Abstract
Background
Serum folate concentration is lower in individuals with the methylenetetrahydrofolate reductase (MTHFR) 677TT genotype than in those with the MTHFR 677CC or 677CT genotypes. Since studies considering folate intake are limited, we examined the association between folate intake and serum folate levels, according to the genotype.
Methods
The subjects comprised 170 Japanese persons (74 males and 96 females) aged 20-75 years who visited a clinic to test for Helicobacter pylori infection. Folate intake was estimated using a semiquantitative food-frequency questionnaire, and serum folate was measured in the residual fasting blood samples of the subjects. MTHFR C677T was genotyped using polymerase chain reaction.
Results
The geometric means of serum folate level were 6.19, 6.20, and 5.17 ng/mL among the 60 participants with the 677CC genotype, 90 participants with the 677CT genotype, and 20 participants with the 677TT genotype, respectively. No difference was noted in the mean folate intake estimated using the food-frequency questionnaire. Regression analysis showed that loge(serum folate) adjusted for age, sex, and loge(folate intake) was significantly lower among those with the 677TT genotype than among those with the 677CT or 677CC genotypes (p = 0.01). The adjusted reduction in serum folate was 20.2% (95% confidence interval, 5.4-32.6%) in the case of the 677TT genotype relative to the levels in the case of the 677CC/677CT genotypes. When folate intake was adjusted for total energy intake, using the residual method, the slope of the regression line for 677TT was smaller than those of the regression lines for 677CC and 677CT.
Conclusion
Individuals with the 677TT genotype may need to consume more folate to maintain serum folate levels similar to those found in individuals with the 677CC/677CT genotypes.
Key words: Eating, Folic Acid, MTHFR C677T, Japanese
INTRODUCTION
Shortage of dietary folate has been proven to elevate homocysteine levels.1-3 Folate supply substantially reduces the plasma homocysteine levels among individuals with hypercysteinemia. Low serum folate and the resultant high plasma homocysteine levels are one of the causes of neural tube defects (NTDs) in children;4,5 neuropsychiatric conditions;6 cardiovascular diseases, including atherosclerosis;7-10 and cancers.11 For the prevention of NTDs, official bodies worldwide recommend that women take 400 µg folate/day before conception and during early pregnancy. However, the implementation of this recommendation is difficult. Although folic acid supplements are effective in optimizing the folate status in women,12 they are not an effective strategy for the primary prevention of NTDs due to poor patient compliance.13 Therefore, mandatory fortification of grain products with folic acid was introduced in several countries, including the United States14 and Canada.15 Despite an impressive decrease in the prevalence of NTDs after this fortification,16,17 the policy remains controversial. The fortification not only delivers the required nutrient levels to the high-risk group but also provides high-dose folate products to a proportion of the general population. Of the greatest concern is the potential for the high intake of folic acid that in turn masks anemia resulting from vitamin B12 deficiency in the elderly, thereby allowing the concomitant irreversible nerve degeneration to go undetected.18 Moreover, the United States and Canada have experienced abrupt reversals of the downward trend in colorectal cancer incidence concurrently with the nationwide fortification of enriched uncooked cereal grains with folic acid.19 A recent large intervention study showed that 1 mg/day folic acid might increase the risk of advanced lesions and adenoma multiplicity.20
In the metabolic pathway involved in the conversion of folate to homocysteine, 10-methylenetetrahydrofolate reductase (MTHFR, EC 1.5.1.20) is one of the key enzymes, others being methionine synthase and cystathionine beta synthase. MTHFR metabolizes 5,10-methylenetetrahydrofolate (THF) to 5-methyl-THF-the primary circulating form of folate. In the gene that codes MTHFR, a C to T polymorphism was reported at position 677 (MTHFR C677T), which causes the substitution of alanine with valine. This substitution leads to a 30% decrease in the enzyme activity in heterozygotes and a 60% decrease in homozygotes.21 The frequency of the T allele approaches 30% in many ethnic groups.22 Several studies have shown that hyperhomocysteinemia was more frequent among individuals with the TT genotype than among those with the CC genotype.1,7,23
Genotype has also been associated with plasma or serum folate concentrations.24-31 However, studies regarding dietary folate intake are relatively rare. Therefore, this study aimed to assess the effect of the MTHFR C677T genotype with adjustments for folate intake. We also examined the association between folate intake and serum folate levels, according to the MTHFR C677T genotype.
METHODS
Study Subjects
The subjects were individuals who visited the Daiko Medical Center, Nagoya University in Nagoya, Japan, and provided written consent to participate in the present study. The Center provided preventive care that was not covered by health insurance. From July 20, 2004 through December 1, 2005, 220 individuals visited the Center to undergo Helicobacter pylori infection tests and subsequent eradication treatment. Those with gastric cancer (n = 8), idiopathic thrombocytopenic purpura (n = 3), or chronic urticaria (n = 5) were excluded from the present study. Among the remaining 204 visitors, 1 person who was 19 years old and 19 persons who did not provide consent were also excluded. Blood for research purposes could not be drawn from 9 persons. The quantity of 4 of the blood samples was insufficient for the measurement of serum folate, and the semiquantitative food-frequency questionnaire (FFQ) was not administered to 1 participant. Analyses were conducted for the remaining 170 participants.
This study was approved by the Nagoya University School of Medicine Ethics Committee (approval No.155, issued on June 2004).
Lifestyle Questionnaire and Biomarker Measurements
Participants were asked to complete a self-administered questionnaire with questions regarding the following: sex, age, supplement use, drug use, alcohol use, smoking, and disease history. Participants who had smoked less than 100 cigarettes in their lifetime were categorized as never smokers, and the rest were categorized as ever smokers. Individuals were categorized as current alcohol drinkers if they drank alcoholic beverages at least once a week and as non-drinkers otherwise. The participants were also asked to complete a validated FFQ developed by Takahashi et al.32 The reproducibility and validity of this FFQ have been measured for most nutrients but not for folate.
The study participants were required to fast overnight for a urea breath test for identifying H. pylori. In the morning, peripheral blood was drawn for anti-H. pylori antibody tests. The blood samples were treated in an indoor laboratory. All serum samples were obtained by centrifugation within 3 h of blood collection, with a few exceptions (maximum 5 h). Hemolysis was not visually observed in any serum sample. The residual sera of the participants were preserved at -40°C and used for serum folate measurement. The serum folate levels were measured using the chemiluminescence enzyme immunoassay method. The laboratory staff was blinded to the genotype of the subjects.
Genotyping
DNA was extracted from the buffy coat and preserved at -40°C, using BioRobot® EZ1 (Qiagen Group, Tokyo). The MTHFR C677T polymorphism was genotyped using polymerase chain reaction (PCR) with confronting two-pair primers.33 Each 25-µL reaction tube contained 50-80 ng DNA, 0.12 mM dNTP, 12.5 pmol of each primer, 0.5 U AmpliTaq Gold (Perkin-Elmer, Foster City, CA), and 2.5 µL 10× PCR buffer, including 15 mM MgCl2. PCR was conducted with initial denaturation at 95°C for 10 min, 30 cycles of denaturation at 95°C for 1 min, annealing at 60°C for 1 min, extension at 72°C for 1 min, and a final extension at 72°C for 5 min. The primers were F1: 5′-AGC CTC TCC TGA CTG TCA TCC-3′, R1: 5′-TGC GTG ATG ATG AAA TCG G-3′, F2: 5′-GAG AAG GTG TCT GCG GGA GT-3′, and R2: 5′-CAT GTC GGT GCA TGC CTT-3′. The length of the amplified DNA fragments was 128 bp for the C allele, 93 bp for the T allele, and 183 bp for the common band.
Statistical Analysis
The distributions of serum folate levels and dietary folate intake were skewed; therefore, natural logarithmic transformation was applied for data analyses. Energy-adjusted dietary folate intake was also estimated using the residual method.34 Log-transformed serum folate was examined using analysis of covariance and linear regression analysis. Hardy-Weinberg equilibrium was applied to test for genotype frequency. All statistics were calculated using the computer program STATA® Version 8.2 (StataCorp LP, College Station, TX). The effect of the interaction between genotype and dietary folate intake on serum folate levels was assessed using a multivariate linear regression model. In this assessment, the log-transformed continuous energy-adjusted residuals of dietary folate intake were used. The p-value for the interaction was calculated using the interaction term of the genotype group multiplied by the continuous log (dietary folate intake). Two-sided P-values less than 0.05 were considered to be statistically significant. Adjustments for multiple comparisons were not performed in any analyses because this was an exploratory study.
RESULTS
A total of 170 Japanese subjects (74 males and 96 females) aged 20-73 years were included in this study. The frequency of the MTHFR C677T polymorphism among subjects with the CC, CT, and TT genotypes was 35.3%, 52.9%, and 11.8%, respectively. In this study, the MTHFR C677T genotype frequency did not deviate from the Hardy-Weinberg equilibrium (P = 0.12).
The characteristics of patients, according to the MTHFR C677T genotype, are shown in Table 1. No difference was present in the geometric mean of folate intake, as estimated using the FFQ: 252.4, 243.8, and 263.0 µg/day in the case of the CC, CT, and TT genotypes, respectively. No significant differences were observed in the age, sex, serum folate, dietary folate intake, dietary vitamin B6 intake, dietary vitamin B12 intake, energy intake, supplement use, smoking status, and alcohol consumption status.
Table 1. Characteristics according to methylenetetrahydrofolate reductase (MTHFR) C677T genotype.
| Characteristics | MTHFR | P* | ||
| CC (n = 60) |
CT (n = 90) |
TT (n = 20) |
||
| (Mean ± standard deviation) | ||||
| Age (years) | 51.1 ± 11.1 | 50.7 ± 11.9 | 47.9 ± 14.5 | 0.57 |
| Vitamin B6 intake (mg/day) | 6.8 ± 3.0 | 6.9 ± 3.0 | 7.5 ± 3.2 | 0.55 |
| Vitamin B12 intake (µg/day) | 265.3 ± 79.2 | 256.0 ± 78.9 | 278.7 ± 97.7 | 0.67 |
| Energy intake (kcal/day) | 1804.3 ± 341.4 | 1805.1 ± 366.0 | 1836.3 ± 492.7 | 0.94 |
| Folate intake (µg/day) | 264.3 ± 79.2 | 256.0 ± 78.9 | 278.7 ± 97.7 | 0.51 |
| (%) | ||||
| Sex (% of males) | 40.0 | 47.8 | 35.0 | 0.46 |
| Vitamin B complex or folic acid supplement users | 5.0 | 3.3 | 5.0 | 0.55 |
| Ever smokers | 5.0 | 14.4 | 15.0 | 0.17 |
| Current alcohol drinkers | 71.7 | 75.6 | 65.0 | 0.72 |
* : Analysis of variance (ANOVA) was used to test the difference in continuous variables, and the chi-square test was used for categorical variables.
The geometric mean of the serum folate level was 6.19 ng/mL among the 60 participants with the 677CC genotype, 6.20 ng/mL among the 90 participants with the 677CT genotype, and 5.17 ng/mL among the 20 participants with the 677TT genotype. Table 2 shows the sex- and age-adjusted means and the standard error of the log-converted serum folate levels according to the MTHFR C677T genotype. No significant difference was detected in the mean serum folate level among the different genotypes. However, among the subjects with a high dietary folate intake (i.e., 50% of all the subjects studied), age-, sex-, and energy-adjusted regression analyses revealed that the log-converted mean was significantly lower in subjects with the TT genotype than in those with the CC genotype (P = 0.01). In contrast, the difference was not significant in the remaining subjects, who had a low dietary folate intake.
Table 2. Age- and sex-adjusted mean and standard error of logarithmic-transformed serum folate level (ng/mL).
| Sex- and age-adjusted* | Sex-, age-, and energy-adjusted† | ||||||
| CC | CT | TT | CC | CT | TT | ||
| All subjects | log(serum folate) | 1.81 ± 0.05 | 1.83 ± 0.04 | 1.64 ± 0.09 | 1.81 ± 0.05 | 1.84 ± 0.04 | 1.63 ± 0.09 |
| (n = 170) | n | 60 | 90 | 20 | 60 | 90 | 20 |
| Dietary folate intake | |||||||
| Low (<255 µg/day) | log(serum folate) | 1.65 ± 0.07 | 1.73 ± 0.06 | 1.49 ± 0.12 | 1.67 ± 0.07 | 1.72 ± 0.06 | 1.63 ± 0.12 |
| (n = 85) | n | 28 | 47 | 9 | 28 | 47 | 9 |
| High (>255 µg/day) | log(serum folate) | 1.97 ± 0.07 | 1.94 ± 0.06 | 1.77 ± 0.13 | 1.94 ± 0.07 | 1.96 ± 0.06 | 1.60 ± 0.11‡ |
| (n = 85) | n | 32 | 43 | 11 | 32 | 43 | 11 |
* : Adjusted for age and sex by using analysis of covariance (ANCOVA)
† : Adjusted for age, sex, and energy intake by ANCOVA
‡ : Siginificantly different from CC genotype: P=0.01
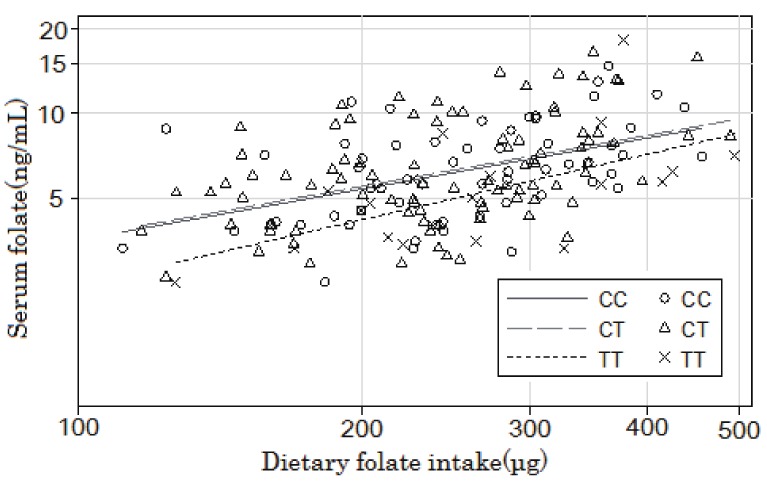
Figure 1 shows the unadjusted regression lines for each MTHFR genotype on a logarithmic scale obtained by plotting the serum folate concentration against the dietary folate intake (non-energy adjusted values). The adjusted analysis for age and sex showed a significant positive relationship between the logarithm of serum folate (y) and that of dietary folate intake (x) in the CC and CT groups (CC: y = 0.56x - 1.38, R2 = 0.29, P for slope = 0.001 and CT: y = 0.44x - 0.87, R2 = 0.29, P for slope = 0.003), whereas the corresponding relationship in the TT group was marginally significant (y = 0.65x - 2.00, R2 = 0.54, P for slope = 0.07). Regression analysis showed that the logarithm of serum folate adjusted for age, sex, and logarithm of folate intake was significantly lower among subjects with the 677TT genotype than among those with the 677CT or 677CC genotypes. The reduction in the serum folate level was 20.2% (95% confidence interval: 5.4-32.6%) for the 677TT genotype relative to those for the 677CC/677CT genotypes.
Figure 1. Regression lines for each MTHFR genotype on a logarithmic scale obtained by plotting the serum folate concentration against the dietary folate intake.
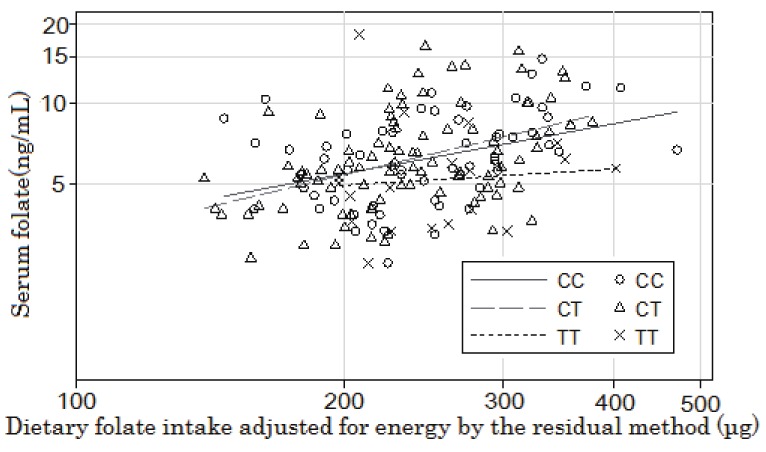
Figure 2 shows the unadjusted regression lines obtained by plotting the logarithmic serum folate levels against the loge(dietary folate intake) values that were energy-adjusted by the residual method for each genotype group. The age- and sex-adjusted slope was significant in both the CC and CT genotype groups (CC: y = 0.55x - 1.91, R2 = 0.29, P for slope = 0.007 and CT: y = 0.59x - 2.00, R2 = 0.31, P for slope = 0.002). The corresponding slope in the TT group was small and not significant (y = 0.09x + 0.80, R2 = 0.83, P for slope = 0.78). However, the slopes of the regression lines adjusted for sex and age did not significantly differ between the CC + CT and TT genotypes (P = 0.13 for the interaction).
Figure 2. Relationship between the MTHFR genotype and the serum folate level and the effect of energy-adjusted dietary folate intake on the latter.
When 7 supplement users were excluded, no substantial changes were seen in these results (data not shown); 6 of them had taken vitamin B supplements not containing folic acid, and 1 had taken folic acid supplements. Similarly, adjustment for alcohol consumption did not substantially change the results (data not shown).
DISCUSSION
This cross-sectional study aimed to evaluate the effect of the MTHFR C677T polymorphism on the association between serum folate levels and dietary folate intake. It has been reported that subjects with the TT genotype of MTHFR C677T have lower serum folate levels; however, it is necessary to compare the levels under conditions of equal dietary folate intake. Our results confirmed that subjects with the TT genotype had lower serum folate levels even after adjustments for the dietary folate intake. The analysis without energy-intake adjustment showed that serum folate levels were lower among subjects with the TT genotype than the levels among subjects with other genotypes; the average reduction was approximately 20%. On the other hand, sex-, age-, and energy-adjusted regression analyses showed that remarkably lower serum folate levels were present in subjects with the TT genotype who had a high folate intake. However, this did not explain which estimates were correct, i.e., the ones in the subjects with high folate intake or in those in the subjects with low folate intake. Previously, 3 studies have reported the association between serum folate and MTHFR C677T while taking dietary folate intake into account; the results were inconsistent. One study reported no difference in the serum folate levels among subjects with different MTHFR genotypes.35 In another study, a remarkable reduction in the serum folate concentration was reported among subjects with the TT genotype and low dietary folate intake.36 The third study reported a significant reduction in serum folate levels among subjects with this genotype who had high dietary folate intake.37 The latter 2 studies estimated dietary folate intake using a validated FFQ, in which energy intake was not adjusted.
In general, nutrient intakes show a positive correlation with the total energy intake. This tendency is particularly strong in the case of the estimates obtained using FFQs. Therefore, when the association between nutrient intake and disease is analyzed, the influence of the total energy intake should be excluded. In the simplest method involving the calculation of nutrient density, the crude nutrient intake is divided by the total energy intake; however, this does not completely remove the influence of total energy intake. Therefore, the method of residuals is often used during linear regression analysis. The residual method appears to be more effective for detecting associations with disease risks. Generally, the residual and nutrient density methods yield similar results.34 The body size of the participants was not adjusted in this analysis. Although there were no extreme observations, an analysis adjusted for energy intake could partly remove the influence of the difference in body size.38 However, this may not be applicable to the identification of dose-response relationships according to the MTHFR genotype.
We determined the MTHFR genotype of 170 individuals and found that 35.3% were homozygous CC, 52.9% were heterozygous CT, and 11.8% were homozygous TT. These frequencies were similar to those reported in other Japanese subjects.39-41 Although the present subjects consisted of visitors to an H. pylori clinic, the results may be extended to the general population in Japan.
Our results suggested that subjects with the TT genotype had approximately 20% lower serum folate levels, based on the sex- and age-adjusted analyses. This indicates that people with the TT genotype may have to consume more dietary folate than people with the CC or CT genotypes. Because the slope in the case of subjects with the TT genotype in the sex- and age-adjusted analyses was 0.65, approximately 1.4 (exp[(log{1/ (1 - 0.2)}/0.65]) times higher intake may be required to ensure that these individuals have the same level of serum folate as those in individuals with other genotypes.
The bioavailability of food folates relative to folic acid supplementation ranges between 10% and 98%, depending primarily on the method of fortification.42-47 The uncertainty regarding folate bioavailability48 is of particular concern in countries where folic acid fortification is not adopted or permitted45 because such countries depend largely on natural food folates to optimize nutritional status. In the United States, folic acid fortification is mandatory and relatively less reliance is placed on natural folate sources. Thus, the dietary recommendations are based mainly on the bioavailability of folic acid added to food, and less on natural food folates, resulting in the introduction of dietary folate equivalents (DFEs).49 The estimated DFE conversion factor of 1.7 is primarily based on a metabolic study in non-pregnant women that estimated the bioavailability of food folates as no more than 50% that of folic acid.45 Another study based on folic acid-fortified food showed that the bioavailability of free folic acid was 85%.50 Very few people use folic acid supplements in Japan. The largest source of dietary folate is vegetables (38-58% of total intake).51 Moreover, dietary folate is different from folic acid, in which the risk of the excess intake is not reported. Therefore, the estimated average requirement of folate may have to be calculated by focusing on individuals with the TT genotype. We must further explore the methods of optimizing folate intake in individuals with the TT genotype.
Our study had several limitations. First, this study had a relatively small sample size because it was an exploratory analysis. The results of subgroup analysis, such as the coefficient for the TT genotype in the non-energy adjusted analysis, were not significant because of the low statistical power (48.7% for a two-sided test with alpha error = 0.05 when the slope for the TT genotype in the population was assumed to be 0.65 and its standard deviation = 0.34). However, the sample size was sufficient for the main purpose, i.e., demonstrating significant differences in the serum folate concentrations among individuals with different MTHFR genotypes after adjusting the folate intake. Second, the reproducibility and validity of the FFQ used in our study have not been reported for folate, although the FFQ is reasonably reproducible and valid for many other nutrients. The correlation coefficients for the intakes of the major sources of folate determined using the FFQs and weighed dietary records for 7 continuous days were moderate: 0.462 for green-yellow vegetables and 0.635 for fruits.32
In conclusion, this study confirmed that Japanese people with the TT genotype had lower serum folate levels than those in people with the CT or CC genotypes, even after the folate intake was adjusted. The slope of the regression line depended on the method used to estimate folate intake, particularly, in the case of people with the TT genotype. These results highlight the need for individuals with the TT genotype to consume slightly more than the recommended folate dose through natural foods or folic acid supplements.
ACKNOWLEDGMENT
This work was supported in part by a Grant-in-Aid for Scientific Research on Special Priority Areas of Cancer from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors are grateful to Ms. Yoko Mitsuda and Ms. Mio Kurata for their technical assistance.
REFERENCES
- 1.Homocysteine Lowering Trialists’ Collaboration . Lowering blood homocysteine with folic acid based supplements: meta-analysis of randomised trials. BMJ 1998; 316: 894-8. 10.1136/bmj.316.7135.894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermeulen EG, Stehouwer CD, Twisk JW, van den Berg M, de Jong SC, Mackaay AJ, et al. Effect of homocysteine-lowering treatment with folic acid plus vitamin B6 on progression of sub-clinical atherosclerosis: a randomised, placebo-controlled trial. Lancet 2000; 355: 517-22. 10.1016/S0140-6736(99)07391-2 [DOI] [PubMed] [Google Scholar]
- 3.Wald DS, Bishop L, Wald NJ, Law M, Hennessy E, Weir D, et al. Randomized trial of folic acid supplementation and serum homocysteine levels. Arch Intern Med 2001; 161: 695-700. 10.1001/archinte.161.5.695 [DOI] [PubMed] [Google Scholar]
- 4.MRC Vitamin Study Research Group . Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991; 338: 131-7. 10.1016/0140-6736(91)90133-A [DOI] [PubMed] [Google Scholar]
- 5.Czeizel E, Dudás I. Prevention of the first occurrence of anencephaly and spina bifida with periconceptional multivitamin supplementation (conclusion). Orv Hetil 1994; 135: 2313-7 (in Hungarian). [PubMed] [Google Scholar]
- 6.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 2002; 346: 476-83. 10.1056/NEJMoa011613 [DOI] [PubMed] [Google Scholar]
- 7.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 2002; 325: 1202. 10.1136/bmj.325.7374.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 1995; 274: 1049-57. 10.1001/jama.1995.03530130055028 [DOI] [PubMed] [Google Scholar]
- 9.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med 1998; 338: 1042-50. 10.1056/NEJM199804093381507 [DOI] [PubMed] [Google Scholar]
- 10.Hankey GJ, Eikelboom JW. Homocysteine and vascular disease. Indian Heart J 2000; 52: S18-26. [PubMed] [Google Scholar]
- 11.Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr 2000; 130: 129-32. [DOI] [PubMed] [Google Scholar]
- 12.Daly S, Mills JL, Molloy AM, Conley M, Lee YJ, Kirke PN, et al. Minimum effective dose of folic acid for food fortification to prevent neural-tube defects. Lancet 1997; 350: 1666-9. 10.1016/S0140-6736(97)07247-4 [DOI] [PubMed] [Google Scholar]
- 13.Wild J, Sutcliffe M, Schorah CJ, Levene MI. Prevention of neural-tube defects. Lancet 1997; 350: 30-1. 10.1016/S0140-6736(05)66239-3 [DOI] [PubMed] [Google Scholar]
- 14.United States Food and Drug Administration Foods Standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Fed Regist 1996; 61: 8781-97. [Google Scholar]
- 15.Health Canada. Food and drug regulations, Amendment Schedule no. 1066. Ottawa: Health Canada; 1997. [Google Scholar]
- 16.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001; 285: 2981-6. 10.1001/jama.285.23.2981 [DOI] [PubMed] [Google Scholar]
- 17.Ray JG, Meier C, Vermeulen MJ, Boss S, Wyatt PR, Cole DE. Association of neural tube defects and folic acid food fortification in Canada. Lancet 2002; 360: 2047-8. 10.1016/S0140-6736(02)11994-5 [DOI] [PubMed] [Google Scholar]
- 18.Savage DG, Lindenbaum J. Folate-cobalamin interactions. In: Bailey LB, editors. Folate in health and disease. New York: Marcel Dekker; 1995. p. 237-85. [Google Scholar]
- 19.Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev 2007; 16: 1325-9. 10.1158/1055-9965.EPI-07-0329 [DOI] [PubMed] [Google Scholar]
- 20.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007; 297: 2351-9. 10.1001/jama.297.21.2351 [DOI] [PubMed] [Google Scholar]
- 21.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995; 10: 111-3. 10.1038/ng0595-111 [DOI] [PubMed] [Google Scholar]
- 22.Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, Renlund M, et al. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world wide. J Med Genet 2003; 40: 619-25. 10.1136/jmg.40.8.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lwin H, Yokoyama T, Date C, Yoshiike N, Kokubo Y, Tanaka H. Are the associations between life-style related factors and plasma total homocysteine concentration different according to polymorphism of 5,10-methylenetetrahydrofolate reductase gene (C677T MTHFR)? A cross-sectional study in a Japanese rural population. J Epidemiol 2002; 12: 126-35. 10.2188/jea.12.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Put NM, Steegers-Theunissen RP, Frosst P, Trijbels FJ, Eskes TK, van den Heuvel LP, et al. Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. Lancet 1995; 346: 1070-1. 10.1016/S0140-6736(95)91743-8 [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Stampfer MJ, Hennekens CH, Frosst P, Selhub J, Horsford J, et al. Methylenetetrahydrofolate reductase polymorphism, plasma folate, homocysteine, and risk of myocardial infarction in US physicians. Circulation 1996; 94: 2410-6. 10.1161/01.CIR.94.10.2410 [DOI] [PubMed] [Google Scholar]
- 26.Nelen WL, Blom HJ, Thomas CM, Steegers EA, Boers GH, Eskes TK. Methylenetetrahydrofolate reductase polymorphism affects the change in homocysteine and folate concentrations resulting from low dose folic acid supplementation in women with unexplained recurrent miscarriages. J Nutr 1998; 128: 1336-41. [DOI] [PubMed] [Google Scholar]
- 27.Harmon DL, Woodside JV, Yarnell JW, McMaster D, Young IS, McCrum EE, et al. The common ‘thermolabile’ variant of methylene tetrahydrofolate reductase is a major determinant of mild hyperhomocysteinaemia. QJM 1996; 89: 571-7. 10.1093/qjmed/89.8.571 [DOI] [PubMed] [Google Scholar]
- 28.Hustad S, Ueland PM, Vollset SE, Zhang Y, Bjorke-Monsen AL, Schneede J. Riboflavin as a determinant of plasma total homocysteine: effect modification by the methylenetetrahydrofolate reductase C677T polymorphism. Clin Chem 2000; 46: 1065-71. [PubMed] [Google Scholar]
- 29.Deloughery TG, Evans A, Sadeghi A, McWilliams J, Henner WD, Taylor LM Jr, et al. Common mutation in methylenetetrahydrofolate reductase. Correlation with homocysteine metabolism and late-onset vascular disease. Circulation 1996; 94: 3074-8. 10.1161/01.CIR.94.12.3074 [DOI] [PubMed] [Google Scholar]
- 30.Schwartz SM, Siscovick DS, Malinow MR, Rosendaal FR, Beverly RK, Hess DL, et al. Myocardial infarction in young women in relation to plasma total homocysteine, folate, and a common variant in the methylenetetrahydrofolate reductase gene. Circulation 1997; 96: 412-7. 10.1161/01.CIR.96.2.412 [DOI] [PubMed] [Google Scholar]
- 31.McQuillan BM, Beilby JP, Nidorf M, Thompson PL, Hung J. Hyperhomocysteinemia but not the C677T mutation of methylenetetrahydrofolate reductase is an independent risk determinant of carotid wall thickening. The Perth Carotid Ultrasound Disease Assessment Study (CUDAS). Circulation 1999; 99: 2383-8. 10.1161/01.CIR.99.18.2383 [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Yoshimura Y, Sekimoto T, Kunii D, Komatsu R, Yamamoto S. Making the food frequency questionnaire based on food group frequency based on food group for intake investigation according to nutrient and food group and validity. Eiyougaku Zasshi 2001; 59: 221-32 (in Japanese) 10.5264/eiyogakuzashi.59.221 [DOI] [Google Scholar]
- 33.Hamajima N, Saito T, Matsuo K, Kozaki K, Takahashi T, Tajima K. Polymerase chain reaction with confronting two-pair primers for polymorphism genotyping. Jpn J Cancer Res 2000; 91: 865-8. 10.1111/j.1349-7006.2000.tb01026.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown CC, Kipnis V, Freedman LS, Hartman AM, Schatzkin A, Wacholder S. Energy adjustment methods for nutritional epidemiology: the effect of categorization. Am J Epidemiol 1994; 139: 323-8. [DOI] [PubMed] [Google Scholar]
- 35.de Bree A, Verschuren WM, Bjørke -Monsen AL, van der Put NM, Heil SG, Trijbels FJ, et al. Effect of the methylenetetrahydrofolate reductase 677C-->T mutation on the relations among folate intake and plasma folate and homocysteine concentrations in a general population sample. Am J Clin Nutr 2003; 77: 687-93. [DOI] [PubMed] [Google Scholar]
- 36.Trinh BN, Ong CN, Coetzee GA, Yu MC, Laird PW. Thymidylate synthase: a novel genetic determinant of plasma homocysteine and folate levels. Hum Genet 2002; 111: 299-302. 10.1007/s00439-002-0779-2 [DOI] [PubMed] [Google Scholar]
- 37.Hiraoka M. Folate intake, serum folate, serum total homocysteine levels and methylenetetrahydrofolate reductase C677T polymorphism in young Japanese women. J Nutr Sci Vitaminol (Tokyo) 2004; 50: 238-45. 10.3177/jnsv.50.238 [DOI] [PubMed] [Google Scholar]
- 38.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997; 65: 1220S-8S. [DOI] [PubMed] [Google Scholar]
- 39.Hiraoka M, Kato K, Saito Y, Yasuda K, Kagawa Y. Genenutrient and gene-gene interactions of controlled folate intake by Japanese women. Biochem Biophys Res Commun 2004; 316: 1210-6. 10.1016/j.bbrc.2004.02.174 [DOI] [PubMed] [Google Scholar]
- 40.Hamajima N, Saito T, Matsuo K, Suzuki T, Nakamura T, Matsuura A, et al. Genotype frequencies of 50 polymorphisms for 241 Japanese non-cancer patients. J Epidemiol 2002; 12: 229-36. 10.2188/jea.12.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyaki K, Murata M, Kikuchi H, Takei I, Nakayama T, Watanabe K, et al. Assessment of tailor-made prevention of atherosclerosis with folic acid supplementation: randomized, double-blind, placebo-controlled trials in each MTHFR C677T genotype. J Hum Genet 2005; 50: 241-8. 10.1007/s10038-005-0247-7 [DOI] [PubMed] [Google Scholar]
- 42.Baker H, Jaslow SP, Frank O. Severe impairment of dietary folate utilization in the elderly. J Am Geriatr Soc 1978; 26: 218-21. 10.1111/j.1532-5415.1978.tb01962.x [DOI] [PubMed] [Google Scholar]
- 43.Tamura T, Stokstad EL. The availability of food folate in man. Br J Haematol 1973; 25: 513-32. 10.1111/j.1365-2141.1973.tb01763.x [DOI] [PubMed] [Google Scholar]
- 44.Colman N, Green R, Metz J. Prevention of folate deficiency by food fortification. II. Absorption of folic acid from fortified staple foods. Am J Clin Nutr 1975; 28: 459-64. [DOI] [PubMed] [Google Scholar]
- 45.Sauberlich HE, Kretsch MJ, Skala JH, Johnson HL, Taylor PC. Folate requirement and metabolism in nonpregnant women. Am J Clin Nutr 1987; 46: 1016-28. [DOI] [PubMed] [Google Scholar]
- 46.Prinz-Langenohl R, Bronstrup A, Thorand B, Hages M, Pietrzik K. Availability of food folate in humans. J Nutr 1999; 129: 913-6. [DOI] [PubMed] [Google Scholar]
- 47.Brouwer IA, van Dusseldorp M, West CE, Meyboom S, Thomas CM, Duran M, et al. Dietary folate from vegetables and citrus fruit decreases plasma homocysteine concentrations in humans in a dietary controlled trial. J Nutr 1999; 129: 1135-9. [DOI] [PubMed] [Google Scholar]
- 48.McNulty H. Increasing evidence in favour of mandatory fortification with folic acid. Br J Nutr 2001; 86: 425-6. 10.1079/BJN2001440 [DOI] [PubMed] [Google Scholar]
- 49.Institute of Medicine, Food and Nutrition Board. Dietary reference intake for thiamine, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- 50.Pfeiffer CM, Rogers LM, Bailey LB, Gregory JF 3rd. Absorption of folate from fortified cereal-grain products and of supplemental folate consumed with or without food determined by using a dual-label stable-isotope protocol. Am J Clin Nutr 1997; 66: 1388-97. [DOI] [PubMed] [Google Scholar]
- 51.Yoshino K, Inagawa M, Oshima M, Yokota K, Umesawa M, Enbo M, et al. Trends in dietary intake of folate, vitamins B6, and B12 among Japanese adults in two rural communities from 1974 through 2001. J Epidemiol 2005; 15: 29-37. 10.2188/jea.15.29 [DOI] [PMC free article] [PubMed] [Google Scholar]