Abstract
Interpersonal attachment is a critical component of the human experience. Pair-bonding ameliorates the severity of several mental and physical diseases. Thus, a better understanding of how the central nervous system responds to and encodes social-buffering during stress is a valuable research enterprise. The prairie vole (Microtus ochrogaster), as a laboratory animal model, provides the gold standard for investigation of the neurobiology underlying attachment. Furthermore, emerging research in voles, additional laboratory rodents, transgenic mice, primates, and humans has provided novel insight into the neurochemical mechanisms underlying the therapeutic effects of social bonds reducing anxiety, depression, and drug abuse liability. In the present review, we highlight the work from this burgeoning field and focus on the role(s) of the neuropeptides oxytocin (OT), vasopressin (AVP), and corticotrophin releasing hormone (CRH) mediating stress buffering. Together, the data suggest that OT underlies social bonding to reduce stress-induced psychological illness while AVP and CRH facilitate arousal to enhance autonomic reactivity, increasing susceptibility to adverse mental and physical health.
Keywords: vasopressin; corticotrophin-releasing hormone; dopamine; paraventricular nucleus of the anterior hypothalamus; posterior dorsal medial amygdala; nucleus accumbens; ventral tegmental area, social bonding; stress; passive stress-coping, autism; depression, anxiety, & drug abuse
Graphical abstract
Introduction
Stable social relationships are necessary for maintaining human health. Conversely, breaking bonds can create severe emotional and physical distress including passive-stress coping states involving anxiety, depression, and vulnerability to drugs of abuse. However, we know little regarding how the brain changes and responds to social attachment and partner loss. Thus, in order to better understand the adverse psychological manifestations caused by breaking pair-bonds, the establishment of an animal model that exhibits strong face validity to human attachment is necessary to investigate basic neurobiological mechanisms underlying the effects of social-buffering modulating the stress response. Because male and female prairie voles (Microtus ochrogaster) form enduring social pair-bonds after mating and extended cohabitation, these rodents provide an innovative animal model system to investigate the neurobiology of social attachment and partner loss. While a laundry list of neurotransmitters, peptides, molecules, and genes in the central nervous system (CNS) has been implicated in attachment behaviour in voles (Young & Wang, 2004), here we focus primarily on the role of neuropeptides modulating bonding behaviour and stress.
Coping represents a psychological state where an individual consciously establishes a set of specific goals to reduce physiological harm during stressful life experiences (Lazarus & Folkman, 1984, Lazarus, 1999). Due to the extreme frequency of adverse life events people endure during life, several definitions and conceptual frameworks have been proposed. There is a vast literature in which the term “coping”, as it pertains to stress, is classified. Here, we focus on the role of sociality within the context of stress social buffering. Social-buffering is a phenomenon whereby socio-sexual interactions protect an individual from adverse physiological and psychological consequences of stress during challenging situations (Lazarus & Folkman, 1984, Lazarus, 1999). This phenomenon is well known in humans, however - only recently, has it begun to be characterized in laboratory animals.
Because mating induces arousal associated with anxiolytic-like states accompanied by sedation and calmness, it was originally hypothesized that sexual activity reduces anxiety because of central oxytocin (OT) release. In support of this hypothesis, in 1989, Flannery & Wieman found that OT was released in the paraventricular nucleus of the anterior hypothalamus (PVN) of male rats courting a sexually-receptive female. These anxiolytic effects of sexual activity were blocked by central administration of an OT receptor antagonist administered directly after mating but had no effects in non-mated male rats. This work demonstrated a critical role of central OT underlying anxiolytic effects associated with mating and social-buffering of the stress response. Later work revealed exogenous central infusions of OT, but not vasopressin (AVP), reduced stress-induced corticosterone (CORT) release and anxiety-like behaviour in female rats (Windle et al., 1997). These data suggested that central OT represents a potent anxiolytic, ameliorating both CORT release and anxiety-like states, and thus may play a significant role in buffering physiological responses to stressful life experiences.
In this review, we begin by discussing previous work examining the role of central OT and related neuropeptides (e.g., AVP and CRH) involved in the stress response. We then focus on more recent studies investigating the direct role of OT and its interactions with other peptidergic systems, classical neurotransmitters, and genes underlying social-buffering of stress in voles and expand the review to include information from similar studies using other laboratory rodents, primates, and humans. We conclude by discussing recent work utilizing OT as a potential therapeutic to alleviate stress-induced psychopathology.
Neuropeptides, Attachment, and the Molecular Genetics of Sociality
The functional significance of long-term pair-bonding has been documented cross-culturally in humans. Individuals living in stable paired relationships live longer than their unpaired, single, counterparts - across demographic groups (House et al., 1988, Lillard & Waite, 1995). A significant number of humans grieving the loss of a family member or intimate partner experience long, and sometimes short - but intense - periods of post-traumatic stress, major depression, panic attacks, and generalized anxiety (Byrne & Raphael, 1999, Kristensen et al., 2012, Onrust & Cuijpers, 2006, Zivin & Christakis, 2007). Social-buffering serves as a potent anxiolytic, while social isolation, separation, or loss of a familiar conspecific predicts poor mental and physical health (Cohen & Wills, 1985, Flannery & Wieman, 1989, Heinrichs et al., 2003, Karelina & DeVries, 2011, Maulik et al., 2010, Smith & Wang, 2012, Smith, Lei, & Wang 2013), increasing the risk of developing debilitating psychological diseases (Byrne & Raphael, 1997, Elwert & Christakis, 2008a, Elwert & Christakis, 2008b, Kristensen et al., 2012, Onrust & Cuijpers, 2006, Rozenzweig et al., 1997, Zivin & Christakis, 2007). Previous clinical research has associated these mental health issues with dysregulation in OT, AVP, and CRH (Dinan et al., 1999, Meyer-Lindenberg et al., 2011, Pitman et al., 1993, Purba et al., 1996, Raadsheer et al., 1994). Thus, elucidating neuropeptidergic mechanisms underlying social-buffering of stress should be an important focus in biomedical research.
Neuropeptides and dopamine promote pair-bond formation and maintenance
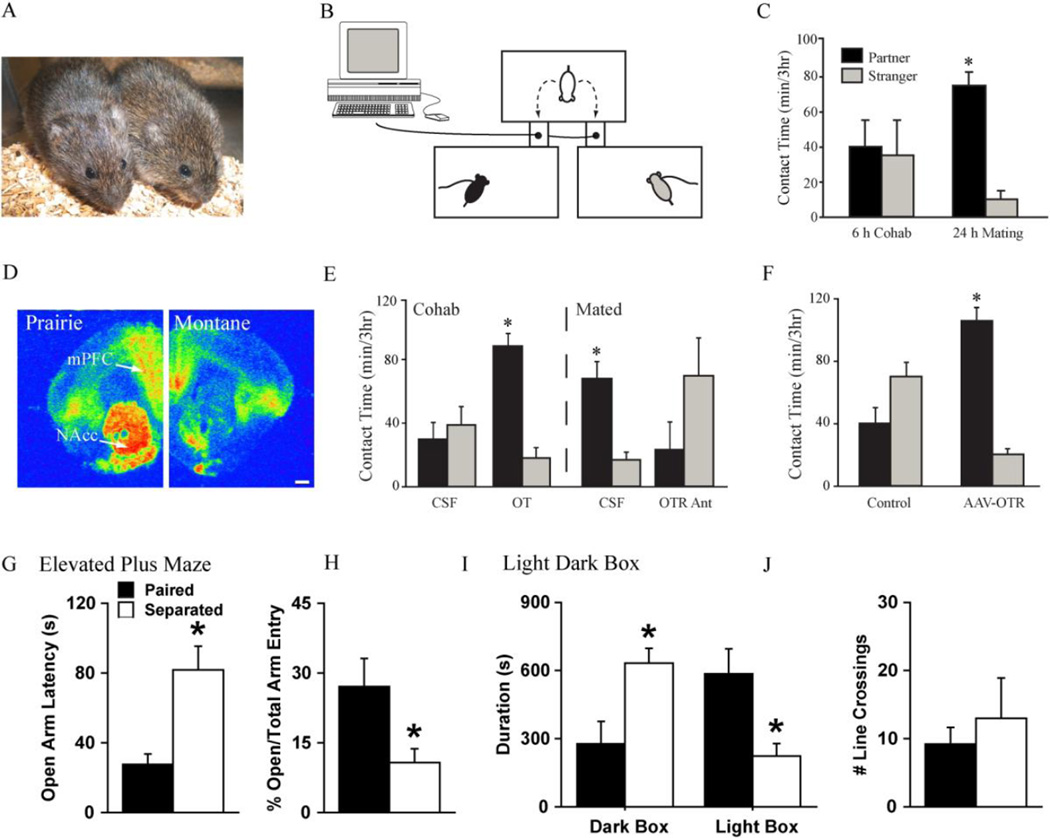
Pair-bonding behaviour in prairie voles has been extensively studied in the field and laboratory settings. After 24-hours of cohabitation, with successful mating, male prairie voles exhibit partner preference toward their female partner but not a stranger (Figure 1 A–C; Dewsbury, 1975, Dewsbury, 1987, Getz & Carter, 1996, Getz et al., 1981, Getz et al., 1993, Gray & Dewsbury, 1973, Williams et al., 1992) and become highly aggressive toward unfamiliar male and female conspecifics (Aragona et al., 2006, Gobrogge et al., 2007, Gobrogge et al., 2009, Insel et al., 1995, Wang et al., 1997, Wang et al., 1996, Winslow et al., 1993). Bonded males can retain their partner preference even after two weeks of separation from a female partner (Insel et al., 1995, Bosch et al., 2009). Field work demonstrates that over 75% of prairie voles maintain their bond throughout life (Getz & Carter, 1996, Getz et al., 2003) even when a female partner dies or abandons their male partner, 80% of males never bond again (Getz et al., 1993).
Figure 1.
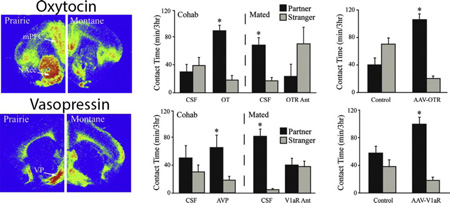
(A) Picture represents a pair-bonded male and female prairie vole exhibiting side-by-side affiliative contact. (B) Automated three-chamber partner preference paradigm. Shoebox size cages are connected by hollow Plexiglas tubes with infrared light sensors to beam breaks enabling automated movement quantification. Socio-sexual behaviours between experimental animal (i.e., white cartoon vole), familiar partner (i.e., black cartoon vole), and unfamiliar stranger conspecific (i.e., gray cartoon vole) are videotaped, recorded by an experimentally-blind observer, and subsequently scored. Affiliation is computed in minutes of side-by-side physical body contact. (C) Male and female prairie voles’ cohabitating without mating for six hours isn’t sufficient to induce a partner preference, as the time spent with a partner and/or stranger is equivalent. Conversely, 24 hours of socio-sexual cohabitation is required to induce partner preference, as mated prairie voles spend significantly more time in side-by-side contact with their familiar partner than with an unfamiliar stranger during a three-hour partner preference test. (D) Vole species differences in oxytocin receptor (OTR) distribution in the medial prefrontal cortex (mPFC) and nucleus accumbens (NAcc) of monogamous prairie (left) and promiscuous montane voles (right). (E) Six hours of non-sexual cohabitation (i.e., Cohab) with intra-NAcc artificial cerebro-spinal fluid (aCSF - control) has no effect on partner preference formation. However, voles micro-injected with OT in the NAcc prior to six hours of nonsexual cohabitation is sufficient to induce a significant partner preference. A full day of socio-sexual cohabitation with mating (Mated) induces a significant partner preference which can be blocked by pretreatment with an intra-NAcc OTR antagonist. (F) Prairie voles infused with an adeno-associated virus (AAV) over-expressing OTRs in the NAcc facilitates partner preference formation in the absence of socio-sexual experience compared to Lac-Z virus controls. (G) Partner separation increased male prairie vole latency to enter the open arm of the elevated plus maze and (H) decreased the percentage of open arm entries versus total arm entries, while partner loss (I) increased time spent in the dark box and decreased light box time in the light dark box test. (J) Partner separation had no effect on locomotor behaviour, as the number of line crossings was equivalent across both separated and paired groups. Bars indicate means ± standard error of the mean. *: p < 0.05, OT = oxytocin, scale bar = 50µm. Adapted from Aragona & Wang 2009, Liu & Wang 2003, Ross et al., 2009b, Sun et al., 2014, Young et al., 2011a.
In prairie voles, mating increases AVP (Wang et al., 1994) and CRH (Bosch et al., 2009) mRNA expressed in neurons of the bed nucleus of the stria terminalis (BNST) yet decreases AVP processes in the lateral septum (LS) (Bamshad et al., 1994) - brain areas where OT acts to reduce stress. Female prairie voles, on-the-other-hand, exposed to olfactory cues display differences in the density of OT receptors (OTRs) in the accessory olfactory bulb (Witt et al., 1991) and exhibit a significantly higher density of OTRs in the medial prefrontal cortex (mPFC) and nucleus accumbens (NAcc) compared to promiscuous montane voles (Figure 1D). Intra-cerebro-ventricular (ICV) infusion of an AVP or OT receptor antagonist reduces partner preference while AVP or OT receptor activation enhances partner preference without mating, in both male (Cho et al., 1999, Winslow et al., 1993) and female (Cho et al., 1999) prairie voles. Site-specific manipulation of neuropeptides in select brain nuclei supports these correlative anatomical findings. For example, micro-infusion of AVP in the LS of sexually naïve males enhances partner preference in the absence of mating (Lim & Young, 2004, Liu et al., 2001). OT release in the NAcc increases when females mate with male prairie voles (Ross et al., 2009a), and OT micro-infusion into the NAcc increases partner preference in the absence of mating. Partner preference can be blocked with concurrent micro-injection of an OTR antagonist in the NAcc (Figure 1E) and prelimbic cortex (PLC), in the absence of mating (Liu & Wang, 2003, Young et al., 2001). Further, sexually naïve males infused ICV, or intra-NAcc, infused with CRH display partner preference can be blocked by co-infusion with CRH receptor antagonist (DeVries et al., 2002, Lim et al., 2007).
Behavioural genetics of partner preference formation and social memory
Neurogenetic research, employing viral-vector-mediated gene transfer methods, has substantiated the role of OT in the NAcc and AVP in the ventral pallidum (VP) in the facilitation of partner preference in both sexes. OTR over-expression in the NAcc of sexually inexperienced female prairie voles enhanced partner preference formation (Figure 1F; Ross et al., 2009b) but had no effect in female meadow voles. Thus, NAcc OTR density correlates positively with affiliation and partner preference in monogamous but not polygamous voles (Ross et al., 2009b). Viral vector expression of AVP receptors (V1aRs) in the VP enhanced partner preference in the absence of mating in male prairie voles (Pitkow et al., 2001). Remarkably, V1aR over-expression in the VP of promiscuous male meadow voles recapitulated a socially monogamous life strategy (Lim et al., 2004).
Neurobiobehavioural Consequences of Stress-Induced Bereavement
Behavioural manifestations and neurochemistry accompanied by partner loss
Because partner loss in humans activates an entire suite of mental and physical health problems (Shear & Shair, 2005), the socially monogamous prairie vole has been utilized to investigate mechanisms associated with bereavement (i.e., bond loss). Several laboratories have demonstrated depressive-like symptomology, by using the prairie vole to model human partner loss-induced depression and anxiety (Bosch et al., 2009, McNeal et al., 2014). In a recent series of experiments, male prairie voles pair-bonded with their female partner for 24-hours and were randomly divided into two groups: (1) remained with their partner or (2) separated from their partner for four weeks. Males separated from their partner exhibited significantly higher levels of anxiety in the elevated plus maze (Figure 1 G&H, (Sun et al., 2014)) and light-dark box (LDB; Figure 1 I, (Sun et al., 2014). Lone males displayed higher levels of depressive-like behaviour in the forced swim test (FST) but didn’t exhibit pair-bonding-induced selective aggression
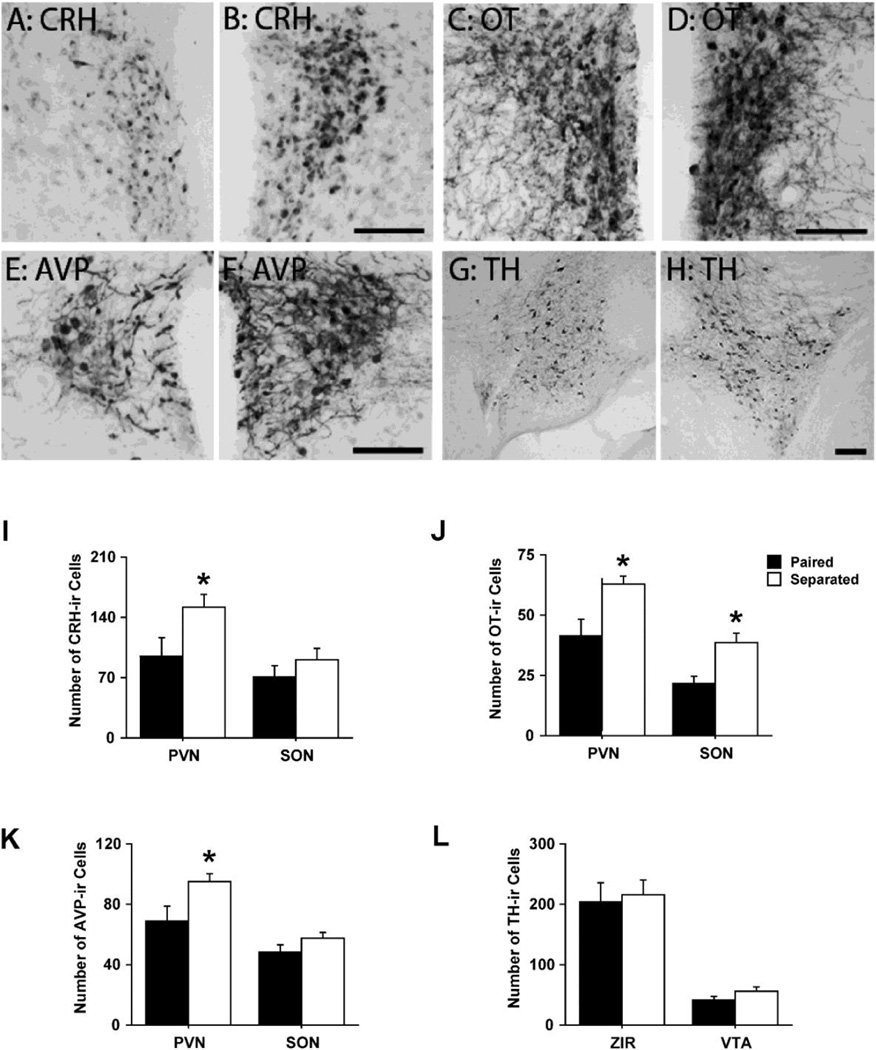
Next, the density of several neuro-peptide/-transmitter systems, previously implicated in pair-bonding behaviour (e.g., OT, CRH, AVP, dopamine (DA), & CORT) in the PVN, medial supra-optic nucleus (mSON), ventral tegmental area (VTA), and rostral zona incerta (ZIR) were immunocytochemically screened through brain sections processed from lone or paired males. Partner loss significantly increased the density of OT-immunoreactive (-ir), AVP-ir, and CRH-ir neurons in the PVN, with no changes in tyrosine hydroxlase-ir (TH) (Figure 2 A–L; Sun et al., 2014). Males displayed robust partner preference two weeks after partner separation but not after four weeks. Body weight and plasma CORT levels were significantly elevated after four weeks of partner separation with no significant changes in plasma OT or AVP (Sun et al., 2014). These data suggest that pair-bond status, post partner loss, may not be affected by pair-bond maintenance because both partner preference and selective aggression extinguished four weeks after partner separation. These results demonstrate a time dependent dissolving of partner preference behaviour in male prairie voles and highlight the prairie vole as a robust animal model to characterize the neurochemical mechanisms underlying bond loss and bereavement.
Figure 2.
(A & B) Photomicrographs depicting corticotrophin releasing hormone immunoreactivity (CRH-ir), (C & D) oxytocin (OT-ir), and (E & F) vasopressin (AVP-ir) neurons and processes in the paraventricular nucleus of the anterior hypothalamus (PVN) and (G & H) tyrosine hydroxylase (TH-ir – rate limiting enzyme in dopamine biosynthesis) neurons and processes in the ventral tegmental area (VTA) from male prairie voles that were either paired (A, C, E & G) with or separated (B, D, F and H) from their familiar female partner. (I) Partner separation significantly increased the number of CRH-ir cells in the PVN but not medial supra-optic nucleus (mSON), (J) increased the number of OT-ir neurons in the PVN and mSON, (K) increased AVP-ir neuronal density in the PVN but not the mSON, and (L) had no effect on TH-ir neuronal density in the rostral zona incerta (ZIR) or VTA. Bars indicate means ± standard error of the mean. *: p < 0.05. Scale bars = 50µm & 100µm. Adapted from Sun et al., 2014.
Neuropeptides and bond loss
Several neuro-peptide/-transmitter systems interact to regulate stress, partner separation, and social memory associated with pair-bonding. OT, CRH, and AVP are produced, transported, and released from parvo- and magno-cellular neurons in the hypothalamus and extra-hypothalamic areas during stress. OT has a single receptor that has been characterized (i.e., OTR) and is coupled to Gq receptors, and when stimulated, enhances neuronal activation and gene expression.
Two CRH receptors have been cloned CRH-R1 and CRH-R2. The intra-cellular signaling cascade and g-protein coupled receptors (Gs/i) vary for each CRH receptor subtype in the CNS and peripheral nervous system (PNS). CRH-R2 has two alternatively spliced forms found in rodents (α and β) and display distinctly different mRNA expression profiles relative to CRH-R1 (Chalmers et al., 1995; Lovenberg et al., 1995b). CRH-R2α is expressed primarily in the CNS (Chalmers et al., 1995; Lovenberg et al., 1995a) while CRH-R2β is found predominantly in the PNS (Chalmers et al., 1995, Lovenberg et al., 1995a). In the pituitary, CRH-R2 is highly expressed in the posterior pituitary lobe with low mRNA levels in the anterior lobe (Kageyama et al., 2003, Van Pett et al., 2000). Both CRH-type receptors bind CRH and urocortins in the CNS, PNS, and pituitary to modulate stress.
AVP has three receptors that have been discovered: V1/2. V1 is further classified into two receptor subtypes V1a & V1b (V3). Similar to CRH receptors, V1a/b vary in g-protein coupling - either to stimulatory (s) or inhibitory (i) g-protein coupled receptors in the CNS. V2 receptors are coupled to G(s) proteins and when activated, cyclic adenosine monophosphate (cAMP) increases – enhancing gene expression and neuronal activity.
Treating pair-bonded males with CRH antagonists has no effect on partner preference formation - even if separated from their female partner (Bosch et al., 2009), while same-sex separation in males doesn’t change PVN-CRH-ir or –mRNA levels (Bosch et al., 2009, Grippo et al., 2007b). In other work, however, CRH pharmacology influences bond formation but not maintenance (Sun et al., 2014). Enhanced CRH-ir neuronal expression in the PVN of males is associated with female partner loss and not social isolation (Figure 2I; Sun et al., 2014). Thus, PVN CRH-ir may be responding to bond loss and not social isolation. Additionally, social separation from a same-sex conspecific doesn’t affect PVN-OT-/AVP-ir neuronal density in male prairie voles (Grippo et al., 2007b, Ruscio et al., 2007). However, partner loss increases PVN-OT-/AVP-ir neuronal density without changing DA-ergic cell expression in the VTA or ZIR in males (Sun et al., 2014, Figure 2 A–L). Thus, a higher density of PVN-OT-/AVP-ir neurons may indicate an overall change in neuropeptide release and/or limited receptor activity associated with a break in pair-bonds.
Central manipulation of V1a-type receptors has been the focus of several studies examining the neurobiology of pair-bonding behaviour in voles (Young et al., 2011a). However, there is strong evidence for a role of AVP V1b-type receptors implicated in social behaviour in a number of elegant studies conducted in the laboratory of Dr. Scott Young at NIMH. In a recent study manipulated V1b receptors in pyramidal neurons of the CA2 region of the hippocampus in AVPR1B knock-out mice - which exhibit a marked reduction in aggressive behaviour. Lentiviral delivery of AVPR1B into the dorsal CA2 region restored the probability of socially motivated attack behaviour in complete AVPR1B knockout mice, without changing patterns of anxiety-like behaviour. Thus, hippocampal AVP V1b-type receptors in the CA2 region are necessary and sufficient to regulate aggression in mice (Pagani et al., 2015), a brain region where stress has a significant impact on neuronal structure and plasticity (McEwen, Nasca, & Gray, 2015).
OT also robustly modulates social behaviour (Churchland & Winkielman, 2012, Feldman, 2012, Gordon et al., 2011) and produces anxiolytic-like effects in rodents (Neumann & Landgraf 2012, Smith & Wang 2012). Blocking OTR in the PVN exacerbates behavioural stress responses (Figure 3 H&I; Smith & Wang 2014b) whereas activation of OTR in the hypothalamic-pituitary-adrenal (HPA) axis (Bosch et al., 2004) and central nucleus of the amygdala (CeA) (Ebner et al., 2005) ameliorates stress reactivity. Further, the HPA axis utilizes CRH and AVP in the PVN to engage arousal in order to increase the production of CORT from the adrenal gland in rodents and cortisol in humans producing anxiogenic responses to environmental stimuli (Armario, 2006, Lightman, 2008). ICV infusion of OT reduces CRH/AVP synthesis and release in the PVN and dampens HPA functioning during stress (Windle et al., 2004, Windle & Shanks, 1997). However, the precise mechanism of how OT acts on the HPA system to reduce CRH/AVP activity and facilitate anti-anxiety-/-depressive-like behaviour is unclear.
Figure 3.
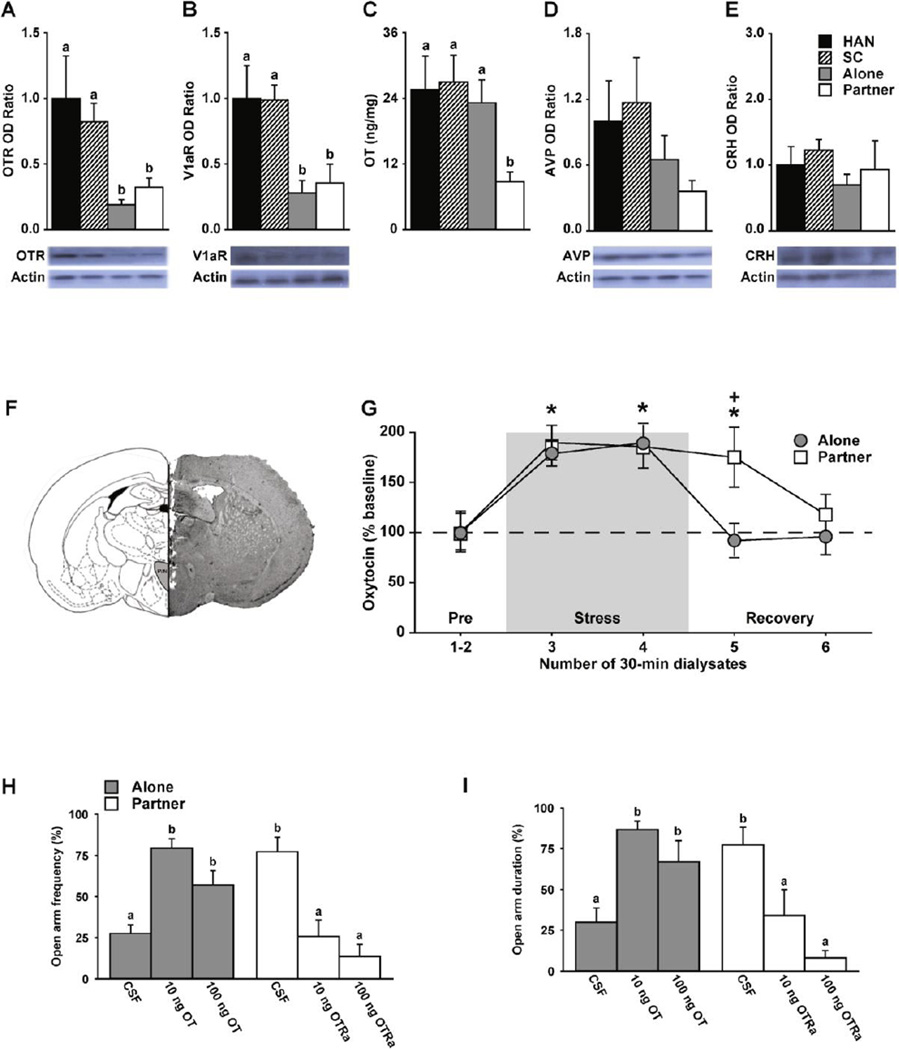
(A) Immobilization stressed female prairie voles (recovered “alone” or with their “partner”) exhibited significantly lower optical density (OD) in western blot detecting oxytocin receptor (OTR) protein in the PVN and (B) V1a receptor (V1aR) protein relative to handled (HAN) and social control (SC) female controls. (C) Recovering from immobilization stress with a familiar partner significantly reduced the level of oxytocin (OT) content in the PVN; while (D, E) no significant protein levels, detected via western blotting, for vasopressin (AVP) or corticotrophin-releasing hormone (CRH) was found between groups. (F) Photomicrograph illustrating microdialysis probe placement in the PVN. (G) Immobilization stress significantly increased OT release in the PVN across both groups of females relative to baseline. Recovery with a partner maintained a significantly higher OT tone 30 minutes after immobilization stress. (H & I) Intra-PVN aCSF infused female voles, recovering alone, exhibited enhanced elevated plus maze anxiety-like behaviour (i.e., reduced open arm frequency (H) and duration (I)) unless they received bilateral intra-PVN-OT (e.g., 10ng/100ng) infusion. Conversely, female voles recovering with their male partner exhibited decreased elevated plus maze anxiety-like behaviour (i.e., increased open arm frequency (H) and duration (I)) relative to females recovering alone. Finally, female voles recovering with their partner infused intra-PVN with a selective OTR antagonist attenuated social-buffering effects on anxiety-like behaviour at both low (10ng) and high (100ng) OT doses. Bars labeled with different letters (A–E, H, & I) differ significantly by post hoc Student Newman-Keuls (SNK) tests of significance examining both main effects or interactions with multiple analysis of variance tests and p value set to < .05. Bars indicate means ± standard error of the mean. Asterisks (*) indicate differences from baseline and the plus sign (+) indicates group differences at a specific time point, determined by post hoc SNK tests examining significant main effects or interactions with multiple analysis of variance tests and p value set to < .05. Adapted from Paxinos & Watson 1998 & Smith & Wang 2014b.
Originally, one theory postulated that OT acted directly on CRH cells in the PVN because OT receptors co-localize on CRH perikarya and suppress action potentials (Bilezikjian & Vale, 1987, Dabrowska et al., 2011, Ludwig & Leng, 2006, Vale et al., 1981). However, the primary effects observed were excitatory (Inenaga & Yamashita, 1986). Additionally, gamma-aminobutyric acid (GABA) signaling provided clues into the inhibitory nature of OT reducing CRH activity in the HPA axis (Cullinan, 2000, Decavel & Van den Pol, 1990, Herman et al., 2002) because PVN-projecting GABA-ergic neurons inhibit stress-induced CRH neuronal firing via GABAA receptor activation (Bali et al., 2005, Bali & Kovacs, 2003, Bartanusz et al., 2004). In support of these working hypotheses, suppression of stress-induced PVN CRH release, via ICV injections of OT, was blocked by a GABAA receptor antagonist (Bulbul et al., 2011). Thus, OT may exert anxiolytic effects indirectly by GABAA inhibition of HPA CRH release. Prior work suggests correlative anxiolytic effects via PVN-OT release or OT micro-injection studies (Smith & Wang, 2012). Furthermore, PVN-OT attenuates stress-induced CRH neuronal activity, reduces plasma CORT, decreases anxiety, and increases GABA-ergic excitability (Smith et al., 2014a). More specifically, concurrent infusion with a GABAA recetor antagonist, bicuculline, blocked stress-inhibition produced by exogenous OT-PVN infusion (Smith et al., 2014a). Thus, PVN-OT may represent a viable therapeutic target to reduce activation of the HPA axis to decrease stress-responsivity.
Neurobiology of Social-buffering and Mammalian Stress Coping
Stress can manifest in several forms. Social separation-induced stress is one specific subtype of stressor which is highly understudied. Relational life events including partner loss, divorce, or infidelity can cause significant mental health problems in humans (Brown, 1989, Dalgard et al., 2006). Social support through friends, family, or formation of new bonds can alleviate some of these deleterious psychological manifestations (Cohen & Wills, 1985, Smith & Wang, 2012, Smith, Lei, & Wang 2013). Tactile contact between mother and her offspring - via suckling - reduces anxiety (Carter & Altemus, 1997) while monogamous romantic long-term relationships decrease the development of stress-related disorders including frequency and magnitude of panic attacks and psychological distress (Cohen & Wills 1985, Smith & Wang, 2012). However, this is a dearth of basic research elucidating the neurochemical mechanisms programming social-buffering of stress through the formation and maintenance of pair-bonds.
Anxiolytic effects of OT are found when social-buffering is used to cope with stress. OT releases in the PVN during social interaction between pair-bonded prairie vole partners representing a natural anxiolytic (Figure 3 F&G; Smith & Wang, 2014b). In other work, PVN-OT is released during mating in male rats (Waldherr & Neumann, 2007) and in lactating female rats nursing (Neumann et al., 1993). Together, data from these studies supports the notion that social interactions induce OT-PVN release and is associated with reduced anxiety and enhanced calmness. It is also important to note that PVN-OT is released during anxiogenic states and stress (e.g., maternal defeat (Bosch et al., 2004), elevated platform (Blume et al., 2008), immobilization (Babygirija et al., 2012, Smith & Wang 2014b), FST (Wigger & Neumann, 2002, Wotjak et al., 1998), and shaker stress (Nishioka et al., 1998)). This dual function of PVN-OT release may serve as a neurochemical thermostat operating as an inhibitory or negative feedback mechanism for stress-induced HPA axis function. Studies using direct pharmacological manipulation provide evidence to support this theory.
Peripheral, or site-specific, treatment with OT decrease stress responding in many animals (e.g., intra-peritoneal (i.p.; Detillion et al., 2004), subcutaneous (Grippo et al., 2009, Petersson et al., 1999), intra-nasal (Ditzen et al., 2009, Heinrichs et al., 2003, Linnen et al., 2012, Parker et al., 2005, Quirin et al., 2011), ICV (Bulbul et al., 2011, Lukas et al., 2011, Norman et al., 2010, Windle et al., 2004, Windle et al., 1997, Zheng et al., 2010), and intra-PVN (Blume et al., 2008, Smith & Wang, 2014b)). The data reported in a recent study demonstrate an anxiolytic effect of PVN-OT infusion attenuating stress-induced activation of PVN-CRH neurons, an increase in circulating CORT, and enhanced anxiety-like behaviour in female prairie voles. These effects were only observed when PVN-OT infusion preceded stress but not after (Smith & Wang, 2014b). These data suggest that PVN-OT serves as an inhibitory rather than a negative feedback mechanism attenuating stress.
Chronic partner loss in prairie voles increases circulating CORT, central CRH mRNA, and depressive-like behaviour after stress challenge (Bosch et al., 2009). It has also been shown in a recent study (Smith & Wang, 2014b) that physical interactions between pair-bonded male and female prairie voles alleviate immobilization stress-induced changes at neuronal, physiological, and behavioural levels. Specifically, stressed female prairie voles (recovered “alone” or with their “partner”) exhibited significantly lower levels of OTRs in the PVN (Figure 3A) and V1aRs (Figure 3B) relative to handled (HAN) and social control (SC) females. Recovering from immobilization stress with a familiar partner significantly reduced the level of OT in the PVN (Figure 3C), while no significant tonic or phasic neuronal release adaptations in AVP or CRH were found between groups (Figure 3 D&E). Because OT release in the PVN was increased during stress recovery with a partner this most likely indicates that decreased PVN-OT is attributed to enhanced OT release.
Prior work shows that during stressful life events, social groups and partners cooperate (Zivin & Christakis, 2007) to reduce stress (Karelina & DeVries, 2011) and mammals are more likely to affiliate, rather than fight, during times of stress. Rats will rescue familiar conspecifics from being restrained - resembling empathic responding (Ben-Ami Bartal et al., 2011), and chimpanzees exhibit unique stress coping via consoling one another following conflict (Fraser et al., 2008). Human and primate females receiving more physical attention from their partner exhibit decreases in stress reactivity (Dunbar, 2010). Empathic responding and physical contact between human partners is positively correlated with OT and inversely related to decreased blood pressure and hypertension (Dunbar, 2010). Mating in male rats causes PVN-OT release which is associated with reduced anxiety (Waldherr & Neumann, 2007) while socio-sexual experience in female prairie voles enhances OT release in the NAcc (Ross et al., 2009a). Suckling rat pups display increases in OT release (Kojima et al., 2012), as do female rats having just given birth (Bosch & Neumann, 2012), and inter-generationally in rat mothers ICV injected with OT whom then lick, groom, and nurse their pups more, which - in turn - increases OT release in these offspring (Pedersen & Boccia, 2002). While the precise location of extra-hypothalamic OT neuronal projections originate is contentiously debated, tract-tracing evidence in prairie voles suggests the PVN as one source (Ross et al., 2009a). ICV OTR blockade attenuates social-buffering of stress-induced depression and anxiety in mice and rats (Norman et al., 2010, Waldherr & Neumann, 2007) while site-specific manipulation in the PVN (Bosch et al., 2004) and CeA (Ebner et al., 2005) show similar results.
Neuropeptide interactions underlying social-buffering of stress
CORT and OT represent two of the first neurotransmitter interactions underlying social memory in prairie voles. Sexually naïve female prairie voles exposed to an unfamiliar male conspecific significantly decreased circulating CORT (DeVries et al., 1995) and central OT release (Ross et al., 2009a). These findings support work illustrating ICV OT infusion significantly decreased circulating CORT, indicating potential OT interactions with the HPA axis underlying attachment and social memory (DeVries et al., 1997a). Similar findings have also been observed in studies examining other forms of social behaviour (Amico et al., 1994, Carter & Altemus, 1999, Chiodera et al., 1991, Kikusui et al., 2006). OT interacts with a structurally and chemically related peptide (only differing by two amino acids), AVP, and both receptor subtypes (Barberis & Tribollet, 1996) regulate social memory in prairie voles. For example, male and female prairie voles infused ICV with OT/AVP induces partner preference after only one hour of social cohabitation without mating. ICV-OT-induced partner preference can be blocked by co-administration with a V1aR antagonist and vice versa (Cho et al., 1999). This work is supported by site-specific infusion of AVP into the LS inducing partner preference in male prairie voles which was blocked by co-administration with an OTR antagonist (Liu et al., 2001).
Neuropeptide/Dopamine interactions programming stress social-buffering
Pair-bonding and the presence of a partner are also regulated by the central DA-ergic reward system, thereby reinforcing mental health and wellness (Aragona & Wang 2004, Neumann, 2009). DA in the rostral NAcc shell facilitates pair-bond formation and maintenance (Aragona et al., 2006), while partner loss disrupts the HPA axis causing dysregulated emotive behaviour similar to that observed in human clinical populations of depressed and anxious-prone patients (Kristensen et al., 2012, Onrust & Cuijpers, 2006). Not surprisingly, OT and AVP work in concert with the mesocorticolimbic DA-ergic system to regulate robust social memory and attachment in prairie voles (Young & Wang, 2004). Activation of NAcc DA-type 2 receptors (D2R) attenuate partner preference formation which can be blocked by simultaneous administration of an OTR antagonist (Liu & Wang, 2003). Together, these findings indicate that both D2R and OTR activation is required in female prairie voles to form robust partner preference and social memory toward a male partner. Promiscuous male meadow voles micro-injected with the prairie vole V1aR promoter in the VP exhibited enhanced V1aR expression which facilitated mating-induced partner preference after 24-hours of successful mating with a female.
These effects were abolished by co-infusion with a D2R antagonist before mating and cohabitation (Lim et al., 2004). Anatomically, NAcc-D2R medium spiny neurons project to the VP (Gerfen, 1992), supporting the hypothesis that AVP and DA interact at the level of the VP to encode robust social memory - even in socially promiscuous voles. Work under review by Smith and colleagues report significant changes in the central DA-ergic systems underlying social-buffering alleviating negative effects of social defeat in female prairie voles. Housing with a male partner for five days after a single social defeat experience (i.e., being an intruder in a resident-intruder test) limits the defeat-induced social avoidance response observed in female prairie voles (Smith et al., 2014a). D2R protein levels in the medial amygdala were also elevated in females post-defeat but only if they recovered with a male partner (Smith et al., 2014a).
Neuropeptide/Classic Neurotransmitter interactions and social stress buffering
Recent work investigated the anxiolytic nature of interactions between PVN-OT and GABA underlying stress-induced HPA activity and anxiety (Smith et al., 2014a). Elevated platform stress (EPS) experience increased CORT while intra-PVN-OT injections 15-minutes prior to, but not immediately following, attenuated EPS-induced-CORT, blocked c-Fos co-localization in PVN-CRH-ir neurons, and activated GABA-ir neurons instead (Smith et al., 2014a). Behaviourally, OT infusion in the PVN prior to EPS significantly reduced anxiety-like behaviour in the elevated plus maze (EPM) while these effects were reversed with co-injection of a GABAA receptor antagonist (Smith et al., 2014a). PVN-OT pretreatment blocked stress activation of the HPA axis via GABA (Smith et al., 2014a). As found previously, stress-induced OT release in the PVN of female prairie voles buffered subsequent stress responding (Figure 3 F&G; Smith & Wang 2014b). These results indicate that OT release in the PVN controls GABA neuronal activity via GABAA receptor-mediated inhibition of the stress response. Similar to most peptides in the CNS, OT can passively diffuse (Ludwig & Leng, 2006, Moos et al., 1984) and/or be coupled to an action potential in cell bodies and dendrites via synaptic release (Van den Pol, 2012). OT is manufactured and synthesized in the PVN and mSON (Ludwig, 1998, Ludwig et al., 1994, Moos et al., 1989, Neumann et al., 1993, Pow & Morris, 1989, Russell et al., 1992) and exogenous OT central infusion excites GABA-ergic cells in select brain regions including the CeA, which has been found to reduce fear and anxiety in rats (Huber et al., 2005, Terenzi & Ingram, 2005). OTRs are co-localized on CRH-ir cells in the PVN (Dabrowska et al., 2011). PVN-CRH neurons receive numerous inputs: 50% representing GABA-ergic synapses confirmed via electrophysiological preparations (Wuarin & Dudek, 1991), electron microscopy (Decavel & Van den Pol, 1990, Decavel & Van den Pol, 1992, Herman et al., 2003, Miklos & Kovacs, 2002), and immunohistochemical studies that reveal an abundance of PVN-CRH neurons co-expressing GABAA receptors (Cullinan, 2000). Direct pharmacological research in rats indicates that activation of these receptors inhibits stress-induced CRH output by blocking CRH mRNA (Bulbul et al., 2011) and release (Hillhouse & Milton, 1989, Plotsky et al., 1987) in the PVN. Smith et al., 2014a provide direct evidence demonstrating that PVN-GABA-ergic neurons respond to OT infusion because intra-PVN-OT injections enhanced GABA-ergic neural responding that was associated with a significant decrease in basal and stress-induced PVN-CRH neuronal activity.
Activation of PVN-CRH neurons enhanced CORT release from the adrenal cortex via ACTH secretion in the pituitary gland (Bilezikjian & Vale, 1987, Ontjes et al., 1977, Vale et al., 1981). Importantly, female prairie voles infused with OT in the PVN exhibited decreased PVN-CRH activity resulting in significantly lower plasma CORT. These anxiolytic effects of OT were attenuated by site-specific infusion of a GABAA receptor antagonist in the PVN (Smith et al., 2014a). PVN-OT infusion blocks basal and stimulated HPA stress-response and anxiety-like behaviour via GABAA receptor-mediated inhibitory neurotransmission in prairie voles. In summary, data generated from our group and others support the notion that social-buffering is mediated by OT neurons localized within the PVN.
Neuronal circuitry programming social-buffering of stress
Previous neuroanatomical data illustrate reciprocal connections in PVN-OT/-CRH neurons that regulate HPA axis functioning. OT and CRH processes form synaptic connections where OT can diffuse or release in the PVN (Ludwig & Leng, 2006) to alter neuronal firing rate properties post-exogenous intra-PVN-OT micro-infusion. In order to precisely examine stress buffering circuitry, until a transgenic prairie vole is generated and made publicly available, future vole research would benefit from performing in-vivo electrophysiological experiments examining either intact or brain slices from prairie voles experiencing long-term bond loss. For example, prior work from Nishijo’s group has previously demonstrated that the presence of a conspecific rat buffered several stress responses to an aversive conditioned stimulus (CS), including freezing and c-Fos activity in the lateral amygdala (LA) of male rats. In a more recent study by the same group, they analyzed freezing behaviour and local field potentials in the LA of fear-conditioned rats in response to the CS and/or in the presence or absence of a familiar conspecific for two consecutive days. The presence of a conspecific animal significantly decreased peak amplitudes of auditory evoked field potentials, gamma oscillations (25–75 Hz), and high frequency oscillations (100–300 Hz) in the LA (Fuzzo et al., 2015). These neuronal responses were positively correlated with freezing duration in fear-conditioned rats (Fuzzo et al., 2015). These data demonstrate the capacity of social-buffering decreasing CS-induced activation of LA neurons to reduce conditioned fear responding.
Neuropeptides and Pair-Bonding as Viable Therapeutics to Alleviate Stress-Induced Bereavement: Clinical Implications and Future Directions
The prairie vole has recently emerged as a valuable model system to examine interactions between natural (e.g., mating & pair-bonding) and artificial (e.g., drugs of abuse) reward uncovering protective or deleterious effects of addiction, withdrawal, and dependence - which all serve as potent stressors. To date, this body of work demonstrates that psychostimulants dysregulate the mesocorticolimbic DA-ergic system underlying pair-bonding behaviours (Liu et al., 2010, Young et al., 2011a, Young et al., 2011b) which can be ameliorated via OT (Young et al., 2014). Thus, the prairie vole represents an innovative animal model to investigate the neurochemical mechanisms underlying interactions between socio-sexual behaviour, social memory, and stress-related psychopathologies. For example, social separation disrupts HPA axis functioning which enhances isolation-induced stress-related dysfunctional social and emotional behaviour in prairie voles (Grippo et al., 2008, Lieberwirth et al., 2012). Smith & Wang, 2014b, also provide strong evidence demonstrating the long-lasting nature of forming a pair-bond in female prairie voles serves as a potent social buffer ameliorating stress responses in the presence of a familiar partner, similar to what has previously been observed in primates and humans (Dunbar, 2010, Fraser et al., 2008). These vole data demonstrate the effects of social support on engaging the PVN-OT system and its requirement to reduce social separation-induced stress relief in female prairie voles and extends prior work in humans showing peripheral OT release during physical interaction between long-term committed couples (Gordon et al., 2011).
Oxytocin, mammalian social buffering, and stress-induced neuronal plasticity
Prairie voles are not the only animal model where OT is being investigated as a potential neurotherapeutic target for the treatment of social-separation stress-related disorders. Similar to familial/social support, OT works as an anxiolytic agent in many mammals. For example, isolated animals exhibit enhanced HPA axis functioning leading to clinical levels of anxiety and depression in socially monogamous laboratory rodents like prairie voles and California mice as well as more generally gregarious animals like rats and/or inbred strains of mice (Detillion et al., 2004, Grippo et al., 2008, Lieberwirth et al., 2012, Norman et al., 2010, Waldherr & Neumann, 2007). Furthermore, central OT micro-infusion ameliorates the negative psychological consequences of social isolation in many laboratory animals including mice, rats, and voles (Detillion et al., 2004, Grippo et al., 2008, Lieberwirth et al., 2012, Norman et al., 2010). Counterintuitively, in other social contexts, socially isolated female prairie voles exhibit depression after psychological stress which engenders anhedonia (i.e., reduced sucrose preference) while contradictory evidence has shown that OT administration during isolation can enhance depressive symptoms (Grippo et al., 2008). Thus, social stress can be alleviated via OT - independent of the social context. Although OT appears to represent a putative therapy for alleviating stress-related disorders and bereavement, the precise neurochemical mechanism of how OT works in the CNS is still unfolding. For example, social and physical stress have both been found to be associated with increased PVN-OT extracellular release (Bosch et al., 2004, Engelmann et al., 2004) from dendrites (Ludwig & Leng, 2006).
Group housing and PVN-OT infusions both decrease stress-induced PVN-CRH neuronal activation in rats (Blume et al., 2008, Windle et al., 2004) whereas social stress caused by isolation can be ameliorated with intra-PVN-OT micro-injection (Blume et al., 2008, Windle et al., 2004). Data from Smith & Wang, 2014b, show that even in the presence of a familiar partner, social-buffering of the stress response doesn’t occur if PVN-OT is blocked via intra-PVN micro-infusion with a selective OTR antagonist (Figure 3 H&I). Thus, these data support the notion that social-buffering reverses adverse effects of social separation stress on dysfunctional psychophysiological behaviour and HPA axis pathology via PVN-OT activation.
Humans experiencing symptoms of major depression exhibit enhanced levels of CORT (Stetler & Miller, 2011, Thase, 2009) and increased expression of PVN-CRH-ir neurons (Raadsheer et al., 1994) facilitated by interactions with the central AVP system (Aguilera & Rabadan-Diehl, 2000, Dinan et al., 1999, Purba et al., 1996, Raadsheer et al., 1994). Thus, increased CORT and PVN-CRH-/AVP-ir may underlie neuroplasticity after repeated stress and enhanced HPA axis priming in male prairie voles experiencing partner loss. Because of the significant alterations in socio-emotional dysregulated behaviour and impaired structural neuronal plasticity caused by partner loss, it may be more beneficial to a species to break a bad bond rather than maintain one (Field, 2006, Stroebe et al., 2010). For example, similar to abstaining from drugs of abuse - maintaining an unhealthy pair-bond (e.g., individuals in co-dependent relationships) can cause severe mental illness by perpetuating an unhealthy partnership and/or “relapsing” with the same or similar partner (Byrne & Raphael, 1997, Byrne & Raphael, 1999, Kristensen et al., 2012, Onrust & Cuijpers, 2006, Zivin & Christakis, 2007). However, the underlying neurochemical mechanisms programming adaptive versus maladaptive bereavement are, at the present time, unknown.
Intra-nasal infusion of OT enhances prosocial communication styles between partners (i.e., increased agreeableness, curiosity, emotional support, and sustained eye-to-eye contact (Ditzen et al., 2009)) and facilitates trust between individuals (Kosfeld et al., 2005). One particular social context, in which prosocial forms of communication are affected - in clinical populations - represents the class of stress-related psychopathologies centered on autism spectrum conditions (Heinrichs et al., 2009, Hollander et al., 2007, McDougle et al., 2005, Yirmiya et al., 2006). Furthermore, germane to the current review, the prairie vole has been established as a translational animal model for human disorders, like autism, characterized by deficits in social communication. For example, prairie voles form unique socio-sexual relationships which aren’t typically observed in humans with autistic spectrum disorders. Thus, because the prairie vole establishes pair-bonds and long-term social memories - all hallmark deficits of individuals on the autistic spectrum - these social behaviours in voles can be neurobiologicaly manipulated in the lab to hopefully reveal neurochemical mechanisms underlying the many behavioural facets of autism. The prairie vole model is also well suited to investigate the neurobiology of anxiety-/depression-like behaviour associated with social loss in adulthood (Bosch et al., 2009, Grippo et al., 2007a).
Oxytocin/GABA interactions, benzodiazepines, anxiety, and stress social-buffering
Several forms of clinical anxiety disorders are primarily treated pharmacologically with GABA positive allosteric modulators (i.e., benzodiazepines) including lorazepam, diazepam, and xanax. Diazepam, for example, binds allosterically to GABAA receptors to dampen neuronal excitability (Hadingham et al., 1993, Luddens & Korpi, 1995, Pritchett & Seeburg, 1990). Anxiety levels are also attenuated by OT and diazepam in several behavioural paradigms: (1) EPM (OT – (Griebel et al., 2000, Smith & Wang 2014b)); (2) LDB (OT - (Waldherr & Neumann, 2007); diazepam - (Griebel, Belzung, 2000)); (3) novel object test (OT - (Veenema et al., 2007); diazepam - (Depino et al., 2008); (4) open field test (OT - (Veenema et al., 2007); diazepam - (Veenema et al., 2007)). OT and diazepam enhance GABA neuronal transmisssion in the PVN, presynaptically via OT release (Smith & Wang 2014b) as well as post-synaptically (Zahner et al., 2007). OT with benzodiazepine treatment may work in concert, with one another, to enhance the efficacy of anti-anxiety treatment. The precise mechanism of OT-GABAA-receptor PVN mediated interactions within the HPA axis to reduce stress responsivity is unclear. Future research would benefit from investigating the underlying connections between these systems for anti-anxiety therapeutic application. Preliminary work demonstrates that both OT and GABA work at the level of the PVN to block stress-induced CORT plasma levels to ameliorate anxiety behaviour: (1) OT, (Smith & Wang 2014b) and (2) GABA, (Marques de Souza & Franci, 2008). Forthcoming work by Smith et al., 2014a, demonstrates that GABAA receptor activity modulates anxiolytic effects of OT in the PVN - providing mechanistic insight regarding neuropeptidergic-classical neurotransmitter interactions modulating social-buffering of stress in monogamous prairie voles.
Neurochemistry underlying interactions between natural/artificial rewards related to stress and social buffering
An emerging field exploring neurobiological interactions between drug abuse, social behaviour, and stress is making headway in the scientific literature (Young et al., 2011b). Addiction can manifest in several forms ranging from drugs of abuse (e.g., cocaine/dextro-amphetamine (d-AMPH), alcohol, nicotine, marijuana, lysergic acid diethylamide (LSD), molly, ecstasy, etc.) to sex, gambling, excessive exercise, overeating, body modification, and codependency in unhealthy and abusive intimate partnerships that can lead to serious mental and/or physical ailments. For example, addiction impairs social behaviour (Strathearn & Mayes, 2010, Young et al., 2011b) – preventing the formation and maintenance of mother-child attachment, and monogamously committed partnerships leading to child neglect/abuse (Wasserman & Leventhal, 1993), decline in marriage rates, infidelity, and increased separation/divorce (Collins et al., 2007, Kaestner, 1995). Because forming pair-bonds engenders social-buffering of stress leading to both physical and psychological health (House et al., 1988, Kiecolt-Glaser & Newton, 2001) - understanding brain areas vulnerable to stress (e.g., amygdala, mPFC, and hippocampus; McEwen, Nasca, & Gray, 2015) and drug abuse usurping natural reward (Young et al., 2011b) is of critical importance in health and disease-related clinical research.
Prior work from our lab has pinpointed the mesocorticolimbic DA-ergic system as a prime neural circuit involved in drugs of abuse hijacking natural rewards like social pair-bonding (Aragona et al., 2006, Liu et al., 2010, Young et al., 2011a, Young et al., 2011b Young et al., 2014). The VTA-NAcc-mPFC DA-ergic projection programs pair-bonding and encodes social memory (Aragona et al., 2006, Gingrich et al., 2000) as well as undergoes significant neuroadapatations involving changes in DA release kinetics and DA receptor (DAR) signaling with drug experience (Henry et al., 1989, Nestler, 2005), which can permanently rewire parts of the CNS controlling natural desire, as observed in dampening motivation for engaging in socio-sexual behaviour (Clemens et al., 2004, Febo & Ferris, 2007, Liu et al., 2010, Ludwig, 1998). However, the roles of prosocial neuropeptides - like OT - have been understudied in the context of drug abuse and their interactions with the DA-ergic mesocorticolimbic system (McGregor et al., 2008).
Oxytocin reinstates pair-bonding when drugs of abuse hijack mesocorticolimbic dopaminergic circuitry
OT has been well known to play a significant role in regulating prosocial behaviour (Melis & Argiolas, 2011) and when it acts in the mesocorticolimbic DA-ergic pathway it mediates the formation and maintenance of pair-bonds (Bosch & Neumann, 2012, Liu & Wang, 2003, Ross et al., 2009b, Young et al., 2001). Drug exposure has been found to alter OT functioning in this reward circuit (Butovsky et al., 2006, Johns et al., 1997a, Johns et al., 1997b, Sarnyai et al., 1992), which varies by drug type, dose, and social context. Together, these data have paved the way for future research examining the role of central OT as a potential treatment for reversing social deficits caused by addiction. OT and DA have begun to be explored as prime candidates underlying natural (i.e., mating, pair-bonding, food, parenting, social play, etc.) and artificial (i.e., psychostimulants, alcohol, opiates, marijuana, nicotine, etc.) reward. A recent paper by Young and colleagues, 2014, provided evidence demonstrating a role for OT in the mPFC reversing psychostimulant-induced deficits in pair-bonding in female prairie voles.
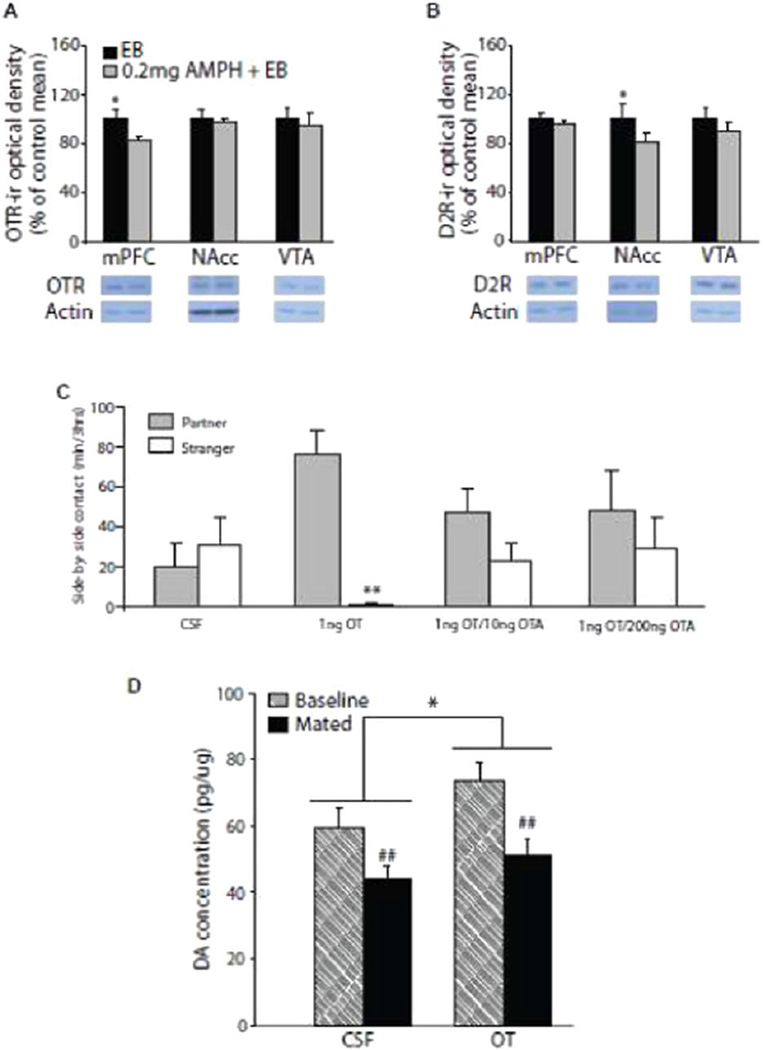
Specifically, we found that repeated i.p. treatment with AMPH blocked mating-induced partner preference in female prairie voles (Figure 4C). Treatment with AMPH also induced significant changes in OT and DA neurochemical signaling in brain regions programming pair-bonds. Repeated AMPH significantly decreased OTR-ir in the mPFC, but not the NAcc or VTA - Figure 4A and D2R-ir in the NAcc, but not the mPFC or VTA - Figure 4B and enhanced NAcc DA levels. PLC infusion of OT, in repeatedly AMPH treated female prairie voles, restored partner preference formation (Figure 4C), which significantly altered DA content in the NAcc (Figure 4D). Importantly, all of these effects were dependent on OTR activation (Figure 4C). Repeated psychostimulant experience impairs pair-bonding behaviour via an OT-mediated neurochemical interaction with the mesocorticolimbic DA-ergic system.
Figure 4.
(A) Western blot data revealed that female prairie voles repeatedly treated with i.p. d-AMPH displayed significantly lower OTR-ir in the mPFC, but not NAcc or VTA, relative to estradiol benzoate (EB)-treated control females. (B) NAcc, but not mPFC or VTA, D2R-ir protein levels were significantly lower in female prairie voles repeatedly treated with AMPH compared to EB-treated controls. OT infusion into the prelimbic cortex (PLC) restores mating-induced partner preference formation in AMPH-treated females. (C) Sexually naïve females infused with aCSF into the PLC did not display partner preference while females injected with 1ng OT intra-PLC, in the absence of mating, displayed robust partner preference and this effect was blocked with concurrent low (10ng OTA) or high (100ng OTA) infusion with the selective OT receptor antagonist. (D) Finally, females infused with OT into the PLC displayed significantly higher levels of dopamine (DA) in the NAcc relative to females treated with intra-PLC aCSF. Furthermore, mated females displayed significantly lower DA levels relative to sexually naïve females (Baseline). Bars indicate means ± standard error of the mean. *p < .05, main treatment effects; ##p < .001, main sample type effects. Adapted from Young et al., 2014.
OT and DA significantly contribute to the formation and maintenance of pair-bonds (Young et al., 2011a) and are altered by exposure to psychostimulants which impairs natural pair-bonding behaviour (Young et al., 2011b). As described above, the data reported by Young and colleagues, 2014, support the notion that interactions between DA and OT in the mesocorticolimbic circuit can restore partner preference formation in drug-exposed animals. Prior work from our laboratory and others firmly established that 24-hours of mating and social cohabitation induce partner preference formation in female prairie voles (Insel & Hulihan, 1995, Williams et al., 1992). Young et al., 2014, demonstrate that pre-exposure to AMPH blocks the formation of mating-induced partner preference. These effects were specific to AMPH because social memory tests were conducted 48-hours after the last AMPH injection when AMPH was completely metabolized. Psychostimulant withdrawal did not have an effect because mating frequency and affiliative behaviour between drug treated and naïve controls was not statistically different. Importantly, these findings in female prairie voles match those found in male prairie voles (Liu et al., 2010) as well as effects of psychostimulant experience affecting soscio-sexual (Clemens et al., 2007), maternal (Febo & Ferris 2007, Johns et al., 1997b, Slamberova et al., 2005), and social play behaviours (Wood et al., 1994).
Repeated AMPH treatment impaired partner preference formation, significantly decreased OTR-ir density in the mPFC, but not the NAcc or VTA (Figure 4A) - replicating prior work from other labs (Johns et al., 2010, Sarnyai et al., 1992). OTR-ir density was decreased in the mPFC after 24-hours of the final AMPH injection when females were paired with a male conspecific and provided ad libitum mating and extended cohabitation prior to partner preference testing. Previous behavioural pharmacological work indicated that OTR activation in the mPFC, PLC subregion, during mating and cohabitation was necessary for pair-bond formation in female prairie voles (Young et al., 2001). Thus, decreased OTRs in the PLC may represent a neurochemical locus underlying AMPH impairment of mating-induced pair-bonding behaviour.
These data are further supported by direct OT micro-infusion into the PLC before pairing which restored partner preference formation in drug treated females. These effects were dependent on PLC-OTR activation (Figure 4C; Young et al., 2014) - extending prior work from other laboratories examining social behaviour (Francis et al., 2000, Olazabal & Young, 2006) and pair-bonding (Ophir et al., 2012, Ross et al., 2009b). PLC-OT micro-infusion increased NAcc-DA concentration relative to artificial cerebrospinal fluid (aCSF) infused controls (Figure 4D), and these results are consistent with previous research illustrating reciprocal and functional connectivity between the PLC and NAcc - both targets of DA-ergic cells bodies in the VTA mesocorticolimbic pathway (Carr & Sesack, 1999). Both electrical and chemical activation of mPFC neurons increases the firing rate of VTA-DA-ergic projection neurons while inactivation inhibits DA burst firing events (Tzschentke, 2001). In summary, PLC-OT infusion increased NAcc DA concentration and restored AMPH-induced deficits in social bonding.
The Future of Social-buffering Stress Research in Prairie Voles
At present, there are no commercially available compounds that physicians and/or Psychiatrists can prescribe patients experiencing debilitating psychological illness resulting from adverse social impairments or bereavement-stress-induced adverse psychological conditions. Because OT administration enhances prosocial behaviour, this particular hormone has recently become a viable target for therapeutic intervention aimed at disorders characterized by marked impairment in social communication and/or bond-loss stress-related psychopathology with several ongoing clinical trials (Miller, 2013). OT underlies socio-sexual behaviour (Carter et al., 2008) and therefore, it has been speculated for decades that it may reduce pathological HPA axis dysfunction.
For example, intra-nasal OT delivery decreases social conflict enhanced by cortisol and increases positive prosocial communication (Ditzen et al., 2009) between human couples during inter-personal conflict, while also facilitating social support reduction during periods of stress-induced CORT release, promoting decreased anxiety and enhanced calmness (Heinrichs et al., 2003, Quirin et al., 2011). Central OT levels are also positively correlated with an increase in positive prosocial communication between intimate partners (Gouin et al., 2010, Holt-Lunstad et al., 2008). Future preclinical work in voles would significantly benefit by expanding the research “tool-kit” by implementing more cellular/molecular approaches currently used in the behavioural neuroscience field (e.g., electrophysiological preparations, optogenetics, DREADDS, AAV-viral-based tract-tracing with CLARITY in order to reveal more concrete neurochemical/-circuit mechanisms altered during bond loss.
As an example, in other work, recent neurogenetic viral-vector-based tract-tracing, optogenetic, and DREADD research in transgenic mice from the laboratories of Dr. Klaus Miczek at Tufts University and Dr. Jamie Maguire at the Tufts School of Medicine have identified a novel CRH micro-circuit associated with social defeat stress (Gobrogge et al., 2015). Specifically, using viral-vector-based tract-tracing by stereotaxically infusing an AAV-Flex-ChR2 virus bilaterally in the ventral-posterior-medial (VPM) region of the posterior VTA (pVTA) in CRH-Cre (Cre recombinase) mice, CRH neurons in the lateral hypothalamus (LH) and dorsal raphe nucleus (DRN) made reciprocal projections with the paranigral (PN) and parainterfasicular (PIF) subnuclei of the VPM. Furthermore, DRN-CRH neuronal dendrites formed a significant number of putative synapses onto DA-ergic cell bodies that co-expressed CRH-type 1 receptors but not CRH-type 2a/b receptors. A significant number of DA-ergic cells in the PN/PIF pVTA co-localized with a post-synaptic density 95 (PSD95) marker, vesicular glutamate transporter 1 (VGLUT1, pre-synaptic label), and DRN CRH/AAV-mCherry (red) dendrites but LH-CRH dendrites did not. Functional activation of the PN/PIF using either optogenetics or Gq(s)-DREADDs in CRH-Cre mice was sufficient to mimic chronic social defeat stress-induced drug-taking behaviour. Together, these data suggest that CRH neurons in the DRN are activated by social defeat stress, releasing CRH in the PN/PIF, which modulates dopamine-dependent behavioural sensitization to psychomotor stimulants. This particular line of work would significantly advance the prairie vole field as well as behavioural neuroscience, in general.
To our knowledge, at the present time, there are two labs attempting to generate transgenic prairie voles: one under the direction of Dr. Larry Young’s group at Emory University in Atlanta (Donaldson et al., 2009) and the second principal investigator Nirao Shah’s group at the University of California at San Francisco (Manoli et al., 2012). When a Cre/LOX (or similar transgenic prairie vole line is generated) - similar studies, as mentioned above, can commence. For example, with a transgenic prairie vole expressing Cre recombinase driven by a specific promoter sequence encoding either OT, AVP, or CRH neuropeptides, one could examine the consequences of modulating molecularly defined regions of only OT, AVP, or CRH neurons and processes in the vole CNS and examine the behavioural consequences of manipulating these systems using optogenetics and/or DREADDs with regard to pair-bonding behaviour and stress social buffering.
In short, OT is a powerful neuropeptide exerting direct regulation of social behaviour and memory (Ross & Young, 2009), while perturbing the OT system has deleterious effects underlying social deficits characterized in several psychiatric conditions including drug addiction, autism spectrum disorders, post-traumatic-stress-disorder and many other forms of stress-induced psychopathology - like anxiety and depression. Therefore, the central OT system represents a clinically viable neurochemical target treatment of social communication deficits (such as those observed in individuals with autism (Hollander et al., 2007)), addiction (McGregor & Bowen, 2012, Strathearn & Mayes, 2010), and social separation-induced anxiety and major depression. Because the central OT system is highly conserved across species (Burkett & Young, 2012, Meyer-Lindenberg et al., 2011, Ross & Young, 2009) and is altered in human addicts (Light et al., 2004), the prairie vole represents a valuable animal model to investigate the effects of pair-bonding and OT on breaking bad-bonds associated with drugs of abuse, conditioned anxiety/panic attacks, and episodic major depression. Finally, future research would benefit from examining the role of pair-bonding and endogenous OT as a viable treatment for drug-induced impairments in devaluing natural reward to restore healthy decision-making and reduce partner loss-induced drug relapse and bereavement in humans (Dawson et al., 2005, Kosten et al., 1987).
Highlights.
Pair-Bonding Buffers Stress via Extra-Hypothalamic Neuropeptide Systems
Neuropeptidergic Interactions Modulate Stress-Mediated Pathology
Attachment and Neuropeptides Serve as Viable Biomedical Therapies for Stress
Acknowledgments
The authors would like to thank Dr. Adam Smith for providing critical feedback and suggested revisions for the manuscript. We would also like to acknowledge Drs. Kimberly Young, Adam Smith, Claudia Lieberwirth, Kelly Lei, and Ping Sun for their significant research contribution to the field with their work investigating the neurobiology of pair-bonding, social-buffering of stress, and drug addiction. This work was supported by NIMHR01-58616 to ZW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no biomedical, financial, or potential conflicts of interest.
Works Cited
- Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regulatory peptides. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- Amico JA, Johnston JM, Vagnucci AH. Suckling-induced attenuation of plasma cortisol concentrations in postpartum lactating women. Endocrine research. 1994;20:79–87. doi: 10.3109/07435809409035858. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang ZX. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair-bonds. Nature neuroscience. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. The prairie vole (Microtus ochrogaster): an animal model for behavioural neuroendocrine research on pair-bonding. ILAR. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Frontiers in behavioural neuroscience. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A. The hypothalamic-pituitary-adrenal axis: what can it tell us about stressors? CNS & neurological disorders drug targets. 2006;5:485–501. doi: 10.2174/187152706778559336. [DOI] [PubMed] [Google Scholar]
- Babygirija R, Bulbul M, Yoshimoto S, Ludwig K, Takahashi T. Central and peripheral release of oxytocin following chronic homotypic stress in rats. Autonomic neuroscience : basic & clinical. 2012;167:56–60. doi: 10.1016/j.autneu.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Bali B, Erdelyi F, Szabo G, Kovacs KJ. Visualization of stress-responsive inhibitory circuits in the GAD65-eGFP transgenic mice. Neuroscience letters. 2005;380:60–65. doi: 10.1016/j.neulet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Bali B, Kovacs KJ. GABA-ergic control of neuropeptide gene expression in parvocellular neurons of the hypothalamic paraventricular nucleus. The European journal of neuroscience. 2003;18:1518–1526. doi: 10.1046/j.1460-9568.2003.02877.x. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, de Vries GJ. Cohabitation alters vasopressin innervation and paternal behaviour in prairie voles (Microtus ochrogaster) Physiology & behaviour. 1994;56:751–758. doi: 10.1016/0031-9384(94)90238-0. [DOI] [PubMed] [Google Scholar]
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Critical reviews in neurobiology. 1996;10:119–154. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Bartanusz V, Muller D, Gaillard RC, Streit P, Vutskits L, Kiss JZ. Local gamma-aminobutyric acid and glutamate circuit control of hypophyseotrophic corticotropin-releasing factor neuron activity in the paraventricular nucleus of the hypothalamus. The European journal of neuroscience. 2004;19:777–782. doi: 10.1111/j.1460-9568.2004.03167.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Decety J, Mason P. Empathy and pro-social behaviour in rats. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikjian LM, Vale WW. Regulation of ACTH secretion from corticotrophs: the interaction of vasopressin and CRF. Annals of the New York Academy of Sciences. 1987;512:85–96. doi: 10.1111/j.1749-6632.1987.tb24952.x. [DOI] [PubMed] [Google Scholar]
- Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. The European journal of neuroscience. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Kromer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behaviour following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Hormones and behaviour. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Brown GW. Life events and illness. London: 1989. [Google Scholar]
- Bulbul M, Babygirija R, Cerjak D, Yoshimoto S, Ludwig K, Takahashi T. Hypothalamic oxytocin attenuates CRF expression via GABA(A) receptors in rats. Brain research. 2011;1387:39–45. doi: 10.1016/j.brainres.2011.02.091. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Young LJ. The behavioural, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology. 2012;224:1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky E, Juknat A, Elbaz J, Shabat-Simon M, Eilam R, Zangen A, Altstein M, Vogel Z. Chronic exposure to Delta9-tetrahydrocannabinol downregulates oxytocin and oxytocin-associated neurophysin in specific brain areas. Molecular and cellular neurosciences. 2006;31:795–804. doi: 10.1016/j.mcn.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Byrne GJ, Raphael B. The psychological symptoms of conjugal bereavement in elderly men over the first 13 months. International journal of geriatric psychiatry. 1997;12:241–251. doi: 10.1002/(sici)1099-1166(199702)12:2<241::aid-gps590>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Byrne GJ, Raphael B. Depressive symptoms and depressive episodes in recently widowed older men. International psychogeriatrics / IPA. 1999;11:67–74. doi: 10.1017/s1041610299005591. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Terminals from the rat prefrontal cortex synapse on mesoaccumbens VTA neurons. Annals of the New York Academy of Sciences. 1999;877:676–678. doi: 10.1111/j.1749-6632.1999.tb09299.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M. Integrative functions of lactational hormones in social behaviour and stress management. Annals of the New York Academy of Sciences. 1997;807:164–174. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M. Integrative functions of lactational hormones in social behaviour and stress management. Cambridge: The MIT Press; 1999. pp. 361–371. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neuroscience and biobehavioural reviews. 1995a;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Taymans SE, Roberts RL, Williams JR, Chrousos GP. Adrenocorticoid hormones and the development and expression of mammalian monogamy. Annals of the New York Academy of Sciences. 1995b;771:82–91. doi: 10.1111/j.1749-6632.1995.tb44672.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Progress in brain research. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. Journal of Neuroscience. 1995;15(10):6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodera P, Salvarani C, Bacchi-Modena A, Spallanzani R, Cigarini C, Alboni A, Gardini E, Coiro V. Relationship between plasma profiles of oxytocin and adrenocorticotropic hormone during suckling or breast stimulation in women. Hormone research. 1991;35:119–123. doi: 10.1159/000181886. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behavioural neuroscience. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behaviour with oxytocin: how does it work? What does it mean? Hormones and behaviour. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens KJ, Cornish JL, Hunt GE, McGregor IS. Repeated weekly exposure to MDMA, methamphetamine or their combination: long-term behavioural and neurochemical effects in rats. Drug and alcohol dependence. 2007;86:183–190. doi: 10.1016/j.drugalcdep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Van Nieuwenhuyzen PS, Li KM, Cornish JL, Hunt GE, McGregor IS. MDMA ("ecstasy"), methamphetamine and their combination: long-term changes in social interaction and neurochemistry in the rat. Psychopharmacology. 2004;173:318–325. doi: 10.1007/s00213-004-1786-x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological bulletin. 1985;98:310–357. [PubMed] [Google Scholar]
- Collins RL, Ellickson PL, Klein DJ. The role of substance use in young adult divorce. Addiction. 2007;102:786–794. doi: 10.1111/j.1360-0443.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- Cullinan WE. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. The Journal of comparative neurology. 2000;419:344–351. doi: 10.1002/(sici)1096-9861(20000410)419:3<344::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–1326. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgard OS, Dowrick C, Lehtinen V, Vazquez-Barquero JL, Casey P, Wilkinson G, Ayuso-Mateos JL, Page H, Dunn G ODIN Group. Negative life events, social support and gender difference in depression: a multinational community survey with data from the ODIN study. Soc Psychiatry Psychiatr Epidemiol. 2006;41:444–451. doi: 10.1007/s00127-006-0051-5. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, Ruan WJ. Recovery from DSM-IV alcohol dependence: United States, 2001–2002. Addiction. 2005;100:281–292. doi: 10.1111/j.1360-0443.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- Decavel C, Van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. The Journal of comparative neurology. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Decavel C, Van den Pol AN. Converging GABA- and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. The Journal of comparative neurology. 1992;316:104–116. doi: 10.1002/cne.903160109. [DOI] [PubMed] [Google Scholar]
- Depino AM, Tsetsenis T, Gross C. GABA homeostasis contributes to the developmental programming of anxiety-related behaviour. Brain research. 2008;1210:189–199. doi: 10.1016/j.brainres.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Detillion CE, Craft TK, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29:1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Cho MM, Cardillo S, Carter CS. Society for Neuroscience Abstract, New Orleans, Louisiana. 1997a [Google Scholar]
- DeVries AC, DeVries MB, Taymans S, Carter CS. Modulation of pair-bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology. 2002;27:705–714. doi: 10.1016/s0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Taymans SE, Carter CS. Social modulation of corticosteroid responses in male prairie voles. Annals of the New York Academy of Sciences. 1997b;807:494–497. doi: 10.1111/j.1749-6632.1997.tb51949.x. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Diversity and adaptation in rodent copulatory behaviour. Science. 1975;190:947–954. doi: 10.1126/science.1188377. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. The comparative psychology of monogamy. Nebr Symp Motiv. 1987;35:1–50. [PubMed] [Google Scholar]
- Dinan TG, Lavelle E, Scott LV, Newell-Price J, Medbak S, Grossman AB. Desmopressin normalizes the blunted adrenocorticotropin response to corticotropin-releasing hormone in melancholic depression: evidence of enhanced vasopressinergic responsivity. The Journal of clinical endocrinology and metabolism. 1999;84:2238–2240. doi: 10.1210/jcem.84.6.5723. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Yang SH, Chan AW, Young LJ. Production of germline transgenic prairie voles (Microtus ochrogaster) using lentiviral vectors. Biology of reproduction. 2009;81(6):1189–1195. doi: 10.1095/biolreprod.109.077529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM. The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neuroscience and biobehavioural reviews. 2010;34:260–268. doi: 10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Ebner K, Bosch OJ, Kromer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behaviour and the release of excitatory amino acids. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- Elwert F, Christakis NA. The effect of widowhood on mortality by the causes of death of both spouses. American journal of public health. 2008a;98:2092–2098. doi: 10.2105/AJPH.2007.114348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwert F, Christakis NA. Wives and ex-wives: a new test for homogamy bias in the widowhood effect. Demography. 2008b;45:851–873. doi: 10.1353/dem.0.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Frontiers in neuroendocrinology. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Febo M, Ferris CF. Development of cocaine sensitization before pregnancy affects subsequent maternal retrieval of pups and prefrontal cortical activity during nursing. Neuroscience. 2007;148:400–412. doi: 10.1016/j.neuroscience.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and social affiliation in humans. Hormones and behaviour. 2012;61:380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Field NP. Continuing bonds in adaptation to bereavement: introduction. Death Stud. 2006;30:709–714. doi: 10.1080/07481180600848090. [DOI] [PubMed] [Google Scholar]
- Flannery RB, Wieman D. Social support, life stress, and psychological disorders: an empirical assessment. Journal of clinical psychology. 1989;45:867–872. doi: 10.1002/1097-4679(198911)45:6<867::aid-jclp2270450606>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of neuroendocrinology. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Fraser ON, Stahl D, Aureli F. Stress reduction through consolation in chimpanzees. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8557–8562. doi: 10.1073/pnas.0804141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzzo F, Matsumoto J, Kiyokawa Y, Takeuchi Y, Ono T, Nishijo H. Social buffering suppresses fear-associated activation of the lateral amygdala in male rats: behavioural and neurophysiological evidence. Frontiers in neuroscience. 2015;9:99. doi: 10.3389/fnins.2015.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends in neurosciences. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Getz LL, Carter CS. Prairie-vole partnerships. Am Sci. 1996;84:56–62. [Google Scholar]
- Getz LL, Carter CS, Gavish L. The mating system of prairie voles, Microtus ochrogaster: field and laboratory evidence for pair-bonding. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- Getz LL, McGuire B, Carter CS. Social behaviour, reproduction, and demography of the praririe vole, Microtus ochrogaster. Ethol Ecol Evol. 2003;15:105–118. [Google Scholar]
- Getz LL, McGuire B, Pizzuto T, Hofman JE, Frase B. Social organization of the prairie vole (Microtus ochrogaster) J. Mammal. 1993;74:44–58. [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang ZX, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behavioural neuroscience. 2000;114:173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Hooper A, Holly E, Han X, DeBold J, Maguire J, Miczek KA. Identification of a novel CRF-VTA microcircuit in the mouse midbrain underlying stress and psychostimulant sensitization. Society for Neuroscience Abstract, Chicago, Illinois. 2015 Abstract control number 14292. [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang ZX. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. The Journal of comparative neurology. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang ZX. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proceedings of the National Academy of Sciences of the United States of America. 2009;N106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Martin C, Feldman R, Leckman JF. Oxytocin and social motivation. Developmental cognitive neuroscience. 2011;1:471–493. doi: 10.1016/j.dcn.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin JP, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, Stowell J, Kiecolt-Glaser JK. Marital behaviour, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology. 2010;35:1082–1090. doi: 10.1016/j.psyneuen.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GD, Dewsbury DA. A quantitative description of copulatory behaviour in prairie voles (Microtus ochrogaster) Brain, behaviour and evolution. 1973;8:426–452. [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology. 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behaviour and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic medicine. 2007a;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioural and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007b;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviours. Biological psychiatry. 2007c;62:1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Pournajafi-Nazarloo H, Sanzenbacher L, Trahanas DM, McNeal N, Clarke DA, Porges SW, Carter CS. Peripheral oxytocin administration buffers autonomic but not behavioural responses to environmental stressors in isolated prairie voles. Stress. 2012;15:149–161. doi: 10.3109/10253890.2011.605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, 2nd, Porges SW, Carter CS. Oxytocin protects against negative behavioural and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviours relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depression and anxiety. 2008;25:E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadingham KL, Wingrove PB, Wafford KA, Bain C, Kemp JA, Palmer KJ, Wilson AW, Wilcox AS, Sikela JM, Ragan CI, Whiting PJ. Role of the beta subunit in determining the pharmacology of human gamma-aminobutyric acid type A receptors. Molecular pharmacology. 1993;44:1211–1218. [PubMed] [Google Scholar]
- Hammack SE, Cooper MA, Lezak KR. Overlapping neurobiology of learned helplessness and conditioned defeat: implications for PTSD and mood disorders. Neuropharmacology. 2012;62:565–575. doi: 10.1016/j.neuropharm.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behaviour. Frontiers in neuroendocrinology. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. The Journal of pharmacology and experimental therapeutics. 1989;251:833–839. [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in neuroendocrinology. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacology, biochemistry, and behaviour. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Milton NG. Effect of noradrenaline and gamma-aminobutyric acid on the secretion of corticotrophin-releasing factor-41 and arginine vasopressin from the rat hypothalamus in vitro. The Journal of endocrinology. 1989;122:719–723. doi: 10.1677/joe.0.1220719. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biological psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham WA, Light KC. Influence of a "warm touch" support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosomatic medicine. 2008;70:976–985. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Ryabinin AE. The CRF system and social behaviour: a review. Frontiers in neuroscience. 2013;7:92. doi: 10.3389/fnins.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Inenaga K, Yamashita H. Excitation of neurones in the rat paraventricular nucleus in vitro by vasopressin and oxytocin. The Journal of physiology. 1986;370:165–180. doi: 10.1113/jphysiol.1986.sp015928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair-bonding: oxytocin and partner preference formation in monogamous voles. Behavioural neuroscience. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Preston S, Winslow JT. Mating in the monogamous male: behavioural consequences. Physiology & behaviour. 1995;57:615–627. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Johns JM, Lubin DA, Walker CH, Meter KE, Mason GA. Chronic gestational cocaine treatment decreases oxytocin levels in the medial preoptic area, ventral tegmental area and hippocampus in Sprague-Dawley rats. Neuropeptides. 1997a;31:439–443. doi: 10.1016/s0143-4179(97)90037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, McMurray MS, Joyner PW, Jarrett TM, Williams SK, Cox ET, Black MA, Middleton CL, Walker CH. Effects of chronic and intermittent cocaine treatment on dominance, aggression, and oxytocin levels in post-lactational rats. Psychopharmacology. 2010;211:175–185. doi: 10.1007/s00213-010-1877-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of short-and long-term withdrawal from gestational cocaine treatment on maternal behaviour and aggression in Sprague-Dawley rats. Developmental neuroscience. 1997b;19:368–374. doi: 10.1159/000111234. [DOI] [PubMed] [Google Scholar]
- Kaestner R. The effects of cocaine and marijuana use on marriage and marital stability. Cambridge, MA: National Bureau of Economic Research; 1995. [Google Scholar]
- Karelina K, DeVries AC. Modeling social influences on human health. Psychosomatic medicine. 2011;73:67–74. doi: 10.1097/PSY.0b013e3182002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychological bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Hairston JE, Devries AC, Nelson RJ. Social environment and steroid hormones affect species and sex differences in immune function among voles. Hormones and behaviour. 1997;32:30–39. doi: 10.1006/hbeh.1997.1402. [DOI] [PubMed] [Google Scholar]
- Kojima S, Stewart RA, Demas GE, Alberts JR. Maternal contact differentially modulates central and peripheral oxytocin in rat pups during a brief regime of mother-pup interaction that induces a filial huddling preference. Journal of neuroendocrinology. 2012;24:831–840. doi: 10.1111/j.1365-2826.2012.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Jalali B, Steidl JH, Kleber HD. Relationship of marital structure and interactions to opiate abuse relapse. The American journal of drug and alcohol abuse. 1987;13:387–399. doi: 10.3109/00952998709001523. [DOI] [PubMed] [Google Scholar]
- Kristensen P, Weisaeth L, Heir T. Bereavement and mental health after sudden and violent losses: a review. Psychiatry. 2012;75:76–97. doi: 10.1521/psyc.2012.75.1.76. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal and coping. New York: Springer; 1984. [Google Scholar]
- Lieberwirth C, Liu Y, Jia X, Wang ZX. Social isolation impairs adult neurogenesis in the limbic system and alters behaviours in female prairie voles. Hormones and behaviour. 2012;62:357–366. doi: 10.1016/j.yhbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA, Boccia M, Brownley KA, Johns JM. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addictive behaviours. 2004;29:1541–1564. doi: 10.1016/j.addbeh.2004.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman SL. The neuroendocrinology of stress: a never ending story. Journal of neuroendocrinology. 2008;20:880–884. doi: 10.1111/j.1365-2826.2008.01711.x. [DOI] [PubMed] [Google Scholar]
- Lillard LA, Waite LJ. Til death do us part: marital disruption and mortality. American Journal of Sociology. 1995;100:1131–1156. [Google Scholar]
- Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang ZX, Young LJ. CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Hormones and behaviour. 2007;51:508–515. doi: 10.1016/j.yhbeh.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Wang ZX, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Linnen AM, Ellenbogen MA, Cardoso C, Joober R. Intranasal oxytocin and salivary cortisol concentrations during social rejection in university students. Stress. 2012;15:393–402. doi: 10.3109/10253890.2011.631154. [DOI] [PubMed] [Google Scholar]
- Liu Y, Aragona BJ, Young KA, Dietz DM, Kabbaj M, Mazei-Robison M, Nestler EJ, Wang ZX. Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1217–1222. doi: 10.1073/pnas.0911998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang ZX. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behavioural neuroscience. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proceedings of the National Academy of Sciences of the United States of America 31. 1995;92(3):836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luddens H, Korpi ER. Biological function of GABAA/benzodiazepine receptor heterogeneity. Journal of psychiatric research. 1995;29:77–94. doi: 10.1016/0022-3956(94)00040-x. [DOI] [PubMed] [Google Scholar]
- Ludwig M. Dendritic release of vasopressin and oxytocin. Journal of neuroendocrinology. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Callahan MF, Neumann I, Landgraf R, Morris M. Systemic osmotic stimulation increases vasopressin and oxytocin release within the supraoptic nucleus. Journal of neuroendocrinology. 1994;6:369–373. doi: 10.1111/j.1365-2826.1994.tb00595.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature Reviews Neuroscience. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behaviour and prevents social avoidance in rats and mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli DS, Subramanyam D, Carey C, Sudin E, Van Westerhuyzen JA, Bales KL, Blelloch R, Shah NM. Generation of induced pluripotent stem cells from the prairie vole. PLoS One. 2012;7(5):e38119. doi: 10.1371/journal.pone.0038119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques de Souza L, Franci CR. GABAergic mediation of of stress-induced secretion of corticosterone and oxytocin, but not prolactin, by the hypothalamic paraventricular nucleus. Life sciences. 2008;83:686–692. doi: 10.1016/j.lfs.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Maulik PK, Eaton WW, Bradshaw CP. The effect of social networks and social support on common mental disorders following specific life events. Acta psychiatrica Scandinavica. 2010;122:118–128. doi: 10.1111/j.1600-0447.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Erickson CA, Stigler KA, Posey DJ. Neurochemistry in the pathophysiology of autism. The Journal of clinical psychiatry. 2005;66(Suppl 10):9–18. [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015 Jun 16; doi: 10.1038/npp.2015.171. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Hormones and behaviour. 2012;61:331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? British journal of pharmacology. 2008;154:358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal N, Scotti MA, Wardwell J, Chandler DL, Bates SL, Larocca M, Trahanas DM, Grippo AJ. Disruption of social bonds induces behavioural and physiological dysregulation in male and female prairie voles. Autonomic neuroscience : basic & clinical. 2014;180:9–16. doi: 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis MR, Argiolas A. Central control of penile erection: a re-visitation of the role of oxytocin and its interaction with dopamine and glutamic acid in male rats. Neuroscience and behavioural reviews. 2011;35:939–955. doi: 10.1016/j.neubiorev.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Miklos IH, Kovacs KJ. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience. 2002;113:581–592. doi: 10.1016/s0306-4522(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Miller G. Neuroscience. The promise and perils of oxytocin. Science. 2013;339:267–269. doi: 10.1126/science.339.6117.267. [DOI] [PubMed] [Google Scholar]
- Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME, Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. The Journal of endocrinology. 1984;102:63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- Moos F, Poulain DA, Rodriguez F, Guerne Y, Vincent JD, Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Experimental brain research. 1989;76:593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature neuroscience. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Neumann ID. The advantage of social living: brain neuropeptides mediate the beneficial consequences of sex and motherhood. Frontiers in neuroendocrinology. 2009;30:483–496. doi: 10.1016/j.yfrne.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviours. Trends in neurosciences. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. Journal of neuroendocrinology. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Anselmo-Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain research. 1998;781:57–61. doi: 10.1016/s0006-8993(97)01159-1. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Karelina K, Morris JS, Zhang N, Cochran M, DeVries CA. Social interaction prevents the development of depressive-like behaviour post nerve injury in mice: a potential role for oxytocin. Psychosomatic medicine. 2010;72:519–526. doi: 10.1097/PSY.0b013e3181de8678. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Hormones and behaviour. 2006;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Onrust SA, Cuijpers P. Mood and anxiety disorders in widowhood: a systematic review. Aging & mental health. 2006;10:327–334. doi: 10.1080/13607860600638529. [DOI] [PubMed] [Google Scholar]
- Ontjes DA, Ways DK, Mahaffee DD, Zimmerman CF, Gwynne JT. ACTH receptors and the effect of ACTH on adrenal organelles. Annals of the New York Academy of Sciences. 1977;297:295–313. doi: 10.1111/j.1749-6632.1977.tb41862.x. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng DJ, Phelps SM. Oxytocin receptor density is associated with male mating tactics and social monogamy. Hormones and behaviour. 2012;61:445–453. doi: 10.1016/j.yhbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani JH, Zhao M, Cui Z, Avram SK, Caruana DA, Dudek SM, Young WS. Role of the vasopressin 1b receptor in rodent aggressive behaviour and synaptic plasticity in hippocampal area CA2. Molecular Psychiatry. 2015;20(4):490–499. doi: 10.1038/mp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Liu Y, Young KA, Zhang Z, Wang ZX. Post-weaning social isolation alters anxiety-related behaviour and neurochemical gene expression in the brain of male prairie voles. Neuroscience letters. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 2005;30:924–929. doi: 10.1016/j.psyneuen.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress. 2002;5:259–267. doi: 10.1080/1025389021000037586. [DOI] [PubMed] [Google Scholar]
- Petersson M, Hulting AL, Uvnas-Moberg K. Oxytocin causes a sustained decrease in plasma levels of corticosterone in rats. Neuroscience letters. 1999;264:41–44. doi: 10.1016/s0304-3940(99)00159-7. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Lasko NB. Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry research. 1993;48:107–117. doi: 10.1016/0165-1781(93)90035-f. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Otto S, Sutton S. Neurotransmitter modulation of corticotropin releasing factor secretion into the hypophysial-portal circulation. Life sciences. 1987;41:1311–1317. doi: 10.1016/0024-3205(87)90211-6. [DOI] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Kenkel W, Mohsenpour SR, Sanzenbacher L, Saadat H, Partoo L, Yee J, Azizi F, Carter CS. Exposure to chronic isolation modulates receptors mRNAs for oxytocin and vasopressin in the hypothalamus and heart. Peptides. 2013;43:20–26. doi: 10.1016/j.peptides.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Seeburg PH. Gamma-aminobutyric acidA receptor alpha 5-subunit creates novel type II benzodiazepine receptor pharmacology. Journal of neurochemistry. 1990;54:1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Archives of general psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Dusing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36:898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behaviour in female prairie voles. Neuroscience. 2009a;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviours in monogamous and polygamous voles. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009b;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behaviour. Frontiers in neuroendocrinology. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenzweig A, Prigerson H, Miller MD, Reynolds CF., 3rd Bereavement and late-life depression: grief and its complications in the elderly. Annual review of medicine. 1997;48:421–428. doi: 10.1146/annurev.med.48.1.421. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Carter CS. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Hormones and behaviour. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Russell JA, Neumann I, Landgraf R. Oxytocin and vasopressin release in discrete brain areas after naloxone in morphine-tolerant and -dependent anesthetized rats: push-pull perfusion study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:1024–1032. doi: 10.1523/JNEUROSCI.12-03-01024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Vecsernyes M, Laczi F, Biro E, Szabo G, Kovacs GL. Effects of cocaine on the contents of neurohypophyseal hormones in the plasma and in different brain structures in rats. Neuropeptides. 1992;23:27–31. doi: 10.1016/0143-4179(92)90006-i. [DOI] [PubMed] [Google Scholar]
- Shear K, Shair H. Attachment, loss, and complicated grief. Developmental psychobiology. 2005;47:253–267. doi: 10.1002/dev.20091. [DOI] [PubMed] [Google Scholar]
- Slamberova R, Charousova P, Pometlova M. Maternal behaviour is impaired by methamphetamine administered during pre-mating, gestation and lactation. Reproductive toxicology. 2005;20:103–110. doi: 10.1016/j.reprotox.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Smith AS, Butler MJ, Liu Y, Wang Z. Social buffering alleviates negative effects of social defeat in female prairie voles (Microtus ochrogaster) Under Review. [Google Scholar]
- Smith AS, Lei K, Wang ZX. Neurobiology of social attachment. In: Charney D, Buxbaum J, Sklar P, Nestler EJ, editors. Neurobiology of Mental Illness. New York: Oxford University Press; 2013. [Google Scholar]
- Smith AS, Tabbaa M, Lei K, Eastham P, Butler MJ, Altshuler R, Linton L, Liu Y, Wang ZX. Local oxytocin tempers anxiety by activating GABA-A receptors in the hypothalamic paraventricular nucleus. Poster Presented at the Society for Neuroscience Meeting; 2014; Washington, DC. 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang ZX. Salubrious effects of oxytocin on social stress-induced deficits. Hormones and behaviour. 2012;61:320–330. doi: 10.1016/j.yhbeh.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang ZX. Hypothalamic oxytocin mediates social buffering of the stress response. Biological psychiatry. 2014b;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosomatic medicine. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Mayes LC. Cocaine addiction in mothers: potential effects on maternal care and infant development. Annals of the New York Academy of Sciences. 2010;1187:172–183. doi: 10.1111/j.1749-6632.2009.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebe M, Schut H, Boerner K. Continuing bonds in adaptation to bereavement: Toward theoretical integration. Clinical psychology review. 2010;30:259–268. doi: 10.1016/j.cpr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, Wang ZX. Breaking bonds in male prairie vole: long-term effects on emotional and social behaviour, physiology, and neurochemistry. Behavioural brain research. 2014;265:22–31. doi: 10.1016/j.bbr.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzi MG, Ingram CD. Oxytocin-induced excitation of neurones in the rat central and medial amygdaloid nuclei. Neuroscience. 2005;134:345–354. doi: 10.1016/j.neuroscience.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Thase ME. Neurobiological aspects of depression. In: Gotlieb IH, Hammen CL, editors. Handbook of depression. New York: Guilford Press; 2009. pp. 187–217. [Google Scholar]
- Tzschentke TM. Pharmacology and behavioural pharmacology of the mesocortical dopamine system. Progress in neurobiology. 2001;63:241–320. doi: 10.1016/s0301-0082(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32:437–450. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behaviour and neural activation in male prairie voles. Brain research. 1997;767:321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain research. 1994;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. The Journal of comparative neurology. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wasserman DR, Leventhal JM. Maltreatment of children born to cocaine-dependent mothers. American journal of diseases of children. 1993;147:1324–1328. doi: 10.1001/archpedi.1993.02160360066021. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Endogenous opioid regulation of stress-induced oxytocin release within the hypothalamic paraventricular nucleus is reversed in late pregnancy: a microdialysis study. Neuroscience. 2002;112:121–129. doi: 10.1016/s0306-4522(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Hormones and behaviour. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behaviour in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair-bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Witt DM, Carter CS, Lnsel TR. Oxytocin receptor binding in female prairie voles: endogenous and exogenous oestradiol stimulation. Journal of neuroendocrinology. 1991;3:155–161. doi: 10.1111/j.1365-2826.1991.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Wood RD, Bannoura MD, Johanson IB. Prenatal cocaine exposure: effects on play behaviour in the juvenile rat. Neurotoxicology and teratology. 1994;16:139–144. doi: 10.1016/0892-0362(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Excitatory amino acid antagonists inhibit synaptic responses in the guinea pig hypothalamic paraventricular nucleus. Journal of neurophysiology. 1991;65:946–951. doi: 10.1152/jn.1991.65.4.946. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, Dina C, Ebstein RP. Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: mediation by socialization skills. Molecular psychiatry. 2006;11:488–494. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang ZX. The neurobiology of pair-bonding: insights from a socially monogamous rodent. Frontiers in neuroendocrinology. 2011a;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Wang Z. The role of mesocorticolimbic dopamine in regulating interactions between drugs of abuse and social behaviour. Neuroscience and behavioural reviews. 2011b;35:498–515. doi: 10.1016/j.neubiorev.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Liu Y, Gobrogge KL, Wang H, Wang ZX. Oxytocin reverses amphetamine-induced deficits in social bonding: evidence for an interaction with nucleus accumbens dopamine. The Journal of neuroscience. 2014;34:8499–8506. doi: 10.1523/JNEUROSCI.4275-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Hormones and behaviour. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang ZX. The neurobiology of pair-bonding. Nature neuroscience. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zahner MR, Li DP, Pan HL. Benzodiazepine inhibits hypothalamic presympathetic neurons by potentiation of GABAergic synaptic input. Neuropharmacology. 2007;52:467–475. doi: 10.1016/j.neuropharm.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Zheng J, Babygirija R, Bulbul M, Cerjak D, Ludwig K, Takahashi T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. American journal of physiology: Gastrointestinal and liver physiology. 2010;299:G946–G953. doi: 10.1152/ajpgi.00483.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin K, Christakis NA. The emotional toll of spousal morbidity and mortality. The American journal of geriatric psychiatry. 2007;15:772–779. doi: 10.1097/JGP.0b013e318050c9ae. [DOI] [PubMed] [Google Scholar]