Abstract
We have developed a novel, light activated drug delivery containers, based on spiropyran doped liquid crystal micro spheres. Upon exposure to UV/violet light, the spiropyran molecules entrapped inside the nematic liquid crystal micro spheres, interconvert from the hydrophobic, oil soluble form, to the hydrophilic, water soluble merocyanine one, which stimulates the translocation of the merocyanine molecules across the nematic liquid crystal-water barrier and results their homogeneous distribution throughout in an aqueous environment. Light controllable switching property and extremely high solubility of spiropyran in the nematic liquid crystal, promise to elaborate a novel and reliable vehicles for the drug delivery systems.
OCIS codes: (160.0160) Materials, (160.3710) Liquid crystals, (160.5335) Photosensitive materials
1. Introduction
Controlled drug delivery systems are gaining increasing importance compared to the traditional forms of drug administration. Delivering drug locally, in the targeted placement and at the controlled portions, is a common way to decrease side effects due to the drug toxicity and, consequently, maximally reduce the undesirable side effects. The drug can either be adsorbed, dissolved, or dispersed throughout the nanoparticle complex or, alternatively, it can be covalently attached to the surface [1]. Assorted types of remotely-triggerable drug delivery systems have been developed, which rely on applying an external stimulus to release the drug load [2]. Ideally, such systems could determine the timing, duration, dosage, and even location of drug release, and could allow remote, noninvasive, repeatable, and reliable switching of therapeutic agent flux [3]. As an example, the thermo sensitive polymers consisting of drugs and gold nanoparticles have been shown to effectively release the embedded drug upon local heating of the gold nanoparticles via near infrared light (NIR) irradiation [4,5]. In other variants of such a system, magnetic nanoparticles have been used in combination with an alternating magnetic field to impose local heating [6,7]. Recently, a wide range of electromagnetic waves has been proposed to control drug release [8,9]. Light-activated drug delivery offers distinct advantages over other stimuli, because it can release a drug at a desired time and place, so that, they hit only targeted cells and not surrounding healthy tissues [10]. At present, different mechanisms are elaborated and proposed that can generally be distinguished based on illumination mode, which may range from femtosecond pulses to continuous wave illumination [11].
In this study, we introduce a new concept of drug delivery system, based on the spiropyran (SP) doped nematic liquid crystal (NLC) micro spheres. The proposed system represents an emulsion formed by the immiscibility between the NLC micro spheres and a water environment. The stable state of SP is a non colored closed molecular form, that can be transformed into their merocyanine (MC) state, upon UV/Violet irradiation. The MC conformation spontaneously tends to come back to the SP state, and this transition can be stimulated by irradiation with visible light or by heating. More specifically, the carbon–oxide bond of the SP molecules is cleaved when it is transformed into the polar-colored MC form [12]. Since the interconversion between the closed SP form and the open MC one, involves a large molecular rearrangement, some compounds of this class does not exhibit photoisomerization in solid-state conditions [13]. In order to efficiently switch SP molecules, require conformational freedom, which usually is not available within the densely packed arrays of molecules in the solid state. Due to the unique properties of liquid crystalline materials, SP doped NLCs are assumed to have many advanced optical characteristics, quite different from those of SP doped isotropic liquids, semiconductors and polymer systems. Further, SP doped NLCs present additional advantageous features: an extremely high solubility of SP in the NLC host, which can vary in the range 3-4% (by weight) without destroying the liquid crystalline phase, a high orientational order parameter for SP molecules provided by the spatial orientation of the NLC host molecules. The widespread utility of the SP switch lies in the fact that SP and MC isomers have vastly different physicochemical properties. The charge separation in MC gives rise to a large electric dipole moment, particularly in comparison with the SP isomer. Density functional theory calculations, as well as electrical interferometry and electrooptical absorption measurements have shown that while the dipole moment of the SP form is in the range of ~4–6 D, this changes drastically to ~14–18 D for the MC form [12]. Therefore, upon the exposure to UV/Violet light, a hydrophobic form of SP molecules undergoes a photoisomerization into the hydrophilic MC form, that can potentially destabilize the lipid membrane of NLC-water interface so that, the MC molecules can escape inside the aqueous medium.
2. Materials and methods
2.1 SP-NLC mixture preparation
To prepare SP-NLC mixture, we used next commercially available compounds: BL-038, as a nematic host (from Merck), 1′,3′,3′-Trimethyl-6-nitro-1′,3′-dihydrospiro[chromene-2,2′-indole] as a photochromic material (from Sigma-Aldrich). It should be noted that the SP, we used in our experiments, is essentially insoluble in water, whereas the solubility in NLC is very high - 3-4%wt. The photo-switching behavior and absorption spectra of the mixtures were investigated with a UV/VIS Spectrometer (AvaSpec 2048, Avantes) at room temperature. For a light induced generation of the polar-colored MC form, we irradiated samples using a 100 W mercury lamp (HG 100 AS, Jelosil) equipped with a 320–410 nm bandpass filter, and the diode pumped solid state laser (Shangai Dream Lasers Technology Co. Ltd.) with a maximum power 50 mW at wavelength λ = 405 nm. For the vigorous agitation of SP-NLC-water emulsion, we used a Vortex test tube mixer, with controlled mixing speed in the range of 0-2800 rpm. To enclose the emulsions, small laboratory glass bottles were used. The images of SP-NLC emulsified micro spheres were obtained with the use of a polarizing microscope coupled to a digital camera (from Polam), and a fluorescence microscope (from Nicon).
At first, a SP doped NLC mixture was prepared by mixing the nematic host and SP material with the following concentration ratio: 96wt.% BL-038 + 4wt.% SP. Prior to preparing the SP doped NLC micro spheres emulsified in water, in order to demonstrate a light stimulated photoisomerization, a macro size drop (3mm) of the SP doped NLC mixture was slowly immersed in the bottle filled with deionized water. Then a laser beam was forwarded to a sphere. During 4 seconds of laser irradiation, a color of the SP doped NLC sphere was dramatically changed from red to dark purple one, Fig. 1.
Fig. 1.
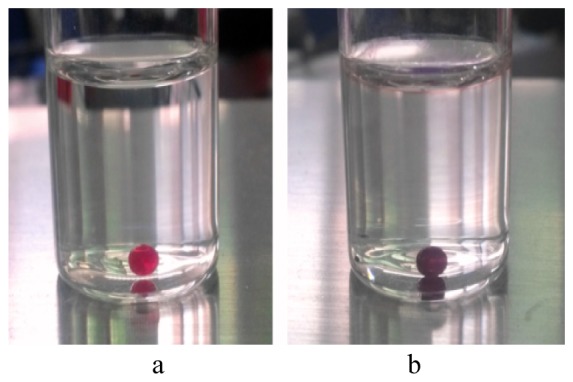
SP-NLC macro sphere in water, before the laser irradiation (a), and after 4s of laser irradiation (b).
2.2 SP-NLC-water emulsion preparation
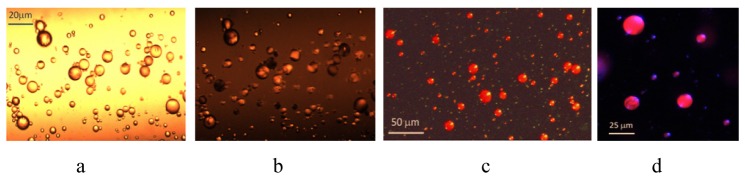
To form a SP-NLC-water emulsion, a mixture consisting of SP-NLC and water, was prepared with the following percentage in weight: 95%wt. (90%wt. water + 10%wt. glycerol) + 5%wt. (96% wt. BL-038 + 4%wt.SP). Glycerol was added to the aqueous phase in order to obtain a homogeneous distribution of SP-NLC micro spheres in water-glycerol matrix and for the emulsion stabilization [14]. Prepared mixture was agitated at 600 rpm, at room temperature for 10 min. As a result, a homogeneous distribution of SP-NLC micro spheres suspended in an aqueous matrix were obtained. The diameters of SP-NLC micro spheres were ranging from 10 to 15μm.
3. Optical measurements
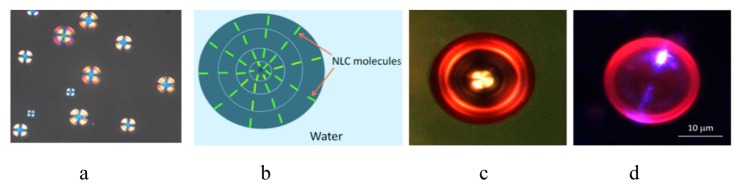
After the preparation, a SP-NLC-water emulsion was sandwiched between two cover glasses and was assembled in an optical cell, whose gap was set to 100μm. Prepared optical cell was placed under the polarizing microscope. In Fig. 2(a), from the top view, shown a distribution of SP-NLC micro spheres inside the water environment. The colors of the micro droplets are produced by the interference between the ordinary and extraordinary rays of transmitting light, which caused by the radial configuration of the NLC molecules inside the sphere, Fig. 2(b). The location of the SP in NLC matrix, causes to the alignment of SP molecules parallel to the host NLC molecules so that, the orientation of SP molecules inside the NLC micro sphere follows the radial configuration of the NLC molecules. To visualize the alignment of SP-MC molecules, we irradiated an optical cell by UV/VIS mercury lamp, equipped with 350-410 nm bandpass filter. A distance from the lamp to the sample was adjusted to 25 cm. The light intensity at the sample, measured by optical power/energy meter, was 0.30 mW/cm2. An exposure time was 5s. During this period a numerous quantity of SP form was photoisomerized to the MC form. In Fig. 2(c), shown an image of SP doped NLC micro sphere between crossed polarizers, when irradiated by a mercury lamp. Red circles correspond to the regions in which the MC molecules are either aligned horizontally or vertically in the field of view, confirming a radial orientation of MC molecules, similar to the orientation of NLC molecules. In Fig. 2(d), is demonstrated the same micro sphere, but image was taken in the reflectance mode.
Fig. 2.
Polarizing microscope observation of SP-NLC micro spheres emulsified in an aqueous medium (a), a schematic representation of the spatial distribution of the molecules inside NLC micro sphere (b) which results a radial director profile with a point defect in the center of the micro sphere. Images of MC-NLC micro sphere upon irradiation of UV/Violet light in the transmittance (c), and reflectance (d) modes respectively.
Chemically, NLC consist of amphiphilic molecules. Therefore, they have two distinctly different characteristics, polar and nonpolar in different parts of the same molecule, that induces a strong homeotropic anchoring at the NLC-aqueous interfaces. The competition between elasticity and interface tension formed around the NLC micro spheres can be controlled by the difference between the polarities of the two liquids. Photoisomerizable molecules that experience a conformational change upon light illumination are promising candidates for the controlling of elastic/tension forces between two liquid mediums. The change of the anchoring condition has a striking effect on the nematic order parameter, which is presented at the interface of the NLC-water phases. In our case, upon UV/Violet illumination, a photoisomerization of SP molecules into MC ones, can potentially disrupt the NLC -water barrier and allow for release of MC molecules. In order to prove that SP-MC photoisomerization is linked to the destabilization of NLC-water barrier and MC molecules are able to pass through this phase boundary, we carried out next measurements. For the experimental manipulation we used five glass bottles filled with just prepared [95%wt (90%wt. water + 10%wt. glycerol) + 5%wt (96%wt.BL-038 + 4%wt. SP)] emulsion. Then each bottle, except one, was irradiated by mercury lamp, equipped with a 350-410 nm band-pass filter. The light intensity, measured at the samples, was about 0.50 mW/cm2. The exposure time for the each bottle was different so that, the first one was irradiated for 4s, second one - for 8s, third one - for 12s and fourth one -for 16s. Immediately, after the light irradiation, each bottle, including non irradiated one, was agitated using a test tube shaker with shaking orbital speed equal 80 rpm. A time of agitation for each bottle was equal to 10s. After this procedure, a non irradiated bottle appears as non colored milk like substance, whereas the color of each irradiated bottle gradually changes from the pale red-orange to the deep red-violet one, Fig. 3.
Fig. 3.
Bottles with SP-NLC-water emulsions: (a) exposure time 0 s. shaking time 10 s. (b) exposure time 4 s. shaking time 10 s. (c) exposure time 8 s. shaking time 10 s. (d) exposure time12 s. shaking time 10 s. (e) exposure time 16 s. shaking time 10 s.
Using a volumetric pipette, from each bottle we extracted a small quantity of the emulsions, which then were injected into the optical cells. A gap between two glass plates was fixed 100 μm. Under the optical microscope, for each optical cell we selected and marked the areas, free of SP-NLC and MC-NLC micro spheres, to minimize a light scattering caused by the micro spheres. By using a UV/Vis spectrometer, we recorded the light intensities passed through the selected areas. Figure 4(a), shows the time-dependent increase of the absorption of five optical cells. First cell was filled with non-irradiated SP-NLC-water emulsion, and other four samples were filled with MC-NLC-water emulsions, after being irradiated by UV lamp with the different exposure times. According to this dependence, a light absorption is gradually increasing with respect to the increasing of exposure time. It is known, that unlike from SP form, an MC form is characterized with fluorescent properties. Figure 4(b), demonstrates a light emission of the MC-NLC-water emulsion, produced by MC molecules.
Fig. 4.
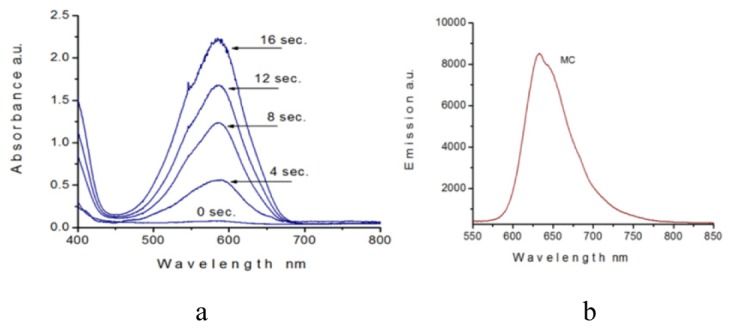
Light absorption as a function of the exposure time for the five optical cells filled with SP-NLC-water and MC-NLC-water emulsions (a). A fluorescence emission from MC-NLC micro spheres (b).
A rate of SP-MC phototransformation and the quantity of MC molecules that penetrate through the NLC-water barrier, may increase dramatically using a laser irradiation. An optical cell filled with SP-NLC-water emulsion was irradiated by the diode laser beam with λ = 405nm and 5s of exposure time. In Fig. 5, shown 150 x150μm size area of the sample, before (a), and after (b), irradiation by a laser beam. Due to light absorption, caused by the MC molecules, an exposed area looks much darker, than that of non-exposed one. We note that not all MC molecules are transferred into the water environment, and some amount of them is permanently located inside the MC-NLC micro spheres. As mentioned above, upon UV irradiation, an emulsion of MC-NLC-water micro spheres emits a bright red light. This fluorescence property of MC molecules is an attractive way for in vivo imaging and tracking of SP-NLC micro container based drug delivery systems. By using a fluorescent microscope, we captured the images of MC-NLC micro droplets emulsified in water Fig. 5(c), 5(d).
Fig. 5.
Images of SP-NLC-water emulsion, before (a), and after (b), exposure to the laser beam. Distribution of MC-NLC micro spheres inside the optical cell, observed under the fluorescent microscope (c,d).
According to our concept, initially, SP molecules are bound to the specific therapeutic drug molecules, and this assemblage physically is entrapped inside the NLC micro containers. Upon exposure to UV light, which results a SP-MC photoisomerization, the combination of MC-drug molecules translocates across the NLC-water barrier and disseminates evenly throughout in an extracellular environment, Fig. 6.
Fig. 6.
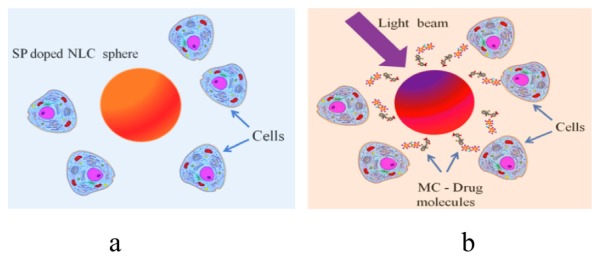
Schematic of light controlled drug release. SP-NLC micro sphere before (a) and after (b) UV activation.
A major drawback is that, the UV/violet irradiation that stimulates a SP-MC photoisomerization, has a limited tissue penetration, because light in this range of the optical spectrum is highly absorbed and scattered by the different kinds of absorbing chromophores, as blood, melanin, fat, yellow pigment, tissue orangels, etc. One way to overcome this obstacle is to introduce a two-photon excitation. This method can be highly advantageous for drug delivery systems because the reduced scattering and absorption of NIR irradiation (750-900nm), results a deep penetration of light in the biological tissues.
5. Conclusion
In summary, we have demonstrated a novel, light controlled drug delivery system, based on SP doped NLC micro spheres. Experimental results have shown that upon exposure to UV/violet light, the photochromic molecules located inside the NLC spheres, experience an interconversion from the hydrophobic, oil soluble, non-polar SP state, to the hydrophilic, water soluble, highly polar MC state. Light induced photoisomerization destabilize NLC-water interface, stimulates the translocation of MC molecules across the NLC- water barrier and results their homogeneous distribution throughout in an aqueous environment. Proposed strategy can be considered as a new platform for the photostimulated drug delivery systems that offer the possibilities of the controlled delivery and release of a wide variety of drugs into the body, at the suitable time and desired site, to fight different kinds of diseases including cancer diseases.
Acknowledgment
This work was supported by Shota Rustaveli National Science Foundation (SRNSF), project number DO/171/8-314/14.
References and links
- 1.Thakor A. S., Gambhir S. S., “Nanooncology: the future of cancer diagnosis and therapy,” CA Cancer J. Clin. 63(6), 395–418 (2013). 10.3322/caac.21199 [DOI] [PubMed] [Google Scholar]
- 2.Shah S., Sasmal P. K., Lee K.-B., “Photo-triggerable hydrogel–nanoparticle hybrid scaffolds for remotely controlled drug delivery,” J. Mater. Chem. B Mater. Biol. Med. 2(44), 7685–7693 (2014). 10.1039/C4TB01436G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timko B. P., Dvir T., Kohane D. S., “Remotely triggerable drug delivery systems,” Adv. Mater. 22(44), 4925–4943 (2010). 10.1002/adma.201002072 [DOI] [PubMed] [Google Scholar]
- 4.Timko B. P., Arruebo M., Shankarappa S. A., McAlvin J. B., Okonkwo O. S., Mizrahi B., Stefanescu C. F., Gomez L., Zhu J., Zhu A., Santamaria J., Langer R., Kohane D. S., “Near-infrared-actuated devices for remotely controlled drug delivery,” Proc. Natl. Acad. Sci. U.S.A. 111(4), 1349–1354 (2014). 10.1073/pnas.1322651111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hribar K. C., Lee M. H., Lee D., Burdick J. A., “Enhanced release of small molecules from near-infrared light responsive polymer-nanorod composites,” ACS Nano 5(4), 2948–2956 (2011). 10.1021/nn103575a [DOI] [PubMed] [Google Scholar]
- 6.Hoare T., Timko B. P., Santamaria J., Goya G. F., Irusta S., Lau S., Stefanescu C. F., Lin D., Langer R., Kohane D. S., “Magnetically triggered Nanocomposite Membranes: a Versatile Platform for Triggered Drug Release,” Nano Lett. 11(3), 1395–1400 (2011). 10.1021/nl200494t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar C. S., Mohammad F., “Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery,” Adv. Drug Deliv. Rev. 63(9), 789–808 (2011). 10.1016/j.addr.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernot S., Klibanov A. L., “Microbubbles in ultrasound-triggered drug and gene delivery,” Adv. Drug Deliv. Rev. 60(10), 1153–1166 (2008). 10.1016/j.addr.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derfus A. M., von Maltzahn G., Harris T. J., Duza T., Vecchio K. S., Ruoslahti E., Bhatia S. N., “Remotely triggered release from magnetic nanoparticles,” Adv. Mater. 19(22), 3932–3936 (2007). 10.1002/adma.200700091 [DOI] [Google Scholar]
- 10.Fan N.-C., Cheng F.-Y., Ho J. A., Yeh C.-S., “Photocontrolled Targeted Drug Delivery: Photocaged Biologically Active Folic Acid as a Light-Responsive Tumor-Targeting Molecule,” Angew. Chem. Int. Ed. Engl. 51(35), 8806–8810 (2012). 10.1002/anie.201203339 [DOI] [PubMed] [Google Scholar]
- 11.Leung S. J., Romanowski M., “Light-activated content release from liposomes,” Theranostics 2(10), 1020–1036 (2012). 10.7150/thno.4847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klajn R., “Spiropyran-based dynamic materials,” Chem. Soc. Rev. 43(1), 148–184 (2014). 10.1039/C3CS60181A [DOI] [PubMed] [Google Scholar]
- 13.Lyergar S., Biewer M. C., “Solid-state interactions in photonic host-guest inclusion complexes,” Cryst. Growth Des. 5(6), 2043–2045 (2005). 10.1021/cg050313b [DOI] [Google Scholar]
- 14.Petriashvili G., De Santo M. P., Chubinidze K., Hamdi R., Barberi R., “Visual micro-thermometers for nanoparticles photo-thermal conversion,” Opt. Express 22(12), 14705–14711 (2014). 10.1364/OE.22.014705 [DOI] [PubMed] [Google Scholar]