Abstract
Context
The frequency of remission of type 2 diabetes achievable with lifestyle intervention is unclear.
Objective
To examine the association of a long-term intensive weight-loss intervention with the frequency of remission from type 2 diabetes to prediabetes or normoglycemia.
Design, Setting, and Participants
Ancillary observational analysis of a 4-year randomized controlled trial (baseline visit, August 2001–April 2004; last follow-up, April 2008) comparing an intensive lifestyle intervention (ILI) with a diabetes support and education control condition (DSE) among 4503 US adults with body mass index of 25 or higher and type 2 diabetes.
Interventions
Participants were randomly assigned to receive the ILI, which included weekly group and individual counseling in the first 6 months followed by 3 sessions per month for the second 6 months and twice-monthly contact and regular refresher group series and campaigns in years 2 to 4 (n=2241) or the DSE, which was an offer of 3 group sessions per year on diet, physical activity, and social support (n=2262).
Main Outcome Measures
Partial or complete remission of diabetes, defined as transition from meeting diabetes criteria to a prediabetes or nondiabetic level of glycemia (fasting plasma glucose <126 mg/dL and hemoglobin A1c <6.5% with no antihyperglycemic medication).
Results
Intensive lifestyle intervention participants lost significantly more weight than DSE participants at year 1 (net difference, −7.9%; 95% CI, −8.3% to −7.6%) and at year 4 (−3.9%; 95% CI, −4.4% to −3.5%) and had greater fitness increases at year 1 (net difference, 15.4%; 95% CI, 13.7%–17.0%) and at year 4 (6.4%; 95% CI, 4.7%–8.1%) (P<.001 for each). The ILI group was significantly more likely to experience any remission (partial or complete), with prevalences of 11.5% (95% CI, 10.1%–12.8%) during the first year and 7.3% (95% CI, 6.2%–8.4%) at year 4, compared with 2.0% for the DSE group at both time points (95% CIs, 1.4%–2.6% at year 1 and 1.5%–2.7% at year 4) (P<.001 for each). Among ILI participants, 9.2% (95% CI, 7.9%–10.4%), 6.4% (95% CI, 5.3%–7.4%), and 3.5% (95% CI, 2.7%–4.3%) had continuous, sustained remission for at least 2, at least 3, and 4 years, respectively, compared with less than 2% of DSE participants (1.7% [95% CI, 1.2%–2.3%] for at least 2 years; 1.3% [95% CI, 0.8%–1.7%] for at least 3 years; and 0.5% [95% CI, 0.2%–0.8%] for 4 years).
Conclusions
In these exploratory analyses of overweight adults, an intensive lifestyle intervention was associated with a greater likelihood of partial remission of type 2 diabetes compared with diabetes support and education. However, the absolute remission rates were modest.
Trial Registration
clinicaltrials.gov Identifier: NCT00017953
DIABETES TRADITIONALLY HAS been considered a progressive, incurable condition wherein the best case scenario after diagnosis is tight metabolic and risk factor management to forestall vascular and neuropathic complications.1 This notion that type 2 diabetes is irreversible is supported by the strong association with genetics and family history, the high prevalence of microvascular complications, and the loss of beta cell mass and function frequently already present at diagnosis.2,3 Despite these observations, 16% of US adults who report a previous diabetes diagnosis take no hypoglycemic medications, and studies of bariatric surgery suggest that many diabetes cases among obese patients can indeed resolve.4–8 Patients diagnosed as having type 2 diabetes frequently ask their physicians whether their condition is reversible, and some physicians may provide hopeful advice that lifestyle change can normalize glucose levels. Ambiguity around the concept of reversibility led to a 2009 American Diabetes Association consensus statement that defined partial remission as hyperglycemia below the diagnostic level for diabetes in the absence of antihyperglycemic medications and defined complete remission as a return to normal glucose levels in the absence of antihyperglycemic therapy.9 However, the rate of remission of type 2 diabetes that may be achieved using nonsurgical approaches has not been reported.
The Look AHEAD (Action for Health for Diabetes) study is perhaps the largest randomized controlled trial of an intensive lifestyle intervention among adults with type 2 diabetes to date.10 Although designed principally to examine the effect of weight loss on cardiovascular disease incidence, it is also a unique opportunity to examine the effect on control and progression of diabetes mellitus. We conducted an ancillary analysis of the Look AHEAD cohort to determine the association of an intensive lifestyle intervention with frequency of partial and complete remission of type 2 diabetes.
METHODS
Study Design, Sample, and Inclusion Criteria
Look AHEAD recruited 5145 overweight adults aged 45 to 76 years with type 2 diabetes at 16 US research centers and randomized them to either an intensive lifestyle-based weight loss intervention (ILI) or a diabetes support and education intervention (DSE).10–12 Yearly clinic visits were conducted over 4 years to assess health status, including glycemic status. Baseline eligibility criteria required a body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 25 or higher (≥27 among those receiving insulin). Look AHEAD excluded participants with particularly high hemoglobin A1c (HbA1c) levels (>11%), blood pressure (>160 mm Hg systolic or >100 mm Hg diastolic), or plasma triglyceride levels (>600 mg/dL). Participants were also excluded if they were unable to perform a maximal graded exercise test or complete 2 weeks of diet and activity self-monitoring. All participants signed a consent form approved by their local institutional review board.
Intervention
Details of the intervention have been described previously.10–12 In short, the ILI included weekly group and individual counseling in the first 6 months, followed by 3 sessions per month for the second 6 months and twice-monthly contact and regular refresher group series and campaigns in years 2 to 4. The ILI aimed to reduce total caloric intake to 1200 to 1800 kcal/d through reductions in total and saturated fat intake and by increasing physical activity levels to a goal of 175 min/wk. Liquid meal replacements were provided to assist dietary goals. Participants in the DSE were offered 3 group sessions each year focusing on diet, physical activity, and social support. Medical care was provided by the participant’s physician independent of the Look AHEAD study. During periods of weight loss, ILI participants who were taking insulin, sulfonylureas, repaglinide, or nateglinide were asked to provide blood glucose measurement records so that Look AHEAD medical staff could determine if reductions in diabetes medications were needed to reduce their risk of hypoglycemia.
Assessments
Self-reported behavioral and demographic risk factors along with measured height, weight, and fasting plasma glucose and HbA1c were assessed at a baseline clinic visit (August 2001–April 2004) and yearly thereafter, with the fourth visit occurring between August 2005 and April 2008. Prescription medications were recorded by trained study technicians at study visits. A maximal graded exercise test was administered at baseline and a sub-maximal exercise test at years 1 and 4. Changes in fitness were computed as the difference between estimated metabolic equivalents at the point that the participants achieved 80% of age-predicted maximal heart rate or a rating of perceived exertion of at least 16 (corresponding to a rating between “hard” and “very hard”) at baseline and at the subsequent assessment.
For these analyses, diabetes was defined as taking diabetes medications or having a fasting plasma glucose level of at least 126 mg/dL or HbA1c of at least 6.5%. (To convert fasting plasma glucose to mmol/L, multiply by 0.0555.) Partial remission of diabetes was defined as a transition from meeting diabetes criteria to a prediabetes level of glycemia (ie, fasting plasma glucose level of 100–126 mg/dL and HbA1c of 5.7%–6.5%) with no antihyperglycemic medication. Complete remission was defined as transition from diabetes criteria to full normalization of glucose (fasting plasma glucose level <100 mg/dL and HbA1c <5.7%) with no antihyperglycemic medication.
Statistical Analyses
Descriptive statistics compared the characteristics of the ILI and DSE groups. Primary analyses compared the yearly prevalence of any remission (partial or complete remission) between participants in the ILI and DSE groups and estimated the prevalence of continuous, sustained remission for at least 2, at least 3, or 4 years. We constructed first-order binary Markov transition models to estimate the yearly transition probabilities in and out of diabetes status. We used multiple imputation to account for missing data elements at year 1 (n=176 [3.9%]), year 2 (n=312 [6.9%]), year 3 (n=335 [7.4%]), and year 4 (n=405 [9.1%]). Imputation of the binary outcome of any remission was conducted using a propensity score approach that accounted for the longitudinal nature of the data.13 Imputations were performed for the DSE and ILI groups separately. The results from 100 imputed data sets were combined to obtain prevalence estimates for yearly remission as well as continuous sustained remission that accounted for both between-and within-imputation variability.
As a post hoc analysis based on the available cases (ie, without imputation), we examined the multivariate association of several predetermined demographic factors (age, sex, race/ethnicity, education), health status factors (history of cardiovascular disease, antihypertensive use, insulin use), baseline risk factors (BMI, HbA1c, fitness), and 2 intervention response variables (1-year weight change, fitness change) with any remission. Since descriptive analyses indicated that most cases of remission occurred between baseline and the year 1 visit and few occurred thereafter, we estimated the probabilities and odds ratios separately for cases of remission occurring in the first year and for those occurring between the first and fourth years. We also tested for interactions with intervention group and present the absolute probability of remission according to these variables for the full sample and by intervention group. All analyses were performed using SAS software, version 9.3 (SAS Institute Inc). A 2-sided P<.05 was considered statistically significant. Since diabetes remission was not a prespecified outcome of Look AHEAD, these analyses were exploratory in nature and no adjustment for multiple testing was applied.
RESULTS
From the original sample of 5145 adults randomized to Look AHEAD, we excluded 355 individuals (6.9%; n=194 ILI and n=161 DSE) who had fasting glucose levels of less than 126 mg/dL and HbA1c levels of less than 6.5% while taking no diabetes medications because they met our definition for non-diabetes at baseline (FIGURE 1). We also excluded 287 participants (5.6%; n=135 ILI and n=152 DSE) who were missing relevant outcome data at all follow-up visits or who underwent bariatric surgery, leaving an analytic sample of 4503 adults (n=2241 in the ILI group and n = 2262 in the DSE group) (Figure 1). Participants excluded because of this combination of factors tended to be younger and more obese and had lower fasting plasma glucose and HbA1c levels, yet were less likely to be taking diabetes or hypertension medications than the primary analytic sample (eTable 1; http://www.jama.com). The Look AHEAD study sample was predominantly middle-aged or older (mean age, 59 years) and of diverse race/ethnicity, education level, and medication status (TABLE 1). Median time since diagnosis was 5 years and the study sample was notably obese at baseline, with a mean BMI of 35.8.
Figure 1.
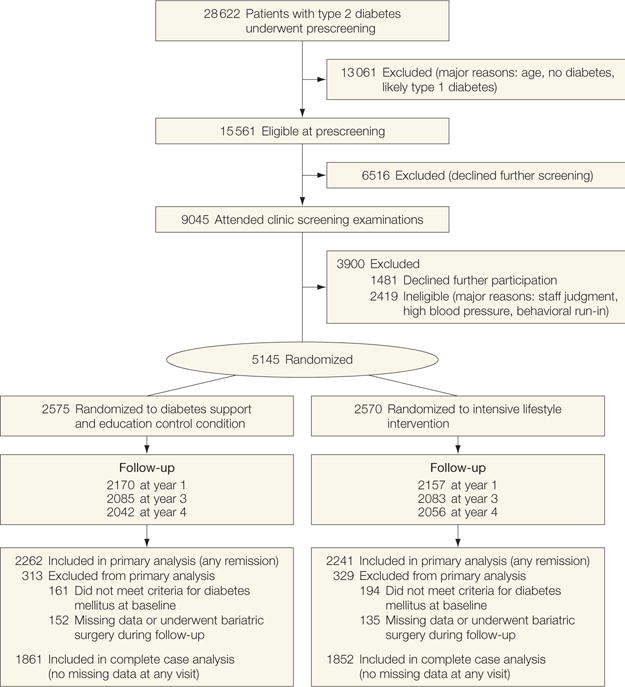
Participant Flow
Table 1.
Baseline Characteristics of the Study Sample According to Intervention Status
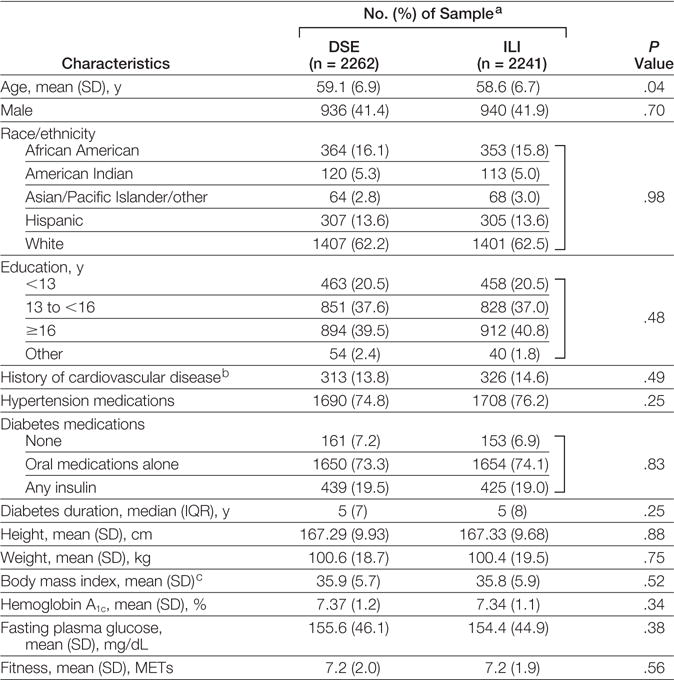
|
Abbreviations: DSE, diabetes support and education; ILI, intensive lifestyle intervention; IQR, interquartile range; MET, metabolic equivalent task.
SI abbreviation: To convert fasting plasma glucose to mmol/L, multiply by 0.0555.
Data are expressed as No. (%) of sample unless otherwise indicated.
Cardiovascular disease history was defined as self-reported history of stroke or myocardial infarction.
Calculated as weight in kilograms divided by height in meters squared.
Participants in the ILI group lost significantly more weight than DSE participants at year 1 (−8.6% [95% CI, −8.9% to −8.4%] vs −0.7% [95% CI, −0.9% to −0.4%]) and at year 4 (−4.7% [95% CI, −5.0% to 4.4%] vs −0.8% [95% CI, −1.1% to −0.5%]) (P<.001 for each) and had greater increases in fitness at both year 1 (20.6% [95% CI, 19.5%–21.8%] vs 5.3% [95% CI, 4.1%–6.4%]) and year 4 (4.9% [95% CI, 3.7%–6.1%] vs 1.5% [95% CI, −2.8% to −0.3%]) (P<.001 for each). The prevalence of complete remission (ie, glucose normalization without medication) was more common in the ILI group than in the DSE group across all years of the study (prevalence ratio, 6.6; 95% CI, 3.3–13.3; P<.001). However, the absolute prevalence was low, ranging from 1.3% (95% CI, 0.9%–1.8%) for ILI vs 0.1% (95% CI, 0%–0.2%) for DSE (P<.001) in year 1 to 0.7% (95% CI, 0.3%–1.0%) for ILI vs 0.2% (95% CI, 0%–0.4%) for DSE in year 4.
In analyses based on multiple imputation, ILI participants were significantly more likely to experience any remission (partial or complete), with a prevalence of 11.5% (95% CI, 10.1%–12.8%) during the first year, decreasing to 7.3% (95% CI, 6.2%–8.4%) during year 4, compared with 2.0% (95% CIs, 1.4%–2.6% at year 1 and 1.5%–2.7% at year 4) in the DSE group at both time points (FIGURE 2) (P<.001 for each year). Accordingly, ratios of the prevalence of remission for ILI vs DSE ranged from 5.8 (95% CI, 4.2–7.9) in year 1 to 3.4 (95% CI, 2.5–4.8) in year 4. The results from the complete case analyses were similar (Figure 2).
Figure 2.
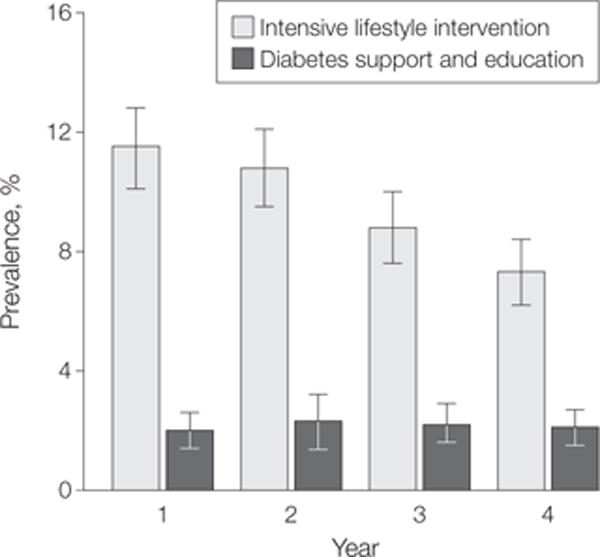
Prevalence of Any Remission (Partial or Complete) by Intervention Condition and Year
Data are prevalence and 95% CIs for any remission (partial or complete). Estimates are based on sample with multiple imputation (n=4503). In complete case analysis (year 1: n = 4327; year 2: n=4191; year 3: n=4168; year 4: n=4098), prevalence estimates with raw cases/denominators were as follows: for intensive lifestyle intervention, year 1: 11.5% (95% CI, 10.1%–12.8%) (247/2157); year 2: 10.4% (95% CI, 9.1%–11.7%) (218/2090); year 3: 8.7% (95% CI, 7.5%–9.9%) (181/2083); and year 4: 7.3% (95% CI, 6.2%–9.4%) (150/2056); for diabetes support and education, year 1: 2.0% (95% CI, 1.4%–2.6%) (43/2170); year 2: 2.3% (95% CI, 1.6%–2.9%) (48/2101); year 3: 2.2% (95% CI, 1.6%–2.8%) (46/2085); and year 4: 2.0% (95% CI, 1.5%–2.7%) (41/2042).
Among those who had a remission, about one-third in the ILI group returned to a clinical diabetes status each year (33.1% [95% CI, 27.4%–39.3%] in year 2; 33.8% [95% CI, 27.9%–40.2%] in year 3; and 31.6% [95% CI, 25.3%–38.6%] in year 4) and close to half of those in the DSE group returned to a clinical diabetes status (52.4% [95% CI, 42.2%–62.3%] in year 2; 45.9% [95% CI, 35.6%–56.6%] in year 3; and 43.8% [95% CI, 32.9%–55.4%] in year 4).
The ILI group was significantly more likely to have continuous, sustained remission (FIGURE 3), as 9.2% (95% CI, 7.9%–10.4%) experienced at least a 2-year remission (vs for DSE, 1.7%; 95% CI, 1.2%–2.3%; P<.001) at some point during follow-up, 6.4% (95% CI, 5.7%–7.4%) had at least a 3-year remission (vs DSE, 1.3%; 95% CI, 0.8%–1.7%), and 3.5% (95% CI, 2.7%–4.3%) had a continuous 4-year remission (vs DSE, 0.5%; 95% CI, 0.2%–0.8%; P = .02) (Figure 3). The results from the complete case analyses were similar (Figure 3).
Figure 3.
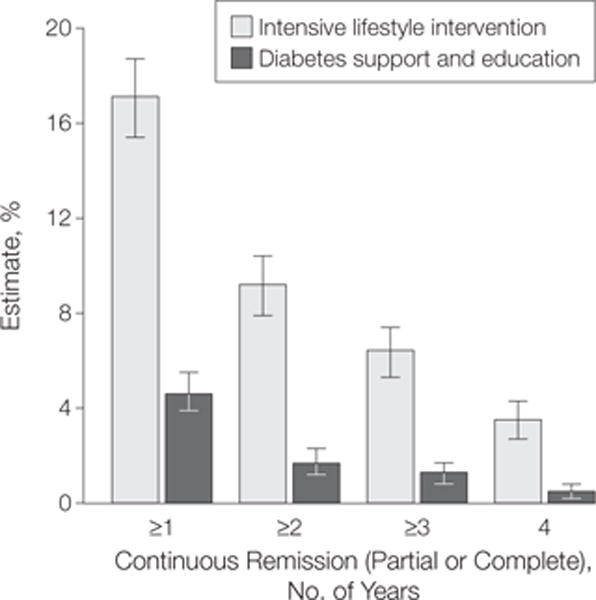
Duration of Any Remission (Partial or Complete) by Intervention Group and Duration of Sustained Remission
Data are estimates and 95% CIs based on sample with multiple imputation (n=4503). Estimates from complete case analysis of persons with no missing data element at any single year (n=3713) were as follows: for intensive lifestyle intervention, year 1: 14.6% (95% CI, 13.0%–16.2%) (271/1852); year 2: 8.2% (95% CI, 6.8%–9.2%) (148/1852); year 3: 5.8% (95% CI, 4.7%–6.8%) (107/1852); and year 4: 3.4% (95% CI, 2.6%–4.2%) (63/1852); for diabetes support and education, year 1: 4.3% (95% CI, 3.4%–5.2%) (80/1861); year 2: 1.6% (95% CI, 1.0%–2.1%) (29/1861); year 3: 1.2% (95% CI, 0.7%–1.7%) (22/1861); and year 4: 0.4% (95% CI, 0.1%–0.7%) (8/1861).
In both univariate and multivariable analyses (based on available case analyses), any remission during the first year was significantly associated with fewer years since diabetes diagnosis, low BMI, low baseline HbA1c, not taking insulin, and greater 1-year weight loss (P<.001) (TABLE 2). A strong fitness improvement was also significantly associated with 1-year remission in univariate analyses (P<.001), but this association was attenuated in multivariate analyses controlling for weight change and all the remaining factors. There were no significant interactions between any of these demographic and health status predictors and intervention condition on their effect on any remission, indicating that the association of the ILI with remission was generally consistent across different strata. The highest probabilities of 1-year remission occurred among intervention participants with less than a 2-year history of diabetes (21.2%; 95% CI, 18.0%–24.7%), more than 6.5% weight loss (16.4%; 95% CI, 14.5%–18.6%) or fitness improvements (15.6%; 95% CI, 13.3%–18.1%), low initial HbA1c values (17.1%; 95% CI, 14.4%–20.3%), and not taking antihypertensive medications (15.2%; 95% CI, 12.3%–18.6%).
Table 2.
Odds Ratios and Probability of Any Remission Associated With Key Demographic and Health Status Predictors for the Overall Sample and by Intervention Groupa
| No. (%) of Sample | Year 1 Remission in Overall Sample, Odds Ratio (95% CI)
|
Unadjusted Probability of Year 1 Remission, % (95% CI)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | P Value | Multivariate | P Value | Overall | DSE | ILI | ||
| Men | 1876 (41.7) | 1 [Reference] | 1 [Reference] | 7.1 (6.0–8.4) | 1.3 (0.8–2.3) | 12.9 (10.8–15.2) | ||
|
| ||||||||
| Women | 2627 (58.3) | 0.90 (0.71–1.15) | .39 | 0.96 (0.71–1.28) | .75 | 6.4 (5.5–7.5) | 2.5 (1.7–3.5) | 10.4 (8.9–12.3) |
|
| ||||||||
| Age tertile, y | ||||||||
| Low: 44 to <56 | 1383 (30.7) | 1 [Reference] | 1 [Reference] | 7.5 (6.2–9.0) | 2.0 (1.2–3.5) | 12.7 (10.4–15.4) | ||
|
| ||||||||
| Middle: 56 to <61 | 1596 (35.4) | 0.85 (0.64–1.13) | .38 | 0.94 (0.67–1.32) | .73 | 6.5 (5.3–7.8) | 1.6 (0.9–2.8) | 11.1 (9.1–13.5) |
|
| ||||||||
| High: ≥61 to 76 | 1524 (33.8) | 0.82 (0.61–1.10) | .38 | 1.03 (0.72–1.48) | .86 | 6.3 (5.1–7.6) | 2.3 (1.5–3.7) | 10.6 (8.5–13.1) |
|
| ||||||||
| Race/ethnicity | ||||||||
| White | 2808 (62.3) | 1 [Reference] | 1 [Reference] | 7.6 (6.6–8.6) | 2.5 (1.8–3.5) | 12.7 (11.0–14.5) | ||
|
| ||||||||
| African American | 717 (15.9) | 0.75 (0.53–1.07) | .12 | 0.97 (0.65–1.46) | .89 | 5.8 (4.3–7.9) | 1.5 (0.6–3.5) | 10.2 (7.4–13.9) |
|
| ||||||||
| American Indian | 233 (5.2) | 0.51 (0.26–1.00) | .05 | 0.83 (0.36–1.91) | .66 | 4.0 (2.1–7.5) | 1.7 (0.4–6.4) | 6.6 (3.2–13.2) |
|
| ||||||||
| Asian/Pacific Islander | 132 (2.9) | 0.92 (0.46–1.81) | .81 | 1.17 (0.50–2.75) | .71 | 7.0 (3.7–12.9) | 3.2 (0.8–12.0) | 10.5 (5.1–20.3) |
|
| ||||||||
| Hispanic | 612 (13.6) | 0.61 (0.40–0.91) | .40 | 0.70 (0.42–1.14) | .15 | 4.7 (3.3–6.8) | NA | 9.4 (6.5–13.3) |
|
| ||||||||
| Diabetes duration tertile, y | ||||||||
| Low: 0 to <2 | 1168 (26.1) | 1 [Reference] | 1 [Reference] | 13.5 (11.6–15.6) | 5.1 (3.5–7.3) | 21.2 (18.0–24.7) | ||
|
| ||||||||
| Middle: 2 to <7 | 1707 (38.2) | 0.42 (0.32–0.54) | <.001 | 0.43 (0.32–0.58) | <.001 | 6.1 (5.0–7.4) | 1.4 (0.8–2.5) | 11.1 (9.1–13.4) |
|
| ||||||||
| High: ≥7 | 1597 (35.7) | 0.15 (0.10–0.22) | <.001 | 0.21 (0.14–0.33) | <.001 | 2.2 (1.6–3.1) | 0.5 (0.2–1.4) | 4.1 (2.9–5.7) |
|
| ||||||||
| Body mass index tertile | ||||||||
| Low: <32.5 | 1501 (33.3) | 1 [Reference] | 1 [Reference] | 7.0 (6.3–8.7) | 2.0 (1.2–3.3) | 12.4 (10.2–15.0) | ||
|
| ||||||||
| Middle: 32.5–37.7 | 1501 (33.3) | 1.02 (0.77–1.35) | .89 | 0.92 (0.66–1.28) | .62 | 7.4 (6.2–8.9) | 1.8 (1.0–3.0) | 13.2 (10.9–15.8) |
|
| ||||||||
| High: >37.7 | 1501 (33.3) | 0.73 (0.54–0.99) | .04 | 0.75 (0.53–1.07) | .11 | 5.4 (4.3–6.7) | 2.2 (1.4–3.6) | 8.8 (6.9–11.1) |
|
| ||||||||
| Hemoglobin A1c tertile, % | ||||||||
| Low: <6.7 | 1300 (28.9) | 1 [Reference] | 1 [Reference] | 10.4 (8.9–12.3) | 3.6 (2.4–5.4) | 17.1 (14.4–20.3) | ||
|
| ||||||||
| Middle: 6.7–7.6 | 1634 (38.2) | 0.70 (0.54–0.90) | <.001 | 0.76 (0.56–1.03) | .07 | 7.5 (6.3–9.0) | 2.0 (1.3–3.3) | 13.0 (10.8–15.5) |
|
| ||||||||
| High: >7.6 | 1569 (35.7) | 0.24 (0.17–0.34) | <.001 | 0.40 (0.27–0.60) | <.001 | 2.7 (2.0–3.6) | 0.7 (0.3–1.5) | 4.9 (3.6–6.7) |
|
| ||||||||
| No insulin use | 2251 (80.7) | 1 [Reference] | 1 [Reference] | 8.1 (7.2–9.0) | 2.3 (1.7–3.1) | 13.8 (12.3–15.6) | ||
|
| ||||||||
| Insulin use | 864 (19.3) | 0.10 (0.05–0.21) | <.001 | 0.23 (0.11–0.51) | <.001 | 0.8 (0.4–1.8) | 0.5 (0.1–1.9) | 1.2 (0.5–2.9) |
|
| ||||||||
| No hypertension medications | 571 (25.25) | 1 [Reference] | 1 [Reference] | 8.3 (6.8–10.1) | 2.0 (1.1–3.6) | 15.2 (12.3–18.6) | ||
|
| ||||||||
| Hypertension medications | 3398 (75.5) | 0.73 (0.56–0.94) | .02 | 0.77 (0.57–1.06) | .11 | 6.2 (5.4–7.1) | 2.0 (1.4–2.8) | 10.3 (8.9–11.9) |
|
| ||||||||
| No cardiovascular disease history | 1949 (86.2) | 1 [Reference] | 1 [Reference] | 7.1 (6.3–8.0) | 2.2 (1.6–3.0) | 12.1 (10.7–13.6) | ||
|
| ||||||||
| Cardiovascular disease history | 639 (14.2) | 0.60 (0.40–0.90) | .01 | 0.91 (0.57–1.46) | .69 | 4.4 (3.0–6.3) | 0.7 (0.2–2.6) | 7.9 (5.4–11.4) |
|
| ||||||||
| 1-y Weight loss tertile, % | ||||||||
| Low: <1 (including weight gain) | 1464 (33.3) | 0.08 (0.05–0.12) | <.001 | 0.15 (0.08–0.27) | <.001 | 1.4 (0.9–2.1) | 1.1 (0.7–1.9) | 2.7 (1.2–5.9) |
|
| ||||||||
| Middle: 1 to 6.5 | 1465 (33.4) | 0.21 (0.16–0.29) | <.001 | 0.37 (0.26–0.53) | <.001 | 3.7 (2.8–4.8) | 2.1 (1.3–3.4) | 5.4 (3.9–7.3) |
|
| ||||||||
| High: >6.5 | 1464 (33.3) | 1 [Reference] | 1 [Reference] | 15.2 (13.4–17.2) | 7.1 (4.9–11.1) | 16.4 (14.5–18.6) | ||
|
| ||||||||
| 1-y Fitness change tertile, % | ||||||||
| Low: <−2.3 | 925 (24.4) | 1 [Reference] | 1 [Reference] | 3.1 (2.1–4.4) | 1.1 (0.5–2.4) | 7.1 (4.7–10.6) | ||
|
| ||||||||
| Middle: 2.3 to <17.9 | 1593 (42.0) | 1.83 (0.77–1.96) | <.001 | 1.23 (0.77–1.96) | .39 | 5.5 (4.5–6.7) | 2.0 (1.3–3.3) | 9.4 (7.5–11.7) |
|
| ||||||||
| High: ≥17.9 | 1274 (33.6) | 4.31 (1.14–2.86) | <.001 | 1.80 (1.14–2.86) | .01 | 12.0 (10.3–13.9) | 3.7 (2.2–6.2) | 15.6 (13.3–18.1) |
Abbreviations: DSE, diabetes support and education; ILI, intensive lifestyle intervention; NA, data not available (no cases).
Category thresholds for diabetes duration, body mass index (calculated as weight in kilograms divided by height in meters squared), hemoglobin A1c, weight change, and fitness change are based on tertiles of the overall sample. Multivariable analyses adjust for intervention group and all variables in the table. Available case analysis is based on sample sizes expressed in second column.
A similar set of predictors (<2-year duration of diabetes, a lower baseline HbA1c, large first-year weight loss, and a large increase in fitness) was also associated with increased longer-term remission (years 2–4). However, the absolute prevalence of remission through years 2 to 4 was relatively rare; even among those with fewer than 2 years since diagnosis, average remission was 3.6% (95% CI, 2.8%–4.7%) for ILI compared with 1.9% (95% CI, 1.4%–2.6%) among DSE participants (eTable 2).
COMMENT
The increasing worldwide prevalence of type 2 diabetes, along with its wide-ranging complications, has led to hopes that the disease can be reversed or prevented.14,15 These analyses of more than 4500 overweight adults with type 2 diabetes confirm that complete remission associated with an intensive lifestyle intervention, when defined by glucose normalization without need for drugs, is rare. However, partial remission, defined as a transition to prediabetic or normal glucose levels without drug treatment for a specific period, is an obtainable goal for some patients with type 2 diabetes. As many as 11.5% of lifestyle intervention participants had partial or complete remission within the first year of intervention and 7% had partial or complete remission after 4 years; these rates were 3 to 6 times those of participants in the DSE condition. Perhaps more important, rates of any remission were notably higher (15%–21%) among persons with substantial weight loss or fitness change, shorter duration of extant diabetes, or a lower HbA1c level at entry and those not using insulin.
The ability to eliminate all diabetes medications while maintaining subdiabetic levels of fasting plasma glucose and HbA1c levels should considerably reduce medication costs, related adverse effects, risks of hypoglycemia, and hyperglycemic symptoms.16,17 Prior studies of medication-based therapy have shown that the reductions in HbA1c and fasting glucose, if sustained, are likely to considerably reduce risks of microvascular complications.18,19 Moreover, the longer-term effect could also be enhanced if the improvements in high-density lipoprotein cholesterol, triglycerides, and systolic blood pressure levels observed in Look AHEAD also extend to a reduction in vascular complications.20 Of note, however, in October 2012, after 8 to 11 years of participant follow-up, the Look AHEAD intervention was stopped by the study sponsor when it was determined that the ILI did not decrease the occurrence of cardiovascular events, the primary trial outcome relative to the DSE group.21 Neither these results nor those of the other pre-specified primary and secondary outcomes of Look AHEAD have been published yet.
The absolute difference in rates of remission observed between the ILI and DSE (9.5 percentage points at year 1; 5.3 percentage points at year 4) were somewhat less than the rate differences generally observed in diabetes prevention studies,22 as the Finnish and US prevention studies observed differences in cumulative incidence between intervention and control participants of 12 to 15 percentage points at 3 to 4 years.22–24 Similarly, a recent analysis of the US Diabetes Prevention Program found rates of 1-time remission from impaired glucose tolerance to normal glucose levels of 23% and 9% for intervention and control groups, or a 14-percentage-point difference.25 These findings, combined with our observations that remission was more common among adults with fewer years of diagnosed diabetes and lower initial HbA1c values, provide indirect evidence that intervention earlier in the natural history of diabetes leads to better outcomes, perhaps by preserving beta cell function and mass in the face of declining insulin action.3 This may influence the ongoing debate about population screening for prediabetes and diabetes, which remains controversial because of the lack of explicit evidence that earlier identification leads to better health outcomes.26,27
Studies of bariatric surgery have observed substantially larger weight loss and rates of partial and complete remission than Look AHEAD,6,7,28 ranging from 27% to 97% depending on the study, surgical approach, and follow-up period. However, long-term benefits on comorbidity have still not been clarified, and bariatric surgery is more invasive and, thus, is unlikely to be a first choice for the majority of people with type 2 diabetes.5–8 Previous studies of both lifestyle and pharmacologic-based interventions have shown significant reductions in HbA1c levels and/or use of antidiabetic medications,29–32 but we are not aware of nonsurgical studies that have examined remission rates based on a combination of medication use and levels of glycemia.
The appropriate definition of diabetes remission remains an area of ambiguity and debate.9 For these analyses, we adopted the American Diabetes Association’s definition, achievement of glycemia below the diabetic range in the absence of active pharmacological treatment or surgical therapy. It could be argued, however, that remission according to glycemic and pharmacologic criteria does not address the underlying health of beta cell function and insulin action and, thus, cannot be used to define a cure for diabetes. Weight regain and failure to maintain changes in diet and physical activity may lead to a deterioration of glycemic control and recurrence of diabetes. We could not fully assess the length of remission in this report because our analyses were limited to 4 years of follow-up.
Our analyses were not conducted using a pure intention-to-treat approach, as participants who underwent bariatric surgery after randomization, had normal glucose levels at baseline, or were missing data were excluded from the analyses. This was a deliberate analytic decision based on the desire to estimate the absolute frequency of diabetes remission that could be attributed to lifestyle intervention. Including even a relatively small number of surgery cases could skew findings because of the high documented frequency of diabetes remission following surgery. This exclusion of surgery cases led to an analytic sample that was slightly older, was more likely to be male, and had worse glycemic control than the broader Look AHEAD study population.
There are additional limitations to our analyses. First, remission was not one of the intended primary objectives of the Look AHEAD study, making these analyses exploratory in nature. We did not adjust our P values for multiple comparisons, which could increase the type 1 error rate. However, we found P<.001 for all of our main comparisons of remission rates, indicating our primary conclusions are robust. Second, the study population was not ideal for this analysis because half of the sample had at least 5 years of diabetes duration, 19% were using insulin, and many had high HbA1c values. These factors would likely make our findings an underestimate of the frequency of remission that may be achieved in a group of individuals identified earlier in their disease progression. Alternatively, Look AHEAD used a more intensive intervention than that commonly used in clinical and community settings, which may overestimate the effect. Third, this study had no assessment of post–glucose challenge beta cell function or insulin action to determine the mechanisms whereby lifestyle intervention may lead toward remission or normalization of glycemic level. This prevented us from evaluating the impact of the intervention on insulin resistance and also means that some of those who were classified as having remission would likely still be classified as having diabetes if the study had included an oral glucose tolerance test. Finally, we cannot rule out the possibility that the physicians of ILI participants were more likely to modify medications than the physicians of DSE participants or, alternatively, physicians were resistant to stopping medications such as metformin even if the glycemic levels of their patients were normal.
In spite of these limitations, this is the largest study to our knowledge to examine the association of a lifestyle intervention with type 2 diabetes remission. Our findings suggest that an intensive lifestyle intervention may be associated with a partial diabetes remission in a subset of patients with type 2 diabetes, particularly those whose diabetes is of short duration, who have lower HbA1c levels, and who do not yet require insulin therapy.
Supplementary Material
Acknowledgments
Funding/Support: This study is supported by the US Department of Health and Human Services through the following cooperative agreements from the National Institutes of Health (NIH): DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (IHS) provided personnel, medical oversight, and use of facilities. Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (M01RR000056), the Clinical Translational Research Center funded by Clinical and Translational Science Award UL1 RR 024153 and NIH grant DK 046204; the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346). The following organizations have committed to making major contributions to Look AHEAD: FedEx Corp; Health Management Resources; LifeScan Inc, a Johnson and Johnson Company; OPTIFAST of Nestle HealthCare Nutrition Inc; Hoffmann-La Roche Inc; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Role of the Sponsor: The primary role of the sponsor (Department of Health and Human Services, including the NIH, Centers for Disease Control and Prevention, and IHS) was to provide funding, administrative oversight, personnel, medical oversight, and use of facilities. In addition, several scientific and administrative representatives of the sponsors contributed to the design and conduct of the overall study as part of the Look AHEAD study group (listed in the eAppendix). Dr Gregg, a representative of one of the cosponsors, conceptualized and led the analysis, interpretation, and writing of this specific manuscript. The decision to publish was made by the Look AHEAD Steering Committee, with no restrictions imposed by the sponsors.
Footnotes
Author Contributions: Dr Chen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gregg, Wagenknecht, Delahanty, Safford, Pi-Sunyer, Wing.
Acquisition of data: Wagenknecht, Clark, Delahanty, Johnson, Safford, Kitabchi, Pi-Sunyer, Wing.
Analysis and interpretation of data: Gregg, Chen, Wagenknecht, Clark, Delahanty, Bantle, Pownall, Johnson, Safford, Wing, Bertoni.
Drafting of the manuscript: Gregg, Pi-Sunyer.
Critical revision of the manuscript for important intellectual content: Gregg, Chen, Wagenknecht, Clark, Delahanty, Bantle, Pownall, Johnson, Safford, Kitabchi, Pi-Sunyer, Wing, Bertoni.
Statistical analysis: Chen.
Obtained funding: Wagenknecht, Pownall, Johnson, Kitabchi, Pi-Sunyer, Wing.
Administrative, technical, or material support: Gregg, Wagenknecht, Johnson, Kitabchi, Pi-Sunyer, Wing.
Study supervision: Gregg, Wagenknecht, Safford.
Conflict of Interest Disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Delehanty reported board membership with Eli Lilly, Boeringer Ingelheim, and Johnson and Johnson and consultancy support from Pfizer. Dr Safford reported receiving consultancy support from diaDexus. No other disclosures were reported.
Disclaimer: The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the IHS or other funding sources.
Online-Only Material: The eAppendix and eTables 1 and 2 are available at http://www.jama.com.
References
- 1.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328(23):1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 3.Kitabchi AE, Temprosa M, Knowler WC, et al. Diabetes Prevention Program Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. Atlanta, GA: Centers for Control and Prevention; 2011. [Google Scholar]
- 5.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256. e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 6.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery vs conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 7.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery vs intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sjöström L. Bariatric surgery and reduction in morbidity and mortality: experiences from the SOS study. Int J Obes (Lond) 2008;32(suppl 7):S93–S97. doi: 10.1038/ijo.2008.244. [DOI] [PubMed] [Google Scholar]
- 9.Buse JB, Caprio S, Ceriello A, et al. How do we define cure of diabetes? Diabetes Care. 2009;32(11):2133–2135. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD Research Group Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24(5):610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 11.Wadden TA, West DS, Delahanty L, et al. Look AHEAD Research Group The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14(5):737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wesche-Thobaben JA. The development and description of the comparison group in the Look AHEAD trial. Clin Trials. 2011;8(3):320–329. doi: 10.1177/1740774511405858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Mehrotra DV, Barnard J. Analysis of incomplete longitudinal binary data using multiple imputation. Stat Med. 2006;25(12):2107–2124. doi: 10.1002/sim.2343. [DOI] [PubMed] [Google Scholar]
- 14.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 16.Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386–399. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 17.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 18.Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75(14):894–903. doi: 10.1016/s0002-9149(99)80683-3. [DOI] [PubMed] [Google Scholar]
- 19.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 20.Wing RR, Look AHEAD Research Group Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: 4-year results of the Look AHEAD trial. Arch Intern Med. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institutes of Health. Weight loss does not lower heart disease risk from type 2 diabetes [press release] 2012 Oct 19; http://www.nih.gov/news/health/oct2012/niddk-19.htm. Accessed November 16, 2012.
- 22.Baker MK, Simpson K, Lloyd B, Bauman AE, Singh MA. Behavioral strategies in diabetes prevention programs: a systematic review of randomized controlled trials. Diabetes Res Clin Pract. 2011;91(1):1–12. doi: 10.1016/j.diabres.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 24.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE, Diabetes Prevention Program Research Group Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379(9833):2243–2251. doi: 10.1016/S0140-6736(12)60525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Echouffo-Tcheugui JB, Ali MK, Griffin SJ, Narayan KM. Screening for type 2 diabetes and dysglycemia. Epidemiol Rev. 2011;33(1):63–87. doi: 10.1093/epirev/mxq020. [DOI] [PubMed] [Google Scholar]
- 27.Griffin SJ, Borch-Johnsen K, Davies MJ, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156–167. doi: 10.1016/S0140-6736(11)60698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolen SD, Chang HY, Weiner JP, et al. Clinical outcomes after bariatric surgery: a 5-year matched cohort analysis in 7 US states. Obes Surg. 2012;22(5):749–763. doi: 10.1007/s11695-012-0595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11–S63. doi: 10.2337/dc12-s011. http://care.diabetesjournals.org/content/35/Supplement_1. Accessed August 1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity vs usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet. 2011;378(9786):129–139. doi: 10.1016/S0140-6736(11)60442-X. [DOI] [PubMed] [Google Scholar]
- 31.Heymsfield SB, Segal KR, Hauptman J, et al. Effects of weight loss with orlistat on glucose tolerance and progression to type 2 diabetes in obese adults. Arch Intern Med. 2000;160(9):1321–1326. doi: 10.1001/archinte.160.9.1321. [DOI] [PubMed] [Google Scholar]
- 32.Kelley DE, Bray GA, Pi-Sunyer FX, et al. Clinical efficacy of orlistat therapy in overweight and obese patients with insulin-treated type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2002;25(6):1033–1041. doi: 10.2337/diacare.25.6.1033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


