Abstract
Introduction:
While level 1 evidence supports the use of neoadjuvant chemotherapy (NAC) for patients with muscle-invasive bladder cancer (MIBC), its uptake has been underwhelming, even in academic centres. Our aim was to determine if the initiation of a multidisciplinary bladder cancer clinic (MDBCC) in 2008 at our institution, where patients are assessed simultaneously by bladder cancer-focused urologists and radiation oncologists with easy access to a medical oncologist, was associated with an increased use of NAC.
Methods:
Patients with MIBC initiating treatment between July 2000 and June 2013 were identified and classified by academic year (July 1 to June 30). Time-series analyses using interventional autoregressive integrated moving average (ARIMA) models with ramp intervention functions were then conducted. A sensitivity analysis was performed on clinical N0 patients.
Results:
The cohort included 278 patients: 168 from 2000–2007 and 110 from 2008–2012 (academic years). Forty-two (15.1%) patients received NAC and 74 (26.6%) received adjuvant chemotherapy (AC). Overall the proportion of patients receiving NAC increased from 7.7% before the MDBCC to 47.6% in 2012 (Interventional ARIMA p=0.036). The results were similar when restricting to cN0 patients (p<0.001). NAC use gradually increased over time regardless of MDBCC attendance, although the proportion of patients receiving NAC appears to have risen more sharply among MDBCC attendees.
Conclusions:
At our institution, the initiation of the MDBCC was temporally associated with increased use of NAC. In addition to multidisciplinary collaboration, having a critical mass of NAC physician advocates and support from institutional leaders are essential to the uptake of NAC.
Introduction
Level 1 evidence supports the use of NAC in patients with MIBC undergoing radical cystectomy (RC);1,2 however, its uptake has been limited, even in academic centres.3–5
Previous studies have demonstrated that multidisciplinary care results in changes in diagnosis and treatment plans for many bladder cancer patients.6,7 Our institution initiated a MDBCC in 2008, providing an opportunity to evaluate whether its initiation impacted the temporal patterns of NAC use in patients undergoing RC for MIBC. Our hypothesis was that NAC would be increasingly used over time, particularly following the initiation of the MDBCC.
Methods
Patients and data sources
After obtaining Ethics Board approval, patients who underwent RC for muscle-invasive urothelial carcinoma of the bladder between July 1, 2000 and June 30, 2013 at a single tertiary-care academic centre were identified. All patients were confirmed as having MIBC by transurethral resection. Patients undergoing salvage RC following failed bladder-conserving chemo-radiation (n=23) and patients with metastatic disease undergoing RC for palliation (n=2) were excluded.
Clinical parameters ascertained by chart review were: age, gender, smoking status, presence of hydronephrosis, year of surgery, clinical and pathological stages, surgical margin status, concurrent carcinoma in-situ (CIS), lymphadenectomy node count, treating urologist, and receipt of NAC or AC.
Exposures
In the MDBCC, a urologic oncologist and radiation oncologist see patients concurrently and are both present during cystoscopic evaluations of patients who are candidates for or who have completed bladder-conserving chemo-radiation therapy for bladder cancer. A medical oncologist is available for consultation as well, although not physically so in the clinic. In this manner, a true multidisciplinary approach to bladder cancer is possible with ensuing rapid consultation and unbiased consensus recommendations for care.
Patients were classified by academic year (July 1–June 30) of initiation of treatment (i.e., RC or NAC) since this best aligned the annual intervals with the events of interest. The MDBCC, initiated in April 2008, was the event of interest (the “intervention”). The 2008 academic year was considered the first year following the introduction of the MDBCC (i.e., “post-intervention”). Although one academic urologist and one medical oncologist with strong interests in bladder cancer and advocates of NAC were recruited in September 2006 and October 2006, respectively, the MDBCC was formally introduced in 2008. Two oncology fellowship-trained urologists, also proponents of NAC, were recruited in July 2011 and July 2012; otherwise there were no meaningful temporal confounders to the best of our knowledge.
Outcomes
The primary outcome was the percent of patients receiving NAC by year. The secondary outcome was the percent of patients receiving AC by year.
Statistical analysis
Cohort characteristics were compared using univariate statistics. Comparisons of NAC and AC use before and after the initiation of the MDBCC were made using the Wilcoxon Rank-Sum test or Chi-Square test. Time series (NAC and AC use by year) were plotted graphically for primary and secondary outcome measures and analyzed using interventional ARIMA models, with a ramp intervention to represent the initiation of the MDBCC. This model tests whether there was an associated change in the event rate per year following a discrete event compared to the expected event rate based on pre-event data.8–11 This approach is more robust than simpler “before vs. after” comparisons since the model intrinsically accounts for confounders that gradually change over time, both measured and unmeasured (e.g., trends in practice patterns), and therefore multivariable adjustment is generally not necessary.8–11 It is only susceptible to temporal confounding from other discrete events that occur in close proximity to the event of interest. To the best of our knowledge, there were none in the present study. Auto-correlation, partial auto-correlation, and inverse auto-correlation plots were used to guide model selection. Auto-correlation and stationarity were assessed using the Ljung-Box Chi-Square statistic and the Augmented Dickey-Fuller test, respectively. Exponential smoothing models were used to create expected post-intervention projections with 95% confidence intervals for comparison to actual values.
To determine whether changes in NAC use over time were different among MDBCC attendees vs. non-attendees, stratified time-series plots by MDBCC attendance were plotted. In order to understand how individual urologists’ use of NAC may have changed over time, before vs. after comparisons were made (relative to the initiation of the MDBCC) stratified by individual urologist. Lastly, given that some patients undergoing RC occasionally had low volume nodal metastases at diagnosis, a sensitivity analysis was performed on cN0 patients.
Statistical analyses were performed using SAS (version 9.3; SAS Institute, Cary, NC).
Results
Cohort characteristics
Between July 1, 2000 and June 30, 2013, 278 patients underwent RC for MIBC (Table 1). There was a greater proportion of cN0 patients in the 2008–2012 group and a greater proportion of cN1 and cNx patients in the 2000–2007 group. Otherwise, there were no significant differences between the pre- and post-intervention groups.
Table 1.
Cohort characteristics
| Parameter | Total n=278 | 2000–2007 n=168 | 2008–2012 n=110 | p value | |
|---|---|---|---|---|---|
| Age in years | Median (IQR) | 69.3 (60.3–75.7) | 70.1 (59.8–75.4) | 68.6 (60.6–77.3) | 0.93 |
| Female sex, n (%) | 59 (21.2) | 36 (21.4) | 23 (20.9) | 0.92 | |
| Heavy Smoking (≥30pck-yrs), n (%) | 101 (36.3) | 63 (37.5) | 38 (34.6) | 0.62 | |
| Hydronephrosis, n (%) | 87 (31.3) | 49 (29.2) | 38 (34.6) | 0.34 | |
| Concurrent CIS, n (%) | 107 (38.5) | 66 (39.3) | 41 (37.3) | 0.74 | |
| cT-stage, n (%) | cTa/Tis/T1/T2 | 222 (79.9) | 131 (78.0) | 91 (82.7) | 0.33 |
| cT3/T4 | 56 (20.1) | 37 (22.0) | 19 (17.3) | ||
| cN-stage, n (%) | cN0 | 176 (63.3) | 92 (54.8) | 84 (76.4) | <0.001 |
| cN1+ | 53 (19.1) | 35 (20.8) | 18 (16.4) | ||
| cNx | 49 (17.6) | 41 (24.4) | 8 (7.3) | ||
| pT-stage, n (%) | pT0 | 16 (5.8) | 10 (6.0) | 6 (5.4) | 0.65 |
| pTa/Tis/T1/T2 | 97 (34.9) | 55 (32.7) | 42 (38.2) | ||
| pT3/T4 | 165 (59.3) | 103 (61.3) | 62 (56.4) | ||
| pN-stage, n (%) | pN0 | 163 (58.6) | 98 (58.3) | 65 (59.1) | 0.30 |
| pN-pos | 107 (38.5) | 63 (37.5) | 44 (40.0) | ||
| pNx | 8 (2.9) | 7 (4.2) | 1 (0.9) | ||
| Positive surgical margin*, n (%) | 18 (6.5) | 10 (6.0) | 8 (7.3) | 0.66 | |
| Node total, median (IQR) | 13.5 (8–20) | 13 (8–20) | 14 (9–20) | 0.39 |
Demographic and clinical characteristics of patients undergoing radical cystectomy between July 1st 2000 and June 30th 2013 compared before and after the initiation of the MDBCC in 2008;
Positive surgical margins are based on permanent sections (paraffin-embedded). Ureteral CIS was not counted as a positive margin; CIS: carcinoma in-situ; MDBCC: multidisciplinary bladder cancer clinic, Std diff : standardized difference.
NAC uptake following the initiation of the MDBCC
Of the 278 patients, 42 (15.1%) received NAC and 74 (26.6%) received AC (Table 2). In univariate comparisons of before vs. after the initiation of the MDBCC, there was a significantly greater proportion of all patients receiving NAC (7.7% vs. 26.4%, p<0.001) and a significantly lower proportion of patients receiving AC (33.9% vs. 15.4%, p<0.001). When evaluating perioperative chemotherapy use on a yearly basis, there was an increase in the percentage of patients receiving NAC per year between the two time periods (8.2% vs. 22.2%), while the percentage of patients receiving AC per year decreased (32.7% vs. 14.3%).
Table 2.
Univariate analysis before vs. after comparisons for NAC and AC
| Type of perioperative chemotherapy administered | 2000–2007 (n=168) | 2008–2012 (n=110) | p value |
|---|---|---|---|
| NAC, n (%) | 13 (7.7) | 29 (26.4) | <0.001 |
| No. receiving NAC/year, median (range) | 1.5 (0–3) | 4 (2–10) | |
| % receiving NAC/year, median (range) | 8.2 (0.0–15.8) | 22.2 (9.1–47.6) | |
| AC, n (%) | 57 (33.9) | 17 (15.4) | <0.001 |
| No. receiving AC/year, median (range) | 7 (3–10) | 3 (1–6) | |
| %receiving AC/year, median (range) | 32.7 (15.8–62.5) | 14.3 (4.2–33.3) |
Use of NAC increased while use of AC decreased after the initiation of the MDBCC.
AC: adjuvant chemotherapy; MDBCC: multidisciplinary bladder cancer clinic; NAC: neoadjuvant chemotherapy.
When restricting analyses to cN0 patients (Supplemental Table 1), the proportion of patients receiving NAC remained significantly increased while AC use significantly decreased, from 2008 onward relative to 2000–2007.
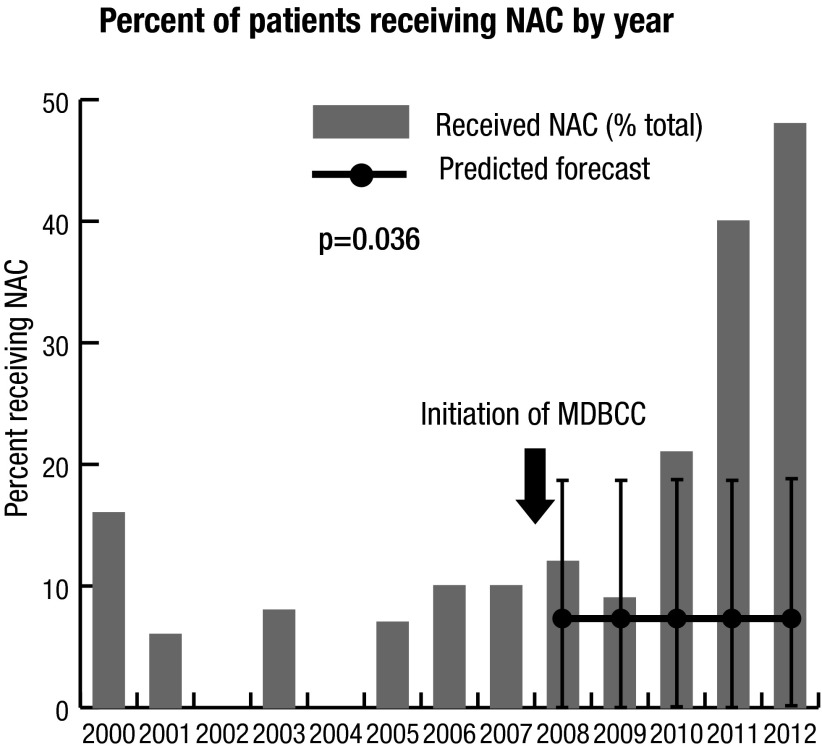
The time series analysis demonstrated that the proportion of patients receiving NAC increased from a mean of 7.7% before the initiation of the MDBCC to 47.6% in 2012 (Fig. 1a, Interventional ARIMA p=0.036). Results were similar when restricting to cN0 patients (p<0.001, supplemental Fig. 1).
Fig. 1a.
Time series plot of percent of patients receiving NAC by year using ARIMA models. There was a significant increase in use of NAC from a mean of 7.7% before the MDBCC to 47.6% in the 2012 academic year. MDBCC: multidisciplinary bladder cancer clinic; NAC: neoadjuvant chemotherapy.
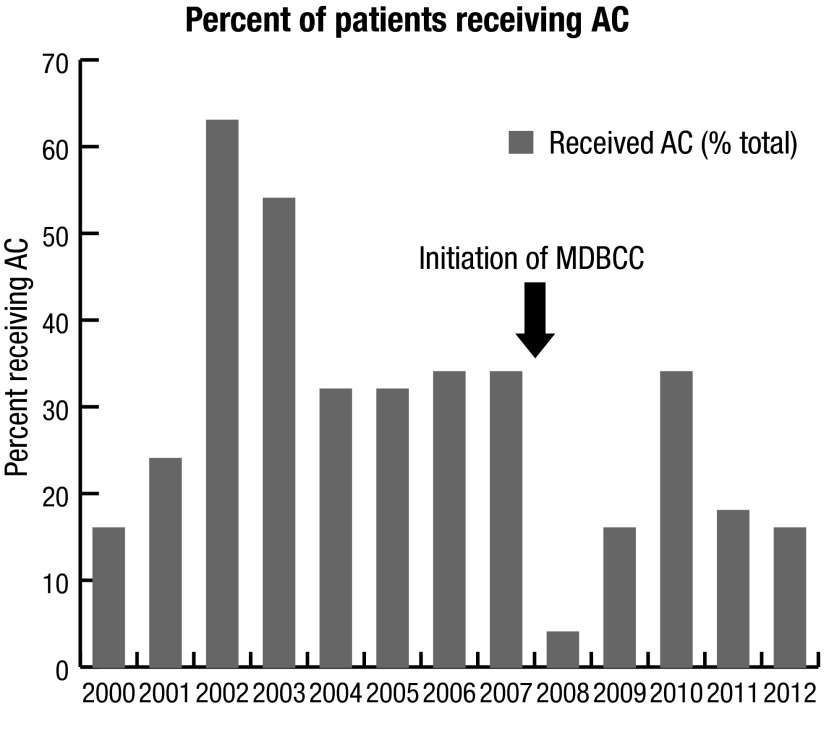
Fig. 1b.
Time series plot of percent of patients receiving AC by year. There was a trend towards decreased use of adjuvant chemotherapy following initiation of MDBCC based on univariate analyses. Our statistical software was unable to fit an ARIMA model to this data; AC: adjuvant chemotherapy; MDBCC: multidisciplinary bladder cancer clinic.
Simultaneously, the use of AC generally decreased over time (Fig 1b); however, it was not possible to fit an ARIMA model to the time series to determine if there was a statistically significant change following the initiation of the MDBCC.
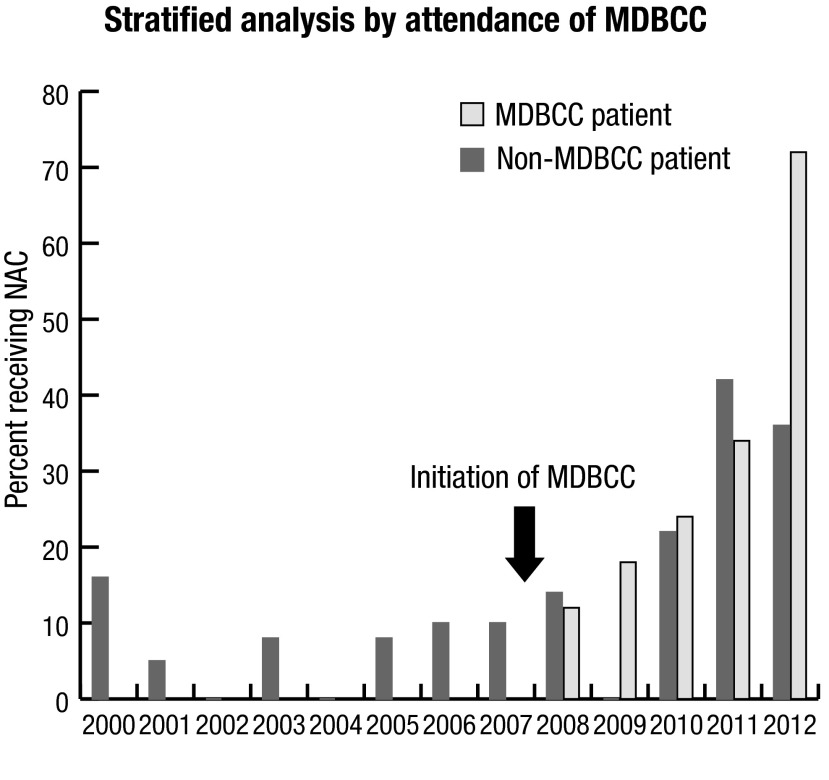
In the time series plot stratified by whether or not patients attended the MDBCC (Fig. 2), NAC use gradually increased over time for both subgroups, regardless of MDBCC attendance, although the proportion of patients receiving NAC appears to have risen more sharply among MDBCC attendees (to a peak of 71%).
Fig. 2.
NAC use by year, stratified by MDBCC attendance. The percentage of patients receiving NAC increased over time, even among patients not attending the MDBCC. MDBCC: multidisciplinary bladder cancer clinic; NAC: neoadjuvant chemotherapy.
The newly recruited uro-oncologists contributed to the institution’s uptake of NAC in later years, however, NAC use increased over time among other urologists as well (Table 3).
Table 3.
NAC use by urologist, before and after initiation of MDBCC
| Urologists | 2000–2007 % receiving NAC (No. receiving NAC/No. of RCs) | 2008–2012 % receiving NAC (No. receiving NAC/No. of RCs) |
|---|---|---|
| Newly recruited urologist #1 (2011) | - | 50.0 (7/14) |
| Newly recruited urologist #2 (2012) | - | 50.0 (1/2) |
| High RC volume urologist #1 | 9.5 (4/42) | 24.0 (6/25) |
| High RC volume urologist #2 | 2.9 (2/69) | 17.9 (7/39) |
| High RC volume urologist #3 | 40.0 (4/10) | 30.8 (8/26) |
| Other urologists | 6.4 (3/47) | 0.0 (0/4) |
Newly recruited urologists had a higher proportion of patients receiving NAC compared to other urologists. MDBCC: multidisciplinary bladder cancer clinic; NAC: neoadjuvant chemotherapy; RC: radical cystectomy.
Discussion
Despite the presence of level 1 evidence supporting the use of NAC in MIBC, use of NAC has been low and only modestly increasing over time.4,5,12 One academic centre found that only 17% of patients undergoing RC for MIBC between 2003 and 2008 received NAC.3 Large population-based studies from Canada5 and the U.S.4 also demonstrate low use of NAC, with 4.4% and 16.9% of patients receiving NAC for MIBC between 2004 and 2008 and 2006 and 2010, respectively. While the use of NAC has increased over time, factors that influence NAC uptake have yet to be elucidated. Identifying such factors may provide insight to facilitate change at different institutions and allow for increased implementation of evidence-based, guideline recommended use of NAC in the treatment of MIBC.
The present study evaluated the temporal patterns of uptake of NAC in patients undergoing RC for MIBC at a tertiary care centre and demonstrated that NAC use increased significantly since the initiation of a MDBCC. Multidisciplinary clinics encompass collaborative patient care by a team of individuals where all diagnostic and treatment options are discussed and tailored for each patient. A recent systematic review found an association between multidisciplinary care and patient survival.13 Potential reasons for these improved outcomes may include a change in diagnosis or better treatment plan following a discussion with members from different specialties. Indeed, a study assessing the diagnostic and treatment decisions in urologic malignancies found that for bladder cancer, there was a change in staging in 23% and change in treatment in 44% of cases.6 Although physicians in the present study were well aware of existing data supporting the use of NAC for MIBC, perhaps the multidisciplinary interactions and explicit discussion of individual patient management catalyzed a change in practice.
Although more pronounced among MDBCC attendees, NAC use increased both among MDBCC attendees and non-attendees, suggesting a more generalized change in practice. In addition to the initiation of the MDBCC, another change that occurred at our institution in more recent years was the recruitment of several urologists and a medical oncologist who were strong advocates of NAC. While these providers used NAC more than other urologists, they were not solely responsible, as the trend for NAC uptake was also seen among other physicians who were using NAC far less frequently before the initiation of the MDBCC. AC use, on the other hand, generally decreased over time, but is still used with a reasonable frequency. While the evidence regarding perioperative chemotherapy is stronger for NAC than AC, both approaches are reasonable as per recent clinical guidelines.14,15 The present study could not assess impact of MDBCC on AC use since we were unable to fit the ARIMA statistical model.
Although our data demonstrate a transition in NAC use at the time of MDBCC initiation, simply forming a MDBCC alone may not result in an increased uptake of NAC across institutions. Rather, it is fundamental to have the leadership and encouragement from bladder cancer opinion leaders and the support and motivation from a critical mass of physicians to start using NAC.
Due to the accumulating evidence for the benefit of NAC in MIBC, there have been corresponding changes in the clinical practice guidelines. In 2005, the European Society for Medical Oncology described NAC as an “investigational treatment” modality,16 while the 2007 guidelines recommended that NAC “should be considered” for MIBC (category 2A recommendation).17 The most recent guidelines published in 2014 encourage the use of NAC (category 1A recommendation).14 Similar changes occurred in the National Comprehensive Cancer Network Clinical Practice Guidelines.15,18 While it is possible that the evolving guidelines were partly responsible for the increase use of NAC in the present study, the wording of recommendations was relatively weak until 2014, which was after the observed increase of NAC use in our series and is, therefore, unlikely to be the primary cause for the drastic uptake of NAC in this study.
Although the initiation of the MDBCC was associated with increased use of NAC, the overall proportion of patients receiving NAC in the most recent year of study was still less than half. However, approximately 24–53% of patients are not eligible to receive cisplatin-based chemotherapy preoperatively based on their renal function status alone,19,20 and one-third of patients may have comorbidities that preclude them from receiving NAC4. Given these data, the ideal proportion of patients who should receive NAC remains unclear.
The present study has limitations. First, given the observational nature of the study, it cannot prove causality. However, the primary analysis used interventional ARIMA models, which are more robust for time-series data than before vs. after analyses or regression analyses. Second, the publication of the SWOG trial in 2003 may partly account for the increased use of NAC in this study. However, the majority of the increase was observed after the initiation of the MDBCC, several years after 2003. Third, we cannot rule out unknown temporal confounders that may have also contributed to NAC uptake, but to the best of our knowledge, there were no other meaningful temporal confounders. Fourth, this study is retrospective in nature and there may be unaccounted factors that may have influenced whether or not patients received NAC. However, it is not expected that accessibility to NAC or patient eligibility to receive NAC have changed over the period of time studied.
Conclusion
At our institution, the initiation of the MDBCC was temporally associated with increased use of NAC. While increased awareness of the data supporting NAC use may have also contributed, NAC uptake only began to rise sharply after the initiation of the MDBCC, years after the publication of trial data supporting NAC in MIBC. Furthermore, guideline recommendations were relatively weak until recently, after the observed rise of NAC uptake in the present study. In addition to multidisciplinary collaboration, having a critical mass of NAC physician advocates and support from institutional leaders are essential to the uptake of NAC.
Time series plot of percent of cN0 patients receiving NAC by year using ARIMA models. There was a significant increase in use of NAC among cN0 patients after the initiation of the MDBCC. MDBCC: multidisciplinary bladder cancer clinic; NAC: neoadjuvant chemotherapy.
Supplemental Table 1.
Univariate before vs. after comparisons for NAC and AC in cN0 patients
| Outcome | 2000–2007 (n=92) | 2008–2012 (n=84) | p value |
|---|---|---|---|
| NAC, n (%) | 7 (7.6) | 20 (23.8) | 0.003 |
| No. receiving NAC/year, median (range) | 1 (0–2) | 4 (1–7) | |
| % receiving NAC/year, median (range) | 7.9 (0.0–18.2) | 26.7 (5.3–43.8) | |
| AC, n (%) | 27 (29.4) | 13 (15.5) | 0.03 |
| No. receiving AC/year, median (range) | 3 (2–5) | 2 (1–6) | |
| %receiving AC/year, median (range) | 28.6 (18.2–50.0) | 10.5 (5.6–40.0) |
Use of NAC increased while use of AC decreased in cN0 patients after the initiation of the MDBCC. AC: adjuvant chemotherapy; MDBCC: multidisciplinary bladder cancer clinic; NAC: neoadjuvant chemotherapy.
Footnotes
Competing interests: The authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 2.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration Advanced bladder cancer meta-analysis C: Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–5. doi: 10.1016/j.eururo.2005.04.006. discussion 205–6. [DOI] [PubMed] [Google Scholar]
- 3.Raj GV, Karavadia S, Schlomer B, et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer. 2011;117:276–82. doi: 10.1002/cncr.25429. [DOI] [PubMed] [Google Scholar]
- 4.Zaid HB, Patel SG, Stimson CJ, et al. Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: Results from the National Cancer Database. Urology. 2014;83:75–80. doi: 10.1016/j.urology.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 5.Booth CM, Siemens DR, Peng Y, et al. Patterns of referral for perioperative chemotherapy among patients with muscle-invasive bladder cancer: A population-based study. Urol Oncol. 2014;32:1200–8. doi: 10.1016/j.urolonc.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Kurpad R, Kim W, Rathmell WK, et al. A multidisciplinary approach to the management of urologic malignancies: Does it influence diagnostic and treatment decisions? Urol Oncol. 2011;29:378–82. doi: 10.1016/j.urolonc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Hermanns T, Wei Y, Bindi B, et al. A multidisciplinary bladder cancer clinic delivers personalized care for complex bladder cancer patients. Eur Urol. 2014;13:e793. doi: 10.1016/S1569-9056(14)60781-8. [DOI] [Google Scholar]
- 8.Bhindi B, Mamdani M, Kulkarni GS, et al. Impact of the U.S. preventive services task force recommendations against PSA screening on prostate biopsy and cancer detection rates. J Urol. 2015;193(5):1519–24. doi: 10.1016/j.juro.2014.11.096. [DOI] [PubMed] [Google Scholar]
- 9.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–51. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton JD. Time series analysis. Princeton University Press; 1994. [Google Scholar]
- 11.Kennedy CE, Turley JP. Time series analysis as input for clinical predictive modeling: Modeling cardiac arrest in a pediatric ICU. Theor Biol Med Model. 2011;8:40. doi: 10.1186/1742-4682-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reardon ZD, Patel SG, Zaid HB, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: A sign of changing tides. Eur Urol. 2015;67:165–70. doi: 10.1016/j.eururo.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong NJ, Wright FC, Gagliardi AR, et al. Examining the potential relationship between multidisciplinary cancer care and patient survival: an international literature review. J Surg Oncol. 2010;102:125–34. doi: 10.1002/jso.21589. [DOI] [PubMed] [Google Scholar]
- 14.Bellmunt J, Orsola A, Leow JJ, et al. Bladder cancer: ESMO practice guidelines for diagnosis, treatment, and followup. Ann Oncol. 2014;25:iii40–8. doi: 10.1093/annonc/mdu223. [DOI] [PubMed] [Google Scholar]
- 15.Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw. 2013;11:446–75. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 16.Kataja VV, Pavlidis N, Force EGT. ESMO minimum clinical recommendations for diagnosis, treatment, and followup of invasive bladder cancer. Ann Oncol. 2005;16:i43–4. doi: 10.1093/annonc/mdi815. [DOI] [PubMed] [Google Scholar]
- 17.Group EGW. Bellmont J, Albiol S. Invasive bladder cancer: ESMO clinical recommendations for diagnosis, treatment, and followup. Ann Oncol. 2007;18:ii38–9. doi: 10.1093/annonc/mdm029. [DOI] [PubMed] [Google Scholar]
- 18.Montie JE, Clark PE, Eisenberger MA, et al. Bladder cancer. J Natl Compr Canc Netw. 2009;7:8–39. doi: 10.6004/jnccn.2009.0002. [DOI] [PubMed] [Google Scholar]
- 19.Thompson RH, Boorjian SA, Kim SP, et al. Eligibility for neoadjuvant/adjuvant cisplatin-based chemotherapy among radical cystectomy patients. BJU Int. 2014;113:E17–21. doi: 10.1111/bju.12274. [DOI] [PubMed] [Google Scholar]
- 20.Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–13. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time series plot of percent of cN0 patients receiving NAC by year using ARIMA models. There was a significant increase in use of NAC among cN0 patients after the initiation of the MDBCC. MDBCC: multidisciplinary bladder cancer clinic; NAC: neoadjuvant chemotherapy.
Supplemental Table 1.
Univariate before vs. after comparisons for NAC and AC in cN0 patients
| Outcome | 2000–2007 (n=92) | 2008–2012 (n=84) | p value |
|---|---|---|---|
| NAC, n (%) | 7 (7.6) | 20 (23.8) | 0.003 |
| No. receiving NAC/year, median (range) | 1 (0–2) | 4 (1–7) | |
| % receiving NAC/year, median (range) | 7.9 (0.0–18.2) | 26.7 (5.3–43.8) | |
| AC, n (%) | 27 (29.4) | 13 (15.5) | 0.03 |
| No. receiving AC/year, median (range) | 3 (2–5) | 2 (1–6) | |
| %receiving AC/year, median (range) | 28.6 (18.2–50.0) | 10.5 (5.6–40.0) |
Use of NAC increased while use of AC decreased in cN0 patients after the initiation of the MDBCC. AC: adjuvant chemotherapy; MDBCC: multidisciplinary bladder cancer clinic; NAC: neoadjuvant chemotherapy.