Abstract
Cardiovascular disease remains the leading cause of mortality in the industrialized world. Despite advances in pharmacotherapy and catheter based interventions, coronary artery bypass grafting remains an essential therapeutic modality. The majority of coronary artery bypass operations, as well as other cardiac surgical procedures require the use of ischemic cardioplegic arrest and cardiopulmonary bypass, both of which result in iatrogenic injury to the vasculature and microcirculation. This injury can manifest as impaired vasorelaxation or vasoconstriction, depending upon the organ system involved, resulting in impaired tissue perfusion and the development of edema. Key to this dysfunction are changes in the following: nitric oxide signaling secondary to changes in eNOS and iNOS expression and activity, cyclooxygenase function with increases in proinflammatory COX-2 activity, alterations in Protein Kinase C and Mitogen Activated Protein Kinase signaling, and an increase in Vascular Endothelial Growth Factor expression increasing vascular permeability and dilatation. This review discusses our current understanding of cardioplegia and cardiopulmonary bypass induced changes in the vasculature, and therapeutic interventions aimed at modulating the altered signaling pathways.
Keywords: Nitric Oxide, Prostaglandins, PKC, Vasodilation, VEGF, Review
2. INTRODUCTION
Cardiovascular disease remains the leading cause of mortality in the industrialized world. Despite advances in pharmacotherapy and catheter-based interventions such as percutaneous transluminal coronary angioplasty and coronary artery stenting, coronary artery bypass grafting (CABG) remains a major therapeutic modality for patients suffering from coronary artery disease (CAD). The majority of CABG operations and other cardiac surgical procedures (valvular, aortic, congenital) utilize ischemic cardioplegic arrest (CP) with cardiopulmonary bypass (CPB) (54) to provide a relatively bloodless and motionless operative field for safe and successful conduct of the procedure. Despite continued refinements in myocardial protection strategies and cardiopulmonary bypass systems, cardiac surgery utilizing CP/CPB is associated with significant systemic vascular dysfunction which can result in impairments to perfusion of the brain, myocardium, lungs, intestinal tract, and skeletal muscle. These perfusion deficits may manifest as neurocognitive deficit, impairments in myocardial pump function secondary to coronary artery vasospasm or impaired coronary microcirculatory vasomotor function, persistent requirement for mechanical ventilatory support, systemic hypotension, and generalized edema secondary to increased vascular permeability.
The etiology of vascular dysfunction after CP/CPB is multi-factorial, involving a complex interaction between inflammatory mediators generated during CPB, endothelial impairments, and alterations in vascular smooth muscle contractility. Central to these changes in the vasculature after CP/CPB lie signaling alterations in nitric oxide synthase (NOS), cyclooxygenase (COX), kinase pathways, and growth factors.
3. NITRIC OXIDE SYNTHASE
Key to the vascular pathophysiology observed after CP/CPB are altered concentrations of nitric oxide (NO) (Figure 1). Nitric oxide is produced in healthy endothelial cells via activation of a constitutive nitric oxide synthase (eNOS), and in a variety of other cell types including activated endothelial cells, inflammatory cells and macrophages, cardiomyocytes, intestinal cells, and vascular smooth muscle cells by the inducible form of nitric oxide synthase, iNOS. Physiologic roles of eNOS, which is responsible for the endothelial production of NO via conversion of L-arginine to L-citrulline, include endothelial-dependent vasorelaxation through activation of guanylate cyclase, inhibition of leukocyte adhesion, and attenuation of platelet activation. In addition to these endothelial effects, eNOS also regulates tone in the vascular smooth muscle to which the endothelium signals, and thus affects medial vasodilatory responses (8).
Figure 1.
Alterations in vascular NOS and COX signaling pathways following cardiac surgery. A) NOS and COX signaling under normal conditions and B) following cardiac surgery Red arrows indicate relative changes following cardiac surgery. Blue arrows depict eNOS associated signaling alterations, green arrows represent iNOS dependent signaling alterations. Abbreviations used: EC – endothelial Cells; VSMC – vascular smooth muscle cells; COX – cyclooxygenase; eNOS – endothelial nitric oxide synthase; iNOS – inducible nitric oxide synthase; RNS/ROS – reactive oxygen/nitrogen species, NO – nitric oxide – sGC – soluble Guanylate Cyclase; cGMP – cyclic Guanosine Monophosphate TXA2 – thromboxane A2; PGF2-alpha – prostaglandin F2-alpha ; PGI2 – prostacyclin; PGE2 – prostaglandin E2.
It is well documented hyperkalemic cardioplegic arrest and ischemia-reperfusion alter endothelial structure and indices of endothelial function, most notably endothelial dependent vasorelaxation (14, 43, 62, 63). The etiology of this impairment in endothelial dependent relaxation is multi-factorial, with experimental studies demonstrating the release of NO from eNOS is reduced after ischemic cardioplegic arrest (23). When assessing eNOS activity via examination of endothelial dependent responses, the defect in function is likely due to changes in cell membrane potential (23, 31), substrate cofactor depletion (5, 12), alterations in the concentration or compartmentalization of intracellular calcium (16, 48), and injury to cell membranes, associated regulatory enzymes, or ion pumps (61). The internal thoracic artery, a common conduit for bypass grafting, actually demonstrates increases in eNOS expression after CPB, which may in part explain the relatively low incidence of vasospasm in this vessel (75). While impaired signal transduction and reduced agonist stimulated production of NO likely contribute to the reduced endothelial-dependent relaxation after cardioplegic arrest, increased degradation or binding of NO through interactions with free radicals may decrease the bioavailability of NO to the vascular smooth muscle (8). Following reperfusion after cardioplegic arrest, increased breakdown of NO occurs from increased oxidative stress secondary to the generation of oxygen-derived free radicals (40), and production is further impaired by exposure of the endothelium to fragments of activated complement (69) (24), activated neutrophils, and macrophages (60). Taken together, the diminshed production, combined with the increase in degradation / free-radical coupling, results in a significant deficit of eNOS generated NO required for homestatic vascular function.
In contradistinction to eNOS, the inducible form of NOS, iNOS, is found in increasing quantities in the myocardium after cardioplegic arrest (20, 73), and to a lesser extent in other organs after CPB including the pulmonary vasculature (28, 57), the mesenteric vasculature (74), and brain (46). In contrast to the low concentrations of NO produced by eNOS which inhibit adhesion molecule expression, cytokine synthesis, and leukocyte adhesion, the large amounts of NO generated by iNOS under stressed local conditions can be toxic and pro-inflammatory (26). This is due to the excess spontaneous reaction of NO with the reactive oxygen radicals released under inflammatory or post-ischemic conditions by stressed endothelial cells and activated leukocytes resulting in formation of peroxynitrite, which may cause cell apoptosis, cell necrosis, and circulatory shock (7). In addition, to the proinflammatory stimulus presented by CPB and enhanced peroxynitrite formation by activated leukocytes, increased plasma nitrotyrosine in blood collected from the coronary sinus indicates enhanced peroxynitrite formation in myocardium following cardioplegia/reperfusion likely due to the associated ischemic insults (30).
There are a number of potential mechanisms of cardiovascular dysfunction induced by excess levels of peroxytnitrite (Figure 1). First peroxynitrite can directly modify numereous proteins through direct nitration/oxidation reactions (reviewed in (52, 70)). Modified proteins of particular interest in cardiovascular perturbations associated with CP/CPB include peroxynitrite-induced loss of function modifications of specific vascular regulators such as eNOS and prostacyclin synthase (58), as well as cystoskeletal and contractile elements in myocytes and/or smooth muscle cells (52). Other general effect of peroxynitrite include induction of apoptosis via upstream activation of apoptotic cell signaling cascades (MAPK’s) resulting in caspase activation, and/or opening of the mitochondrial permeability transition pore and associated subsequent apoptotic effects (11, 15, 52). Peroxynitrite also causes irreversible inhibition of many proteins in the mitochondrial respiratory chain resulting in reduced ATP formation and metabolic alterations (55). Another major mechanism of peroxynitrite-induced cell damage is the production of single-strand DNA breaks which results in activation of the DNA repair enzyme poly-ADP ribose polymerase (PARP) subsequently depleting ATP levels and initiation of necrotic cell death (71).
iNOS expression can also be elevated by increased circulating levels of tumor necrosis factor-alpha (TNF-alpha), interleukin (IL)-6, IL-8, and other cytokines liberated during CPB (20) (22). In the pulmonary circulation, eNOS expression decreases significantly, but no significant changes occur in iNOS expression (57). The resulting deficit, combined with increased cyclooxygenase expression (as discussed in Section 4), can lead to significant impairments in pulmonary vasodilatation, manifesting as increased pulmonary vascular resistance. Interestingly, expression of eNOS mRNA is increased in the kidney and cerebral cortex of rats subjected to CPB (47), but the functional significance of this remains unclear.
4. CYCLOOXYGENASE
Cyclooxygenase (COX) acts in a two-step conversion process of arachidonic acid (AA) (51) to form prostaglandins (PG). Initially, COX converts AA to a cyclic endoperoxide (PGG2) by the action of COX-1 or COX-2. This is subsequently followed by cleavage (via a peroxidase) to yield endoperoxide (PGH2). These intermediate products of AA metabolism by COX are unstable and rapidly converted to prostaglandins (PGE2, PGF2, thromboxane A2, PGI2) by specific isomerase enzymes.
The expression of COX-2 (common in the endothelium of patients with coronary artery disease) is enhanced in the reperfusion phase following blood cardioplegia during coronary artery bypass graft surgery, while COX-1 expression remains unchanged (50, 76). Consequently, prostaglandin release (likely thromboxane A2) is stimulated which then activates the contractile response of coronary arterioles to serotonin (Figure 1). The upregulation of COX-2 by CP/CPB also results in the generation of predominantly vasoconstrictive prostaglandins resulting in atrial and ventricular microvascular constriction (49, 50, 62). This enhanced response is due to an increased production and release of contractile prostanoids since the response is inhibited in the presence of either indomethacin or NS398, a selective inhibitor of COX-2, as well as the thromboxane A2 synthase inhibitor U63557A (49, 50). This may lead to coronary microvessel spasm, which potentially, can contribute to myocardial ischemia and injury after surgery (Figure 1). Additionally, these prostoglandins can regulate vascular permeability in a manner similar to that of NO in part through activation of tyrosine kinase receptors and mitogen activated protein (MAP) kinases (53). The inducing factors leading to increased expression of COX-2 are most likely myocardial hypoxia and ischemia which occur during cardioplegic arrest as well as the exposure of the myocardium and blood vessels to inflammatory cytokines. In contrast to iNOS, which is not regulated by agonist stimulation or by intracellular calcium concentration, there is evidence that COX-2 is regulated by agonists such as serotonin (49, 50). Separating the effects of NO and PG when discussing changes in vasomotor activity and permeability during cardiac surgery remains difficult as they are often synergistic and complementary in their actions. In addition to coronary changes in COX-2 expression, experimental models utilizing rats undergoing CPB have demonstrated COX-2 expression is significantly up-regulated in the cerebral cortex after CPB, a finding which may have clinical implications relating to neurocognitive function after cardiac surgery (33). Increased release of constrictor prostanoids have also been implicated in the alterations in pulmonary microvascular responses after CPB (66). The mechanism underlying these findings may relate to increases in COX-2 mRNA and protein expression (no changes in COX-1 expression were found), which have been found in the lungs and pulmonary vasculature after CP/CPB, with concominant increases in pulmonary microvascular vasoconstriction in response to serotonin. These increases in vasoconstrictive responses were partially inhibited by the COX-2 inhibitor NS398, but not the thromboxane synthase inhibitor U63557A (57). These findings implicate increases in COX-2 expression as a possible mechanism for the increased pulmonary vascular resistance often observed after CPB.
5. KINASE PATHWAYS
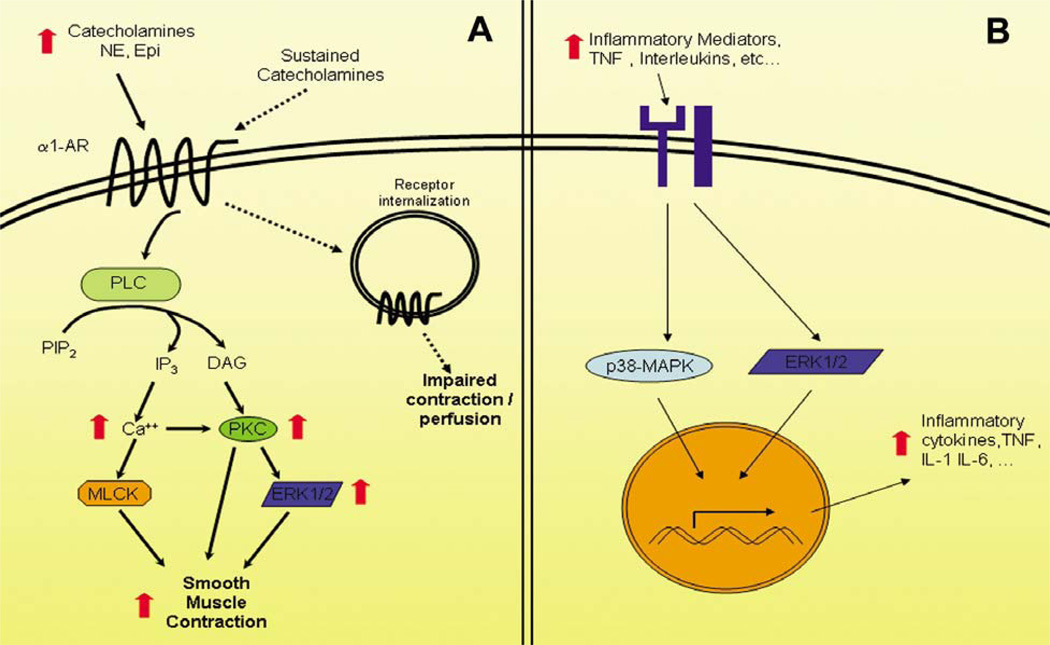
Much of the vascular dysfunction observed in the coronary and systemic circulation after CP/CPB relates to impairments in vascular tone and responsiveness to adrenergic agonists (Figure 2A). These impairments, which can range from diminished to excess vasoconstriction culminate in insufficient tissue perfusion (20, 80, 82). Key to this derangement are Protein Kinase C (PKC), and the downstream Mitogen Activated Protein Kinases (MAPK). The PKC family consists of 12 serinethreonine kinases, of which PKC Alpha has been identified as the predominant isoform present in the human coronary and skeletal microcirculation (67). Stimulation of the alpha-1 adrenoreceptor (G-protein receptor) results in activation of Phospholipase C resulting in conversion of phosphoinositolphosphate-2 (PIP2), liberating 1,4,5-triphosphate (IP3), diacylgylcerol (DAG) and calcium (59). DAG and calcium serve to activate PKC, which can augment smooth muscle contraction directly through increasing intracellular calcium (45), decreasing activity of myosin light chain phosphatase (68), or increasing myofilament sensitization to calcium, independent of increases in cytosolic calcium concentration (32). Activation of PKC also leads to activation of the MAPK ERK 1/2 (27, 78). MAPK are serine-threonine kinases involved in vasomotor function and vascular permeability and can be found in endothelial cells and vascular smooth muscle (39). Multiple stimuli have been shown to activate MAPK, including ischemia, shear stress, and vasoactive agents. The three major MAPK families implicated in cardiovascular signaling thus far include: the extracellular signal-regulated kinases (ERK), the c-Jun-NH2-terminal protein kinases (JNK), and p38 kinase. Of the three, ERK 1/2 is thought to play the most significant role in postoperative vascular dysfunction, given it can regulate endothelial cell permeability / edema formation (10, 35), myogenic tone (39) and contractile responses of microvessels to phenylephrine and vasopressin (36).
Figure 2.
Alterations in (A) catecholamine signaling and (B) inflammatory mediators following cardiac surgery. A) Red arrows indicate relative changes shown to be increased following cardiac surgery. Dotted arrows represent effects associated with sustained catecholamine exposure. Early during reperfusion increased catecholamines promote smooth muscle contraction through increased activation of components of adrenergic signaling. Sustained catecholamine exposure results in down regulation of receptor signaling and impaired perfusion. B) Numerous inflammatory mediators from white cells subjected to CPB can produce increased production of inflammatory mediators from the target vascular tissues. Abbreviations: NE – norepinephrine; Epi – Epinephrine; a1-AR – alpha 1 adrenergic receptor; PLC – phospholipase C; PIP2 – phosphatidyl inositol 4,5 bisphosphate; IP3 – inositol tri-phosphate; DAG – Diacylglycerol; PKC – protein kinase C; MLCK – myosin light chain kinase; ERK – extracellular signal regulated kinase; p38-MAPK – p38 mitogen activated protein kinase, TNF – tumor necrosis factor; IL – interleukin.
Postoperatively, the endogenous adrenergic stress response to CPB results in a release of vasoactive catecholamines, which act on alpha-1-adrenoreceptors, including norepinephrine and epinephrine (34, 79). A sustained increase in circulating levels of catecholamines in vivo, or prolonged exposure to catecholamines in vitro results in subsequent loss of alpha-1-adrenoreceptor mediated vascular smooth muscle cell contraction, and diminished IP3 turnover, which results in decreased concentrations DAG and calcium, both of which are required for conventional PKC activation (41, 56). The resulting lack of DAG and calcium may account for the decreases in PKC activity seen in coronary and skeletal microvessels after CP/CPB (67). Decreases in PKC activity, which functions in activating MAPK, specifically ERK 1/2 (27, 78), can lead to decreases in ERK 1/2 activity as has been demonstrated after CP/CPB in the coronary and skeletal microcirculation (36, 39), culminating in impaired microvascular myogenic tone and vasoconstriction.
Compounding the vascular dysfunction induced by the effects of CP/CPB on kinase activity, are signal transduction mechanisms triggered by a broad range of effectors including TNF-alpha, which is itself elevated after CP/CPB (44), and can lead to ERK 1/2 and MAPK activation in turn inducing IL-6 production (29), leading to a cascade of inflammatory events that include leukocytosis, thrombosis, and lymphocyte activation (77). The predominance of TNF-alpha’s stimulatory effect on MAPK remains unknown as the overall affect of CP/CPB on ERK 1/2 seems to be stimulatory (Figure 2B). In the pulmonary circulation, rapid induction of ERK 1/2 (37) leads to enhanced vasoconstriction likely contributing to the elevations in pulmonary vascular resistance commonly seen after CP/CPB. In the mesenteric microcirculation, key to the pathophysiology of mesenteric ischemia, ERK 1/2 activity and expression levels are markedly increased after CPB with significant augmentation of responses to phenylephrine (38).
6. GROWTH FACTORS
The predominant growth factor involved in vascular changes after CP/CPB has been identified as Vascular Endothelial Growth Factor (VEGF), a potent vasodilator and inducer of vascular permeability operating through the tyrosine kinase regulated release of NO (64, 72). The expression of VEGF and that of it’s receptor VEGFR-1, are significantly up-regulated after CP and reperfusion, resulting in increased coronary microvascular relaxation responses (6, 64, 72, 75). Conversely, coronary microvascular relaxation responses to adenosine diphosphate (ADP), an endothelial-dependent vasodilator, are unchanged after CP, suggesting the up-regulation of VEGF receptors on the coronary endothelium is selective and may play a role in mediating perioperative increases in vascular permeability. Porcine studies involving CP/CPB have demonstrated increases in VEGF in the lungs after CPB, possible contributing to the post-operative lung edema observed in patients after CPB (65). The increases in circulating levels of VEGF after CP/CPB have been correlated with clinical cardiovascular impairments, as well as the development of capillary leak syndrome (1, 21).
Basic fibroblast growth factor (bFGF), another growth factor capable of inducing micrvascular relaxation, increases in concentration in the plasma after CP/CPB (2), but remains unchanged in the myocardium and skeletal muscle (72). The coronary and skeletal microvascular relaxation responses to bFGF also remain unaltered after CP/CPB, indicating it may play little, if any role in postoperative vasomotor dysfunction after CP/CPB (72).
7. PERSPECTIVE
The clinical manifestations of CP/CPB induced vascular dysfunction are wide ranging (Table 1). We commonly observe decreased basal tone and decreased alpha-adrenergic microvascular responses in the peripheral/skeletal muscle vascular beds which manifests as systemic hypotension, often necessitating the use of adrenergic vasopressors to maintain adequate tissue perfusion pressure (36, 72, 73). In the coronary circulation, impairments in smooth muscle contraction lead to decreased coronary vascular tone in certain patients (67, 81), whereas decreased endothelial-mediated relaxation can lead to an increased propensity to spasm in others (49, 50). The pulmonary circulation demonstrates increased microvascular contractile responses, which in addition to edema from increased endothelial permeability, result in increased pulmonary vascular resistance and shunting (24, 57). Similar increases in vasoconstrictor responses arise in the mesenteric microcirculation (38) predisposing to the development of mesenteric ischemia, particularly when vasoactive drugs are administered to regulate blood pressure after CPB (3).
Table 1.
Location specific changes in vasomotor regulation following cardiac surgery
| Circulatory Bed | Vascular Response | Potential Mechanism |
|---|---|---|
| Coronary | Impaired vasomotor regulation. Increased or decreased vascular tone Vasospasm | Altered NO and COX signaling, increased VEGF Insults associated with cardioplegic arrest |
| Pulmonary | Enhanced SM and EC contraction Increased permeability and vascular tone | Inflammatory signals associated with CPB ERK and PKC activity Altered eNOS activity |
| Peripheral (Skeletal) | Hypotension/ impaired vasomotor regulation | Altered NO and COX signaling Sustained catecholamines |
| Gastrointestinal (mesenteric) | Enhanced constriction | Inflammatory signals associated with CPB ERK and PKC activity Altered NO and COX signaling |
Clinical interventions to limit the degree of vascular dysfunction have attempted to manipulate the previously described alterations after CP/CPB. The increased expression of COX-2 and enhanced contractile response of coronary arterioles to serotonin after CPB and cardioplegia (49, 50) indicate the improvements in coronary bypass graft patency obtained from the perioperative administration of aspirin may not only derive from its effects on prevention of platelet aggregation and thrombus formation (17, 25, 42), but also from the prevention of coronary spasm and preservation of microvascular flow as a result of COX-2 inhibition. The advantages of blood over crystalloid cardioplegia in emergency or high-risk cases may result from the inhibitory effects of blood on oxygen-derived free radical generation, improved coronary endothelial oxygenation, enhanced buffering capacity from histidine and other blood proteins, and better preservation of the morphology of coronary endothelial cells (60, 62). The administration of glucocorticosteroids during CPB was thought to theoretically block the effects of inflammatory cytokines and the expression of iNOS and COX-2, but has not resulted in significant clinical benefit (4, 9). Attempts to improve the bioavailability of NO in the coronary circulation have led to multiple studies investigating the supplementation of L-arginine to cardioplegic solutions. These trials have demonstrated benefits to L-arginine supplementation, including reduced release of biochemical markers of myocardial damage (13), reduced IL-2 receptor, IL-6, and TNF-alpha expression (18), and better hemodynamic performance with shorter intensive care unit stays (19). Additional investigation is needed to further examine the impact of these and other mechanistically based interventions on the clinical outcomes after cardiac surgery utilizing cardioplegic arrest and cardiopulmonary bypass.
8. CONCLUSION
Cardioplegic arrest and cardiopulmonary bypass are associated with marked systemic changes in vascular function which can lead to significant derangements in end-organ perfusion. Central to these alterations are changes in expression of nitric oxide synthases, increases in COX-2 signaling, alterations in PKC and MAPK signaling, and increased VEGF induced vasorelaxation and endothelial permeability. Therapeutic interventions aimed at these targets have met with limited proven success thus far, with the exception of aspirin therapy which may prevent of coronary spasm and preserve microvascular flow as a result of COX-2 inhibition. Despite the dramatic degree of change in vascular physiology observed after cardiac surgery utilizing cardioplegic arrest and cardiopulmonary bypass, the vast majority of patients are able to undergo surgery safely without significant complication.
Acknowledgments
Funding relating to this work was provided by NIH grant HL46716 (F.W.S) and NIH grant T-32HL076130-02 (N.R.S., R.T.C.) and the Irving Bard Memorial Fellowship (N.R.S., R.T.C.).
Abbreviations
- AA
Arachidonic Acid
- bFGF
basic Fibroblast Growth Factor
- CABG
Coronary Artery Bypass Grafting
- CAD
Coronary Artery Disease
- cGM
cyclic Guanosine Monophosphate
- CP
Cardioplegia
- CPB
Cardiopulmonary Bypass
- COX
cyclooxygenase
- DAG
Diacylglycerol
- ERK
Extracellular Signal Related Kinase
- JNK: c-Jun
Nh2-terminal protein kinase
- Il
Interleukin
- IP3
inositol 1,4,5-tri phosphate
- eNOS
endothelial Nitric Oxide Synthase
- iNOS
inducible Nitric Oxide Synthase
- MAPK
mitogen activated protein kinase
- MLCK
Myosin Light Chain Kinase
- NO
Nitric Oxide
- PG
prostaglandin
- PIP2
phosphoinositolphosphate-2
- PKC
Protein Kinase C
- PKG
cGMP Dependent Protein Kinase
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- TNF
Tumor Necrosis Factor
- TXAa
Thromboxane A2
- VEGF
Vascular Endothelial Growth Factor
Footnotes
The authors have no conflicts of interest relating to this work.
REFERENCES
- 1.Parolari A, Alamanni F, Polvani G, Agrifoglio M, Chen YB, Kassem S, Veglia F, Tremoli E, Biglioli P. Meta-analysis of randomized trials comparing off-pump with on-pump coronary artery bypass graft patency. Ann Thorac Surg. 2005;80(6):2121–2125. doi: 10.1016/j.athoracsur.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sellke FW, Shafique T, Schoen FJ, Weintraub RM. Impaired endothelium-dependent coronary microvascular relaxation after cold potassium cardioplegia and reperfusion. J Thorac Cardiovasc Surg. 1993;105(1):52–58. [PubMed] [Google Scholar]
- 4.Sellke FW, Shafique T, Johnson RG, Dai HB, Banitt PF, Schoen FJ, Weintraub RM. Blood and albumin cardioplegia preserve endothelium-dependent microvascular responses. Ann Thorac Surg. 1993;55(4):977–985. doi: 10.1016/0003-4975(93)90130-a. [DOI] [PubMed] [Google Scholar]
- 5.Cartier R, Pellerin M, Hollmann C, Pelletier LC. Effects of pressure and duration of hyperkalemic infusions on endothelial function. Ann Thorac Surg. 1993;55(3):700–705. doi: 10.1016/0003-4975(93)90278-p. [DOI] [PubMed] [Google Scholar]
- 6.Mankad PS, Chester AH, Yacoub MH. Role of potassium concentration in cardioplegic solutions in mediating endothelial damage. Ann Thorac Surg. 1991;51(1):89–93. doi: 10.1016/0003-4975(91)90457-2. [DOI] [PubMed] [Google Scholar]
- 7.Engelman DT, Watanabe M, Engelman RM, Rousou JA, Flack JE, 3rd, Deaton DW, Das DK. Constitutive nitric oxide release is impaired after ischemia and reperfusion. J Thorac Cardiovasc Surg. 1995;110(4 Pt 1):1047–1053. doi: 10.1016/s0022-5223(05)80173-4. [DOI] [PubMed] [Google Scholar]
- 8.He GW, Yang CQ. Hyperkalemia alters endothelium-dependent relaxation through non-nitric oxide and noncyclooxygenase pathway: a mechanism for coronary dysfunction due to cardioplegia. Ann Thorac Surg. 1996;61(5):1394–1399. doi: 10.1016/0003-4975(96)00086-0. [DOI] [PubMed] [Google Scholar]
- 9.Andrasi TB, Soos P, Bakos G, Stumpf N, Blazovics A, Hagl S, Szabo G. L-arginine protects the mesenteric vascular circulation against cardiopulmonary bypass-induced vascular dysfunction. Surgery. 2003;134(1):72–79. doi: 10.1067/msy.2003.208. [DOI] [PubMed] [Google Scholar]
- 10.Cakir O, Oruc A, Eren S, Buyukbayram H, Erdinc L, Eren N. Does sodium nitroprusside reduce lung injury under cardiopulmonary bypass? Eur J Cardiothorac Surg. 2003;23(6):1040–1045. doi: 10.1016/s1010-7940(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 11.Cavallo MJ, Dorman BH, Spinale FG, Roy RC. Myocyte contractile responsiveness after hypothermic, hyperkalemic cardioplegic arrest. Disparity between exogenous calcium and beta-adrenergic stimulation. Anesthesiology. 1995;82(4):926–939. doi: 10.1097/00000542-199504000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Meldrum DR, Cleveland JC, Jr, Sheridan BC, Rowland RT, Banerjee A, Harken AH. Cardiac surgical implications of calcium dyshomeostasis in the heart. Ann Thorac Surg. 1996;61(4):1273–1280. doi: 10.1016/0003-4975(95)00952-3. [DOI] [PubMed] [Google Scholar]
- 13.Sellke FW, Friedman M, Dai HB, Shafique T, Schoen FJ, Weintraub RM, Johnson RG. Mechanisms causing coronary microvascular dysfunction following crystalloid cardioplegia and reperfusion. Cardiovasc Res. 1993;27(11):1925–1932. doi: 10.1093/cvr/27.11.1925. [DOI] [PubMed] [Google Scholar]
- 14.Toprak V, Sirin BH, Tok D, Ozbilgin K, Saribulbul O. The effect of cardiopulmonary bypass on the expression of inducible nitric oxide synthase, endothelial nitric oxide synthase, and vascular endothelial growth factor in the internal mammary artery. J Cardiothorac Vasc Anesth. 2006;20(1):63–67. doi: 10.1053/j.jvca.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Kukreja RC, Hess ML. The oxygen free radical system: from equations through membrane-protein interactions to cardiovascular injury and protection. Cardiovasc Res. 1992;26(7):641–655. doi: 10.1093/cvr/26.7.641. [DOI] [PubMed] [Google Scholar]
- 16.Stahl GL, Reenstra WR, Frendl G. Complement-mediated loss of endothelium-dependent relaxation of porcine coronary arteries. Role of the terminal membrane attack complex. Circ Res. 1995;76(4):575–583. doi: 10.1161/01.res.76.4.575. [DOI] [PubMed] [Google Scholar]
- 17.Friedman M, Wang SY, Stahl GL, Johnson RG, Sellke FW. Altered beta-adrenergic and cholinergic pulmonary vascular responses after total cardiopulmonary bypass. J Appl Physiol. 1995;79(6):1998–2006. doi: 10.1152/jappl.1995.79.6.1998. [DOI] [PubMed] [Google Scholar]
- 18.Sellke FW, Boyle EM, Jr, Verrier ED. Endothelial cell injury in cardiovascular surgery: the pathophysiology of vasomotor dysfunction. Ann Thorac Surg. 1996;62(4):1222–1228. doi: 10.1016/0003-4975(96)00538-3. [DOI] [PubMed] [Google Scholar]
- 19.de Vera ME, Shapiro RA, Nussler AK, Mudgett JS, Simmons RL, Morris SM, Jr, Billiar TR, Geller DA. Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: initial analysis of the human NOS2 promoter. Proc Natl Acad Sci U S A. 1996;93(3):1054–1059. doi: 10.1073/pnas.93.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tofukuji M, Stahl GL, Agah A, Metais C, Simons M, Sellke FW. Anti-C5a monoclonal antibody reduces cardiopulmonary bypass and cardioplegia-induced coronary endothelial dysfunction. J Thorac Cardiovasc Surg. 1998;116(6):1060–1068. doi: 10.1016/S0022-5223(98)70059-5. [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Li J, Metais C, Bianchi C, Sellke F. Increased pulmonary vascular contraction to serotonin after cardiopulmonary bypass: role of cyclooxygenase. J Surg Res. 2000;90(2):138–143. doi: 10.1006/jsre.2000.5869. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi Y, Sawa Y, Fukuyama N, Nakazawa H, Matsuda H. Inducible nitric oxide production is an adaptation to cardiopulmonary bypass-induced inflammatory response. Ann Thorac Surg. 2001;72(1):149–155. doi: 10.1016/s0003-4975(01)02637-6. [DOI] [PubMed] [Google Scholar]
- 23.Tofukuji M, Stahl GL, Metais C, Tomita M, Agah A, Bianchi C, Fink MP, Sellke FW. Mesenteric dysfunction after cardiopulmonary bypass: role of complement C5a. Ann Thorac Surg. 2000;69(3):799–807. doi: 10.1016/s0003-4975(99)01408-3. [DOI] [PubMed] [Google Scholar]
- 24.Mayers I, Hurst T, Radomski A, Johnson D, Fricker S, Bridger G, Cameron B, Darkes M, Radomski MW. Increased matrix metalloproteinase activity after canine cardiopulmonary bypass is suppressed by a nitric oxide scavenger. J Thorac Cardiovasc Surg. 2003;125(3):661–668. doi: 10.1067/mtc.2003.38. [DOI] [PubMed] [Google Scholar]
- 25.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54(4):469–487. [PubMed] [Google Scholar]
- 26.Becker BF, Kupatt C, Massoudy P, Zahler S. Reactive oxygen species and nitric oxide in myocardial ischemia and reperfusion. Z Kardiol. 2000;89(Suppl 9) doi: 10.1007/s003920070037. IX/88-91. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi Y, Sawa Y, Ohtake S, Fukuyama N, Nakazawa H, Matsuda H. Peroxynitrite formation from human myocardium after ischemia-reperfusion during open heart operation. Ann Thorac Surg. 2001;72(2):571–576. doi: 10.1016/s0003-4975(01)02668-6. [DOI] [PubMed] [Google Scholar]
- 28.Pacher P, Beckman JS, Liaudet L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. 2007;6(8):662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt P, Youhnovski N, Daiber A, Balan A, Arsic M, Bachschmid M, Przybylski M, Ullrich V. Specific Nitration at Tyrosine 430 Revealed by High Resolution Mass Spectrometry as Basis for Redox Regulation of Bovine Prostacyclin Synthase. J Biol. Chem. 2003;278(15):12813–12819. doi: 10.1074/jbc.M208080200. [DOI] [PubMed] [Google Scholar]
- 31.Brookes PS, Darley-Usmar VM. Role of calcium and superoxide dismutase in sensitizing mitochondria to peroxynitrite-induced permeability transition. Am J Physiol Heart Circ Physiol. 2004;286(1):H39–H46. doi: 10.1152/ajpheart.00742.2003. [DOI] [PubMed] [Google Scholar]
- 32.Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. Cytochrome c Nitration by Peroxynitrite. J Biol. Chem. 2000;275(28):21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 33.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radical Biology and Medicine. 2002;33(11):1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 34.Szabo C, Zingarelli B, O'Connor M, Salzman AL. DNA strand breakage, activation of poly (ADPribose) synthetase, and cellular energy depletion are involved in the cytotoxicity in macrophages and smooth muscle cells exposed to peroxynitrite. Proceedings of the National Academy of Sciences. 1996;93(5):1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Downing SW, Edmunds LH., Jr Release of vasoactive substances during cardiopulmonary bypass. Ann Thorac Surg. 1992;54(6):1236–1243. doi: 10.1016/0003-4975(92)90113-i. [DOI] [PubMed] [Google Scholar]
- 36.Mazer CD, Briet F, Blight KR, Stewart DJ, Robb M, Wang Z, Harrington AM, Mak W, Li X, Hare GM. Increased cerebral and renal endothelial nitric oxide synthase gene expression after cardiopulmonary bypass in the rat. J Thorac Cardiovasc Surg. 2007;133(1):13–20. doi: 10.1016/j.jtcvs.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 37.Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 38.Metais C, Li J, Simons M, Sellke FW. Serotonin-induced coronary contraction increases after blood cardioplegia-reperfusion: role of COX-2 expression. Circulation. 1999;100(19 Suppl):II328–II334. doi: 10.1161/01.cir.100.suppl_2.ii-328. [DOI] [PubMed] [Google Scholar]
- 39.Uotila P, Saraste A, Vahasilta T, Kentala E, Savunen T. Stimulated expression of cyclooxygenase-2 in porcine heart after bypass circulation and cardioplegic arrest. Eur J Cardiothorac Surg. 2001;20(5):992–995. doi: 10.1016/s1010-7940(01)00930-7. [DOI] [PubMed] [Google Scholar]
- 40.Metais C, Bianchi C, Li J, Simons M, Sellke FW. Serotonin-induced human coronary microvascular contraction during acute myocardial ischemia is blocked by COX-2 inhibition. Basic Res Cardiol. 2001;96(1):59–67. doi: 10.1007/s003950170078. [DOI] [PubMed] [Google Scholar]
- 41.Parenti A, Morbidelli L, Cui XL, Douglas JG, Hood JD, Granger HJ, Ledda F, Ziche M. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem. 1998;273(7):4220–4226. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- 42.Hindman BJ, Moore SA, Cutkomp J, Smith T, Ross-Barta SE, Dexter F, Brian JE., Jr Brain expression of inducible cyclooxygenase 2 messenger RNA in rats undergoing cardiopulmonary bypass. Anesthesiology. 2001;95(6):1380–1388. doi: 10.1097/00000542-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 43.Shafique T, Johnson RG, Dai HB, Weintraub RM, Sellke FW. Altered pulmonary microvascular reactivity after total cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1993;106(3):479–486. [PubMed] [Google Scholar]
- 44.Wang SY, Stamler A, Li J, Johnson RG, Sellke FW. Decreased myogenic reactivity in skeletal muscle arterioles after hypothermic cardiopulmonary bypass. J Surg Res. 1997;69(1):40–44. doi: 10.1006/jsre.1997.5020. [DOI] [PubMed] [Google Scholar]
- 45.Wang SY, Friedman M, Johnson RG, Weintraub RM, Sellke FW. Adrenergic regulation of coronary microcirculation after extracorporeal circulation and crystalloid cardioplegia. Am J Physiol. 1994;267(6 Pt 2):H2462–H2470. doi: 10.1152/ajpheart.1994.267.6.H2462. [DOI] [PubMed] [Google Scholar]
- 46.Sodha NR, Feng J, Clements RT, Bianchi C, Boodhwani M, Ramlawi B, Mieno S, Khabbaz KR, Sellke FW. Protein kinase C alpha modulates microvascular reactivity in the human coronary and skeletal microcirculation. Surgery. 2007;142(2):243–252. doi: 10.1016/j.surg.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci (Lond) 1999;96(4):313–326. [PubMed] [Google Scholar]
- 48.Martinez MC, Randriamboavonjy V, Ohlmann P, Komas N, Duarte J, Schneider F, Stoclet JC, Andriantsitohaina R. Involvement of protein kinase C, tyrosine kinases, and Rho kinase in Ca(2+) handling of human small arteries. Am J Physiol Heart Circ Physiol. 2000;279(3):H1228–H1238. doi: 10.1152/ajpheart.2000.279.3.H1228. [DOI] [PubMed] [Google Scholar]
- 49.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372(6503):231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 50.Hill MA, Falcone JC, Meininger GA. Evidence for protein kinase C involvement in arteriolar myogenic reactivity. Am J Physiol. 1990;259(5 Pt 2):H1586–H1594. doi: 10.1152/ajpheart.1990.259.5.H1586. [DOI] [PubMed] [Google Scholar]
- 51.Hattori Y, Kakishita H, Akimoto K, Matsumura M, Kasai K. Glycated serum albumin-induced vascular smooth muscle cell proliferation through activation of the mitogen-activated protein kinase/extracellular signalregulated kinase pathway by protein kinase C. Biochem Biophys Res Commun. 2001;281(4):891–896. doi: 10.1006/bbrc.2001.4436. [DOI] [PubMed] [Google Scholar]
- 52.Velarde V, Jenkins AJ, Christopher J, Lyons TJ, Jaffa AA. Activation of MAPK by modified low-density lipoproteins in vascular smooth muscle cells. J Appl Physiol. 2001;91(3):1412–1420. doi: 10.1152/jappl.2001.91.3.1412. [DOI] [PubMed] [Google Scholar]
- 53.Khan TA, Bianchi C, Ruel M, Voisine P, Li J, Liddicoat JR, Sellke FW. Mitogen-activated protein kinase inhibition and cardioplegia-cardiopulmonary bypass reduce coronary myogenic tone. Circulation. 2003;108(Suppl 1):II348–II353. doi: 10.1161/01.cir.0000087652.93751.0e. [DOI] [PubMed] [Google Scholar]
- 54.Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW, 2nd, Duran WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol. 2003;284(1):H92–H100. doi: 10.1152/ajpheart.00330.2002. [DOI] [PubMed] [Google Scholar]
- 55.Jaffee BD, Manos EJ, Collins RJ, Czerniak PM, Favata MF, Magolda RL, Scherle PA, Trzaskos JM. Inhibition of MAP kinase kinase (MEK) results in an anti-inflammatory response in vivo. Biochem Biophys Res Commun. 2000;268(2):647–651. doi: 10.1006/bbrc.2000.2184. [DOI] [PubMed] [Google Scholar]
- 56.Khan TA, Bianchi C, Araujo EG, Ruel M, Voisine P, Li J, Liddicoat JR, Sellke FW. Cardiopulmonary bypass reduces peripheral microvascular contractile function by inhibition of mitogen-activated protein kinase activity. Surgery. 2003;134(2):247–254. doi: 10.1067/msy.2003.229. [DOI] [PubMed] [Google Scholar]
- 57.Hoar PF, Stone JG, Faltas AN, Bendixen HH, Head RJ, Berkowitz BA. Hemodynamic and adrenergic responses to anesthesia and operation for myocardial revascularization. J Thorac Cardiovasc Surg. 1980;80(2):242–248. [PubMed] [Google Scholar]
- 58.Wallach R, Karp RB, Reves JG, Oparil S, Smith LR, James TN. Pathogenesis of paroxysmal hypertension developing during and after coronary bypass surgery: a study of hemodynamic and humoral factors. Am J Cardiol. 1980;46(4):559–565. doi: 10.1016/0002-9149(80)90503-2. [DOI] [PubMed] [Google Scholar]
- 59.Rosenbaum JS, Zera P, Umans VA, Ginsburg R, Hoffman BB. Desensitization of aortic smooth muscle contraction in rats harboring pheochromocytoma. J Pharmacol Exp Ther. 1986;238(2):396–400. [PubMed] [Google Scholar]
- 60.Lurie KG, Tsujimoto G, Hoffman BB. Desensitization of alpha-1 adrenergic receptor-mediated vascular smooth muscle contraction. J Pharmacol Exp Ther. 1985;234(1):147–152. [PubMed] [Google Scholar]
- 61.Marano CW, Garulacan LA, Laughlin KV, Igidbashian L, Trace C, Goldman SM, Sutter FP, Reichard GA, Jr, Mullin JM. Plasma concentrations of soluble tumor necrosis factor receptor I and tumor necrosis factor during cardiopulmonary bypass. Ann Thorac Surg. 2000;70(4):1313–1318. doi: 10.1016/s0003-4975(00)01932-9. [DOI] [PubMed] [Google Scholar]
- 62.Hayashi Y, Sawa Y, Nishimura M, Tojo SJ, Fukuyama N, Nakazawa H, Matsuda H. P-selectin participates in cardiopulmonary bypass-induced inflammatory response in association with nitric oxide and peroxynitrite production. J Thorac Cardiovasc Surg. 2000;120(3):558–565. doi: 10.1067/mtc.2000.108593. [DOI] [PubMed] [Google Scholar]
- 63.Van den Berghe G. Novel insights into the neuroendocrinology of critical illness. Eur J Endocrinol. 2000;143(1):1–13. doi: 10.1530/eje.0.1430001. [DOI] [PubMed] [Google Scholar]
- 64.Khan TA, Bianchi C, Araujo EG, Ruel M, Voisine P, Sellke FW. Activation of pulmonary mitogen-activated protein kinases during cardiopulmonary bypass. J Surg Res. 2003;115(1):56–62. doi: 10.1016/s0022-4804(03)00236-1. [DOI] [PubMed] [Google Scholar]
- 65.Khan TA, Bianchi C, Ruel M, Feng J, Sellke FW. Differential effects on the mesenteric microcirculatory response to vasopressin and phenylephrine after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2007;133(3):682–688. doi: 10.1016/j.jtcvs.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 67.Tofukuji M, Metais C, Li J, Franklin A, Simons M, Sellke FW. Myocardial VEGF expression after cardiopulmonary bypass and cardioplegia. Circulation. 1998;98(19 Suppl):II242–II246. discussion II247–8. [PubMed] [Google Scholar]
- 68.Sellke FW, Wang SY, Stamler A, Lopez JJ, Li J, Simons M. Enhanced microvascular relaxations to VEGF and bFGF in chronically ischemic porcine myocardium. Am J Physiol. 1996;271(2 Pt 2):H713–H720. doi: 10.1152/ajpheart.1996.271.2.H713. [DOI] [PubMed] [Google Scholar]
- 69.Arab S, Konstantinov IE, Boscarino C, Cukerman E, Mori A, Li J, Liu PP, Redington AN, Coles JG. Early gene expression profiles during intraoperative myocardial ischemia-reperfusion in cardiac surgery. J Thorac Cardiovasc Surg. 2007;134(1):74–81. 81 e1–81 e2. doi: 10.1016/j.jtcvs.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 70.Serraf A, Aznag H, Baudet B, Detruit H, Seccatore F, Mazmanian MG, Planche C. Pulmonary vascular endothelial growth factor and nitric oxide interaction during total cardiopulmonary bypass in neonatal pigs. J Thorac Cardiovasc Surg. 2003;125(5):1050–1057. doi: 10.1067/mtc.2003.402. [DOI] [PubMed] [Google Scholar]
- 71.Denizot Y, Guglielmi L, Cornu E, Nathan N. Alterations in plasma angiogenic growth factor concentrations after coronary artery bypass graft surgery: relationships with post-operative complications. Cytokine. 2003;24(1–2):7–12. doi: 10.1016/s1043-4666(03)00241-2. [DOI] [PubMed] [Google Scholar]
- 72.Abrahamov D, Erez E, Tamariz M, Dagan O, Pearl E, Abrahamov Y, Gendel B, Desai N, Kats J, Vidne B, Barak V. Plasma vascular endothelial growth factor level is a predictor of the severity of postoperative capillary leak syndrome in neonates undergoing cardiopulmonary bypass. Pediatr Surg Int. 2002;18(1):54–59. doi: 10.1007/s003830200012. [DOI] [PubMed] [Google Scholar]
- 73.Abramov D, Erez E, Dagan O, Abramov Y, Pearl E, Veena G, Katz J, Vidne BA, Barak V. Increased levels of basic fibroblast growth factor are found in the cross-clamped heart during cardiopulmonary bypass. Can J Cardiol. 2000;16(3):313–318. [PubMed] [Google Scholar]
- 74.Wang SY, Friedman M, Johnson RG, Zeind AJ, Sellke FW. Adenosine triphosphate-sensitive K+ channels mediate postcardioplegia coronary hyperemia. J Thorac Cardiovasc Surg. 1995;110(4 Pt 1):1073–1082. doi: 10.1016/s0022-5223(05)80177-1. [DOI] [PubMed] [Google Scholar]
- 75.Allen KB, Salam AA, Lumsden AB. Acute mesenteric ischemia after cardiopulmonary bypass. J Vasc Surg. 1992;16(3):391–395. discussion 395–6. [PubMed] [Google Scholar]
- 76.Chesebro JH, Clements IP, Fuster V, Elveback LR, Smith HC, Bardsley WT, Frye RL, Holmes DR, Jr, Vlietstra RE, Pluth JR, Wallace RB, Puga FJ, Orszulak TA, Piehler JM, Schaff HV, Danielson GK. A platelet-inhibitor-drug trial in coronary-artery bypass operations: benefit of perioperative dipyridamole and aspirin therapy on early postoperative vein-graft patency. N Engl J Med. 1982;307(2):73–78. doi: 10.1056/NEJM198207083070201. [DOI] [PubMed] [Google Scholar]
- 77.Gavaghan TP, Gebski V, Baron DW. Immediate postoperative aspirin improves vein graft patency early and late after coronary artery bypass graft surgery. A placebo-controlled, randomized study. Circulation. 1991;83(5):1526–1533. doi: 10.1161/01.cir.83.5.1526. [DOI] [PubMed] [Google Scholar]
- 78.Mangano DT. Aspirin and mortality from coronary bypass surgery. N Engl J Med. 2002;347(17):1309–1317. doi: 10.1056/NEJMoa020798. [DOI] [PubMed] [Google Scholar]
- 79.Boscoe MJ, Yewdall VM, Thompson MA, Cameron JS. Complement activation during cardiopulmonary bypass: quantitative study of effects of methylprednisolone and pulsatile flow. Br Med J (Clin Res Ed) 1983;287(6407):1747–1750. doi: 10.1136/bmj.287.6407.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andersen LW, Baek L, Thomsen BS, Rasmussen JP. Effect of methylprednisolone on endotoxemia and complement activation during cardiac surgery. J Cardiothorac Anesth. 1989;3(5):544–549. doi: 10.1016/0888-6296(89)90150-6. [DOI] [PubMed] [Google Scholar]
- 81.Carrier M, Pellerin M, Perrault LP, Bouchard D, Page P, Searle N, Lavoie J. Cardioplegic arrest with L-arginine improves myocardial protection: results of a prospective randomized clinical trial. Ann Thorac Surg. 2002;73(3):837–841. doi: 10.1016/s0003-4975(01)03414-2. discussion 842. [DOI] [PubMed] [Google Scholar]
- 82.Colagrande L, Formica F, Porta F, Brustia M, Avalli L, Sangalli F, Muratore M, Paolini G. L-arginine effects on myocardial stress in cardiac surgery: preliminary results. Ital Heart J. 2005;6(11):904–910. [PubMed] [Google Scholar]
- 83.Colagrande L, Formica F, Porta F, Martino A, Sangalli F, Avalli L, Paolini G. Reduced cytokines release and myocardial damage in coronary artery bypass patients due to L-arginine cardioplegia supplementation. Ann Thorac Surg. 2006;81(4):1256–1261. doi: 10.1016/j.athoracsur.2005.10.003. [DOI] [PubMed] [Google Scholar]