Abstract
Sarcoidosis is a systemic granulomatous disorder of unknown origin that affects the lungs and mediastinal lymph nodes in most patients. The coexistence of sarcoidosis and breast cancer has been reported. An unfortunate consequence of the presence of both entities in the same patient is the risk of misdiagnosis. We report the case of a 70-year-old female with T1N0 cancer of the right breast that was initially diagnosed as stage IV because of mediastinal positron-emission tomography -positive lymphadenopathy. Biopsy of a mediastinal lymph node allowed us to diagnose sarcoidosis and correctly stage her disease as stage I breast cancer.
Keywords: Breast cancer, sarcoidosis, positron-emission tomography/computed tomography, metastasis
INTRODUCTION
Sarcoidosis is a disease involving abnormal collections of granulomas that can form as nodules in multiple organs, mostly located in the lungs and mediastinal lymph nodes. Common symptoms include cough, dyspnea, fatigue, weight loss, and skin lesions such as erythema nodosum; clinically, patients could be asymptomatic (1–3). The incidence of sarcoidosis in cancer patients is reported to be higher than that in the general population (4). The relationship between sarcoidosis and carcinogenesis is unclear, and immune dysfunction may facilitate both disorders (5).
We report a case of a patient with early-stage breast cancer with no previous diagnosis of sarcoidosis, in whom metastatic disease was suspected based on positron-emission tomography (PET)/computed tomography (CT) findings that were proven upon biopsy to be consistent with sarcoidosis.
CASE PRESENTATION
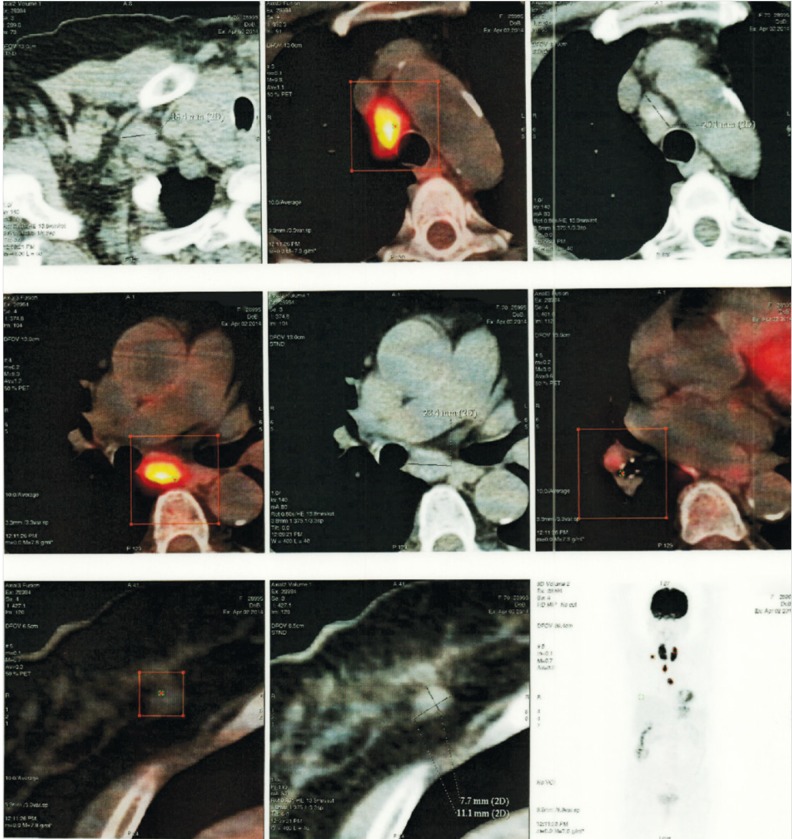
A 70-year-old female arrived at our clinic for a routine visit without any complaints or significant health problems. She was admitted to our clinic for an annual routine mammographic and ultrasonography evaluation. At physical examination, there were no palpable mass and axillary lymph nodes. She had no family history of breast cancer, no personal history of cancer, no previous breast biopsies or surgeries, and no use of hormone replacement. Mammography revealed that further evaluation was needed (BIRADS 0). Right breast ultrasound findings revealed a hypoechoic, heterogeneous area and/or a mass with an irregular margin at the 6 o’clock position, 2 cm from the nipple areola. Ultrasound-guided core biopsy was performed to examine the hypoechoic, heterogeneous mass. Core biopsy showed invasive ductal carcinoma that was estrogen receptor positive, progesterone receptor negative, and human epidermal growth factor receptor 2 (HER2) negative. Thus, she underwent initial staging using PET/CT, which showed a hypermetabolic lesion at the lower part of the right breast. Multiple mediastinal hypermetabolic lymphadenopathies indicated metastasis (Figure 1). There were no abnormal axillary nodes. Thus, we requested for a nodal biopsy to be conducted for a definitive diagnosis. She had no complaints of cough, dyspnea, fever, or hemoptysis. Pulmonary function tests were normal. There was no abnormal finding on the chest radiograph (Figure 2). She underwent mediastinoscopy and biopsy of the right paratracheal nodes. Histological examination of the paratracheal nodes showed noncaseating granulomas consistent with sarcoidosis (Figure 3). Staining and culturing for bacteria, fungus, and acid-fast bacilli were negative.
Figure 1.
Positron-emission tomography/computed tomography showing intense uptake mediastinal lymphadenopathies and right breast mass
Figure 2.
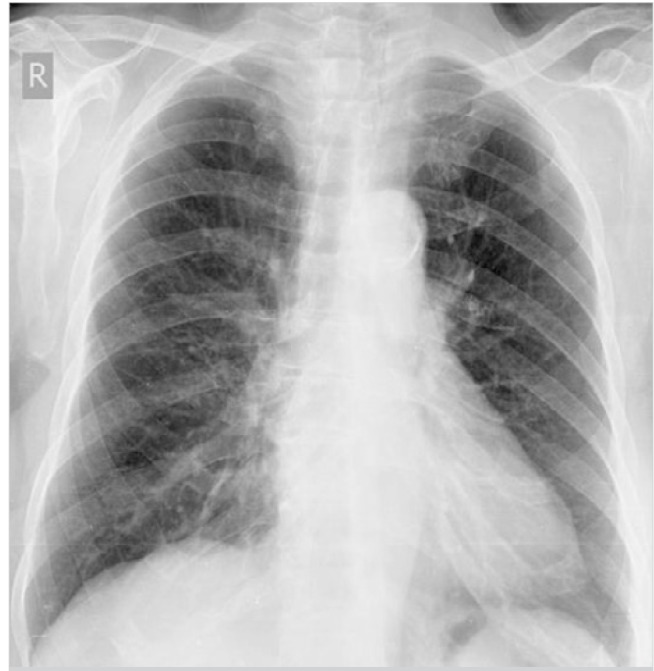
There was no abnormal finding on the posteroanterior chest X-ray
Figure 3.
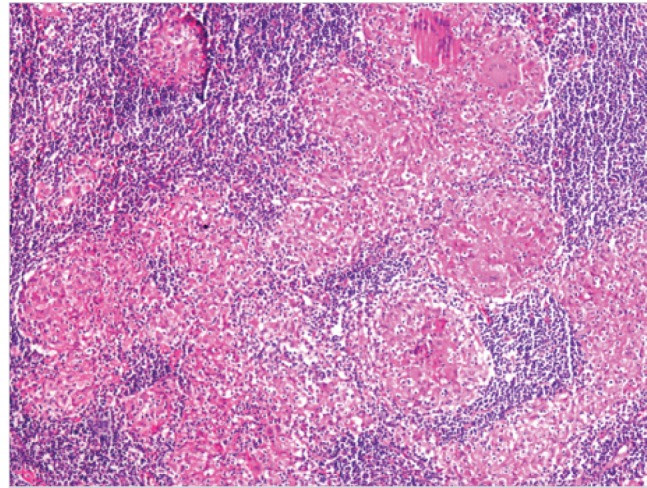
Mediastinoscopy and biopsy of the paratracheal lymphadenopathies showed noncaseating granulomas consistent with sarcoidosis (H&E x100 magnification)
The treatment options were explained to the patient. Although breast-conserving surgery and sentinel lymph node biopsy were offered to the patient, she refused this treatment option. Modified radical mastectomy was performed instead of the technique offered above. The surgery was completed without any complication. The specimen size was 1.4 cm, and the pathological result was invasive ductal carcinoma that was estrogen receptor positive, progesterone receptor negative, and HER2 negative. Axillary dissection was performed; 14 lymph nodes were dissected and all of them were negative. The patient was discharged on the third postoperative day. No additional application was suggested except 1 mg of anastrozole daily for 5 years. The patient was informed and written consent was obtained.
DISCUSSION
Sarcoidosis and breast carcinoma are two distinct diseases that affect females. Pulmonary sarcoidosis in breast cancer patients is a rare phenomenon. It can present either at the same time or in a sequence similar to the clinical and imaging features of sarcoidosis and metastasis of breast carcinoma, leading to misdiagnosis and incorrect treatment (1, 6). Sarcoidosis and breast cancer occur most commonly in middle-aged females.
Although there has been much speculation regarding the relationship between cancer and sarcoidosis, it remains unclear. Patients with sarcoidosis have been reported to be at an increased risk for malignancies, particularly those of the lung, bone, small intestine, and liver. The estimated relative risk for breast cancer has been reported to be slightly higher in sarcoidosis than that in other chronic diseases (7).
PET/CT has been shown to be superior to conventional imaging techniques in the detection of distant metastases in patients with stage II–III breast cancer (8). However, false-positive 18F-fluorodeoxyglucose (FDG) uptake or false-negative PET/CT scans are frequently encountered. Many nonmalignant conditions with active granulomatous processes, such as tuberculosis, fungal infections, and sarcoidosis, have been reported to accumulate FDG and cause false-positive PET scans for malignancy (9). During the preoperative period with early stage breast cancer, standard use of PET/CT for initial staging is not recommended because of the suboptimal axillary sensitivity. The added radiation dose and high costs further limit its use as a standard initial staging procedure. Although sensitivity is suboptimal, specificity is consistently high for axillary lymph nodes and it has an excellent positive predictive value, confirming that an immediate axillary dissection instead of sentinel lymph node biopsy is reasonable in case of high FDG uptake at axillary nodes. However, sentinel lymph node biopsy remains mandatory in axilla with no or slightly increased FDG uptake (8). We sometimes perform PET/CT in our daily practice for initial staging.
Differentiating sarcoidosis from malignant neoplasms is sometimes difficult, particularly when intense FDG uptake is detected in the lymph nodes of patients with known malignancy (4). Regarding breast cancer, axillary lymph nodes are known to be the primary site of spread, with axillary lymph node dissection previously being a standard part of treatment along with mastectomy. Breast cancer can metastasize to intrathoracic lymph nodes, but isolated mediastinal involvement is extremely rare (2, 10). Therefore, tissue biopsy is essential for differentiating metastasis from other diseases. In patients with known malignancy and mediastinal lymphadenopathy suggestive of sarcoidosis, mediastinoscopy is more definitive than bronchoscopy (10).
CONCLUSION
We have presented the case of a patient with early-stage breast cancer and no previous diagnosis of sarcoidosis, who, after FDG-PET/CT for initial staging of her breast cancer, was found to have mediastinal lymph node involvement. A biopsy of a mediastinal lymph node allowed us to diagnose sarcoidosis and correctly stage her disease as stage I.
It is important to consider that, in patients with breast cancer who are found to have positive FDG-PET findings in the mediastinal lymph nodes, these nodes may not be metastatic lymph nodes but may be granulomatous nodules. To prevent misdiagnosis and overtreatment, a tissue diagnosis is strongly recommended.
Footnotes
Informed Consent: Written informed consent was obtained from patient who participated in this case.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.A., N.A.; Design - B.H.; Supervision - M.A.; Analysis and/or Interpretation - M.A., B.H.; Writer - N.A., M.A.; Critical Review - N.A., B.H.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Tolaney SM, Colson YL, Gill RR, Schulte S, Duggan MM, Shulman LN, et al. Sarcoidosis mimicking metastatic breast cancer. Clin Breast Cancer. 2007;7:804–810. doi: 10.3816/CBC.2007.n.044. http://dx.doi.org/10.3816/CBC.2007.n.044. [DOI] [PubMed] [Google Scholar]
- 2.Akhtari M, Quesada JR, Schwartz MR, Chiang SB, Teh BS. Sarcoidosis presenting as metastatic lymphadenopathy in breast cancer. Clin Breast Cancer. 2014;14:107–110. doi: 10.1016/j.clbc.2014.05.001. http://dx.doi.org/10.1016/j.clbc.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Shin HC, Choe JW, Ryu HS, Kim HS, Shin JW, Kim YS, et al. Sarcoidosis mimicking metastatic breast cancer in Korean woman with breast cancer. Breast J. 2014;20:198–199. doi: 10.1111/tbj.12245. [DOI] [PubMed] [Google Scholar]
- 4.Ito T, Okada T, Murayama K, Hanamura T, Kanai T, Mochizuki Y, et al. Two cases of sarcoidosis discovered accidentally by positron emission tomography in patients with breast cancer. Breast J. 2010;16:561–563. doi: 10.1111/j.1524-4741.2010.00961.x. http://dx.doi.org/10.1111/j.1524-4741.2010.00961.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Lee SY, Oh SC, Choi CW, Kim JS, Seo JH. Case report of pulmonary sarcoidosis suspected to be pulmonary metastasis in a patient with breast cancer. Cancer Res Treat. 2014;46:317–321. doi: 10.4143/crt.2014.46.3.317. http://dx.doi.org/10.4143/crt.2014.46.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viswanath L, Pallade S, Krishnamurthy B, Naveen T, Preethi BL, Pramod KP, et al. Darier-roussy sarcoidosis mimicking metastatic breast cancer. Case Rep Oncol. 2009;2:251–254. doi: 10.1159/000262412. http://dx.doi.org/10.1159/000262412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Askling J, Grunewald J, Eklund A, Hillerdal G, Ekbom A. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med. 1999;160:1668–1672. doi: 10.1164/ajrccm.160.5.9904045. http://dx.doi.org/10.1164/ajrccm.160.5.9904045. [DOI] [PubMed] [Google Scholar]
- 8.Koolen BB, van der Leij F, Vogel WV, Rutgers EJ, Vrancken Peeters MJ, Elkhuizen PH, et al. Accuracy of 18F-FDG PET/CT for primary tumor visualization and staging in T1 breast cancer. Acta Oncol. 2014;53:50–57. doi: 10.3109/0284186X.2013.783714. http://dx.doi.org/10.3109/0284186X.2013.783714. [DOI] [PubMed] [Google Scholar]
- 9.Ataergin S, Arslan N, Ozet A, Ozguven MA. Abnormal 18F-FDG uptake detected with positron emission tomography in a patient with breast cancer: A case of sarcoidosis and review of the literature. Case Rep Med. 2009;2009:785047. doi: 10.1155/2009/785047. http://dx.doi.org/10.1155/2009/785047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urschel JD, Loewen GM, Sarpel SC. Metastatic breast cancer masquerading as sarcoidosis. Am J Med Sci. 1997;314:124–125. doi: 10.1097/00000441-199708000-00016. http://dx.doi.org/10.1097/00000441-199708000-00016. [DOI] [PubMed] [Google Scholar]