S A. 2002;99:13102–13107. doi: 10.1073/pnas.192353499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sleeper MM, Fornasari B, Ellinwood NM, et al. Gene therapy ameliorates cardiovascular disease in dogs with mucopolysaccharidosis VII. Circulation. 2004;110:815–820. doi: 10.1161/01.CIR.0000138747.82487.4B. [DOI] [PubMed] [Google Scholar]
- 14.Traas AM, Wang P, Ma X, et al. Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy. Mol Ther. 2007;15:1423–1431. doi: 10.1038/sj.mt.6300201. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler R, Bercury SD, Fidler J, et al. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid-alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum Gene Ther. 2008;19:609–621. doi: 10.1089/hum.2008.010. [DOI] [PubMed] [Google Scholar]
- 16.Mah C, Pacak CA, Cresawn KO, et al. Physiological correction of Pompe disease by systemic delivery or adeno-associated virus serotype 1 vectors. Mol Ther. 2007;15:501–507. doi: 10.1038/sj.mt.6300100. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler R, Cherry M, Barbon CM, et al. Correction of the biochemical and functional deficits in Fabry mice following AAV8-mediated hepatic expression of alpha-galactosidase. A. Mol Ther. 2007;15:492–500. doi: 10.1038/sj.mt.6300066. [DOI] [PubMed] [Google Scholar]
- 18.Turecek F, Scott CR, Celb MH. Tandem mass spectrometry in the detection of inborn errors of metabolism for newborn screening. Methods Mol Biol. 2007;359:143–157. doi: 10.1007/978-1-59745-255-7_10. [DOI] [PubMed] [Google Scholar]