Abstract
IMPORTANCE
Standard molecularly based strategies to predict and/or prevent oral cancer development in patients with oral premalignant lesions (OPLs) are lacking.
OBJECTIVE
To test if the epidermal growth factor receptor inhibitor erlotinib would reduce oral cancer development in patients with high-risk OPLs defined by specific loss of heterozygosity (LOH) profiles. Secondary objectives included prospective determination of LOH as a prognostic marker in OPLs.
DESIGN
The Erlotinib Prevention of Oral Cancer (EPOC) study was a randomized, placebo-controlled, double-bind trial. Accrual occurred from November 2006 through July 2012, with a median follow-up time of 35 months in an ambulatory care setting in 5 US academic referral institutions. Patients with OPLs were enrolled in the protocol, and each underwent LOH profiling (N = 379); they were classified as high-risk (LOH-positive) or low-risk (LOH-negative) patients based on their LOH profiles and oral cancer history. The randomized sample consisted of 150 LOH-positive patients.
INTERVENTIONS
Oral erlotinib treatment (150mg/d) or placebo for 12 months.
MAIN OUTCOMES AND MEASURES
Oral cancer–free survival (CFS).
RESULTS
A total of 395 participants were classified with LOH profiles, and 254 were classified LOH positive. Of these, 150 (59%) were randomized, 75 each to the placebo and erlotinib groups. The 3-year CFS rates in placebo- and erlotinib-treated patients were 74%and 70%, respectively (hazard ratio [HR], 1.27; 95%CI, 0.68–2.38; P = .45). The 3-year CFS was significantly lower for LOH-positive compared with LOH-negative groups (74%vs 87%, HR, 2.19; 95%CI, 1.25–3.83; P = .01). Increased EGFR gene copy number correlated with LOH-positive status (P < .001) and lower CFS (P = .01). The EGFR gene copy number was not predictive of erlotinib efficacy. Erlotinib-induced skin rash was associated with improved CFS (P = .01).
CONCLUSIONS AND RELEVANCE
In this trial, LOH was validated as a marker of oral cancer risk and found to be associated with increased EGFR copy number (the target of the intervention). Erlotinib did not, however, improve CFS in high-risk patients with LOH-positive or high-EGFR-gene-copy-number OPLs. These results support incorporation of LOH testing as a prognostic tool in routine clinical practice but do not support erlotinib use in this setting.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00402779
Oral premalignant lesions (OPLs), often clinically recognized as leukoplakia or erythroplakia, are considered risk factors for development of oral cancer. Oral premalignant lesions are a clinical manifestation of what can be a diffuse process of field cancerization. In patients with treated head and neck cancers, field cancerization contributes to development of second primary tumors, a significant cause of morbidity and mortality.1 Systemic chemoprevention has been proposed as a method to address the entire mucosal field at risk to halt or reverse carcinogenesis.2 However, a standard head neck cancer chemoprevention approach has yet to be developed, despite extensive previous clinical investigations of various retinoids,3–7 interferon,8 celecoxib,9 ONYX-015,10 and Bowman Birk inhibitor concentrate,11 among other candidate preventive drugs.
The epidermal growth factor receptor (EGFR) plays a critical role in oral epithelial carcinogenesis and is the only validated molecular target for which an agent (ie, the monoclonal antibody cetuximab) has been approved to treat head and neck squamous cell carcinomas (HNSCCs).12,13 However, the intravenous weekly administration of cetuximab imposes a limitation for long-term preventive use. In contrast, EGFR tyrosine kinase inhibitors (eg, erlotinib) are dosed orally and have demonstrated modest activity against invasive HNSCC,14–16 rendering them reasonable choices for evaluation for oral cancer chemoprevention.
Even within patients with oral premalignant lesions, the reported risk of malignant transformation is highly variable,17 and applying a chemoprevention strategy is best justified if focused on individuals at highest cancer risk.18 To date, no robust prognostic marker has been developed to guide routine clinical management. Our group and others19–21 have reported that specific loss of heterozygosity (LOH) profiles in OPLs were associated with an increased risk of cancer in patients with or without a history of oral cancer in retrospective case-control and prospective cohort observational studies.
We designed the Erlotinib Prevention of Oral Cancer (EPOC) randomized, placebo-controlled trial to test the hypothesis that targeting EGFR in patients with high-risk OPLs, defined by specific LOH profiles, would prevent oral cancer. In contrast to previous studies, to our knowledge this is the first trial of OPLs designed with a definitive primary end point of oral cancer development—a critical aspect, given the lack of correlation between premalignant lesion response and oral cancer–free survival (CFS).5 This is also the first randomized clinical trial in the entire cancer prevention field to utilize molecular risk assessment as a core selection strategy, thus attempting to develop a chemopreventive approach based on precision medicine.
Methods
The EPOC trial was a randomized, double-blind, placebo-controlled, multicenter study involving 5 institutions in the United States. The study was approved by the institutional review boards of each institution. The trial protocol is available for review in Supplement 1. Written informed consent was obtained from all patients prior to participation.
Eligibility
Participants were eligible for LOH screening if they had histologic evidence of oral premalignant lesions within 1 year prior to enrollment, with or without a history of oral cancer. Patients with a premalignant lesion at the margins of a cancer resection were eligible. The EPOC trial used a convergent trial design in which individuals with premalignant lesions with or without previously treated oral cancers were included in the same trial, with LOH used as the common high-risk eligibility criterion, but oral cancer history was accounted for as a stratification factor.18,22
Molecular risk assessment was based on LOH profiles in the OPL and was performed centrally real time at MD Anderson Cancer Center19–21 (eMethods in Supplement 2). Treating physicians were notified of the results via a web-based system. High-risk LOH profiles (LOH-positive cases) were defined as LOH at 3p14 and/or 9p21 in participants with a history of oral cancer or LOH at 3p14 and/or 9p21 plus an additional chromosomal site (17p, 8p, 11p, 4q, or 13q) in participants without a history of oral cancer, based on previously published data.20,21 All other cases were considered LOH negative.
Only LOH-positive patients were eligible for randomization in EPOC. Additional main eligibility criteria included Eastern Cooperative Oncology Group performance status less than 2, normal organ and bone marrow function, and no active invasive cancer within the previous 2 years (with the exception of oral cancers and nonmelanoma skin cancers). One informed consent document was used for molecular testing and participation in the active treatment portion of the protocol. However, prior to randomization, eligible patients signed the consent form again if the time from initial consent to randomization was greater than 1 month, according to institutional policies.
Treatment Allocation and Study Procedures
Eligible patients were stratified by prior oral cancer status and by registration site. Within each stratum, the Pocock-Simon dynamic allocation method was applied to achieve balanced randomization with respect to smoking status (current, former, and never smoker) and nonsteroidal anti-inflammatory drug use. Subjects were randomized to the erlotinib arm or the placebo arm with equal probability by a centralized computer system. The investigators, clinicians, and patients were blinded to the treatment arm assignments.
Treatment with erlotinib (at a starting dose of 150 mg/d orally) or matching placebo was initiated in a double-blind fashion after randomization. Dose reductions for erlotinib to 100 mg/d orally and subsequently 50 mg/d orally were allowed in accordance with protocol-defined toxic effects criteria (trial protocol in Supplement 1).
Treatment continued for a maximum of 12 months, unless participants developed oral cancer or intolerable toxic effects or withdrew treatment consent (see eMethods in Supplement 2 for further details). Rationale for the 1-year treatment period was based on the expectation of a therapeutic effect of promoting cell death and elimination of LOH-positive premalignant clones during use of the EGFR inhibitor, balanced by the ability of relatively asymptomatic patients to tolerate full therapeutic doses of the drug (with potential for adverse effects) for an extended period in the prevention setting.
The EGFR gene copy number was evaluated in pretreatment biopsy specimens by fluorescence in situ hybridization, as previously described23 (eMethods in Supplement 2).
Statistical Considerations
The primary end point of the study was oral CFS, defined as time from randomization to development of histologically confirmed oral cancer or death in the intent-to-treat population. For events that had not occurred by the time of the analysis, times were censored at the last contact at which the patient was known to be alive and oral cancer free. The distribution of CFS was estimated by the Kaplan-Meier method. A stratified log-rank test (stratified by prior oral cancer status) was used for the comparison of CFS between the treatment and placebo group. A log-rank test was performed to compare differences between groups.
The Cox proportional hazards regression model was used to incorporate potential prognostic factors including age, sex, smoking history, alcohol use, number of prior oral cancers, time from last oral cancer to the randomization, histologic findings, and treatment assignment as covariates. The interaction effect between treatment and a covariate such as EGFR gene copy number on CFS was evaluated using the Cox proportional hazards regression model by including the main effect terms of treatment and EGFR gene copy number and the interaction term. The proportional hazards assumption for the Cox proportional hazards model was evaluated. No model violations were detected.
Regression diagnostics (eg, generalized residuals, Martingale residuals, and Shoenfeld residuals)were examined to ensure that the models were appropriate. The Fisher exact test was used to compare EGFR gene copy number between LOH-positive and LOH-negative groups. Exploratory landmark analysis was performed to investigate the relationship between grade 2 or higher rash at 1month and improved oral CFS in the erlotinib arm, consistent with previous studies.24
The proposed sample size was 150 randomized patients, which was calculated based on the following assumptions: 100 patients with and 50 patients without a history of oral cancer; accrual over a 2-year period, with an additional 2.5 years of follow-up; and oral cancer rates of 65% and 35% at 3 years in patients with21 and without19 a history of oral cancer, respectively. With these parameters, the study would have 85% power to detect a 40% erlotinib-induced reduction in the 3-year oral cancer rate (corresponding to a hazard ratio [HR] of 0.47), with a 2-sided type I error rate of .05. Subgroup analyses of the efficacy of erlotinib in patients with and without a history of oral cancer were prespecified. We aimed at a relatively large treatment effect that could optimize benefit-risk and benefit-cost ratios. We expected erlotinib to be associated with moderate adverse effects and significant costs. Therefore, we reasoned that further clinical development of this drug in the prevention setting would only be justified if cancer incidence could be reduced in a high proportion of patients.
Two interim analyses for efficacy monitoring were planned, at the end of years 2.5 and 3.5. However, we conducted only 1 interim analysis as planned at the time when 23 events were observed. This first interim analysis found no statistically significant difference in CFS at the .0005 level between the 2 treatment groups, so the study was not terminated early. This interim analysis had minimal effect on the significance level of the final analysis. The P values were compared with the O’Brien-Fleming boundary, which has already taken the multiple testings under consideration. We did not perform the second planned interim analysis because the number of events never reached 49 at the planned time for the second interim analysis while the trial was ongoing. The final analysis was to be performed at the end of year 4.5, but this was delayed to year 7 to allow at least 12 months’ follow-up after randomization of the last participant.
The study was monitored by the MD Anderson Data Safety Monitoring Board (DSMB) every 6 to 12 months and was allowed to proceed until its completion. The timing of database freeze was approved by the DSMB, with the assumption that the longer-than-expected recruitment period leading to extended follow-up of patients initially enrolled in the study would compensate for the shorter follow-up time for later enrollees, thus minimizing further delays in the readout of the primary end point.
Results
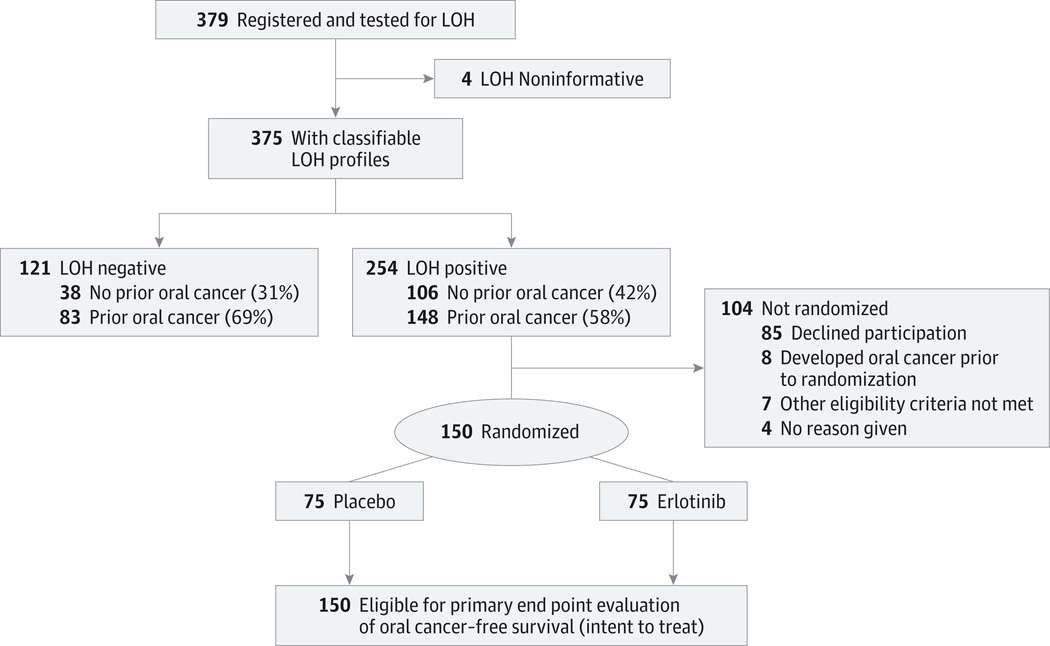
From November 2006 until July 2012, 379 participants were consented, registered, enrolled, and tested for LOH. Four participants had noninformative LOH results and were excluded from further analyses, yielding 375 participants classified as either LOH negative (n = 121, 32%) or LOH positive (n = 254, 68%). Of the 254 LOH-positive patients, 150 (59%) were randomized to erlotinib (n = 75,50%)or placebo (n = 75,50%) and initiated treatment. All randomized patients were eligible for evaluation of the primary end point of oral CFS (Figure 1). At the time of the analysis, 78%of the censored patients had been followed for 2 years or longer.
Figure 1. Flow Diagram of Study Enrollment.
LOH indicates loss of heterozygosity.
Patient Characteristics
The characteristics of enrolled patients according to LOH and randomization status are described in the Table and eTable 1 in Supplement 2.
Table.
Demographics of the LOH-Positive Study Participants
| Characteristic | Participants, No. (%) | |
|---|---|---|
| Placebo (n = 75) |
Erlotinib (n = 75) |
|
| Age, median, y | 57 | 57 |
| Sex | ||
| Female | 33 (44) | 32 (43) |
| Male | 42 (56) | 43 (57) |
| Smoking status | ||
| Current | 9 (12) | 9 (12) |
| Former | 31 (41) | 33 (44) |
| Never | 35 (47) | 33 (44) |
| Alcohol use | ||
| No | 8 (11) | 6 (8) |
| Yes | 67 (89) | 69 (92) |
| Light drinkinga | 37 (55) | 42 (61) |
| Moderate/heavy drinkinga | 12 (18) | 10 (14) |
| Missing alcohol amounta | 18 (27) | 17 (25) |
| Prior oral cancers, No. | ||
| 0 | 32 (43) | 33 (44) |
| 1 | 37 (49) | 31 (41) |
| ≥2 | 6 (8) | 11 (15) |
| Time from last oral cancer, y | ||
| ≤1 | 28 (37) | 24 (32) |
| 1–3 | 7 (9) | 10 (13) |
| >3 | 8 (11) | 7 (9) |
| Histologic finding | ||
| Hyperkeratosis/hyperplasia | 20 (27) | 18 (24) |
| Mild/moderate dysplasia | 42 (56) | 48 (64) |
| Severe dysplasia | 11 (15) | 8 (11) |
| Missing | 2 (3) | 1 (1) |
Abbreviation: LOH, loss of heterozygosity.
Light drinking was defined as intake of up to 1 drink per day for women and up to 2 drinks per day for men; moderate/heavy drinking, intake of more than 1 drink per day for women or 2 drinks per day for men. Percentages were calculated among alcohol users only.
Effect of Erlotinib on Oral CFS
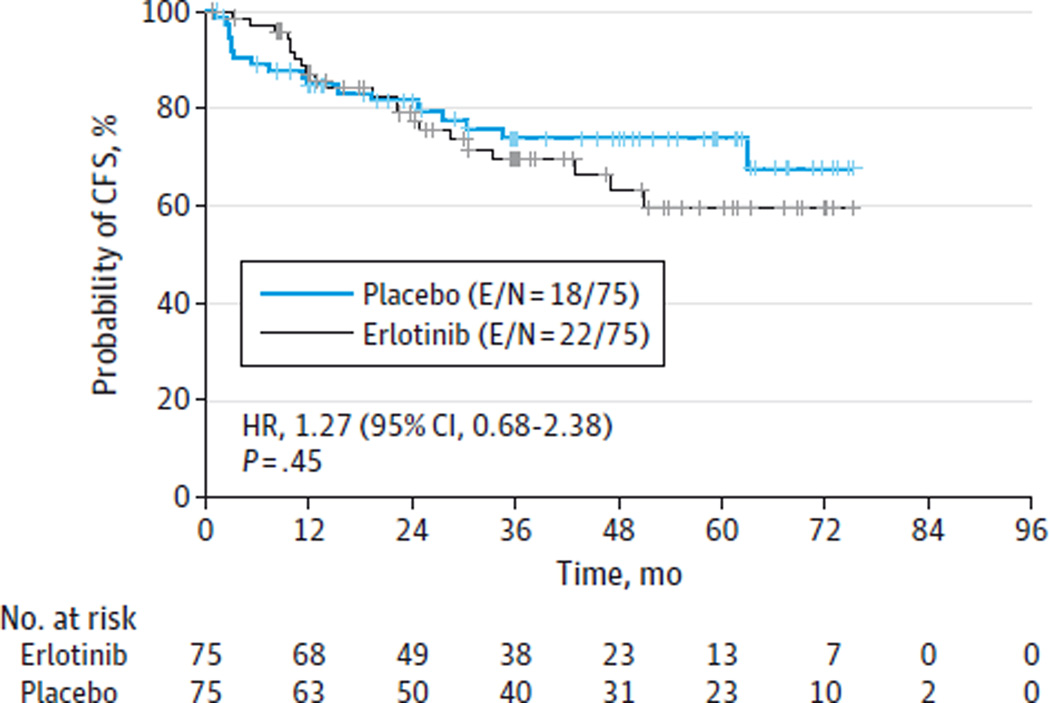
After a median follow-up time of 35 months for the surviving participants, 40 patients developed oral cancer, yielding a cumulative incidence of oral cancer of 27% in the randomized population. There were no statistically significant differences in oral CFS between the placebo and erlotinib groups (P = .45, stratified log-rank test, Figure 2). There was a trend toward improved oral CFS during the initial 12-month treatment period for the erlotinib group, but this effect was not statistically significant or sustained. The 3-year oral CFS rates were 74% and 70% for the placebo- and erlotinib-treated patients, respectively (HR, 1.27; 95% CI, 0.68–2.38; P = .45, stratified Cox proportional hazards model). There were 10 deaths in the randomized population (6 in the erlotinib group [4 from oral cancer, 1 from lung cancer, 1 from brain cancer], 4 in the placebo group [3 from oral cancer, 1 unknown]).
Figure 2. Oral Cancer–Free Survival (CFS) in Study Participants With Loss of Heterozygosity in the Erlotinib vs Placebo Study Arms.
E/N indicates number of participants who experienced a cancer or death event/total number of participants; HR, hazard ratio.
Toxic Effects and Treatment Characteristics
eTable 2 in Supplement 2 describes the grade 2 and 3 toxic effects that occurred in 10% or more of the participants. As expected, erlotinib-treated patients experienced more dermatologic toxic effects, diarrhea, fatigue, and mucositis than did those in the placebo group. There were only 2 other grade 3 toxic effects (cellulitis and dehydration) and 1 grade 4 toxic effect (diarrhea), occurring in 1 erlotinib-treated patient each. There were no treatment-related deaths. Dose reductions were required in 45% of patients randomized to erlotinib and 1% of those receiving placebo (eTable 3 in Supplement 2). The main reason for dose reductions and/or treatment interruptions was adverse events. Dose intensity and compliance, as assessed by pill counts, are reported in eTable 3 in Supplement 2. Nonsteroidal anti-inflammatory drugs were used by 20 randomized patients during the 36-month trial period.
LOH as a Marker of Oral Cancer Risk
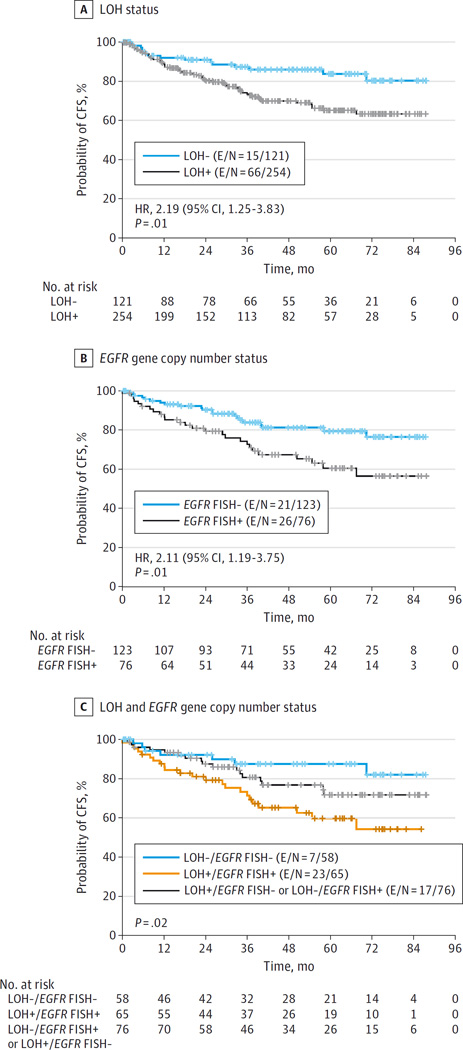
There was a statistically significant difference in 3-year oral CFS rate between the LOH-positive group (74%) and the LOH-negative group (87%) (HR, 2.19; 95% CI, 1.25–3.83; P = .01) (Figure 3A). On multivariate analysis with adjustments by number of prior oral cancers, histologic findings, and treatment, the difference in CFS between LOH-positive and LOH-negative groups remained significant (HR, 2.20; 95% CI, 1.16–4.19; P = .02) (eTable 4 in Supplement 2).
Figure 3. Oral Cancer–Free Survival (CFS) According to 3 Study Parameters.
E/N indicates number of participants who experienced a cancer or death event/total number of participants; FISH, fluorescence in situ hybridization; HR, hazard ratio; LOH, loss of heterozygosity.
Exploratory Analyses
There was a statistically significant association between EGFR gene copy number and LOH-positive status (P < .001) (eTable 5 in Supplement 2), providing further rationale for EGFR targeting in this patient population. Increased EGFR gene copy number was associated with inferior oral CFS (HR, 2.11; 95% CI, 1.19–3.75; P = .01) (Figure 3B). The combination of LOH-positive status and increased EGFR gene copy number conferred the highest cancer risk (P = .02) (Figure 3C).
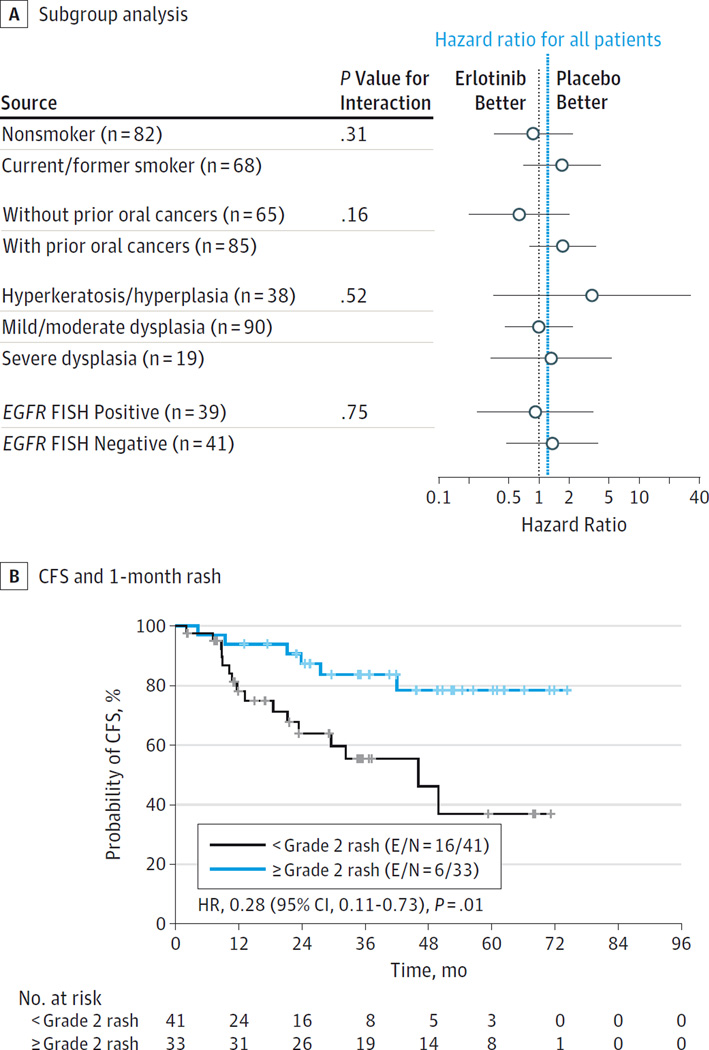
Figure 4A depicts erlotinib effects on oral CFS in subgroups of interest. No clear benefit from erlotinib was identified in any subgroup, including increased EGFR gene copy number, with P > .05 for all interaction tests.
Figure 4. Oral Cancer–Free Survival (CFS) in Erlotinib-Treated Participants.
A, Effects of erlotinib on subgroups of interest. B, Landmark analysis of the association between CFS and 1-month rash (n = 74; 1 patient randomized to the erlotinib arm withdrew study consent before the 1-month landmark). E/N indicates number of participants who experienced a cancer or death event/total number of participants; FISH, fluorescence in situ hybridization; HR, hazard ratio; LOH, loss of heterozygosity.
An exploratory landmark analysis found a significant association between grade 2 or higher rash at 1 month and improved oral CFS in the erlotinib arm (84% vs 55% 3-year CFS; P = .01), (Figure 4B). No associations between oral CFS and dose intensity were identified (eFigure in Supplement 2).
Discussion
To our knowledge, the EPOC trial represents the first molecularly based precision medicine trial design in cancer prevention, and the first study of OPLs to use cancer as the primary end point. This trial established that targeting EGFR with erlotinib in patients with high-risk OPLs did not improve oral CFS. The EPOC trial established LOH as the most robust OPL prognostic molecular marker prospectively validated to date. We observed a novel association between LOH-positive status and increased EGFR gene copy number and identified EGFR gene copy number as a marker of oral cancer risk. However, EGFR gene copy number (the target of erlotinib) was not a predictive marker of drug efficacy in this setting.
We chose the EGFR inhibitor erlotinib as the agent for this trial because EGFR plays a critical role in oral carcinogenesis and is the only clinically validated molecular target in head and neck cancer therapy. Gain in EGFR gene copy number in OPLs was correlated with an increased risk of oral cancer development in a retrospective analysis.23 In mouse models of OPLs, treatment with erlotinib reduced development of premalignant and malignant lesions.25 Early-phase clinical trials suggest that targeting EGFR26,27 or EGFR downstream signaling (eg, cyclin D1)8 may be effective in treating OPLs. However, the EPOC trial did not demonstrate an oral CFS benefit (even in lesions with increased EGFR gene copy number) from erlotinib in LOH-positive participants despite a trend toward delayed cancer development during the 12-month erlotinib intervention.
The EPOC trial found that the erlotinib-induced rash was a strong positive predictor of improved CFS, which is consistent with data from cancer therapy trials.24,28,29 Rash is considered a pharmacodynamic marker of EGFR-inhibitor drug exposure.24,30 The high rate of dose reductions owing to occurrence of toxic effects, however, suggests that increased doses of erlotinib may not be tolerated in this setting. Alternatively, EGFR-inhibitor rash may reflect host-derived prognostic factors regardless of drug level, as suggested by recent studies of specific gene polymorphisms30 and innate immunity in animal models.31
We observed an association of the core selection criteria for EPOC (LOH profiles) with the target of the drug intervention (EGFR). Although EGFR gene copy number gain in this setting could be due to a nonspecific manifestation of genomic instability (marked by the phenomenon of LOH), there is also biologic plausibility for a mechanistic link to the specific LOH loci included in EPOC eligibility, such as 3p14 (FHIT gene), 9p21 (p16INK4a/CDKN2A gene), and 17p (TP53 gene). In head and neck cancer, cyclin D1 overexpression (a downstream target of EGFR) seems to precede and enhance permissiveness for genomic instability and gene amplification,32 which could potentially explain our findings of a correlation between LOH-positive (and genomically unstable) lesions and increased EGFR gene copy number. Based on recent findings33,34 and our present data, one can postulate that genotoxic or replicative stress (whether mediated or not by EGFR)may lead to cyclin D1 activation and genomic instability resulting in either the loss of chromosomal fragments, including key “caretaker” tumor suppressor genes (eg, TP53 and CDKN2A), or gain and loss of whole chromosomes (eg, chromosome 7 polysomy) and EGFR amplification.35 Increased EGFR copy number could, in turn, serve as a positive feedback loop, stimulating cell proliferation further, particularly within the context of p16INK4a loss (as reflected by LOH at 9p21),34,36 thus leading to growth advantage, selection, and malignant transformation. Indeed, non–human papillomavirus head and neck cancers are characterized by amplification of EGFR (7p12) and CYCLIN D1 (11q13) and deletion of FHIT (3p14) andCDKN2A (9p21).37 Likewise, a correlation of EGFR gene amplification andCDKN2A deletion has also been reported in glioblastomas.38
Regardless of mechanism, lesions with both LOH and EGFR gain were at highest cancer risk. The 2-hit (LOH and EGFR/chromosome 7 polysomy) genomic instability-driven model may explain the lack of erlotinib efficacy found in the EPOC trial. These high-risk OPLs may have already accumulated additional changes leading to the persistent activation of signaling pathways operating downstream or independently from EGFR. For example, LOH in 3p is often accompanied by copy number gains in 3q26,39 an amplicon that includes the PIK3CA gene encoding the catalytic subunit of PI3Kα. Amplification of PIK3CA may in turn activate PI3K/AKT/mTOR signaling, thus bypassing EGFR inhibition.40
Conclusions
In summary, despite the importance of EGFR biology to head and neck neoplasia and the strength of the preclinical and preliminary clinical data, erlotinib did not reduce the risk of oral cancer in patients with LOH-positive OPLs, including lesions with increased EGFR gene copy number. Nonetheless, the EPOC trial demonstrated prospectively the role of LOH as a marker of cancer risk in OPLs and supports its use as a prognostic tool in clinical practice. The novel approach in the EPOC trial of selecting a molecularly defined high-risk population most likely to benefit, allowing the first streamlined OPL trial designed with the clinically meaningful definitive end point of invasive cancer, is a new paradigm for future precision cancer chemoprevention trials.
Supplementary Material
At a Glance.
This randomized, placebo-controlled trial tested if erlotinib would reduce oral cancer risk in patients with high-risk oral premalignant lesions defined by specific loss of heterozygosity (LOH) profiles.
Erlotinib did not reduce oral cancer development.
The EGFR gene copy number (the target of the intervention) was prognostic (and additive with LOH) but was not predictive of erlotinib efficacy.
LOH was prospectively validated as a prognostic marker of cancer risk; the 3-year oral cancer-free survival rates were 74% and 87% for LOH-positive and LOH-negative groups, respectively (hazard ratio, 2.19; 95%CI, 1.25–3.83; P = .01).
Erlotinib-induced skin rash was associated with improved cancer-free survival (P = .01).
Acknowledgments
Funding/Support: This work was financially supported by OSI Pharmaceuticals, by National Cancer Institute grants P01 CA106451 (Dr Lippman), P50 CA097007 (Dr Lippman), and P30 CA016672, by Cancer Prevention and Research Institute of Texas grant RP140464 (Dr William), and by Conquer Cancer Foundation Career Development Award (Dr William). OSI Pharmaceuticals also provided drug supply.
Role of the Funder/Sponsor: The NCI and OSI Pharmaceuticals approved the design of the study, and OSI Pharmaceuticals reviewed the data presented in this manuscript. In all other aspects, the funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: The authors are indebted to the research nurses and data managers for the assistance they provided and to Waun Ki Hong, MD, and Eva Szabo, MD, for their thoughtful discussions and support and to Sunita Patterson for editorial assistance. None of these persons received compensation for their contributions except in the normal course of their employment.
Footnotes
Supplemental content at jamaoncology.com
Author Contributions: Drs William and Lippman had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: William, Papadimitrakopoulou, Lee, Mao, Boyle, Shin, Kim, Lippman.
Acquisition, analysis, or interpretation of data: William, Papadimitrakopoulou, Lee, Mao, Cohen, Lin, Gillenwater, Martin, Lingen, Shin, Vigneswaran, Shinn, Heymach, Wistuba, Tang, Kim, Saintigny, Blair, Meiller, Gutkind, Myers, El-Naggar, Lippman.
Drafting of the manuscript: William, Papadimitrakopoulou, Lee, Cohen, Lin, Shinn, Gutkind, Lippman.
Critical revision of the manuscript for important intellectual content: William, Papadimitrakopoulou, Lee, Mao, Cohen, Gillenwater, Martin, Lingen, Boyle, Shin, Vigneswaran, Heymach, Wistuba, Tang, Kim, Saintigny, Blair, Meiller, Myers, El-Naggar, Lippman.
Statistical analysis: William, Lee, Lin, Meiller.
Obtained funding: William, Papadimitrakopoulou, Lippman.
Administrative, technical, or material support: William, Mao, Cohen, Martin, Lingen, Boyle, Shin, Vigneswaran, Shinn, Heymach, Tang, Kim, Blair, Meiller, Myers, El-Naggar, Lippman.
Study supervision: William, Papadimitrakopoulou, Mao, Shin, Heymach, Wistuba, Lippman.
Conflict of Interest Disclosures: Dr Wistuba has served on the advisory board for Genentech/Roche, Ventana, GlaxoSmithKline, Celgene, Bristol-Myers Squibb, Synta, Clovis, and AstraZeneca and participated in educational activities for Boehringer Ingelheim, Pfizer, and Medscape. Dr Kim has served on the advisory board for Celgene and Eli Lilly.
No other disclosures are reported.
REFERENCES
- 1.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328(3):184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 2.Hong WK, Lippman SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323(12):795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 3.Hong WK, Endicott J, Itri LM, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315(24):1501–1505. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 4.Lippman SM, Batsakis JG, Toth BB, et al. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328(1):15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 5.Papadimitrakopoulou VA, Lee JJ, William WN, Jr, et al. Randomized trial of 13-cis retinoic acid compared with retinyl palmitate with or without beta-carotene in oral premalignancy. J Clin Oncol. 2009;27(4):599–604. doi: 10.1200/JCO.2008.17.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lippman SM, Lee JJ, Martin JW, et al. Fenretinide activity in retinoid-resistant oral leukoplakia. Clin Cancer Res. 2006;12(10):3109–3114. doi: 10.1158/1078-0432.CCR-05-2636. [DOI] [PubMed] [Google Scholar]
- 7.Lotan R, Xu XC, Lippman SM, et al. Suppression of retinoic acid receptor-beta in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med. 1995;332(21):1405–1410. doi: 10.1056/NEJM199505253322103. [DOI] [PubMed] [Google Scholar]
- 8.Izzo JG, Papadimitrakopoulou VA, Liu DD, et al. Cyclin D1 genotype, response to biochemoprevention, and progression rate to upper aerodigestive tract cancer. J Natl Cancer Inst. 2003;95(3):198–205. doi: 10.1093/jnci/95.3.198. [DOI] [PubMed] [Google Scholar]
- 9.Papadimitrakopoulou VA, William WN, Jr, Dannenberg AJ, et al. Pilot randomized phase II study of celecoxib in oral premalignant lesions. Clin Cancer Res. 2008;14(7):2095–2101. doi: 10.1158/1078-0432.CCR-07-4024. [DOI] [PubMed] [Google Scholar]
- 10.Rudin CM, Cohen EE, Papadimitrakopoulou VA, et al. An attenuated adenovirus, ONYX-015, as mouthwash therapy for premalignant oral dysplasia. J Clin Oncol. 2003;21(24):4546–4552. doi: 10.1200/JCO.2003.03.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong WB, Taylor TH, Kennedy AR, et al. Bowman Birk inhibitor concentrate and oral leukoplakia: a randomized phase IIb trial. Cancer Prev Res (Phila) 2013;6(5):410–418. doi: 10.1158/1940-6207.CAPR-13-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 13.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 14.Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22(1):77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 15.Cohen EE, Kane MA, List MA, et al. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11(23):8418–8424. doi: 10.1158/1078-0432.CCR-05-1247. [DOI] [PubMed] [Google Scholar]
- 16.Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected] J Clin Oncol. 2009;27(11):1864–1871. doi: 10.1200/JCO.2008.17.0530. [DOI] [PubMed] [Google Scholar]
- 17.Arduino PG, Surace A, Carbone M, et al. Outcome of oral dysplasia: a retrospective hospital-based study of 207 patients with a long follow-up. J Oral Pathol Med. 2009;38(6):540–544. doi: 10.1111/j.1600-0714.2009.00782.x. [DOI] [PubMed] [Google Scholar]
- 18.William WN, Jr, Heymach JV, Kim ES, Lippman SM. Molecular targets for cancer chemoprevention. Nat Rev Drug Discov. 2009;8(3):213–225. doi: 10.1038/nrd2663. [DOI] [PubMed] [Google Scholar]
- 19.Mao L, Lee JS, Fan YH, et al. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996;2(6):682–685. doi: 10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 20.Rosin MP, Cheng X, Poh C, et al. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin Cancer Res. 2000;6(2):357–362. [PubMed] [Google Scholar]
- 21.Rosin MP, Lam WL, Poh C, et al. 3p14 and 9p21 loss is a simple tool for predicting second oral malignancy at previously treated oral cancer sites. Cancer Res. 2002;62(22):6447–6450. [PubMed] [Google Scholar]
- 22.Abbruzzese JL, Lippman SM. The convergence of cancer prevention and therapy in early-phase clinical drug development. Cancer Cell. 2004;6(4):321–326. doi: 10.1016/j.ccr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Taoudi Benchekroun M, Saintigny P, Thomas SM, et al. Epidermal growth factor receptor expression and gene copy number in the risk of oral cancer. Cancer Prev Res (Phila) 2010;3(7):800–809. doi: 10.1158/1940-6207.CAPR-09-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peréz-Soler R, Saltz L. Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol. 2005;23(22):5235–5246. doi: 10.1200/JCO.2005.00.6916. [DOI] [PubMed] [Google Scholar]
- 25.Leeman-Neill RJ, Seethala RR, Singh SV, et al. Inhibition of EGFR-STAT3 signaling with erlotinib prevents carcinogenesis in a chemically-induced mouse model of oral squamous cell carcinoma. Cancer Prev Res (Phila) 2011;4(2):230–237. doi: 10.1158/1940-6207.CAPR-10-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin DM, Zhang H, Saba NF, et al. Chemoprevention of head and neck cancer by simultaneous blocking of epidermal growth factor receptor and cyclooxygenase-2 signaling pathways: preclinical and clinical studies. Clin Cancer Res. 2013;19(5):1244–1256. doi: 10.1158/1078-0432.CCR-12-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Califano JA, Ferris RL, Epstein JB, et al. A phase II trial of cetuximab in high-risk premalignant lesions of the upper aerodigestive tract. J Clin Oncol. 2012;30(suppl) abstract 5528. [Google Scholar]
- 28.Cohen EE, Halpern AB, Kasza K, Kocherginsky M, Williams R, Vokes EE. Factors associated with clinical benefit from epidermal growth factor receptor inhibitors in recurrent and metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2009;45(10):e155–e160. doi: 10.1016/j.oraloncology.2009.05.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bar-Ad V, Zhang Q, Harari PM, et al. The correlation between the severity of cetuximab-induced rash and clinical outcome for patients with head and neck carcinoma treated with chemoradiotherapy (CRT) plus cetuximab: the RTOG experience. J Clin Oncol. 2014;32 abstract 6025. [Google Scholar]
- 30.Liu S, Kurzrock R. Toxicity of targeted therapy: Implications for response and impact of genetic polymorphisms. Cancer Treat Rev. 2014;40(7):883–891. doi: 10.1016/j.ctrv.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Lacouture ME, Rodeck U. Skinflammation and drug toxicity--a delicate balance. Sci Transl Med. 2013;5(199):199fs33. doi: 10.1126/scitranslmed.3006993. [DOI] [PubMed] [Google Scholar]
- 32.Izzo JG, Papadimitrakopoulou VA, Li XQ, et al. Dysregulated cyclin D1 expression early in head and neck tumorigenesis: in vivo evidence for an association with subsequent gene amplification. Oncogene. 1998;17(18):2313–2322. doi: 10.1038/sj.onc.1202153. [DOI] [PubMed] [Google Scholar]
- 33.Burrell RA, McClelland SE, Endesfelder D, et al. Replication stress links structural and numerical cancer chromosomal instability. Nature. 2013;494(7438):492–496. doi: 10.1038/nature11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability: an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11(3):220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 35.Vogt N, Lefèvre SH, Apiou F, et al. Molecular structure of double-minute chromosomes bearing amplified copies of the epidermal growth factor receptor gene in gliomas. Proc Natl Acad Sci U S A. 2004;101(31):11368–11373. doi: 10.1073/pnas.0402979101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott KM, Zhang J, Holst CR, Kozakiewicz BK, Singla V, Tlsty TD. p16(INK4a) prevents centrosome dysfunction and genomic instability in primary cells. PLoS Biol. 2006;4(3):e51. doi: 10.1371/journal.pbio.0040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keck MK, Zuo Z, Khattri A, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21(4):870–881. doi: 10.1158/1078-0432.CCR-14-2481. [DOI] [PubMed] [Google Scholar]
- 38.Brennan CW, Verhaak RG, McKenna A, et al. TCGA Research Network. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gollin SM. Cytogenetic alterations and their molecular genetic correlates in head and neck squamous cell carcinoma: a next generation window to the biology of disease. Genes Chromosomes Cancer. 2014;53(12):972–990. doi: 10.1002/gcc.22214. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Martin D, Molinolo AA, et al. mTOR co-targeting in cetuximab resistance in head and neck cancers harboring PIK3CA and RAS mutations. J Natl Cancer Inst. 2014;106(9) doi: 10.1093/jnci/dju215. dju215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.