Abstract
Activation of proinflammatory macrophages plays an important role in the pathogenesis of insulin resistance, type 2 diabetes, and atherosclerosis. Previous work using high fat-fed mice has shown that ablation of the adipocyte fatty acid binding protein (FABP4/aP2) in macrophages leads to an antiinflammatory state both in situ and in vivo, and the mechanism is linked, in part, to increased intracellular monounsaturated fatty acids and the up-regulation of uncoupling protein 2. Here, we show that loss of FABP4/aP2 in macrophages additionally induces sirtuin 3 (SIRT3) expression and that monounsaturated fatty acids (C16:1, C18:1) lead to increased SIRT3 protein expression. Increased expression of SirT3 in FABP4/aP2 null macrophages occurs at the protein level with no change in SirT3 mRNA. When compared with controls, silencing of SIRT3 in Raw246.7 macrophages leads to increased expression of inflammatory cytokines, inducible nitric oxide synthase and cyclooxygenase 2. In contrast, loss of SIRT3 in FABP4/aP2-deficient macrophages attenuates the suppressed inflammatory signaling, reduced reactive oxygen species production, lipopolysaccharide-induced mitochondrial dysfunction, and increased fatty acid oxidation. These results suggest that the antiinflammatory phenotype of FABP4/aP2 null mice is mediated by increased intracellular monounsaturated fatty acids leading to the increased expression of both uncoupling protein 2 and SirT3.
The prevalence of syndromes associated with obesity, including insulin resistance, hypertension, and dyslipidemia, have increased over the last decade (1, 2). High saturated fat or “Western” diets and lack of exercise contribute to the epidemic of the metabolic syndrome (1, 3, 4). At the molecular level, multiple pathways are involved in the pathogenesis of metabolic diseases, including lipotoxicity and chronic inflammation in multiple tissues such as liver, muscle, and adipose (5–8). Importantly, the infiltration and activation of immune cells such as T cells and macrophages play an essential role in the development of insulin resistance in adipose tissue (9, 10). Of these activated immune cells, macrophages play an integral role in the production of inflammatory cytokines as well as the development of oxidative stress in adipose tissue (8).
Macrophage lipid metabolism is critical in mediating adipose tissue inflammation and oxidative stress (11, 12). Work from our laboratory and many others have shown that the adipocyte fatty acid binding protein (FABP4, also known as aP2) plays an important role in the activation of macrophage inflammation (13–16). Ablation of FABP4/aP2 (AKO) in macrophages alone is sufficient to protect mice from diet-induced atherosclerosis and dyslipidemia (14, 16). FABP4/aP2 is a small 15-kDa lipid chaperone involved in intracellular fatty acid trafficking but recently has been shown to be secreted into the extracellular environment (17, 18). Deficiency of FABP4/aP2 leads to suppressed inflammation, decreased endoplasmic reticulum stress, and decreased nuclear factor kappa light-chain enhancer of activated B cells activation in macrophages (13, 19, 20). Consistent with its role in ameliorating metabolic disorder in mice, a genetic variant at the promoter region of human FABP4/aP2, which leads to decreased expression, has been associated with reduced risk of coronary disease and type 2 diabetes (21).
Previous work has shown that the monounsaturated fatty acids palmitoleate and oleate are specifically increased in FABP4/aP2-deficient macrophages and are linked to the selective up-regulation of uncoupling protein 2 (UCP2) (22). Increased expression of UCP2 in macrophages attenuates oxidative stress and reduces inflammatory signaling. However, increased UCP2 expression alone is not sufficient to explain the increased fatty acid oxidation and resistance from lipopolysaccharide (LPS)-induced mitochondrial dysfunction measured in FABP4/aP2-deficient cells, suggesting additional, unappreciated regulatory mechanisms. Here, we report that sirtuin 3 (SIRT3), a member of the sirtuin family, is specifically up-regulated in macrophages deficient in FABP4/aP2 and that monounsaturated fatty acids can induce SIRT3 up-regulation at the protein level. The resulting increase of SIRT3 expression in macrophages is linked to suppressed inflammatory signaling as well as decreased reactive oxygen production and increased fatty acid oxidation in FABP4/aP2-deficient macrophages. Furthermore, SIRT3 up-regulation protects FABP4/aP2-deficient macrophages from LPS-induced mitochondrial dysfunction.
Research Design and Methods
Cell culture
Raw264.7 macrophages and SIRT3 knockdown Raw264.7 macrophages were maintained in DMEM (Invitrogen) with 10% fetal bovine serum. FABP4/aP2 knockout (AKO), wild-type (WT) and SIRT3 knockdown FABP4/aP2-deficient macrophages were maintained in RPMI 1640 (Invitrogen) with 5% fetal bovine serum.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was isolated using TRIzol reagent (Invitrogen). cDNA synthesis was performed by using iScript according to the manufacturer's protocol (Bio-Rad). Quantitative real-time polymerase chain reaction amplification used a Bio-Rad CFX 96 real-time system with a SYBR green Supermix (Bio-Rad). Transcription factor II E was used as an internal control to normalize expression. Primer sequences are provided in Table 1.
Table 1.
Sequences of Primers Used for Quantitative RT-PCR
| Target | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| Sirt3 | GCTGCTTCTGCGGCTCTATAC | GAAGGACCTTCGACAGACCGT |
| TNFα | AGCCGATGGGTTGTACCTTGTCTA | TGAGATAGCAAATCGGCTGAGGGT |
| Mcp1 | TCACCTGCTGCTACTCATTCACCA | TACAGCTTCTTTGGGACACCTGCT |
| Mmp9 | CCCACATTTGACGTCCAGAGAAGAA | GTTTTTGATGCTATTGCTGAGATCCA |
| TFIIE | CAAGGCTTTAGGGGACCAGATAC | CATCCATTGACTCCACAGTGACAC |
TFIIE, transcription factor II E.
Silencing of Sirt3 in macrophages
Raw264.7 macrophages were transduced with a short hairpin RNA (shRNA) lentivirus targeting either green fluorescent protein (GFP) or SIRT3 as described previously (23). GFP and Sirt3 (GenBank accession number NP_001120823) targeting sequences were obtained from Open Biosystems: Sirt3 (kd-1), 5′-CCGGGCCATCTTTGAACTTGGCTTTCTCGAGAAAGCCAAGTTCAAAGATGGCTTTTTG-3′; GFP, 5′-AACGTACGCGGAATACTTCGA-3′. Because SIRT3 is highly expressed in FABP4/aP2−/− macrophages (AKO) compared with WT cells, 2 rounds of lentivirus infection were required to obtain significant silencing. To that end, FABP4/aP2−/− macrophages were first transduced with kd-1 followed by an additional shRNA lentivirus (kd-2), 5′-CCGGCCTACTCCATATGGCTGACTTCTCGAGAAGTCAGCCATATGGAGTAGGTTTTTG-3′.
Stromal vascular fraction (SVF) isolation
Isolation of SVF was performed as described in Xu et al (22). Briefly, epididymal fat pads were dissected from WT and FABP4/aP2 knockout (AKO) mice (15-wk-old male C57BL/6J mice maintained on high saturated fat diet [BioServe F3282] for 12 wk), minced and digested in Krebs-Ringers-HEPES (KRH) buffer supplemented with type I collagenase (Worthington) and BSA for 1 hour at 37°C. The mixture was filtered with 100-μm pore size nylon cell strainer (Falcon) to remove undigested tissues. The SVF was collected by centrifugation at 500g for 10 minutes. After washing with KRH buffer, the SVF was either resuspended in TRIzol reagent for RNA isolation or in cell lysis buffer supplemented with protease inhibitors for protein assays. All experimental procedures using animals were reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Mitochondrial isolation and β-oxidation
Mitochondrial isolation were carried out as described in Xu et al (22). Briefly, cells were scraped into ice-cold mitochondrial isolation buffer (20mM Tris [pH 7.4], 220mM mannitol, 70mM sucrose, 1mM EDTA, and 0.1mM EGTA) and supplemented with protease inhibitors. Cells were then lysed with 20 strokes of a Dounce homogenizer and homogenates centrifuged at 700g for 10 minutes to remove nuclei and unbroken cells. Mitochondria were pelleted by centrifugation at 10 000g for 15 minutes at 4°C. Fatty acid oxidation was carried out as described previously (22).
Measurement of reactive oxygen species (ROS)
ROS production was measured by incubating cells with cell permeable 2′, 7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Invitrogen). Briefly, cells were washed with PBS, and incubated in 1 mL of KRH buffer (pH 7.4) with 10μM final concentration of H2DCFDA for 30 minutes. Then, cells were washed with PBS and harvested into 300 μL KRH buffer. Of each sample, 150 μL were loaded into a 96-well plate, and fluorescence was measured using a microplate reader with excitation at 488 nm and emission at 535 nm.
Immunoblot analysis
Cells were lysed with radioimmunoprecipitation assay buffer supplemented with protease inhibitors (Calbiochem). A total of 50 μg of protein from each sample were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. After blocking with Odyssey blocking buffer (Li-Cor Biosciences), membranes were incubated with primary antibody overnight at 4°C. Membranes were washed and incubated with secondary antibody conjugated to Li-Cor IRDye for 1 hour and visualized using Odyssey infrared imaging (Li-Cor Biosciences). The primary antibodies used were anti-SIRT3 (Cell Signaling), anti-FABP4, anti-cyclooxygenase-2 (Cox2) (BD Transduction Laboratories), anti-inducible nitric oxide synthase (iNOS) (BD Transduction Laboratories), antisuperoxide dismutase 2 (SOD2) (Cell Signaling), antilong-chain acyl-Coenzyme A dehydrogenase (LCAD) (Abcam), antiacetylated lysine (Cell Signaling), anti-β-actin (Sigma-Aldrich), and anti-ATP synthase-α-subunit (MitoSciences).
Immunoprecipitation
Cells were washed twice with cold PBS and scraped into 1 mL of lysis buffer (20mM Tris [pH 7.4], 150mM NaCl, 1mM EDTA, 1mM EGTA, and 1% Triton X-100) supplemented with protease inhibitors and incubated for 10 minutes at 4°C. After centrifugation at 13 500 rpm for 10 minutes, supernatants were transferred to a new tube, and the protein concentration was determined. A total of 800 μg of protein lysates were precleared by incubation with 30 μL protein G beads for 4 hours. After centrifugation, supernatants were transferred to a new tube, and 30 μL of a protein G bead slurry were added to each tube together with primary antibody or antirabbit IgG control antibody. After rotating overnight at 4°C, the pelleted beads were washed 5 times with 500 μL lysis buffer and resuspended in radioimmunoprecipitation assay buffer. Precipitated proteins were released from the IgG beads by boiling the samples for 10 minutes and loaded onto a SDS-PAGE gel for separation.
Cellular respiratory assay
Macrophage respiratory assay was performed on a XF24 Analyzer (Seahorse Biosciences). Macrophages were plated on V7 microplates at a density of 200 000 cells per well a day before the assay. On the day of the experiment, cells were treated either with vehicle or LPS (100 ng/mL) for 6 hours. The cells were then washed and incubated with assay media. During the assay, cells were exposed to compounds in the following order: 2 μM oligomycin, 0.4 μM FCCP, and 4μM antimycin A.
Statistical analysis
All data in the article are expressed as mean ± SEM. Statistical significance was determined using an unpaired 2-tailed Student's t test.
Results
Loss of FABP4/aP2 in macrophages increases SIRT3 expression
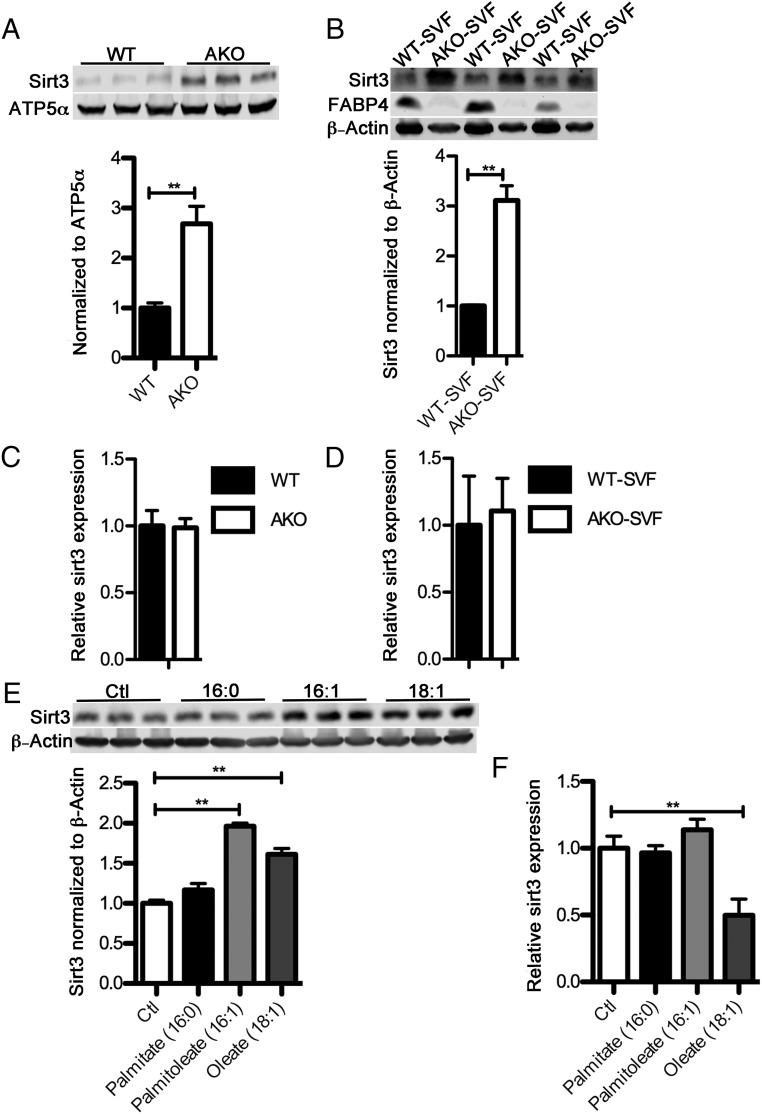
Previous work by Xu et al (22) as well as Erbay et al (20) and Coe et al (24) has shown that loss of FABP4/aP2 alters the intracellular level and composition of fatty acids in both macrophages and adipocytes resulting in the accumulation of monounsaturated fatty acids, particularly C16:1 and C18:1. Interestingly, consumption of monounsaturated fatty acids, which are a major component of the Mediterranean diet, are associated with improved insulin sensitivity and decreased inflammation in both humans and mice (25). In muscle, oleic acid treatment can activate the SIRT1-peroxisome proliferator-activated receptor γ coactivator 1-α axis to increase fatty acid oxidation (26). More recently, Liu et al reported that in monocytes, SIRT1 activation leads to an increase of both expression and activation of SIRT3, thus restoring immunometabolic homeostasis (27). Therefore, we hypothesized that loss of FABP4/aP2 will increase SIRT3 expression via an increased level of monounsaturated fatty acids. To test this hypothesis, we evaluated the protein level of SIRT3 in both WT and FABP4/aP2−/− (AKO) macrophages derived from WT and FABP4/aP2−/− mice, respectively. SIRT3 expression in FABP4/aP2−/− macrophages was more than doubled compared with that in WT (Figure 1A). To further confirm that the loss of FABP4/aP2 leads to up-regulation of SIRT3 in macrophages, we isolated the SVF from visceral adipose tissue of male high-fat diet (HFD)-fed WT and AKO mice. The level of SIRT3 was increased about 3-fold in AKO-SVF compared with that of WT (Figure 1B). Interestingly, the level of Sirt3 mRNA did not change between WT and FABP4/aP2−/− cell lines or SVF fractions indicating the difference of SIRT3 expression came from regulation at the translational or posttranslational level (Figure 1, C and D). To explore the potential role of monounsaturated fatty acids on SIRT3 expression, Raw264.7 macrophages were treated with either monounsaturated fatty acids (MUFAs) (palmitoleate and oleate) or palmitate. The results showed that MUFA treatment could induce SIRT3 expression in macrophages but not the saturated fatty acid palmitate (Figure 1E). Similar to the results in experimental mice, the increase in protein was not accompanied by an increase in SIRT3 mRNA (Figure 1F). Taken together, these results suggest that the increased monounsaturated fatty acids, due to the genetic ablation of FABP4/aP2, may be responsible for the increased SIRT3 expression in macrophages.
Figure 1.
Loss of FABP4/aP2 increases SIRT3 expression. A, SIRT3 protein level normalized to ATP5α in the mitochondrial fraction of WT and FABP4/aP2−/− (AKO) macrophages. B, SIRT3 protein level normalized to β-actin in the SVF of epididymal adipose tissue obtained from HFD-fed WT and FABP4/aP2 (AKO) null mice. C, SIRT3 mRNA level in WT and AKO macrophages. D, SIRT3 mRNA level in the SVF of epididymal adipose tissue obtained from HFD-fed WT and AKO mice. E, SIRT3 protein expression in Raw264.7 macrophages treated with 300μM palmitate, palmitoleate, or oleate for 36 hours. F, SIRT3 mRNA level in Raw264.7 macrophages treated with 300μM palmitate, palmitoleate, or oleate for 36 hours. Fatty acids were added in complex to BSA at a molar ratio of 4:1 (FFA/BSA); *, P < .05; **, P < .01; n = 3–6 per group.
SIRT3 expression is antiinflammatory in macrophages
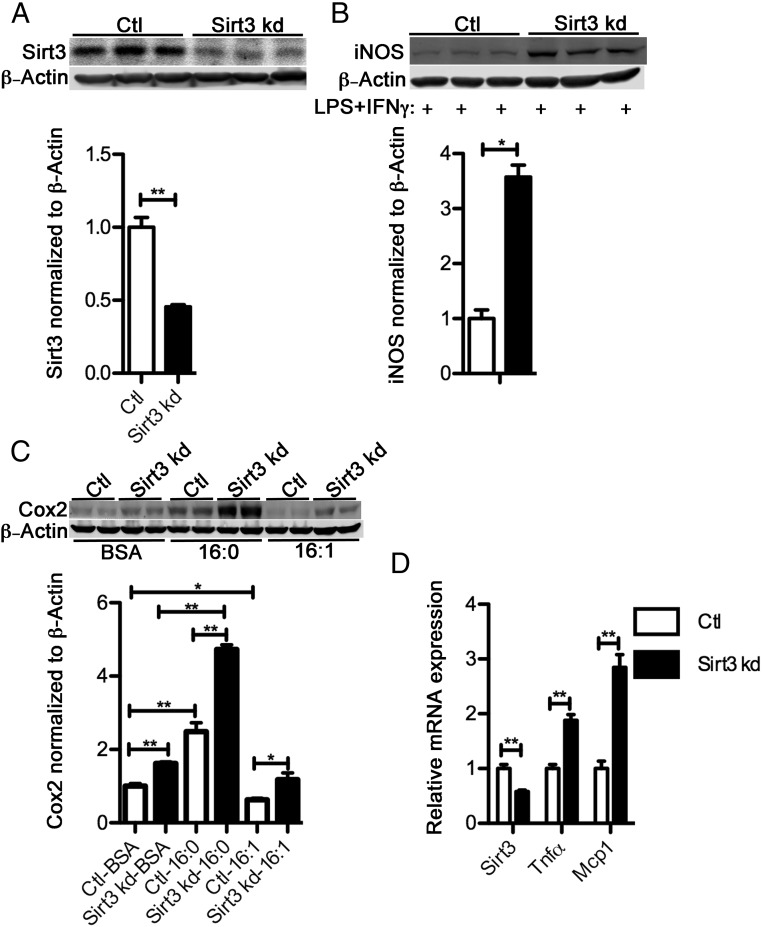
Diet-induced obesity is accompanied by decreased SIRT3 expression in multiple tissues, including liver, skeletal muscle, heart, and pancreas (28–32). Both genetic knockout animal models and clinical studies of persons with type-2 diabetes have shown the important role of SIRT3 in maintaining insulin sensitivity in various tissues and organs (29, 30, 32). However, the role of SIRT3 in tissue macrophages has not been well defined, having been studied primarily in a sepsis model (27). To illustrate the relationship between SIRT3 expression and macrophage inflammation, we silenced Sirt3 in Raw264.7 macrophages by infecting cells with lentivirus encoding a SIRT3-targeted shRNA. The knockdown cell line expressed about 50% of SIRT3 protein compared with the control cells (Figure 2A). Cotreatment of Sirt3 silenced macrophage cells with LPS and interferon (IFN)γ induced a significantly higher level of iNOS expression in Sirt3 knockdown Raw264.7 macrophages compared with control cells (Figure 2B). In addition, macrophages treated with palmitate or palmitoleate, which have been shown to prime macrophages towards a pro- or antiinflammatory state, respectively, regulated inflammatory marker genes. Decreased expression of SIRT3 led to increased palmitate-induced Cox2 expression, and compromised the ability of palmitoleate to reduce Cox2 expression (Figure 2C). Additionally, Sirt3 knockdown Raw264.7 macrophages expressed higher transcript levels of the inflammatory cytokines TNFα and monocyte chemotactic protein 1 (Figure 2D). In summary, loss of SIRT3 in macrophages results in an elevated inflammatory state.
Figure 2.
Loss of SIRT3 is proinflammatory in macrophages. A, SIRT3 expression in SIRT3 knockdown Raw264.7 macrophages (Sirt3 kd) and control knockdown cells (Ctl). B, iNOS abundance measured by Western blotting in Ctl and Sirt3 kd macrophages cotreated with LPS (100 ng/mL) and IFNγ (10 U) for 14 hours. C, Cox2 abundance in palmitate or palmitoleate (300μM) (4:1 fatty acids to BSA)-treated Ctl and Sirt3 kd macrophages. D, mRNA levels of Sirt3, Tnfα, and Mcp1 in Ctl and Sirt3 kd macrophages; *, P < .05; **, P < .01; n = 3 per group.
SIRT3 mediates the decreased inflammatory signaling in FABP4/aP2−/− macrophages
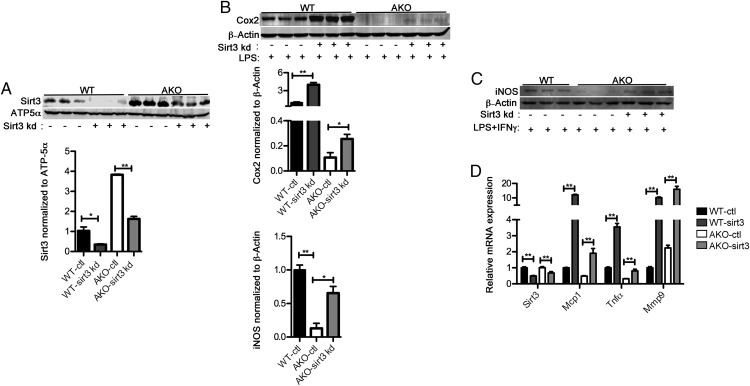
Loss of FABP4/aP2 reduces macrophage inflammatory markers such as Cox2 and iNOS (13). In order to determine whether SIRT3 plays a role in the suppressed inflammatory signaling in FABP4/aP2−/− macrophages, we silenced SIRT3 (sirt3 kd) in both WT and FABP4/aP2−/− (AKO) backgrounds (Figure 3A). LPS treatment induced higher expression of Cox2 in both WT-sirt3 kd and AKO-sirt3 kd macrophages compared with their corresponding control cell lines. The basal expression of SIRT3 was similar between WT and AKO-sirt3 kd macrophages; however, the expression of Cox2 was still much lower in AKO-sirt3 kd (Figure 3B). Therefore, SIRT3 expression in AKO is partially responsible for the reduced inflammatory signaling. Similarly, knockdown of Sirt3 in FABP4/aP2−/− cells dramatically increased LPS and IFNγ-induced iNOS expression (Figure 3C). Additionally, genetic knockdown of Sirt3 increased the basal expression of the inflammatory cytokines, MCP1, TNFα, and matrix metalloproteinase 9 mRNA in WT and FABP4/aP2−/− cells compared with their respective controls (Figure 3D). These results suggest that increased SIRT3 expression in FABP4/aP2−/− macrophages is at least partially responsible for the reduced inflammatory signaling.
Figure 3.
SIRT3 up-regulation mediates the decreased inflammation in FABP4/aP2−/− macrophages. A, SIRT3 protein expression in the mitochondrial fraction of WT-ctl, WT-sirt3 kd, AKO-ctl, and AKO-sirt3 kd macrophages. B, Cox2 abundance determined by Western blotting in WT-ctl, WT-sirt3 kd, AKO-ctl, and AKO-sirt3 kd cells treated with LPS (100 ng/mL) for 16 hours. C, iNOS abundance determined by Western blotting in WT-ctl, AKO-ctl, and AKO-sirt3 kd cells cotreated with LPS+IFNγ for 18 hours. D, mRNA level of SIRT3 and inflammatory cytokines in WT-ctl, WT-sirt3 kd, AKO-ctl, and AKO-sirt3 kd macrophages; *, P < .05; **, P < .01; n = 3–6 per group.
SIRT3 expression mediates the protective effect of FABP4/aP2 deficiency on LPS-induced mitochondrial dysfunction
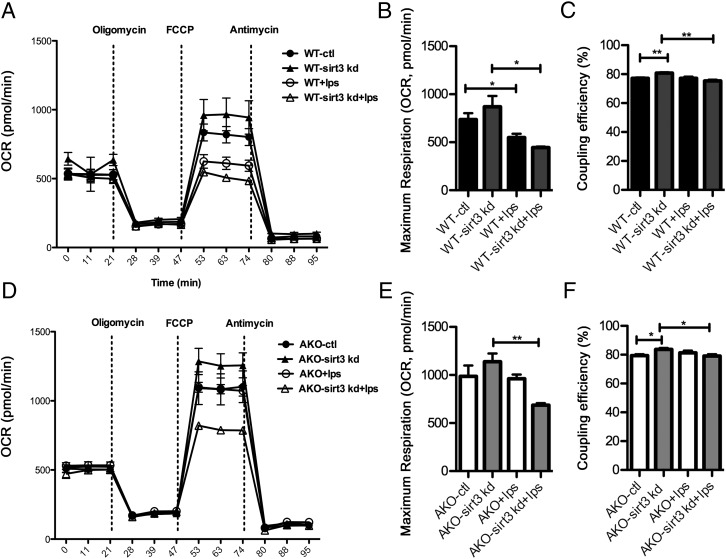
Genetic ablation of FABP4/aP2 has been shown to protect macrophages from LPS-dependent loss of maximum respiration capacity, independent of UCP2 expression (22). In order to determine whether the increased expression of SIRT3 in FABP4/aP2−/− macrophages could mediate the suppression of LPS-induced mitochondrial dysfunction, both WT-sirt3 kd and AKO-sirt3 kd, as well as their control cells, were treated with LPS for 6 hours, and cellular respiration was measured. Consistent with our previous report (22), LPS treatment induced a decrease of maximum respiration in WT cells, whereas FABP4/aP2−/− macrophages were protected from this effect (Figures 4, A, B and D, E). Knockdown of Sirt3 rendered WT macrophages more susceptible to a LPS-induced decrease of maximum respiration (Figure 4B). Moreover, knockdown of Sirt3 in FABP4/aP2−/− macrophages led to a similar reduction of maximum respiration by LPS treatment, thus eliminating the protection seen in the FABP4/aP2−/− macrophages (Figure 4E). Interestingly, coupling efficiency was increased after Sirt3 knockdown in WT and FABP4/aP2−/− macrophages and decreased in Sirt3 knockdown cells after LPS treatment (Figure 4, C and F). No difference of basal respiration or ATP turnover was observed (data not shown). Therefore, up-regulation of SIRT3 in FABP4/aP2−/− macrophages is responsible for changes in mitochondrial respiration in response to LPS treatment.
Figure 4.
SIRT3 expression protects macrophages from LPS-induced mitochondrial dysfunction. A, Oxygen consumption rate in WT-ctl and WT-sirt3 kd macrophages treated with or without LPS (100 ng/mL) for 6 hours (2μM oligomycin, 0.4μM FCCP, 4μM antimycin). B, Maximum respiration in WT-ctl and WT-sirt3 kd macrophages treated with or without LPS (100 ng/mL) for 6 hours. C, Coupling efficiency in WT-ctl and WT-sirt3 kd macrophages treated with or without LPS (100 ng/mL) for 6 hours. D, Oxygen consumption rate in AKO-ctl and AKO-sirt3 kd macrophages treated with or without LPS (100 ng/mL) for 6 hours (2μM oligomycin, 0.4μM FCCP, 4μM antimycin). E, Maximum respiration in AKO-ctl and AKO-sirt3 kd macrophages treated with or without LPS (100 ng/mL) for 6 hours. F, Coupling efficiency in AKO-ctl and AKO-sirt3 kd macrophages treated with or without LPS (100 ng/mL) for 6 hours; *, P < .05; **, P < .01; n = 5 per group.
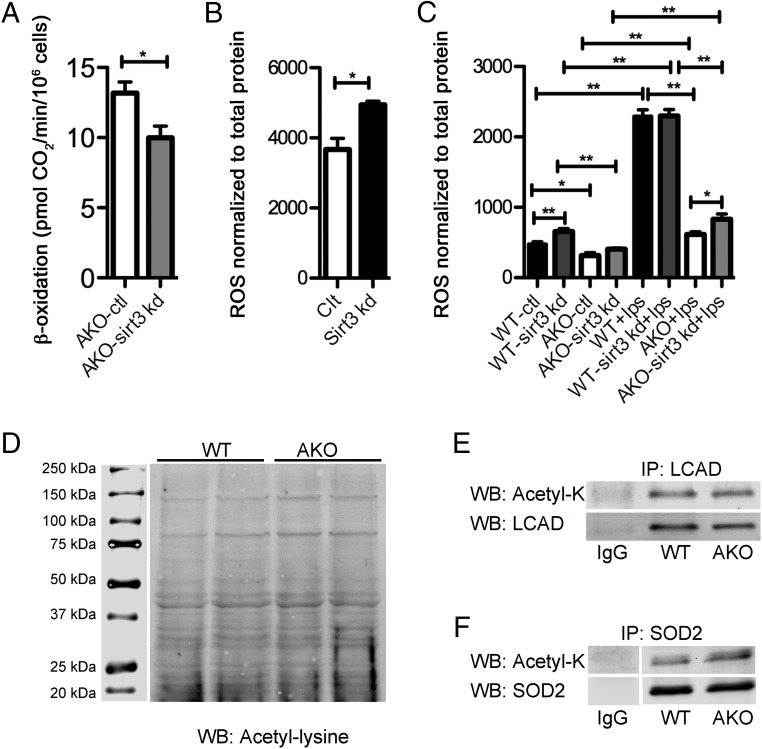
SIRT3 expression is responsible for fatty acid oxidation and ROS production in macrophages independent of lysine acetylation changes
Among the 7 members in the sirtuin family, SIRT3 plays a major role in regulating mitochondrial function by affecting various aspects of mitochondrial metabolism, including mitochondrial respiration, fatty acid oxidation, and the tricarboxylic acid cycle flux (33–35). Consistent with the report that SIRT3 regulates fatty acid oxidation (34), Sirt3 knockdown in FABP4/aP2−/− macrophages decreased fatty acid oxidation (Figure 5A). Another prominent role of SIRT3 is controlling ROS production (33). Consistently, knockdown of Sirt3 increased ROS production in Raw264.7 and WT macrophages (Figure 5, B and C). Because our previous work has shown that FABP4/aP2−/− macrophages have a significantly lower level of oxidative stress (22), we speculate that the increased SIRT3 expression may be responsible for the decreased ROS level. Consistent with our previous report, the ROS level was significantly lower in FABP4/aP2−/− macrophages compared with that in the WT (Figure 5C). Upon LPS stimulation, there was a significant increase of ROS in WT, but not FABP4/aP2−/− macrophages. Importantly, upon LPS stimulation, AKO-sirt3 kd cells had a significant increase in ROS production compared with AKO macrophages; however, the ROS levels were still considerably lower than that in WT macrophages in response to LPS stimulation (Figure 5C). These data indicate up-regulation of SIRT3 is at least partially responsible for the decreased ROS in FABP4/aP2−/− macrophages in response to LPS treatment. Despite the increased SIRT3 expression in FABP4/aP2−/− macrophages, we did not detect any substantive difference in the mitochondrial acetylome or the acetylation of classic SIRT3 targets, LCAD and SOD2 (Figure 5, D–F).
Figure 5.
Increased SIRT3 expression mediates increased β-oxidation and decreased oxidative stress in FABP4/aP2−/− macrophages independent of protein acetylation. A, β-Oxidation measured in AKO-ctl and AKO-sirt3 kd macrophages. B, ROS measured by H2DCFDA in Control and Sirt3 kd Raw264.7 macrophages. C, ROS measured by H2DCFDA in WT-ctl, WT-sirt3 kd, AKO-ctl, and AKO-sirt3 kd macrophages treated with or without LPS (100 ng/mL) for 6 hours. D, Mitochondrial protein acetylation in WT and AKO macrophages. E, Analysis of acetylated and total LCAD after immunoprecipitation from WT and AKO macrophages. F, Evaluation of acetylated and total SOD2 immunoprecipitated from WT and AKO macrophages; *, P < .05; **, P < .01; n = 3–6 per group.
Discussion
Insulin resistance in peripheral tissues such as liver, adipose, and muscle is a major feature in the pathogenesis of cardiovascular disease, fatty liver, and type 2 diabetes (1, 5). The progression of insulin resistance is accompanied with macrophage infiltration and chronic inflammatory activation in multiple tissues (12, 36–38). Mouse models which either reduce macrophage recruitment in adipose tissue or suppress macrophage inflammation have been show to protect the animal from HFD-induced insulin resistance (39–41). One of the most well-characterized mouse models that affect these inflammatory events is genetic deletion of FABP4/aP2. Despite a similar level of adiposity, FABP4/aP2−/− mice are protected from diet-induced insulin resistance (42). Interestingly, macrophage specific deletion of FABP4/aP2 is sufficient to prevent the development of atherosclerosis in the apolipoprotein E-deficient mouse model (14). More detailed studies have shown that loss of FABP4/aP2 could suppress inflammatory signaling in macrophages, which is a major reason for the metabolic benefits of FABP4/aP2 deficiency (13, 16, 43, 44). Recent work from our laboratory has shown that induction of UCP2 expression is a major mediator for the decreased inflammation and endoplasmic reticulum stress responses observed in FABP4/aP2−/− macrophages (22). However, up-regulation of UCP2 does not explain the increased fatty acid oxidation and improved mitochondrial function in FABP4/aP2−/− macrophages. Previous reports demonstrating a MUFA-SIRT1-peroxisome proliferator-activated receptor γ coactivator 1-α-SIRT3 axis, as well as our own observation that monounsaturated fatty acids are specifically increased in FABP4/aP2−/− macrophages, led us to speculate that SIRT3 expression might be altered in FABP4/aP2−/− macrophages (26, 27). Indeed, the results herein showed a dramatic increase of SIRT3 protein, but not mRNA, after loss of macrophage FABP4/aP2. Additionally, silencing of Sirt3 in FABP4/aP2−/− macrophages decreased fatty acid oxidation. More importantly, our results also demonstrated for the first time that MUFAs, but not palmitate, could induce SIRT3 expression in macrophages. By knocking down Sirt3 in macrophages, the results here revealed an important role of SIRT3 in the suppression of macrophage inflammation. Induction of SIRT3 is required for the antiinflammatory role of palmitoleate, as knockdown of Sirt3 compromised the reduction of Cox2 expression caused by palmitoleate treatment.
In parallel with the identification of UCP2 as a major mediator of the decreased inflammation in FABP4/aP2−/− macrophages, the loss of SIRT3 also restored the inflammation in FABP4/aP2-deficient cells similar to WT macrophages. Interestingly, SIRT3 expression has been shown to be correlated with UCP2 levels in hepatocytes cultured in high glucose and in this system, inhibition of SIRT3 also leads to decreased UCP2 expression (45). Therefore, it is likely that SIRT3 acts as an upstream regulator of UCP2 and exerts some of its action by suppressing inflammation in macrophages through UCP2.
SIRT3 localizes primarily to mitochondria and regulates the activity of a number of enzymes involved in some major metabolic pathways, including tricarboxylic acid cycle, the urea cycle, and fatty acid metabolism (33–35). Given the prominent role of SIRT3 in mitochondrial function, it was not surprising to find that the up-regulation of SIRT3 is an important contributor to the improved mitochondrial function observed in FABP4/aP2−/− macrophages (22). Although FABP4/aP2−/− macrophages are protected from LPS-induced loss of maximum respiration capacity, knockdown of SIRT3 potentiated LPS-induced mitochondrial dysfunction. Consistent with SIRT3's role as a suppressor of oxidative stress, loss of SIRT3 increased ROS production both basally and in response to LPS in macrophages.
Paradoxically, despite the dramatic up-regulation of SIRT3, we were not able to detect any difference of global mitochondrial protein lysine acetylation or in the acetylation status of classic targets of SIRT3 such as LCAD and SOD2. One potential explanation is that SIRT3 exerts its role in inflammation, ROS control, and mitochondrial function through a mechanism other than protein deacetylase activity in macrophages (46). It is also possible that the acetylation status of the SIRT3 targets analyzed here is affected by other pathways in addition to SIRT3. Generally, SIRT3 is considered as a ROS suppressor mainly by regulating SOD2 acetylation status (47). However, we did not detect any difference of SOD2 expression or acetylation between WT and FABP4/aP2−/− macrophages (Figure 5). Although we speculate that SIRT3 serves as an oxidative stress suppressor in macrophages by regulating UCP2 expression, further work is required to validate this point.
In summary, the results presented herein demonstrate that SIRT3 is up-regulated in FABP4/aP2-deficient macrophages, potentially via increased levels of palmitoleic acid. The increased SIRT3 expression mediates decreased inflammation and intracellular ROS as well as the improved mitochondrial function in FABP4/aP2−/− macrophages. However, increased SIRT3 expression did not affect global acetylation status of mitochondrial protein in FABP4/aP2−/− macrophages.
Acknowledgments
We thank the members of the Bernlohr laboratory for helpful discussions during the study and preparation of the manuscript. We also thank Dr Douglas Mashek and Dr Salmanan Kahn for several helpful suggestions.
This work was supported by National Institutes of Health Grants R01 DK053189 (to D.A.B.) and T32 AG029796 (to K.A.S.) and the Minnesota Nutrition and Obesity Center (NIH Grant P30 DK050456). This work was also supported by the Minnesota Supercomputing Institute.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AKO
- FABP4/aP2 knock out
- Cox2
- cyclooxygenase-2
- FABP
- fatty acid binding protein
- FABP4/aP2
- adipocyte fatty acid binding protein
- FCCP
- carbonyl cyanide 4-(trifluoromethoxy) phenyl hydrazone
- GFP
- green fluorescent protein
- H2DCFDA
- 2′, 7′-dichlorodihydrofluorescein diacetate
- HFD
- high-fat diet
- KRH
- Krebs-Ringers-HEPES
- IFN
- interferon
- iNOS
- inducible nitric oxide synthase
- LCAD
- long-chain acyl-CoA dehydrogenase
- LPS
- lipopolysaccharide
- MUFA
- monounsaturated fatty acids
- ROS
- reactive oxygen species
- shRNA
- short hairpin RNA
- SIRT3
- sirtuin 3
- SOD2
- superoxide dismutase 2
- SVF
- stromal vascular fraction
- TNF
- tumor necrosis factor
- UCP2
- uncoupling protein 2
- WT
- wild type.
References
- 1. Reaven GM. Banting Lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. [DOI] [PubMed] [Google Scholar]
- 2. Jane M, Foster J, Hagger M, Pal S. Using new technologies to promote weight management: a randomised controlled trial study protocol. BMC Public Health. 2015;15(1):509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zimmet PZ. The pathogenesis and prevention of diabetes in adults. Genes, autoimmunity, and demography. Diabetes Care. 1995;18(7):1050–1064. [DOI] [PubMed] [Google Scholar]
- 4. Feldeisen SE, Tucker KL. Nutritional strategies in the prevention and treatment of metabolic syndrome. Appl Physiol Nutr Metab. 2007;32(1):46–60. [DOI] [PubMed] [Google Scholar]
- 5. Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132(6):2169–2180. [DOI] [PubMed] [Google Scholar]
- 6. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruskovska T, Bernlohr DA. Oxidative stress and protein carbonylation in adipose tissue - implications for insulin resistance and diabetes mellitus. J Proteomics. 2013;92:323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–949. [DOI] [PubMed] [Google Scholar]
- 9. Lynch L, Nowak M, Varghese B, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37(3):574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol Metab. 2012;23(8):407–415. [DOI] [PubMed] [Google Scholar]
- 11. Afonso Mda S, Castilho G, Lavrador MS, et al. The impact of dietary fatty acids on macrophage cholesterol homeostasis. J Nutr Biochem. 2014;25(2):95–103. [DOI] [PubMed] [Google Scholar]
- 12. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72(1):219–246. [DOI] [PubMed] [Google Scholar]
- 13. Hertzel AV, Hellberg K, Reynolds JM, et al. Identification and characterization of a small molecule inhibitor of fatty acid binding proteins. J Med Chem. 2009;52(19):6024–6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Makowski L, Boord JB, Maeda K, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7(6):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maeda K, Cao H, Kono K, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1(2):107–119. [DOI] [PubMed] [Google Scholar]
- 16. Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor γ and IκB kinase activities. J Biol Chem. 2005;280(13):12888–12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao H, Sekiya M, Ertunc ME, et al. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 2013;17(5):768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ertunc ME, Sikkeland J, Fenaroli F, et al. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J Lipid Res. 2015;56(2):423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shum BO, Mackay CR, Gorgun CZ, et al. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J Clin Invest. 2006;116(8):2183–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erbay E, Babaev VR, Mayers JR, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15(12):1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tuncman G, Erbay E, Hom X, et al. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci USA. 2006;103(18):6970–6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu H, Hertzel AV, Steen KA, Wang Q, Suttles J, Bernlohr DA. Uncoupling lipid metabolism from inflammation through fatty acid binding protein-dependent expression of UCP2. Mol Cell Biol. 2015;35(6):1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Curtis JM, Grimsrud PA, Wright WS, et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59(5):1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coe NR, Simpson MA, Bernlohr DA. Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J Lipid Res. 1999;40(5):967–972. [PubMed] [Google Scholar]
- 25. Kien CL. Dietary interventions for metabolic syndrome: role of modifying dietary fats. Curr Diab Rep. 2009;9(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim JH, Gerhart-Hines Z, Dominy JE, et al. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1α complex. J Biol Chem. 2013;288(10):7117–7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu TF, Vachharajani V, Millet P, Bharadwaj MS, Molina AJ, McCall CE. Sequential actions of SIRT1-RELB-SIRT3 coordinate nuclear-mitochondrial communication during immunometabolic adaptation to acute inflammation and sepsis. J Biol Chem. 2015;290(1):396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirschey MD, Shimazu T, Jing E, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44(2):177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lantier L, Williams AS, Williams IM, et al. SIRT3 is crucial for maintaining skeletal muscle insulin action and protects against severe insulin resistance in high-fat-fed mice. Diabetes. 2015;64(9):3081–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fernandez-Marcos PJ, Jeninga EH, Canto C, et al. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Sci Rep. 2012;2:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caton PW, Richardson SJ, Kieswich J, et al. Sirtuin 3 regulates mouse pancreatic β cell function and is suppressed in pancreatic islets isolated from human type 2 diabetic patients. Diabetologia. 2013;56(5):1068–1077. [DOI] [PubMed] [Google Scholar]
- 32. Paulin R, Dromparis P, Sutendra G, et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab. 2014;20(5):827–839. [DOI] [PubMed] [Google Scholar]
- 33. Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12(6):662–667. [DOI] [PubMed] [Google Scholar]
- 34. Hirschey MD, Shimazu T, Goetzman E, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao S, Xu W, Jiang W, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327(5968):1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harman-Boehm I, Blüher M, Redel H, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92(6):2240–2247. [DOI] [PubMed] [Google Scholar]
- 37. Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18(3):363–374. [DOI] [PubMed] [Google Scholar]
- 38. Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 2009;276(20):5738–5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scheja L, Makowski L, Uysal KT, et al. Altered insulin secretion associated with reduced lipolytic efficiency in aP2−/− mice. Diabetes. 1999;48(10):1987–1994. [DOI] [PubMed] [Google Scholar]
- 40. Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274(5291):1377–1379. [DOI] [PubMed] [Google Scholar]
- 43. Erbay E, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid binding proteins in metabolic syndrome. Curr Atheroscler Rep. 2007;9(3):222–229. [DOI] [PubMed] [Google Scholar]
- 44. Furuhashi M, Tuncman G, Görgün CZ, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447(7147):959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gounden S, Phulukdaree A, Moodley D, Chuturgoon A. Increased SIRT3 expression and antioxidant defense under hyperglycemic conditions in HepG2 cells. Metab Syndr Relat Disord. 2015;13(6):255–263. [DOI] [PubMed] [Google Scholar]
- 46. Feldman JL, Baeza J, Denu JM. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem. 2013;288(43):31350–31356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tao R, Coleman MC, Pennington JD, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40(6):893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]