Abstract
SCOT-HEART and PROMISE represent the 2 largest and most comprehensive cardiovascular imaging outcome trials in patients with stable chest pain, and provide significant insights into patient diagnosis, management, and outcomes. These trials are particularly timely, given the well-recognized knowledge gaps and widespread use of noninvasive imaging. The overall goal of this review is to distill the data generated from these 2 pivotal trials to better inform the practicing clinician in the selection of noninvasive testing for stable chest pain. Similarities and differences between SCOT-HEART and PROMISE are highlighted, and clinical and practical implications discussed. Both trials show that CCTA should have a greater role in the diagnostic pathway of patients with stable chest pain.
Keywords: Angina Pectoris, Coronary Artery Disease, Noninvasive Cardiac Imaging, Patient Selection
Introduction
Angina is highly prevalent in the general population and increases with age in both sexes, occurring in 10% to 11% of those >80 years of age (1). New-onset, stable chest pain is a common clinical problem that results in approximately 4 million stress tests annually in the United States (2). At the same time, patients diagnosed with noncardiac chest pain account for a third of patients who subsequently die from cardiovascular disease or have an acute coronary syndrome during 5 years of follow-up (3). Therefore, despite several decades of noninvasive cardiovascular testing development and experience, improved diagnostic accuracy and risk stratification is still needed (4).
Significant variations in diagnostic strategies between European countries and the U.S. are well-documented and may be related to differences in health care systems, access to testing technologies, and risk tolerance (2,5,6). Furthermore, variation may be explained by the limited information on health-related outcomes in this stable, undiagnosed population, and there is little consensus about which test is preferable, or even when one is required (7-9). Major U.S. and European guidelines differ fairly substantially in their fundamental approach to determining the pretest probability (PTP) of coronary artery disease (CAD) in symptomatic patients and how to proceed with test selection. Furthermore, both U.S. and European measures markedly overestimate PTP rates (10).
To address these issues systematically, 2 large multicenter, open-label, randomized controlled trials explored the diagnostic evaluation of patients with symptoms that may represent coronary heart disease (CHD). The SCOT-HEART (Scottish COmputed Tomography of the HEART) (11) and PROMISE (PROspective Multicenter Imaging Study for Evaluation of chest pain) (12) trials sought to address an evidence gap in noninvasive testing in stable chest pain, an area in which few randomized trials have been conducted. (7,13). Each examined the potential role of coronary computed tomography angiography (CCTA). Similarities between SCOT-HEART and PROMISE make it tempting to combine these studies (14,15). However, several salient differences in study populations and endpoints are critical to understanding the implications of each.
How do we best incorporate the results of 2 pivotal trials, SCOT-HEART and PROMISE, into current practice to provide optimal care for our patients? This review thus aims to provide a context for approaching non-noninvasive imaging by:
Describing the historically unmet clinical need for outcomes research in cardiovascular imaging;
Enumerating similarities and differences between SCOT-HEART and PROMISE;
Briefly summarizing other, very recent trial results or ongoing trials;
Providing a unified set of conclusions, drawing upon the findings of both SCOT-HEART and PROMISE.
An historical unmet need for cardiovascular imaging outcomes trials
Despite the routine use of noninvasive testing for patients with stable chest pain of suspected cardiac etiology over the last several decades, until 2015, no large-scale randomized trials had evaluated the diagnosis, management, and outcomes of these patients. Most recent clinical trials for CCTA focused on assessing its accuracy and comparability for identification of CHD (16,17), or its effect on management of low-risk patients presenting to the emergency department with acute chest pain (18). However, few (if any) randomized studies directly compared the various anatomic and functional testing options in patients with stable chest pain using clinical endpoints.
Hierarchy of evidence in cardiovascular imaging outcomes research
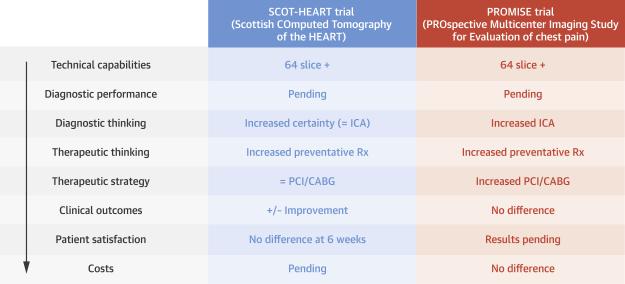
In 1991, Fryback and Thornbury devised hierarchical levels of diagnostic test evidence (19). This commonly-cited model for efficacy in imaging describes 6 hierarchical tiers of evidence: 1) technical efficacy; 2) diagnostic accuracy; 3) diagnostic thinking; 4) therapeutic efficacy; 5) patient outcomes; and 6) societal efficacy, including cost-effectiveness. Yet, only 1% of the over 700 recommendations for cardiovascular imaging in American College of Cardiology and American Heart Association guidelines are on the basis of Level of Evidence: A (20). Recent randomized trials have assessed the impact of CCTA versus usual care among patients with suspected acute coronary syndromes in the emergency department, primarily with safety (21), hospital length-of-stay (18), and cost-effectiveness (22) endpoints, and are not included in this review. The results of SCOT-HEART and PROMISE are, therefore, significant in that they are the first large-scale randomized cardiovascular imaging trials to evaluate clinical outcomes in patients with stable chest pain. Their results may also now be critically evaluated in the context of this model (Figure 1).
Figure 1. Diagnostic Strategies for the Evaluation of Chest Pain.
The left-hand column shows the Fryback and Thornbury model for assessing diagnostic test evidence on the basis of hierarchical levels of clinical outcomes (19). These levels range from technical quality and diagnostic/prognostic accuracy to establishing a test's impact on clinical decision-making to societal outcomes. The findings of both trials are summarized in subsequent columns, providing a comparison of the types of evidence provided by each, as well as the results. CABG = coronary artery bypass graft; ICA = invasive coronary angiography; PCI = percutaneous coronary intervention; Rx = prescription.
Key findings from SCOT-HEART and PROMISE
SCOT-HEART enrolled 4,146 patients with stable chest pain to CCTA in addition to usual care (which generally included electrocardiogram [ECG] stress testing) or to usual care alone (11). The trial used an upstream primary endpoint related to diagnostic thinking, certainty of the attribution of symptoms to CAD, which showed an increase in the CCTA group (relative risk: 1.79; 95% CI: 1.62 to 1.96), as did the secondary endpoint of certainty of diagnosis of CAD (2.56; 95% CI: 2.33 to 2.79). The clinical outcomes-related secondary endpoint of the rate of cardiovascular death or myocardial infarction (MI) appeared to be reduced in the CCTA group at 20 months (0.62, 95% CI: 0.38 to 1.01; p = 0·0527), although the overall event rates were low in both arms, reflecting the inclusion of a large number of patients without CHD.
The larger PROMISE trial randomly assigned 10,003 symptomatic, stable outpatients requiring evaluation for suspected CAD to either CCTA or functional stress testing (exercise treadmill testing [ETT], nuclear stress testing, or stress echocardiography), with a median follow-up of 25 months (12). The event-related composite primary endpoint (death, MI, hospitalization for unstable angina, or major CV procedural complication) occurred at similar rates in the CCTA and functional testing groups (3.3% and 3.0%), which was lower than previously established historical rates. More patients in the CCTA group underwent cardiac catheterization within 90 days after randomization (12.2% vs. 8.1%), but the secondary endpoint of the frequency of catheterization showing no obstructive CAD was significantly lower in the CCTA group (6.2% vs. 3.2%). Furthermore, among patients randomized to an intended nuclear test strata, the mean cumulative radiation exposure was lower in the CCTA group compared with the functional testing group (12.0 ± 8.4 versus 14.1 ± 7.6 mSv). This included all downstream radiation within 90 days, including that associated with cardiac catheterization, and is particularly intriguing, since more CCTA patients received cardiac catheterization.
SCOT-HEART and PROMISE: Similarities
Tables 1 and 2 summarize the similarities between the 2 trials. First, both trials recruited symptomatic patients requiring nonemergent evaluation. Secondly, patients in both trials had a high burden of cardiovascular risk factors, including small numbers of those with prior peripheral and cerebrovascular disease, and were felt to have a 50% chance of having CHD. Consistent with this, nearly half of the patients in both trials received aspirin and statin therapy at baseline.
Table 1.
SCOT-HEART and PROMISE: Trial characteristics.
| SCOT-HEART | PROMISE | |
|---|---|---|
| Country | UK | United States and Canada |
| Comparators | CCTA + standard of care vs. standard of care | CCTA vs. functional stress test |
| Trial design | Open-label | Open-label |
| Recruiting centers | 12 | 193 |
| Length of follow-up | 20 months | 25 months |
| Sample size | 4,146 | 10,003 |
| Primary endpoint | Certainty of diagnosis of angina due to coronary heart disease | Death, nonfatal MI, hospitalization for unstable angina, major procedural complications (anaphylaxis, stroke, major bleeding, and renal failure) |
| Follow-up | National Health Record systems | Mail and telephone |
CCTA = cardiac computed tomography angiography; MI = myocardial infarction.
Table 2.
SCOT-HEART and PROMISE: Participant Profiles
| SCOT-HEART | PROMISE | |
|---|---|---|
| Participant Characteristics | ||
| Age (yrs) | 57 ± 10 | 61 ± 8 |
| Women | 44% | 53% |
| Body-mass index | 30 ± 6 | 31 ± 6 |
| Hypertension | 34% | 65% |
| Diabetes mellitus | 11% | 21% |
| Hyperlipidemia | 53% | 68% |
| Family history | 41% | 32% |
| Past or current smoking | 53% | 51% |
| Prior CHD | 9% | 0% |
| Prior CVD/PAD | 4% | 6% |
| Baseline Treatments | ||
| Aspirin | 49% | 45% |
| Statin | 43% | 45% |
| ACE/ARB | 17% | 44% |
| Beta blocker | 33% | 25% |
| Baseline Symptoms | ||
| Angina | ||
| Typical | 35% | 12% |
| Atypical | 24% | 78% |
| Nonanginal | 41% | 11% |
| Baseline Risk | ||
| Framingham 10-yr risk | 17 ± 12% | 22 ± 15% |
| Pretest probability of obstructive CHD | 47% | 53% |
| Selection of Functional Testing | ||
| ECG | 85% | 10%* |
| MPI | 9% | 68%* |
| Echo | 1% | 22%* |
| Planned invasive coronary angiography at baseline | 12% | 0% |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CHD = coronary heart disease; CVD = cardiovascular disease; ECG = electrocardiogram; Echo = echocardiography; MPI = myocardial perfusion imaging; PAD = peripheral arterial disease.
Thirdly, the interventions in both trials were similar: a comparison of CCTA to usual care early in the evaluation of patients with suspected CHD. Importantly, neither included a “no-testing” arm, an option some support because of the trials’ low event rates. Both trials followed patients for up to 4 years (median 20 to 25 months) and had low rates of adverse events attributable to CCTA which, when they did occur, were mild and self-limiting. Both trials saw similar rates of the use of invasive coronary angiography in the CCTA group at 6 weeks (SCOT-HEART, 12%) and 90 days (PROMISE, 12%). CCTA was associated with increased use of percutaneous coronary intervention and coronary artery bypass graft surgery, although this was statistically nonsignificant in SCOT-HEART (p = 0.06).
Fourthly, event rates in both trials were low, with large proportions of patients having normal or near normal coronary arteries and already receiving excellent preventative therapy at baseline. In SCOT-HEART, the overall rate of all-cause death and nonfatal MI was 2.3% at a median follow-up of 1.7 years (1.35%/year), whereas the overall rate of the same 2 endpoints in PROMISE was 2.2% at 2.1 years (1.05%/year). Although CCTA did not improve the primary endpoint in PROMISE (all-cause death, nonfatal MI, hospitalization for unstable angina, and major procedural complications), its use in both trials was associated with lower MI rates that were of borderline statistical significance. The influence of CCTA use on MI rates is suggested by the divergence of the event curves in SCOT-HEART beginning at 6 weeks, a time point which is attributable to the delay in obtaining and acting on the CCTA result. Indeed, landmark analyses in this trial suggest that CCTA may lead to a halving of MI rates.
SCOT-HEART and PROMISE: Differences
A number of notable differences between the trials are summarized in Tables 1 and 2. First, PROMISE was the larger trial, included centers over a much larger geographical area in North America, and had broader inclusion criteria, although it did exclude known CHD or a recent CHD evaluation. SCOT-HEART was conducted within a single health care system, with a more focused patient group and care pathway. Secondly, and perhaps most importantly, 85% of SCOT-HEART participants had already undergone ETT and all had a care plan established on the basis of these results, as well as clinical assessment. Because of an inconclusive treadmill ECG, only 10% of patients were referred for further stress imaging (9% radionuclide perfusion imaging and 1% stress echocardiography), whereas a further 12% had already been referred for invasive coronary angiography prior to randomization. In contrast, PROMISE patients underwent initial diagnostic evaluation before any testing or care plan was formulated, and were randomized to either an initial, pre-specified stress test or CCTA. These differences in trial design allowed SCOT-HEART to evaluate changes in diagnostic thinking, including changes in diagnostic certainty and frequency, and in plans for testing and subsequent care within the CCTA arm. In contrast, outcomes in PROMISE were on the basis of long-term comparisons of clinical events. It is widely recognized that clinical outcomes, rather than surrogates, should be the standard for cardiovascular trials. However, the event rates in both trials show that hard endpoints are challenging to meet in this low-risk population. This may have contributed to the neutral overall PROMISE results, with some dismissing the trial as underpowered. However, the importance of the PROMISE findings must be also be considered in the context of the large patient population and pragmatic design reflecting contemporary clinical practice.
Thirdly, the trial populations were distinct. PROMISE participants were slightly older, included more women, and had higher rates of cardiovascular risk factors at baseline, especially hypertension and diabetes mellitus. In PROMISE, 88% of participants had chest pain (72%) or an anginal equivalent (16%), and 10% had typical angina compared with 100% with chest pain and 35% with typical angina for SCOT-HEART, which, unlike PROMISE, also included some patients (9%) with known CHD. SCOT-HEART also included patients at very low risk, with 41% having nonanginal chest pain compared with only 11% in PROMISE.
Fourthly, stress testing in PROMISE was dominated by radionuclide perfusion imaging, whereas exercise ECG testing was the norm in SCOT-HEART. Notably, the choice of functional imaging over exercise ECG testing for the majority of PROMISE subjects demonstrates a disconnect between North American practice patterns and guideline recommendations for patients with an intermediate PTP of CAD who are able to exercise and have an interpretable ECG (8). Radiation doses for CCTA were lower in SCOT-HEART, perhaps reflecting the restriction of imaging to 3 centers that had a greater use of prospective gating, single heartbeat angiography, and iterative reconstruction algorithms to reduce radiation exposure, as compared with the diverse settings and pragmatic testing design used in PROMISE.
Fifthly, PROMISE saw a doubling of coronary revascularization rates in patients randomized to receive CCTA, whereas SCOT-HEART saw an increase of approximately 20%. However, this likely reflects that no patients were scheduled for an invasive coronary angiogram at randomization in PROMISE, whereas 1 in 8 patients in SCOT-HEART were already scheduled for this test. The revascularization rates in SCOT-HEART were nearly twice those in PROMISE, likely reflecting the 2 to 3-fold higher rates of obstructive CAD and the inclusion of patients with more severe symptoms in the SCOT-HEART population.
Finally, the 2 trials had markedly different primary and secondary endpoints. SCOT HEART assessed the certainty of the diagnosis of angina due to CHD, whereas PROMISE assessed a composite of major adverse cardiac events and safety. However, both trials assessed a variety of measures of test outcomes along the same hierarchical continuum of diagnostic test performance (19) (Figure 1). Nevertheless, by assessing changes in diagnostic thinking, the primary endpoint in SCOT-HEART was more upstream and independent of subsequent care choices than in PROMISE. This aspect was examined indirectly in PROMISE by the rate of referral to invasive catheterization. Only PROMISE pre-specified an endpoint of the rate of invasive catheterization, which did not show obstructive CHD and overall radiation exposure.
Practical and clinical implications from SCOT-HEART and PROMISE
Contemporary patients with stable chest pain appear to be at low risk of clinical events, regardless of diagnostic test choice
SCOT-HEART and PROMISE extend prior observations that contemporary patients with stable chest pain are at lower risk for clinical events than previously believed, despite intermediate PTP of obstructive disease, according to traditional scoring systems (Central Illustration). They also confirm findings from prior observational studies of high rates of nonobstructive CAD on invasive angiography, which may speak to the difficulty of clinical assessment, including the crucial step of patient selection for invasive testing (23). The studies also corroborate several reports showing that more than two-thirds of noninvasive tests performed in patients with stable chest pain of suspected ischemic etiology are normal or that show nonobstructive CAD disease, and that many of these patients will not experience an untoward clinical event (24-26). However, only an anatomic approach can identify nonobstructive disease, which is associated with event rates similar to obstructive, single-vessel disease (27). Finally, both trials demonstrate that both anatomic and functional strategies resulted in few safety endpoints related to either testing arm or downstream events, such as cardiac catheterization, and relatively low levels of radiation exposure. These findings are important, given recent concerns about inappropriate cardiac testing to prevent unnecessary risk to patients (2,28,29).
Central Illustration.
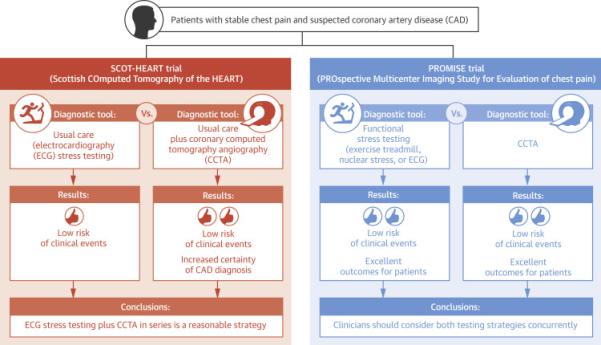
Comparison of diagnostic tools the Evaluation of Chest Pain: Levels of Evidence of SCOT HEART and PROMISE. Main results and conclusions from the SCOT-HEART and PROMISE trials.
A continued role for functional stress testing
Stress testing will continue to play an important and highly appropriate frontline role in our assessment of stable, symptomatic patients. However, despite widespread adaptation into practice, stress testing had not previously undergone the same rigorous assessment for determining the impact of a diagnostic test on downstream clinical endpoints that both stress testing and CCTA have now undergone with these 2 trials. For PROMISE, there was a head-to-head comparison of stress versus anatomic testing in which CCTA did not improve outcomes compared to functional testing. Both strategies resulted in acceptable (if not excellent) outcomes for our patients. For SCOT-HEART, stress testing and CCTA were performed sequentially, and therefore integrated as part of a care pathway. This trial's findings suggest that stress testing alone will provide a somewhat different diagnostic formulation in these higher-risk patients. Overall, event rates were low and the incremental benefit of investigating the lowest-risk patients may be questioned. For example, if CCTA were used indiscriminately, SCOT-HEART suggests that approximately 100 CCTAs would need to be performed to prevent 1 MI.
Moving forward: CCTA as a reasonable first choice for routine assessment of patients with stable chest pain
When stress imaging and CCTA are compared head-to-head, the PROMISE trial demonstrated similar clinical outcomes compared to usual care, but better patient selection for invasive coronary angiography in the CCTA arm on the basis of finding obstructive CAD (Figure 2). Compared to standard of care, SCOT-HEART demonstrated that using exercise ECG and CCTA in series clarified the diagnosis of angina due to CHD and altered patient management (selection of invasive coronary angiography and preventative therapies). Both trials demonstrated that CCTA could be performed safely at acceptable radiation doses. Although not statistically significant, trends for reductions in clinical events may be plausibly related to changes in medical and revascularization therapies.It should also be remembered that for the patient, discontinuation of unnecessary investigations and treatments is greatly valued, despite its limited impact on clinical outcomes.
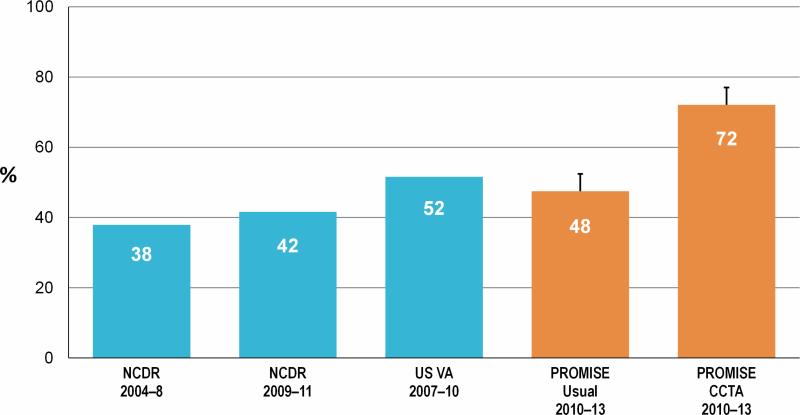
Figure 2. Proportion of Patients With Obstructive Coronary Artery Disease Found on Elective Cardiac Catheterization Following Noninvasive Testing for Suspected Cardiac Chest Pain Across Multiple Studies.
Obstructive coronary disease was defined as having at least 1 stenosis of > 50% of an epicardial coronary artery measuring ≥2 mm in diameter (12,41-43). Dates represent the time frames during which patient data was accrued. CCTA = coronary computed tomography angiography; FFRCT = fractional flow reserve computed tomography NCDR = National Cardiovascular Data Registry; PROMISE = PROspective Multicenter Imaging Study for Evaluation of chest pain trial; Outcome and Resource Impacts; VA = Veterans Affairs.
Putting it all together: consider concurrent CCTA and functional testing
If the patient with stable chest pain and an intermediate pretest probability of CAD is a potential revascularization candidate and is eligible for both anatomic and functional testing strategies (i.e., does not have a contrast allergy), the clinician should consider both testing strategies concurrently. This would require a reexamination of current U.S. guidelines, which recommend ETT as the first diagnostic test of choice and recommend CCTA only in patients who cannot undergo stress testing (8), as well as current U.S. clinical practice, which skews towards use of stress radionuclide imaging as the first test. Notably, the SCOT-HEART results favor the sequential combination of ETT and CCTA as a first-line strategy that enhances diagnostic certainty and shows a trend towards reducing events over ETT alone.
Other imaging modality-specific features should also be considered. For example, CCTA may be favored if additional thoracic CT imaging is required; for example, a triple or double rule-out in suspected pulmonary embolism (D-dimer positive) and aortic dissection, or if an intra-thoracic pathology is suspected, such as pericardial disease (30), if there is a suspected coronary anomaly (31), or if the diagnosis of nonobstructive or obstructive CAD alone would result in an intensification or change in medical therapy (32-34). Stress echocardiography or CMR may be favored if evaluation of a radiation-sensitive population is required; for example, sex and age or previous radiation exposure history (30), or if valvular, pericardial, or congenital abnormality is also suspected. ETT alone, without imaging, may be favored in the evaluation of radiation-sensitive population (30), in a very low-risk group, or to help mitigate cost.
An evolving field: recent and ongoing cardiovascular imaging outcomes studies for low-risk chest pain
Other studies published within the last year indicate growing recognition of the importance of improving imaging outcomes evidence for low/intermediate-risk stable chest pain (Table 3). The CAPP (Cardiac CT for the Assessment of Pain and Plaque) trial randomized 500 patients with troponin-negative stable chest pain and without known CAD to CCTA versus exercise ECG (35). At 12 months of follow-up, CCTA was associated with improved angina symptoms (measured by the Seattle Angina Questionnaire) and resulted in fewer investigations and rehospitalizations compared with exercise ECG. Levsky et al. randomized 400 patients with acute chest pain admitted to telemetry-monitored wards of an inner-city medical center to CCTA versus radionuclide stress myocardial perfusion imaging (MPI) (36). They found no significant differences in outcomes or resource utilization between the CCTA and MPI groups at 40 months of follow-up. Compared with MPI, CCTA was also associated with less radiation exposure and a more positive patient experience. The CATCH (CArdiac cT in the treatment of acute CHest pain) trial randomized 600 patients referred initially for ACS, who had normal or nondiagnostic ECG, 2 normal measures of troponins, and were discharged following 24 h of in-hospital clinical observation to either a CCTA-guided strategy or standard of care (37). Following a median follow-up of nearly 19 months, patients in the CCTA-guided arm had improved clinical outcomes compared with the standard-care group (hazard ratio [HR]: 0.62; 95% CI: 0.40 to 0.98]), driven by nonfatal outcomes (38). Finally, a prospective, but nonrandomized study, PLATFORM (Prospective Longitudinal Trial of FFRCT: Outcome and Resource Impacts), recently reported results for clinical outcomes (39) and resource utilization (40), comparing standard-of-care to care guided by CTA-derived fractional flow reserve or FFRCT. In patients for whom invasive testing was planned, use of FFRCT reduced the primary endpoint of the rate of coronary angiography showing no stenosis from ≥50% to 12% versus 73% with usual care (p < 0.0001) and was cost saving.
Table 3.
Selected Prospective Noninvasive Imaging Outcomes Studies for the Evaluation of Low/Intermediate-Risk Chest Pain Patients
| Trial | N | Country | Setting | Study Population | Randomization Arms | Primary Outcome Endpoint(s) | Study Completion* |
|---|---|---|---|---|---|---|---|
| Recently completed studies | |||||||
| CAPP (35) | 500 | United Kingdom | Stable chest pain | ≥18 yrs of age; no known CAD | CCTA vs. exercise stress testing | Change in Seattle Angina Questionnaire score (at 12 months) | Published 2015 |
| Levsky et al. (36) | 400 | United States | Admitted patients | No known CAD; intermediate-risk chest pain | CCTA vs. radionuclide stress MPI | Cardiac catheterization without revascularization (at 40 months) | Published 2015 |
| CATCH (37,38)% Primary and long term follow-up studies published |
600 | Denmark | Admitted, but discharged within 24 h | ≥18 yrs; ECG nondiagnostic for ACS; no prior CABG | CCTA vs. usual care once discharged | Referral rate for cardiac catheterization and PPV of significant CAD, revascularization (at 120 days); cardiac death; MI; hospitalization for UA; revascularization; and readmission for chest pain (19 months) | Published 2015 |
| PLATFORM (39,40)† Primary and economic/quality of life studies published |
584 | Europe | Stable chest pain | ≥18 yrs of age; no known CAD; intermediate likelihood of CAD | Planned noninvasive test vs. planned ICA; further subdivided into standard noninvasive testing (usual care) or FFRCT guided | Rate of ICA that showed no obstructive CAD (at 90 days) | Published 2015 |
| Ongoing studies | |||||||
| Levsky et al. (44) (NCT01384448) | 400 | United States | ER | Low to intermediate-risk per D-F; no known CAD | CCTA vs. stress echo | Hospital admission (at 1 month) | June 2017 |
| Kim et al. (NCT01770444) | 681 | South Korea | ER | 20-55 yrs of age; no known CAD | Low-dose CCTA (dedicated cardiac imaging protocol, prospective gating, no CAC) vs. conventional CCTA | Death or MI (at 1 month) | February 2016 |
| Gurunathan et al. (NCT02346565) | 450 | United Kingdom | Stable chest pain | Female; ≥30 yrs of age; no known CAD | Exercise stress testing vs. stress echo | CV death or nonfatal MI (at 2 yrs) | June 2018 |
| PERFECT (NCT01604655) | 500 | United States | Admitted patients | ≥45 yrs of age; ECG nondiagnostic for ACS; troponin-negative × 1 | CCTA vs. SPECT or stress echo | Time to discharge; change in medical regimen; downstream testing; and hospitalization (at 2 years) | December 2015 |
| CATCH2 (NCT02014311) | 600 | Denmark | Admitted patients | ≥50 yrs of age; ≥1 CV risk factor; troponin-negative; no or nondiagnostic ECG changes (LVH, BBB, paced) | CCTA + CT perfusion vs. CCTA alone | Revascularization among patients referred for coronary angiography (at 60 days) | June 2016 |
| RAPID-CTCA (NCT02284191) | 2,500 | United Kingdom | ER or medical assessment unit | ≥18 yrs of age; ECG abnormalities or history of IHD or troponin-positive | CCTA + standard care vs. standard care alone | Death or recurrent MI (at 1 yr) | June 2018 |
| CRESCENT (NCT01393028) | 350 | Netherlands | Stable chest pain | ≥18 years; no known CAD, no prior revascularization, no normal invasive or noninvasive test within past year | CCTA vs. standard care | Reduction of chest pain symptoms; ESC class IA revascularizations | August 2014 |
| CRESCENT2 (NCT02291484) | 250 | Netherlands | Stable chest pain | ≥18 yrs of age; >10% pretest probability of CAD | Comprehensive cardiac CT (CAC, CCTA, CT perfusion) vs. standard care | Rate of negative invasive angiograms (at 6 months) | December 2015 |
clinicaltrials.gov, accessed August 27, 2015. For ongoing studies, included only open trials currently enrolling or recently completed (and not published) with a clinical outcome as the primary endpoint. Excluded trials with unknown status, or acute coronary syndrome, including those studies requiring a positive cardiac biomarker for study inclusion. Search terms: chest pain PLUS stress test or imaging or CT or nuclear or echo or MRI.
Prospective, consecutive cohort study
ACS = acute coronary syndrome; BBB = bundle branch block; CABG = coronary artery bypass graft; CAC = coronary artery calcium; CAD = coronary artery disease; CT = computed tomography; ER = emergency room; ESC = European Society of Cardiology; FFR = fractional flow reserve; ICA = invasive coronary angiography; IHD = ischemic heart disease; LVH = left ventricular hypertrophy; MRI = magnetic resonance imaging; PPV = positive predictive value; SPECT = single-photon emission computed tomography; UA = unstable angina. Other abbreviations as in Tables 1 and 2.
Several ongoing imaging studies have clinical outcomes as endpoints, with the majority focused on CCTA (Table 3). To create this summary, we searched clinicaltrials.gov for imaging trials for the evaluation of chest pain with a clinical outcome as the primary endpoint (i.e., not diagnostic accuracy), excluding trials with unknown status or evaluating acute coronary syndromes that required a positive cardiac biomarker for inclusion. Most of these trials will comprise patients with low/intermediate-risk chest pain (cardiac biomarker-negative), except for the RAPID-CTCA (Rapid Assessment of Potential Ischaemic Heart Disease With CTCA) trial. Whereas some of these trials included patients with acute chest pain of low/intermediate risk, all evaluated clinical outcomes.
Design of future trials in diagnostic testing
Given the low event rates and similar outcomes in SCOT-HEART and PROMISE, future trials should explore better methods for patient risk stratification and test selection. An important challenge is to identify, with a high level of certainty and safety, very low-risk patients, who may not require immediate noninvasive testing, and very high-risk patients, for whom cardiac catheterization would be the most appropriate first test; both strategies require prospective evaluation. The recently published PLATFORM study evaluates a novel technology, and, as results differed in patients with a planned initial noninvasive versus invasive approach, adds the concept of customized imaging on the basis of patient population. Trials of other emerging CCTA technologies that noninvasively evaluate lesion hemodynamics or myocardial perfusion are also needed. Finally, a diagnostic strategy will ideally not only clarify the diagnosis and direct subsequent care, but also maximize efficiency and patient outcomes at the lowest cost.
Conclusions
SCOT-HEART and PROMISE remain the largest and most comprehensive imaging outcome trials to date. The cardiovascular community will continue to learn valuable lessons about the diagnosis, management, and outcome of cardiovascular patients from their complex datasets for years to come. These 2 large randomized trials and several smaller ones have shown CCTA to be a useful clinical tool (35-38) (Central Illustration). CCTA now appears to have a proven role in management of patients in whom there is uncertainty about the diagnosis of CHD. Clinicians should consider both CCTA and functional imaging when evaluating eligible patients.
Acknowledgments
Dr. Fordyce has received support from the Clinician Investigator Program of the University of British Columbia.
Abbreviations
- CAD
coronary artery disease
- CHD
coronary heart disease
- CCTA
coronary computed tomography angiography
- ECG
electrocardiogram
- ETT
exercise treadmill test
- MI
myocardial infarction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Dr. Fordyce has reported that he has no relationships relevant to the contents of this paper to disclose. Dr. Newby has reported receiving honoraria and consultancy fees from Toshiba Medical Systems and is the Chief Investigator of the SCOT-HEART trial. Dr. Douglas has reported receiving research funding from HeartFlow and is the Primary Investigator of the PROMISE trial
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Ladapo JA, Blecker S, Douglas PS. Physician decision making and trends in the use of cardiac stress testing in the United States: an analysis of repeated cross-sectional data. Ann Intern Med. 2014;161:482–90. doi: 10.7326/M14-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sekhri N, Feder GS, Junghans C, et al. How effective are rapid access chest pain clinics? Prognosis of incident angina and non-cardiac chest pain in 8762 consecutive patients. Heart. 2007;93:458–63. doi: 10.1136/hrt.2006.090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle RM. Value of rapid-access chest pain clinics. Heart. 2007;93:415–6. doi: 10.1136/hrt.2006.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly CA, Clemens F, Sendon JLL, et al. The clinical characteristics and investigations planned in patients with stable angina presenting to cardiologists in Europe: from the Euro Heart Survey of Stable Angina. Eur Heart J. 2005;26:996–1010. doi: 10.1093/eurheartj/ehi171. [DOI] [PubMed] [Google Scholar]
- 6.Shaw LJ, Min JK, Hachamovitch R, et al. Cardiovascular imaging research at the crossroads. J Am Coll Cardiol Img. 2010;3:316–24. doi: 10.1016/j.jcmg.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence [December 4, 2015];Chest pain of recent onset: assessment and diagnosis [CG95] 2010 Available at: https://www.nice.org.uk/guidance/cg95. [PubMed]
- 8.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Task Force Members. Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 10.Cheng VY, Berman DS, Rozanski A, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM). Circulation. 2011;124:2423–32, 1-8. doi: 10.1161/CIRCULATIONAHA.111.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SCOT-HEART investigators CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–91. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 12.Douglas PS, Hoffmann U, Patel MR, et al. PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mowatt G, Cummins E, Waugh N, et al. Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease. Health Technol Assess. 2008;12:iii–iv, ix-143. doi: 10.3310/hta12170. [DOI] [PubMed] [Google Scholar]
- 14.Schulman-Marcus J, Danad I, Truong QA. State-of-the-art updates on cardiac computed tomographic angiography for assessing coronary artery disease. Curr Treat Options Cardiovasc Med. 2015;17:398. doi: 10.1007/s11936-015-0398-6. [DOI] [PubMed] [Google Scholar]
- 15.Huynh K. Imaging. Effect of CT coronary angiography on the diagnosis and outcomes of patients with CAD. Nat Rev Cardiol. 2015;12:258. doi: 10.1038/nrcardio.2015.47. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder S, Achenbach S, Bengel F, et al. Cardiac computed tomography: indications, applications, limitations, and training requirements. Eur Heart J. 2008;29:531–56. doi: 10.1093/eurheartj/ehm544. [DOI] [PubMed] [Google Scholar]
- 17.Layritz C, Schmid J, Achenbach S, et al. Accuracy of prospectively ECG-triggered very low-dose coronary dual-source CT angiography using iterative reconstruction for the detection of coronary artery stenosis: comparison with invasive catheterization. Eur Heart J Cardiovasc Imaging. 2014;15:1238–45. doi: 10.1093/ehjci/jeu113. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann U, Truong QA, Schoenfeld DA, et al. ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making. 1991;11:88–94. doi: 10.1177/0272989X9101100203. [DOI] [PubMed] [Google Scholar]
- 20.Douglas PS. Improving imaging: our professional imperative. J Am Coll Cardiol. 2006;48:2152–5. doi: 10.1016/j.jacc.2006.04.107. [DOI] [PubMed] [Google Scholar]
- 21.Litt HI, Gatsonis C, Snyder B, et al. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393–403. doi: 10.1056/NEJMoa1201163. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein JA, Chinnaiyan KM, Abidov A, et al. CT-STAT Investigators. The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58:1414–22. doi: 10.1016/j.jacc.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 23.Douglas PS, Patel MR, Bailey SR, et al. Hospital variability in the rate of finding obstructive coronary artery disease at elective, diagnostic coronary angiography. J Am Coll Cardiol. 2011;58:801–9. doi: 10.1016/j.jacc.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Hachamovitch R, Berman DS, Kiat H, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93:905–14. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 25.Bangalore S, Gopinath D, Yao SS, et al. Risk stratification using stress echocardiography: incremental prognostic value over historic, clinical, and stress electrocardiographic variables across a wide spectrum of bayesian pretest probabilities for coronary artery disease. J Am Soc Echocardiogr. 2007;20:244–52. doi: 10.1016/j.echo.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Mudrick DW, Cowper PA, Shah BR, et al. Downstream procedures and outcomes after stress testing for chest pain without known coronary artery disease in the United States. Am Heart J. 2012;163:454–61. doi: 10.1016/j.ahj.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho I, Chang HJ, Sung JM, et al. CONFIRM Investigators Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry (coronary CT angiography evaluation for clinical outcomes: an international multicenter registry). Circulation. 2012;126:304–13. doi: 10.1161/CIRCULATIONAHA.111.081380. [DOI] [PubMed] [Google Scholar]
- 28.Patel MR, Bailey SR, Bonow RO, et al. ACCF/SCAI/AATS/AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 appropriate use criteria for diagnostic catheterization: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;59:1995–2027. doi: 10.1016/j.jacc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Raff GL, Chinnaiyan KM, Cury RC, et al. SCCT guidelines on the use of coronary computed tomographic angiography for patients presenting with acute chest pain to the emergency department: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:254–71. doi: 10.1016/j.jcct.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Roberts WT, Bax JJ, Davies LC. Cardiac CT and CT coronary angiography: technology and application. Heart. 2008;94:781–92. doi: 10.1136/hrt.2007.116392. [DOI] [PubMed] [Google Scholar]
- 32.Cheezum MK, Hulten EA, Smith RM, et al. Changes in preventive medical therapies and CV risk factors after CT angiography. J Am Coll Cardiol Img. 2013;6:574–81. doi: 10.1016/j.jcmg.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Hulten E, Bittencourt MS, Singh A, et al. Coronary artery disease detected by coronary computed tomographic angiography is associated with intensification of preventive medical therapy and lower low-density lipoprotein cholesterol. Circ Cardiovasc Imaging. 2014;7:629–38. doi: 10.1161/CIRCIMAGING.113.001564. [DOI] [PubMed] [Google Scholar]
- 34.Pursnani A, Schlett CL, Mayrhofer T, et al. Potential for coronary CT angiography to tailor medical therapy beyond preventive guideline-based recommendations: insights from the ROMICAT I trial. J Cardiovasc Comput Tomogr. 2015;9:193–201. doi: 10.1016/j.jcct.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKavanagh P, Lusk L, Ball PA, et al. A comparison of cardiac computerized tomography and exercise stress electrocardiogram test for the investigation of stable chest pain: the clinical results of the CAPP randomized prospective trial. Eur Heart J Cardiovasc Imaging. 2015;16:441–8. doi: 10.1093/ehjci/jeu284. [DOI] [PubMed] [Google Scholar]
- 36.Levsky JM, Spevack DM, Travin MI, et al. Coronary computed tomography angiography versus radionuclide myocardial perfusion imaging in patients with chest pain admitted to telemetry: a randomized trial. Ann Intern Med. 2015;163:174–83. doi: 10.7326/M14-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linde JJ, Kofoed KF, Sørgaard M, et al. Cardiac computed tomography guided treatment strategy in patients with recent acute-onset chest pain: results from the randomised, controlled trial: CArdiac cT in the treatment of acute CHest pain (CATCH). Int J Cardiol. 2013;168:5257–62. doi: 10.1016/j.ijcard.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Linde JJ. Long-term clinical impact if coronary CT angiography in patients with recent acute-onset chest pain: The randomized controlled CATCH trial. J Am Coll Cardiol Img. 2015 Nov 5; doi: 10.1016/j.jcmg.2015.07.015. [E-pub ahead of print], http://dx.doi.org/10.1016/j.jcmg.2015.07.015. [DOI] [PubMed]
- 39.Douglas PS, Pontone G, Hlatky MA, et al. PLATFORM Investigators. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFRct: outcome and resource impacts study. Eur Heart J. 2015 Sep 1; doi: 10.1093/eurheartj/ehv444. [E-pub ahead of print], http://dx.doi.org/10.1093/eurheartj/ehv444. [DOI] [PMC free article] [PubMed]
- 40.Hlatky MA, De Bruyne B, Pontone G, et al. PLATFORM Investigators. Quality-of-life and economic outcomes of assessing fractional flow reserve with computed tomography angiography: PLATFORM. J Am Coll Cardiol. 2015;66:2315–23. doi: 10.1016/j.jacc.2015.09.051. [DOI] [PubMed] [Google Scholar]
- 41.Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel MR, Dai D, Hernandez AF, et al. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am Heart J. 2014;167:846–52. e2. doi: 10.1016/j.ahj.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Bradley SM, Maddox TM, Stanislawski MA, et al. Normal coronary rates for elective angiography in the Veterans Affairs Healthcare System: insights from the VA CART program (Veterans Affairs Clinical Assessment Reporting and Tracking). J Am Coll Cardiol. 2014;63:417–26. doi: 10.1016/j.jacc.2013.09.055. [DOI] [PubMed] [Google Scholar]
- 44.Levsky JM, Haramati LB, Taub CC, et al. Rationale and design of a randomized trial comparing initial stress echocardiography versus coronary CT angiography in low-to-intermediate risk emergency department patients with chest pain. Echocardiography. 2014;31:744–50. doi: 10.1111/echo.12464. [DOI] [PubMed] [Google Scholar]