Abstract
Introduction:
Surgical volume can affect several outcomes following radical prostatectomy (RP). We examined if surgical volume was associated with novel categories of treatment-related complications following RP.
Methods:
We examined a population-based cohort of men treated with RP in Ontario, Canada between 2002 and 2009. We used Cox proportional hazard modeling to examine the effect of physician, hospital and patient demographic factors on rates of treatment-related hospital admissions, urologic procedures, and open surgeries.
Results:
Over the study interval, 15 870 men were treated with RP. A total of 196 surgeons performed a median of 15 cases per year (range: 1–131). Patients treated by surgeons in the highest quartile of annual case volume (>39/year) had a lower risk of hospital admission (hazard ratio [HR]=0.54, 95% CI 0.47–0.61) and urologic procedures (HR=0.69, 95% CI 0.64–0.75), but not open surgeries (HR=0.83, 95% CI 0.47–1.45) than patients treated by surgeons in the lowest quartile (<15/year). Treatment at an academic hospital was associated with a decreased risk of hospitalization (HR=0.75, 95% CI 0.67–0.83), but not of urologic procedures (HR=0.94, 95% CI 0.88–1.01) or open surgeries (HR=0.87, 95% CI 0.54–1.39). There was no significant trend in any of the outcomes by population density.
Conclusions:
The annual case volume of the treating surgeon significantly affects a patient’s risk of requiring hospitalization or urologic procedures (excluding open surgeries) to manage treatment-related complications.
Introduction
There is large variation in the rate of complications among surgeons who perform RP for prostate cancer.1,2 Hospital length of stay, rates of perioperative complications, transfusions, incontinence, and erectile dysfunction (ED) are higher among patients treated by lower volume surgeons.2–9 We previously described novel treatment-related complications among patients who underwent surgery or radiation among a large, population-based cohort.10 We showed that rates of treatment-related hospital admissions, minimally invasive urologic procedures, gastrointestinal endoscopic procedures, and major surgical procedures ranged from 1–34%.10
It would be of interest to determine whether surgeon volume and practice setting affect the rates of these newly described complications. We examined 15 870 men who underwent surgery alone among this cohort and examined whether surgeon volume significantly affects rates of these complications after adjustment for confounders such as population density and treatment at an academic or community-based hospital.
Methods
Study subjects
A detailed description of the study subjects has been previously published.10 In short, we included all patients who underwent open radical prostatectomy for localized prostate cancer between January 1, 2002 and December 31, 2009 in Ontario, Canada. All medical procedures in Ontario are reimbursed by the government through the Ontario Health Insurance Plan (OHIP). The OHIP fee codes are listed for specific procedures with specific indications. We linked the OHIP fee code for RP ($651) to patients who were diagnosed with prostate cancer from the Ontario Cancer Registry to identify those who had surgery for prostate cancer within one year of diagnosis. We excluded patients who underwent laparoscopic or robotic RP and those who had radiotherapy after radical prostatectomy. We linked records from the OHIP physician claims database, the Canadian Institute for Health Information (CIHI) Discharge Abstract Database (DAD), the Ontario Cancer Registry, and the Registered Person Database.
The study protocol was approved by the Research Ethics Board at Sunnybrook Health Science Centre.
Outcome measures
We examined three outcome measures for treatment-related complications: 1) hospital admissions to manage a treatment-related problem; 2) minimally invasive urological procedures; and 3) open surgical procedures related to the urinary tract, rectum, and anus. The definition of each complication category has previously been published.10 For each patient, we identified the first outcome for each of the three outcome measures using the CIHI DAD for hospital admissions, OHIP fee codes for surgical and endoscopic procedures, and the CIHI Same Day Surgery database for percutaneous procedures. We did not measure repeat procedures or complications and analyzed only time to first complication.
Exposure and covariate definition
We examined surgeon volume of open radical prostatectomies per year, population density, practice setting (university-affiliated vs. community-based practice), patient age, and comorbidity. To estimate population density, we used 14 distinct geographic areas where healthcare is delivered within Ontario called Local Health Integration Networks (LHINs).11 Each LHIN comprises a geographic jurisdiction and is overseen by a committee that decides how health services are provided within the region. We used the Johns Hopkins University ACG case Mix System to measure comorbidity. We used the sum of aggregated disease groups (ADG), which forms a high level classification scheme for groups of diseases and disorders.12
Data analysis
We performed Cox proportional hazard modeling to estimate the hazard ratio (HR) of the three different complication outcome groups for several covariates, including age at prostatectomy, comorbidity level, individual physician volume of RP, hospital setting, and LHIN population density. Age was dichotomized (≤60 years vs. >60 years). Comorbidity was considered as continuous variable based on the ADG score. Physician volume of the number of RPs was divided into quartiles (≤15 cases/year, 15–23 cases/year, 24–38 cases/year, and ≥39 cases/year). Since the volume of each physician may vary from year to year, for each surgeon we assigned the average number of RPs done annually in the last three years before the date of prostatectomy, including lookback, where necessary. The hospital setting in which the procedure was performed was dichotomized (university-affiliated vs. community hospitals). Where this data was unavailable, it was coded as unknown in order to preserve the record in the analysis. The population density for each of the 14 LHINs was grouped into quartiles: <26 people per km2, 44–85 people/km2, 97–324 people/km2, and >549 people/km2.
Results
A total of 15 870 patients underwent RP in Ontario between 2002 and 2009 that met our inclusion criteria. Demographics of this group have previously been published.10,13,14 In short, the patients had mean age 61.5 years (standard deviation: 6.58 years) with median ADG score 5 (interquartile range [IQR]: 3–6). Median followup ranged from 3.0 years (IQR: 1.1–5.2 years) for genitourinary procedures to 4.4 years (IQR: 2.8–6.3 years) for open surgical procedures.
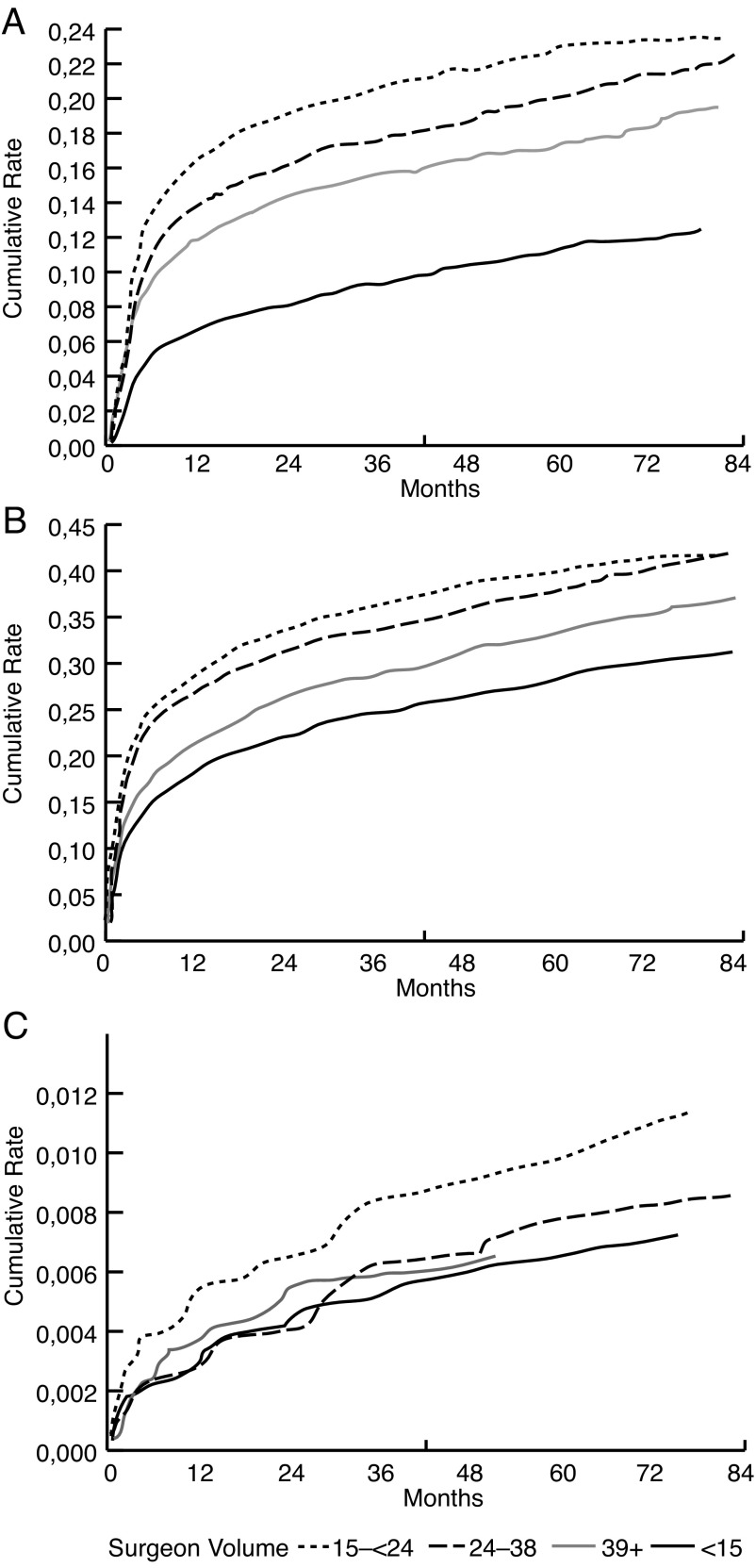
Over the study period, the five-year Kaplan-Meier cumulative incidence rate of minimally invasive urologic procedures was 34.2% (95% CI 33.4–35.0; Fig. 1) with cystoscopy being the most common procedure performed (58%). The five-year rate of hospital admission to manage a treatment-related complication was 17.5% (95% CI 16.9–18.1; Fig. 1) with 72% being for urinary obstruction. The open surgical procedure rate was the lowest at 0.8% (95% CI 0.6–0.9; Fig. 1), with cystotomy being the most common procedure (Table 1).
Fig. 1.
Kaplan-Meier cumulative incidence for three outcome measures: (a) hospital admissions (log-rank p value<0.0001); (b) minimally-invasive urologic procedures (log-rank p value<0.0001); and (c) open surgical procedures (log-rank p value=0.26).
Table 1.
Specific breakdown of procedures and diagnoses comprising each complication category
| Complication | Frequency (%) n=15 870 |
|---|---|
| Admission to hospital | 2749 (17) |
| Genitourinary or gastrointestinal fistula | 30 (1.1) |
| Genitourinary bleeding | 165 (6) |
| Renal insufficiency | 45 (1.6) |
| Infection | 370 (13.5) |
| Urinary obstruction | 2000 (72.8) |
| Bladder stone | 139 (5.1) |
| Minimally invasive urological procedures | 5368 (33.8) |
| Cystoscopy | 3103 (57.8) |
| Catheterization | 1184 (22.1) |
| Urethral dilation or incision | 1014 (18.9) |
| Calculi or clot removal | 67 (1.2) |
| Open surgical procedures | 115 (0.7) |
| Ureteric re-implant | <6* |
| Cystotomy | 124 (76) |
| Open bladder neck repair | <6* |
| Genitourinary or gastrointestinal fistula repair | 39 (24) |
| Cystectomy and conduit | <6* |
| Open lymphocele drainage | <6* |
True counts and rates suppressed for privacy reasons.
A total of 196 surgeons performed all RPs within the province over the study interval. The median number of surgeries performed per surgeon was 15 per year (mean 19, range: 1–131). The majority of RPs were done in a community-based hospital (n=8097, 51%) while the remaining were done in a university-affiliated hospital (n=4850, 31%) and we were unable to ascertain hospital status for 2923 patients (18%). There was a positive correlation between surgeon volume and academic hospital setting (Pearson correlation coefficient 0.31, p<0.0001).
The LHIN areas were grouped into four categories according to their population density. Ten percent (10%) of RPs were done in LHINs in the bottom quartile of population density, 30% in the second quartile, 35% in the third quartile, and 26% in LHINs in the highest quartile of population density.
Age and level of comorbidity were strong predictors for developing a treatment-related complication (Table 2). Patients treated by high-volume surgeons had significantly lower rates of hospital admissions and urologic procedures, while a non-significant trend was observed for open surgical procedures (Table 2). The adjusted HR of hospital admission for patients who had surgeons who performed more than 39 RPs per year was 0.54 (95% CI 0.47–0.61, p<0.0001), compared to patients who had surgeons who performed less than 15 RPs per year, with a clear linear trend by quartiles (Table 2). Similarly, the rates of minimally invasive urological procedures decreased significantly with patients who had surgeons with high surgical volumes (adjusted HR 0.69, 95% CI 0.64–0.75, p<0.0001; Table 2). No such trend was seen for open surgeries (Table 2). The risk of hospital admission was significantly lower in patients treated at academic hospitals than community hospitals, with an adjusted hazard ratio of 0.75 (95% CI 0.67–0.83, p<0.0001). Rates of minimally invasive urologic procedures and open surgeries did not statistically differ according to hospital type. There was no clear trend in the outcome measures based on population density.
Table 2.
Multivariable analysis using Cox proportional hazard modeling to examine factors predicting the development of complications following radical prostatectomy
| Treatment-related hospital admission | Minimally invasive urologic procedures | Open surgical procedures | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Age (>60 years vs. <60 years) | 1.20 | 1.10–1.30 | <0.0001 | 1.19 | 1.12–1.26 | <0.0001 | 1.18 | 0.79–1.75 | 0.4278 |
| Comorbidity (Total ADG) | 1.08 | 1.06–1.10 | <0.0001 | 1.08 | 1.06–1.09 | <0.0001 | 1.11 | 1.02–1.20 | 0.0175 |
| Surgeon volume (RP/year) | |||||||||
|
|
|||||||||
| ≤15 | Referent | Referent | Referent | ||||||
|
|
|||||||||
| 15–23 | 0.85 | 0.77–0.94 | 0.0018 | 0.97 | 0.90–1.04 | 0.3366 | 0.87 | 0.53–1.44 | 0.5940 |
| 24–38 | 0.74 | 0.67–0.82 | <0.0001 | 0.77 | 0.71–−0.83 | <0.0001 | 0.71 | 0.42–1.19 | 0.1908 |
| ≥39 | 0.54 | 0.47–0.61 | <0.0001 | 0.69 | 0.64–0.75 | <0.0001 | 0.83 | 0.47–1.45 | 0.5041 |
| LHIN population density | |||||||||
|
|
|||||||||
| <26 | Referent | Referent | Referent | ||||||
|
|
|||||||||
| 44–85 | 0.48 | 0.41–0.56 | <0.0001 | 0.85 | 0.77–0.95 | 0.0038 | 0.31 | 0.17–0.57 | 0.0002 |
| 97–324 | 0.94 | 0.82–1.07 | 0.3452 | 1.10 | 0.99–1.22 | 0.0796 | 0.42 | 0.24–0.75 | 0.0030 |
| >549 | 1.06 | 0.92–1.21 | 0.4333 | 1.30 | 1.18–1.45 | <0.0001 | 0.58 | 0.33–1.03 | 0.0610 |
| Hospital setting (community vs. academic) | 0.75 | 0.67–0.83 | <0.0001 | 0.94 | 0.88–1.01 | 0.1010 | 0.87 | 0.54–1.39 | 0.5502 |
ADG: aggregated disease groups; CI: confidence interval; HR: hazard ratio; RP: radical prostatectomy.
Discussion
In this large, population-based cohort of patients treated with open radical prostatectomy, we demonstrated that patients treated by high-volume surgeons have a lower risk of requiring hospitalization or minimally invasive urologic procedures to manage treatment-related complications.
Previous studies have shown that increasing surgical volume of the treating surgeon is inversely correlated with several measures of morbidity and mortality, including postoperative mortality, length of stay, transfusion rates, positive surgical margins rate, and recurrence rates.7 We recently showed that high surgical volume was associated with lower rates of artificial urinary sphincter insertion for the treatment of post-prostatectomy incontinence among a similar population-based cohort.8
Increasing hospital volume has been shown to decrease several important endpoints, including mortality, perioperative complications, transfusions, and recurrence-free survival in a systematic review,7 while an older review showed that higher surgeon volume was associated with decreased length of stay and risk of long-term incontinence or urologic complications, but not surgery-related mortality or positive margin rates.15 Begg et al examined patients in the SEER-Medicare registry and found that surgical volume (both at the individual and hospital level) was inversely related to postoperative complications and late urologic complications, but not surgery-related death.1 Similarly, among 5923 patients in the SEER-Medicare database, Lowrance et al showed that surgeon volume was inversely related to bladder neck and urethral stricture rates at one year following surgery.16 These results are consistent with our findings, though ours come from a much larger cohort and include patients of all ages while the previous reports were limited to patients over the age of 65 due to limitations of the Medicare databases.
We found that patients treated at academic hospitals had lower rates of hospital admissions, though not of urologic procedures or open surgeries. However, this finding must be considered in the context in which it was derived. Hospitals with residency and fellowship teaching programs have been shown to have higher annual case volumes than non-teaching hospitals17 and this is supported in our data, with a positive correlation between surgical volume and academic hospital setting. We then assessed the independent effect of academic hospital status after accounting for surgical volume. While initial studies showed that the protective effect of hospital volume does not persist after accounting for surgeon volumes,18 more recent reports have shown that treatment at an academic institution was protective even after accounting for case volume.19 Therefore, patients treated by high-volume surgeons at academic centres would be expected to derive benefit from both of these factors.
Beyond surgeon and hospital volume and hospital setting, surgeon fellowship training may influence the rate of these complications. Assessing positive margin rates rather than complication rates, Nayak et al recently showed that patients treated by uro-oncology fellowship-trained surgeons had lower rates of positive surgical margins, though there was no difference between non-fellowship-trained surgeons practicing in academic and community centres.20 We did not assess surgeon training in this analysis.
The main strength of this study is the comprehensiveness of the data provided due to the simple payer system in Ontario (OHIP). This allows us to capture all complications following treatment regardless of where in the province the patient sought care. This is of particular relevance for patients who seek care at centres of excellence — in single institution studies, these patients may receive care for complications at local hospitals and thus lead to underestimates of complication rates. We excluded patients treated with postoperative radiotherapy in order to better ascertain the influence of surgeon characteristics on these outcomes. However, we have shown that postoperative radiation increases the risk of each of these complications in this cohort.13
There are a number of limitations which result from the administrative nature of our data. We lack information on grade, stage, and preoperative prostate-specific antigen (PSA). The influence of local tumour characteristics on these complications is not well-characterized, though one may hypothesize that surgeons will be more aggressive in their treatment of higher-grade or -stage tumours, which may result in higher complication rates. We also lack data on patient characteristics, such as body mass index and prostate volume, which may influence complication rates. We were unable to assess complications following robotic prostatectomy, as it was not widely adopted in Ontario during the study interval. Finally, due to the administrative nature of the data analyzed, complications were defined based on procedures and diagnoses that act as surrogate for the actual complication. As a result, for some procedures, attribution to cancer treatment could not unequivocally be determined, as the procedure could be done for another reason. This may have led to overestimation of the incidence of outcomes. Chart review will be required to address this.
Conclusions
Patients undergoing RP have lower rates of treatment-related complications requiring hospital admission and urologic procedures when treated by surgeons with higher annual RP case volumes. These data support the idea of centralization of prostate cancer care.
Acknowledgments
This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Dr. Saskin had access to the raw data. The corresponding and senior authors had full access to all the data and take responsibility for the integrity of the data and the accuracy of the data analysis. This study was solely funded by the Ajmera Family Chair in Urologic Oncology awarded to the corresponding author. The funding source had no role in any part of the study.
The authors thank David Wu and Nicholas Gnidziejko for their assistance in the preparation of figures for this manuscript.
Footnotes
Competing interests: The authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–44. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 2.Eastham JA, Kattan MW, Riedel E, et al. Variations among individual surgeons in the rate of positive surgical margins in radical prostatectomy specimens. J Urol. 2003;170:2292–5. doi: 10.1097/01.ju.0000091100.83725.51. [DOI] [PubMed] [Google Scholar]
- 3.Choi WW, Gu X, Lipsitz SR, et al. The effect of minimally invasive and open radical prostatectomy surgeon volume. Urol Oncol. 2012;30:569–76. doi: 10.1016/j.urolonc.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Dash A, Dunn RL, Resh J, et al. Patient, surgeon, and treatment characteristics associated with homologous blood transfusion requirement during radical retropubic prostatectomy: Multivariate nomogram to assist patient counseling. Urology. 2004;64:117–22. doi: 10.1016/j.urology.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Hu JC, Wang Q, Pashos CL, et al. Utilization and outcomes of minimally invasive radical prostatectomy. J Clin Oncol. 2008;26:2278–84. doi: 10.1200/JCO.2007.13.4528. [DOI] [PubMed] [Google Scholar]
- 6.Leibman BD, Dillioglugil O, Abbas F, et al. Impact of a clinical pathway for radical retropubic prostatectomy. Urology. 1998;52:94–9. doi: 10.1016/S0090-4295(98)00130-7. [DOI] [PubMed] [Google Scholar]
- 7.Trinh QD, Bjartell A, Freedland SJ, et al. A systematic review of the volume-outcome relationship for radical prostatectomy. Eur Urol. 2013;64:786–98. doi: 10.1016/j.eururo.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nam RK, Herschorn S, Loblaw DA, et al. Population based study of long-term rates of surgery for urinary incontinence after radical prostatectomy for prostate cancer. J Urol. 2012;188:502–6. doi: 10.1016/j.juro.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2004;96:1358–67. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 10.Nam RK, Cheung P, Herschorn S, et al. Incidence of complications other than urinary incontinence or erectile dysfunction after radical prostatectomy or radiotherapy for prostate cancer: A population-based cohort study. Lancet Oncol. 2014;15:223–31. doi: 10.1016/S1470-2045(13)70606-5. . [DOI] [PubMed] [Google Scholar]
- 11.Bender JL, Wiljer D, Matthew A, et al. Fostering partnerships in survivorship care: Report of the 2011 Canadian genitourinary cancers survivorship conference. J Cancer Surviv. 2012;6:296–304. doi: 10.1007/s11764-012-0220-3. [DOI] [PubMed] [Google Scholar]
- 12. The Johns Hopkins ACG® Case-Mix System Reference Manual V.
- 13.Wallis CJ, Cheung P, Herschorn S, et al. Complications following surgery with or without radiotherapy or radiotherapy alone for prostate cancer. Br J Cancer. 2015;112:977–82. doi: 10.1038/bjc.2015.54. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallis CJ, Herschorn S, Saskin R, et al. Complications after radical prostatectomy or radiotherapy for prostate cancer: Results of a population-based, propensity score-matched analysis. Urology. 2015;85:621–8. doi: 10.1016/j.urology.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Wilt TJ, Shamliyan TA, Taylor BC, et al. Association between hospital and surgeon radical prostatectomy volume and patient outcomes: a systematic review. J Urol. 2008;180:820–8. doi: 10.1016/j.juro.2008.05.010. discussion 828–9. [DOI] [PubMed] [Google Scholar]
- 16.Lowrance WT, Elkin EB, Jacks LM, et al. Comparative effectiveness of prostate cancer surgical treatments: A population-based analysis of postoperative outcomes. J Urol. 2010;183:1366–72. doi: 10.1016/j.juro.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trinh QD, Sun M, Kim SP, et al. The impact of hospital volume, residency, and fellowship training on perioperative outcomes after radical prostatectomy. Urol Oncol. 2014;32:29.e13–20. doi: 10.1016/j.urolonc.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu JC, Gold KF, Pashos CL, et al. Role of surgeon volume in radical prostatectomy outcomes. J Clin Oncol. 2003;21:401–5. doi: 10.1200/JCO.2003.05.169. [DOI] [PubMed] [Google Scholar]
- 19.Trinh QD, Schmitges J, Sun M, et al. Radical prostatectomy at academic versus nonacademic institutions: A population-based analysis. J Urol. 2011;186:1849–54. doi: 10.1016/j.juro.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 20.Nayak JG, Drachenberg DE, Mau E, et al. The impact of fellowship training on pathological outcomes following radical prostatectomy: A population-based analysis. BMC Urol. 2014;14:82. doi: 10.1186/1471-2490-14-82. [DOI] [PMC free article] [PubMed] [Google Scholar]