Abstract
The aim of this review was to evaluate the clinical effectiveness of fractional exhaled nitric oxide (FeNO) measured in a clinical setting for the management of asthma in adults.
13 electronic databases were searched and studies were selected against predefined inclusion criteria. Quality assessment was conducted using QUADAS-2. Class effect meta-analyses were performed.
Six studies were included. Despite high levels of heterogeneity in multiple study characteristics, exploratory class effect meta-analyses were conducted. Four studies reported a wider definition of exacerbation rates (major or severe exacerbation) with a pooled rate ratio of 0.80 (95% CI 0.63–1.02). Two studies reported rates of severe exacerbations (requiring oral corticosteroid use) with a pooled rate ratio of 0.89 (95% CI 0.43–1.72). Inhaled corticosteroid use was reported by four studies, with a pooled standardised mean difference of −0.24 (95% CI −0.56–0.07). No statistically significant differences for health-related quality of life or asthma control were found.
FeNO guided management showed no statistically significant benefit in terms of severe exacerbations or inhaled corticosteroid use, but showed a statistically significant reduction in exacerbations of any severity. However, further research is warranted to clearly define which management protocols (including cut-off points) offer best efficacy and which patient groups would benefit the most.
Short abstract
FeNO testing for adult asthma management may confer clinical benefit, but research is needed to establish its role http://ow.ly/WGWkx
Introduction
Asthma is a chronic disorder of the airways, caused primarily by inflammatory processes and bronchoconstriction. Poorly controlled asthma can have a significant impact on the quality of life of the affected individual and their family. An estimated 5.4 million people in the UK are currently receiving treatment for asthma [1, 2]. Despite the high prevalence rates, deaths resulting from asthma are uncommon.
The pharmacological management of asthma in adults aims to control symptoms (including nocturnal symptoms and exercise induced asthma), prevent exacerbations and achieve the best possible lung function, with minimal side-effects of treatment. Inhaled corticosteroids (ICSs) are the main treatment for asthma, and although at low dosage the side-effects are few, high dosage or long-term use of ICS is associated with an increased risk of systemic side-effects [3]. The current British guidelines on the management of asthma recommend a stepwise approach, with escalation of medication until control is reached or stepping down when control is good [4]. However, in certain cases there is suspected over- and under-treatment.
Fractional exhaled nitric oxide (FeNO) is a noninvasive biomarker of airway inflammation in asthma. High FeNO in the breath of patients with symptoms of asthma are correlated with eosinophilic airway inflammation (a distinct corticosteroid responsive phenotype of asthma) [5–7]. The presence of eosinophils may be used to direct treatment as patients without eosinophilic inflammation are thought to be less responsive to ICS treatment [8]. Therefore, in order to reach a balance between treatment and control, the addition of FeNO monitoring might allow optimisation of treatment in the different disease phenotypes. Existing reviews of FeNO monitors suggest some benefits associated with FeNO [9–11]; however, none were statistically conclusive. In addition, these reviews focused on number of people with an exacerbation, inappropriately included the cohort of pregnant women in the meta-analysis (pregnancy can substantially affect the course of asthma) [12] and are out-of-date. To address these limitations we have updated an existing review [9], with the addition of three new studies [13–16], to determine the potential role of FeNO monitors in the management and monitoring of asthma in adults. This systematic review was undertaken to inform a UK National Institute for Health and Care Excellence appraisal which included an assessment of the use of the electrochemical FeNO monitors NIOX MINO (Aerocrine AB, Solna, Sweden), NIOX VERO (Aerocrine AB) and NObreath (Bedfont Scientific Ltd, Maidstone, UK) in the diagnosis and management of asthma [17, 18].
Methods
A systematic review was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [19].
Data sources and searches
13 electronic databases and research registers were searched (including MEDLINE and the Cochrane Library) between March and April 2013, with update searches conducted in September 2013 and November 2014. Terms for NIOX VERO, a new FeNO device, were added to the strategy in August 2013. The search strategy used free-text terms and subject headings for the tests (e.g. NIOX MINO, NObreath and FeNO) combined with keywords for the condition (i.e. asthma or lower respiratory tract symptoms). No language restrictions were applied. As part of updating an earlier systematic review [9], searches were limited by date from 2009 (the last search date from the earlier review). Searches were supplemented by hand-searching reference lists of relevant studies and contact with experts in the field. Further details of the search strategy are provided in the online supplementary appendix 1.
Study selection
All titles were examined for inclusion by one reviewer and any citations that did not meet the inclusion criteria (e.g. non-human or unrelated to asthma) were excluded. All abstracts and full-text articles were then examined independently by two reviewers. Any disagreements in the selection process were resolved through discussion. Details of the selection criteria are provided in table 1. This review focuses on studies relating to adults only. Details of FeNO for the management of asthma in children have been published elsewhere [21].
TABLE 1.
Study selection criteria
| Inclusion | Exclusion | |
| Population | Adults (≥18 years) with diagnosis of asthma including pregnant women. | Studies that included cohorts with a mean age <18 years of age Recruited patients were not diagnosed with asthma Animal models Unselected specific population (e.g. firefighters, obese or athletes) |
| Intervention | Studies that measured FeNO according to the ATS 2005 criteria [20] for the management of asthma, either with or without other indicators of asthma control. ATS criteria relating to multiple testing were relaxed to allow inclusion of studies that operated electrochemical devices in line with the manufacturer's instructions, which state only one test is required. Studies where monitoring was performed at home were excluded as this was not within the scope of the assessment. | Device which is not validated for measuring FeNO Offline measurements Studies where FeNO is measured on a more regular basis (i.e. not during a routine annual review) |
| Comparator | Studies comparing the intervention to any other management strategy that does not utilise FeNO measurements. | Includes the use of FeNO measurement as part of the management strategy |
| Outcome | Primary outcome of interest included incidence of acute exacerbation (any definition of exacerbation severity was acceptable, including “use of oral corticosteroids”), inhaled corticosteroid use, unscheduled contact with healthcare officials, hospitalisations and emergency department visits expressed or calculable as rates per person year or as the number of patients experiencing exacerbations. These outcomes were chosen as they have the greatest impact both clinically and economically. Other outcomes included clinical complications associated with acute exacerbation, asthma control and symptoms, adverse events, health-related quality of life, mortality and compliance. |
Does not report data on FeNO-guided step-up/step-down therapy Measure of alveolar nitric oxide or nasal nitric oxide |
| Study type | Randomised controlled trials. | Preclinical and biological studies Editorials and opinion pieces Studies only published in languages other than English |
FeNO: fractional exhaled nitric oxide; ATS: American Thoracic Society.
Data abstraction
Data relating to study design, patient characteristics and outcomes were extracted by one reviewer into a standardised data extraction form and independently checked for accuracy by a second reviewer. Any discrepancies were resolved through discussion. Where necessary, study authors were contacted for missing information or additional data.
Assessment of methodological quality
The methodological quality of each included study was assessed according to the Cochrane Collaboration's tool for assessing the risk of bias in randomised controlled trials (RCTs) [22]. The studies were assessed by one reviewer and independently checked by another.
Data synthesis and analysis
Data were tabulated and discussed in a narrative review. Meta-analyses were planned, where appropriate, to estimate a summary measure of effect on relevant outcomes using the methods documented in the Cochrane Handbook [22, 23]. For rate outcomes, rates per person year were the preferred outcome metric, as this accounts for multiple events in a single patient. The generic inverse variance method was used to meta-analyse rate ratios using Review Manager software (Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). For continuous outcomes, a standardised mean difference analysis was conducted where outcomes were not reported in a standardised way. In all cases, fixed effects were used first, and random effects applied if the I2 statistic indicated that heterogeneity was moderate or high. This was judged to be the case at >40%. Studies in pregnant women were analysed separately as FeNO may be affected by pregnancy [12].
Results
Trial flow
Of the 5354 citations identified, three RCTs [13, 14, 16] met the inclusion criteria and were added to the three existing trials [24–26] identified in the previous systematic reviews [9, 11]. The majority of the excluded articles did not use FeNO to guide step-up/step-down therapy or the study design was not an RCT. A summary of the process of identifying and selecting the relevant literature can be found in online supplementary appendix 2.
Characteristics of included studies
Table 2 presents the study characteristics of the six included studies [13, 14, 16, 24–26]. All the included studies compared FeNO-guided asthma management to non-FeNO-guided management and all patients were recruited in primary care, except for Calhoun et al. [13], where the recruitment setting was unclear. The device used to measure FeNO was not clearly reported in three studies. Most studies were of a small to moderate size, with the number of patients ranging from 94 [24] to 611 [16]. All studies recruited adults of either sex [13, 14, 16, 24, 25], apart from Powell et al. [26], which recruited only pregnant women. The comparability of study populations in terms of severity at baseline is difficult to determine as different scales for severity and different metrics for medication use were reported. Inclusion and exclusion criteria suggest that at least four studies [13, 14, 24, 26] recruited populations with mild to moderate asthma; while the other two studies [16, 25] included a broader spectrum of severity. However, overall the patient population is predominantly milder asthmatics (mean forced expiratory volume in 1 s (FEV1) range 81–96% predicted). In addition, no studies followed the same timeline, visit frequency, management protocols, number and points of FeNO cut-offs, and treatment doses varied across the included studies (table 3).
TABLE 2.
Study and population characteristics
| First author [ref.] | Country, funding details | Study design | Inclusion/exclusion criteria | Subjects analysed/ recruited n/N | Age years | Males n/N (%) | Spirometry | Severity | FeNO |
Smokers; Atopic; Medication use |
| Smith [24] | New Zealand, Mixed funding# including equipment from Aerocrine |
RCT: single blind, single centre, placebo-controlled | Chronic asthma [27] managed in primary care; regular ICS for ≥6 months, no dose change in previous 6 weeks. If could not tolerate removal of LABA during run-in allowed to participate if could tolerate a fixed dose. Exclusions: ≥4 courses oral prednisone in previous 12 months; admission to hospital for asthma in previous 6 months; ever admitted to IC for asthma; smokers (current or ex-) with a history of >10 pack-years. |
94/110 WBR: 13; Intervention group: 46/48 Control group: 48/49 |
Mean age 44.8 (range 12–73) | 41/110 (37.3%) | Mean (range) FEV1 % pred
Intervention group: 86.4 (80.6–92.2) Control group: 83.1 (76.5–89.7) |
Mean (95% CI) symptom score¶
Intervention group: 0.6 (0.4–0.8) Control group: 0.8(0.6–1.1) |
GM (95% CI) FeNO 250 mL+ Intervention group: 7.8 (6.6–9.3) Control group: 6.4 (5.5–7.5) |
Smokers: None Atopic: NR Medication use: Bronchodilator use, mean per day over the previous 7 days (95% CI) Intervention group: 0.5 (0.2–0.8) Control group: 0.6 (0.3–0.8) ICS use NR |
| Shaw [25] | UK, Asthma UK grant, speakers fees reported, but not from Aerocrine |
RCT: single blind, parallel group | GP diagnosis of asthma with ≥1 prescription for anti-asthma medication in the past 12 months. Current nonsmokers with a past smoking history of <10 pack-years. Exclusions: poorly compliant; those with a severe asthma exacerbation (needing prednisolone) in the previous 4 weeks. |
118 (ITT LOCF)/119 WBR: 1 Intervention group: 58 Control group: 60 |
Adults >18 years Mean age NR |
54/118 (46%) | Mean±sd FEV1 % pred
Intervention group: 81.4±20.9 Control group: 84.9±20.1 Mean±sd FEV1/FVC Intervention group: 71±10.7 Control group: 72±9.9 |
Mean±sd Juniper score
Intervention group: 1.32±0.65 Control group: 1.26±0.75 |
GM (68% CI) log FeNO
Intervention group: 29.2 (14.0–61.0) Control group: 31.2 (13.3–73.1) |
Ex-smokers: Intervention group: 22% Control group: 25% Atopic:78 (66.1%) out of 118 Medication use: Mean±sd ICS daily dose Intervention group: 697±708 µg Control group: 652±533 μg |
| Syk [14] | Sweden, Mixed funding#, some from Aerocrine |
RCT: open label, parallel group, multicentre | Doctor's diagnosis of asthma and ICS treatment for ≥ 6 months, IgE sensitisation to at least one major airborne perennial allergen. Nonsmokers for ≥1 year and with smoking history of <10 pack-years. Patients all had mild to moderate asthma. | 165/187 WBR: 6 Intervention group: 87/93 Control group: 78/88 |
Adults (18–64 years) Mean±sd 41±12.4 |
94/181 (51.9%) | Mean±sd FEV1 % pred Intervention group: 84.3±14.1 Control group: 83.7±12.5 Mean±sd FEV1/FVC Intervention group: 0.78±0.08 Control group: 0.79±0.08 |
NR | GM (95% CI) FeNO ppb Intervention group: 22.0 (19.3–25.2) Control group: 21.6 (18.7–25.0) |
Smokers: None Atopic:165 (100%) out of 165 Medication use: Median (IQR) budesonide equivalent ICS dose 400 (400–800) µg·day−1 LABA before study entry 54 (30.0%) out of 180 |
| Calhoun [13] | USA, Mixed funding#, equipment from Aerocrine |
RCT: multiply-blinded, multicentre study | Mild to moderate asthmatics, well controlled persistent asthma with compliance rates ≥75%, who could tolerate treatment of two puffs twice daily of beclomethasone HFA (40 μg·puff−1) during the 2 week run-in period. | 363 recruited to trial WBR: 21 Intervention group: 115/115§ Control group: 114/114ƒ Other study arm (not included in review): 113/113 |
Mean±sd: Intervention group: 34.8±11.3; Control group: 34.2±11.9 |
75/229 (32.8%) | Mean±sd FEV1 % pred Intervention group: 86.3±10.4 Control group: 87.7±12.1 |
Mean±sd ACQ score
Intervention group: 0.79±0.54 Control group: 0.72±0.50 Mean±sd AQLQ score Intervention group: 6.16±0.77 Control group: 6.27± 0.76 Mean±sd ASUI score Intervention group: 0.88±0.12 Control group: 0.90±0.10 |
GM±sd
FeNO ppb Intervention group: 18.88±0.66 Control group: 21.38±0.62 |
Smokers: NR Atopic: 196 (85.6%) out of 229 Medication use: Albuterol rescue use median (IQR) Intervention group: 0.07 (0–0.43) Control group: 0.04 (0–0.29) |
| Honkoop [16] | The Netherlands, Mix of non-commercial grants and funding from Aerocrine |
RCT; cluster design | From protocol: doctor's diagnosis of asthma; who need ICS as controller medication (step 2–4 GINA guidelines); ICS ≥3 months in the previous year; no exacerbation of asthma within 1 month before entry. Exclusions: daily or alternate day oral corticosteroid therapy for at least 1 month before entering into the study. | 611 randomised Other data NR Intervention group: 189/205 Controlled asthma: 219/232 Partly controlled asthma: 203/210 |
Mean±sd age: 39.4±9.5 Intervention group: 39.5±9.3 Controlled asthma: 38.9±9.3 Partly controlled asthma: 39.9±9.8 |
190/611 (31%) Intervention group: 27.7% Controlled asthma: 31.6% Partly controlled asthma: 34.2% |
Mean±sd FEV1 % pred Intervention group: 93.1±17.0 Controlled asthma: 92.4±17.2 Partly controlled asthma: 93.0±17.0 |
Mean±sd ACQ score Intervention group: 0.99±0.73 Controlled asthma: 1.08±0.84 Partly controlled asthma: 0.93±0.80 |
Mean±sd
FeNO ppb Intervention group: 24.5±21.7 Controlled asthma: 27.3±30.4 Partly controlled asthma: 24.7±29.8 |
Smokers: Intervention group: 14% Controlled asthma: 13% Partly controlled asthma: 16% Atopic: 322 (54%) out of 611 Medication use: LABA: Intervention group: 47% Controlled asthma: 49% Partly controlled asthma: 52% Mean±sd beclomethasone equivalent dose: Intervention group: 853±642 μg Controlled asthma: 831±701 μg Partly controlled asthma: 825±639 μg |
| Powell [26] | Australia, Mixed funding, lecture fees from Aerocrine Powell [26] |
RCT: double-blind, parallel group, multicentre | Doctor's diagnosis confirmed by respiratory physician's diagnosis of asthma. Nonsmoking pregnant women between 12 and 20 weeks gestation with doctor's diagnosis of asthma and who were using inhaled therapy in last year. | 203/242 WBR: 22 Intervention group: 100/111 Control group: 103/109 |
Pregnant adults >18 years Mean±sd age 28±5.4 |
0/220 (0%) | Mean (95% CI) FEV1 % pred Intervention group: 95.1 (92.8–97. 4) Control group: 96.1 (93.5–98.7) Mean (95% CI) FEV1/FVC Intervention group: 79.7 (75.4–78.0) Control group: 80.63 (79.3–82.0)0/220 (0%) |
Median (IQR) AQLQ-M Intervention group: 0.8 (0.4–1.5) Control group: 1.0 (0.5–1.6) Mean ACQ score (read off graph) Intervention group: 0.98 Control group: 1.01 |
Median (IQR) FeNO ppb Intervention group: 13.9 (6.6–32.0) Control group: 13.1 (7.5–24.0) |
Ex-smokers: 80 (39.4%) out of 203 Atopic: 156 (75.7%) out of 206 Medication use: Median (IQR) days β2-agonist in the past week Intervention group: 1.0 (0–5) Control group: 2.0 (0–6) ICS users Intervention group: 46 (41.4%) out of 111 Control group: 47 (43.1%) out of 109 Median (IQR) BDP equivalent ICS dose (µg per day) Intervention group: 800 (400–800) Control group: 800 (400–1600) |
FeNO: fractional exhaled nitric oxide; RCT: randomised controlled trial; ICS: inhaled corticosteroid; LABA: long-acting β2-agonist; IC: intensive care; WBR: withdrew before randomisation; FEV1: forced expiratory volume in 1 s; GM: geometric mean; NR: not reported; GP: general practitioner; ITT: intention to treat; LOCF: last observation carried forward; FVC: forced vital capacity; IQR: interquartile range; HFA: hydrofluoroalkanes; ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; ASUI: Asthma Symptom Utility Index; GINA: Global Initiative for Asthma; AQLQ-M; Asthma Quality of Life Questionnaire-Marks; BDP: beclomethasone dipropionate. #: mix of industry and non-industry funding, e.g. research council grants. ¶: daily score over the previous 7 days. Asthma symptoms were scored for each 24-h period as follows: 0, indicated no symptoms; 1, symptoms for one short period; 2, symptoms for two or more short periods; 3, symptoms most of the time that did not affect normal daily activities; 4, symptoms most of the time that did affect normal daily activities; and 5, symptoms so severe as to disrupt daily activities. +: FeNO measured at 250 mL·s−1 gives lower values than FeNO at 50 mL·s−1. §: 37 withdrew, imputation method NR. ƒ: 13 withdrew, imputation method NR.
TABLE 3.
Description of management strategies
| First author [ref.] | Basis for decisions | Treatments indicated | ||
| Intervention | Control | Intervention | Control | |
| Smith [24] |
FeNO, with a safety measure based on symptoms, bronchodilator use and spirometry FeNO <35 ppb (equivalent at 50 mL·s−1) defined as controlled asthma FeNO ≥35 ppb defined as uncontrolled asthma Safety measure: if one or more of the following clinical criteria are met, increase one step: 1) Symptom score for previous 7 days ≥1 point more than mean during run-in and minimum score of 2 out of 5 2) Nocturnal wakening on ≥3 nights per week more than mean during run-in 3) Mean daily bronchodilator use ≥3 times that of mean during run-in and minimum use of 15 occasions during prior 7 days 4) Diurnal peak flow variation ≥30% and/or FEV1 of <85% of baseline |
GINA 2002: symptoms, bronchodilator use, spirometer | Dose steps: placebo, inhaled fluticasone 100 µg, 250 µg, 500 µg, 750 µg and 1000 µg Phase 1: until optimal dose reached Phase 2: up titrate one step at a time; down titrate if controlled for two visits, but not lower than optimal dose Patients had personalised self-management plans, which instructed them to take oral prednisone 40 mg per day when morning peak flows fell below 70% of mean run-in values, until it reached >85%, at which time they took 20 mg per day for the same number of days |
As for intervention, but without the personalised management plan |
| Shaw [25] |
FeNO plus symptoms (Juniper score) Exhaled nitric oxide <16 ppb on first occasion or exhaled nitric oxide 16–26 ppb on second occasion with 1) Juniper score ≤1.57: step-down anti-inflammatory treatment, step-down bronchodilator treatment once off steroids. 2) Juniper score >1.57: step-down anti-inflammatory treatment, step-up bronchodilator treatment Exhaled nitric oxide >26 ppb with 1) Juniper score ≤1.57: step-up anti-inflammatory treatment, no change in bronchodilator treatment 2) Juniper score >1.57: step-up anti-inflammatory treatment, step-up bronchodilator treatment once on maximum anti-inflammatory treatment Safety measure: patients on 2000 µg beclomethasone per day with >26 ppb FeNO and had not fallen to 60% of baseline had sputum checked. If no eosinophilic inflammation, treatment reduced stepwise, unless FeNO increased by >60% of baseline. |
BTS/SIGN guidelines using Juniper scale to score symptoms: 1) treatment doubled if score >1.57 2) treatment halved if score <1.57 for 2 consecutive months |
Hierarchy of anti-inflammatory treatment: 1) Low dose ICS (100–200 μg BDP twice daily) 2) Moderate dose ICS (200–800 μg BDP twice daily) 3) High dose ICS (800–2000 μg BDP twice daily) 4) High dose ICS (800–2000 μg BDP twice daily) plus LTRA 5) Higher dose ICS (2000 μg BDP twice daily) plus LTRA 6) Higher dose ICS (2000 μg BDP twice daily) plus LTRA plus oral prednisolone 30 mg for 2 weeks, then titrate the dose reducing by 5 mg·week−1 Hierarchy of bronchodilator treatment 1) SABA as needed 2) LABA 3) LABA plus theophylline 4) LABA plus theophylline plus nebulised bronchodilator |
Step 1: SABA as required Step 2: Add ICS 200–800 μg·day−1 BDP equivalent Step 3: Add inhaled LABA Step 4: increase ICS up to 2000 μg·day−1 and addition of fourth drug, e.g. LTRA, theophylline or LABA Step 5: oral prednisolone, high dose ICS, refer to specialist care |
| Syk [14] |
FeNO only FeNO <19 ppb (men), <21 ppb (women): decrease one step FeNO 19–23 ppb (men), 21–25 ppb (women): no change FeNO ≥24 ppb (men), ≥26 ppb (women): increase one step (no change in treatment step if on step 4 or 5 and using ≤2 inhalations of SABA per week) FeNO ≥30 ppb (men), ≥32 ppb (women): increase two steps (only if on treatment step 1) Grey zone of 5 ppb applied to avoid frequent dose changes |
Symptoms, lung function, β-agonist use (usual care) | Steps 1–6: Budesonide (µg·day−1): 0, 200, 400, 800, 800+LTRA, 1600+LTRA Fluticasone (µg·day−1): 0, 100, 250, 500, 500+LTRA, 1000+LTRA Mometasone (µg·day−1): 0, 100, 200, 400, 400+LTRA, 800+LTRA |
Assume same doses as intervention |
| Calhoun [13] |
FeNO only Well controlled, FeNO <22 ppb: down one level Controlled, FeNO 22–35 ppb: maintain level Under-controlled, FeNO >35 ppb: up 1 level |
NHLBI guidelines (USA version of SIGN guidelines) | Dosing beclomethasone HFA: Level 1=0 μg per day Level 2=80 μg once daily Level 3=160 μg twice daily Level 4=320 μg twice daily Level 5=640 μg twice daily |
As intervention |
| Honkoop [16] | ACQ and FeNO Where ACQ ≤0.75 with 1) FeNO ≤25 ppb, step down 2) FeNO >25 ppb and <50 ppb, no change 3) FeNO ≥50 ppb, step up Where ACQ >0.75 and <1.50 with 1) FeNO ≤25 ppb: and time <3 months, no change, or change to LABA; if time >3 months, step down ICS 2) FeNO >25 ppb and <50 ppb: step-up (treatment choice) 3) FeNO ≥50 ppb, step-up ICS by one level Where ACQ ≥1.50 with 1) FeNO ≤25 ppb: step-up LABA 2) FeNO >25 ppb and <50 ppb: step-up (treatment choice) 3) FeNO ≥50 ppb: step-up ICS by two levels |
ACQ scores Strict strategy ACQ ≤0.75: <3 months, no change; > 3 months, step-down ACQ >0.75 and <1.50: Step-up: treatment choice ACQ ≥1.50: Step-up: treatment choice Sufficient strategy ACQ ≤0.75: Step-down ACQ >0.75 and <1.50: No change ACQ ≥1.50: Step-up: treatment choice |
Step 1: SABA as needed Step 2: low-dose ICS; or LTRA Step 3: low-dose ICS + LABA; or medium- or high-dose ICS; or low-dose ICS+LTRA Step 4: Add one or more of medium- or high-dose ICS + LABA, and/or LTRA Step 4: Add one or both of OCS (lowest dose), anti-IgE treatment |
As intervention for both strategies |
| Powell [26] |
FeNO concentration use to adjust dose of ICS ACQ used to adjust dose of LABA FeNO >29 ppb: ICS increase one step, LABA no change FeNO 16–29 ppb and ACQ ≤1.5: ICS no change, LABA no change FeNO 16–29 ppb and ACQ >1.5: ICS no change, LABA increase one step FeNO <16 ppb and ACQ ≤1.5: ICS decrease one step, LABA no change FeNO <16 ppb and ACQ >1.5: ICS decrease one step, LABA increase one step If a patient had undergone two ICS dose increments and FeNO remained >29 ppb, ICS was not increased further. If still symptomatic (ACQ >1.5) formoterol 6 µg twice daily was added. For patients taking formoterol, the ICS dose could never be 0, but would be reduced to 100 µg twice daily. Patients who remained uncontrolled at maximum doses were referred to a respiratory physician. |
ACQ-guided Well controlled asthma, ACQ <0.75: reduce treatment one step Partially controlled asthma, ACQ 0.75–1.50: no treatment change Uncontrolled asthma, ACQ >1.5: increase one step Those at maximum dose were referred to a respiratory physician |
Steps 1–5 ICS: budesonide 0, 100, 200, 400 or 800 µg twice daily, respectively LABA: Step 1: salbutamol as required Step 2–5: formoterol 6, 12, 24 or 24 µg twice daily, respectively |
Step 1: salbutamol as required Step 2: budesonide 200 µg twice daily plus salbutamol as required Step 3: budesonide 400 µg twice daily plus salbutamol as required Step 4: budesonide 400 µg and formoterol 12 µg twice daily Step 5: budesonide 800 µg twice daily and formoterol 24 µg twice daily |
FeNO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in 1 s; GINA: Global Initiative for Asthma; BTS: British Thoracic Society; SIGN: Scottish Intercollegiate Guidelines Network; HFA: hydrofluoroalkanes; ICS: inhaled corticosteroid; BDP: beclomethasone dipropionate; LTRA: leukotriene receptor antagonist; SABA: short-acting β2-agonist; LABA: long-acting β2-agonist; NHLBI: National Heart, Lung and Blood Institute; ACQ: Asthma Control Questionnaire; OCS: oral corticosteroid.
Risk of bias within studies
Table 4 summarises the methodological quality of the included studies. Generally, two studies [25, 26] performed well receiving a positive assessment of at least six of the seven quality items. The most frequently identified potential sources of a high risk of bias concerned “other biases” related to the receipt of commercial funding (67%) [13, 14, 16, 24]. A high number of publications poorly reported the following aspects: random sequence generation (33%) [13, 24], allocation concealment (33%) [13, 24] and blinding of outcome assessment (50%) [13, 24, 25]. It should be noted that poor performance in quality assessment for the study by Syk et al. [14] was due to its open label study design, which was necessary to influence patients' adherence to treatment and to capture these clinically valuable effects.
TABLE 4.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
| First author [ref.] | Methodological quality assessment: randomised controlled trials | ||||||
| Random sequence generation (selection bias) | Allocation of treatment concealed | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other biases (e.g. commercial funding) | |
| Calhoun [13] | U | U | L | U | U | H | H |
| Honkoop [16] | L | L | H | H | L | L | H |
| Powell [26] | L | L | L | L | L | L | U |
| Shaw [25] | L | L | L | U | L | L | L |
| Smith [24] | U | U | U | U | L | L | H |
| Syk [14] | L | L | H | H | L | H | H |
L: low risk of bias; H: high risk of bias; U: unclear risk of bias.
Outcomes and synthesis of results
Despite wide variation in all aspects of study design across the five studies [13, 14, 16, 24, 25] (excluding the study on pregnant women) [26]; exploratory meta-analyses were conducted where possible for all relevant outcomes (table 5).
TABLE 5.
Exacerbations and inhaled corticosteroid (ICS) use in adult patients with or without fractional exhaled nitric oxide (FeNO)-guided management
| First author [ref.] | Time of outcome | Definition of outcomes | Subjects n | Exacerbations per person year | Between group comparison | ICS use | Between group difference# |
| Smith [24] | 3–12 months optimisation (exacerbation rates not reported for this period) plus 12 months titration | Minor: global daily asthma score¶ of two on ≥2 consecutive days | 94 | Intervention group+: 0.36 Control group+: 0.75 |
p=0.24 | Final value ICS use§ Intervention Baseline: mean 411 μg per day (95% CI 344–478) End of phase 2: mean 370 µg per day (95% CI 263–477) Control Baseline: mean 491 μg per day (95% CI 403–579) End of phase 2: mean 641 µg per day (95% CI 526–756) |
Mean difference −270 µg per day (95% CI −112– −430, p=0.003) |
| Major: global daily asthma score¶ of three on ≥2 consecutive days (or in 1 day, in the context of a minor exacerbation) Major exacerbation or medical emergency: global daily asthma score¶ of four in 1 day |
Intervention group+: 0.13 Control group+: 0.14 |
p=0.91 | |||||
| Any minor or major exacerbation | Intervention group: 0.49 (95% CI 0.20–0.78) Control group: 0.90 (95% CI 0.31–1.49) |
−45.6% (95% CI −78.6–54.5, p=0.27) NS | |||||
| Course of oral prednisone | Intervention group: 0.48 Control group: 0.60 |
p=0.60 | |||||
| Shaw [25] | 12 months | Course of OCS or antibiotics | 118 | Intervention group: 0.33 (sd 0.69) Control group: 0.42 (sd 0.79) |
−21% (95% CI −57–43%, p=0.43) | Final value ICS useƒ Intervention: 557 µg Control: 895 µg |
Mean difference −338 µg per day (95% CI −640– −37 µg, p= 0.028) Total used in study (AUC): 11% greater in FeNO group (95% CI −15–37%) |
| Syk [14] | End-points analysed from visit 2 to visit 6 (2–4 weeks, 12 months) | Moderate exacerbation: need to step-up controller treatment for at least 2 days with or without clinic visit Prophylactic use before pollen season excluded |
165 | Intervention group: 0.1 Control group: 0.325 |
NR | ICS use¶¶ Intervention Median 0 (IQR −400–400) Baseline: mean 604 (se 370) Final value: 586 (se 454) Control 0 (IQR −200– 200) Baseline: mean 626 (se 391) Final value: 540 (se 317) |
0.945 |
| Severe exacerbation ##: worsening requiring a course of OCS | Intervention group: 0.113 Control group: 0.0875 |
NS | |||||
| Moderate or severe exacerbation | Intervention group: 0.22 Control group: 0.41 |
p=0.024 | |||||
| Calhoun [13] | 9 months | Exacerbation: unscheduled medical contact for increased asthma symptoms that results in the use of OCS, increased ICS or additional medication for asthma | 229 | Intervention group: 0.21 (97.5% CI 0.1–0.32) Control group: 0.23 (97.5% CI 0.1–0.37) |
“Did not differ” | ICS use (unclear if mean over whole study or final value)ƒ Intervention Mean 1617 µg·month−1 Control Mean 1610 µg·month−1 |
NR |
| Treatment failure defined as exacerbation or loss of control++ | Intervention group: 0.27 (97.5% CI 0.14–0.39) Control group: 0.43 (97.5% CI 0.23–0.64) |
“Were not different” | |||||
| Honkoop [16] | 12 months | Severe exacerbation: course of oral prednisone, hospitalisation and/or emergency department visit | 611 | Intervention group: 0.19 (95% CI 0.11–0.29) Control group Strict: 0.29 (95% CI 0.17–0.40) Sufficient: 0.29 (95% CI 0.15–0.43) |
Odds ratio versus Strict: 0.64 (95% CI 0.27–1.56) Sufficient: 0.79 (95% CI 0.32–1.92) |
NR | NR |
| Unscheduled healthcare utilisation: hospitalisation and/or emergency department visit | Number of visits Intervention group: 3 Controlled asthma: strict 5 Partly controlled asthma: sufficient 9 |
Odds ratio versus Strict: 0.61 (95% CI 0.14–2.58) Sufficient: 0.37 (95% CI 0.10–1.38) |
NS: nonsignificant difference; OCS: oral corticosteroid; AUC: area under curve; NR: not reported; IQR: interquartile range; PEFR: peak expiratory flow rate. #: Expressed as intervention minus control (negative values indicate lower FeNO). ¶: Asthma scores were as follows. 0 (stable): morning PEFR >75% of best PEFR in 14-day run-in period without deterioration in any symptom scores. 1 (mildly unstable): one or more of the following a) bronchodilator use on two or more occasions in 24 h more than the rounded mean number of occasions during the run-in period; b) increase in symptom score of 1 point or more as compared with rounded mean during run-in period; c) onset of or increase in nocturnal waking by one or more times in the previous seven nights more than rounded mean number of times during the run-in period, or morning PEFR of 61–75% without deterioration in any of the above categories. 2 (minor deterioration): morning PEFR of 61–75% of best PEFR during the run-in period and one or more criteria for an asthma score of 1; or morning PEFR of 41–60% without deterioration in any criteria for an asthma score of 1. 3 (major deterioration): morning PEFR of 41–60% of best PEFR during run-in period and one or more criteria for an asthma score of 1. 4 (major exacerbation or medical emergency): morning PEFR of 40% or less than best PEFR during run-in period regardless of symptoms, or attendance at clinician's office or emergency department because of severe asthma. +: Estimated off graph. §: Fluticasone or the equivalent. ƒ: Beclomethasone diproprionate or equivalent. ##: American Thoracic Society/European Respiratory Society Task Force Criteria 2009. ¶¶: Budesonide equivalent. ++: At-home measurements: 1) Pre-bronchodilator AM peak expiratory flow (PEF) of <65% of baseline on two consecutive mornings, scheduled measurements. 2) Post-bronchodilator PEF of <80% of baseline despite 60 min of rescue β-agonist treatment. 3) Post-bronchodilator PEF may be taken at any time of day, an increase in albuterol use of more than 8 puffs per 24 h over baseline use for a period of 48 h, or more than 16 puffs per 24 h for more than 48 h. In-clinic measurements: 1) Pre-bronchodilator forced expiratory volume in 1 s (FEV1) values on two consecutive sets of spirometric determinations, measured 24–72 h apart, that are <80% of the baseline pre-bronchodilator value (baseline value for adherence period: FEV1 value at visit 3; baseline for randomisation period: FEV1 value at visit 4). All participants found to have an FEV1 of <80% of baseline at any centre visit but who are not considered to meet treatment failure or exacerbation criteria must be seen again within 72 h to have FEV1 measured. 2) Physician judgment for patient safety. 3) Patient dissatisfaction with asthma control achieved by study regimen. 4) Requirement for open-label ICSs or another (nonsystemic corticosteroid) new asthma medication (e.g. montelukast) without the addition of systemic corticosteroids.
Healthcare utilisation
Unscheduled healthcare utilisation, defined as emergency department/accident and emergency visits, out-of-hours general practitioner's surgery visits or hospitalisation, was only reported in Honkoop et al. [16]. Although the result showed improvement in healthcare utilisation with FeNO management (table 5), this was not statistically significant for all comparisons (p>0.05). In the remaining four studies [13, 14, 24, 25], unscheduled healthcare utilisation was included as either treatment failure or severe exacerbations (see later), since exacerbations of asthma can lead to both unscheduled healthcare utilisation and the need for a course of oral corticosteroids (OCSs).
Severe exacerbations
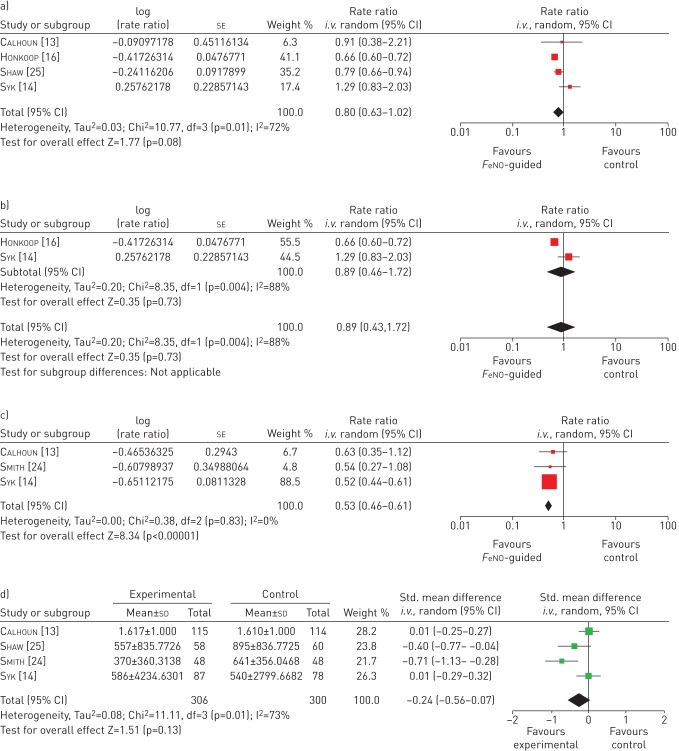
This outcome was defined differently across studies (table 5). Syk et al. [14] and Honkoop et al. [16] defined it as “worsening requiring a course of OCS”; Shaw et al. [25] defined it as “exacerbations resulting in the use of OCS or antibiotics”; and Calhoun et al. [13] reported it as “exacerbations”, which included exacerbations leading to OCS use, increased ICS use or additional medication for asthma. A meta-analysis of four studies (the study of Smith et al. [24] was not included as follow-up data were not calculable as rates per person year) showed that severe exacerbations (while statistically not significant) were less likely in the FeNO-guided-management group compared with the control group (figure 1a), with rate ratio of 0.80 (95% CI 0.63–1.02; p=0.08).
FIGURE 1.
Random effects meta-analysis. a) Effects of fractional exhaled nitric oxide (FeNO)-guided asthma management on major/severe exacerbation rates. b) Number of severe exacerbations resulting in the use of oral corticosteroids. c) Effects of FeNO-guided asthma management on the composite outcome of all exacerbation and treatment failure rates. d) Effects of FeNO-guided asthma management on mean inhaled corticosteroids use (standardised (Std) mean difference analysis).
Severe exacerbations resulting in the use of OCS
Analysis of studies reporting the number of severe exacerbations resulting in the use of OCS (figure 1b) was limited to only two studies [14, 16], which showed opposite directions of effect. This may be due to variations in the step-up/step-down protocols employed in the studies, or due to the populations being slightly different.
Moderate and minor exacerbations
Two studies [14, 24] reported data on less severe exacerbations; however, this data was not amenable to meta-analysis due to unreported data (table 5). Both studies observed lower rates of minor/moderate asthma exacerbations in the intervention group compared with the control group. In Smith et al. [24], the rate was 0.36 versus 0.75 (p=0.24) and in Syk et al. [14], 0.1 versus 0.325 events per person year respectively (p-value not reported).
Composite of all exacerbations and failure rates
Three studies reported composite outcomes that were considered to be broadly similar and represent what may be termed “treatment failure” (table 5). In Smith et al. [24] and Syk et al. [14] this was “any major or minor exacerbation”, while in Calhoun et al. [13] it was exacerbation or any loss of control by a variety of measures. A meta-analysis of these studies (fig. 1c) showed a statistically significant effect in favour of using FeNO-guided management in adults, with a rate ratio of 0.53 (95% CI 0.46–0.61; p<0.00001). However, due to high degree of heterogeneity in composite outcomes, the effect is therefore liable to high risk of bias.
ICS use
Four studies reported some data on ICS use [13, 14, 24, 25]; however, outcomes were not reported in a standardised manner (table 5). As shown in figure 1d, a meta-analysis using the standardised mean difference analysis showed a beneficial overall effect of −0.24 (95% CI −0.56–0.07) in favour of FeNO-guided management; however, the findings were not statistically significant (p=0.13).
Relationship between ICS use, step-up/step-down protocol and exacerbations
A post hoc analysis was undertaken to examine the relationship between ICS use, exacerbations and which step-up/step-down approach was used. A summary of the data is presented in table 6. Two studies that used FeNO levels in conjunction with symptoms showed a statistically significant decrease in ICS use in the FeNO-guided management groups and a nonsignificant decrease in any type of exacerbation [24, 25], thus indicating improved management overall. By contrast, the studies which managed asthma based on FeNO levels alone were less clear. Syk et al. [14] reported no change in ICS use and a nonsignificant decrease in moderate exacerbation and a nonsignificant increase in severe exacerbation, but a significant decrease in any exacerbation. Calhoun et al. [13] reported no difference in ICS use and exacerbations.
TABLE 6.
Relationship between inhaled corticosteroid (ICS) use, step-up/step-down protocol and exacerbations
| First author [ref.] | Management plan | Severity of population | Treatment | Atopic | Exacerbation | ICS use | ||
| Any | Major | Minor | ||||||
| Smith [24] | FeNO + symptom-based safety protocol | Excluded severe | ICS | NR | NS decrease | NS decrease | NS decrease | SS decrease |
| Shaw [25] | FeNO + symptoms | Recent severe exacerbations excluded | ICS, LTRA, bronchodilator | 66% | NR | NS decrease | NR | SS decrease |
| Syk [14] | FeNO only | Mild to moderate | ICS, LTRA | 100% | SS decrease | NS increase | NS decrease (moderate) | No change |
| Calhoun [13] | FeNO only | Mild to moderate | ICS | 86% | No change | No change | NR | No change |
| Honkoop [16] | FeNO + symptoms | Excluded those taking OCS every day/every other day | ICS, SABA, LABA, LTRA, OCS | 54% | NR | NS decrease | NR | NR |
FeNO: fractional exhaled nitric oxide; NR: not reported; NS: nonsignificant; SS: statistically significant; LTRA: leukotriene receptor antagonist; OCS: oral corticosteroid; SABA: short-acting β2-agonist; LABA: long-acting β2-agonist.
Other outcomes
Health-related quality of life was infrequently reported. Three studies [13, 14, 16] used versions of the Asthma Quality of Life Questionnaire to measure quality of life. Two studies showed no effect in the global score (pooled standardised mean difference: 0.00 (95%CI −0.20–0.20); p=0.96) [13, 16]. However, one study investigated domains and found a statistically significant difference in the symptoms score (p=0.041) with a between group difference in change from baseline of 0.10 in favour of FeNO management [14]. Asthma control was reported in all studies, but showed no statistically significant difference. Further details on other outcomes are summarised in online supplementary appendix 3.
Efficacy of FeNO in pregnant women
One study reported the efficacy of FeNO-guided management of asthma in pregnant women [26]. The composite outcome of all exacerbations was statistically significantly reduced in the intervention arm, with an incidence rate ratio of 0.496 per pregnancy (95% CI 0.325–0.755; p=0.001). This difference was mostly driven by the rate of OCS use and the rate of doctors’ visits during pregnancy (table 7). Mean OCS use in the FeNO and control arm was 0.08 (95% CI 0.03–0.133) and 0.19 (95% CI 0.08–0.31), respectively (p=0.042). Similarly, the rate of doctors’ visits was 0.26 (95% CI 0.16–0.36) in the FeNO arm and 0.56 (95% CI 0.40–0.72) in the control arm with a p-value of 0.002 in favour of FeNO management. Other components of the exacerbation outcome (hospitalisations and emergency room/labour ward visits) did not differ between groups. The change in mean value from baseline to final visit for ICS use decreased by 210 µg·day−1 in the intervention arm and increased by 50 µg·day−1 in the control arm. The difference was statistically significant in favour of FeNO management (p=0.043). However, overall more patients received ICS (68% versus 42%) in the FeNO group than in the control group by the end of the study. Other outcomes are summarised in table 7.
TABLE 7.
Pregnant women: all outcomes
| Time of outcome | Definition of outcomes | Intervention | Control | Between group comparison |
| Exacerbations# | Exacerbations: an unscheduled visit to a doctor, presentation to the emergency room or admission to hospital, or when OCS used Events separated by 7 days or more were counted as a second event |
0.288 per pregnancy (mean±sd study time 17.8±5.5 weeks) | 0.615 per pregnancy (mean study time 18.8±3.8 weeks) | Incidence rate ratio 0.496 (95% CI 0.325–0.755), p=0.001 |
| Mean (95% CI) OCS use | 0.08 (0.03–0.133) | 0.19 (0.08–0.31) | p=0.042 | |
| Mean (95% CI) hospitalisations | 0 (0–0) | 0.03 (−0.004–0.06) | p=1.0 | |
| Mean (95% CI) emergency room/labour ward visits | 0.04 (0.001–0.07) | 0.02 (−0.01–0.04) | p=0.399 | |
| Mean (95% CI) unplanned or unscheduled doctors' visits | 0.26 (0.16–0.36) | 0.56 (0.40–0.72) | p=0.002 | |
| ICS use | Difference in means (from baseline to last visit) (read off graph): | −210 µg·day−1 | 50 µg·day−1 | p=0.043 |
| Median (IQR) BDP equivalent ICS dose (µg·day−1) | 200 (0–400) | 0 (0–800) | p=0.079 | |
| Users | 76 (68.5%) out of 111 | 46 (42.2%) out of 109 | p<0.0001 | |
| Other outcomes | Median (IQR) HRQoL | |||
| SF-12 physical summary (low 0, high 100): | 47.7 (40.8–52.0) | 46.9 (38.2–51.8) | p=0.89 | |
| SF-12 mental summary (low 0, high 100): | 56.9 (50.2–59.3) | 54.2 (46.1–57.6) | p=0.037 | |
| AQLQ-M: total score (good 0, poor 10): | 0.75 (0.38–1.25) | 0.81 (0.38–1.63) | p=0.54 | |
| Asthma control: mean±sd ACQ | 0.56±0.67 | 0.72±0.80 | p=0.046 | |
| Median (IQR) β2-agonist use in past week | 0 (0–3) | 1 (0–5) | p=0.024 | |
| LABA users | 45 (40.5%) out of 111 | 19 (17.4%) out of 109 | p<0.0001 | |
| Adverse events, mortality, compliance and test failure rates | NR | NR | NR |
OCS: oral corticosteroids; IQR: interquartile range; BDP: beclomethasone diproprionate; ICS: inhaled corticosteroid; HRQoL: health-related quality of life; SF-12: short form 12; AQLQ-M: Asthma Quality of Life Questionnaire-Marks; ACQ: Asthma Control Questionnaire; LABA: long-acting β2-agonist; NR: not reported. #: time of outcome was monthly until birth (maximum ∼30 weeks). Information from [26].
Discussion
In this systematic review, six RCTs were identified that assessed the use of FeNO for the management of asthma in adults [13, 14, 16, 24–26]. In general, using exploratory meta-analysis, a fall in exacerbation rates per person year were observed, but none were statistically significant apart from the composite of all exacerbations and failure rates. However, the findings should be interpreted with caution due to the high degree of heterogeneity in the outcome definition. The effects on ICS use were heterogeneous, although the direction of the effect was towards a decrease in ICS use. The effect on healthcare utilisation was not statistically significant; however, as this outcome was only reported in one low quality study [16], to base any conclusion on this could be misleading. The use of FeNO to guide asthma management in pregnant women in the second trimester appears to be as effective, if not more so, than in other adults [26], and appears to reduce exacerbations and ICS use, but by the end of the study more patients in FeNO group had received ICS. The differences in outcome between studies may have occurred due to some step-up/step-down protocols being better at decreasing ICS use than others, or may be due to the characteristics of the study populations. Other potential factors as to why the FeNO monitoring studies have been predominately negative could be due to the difference in severity of asthma at baseline, different treatment strategies used (i.e. some studies controlled only ICS while some also controlled other medications), differences in the number and points of FeNO cut-off used, and also the comparator groups did not all use the same algorithm.
There are at least two previous systematic reviews on the effectiveness of FeNO monitoring to guide management [9, 11]. Petsky et al. [9] compared adjustments of asthma therapy based on FeNO with conventional methods (typically clinical symptoms and spirometry). The review suggested some benefits associated with FeNO for several outcomes, in particular the number of subjects with >1 exacerbation, exacerbation rates, FEV1 % predicted at final visit and geometric change in FeNO from baseline; however, none of these results were statistically conclusive. FeNO appeared to have some beneficial effect on symptom score (mean difference: −0.14, 95% CI −0.42–0.14) and lowered ICS dose (mean difference: −450.03 μg, 95% CI −676.73– −223.34 μg). Furthermore, there was substantial clinical heterogeneity among the study cohorts, with no two studies using exactly the same step-up/step-down protocols. There is some agreement between the review by Petsky et al. [9] and our own review, especially relating to the lack of statistically significant effects in most outcomes. The strength of our review lies in the inclusion of subsequently published studies (Calhoun et al. [13], Syk et al. [14] and Honkoop et al. [16]), the focus on exacerbation rates rather than number of people with an exacerbation, and the prior separation of pregnant women into a different subgroup. The second review by Donohue and Jain [11] updated the meta-analyses of the number of patients with >1 exacerbation and exacerbation rates from the aforementioned Cochrane review [9], and included a study in pregnant women [26]. Inclusion of this study resulted in improvements on all measures of exacerbations (mean difference: −0.27, 95% CI −0.42– −0.12), and the relative rate of asthma exacerbations (relative rate: 0.57, 95% CI 0.41–0.80). However, since it is known that pregnancy can substantially affect the course of asthma [12], it was arguably inappropriate to include the cohort of pregnant women in a meta-analysis of adults with asthma.
One of the putative benefits of using FeNO for the management of asthma is the identification of patients for whom increased ICS use will not improve control. These patients are likely to present with symptoms, which would indicate an increase in pharmaceutical management under standard clinical guidelines, and under most of the FeNO protocols that have been studied to date, whereas they may be better treated with other asthma control medications. A key limitation is therefore the paucity of studies that allowed step-down of ICS to be performed on the basis of low FeNO values alone. Only two studies [13, 14] and the study in pregnant women [26] included such a strategy, and only Powell et al. [26] made provision for adjusting other treatments which may offer superior control in these patients in response to their reported symptomatology. We did not plan or perform a sensitivity analysis of this data, but did present a rudimentary analysis of the relationship between ICS use, management protocols and exacerbations (table 6). It is interesting to note that the two studies that managed patients on the basis of FeNO only (Syk et al. [14] and Calhoun et al. [13]) did not report any change in ICS use, which is perhaps contrary to expectations, or in severe exacerbations. However, Syk et al. [14] did report a fall in exacerbations overall. In comparison, the two studies that managed patients on the basis of FeNO and symptoms (Smith et al. [24] and Shaw et al. [25]) reported a statistically significant decrease in ICS use and a nonsignificant decrease in exacerbations. This perhaps indicates a shift in treatment patterns, with better targeting of treatment with the addition of FeNO to the patients who will benefit most. In addition, although there was no significant difference in compliance with treatment between the FeNO management and control group, there is a potential that FeNO may help improve compliance with ICS use.
There are a number of limitations to our review which warrant caution in its interpretation to clinical practice. The evidence from the included studies are of low quality and there is significant heterogeneity in all aspects of study design across the studies, including patient characteristics, outcome definitions, FeNO cut-off points and in management protocols, hence an exploratory meta-analysis was used to overcome these differences. In addition, the management plan used in some studies did not reflect real life practice, for example in the study by Smith et al. [24], long-acting β2-agonist (LABA) was not used and patients underwent a step-down therapy approach in the pre-study phase. It is noteworthy that LABA in combination with ICS are key steps in asthma management. The equivalence of devices is assumed and this may not hold true in practice. As such, FeNO cut-off values as reported in the primary research may not be applicable to measurements using other devices. Smoking affects FeNO levels and majority of the patients in this review were nonsmokers, hence it is not clear if the results can be generalised to the smoking population. Also, the average age of patients in this review was around 40 years old. However, the majority of asthma deaths occur in older people with severe disease. All the included studies recruited patients that were stable during the run-in period and excluded the more severe/difficult patients with recent hospital admissions. So, by definition, some of the real life “difficult” patients, who require more help, were excluded. Finally, the criteria used for the diagnosis of asthma across the included studies varied with limited data and as recent studies have reported the potential of overdiagnosis of asthma, this may have implications for the results. It is important to note that these limitations are principally sourced in the evidence base, rather than the methods used to interrogate and evaluate it. One should also bear in mind that the addition of FeNO to the current management strategy will require change in organisation and to the philosophy of care in self-management.
Conclusion
FeNO guided management showed no statistically significant benefit in terms of severe exacerbations or ICS use, but showed a statistically significant reduction in exacerbations of any severity. Due to heterogeneity in the studies it was not possible to draw any firm conclusions as to which management protocol or cut-off points offer the best efficacy. Further research is required to investigate the best way to use FeNO in the management of asthma, which management protocol and cut-offs to use; to establish which patient groups are likely to benefit from FeNO monitoring, e.g. individuals with atopy, frequent exacerbations or those with poor adherence; and how treatment effect will progress over time. Larger, well designed RCT studies, taking into account issues such as severity as defined by previous exacerbations, blinding and approximating to routine care are warranted to clearly define the role of FeNO in clinical practice.
Acknowledgements
We would like to thank John W. Stevens (School of Health and Related Research, University of Sheffield, Sheffield, UK) for providing statistical support.
Footnotes
This article has supplementary material available from erj.ersjournals.com
Support statement: This project was funded by the National Institute for Health Research Health Technology Assessment (NIHR HTA) Programme (project number: 12/60/01) as part of a review on “Measurement of exhaled nitric oxide concentration in asthma; NIOX MINO, NIOX VERO and Nobreath”, and published as part of a full report in Health Technology Assessment (PROSPERO registration number: CRD42013004149 (www.crd.york.ac.uk/prospero/)). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, CCF, NETSCC, the NIHR HTA programme or the UK Department of Health. Funding information for this article has been deposited with FundRef.
Conflict of interest: Disclosures can be found alongside the online version of this article at erj.ersjournals.com
References
- 1.Royal College of Physicians. Why Asthma Still Kills: the National Review of Asthma Deaths (NRAD). www.asthma.org.uk/globalassets/campaigns/nrad-full-report.pdf Date last accessed: June 1, 2015. Date last updated: May, 2014.
- 2.British Thoracic Society (BTS). The Burden of Lung Disease. 2nd Edn. A statistics report from the British Thoracic Society. www.brit-thoracic.org.uk/document-library/delivery-of-respiratory-care/burden-of-lung-disease/burden-of-lung-disease-2006/ Date last accessed: accessed 1 June 2015. Date last updated: 2006.
- 3.Dahl R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med 2006; 100: 1307–1317. [DOI] [PubMed] [Google Scholar]
- 4.British Thoracic Society (BTS), Scottish Intercollegiate Guidelines Network (SIGN). British Guideline on the Management of Asthma: a National Clinical Guideline. Revised 2014. www.brit-thoracic.org.uk/guidelines-and-quality-standards/asthma-guideline/ Date last accessed: June 2, 2015. Date last updated: October 8, 2014.
- 5.Dweik RA, Boggs PB, Erzurum SC, et al. . An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med 2011; 184: 602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schleich FN, Seidel L, Sele J, et al. . Exhaled nitric oxide thresholds associated with a sputum eosinophil count ≥3% in a cohort of unselected patients with asthma. Thorax 2010; 65: 1039–1044. [DOI] [PubMed] [Google Scholar]
- 7.Pavord ID, Shaw DE, Gibson PG, et al. . Inflammometry to assess airway diseases. Lancet 2008; 372: 1017–1019. [DOI] [PubMed] [Google Scholar]
- 8.Berry M, Morgan A, Shaw DE, et al. . Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 2007; 62: 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petsky HL, Cates CJ, Li A, et al. . Tailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adults. Cochrane Database Syst Rev 2009; 4: CD006340. [DOI] [PubMed] [Google Scholar]
- 10.Petsky HL, Cates CJ, Lasserson TJ, et al. . A systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils) asthma in children and adults. Thorax 2012; 67: 199–208. [DOI] [PubMed] [Google Scholar]
- 11.Donohue JF, Jain N. Exhaled nitric oxide to predict corticosteroid responsiveness and reduce asthma exacerbation rates. Respir Med 2013; 107: 943–952. [DOI] [PubMed] [Google Scholar]
- 12.Tan KS, Thomson NC. Asthma in pregnancy. Am J Med 2000; 109: 727–733. [DOI] [PubMed] [Google Scholar]
- 13.Calhoun WJ, Ameredes BT, King TS, et al. . Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA 2012; 308: 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syk J, Malinovschi A, Johansson G, et al. . Anti-inflammatory treatment of atopic asthma guided by exhaled nitric oxide: a randomized controlled trial. J Allergy Clin Immunol Pract 2013; 1: 639–648. [DOI] [PubMed] [Google Scholar]
- 15.Honkoop P, Loijmans R, Termeer E, et al. . A cluster randomized trial comparing strict, partial, and FeNO-guided asthma control strategies in primary care. Eur Respir J 2013; 42: Suppl. 57, abstract 1710. [Google Scholar]
- 16.Honkoop PJ, Loijmans RJB, Termeer EH, et al. . Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol 2015; 135: 682–688. [DOI] [PubMed] [Google Scholar]
- 17.National Institute for Health and Care Excellence (NICE). Measuring fractional exhaled nitric oxide concentration in asthma: NIOX MINO, NIOX VERO and NObreath. NICE diagnostics guidance 12. www.nice.org.uk/guidance/dg12 Date last accessed: June 10, 2015. Date last updated: April, 2014.
- 18.Harnan SE, Tappenden P, Essat M, et al. . Measurement of exhaled nitric oxide concentration in asthma: a systematic review and economic evaluation of NIOX MINO, NIOX VERO and NObreath. Health Technol Assess 2015; 19: 1–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005; 171: 912–930. [DOI] [PubMed] [Google Scholar]
- 21.Gomersall T, Harnan S, Essat M, et al. . A systematic review of fractional exhaled nitric oxide in the routine management of childhood asthma. Pediatr Pulmonol 2015. [In press; DOI: 10.1002/ppul.23371]. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 The Cochrane Collaboration, 2011. [Google Scholar]
- 23.Centre for Reviews and Dissemination. Systematic Reviews. CRD's Guidance for Undertaking Reviews in Health Care York, Centre for Reviews and Dissemination, 2009. [Google Scholar]
- 24.Smith AD, Cowan JO, Brassett KP, et al. . Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med 2005; 352: 2163–2173. [DOI] [PubMed] [Google Scholar]
- 25.Shaw DE, Berry MA, Thomas M, et al. . The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med 2007; 176: 231–237. [DOI] [PubMed] [Google Scholar]
- 26.Powell H, Murphy VE, Taylor DR, et al. . Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet 2011; 378: 983–990. [DOI] [PubMed] [Google Scholar]
- 27.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis 1987; 136: 225–244. [DOI] [PubMed] [Google Scholar]