Abstract
Leptin triggers signaling events with significant transcriptional responses that are essential to metabolic processes affecting obesity and glucose disposal. We asked whether hexamethylene bis-acetamide inducible-1 (Hexim1), an inhibitor of RNA II polymerase-dependent transcription elongation, regulates leptin-Janus kinase 2 signaling axis in the hypothalamus. We subjected C57BL6 Hexim1 heterozygous (HT) mice to high-fat diet and when compared with wild type, HT mice were resistant to high-fat diet-induced weight gain and remain insulin sensitive. HT mice exhibited increased leptin-pY705Stat3 signaling in the hypothalamus, with normal adipocyte size, increased type I oxidative muscle fiber density, and enhanced glucose transporter 4 expression. We also observed that normal Hexim1 protein level is required to facilitate the expression of CCAAT/enhancer-binding proteins (C/EBPs) required for adipogenesis and inducible suppressor of cytokine signaling 3 (SOCS) expression. Further support on the role of Hexim1 regulating C/EBPs during adipocyte differentiation was shown when HT 3T3L1 fibroblasts failed to undergo adipogenesis. Hexim1 selectively modulates leptin-mediated signal transduction pathways in the hypothalamus, the expression of C/EBPs and peroxisome proliferator-activated receptor-γ (PPAR γ) in skeletal muscle and adipose tissue during the adaptation to metabolic stress. We postulate that Hexim1 might be a novel factor involved in maintaining whole-body energy balance.
Leptin is an important regulator of metabolic processes that affect energy balance essential to normal regulation of food intake and body weight (1). Leptin, working through signal transduction and activator of transcription (Stat3), can induce transcription of several target genes that affect energy balance. Among these genes, suppressor of cytokine signaling 3 (SOCS) a transcription factor that triggers a negative feedback loop through direct interaction with Janus kinase 2 (Jak2) and the leptin receptor (2, 3). Haploinsufficiency of SOCS3 in mice was resistance to high-fat diet (HFD)-induced obesity and diabetes (4), whereas SOCS3 overexpression is associated with leptin and insulin resistance in skeletal muscle (5). Accordingly, changes in SOCS3 expression and/or Jak2 activity have been shown to greatly influence leptin function in energy balance (6).
A major source of leptin is adipose tissue. Adipocytes store excess of energy as triglycerides and are crucial for maintaining energy and metabolic homeostasis (7). Transcription factors CCAAT/enhancer-binding proteins (C/EBPs) (C/EBPα, C/EBPβ, and C/EBPδ) and peroxisome proliferator-activated receptor (PPAR)γ play essential roles in the formation of adipose tissue. This process, adipogenesis, is characterized by a distinct temporal pattern of transcription factors expression in which C/EBPβ and C/EBPγ appear early followed by C/EBPα (8). Given the role of leptin and these transcription factors in adipogenesis, a major causative factor in obesity, a better understanding of the signaling and transcriptional regulatory networks that control the formation and function of adipose tissue will be key to providing further insights into the cause and prevention of obesity.
We have been studying the role of hexamethylene bis-acetamide inducible-1 (Hexim1), a regulator of gene expression via control of transcription elongation via inhibition of RNA polymerase II (9) that likely includes the expression of adipogenic genes. Hexim1 deletion is lethal past embryonic day 16.5 after coitum (10), whereas Hexim1 heterozygous (HT) mice are viable and develop normally unless they are subjected to stress (11–13). RNA polymerase II activity, transcription elongation, and regulatory components such as Hexim1 are present in every eukaryotic cell (14); however, little is known regarding their role during metabolic stress. To study Hexim1's role during the transcriptional response to metabolic stress, we used heterozygous (HT) Hexim1 knockout mice and subjected these mice to HFD. Affymetrix array analysis revealed impaired expression of C/EBPs and impaired inducible expression of SOCS3 gene in the HT background as compared with wild type (WT). These results suggested that Hexim1 is involved in controlling SOCS3 gene expression during the genomic stress response to HFD.
Structural analysis of Hexim1 protein revealed the presence of conserved YXXL motifs that are present in the Jak2 tyrosine kinase (see Supplemental Figure 1) and the Src Homology 2B adaptor protein 1 (SH2B1). YXXL motifs containing proteins, including Jak2 kinase and SH2B1, play an important role in metabolic adaptation (15). Given the established role of SOCS3 expression in metabolism (6, 16), and C/EBPs in adipogenesis (17), we asked whether Hexim1 can also play a role in the pathophysiology of obesity and diabetes, collectively referred to as diabesity.
Materials and Methods
Animals and diets
All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication 85-23, revised 1996) and were approved by the institutional Animal Care and Use Committee at State University of New York Downstate Medical School. HT mice were backcrossed onto a pure C57BL/6 background for more than 8 generations (12). Male mice were maintained in groups of 4 in a climate-controlled room with a 12-hour light, 12-hour dark cycle and weighed weekly.
Diet treatment
Ten weeks old littermate male WT and HT mice were fed HFD pellets (Diet D12492; Research Diets, Inc) or standard chow pellets (5010 Lab diet) for 14 weeks. HFD provided approximately 60% of calories as lipids, whereas standard chow provided only 12%.
Diet and chronic leptin treatment
A parallel study of similar age littermates of WT and HT were fed HFD (Diet D12492; Research Diet, Inc) and subjected to twice daily ip injections of leptin (2-μg/g body weight) for 14 weeks.
Daily food intake
Individual WT and HT mice littermates of similar age and weight (8 wk old and about 20 g) maintained in single cages were fed HFD for 14 days. We measured daily food intake and weight gain using mouse eatometers. Food intake is expressed per gram of body weight.
Blood chemistry
After overnight fast, blood was collected via tail vein clipping; total plasma cholesterol and triglyceride levels were measured using commercial kits (Thermo Trace), and plasma insulin using the rat/mouse insulin ELISA kit (EZRMI-13k) from Millipore. Mouse plasma leptin and adiponectin levels were measured by ELISA (Quantikine mouse/rat [MOB00] from R&D Systems and EZMADP-60K from Millipore). Blood glucose was measured using Accu-check Aviva blood glucose monitor.
Metabolic studies
The morning after an overnight fast, on separate days, WT and HT mice littermates fed HFD for 12 weeks had ip glucose and insulin tolerance tests with 1-g glucose/kg or 0.75-U insulin/kg body weight with sampling performed immediately before treatment (0 time), and 15, 30, 60, and 120 minutes after treatment. Glucose tolerance and insulin sensitivity were calculated by area under the curve (AUC) and insulin resistance according to the homeostatic model of assessment of insulin resistance: fasting glucose level (mg/dL) × fasting insulin level (ng/mL) ÷ 22.5.
Leptin signaling
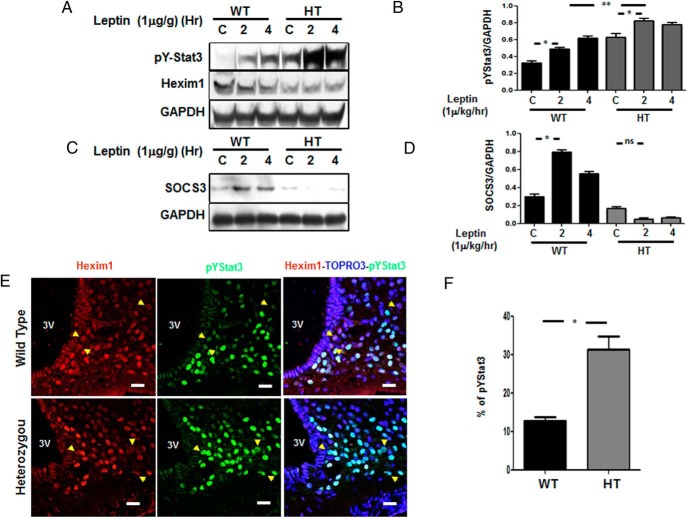
Ten-week-old littermate male (n = 4) WT and HT mice were fasted overnight before ip injection of leptin (1-μg/g body weight; Sigma-Aldrich). Brains were harvested and the hypothalamus dissected at 3 time points (0, 2, and 4 h), and immediately frozen in liquid nitrogen. The whole tissue protein extracts were used at each time point and antibodies for pY-Stat3 (1:1000; Cell Signaling), Hexim1 (1:1000; Abcam), and SOCS3 (1:1000; Cell Signaling) levels were determined by Western blotting.
Western blotting and immunoprecipitation
The tissues (hypothalamus, skeletal muscle) or cells (HEK293, 3T3-L1) were homogenized with a glass tissue grinder in ice-chilled buffer A (10 mmol/L HEPES [pH 7.9], 1.5 mmol/L MgCl2, 10 mmol/L KCl, 200 mmol/L NaCl, and 0.2 mmol/L EDTA) supplemented with 1 mmol/L 1,4-Dithiothreitol, protease inhibitor mixture (P-8340; Sigma), 40-U/mL RNasin (Promega), phosphatase inhibitor mixtures (P2850 and P5726; Sigma), and 0.1% Nonidet P-40. The extracts were subjected to sequential centrifugations for 5 minutes each at 500g and 9000g at 4°C. The final supernatant was used in all experiments. Protein concentration was determined using Bio-Rad Bradford assay. The blots were blocked in Tris-buffered saline, 0.1% Tween 20 with 5% BSA powder for 1 hour at room temperature. After incubation with the primary antibody, detection was performed using secondary horseradish peroxide-coupled antibody and enhanced chemiluminescence according to the recommendations of the supplier (Amersham Biosciences). The following antibodies were used: rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2000) (Santa Cruz Biotechnology, Inc), rabbit anti-SOCS3, rabbit anti-pY705-Stat3, rabbit anti-pY1007/1008 Jak2, rabbit anti-C/EBPs (α and β), and rabbit anti-PPARγ were used at a dilution (1:1000; Cell Signaling Technology). Mouse antimyc (1:2000; Sigma-Aldrich) and rabbit anti-Hexim1 (1:1000; Abcam). Quantification of Western blottings density was performed with ImageJ (NIH).
Tissue immunohistochemistry
Brains were perfused with 4% (wt/vol) paraformaldehyde in PBS. Each brain was quickly dissected and maintained in paraformaldehyde for 24 hours at 4°C. The fixed brains were transferred to 30% (wt/vol) sucrose in PBS at 4°C, embedded in optimal cutting temperature medium, and frozen at −80°C until the time of sectioning. Using a cryostat (Leica), we obtained 20-μm frozen coronal sections that were subsequently processed for immunofluorescence staining. The sections were pretreated with 1% NaOH and 1% hydrogen peroxide for 20 minutes at room temperature, followed by 0.3M glycine in PBS for 10 minutes at room temperature. Treated sections were blocked for 2 hours at room temperature in 0.3% (vol/vol) Triton X-100, 3% (wt/vol) BSA, 5% (vol/vol) goat serum in PBS, and then incubated overnight at 4°C with one of the following primary antibodies: mouse anti-Hexim1 (1:50; from Santa Cruz Biotechnology, Inc), rabbit anti-SOCS3 (1:50), and rabbit antiphospho-Tyr705-Stat3 (1:50) from Cell Signaling. Primary antibodies were incubated with goat antimouse Alexa Fluor 594 (5 μg/mL) (Invitrogen) or goat antirabbit Alexa Fluor 488 (5 μg/mL) (Invitrogen). Nuclear staining was obtained with TOPRO3 (1μM in PBS) (Invitrogen).
Gastrocnemius skeletal muscle was dissected, fixed, and maintained in paraformaldehyde for 24 hours at 4°C or immediately frozen in liquid nitrogen. The fixed gastrocnemius was paraffinized by ethanol dehydration and embedded in paraffin blocks in a vacuum oven at 60°C. Sections (10 μm) were cut using a microtome and mounted on positively charged microscopic slides (Polysciences, Inc) to ensure adhesion. The sections were then deparaffinized (3 × 10 min) in xylene, rehydrated in water (3 × 100% ethanol, 95% ethanol, 70% ethanol, 50% ethanol, 10 min each), and incubated in citrate-EDTA buffer at 95°C in a water bath for 20 minutes for antigen retrieval. The sections were then blocked for 2 hours at room temperature in 0.3% (vol/vol) Triton X-100, 3% (wt/vol) BSA, and 5% (vol/vol) goat serum in PBS before overnight incubation at 4°C with one of 2 primary antibodies: monoclonal antislow skeletal myosin clone NOQ7 (1:500) from Sigma, or rabbit anti-Glut4 (1:50) from Millipore. Primary antibodies were incubated with goat antimouse Alexa Fluor 594 (5 μg/mL) (Invitrogen) or goat antirabbit Alexa Fluor 488 (5 μg/mL) (Invitrogen). Nuclear staining was obtained with TOPRO3 (1μM in PBS) (Invitrogen).
Subcutaneous inguinal adipose tissues were dissected and maintained in paraformaldehyde for 24 hours at 4°C; the fixed adipose tissue was transferred to 30% (wt/vol) sucrose in PBS at 4°C, embedded in optimal cutting temperature medium, and frozen at −80°C. We obtained 10-μm sections stained with hematoxylin/eosin staining (Sigma-Aldrich). The hematoxylin/eosin slides were used to acquire adipocyte diameter in 300 adipocytes/group using the software ImageJ (NIH). Adipocyte tissue was also frozen in liquid nitrogen and used to determine triglycerides content. To this end, adipose tissue (200–400 mg) was homogenized in lysis buffer and centrifuged at 10 000g for 10 minutes. Protein concentration was determined and equal amount of protein was used to determine triglycerides content expressed as mg/g tissue following the procedure described in Infinity triglycerides (18).
Transfection of HEK293 cells
The plasmid constructs for leptin receptor and TELJak2 have been previously reported (19, 20). HEK293 cells (5 × 105) were plated and after 24 hours transfected with 1 μg of a plasmid expressing HA-TELJak2 cDNA with or without 1 μg of a plasmid expressing myc-Hexim1 cDNA, maintained overnight in serum-free medium with or without the Jak2 kinase inhibitor Z3 (30μM), and after 24 hours, each transfection set was collected and total protein extracts analyzed by immunoprecipitation using antimyc antibody (Sigma) and Catch and Release from Upstate Biotechnology followed by Western blotting. Additionally, direct Western blot analysis was performed to evaluate Z3 inhibition of Jak2-mediated Hexim1 tyrosine phosphorylation at residues pY167Hexim1 (1:1000) and pY291Hexim1 (1:1000). Rabbit affinity purified antibodies anti-pY167Hexim1 and anti-pY291Hexim1 were made by ImmunoKontact using the tyrosine phosphorylated peptides (skkkrhwkpy167y168kltwee) and (skqelikey291lelekcls), respectively.
Leptin receptor and Hexim1 transfection
Briefly, 5 × 105 HEK293 cells were plated and, after 24 hours, transfected with 1-μg HA-Ob-Rb with or without 1-μg myc-Hexim1 or a Hexim1 protein carrying substitution mutation in both YXXL motifs (YY161TG/Y291E-Hexim1). After 24 hours, transfected cells were maintained in serum-free medium, and then mouse leptin (100 ng/mL) was added for 30 minutes, 1 hour, and 2 hours. HEK293 cells were collected and pY705Stat3 induction analyzed by Western blotting, and the quantification of density was performed with ImageJ (NIH) (Supplemental Figure 2).
Induction of adipocyte differentiation
3T3L1 cells were purchased from ATCC (3T3L1 CL-173). We derived HT 3T3L1-HT by homologous recombination using a previously reported Neo-Hexim1 cassette (10). Confluent 3T3L1 and 3T3L1-HT cells were transferred to differentiation medium (d 0) (DMEM containing 10% fetal calf serum, 0.5mM 3-isobutyl-1-methyl xanthine, 2μM dexamethasone, and 5 μg/mL of insulin). Two days later (d 2), the differentiation medium was replaced with a maintenance medium (DMEM supplemented with 10% fetal calf serum and 5 μg/mL of insulin), which was renewed every other day. At day 8 of differentiation, adipose drops in the cells were stained with Oil Red-O and observed under a Nikon Microphot SA microscope. Cells were collected at 0, 2, 4, 6, and 8 days. Total cell extracts were analyzed by Western blotting using rabbit antibodies against C/EBPα, PPARγ, acetyl CoA carboxylase, and fatty acid synthase at a 1:1000 dilutions (Cell Signaling). The dilutions used for rabbit antibody against Hexim1 (1:1000) from Abcam and rabbit antibodies against GAPDH (1:2000) or actin (1:1000) from Santa Cruz Biotechnology, Inc.
Statistical analysis
Results are expressed as mean ± SEM. Comparisons among groups were obtained by one- or two-way ANOVA followed by Bonferroni's multiple comparison post hoc test. Comparison between 2 groups was obtained using paired Student's t test. GraphPad Prism5 software was used for statistical analysis. P < .05 was considered statistically significant.
Results
Resistance to HFD-induced metabolic obesity in HT mice
When placed on HFDs, HT mice exhibited significant differences from WT mice with respect to weight and weight gain, glucose disposal and insulin resistance, cholesterol and triglyceride levels, and adipocyte size.
For body weight and weight gain, HT mice placed on a HFD exhibited lowered body weight compared with WT on HFD (Table 1). For cholesterol, triglyceride levels, and adipocyte size, both HT and WT mice showed an increase in plasma cholesterol with the HFD. Baseline plasma triglyceride levels are higher in chow-fed HT mice vs WT mice. However, WT but not HT mice show significantly increased levels of plasma triglycerides when placed on a HFD (Table 1). Plasma leptin and triglycerides levels were higher in HT than WT mice in both chow-fed and HFD mice (Table 1). Adipocyte triglyceride content was lower in the HT background irrespective of diet. On chow diet, WT and HT mice exhibited similar adipocyte diameters, whereas they significantly increased in WT-HFD mice.
Table 1.
Metabolic Profile in WT and Hexim1 HT Mice HFD
| WT | HT | WT-HFD | HT-HFD | |
|---|---|---|---|---|
| n = 10 | n = 10 | n = 8 | n = 8 | |
| Body weight (g) | 31 ± 0.4 | 29.1 ± 0.3 | 44.1 ± 0.8 | 35.7 ± 1.2b |
| Glucose (mg/dL) | 129 ± 5.8 | 108 ± 4.4 | 231 ± 8.3 | 126 ± 8.8b |
| Insulin (ng/mL) | 0.38 ± 0.08 | 0.4 ± 0.02 | 1.36 ± 0.2 | 0.4 ± 0.05b |
| HOMA-IR | 2.2 ± 1.3 | 1.9 ± 0.6 | 14 ± 3.0 | 2.2 ± 0.4b |
| Cholesterol (mg/dL) | 77 ± 7.0 | 86 ± 9.4 | 201 ± 11.7c | 200 ± 10.5b,d |
| Triglycerides (mg/dL) | 36.0 ± 3.1 | 51 ± 5.6a | 62 ± 3.2c | 47 ± 2.7b |
| Leptin (ng/mL) | 2.1 ± 0.2 | 3.6 ± 0.4a | 4.8 ± 0.6 | 6.7 ± 0.7b |
| Adiponectin (ng/mL) | 4.2 ± 0.4 | 6.7 ± 0.6a | 5.3 ± 0.2 | 7.1 ± 0.3b |
| Adipocyte cell size (μm2) | 6892 ± 558 | 5470 ± 386 | 15 397 ± 1029 | 9015 ± 760b |
| Triglycerides (mg/mg protein) | 40 ± 5.0 | 26 ± 1.0 | 66.2 ± 5.7 | 38.2 ± 8.4b |
Values are the mean ± SEM for each group. HOMA-IR, homeostasis model assessment of insulin resistance.
P < .05 vs WT mice.
P < .05 vs WT-HFD mice
P < .05 vs WT mice.
P < .05 vs HT mice.
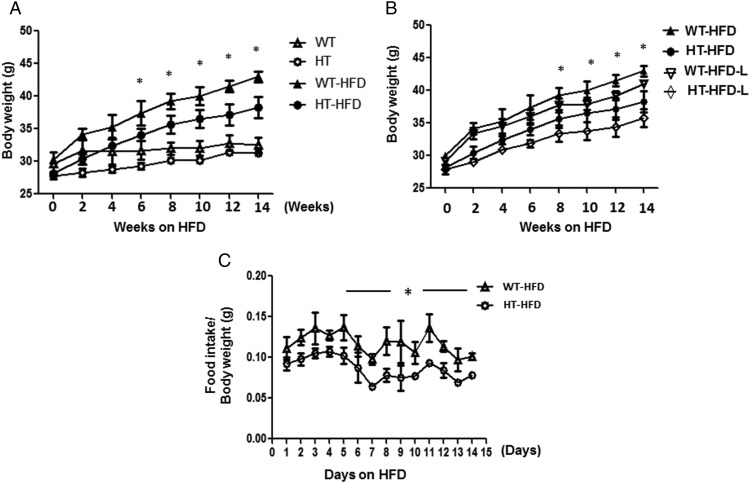
Weight gain in WT and HT fed chow diet was similar in both genetic backgrounds (31 ± 0.4 and 29.1 ± 0.3 g, respectively; P > .05). However, HT mice gained significantly less weight than WT mice after 6 weeks on a HFD (Figure 1A). Additionally, another group of WT and HT mice subjected to HFD and leptin during 14 weeks showed significantly less body weight gain in HT mice after 8 weeks as compared with WT (Figure 1B). HT mice had significantly less total food consumption: 136.5 g compared with 191.3 g for the WT: P < .0001 (Figure 1C). After adjusting for body weight, we observed that HT mice consumed less HFD daily than WT mice (Figure 1C), mean ± SEM; WT 2.5 ± 0.2 g and HT 1.6 ± 0.13 g; *, P < .05 WT-HFD vs HT-HFD. However, WT and HT show no differences in daily consumption of chow food, mean ± SEM. WT 2.1 ± 0.07 g and HT 1.9 ± 0.05 g, WT vs HT; *, P > .05.
Figure 1.
HT mice have lower body weight gain and greater glucose disposal compared with WT mice after feeding a HFD for 14 weeks. A, Body weight gain during 14 weeks of a HFD in WT (▵), HT (○), WT-HFD (▴), and HT-HFD (•) mice, n = 10 per group. Mean ± SEM, two-way ANOVA and Bonferroni's post hoc tests WT-HFD vs HT-HFD; *, P < .05. B, Body weight gain due to HFD and chronic treatment with leptin (L), WT-HFD (▴), HT-HFD (•),WT-HFD+L (▿), HT-HFD+L (♢) mice, n = 10 per group. Mean ± SEM, two-way ANOVA and Bonferroni's post hoc tests WT-HFD+L vs HT-HFD+L; *, P < .05. C, The graph shows the ratio between daily food intake/body weight of WT (Δ) and HT (○) (n = 5 per group). Mean ± SEM; WT vs HT; *, P < .0001 obtained by paired t test.
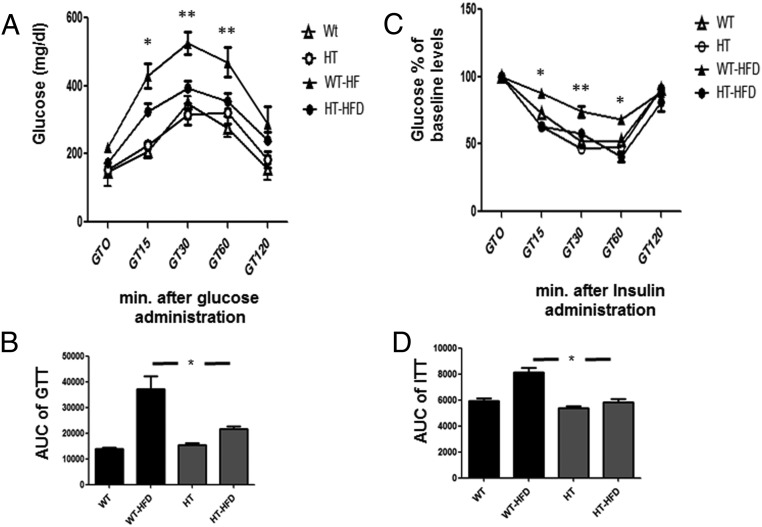
For glucose disposal and insulin resistance, HT mice placed on a HFD appeared to maintain normal glucose disposal and insulin sensitivity as compared with WT mice that went on to develop hyperglycemia and hyperinsulinemia. Glucose and insulin tolerance tests showed that WT mice on a HFD developed glucose resistance (WT-HFD vs HT-HFD; *, P < .05) and insulin resistance (WT-HFD vs HT-HFD; *, P < .001) (Figure 2, A and C), whereas HT mice on a HFD maintained glucose and insulin sensitivity (HT vs HT-HFD; *, P > .05) (see AUC for glucose tolerance test [GTT] and insulin tolerance test [ITT]) (Figure 2, B and D, respectively).
Figure 2.
HT mice have a greater glucose disposal compared with WT mice after feeding HFD for 14 weeks. A, Glucose tolerance test WT (Δ), HT (○), WT-HFD (▴), and HT-HFD (•) in mice, n = 5 per group. Mean ± SEM. One-way ANOVA and Bonferroni's post hoc test WT-HFD vs HT-HFD; *, P < .05 (GT15), WT-HFD vs HT-HFD; **, P < .001 (GT30 and GT60). B, AUC, AUC analysis of glycemia profile of glucose tolerance test (GTT) after 12 weeks of diet regimen. Values are expressed as mean ± SEM; *, P < .05. WT-HFD vs HT-HFD. C, Insulin tolerance test WT (Δ), HT (○), WT-HFD (▴), and HT-HFD (•) in mice, n = 5 per group. Mean ± SEM. One-way ANOVA and Bonferroni's post hoc test WT-HFD vs HT-HFD; *, P < .001 (GT15 and GT60), WT-HFD vs HT-HFD; **, P < .01 (GT30). D, AUC analysis of glycemia profile of insulin tolerance test (ITT) after 12 weeks of HFD. Values are expressed as mean ± SEM; *, P < .05. WT-HFD vs HT-HFD.
Hexim1 haploinsufficiency shows significant metabolic differences in skeletal muscle
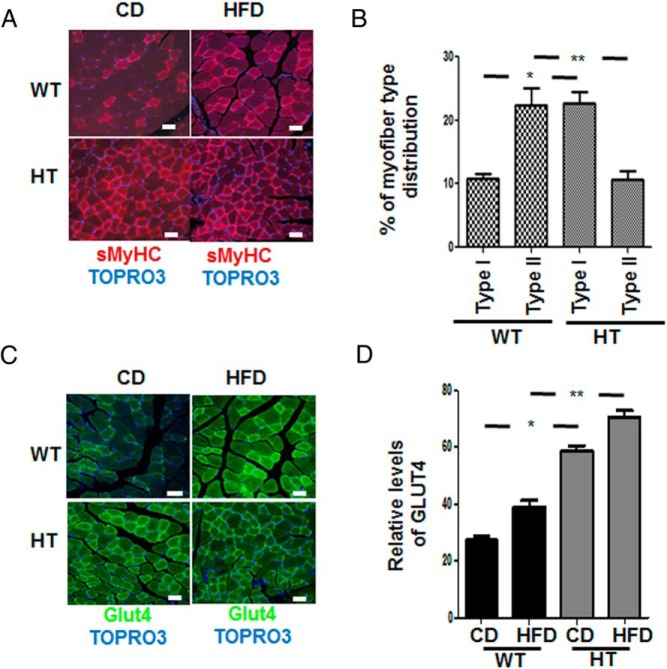
HT mice had a higher density of type I slow myosin heavy chain (sMHC) protein (Figure 3A), and the expression of sMHC was not affected by diet in either background (see below). There were more glycolytic fiber types (type II) in WT vs oxidative (type I) in the HT background (Figure 3B).
Figure 3.
Muscle fiber type switch and enhanced detection of Glut4 in gastrocnemius muscle of HT mice. A, Antimouse sMHC followed by antimouse-labeled 594 confocal immunofluorescence staining of the gastrocnemius muscle of chow-fed (CD) and HFD-fed mice. B, The figure shows the increase in myofiber type switch in the HT mice compared with WT mice. A total of 400 fibers were counted per group (white bar, 40 μm), expression of muscle fibers type I in WT vs HT data represent the mean ± SEM; *, P < .05, and type II WT vs HT; **, P < .002. C, Antirabbit Glut4 followed by antirabbit-labeled 488 in gastrocnemius muscle tissue. Nuclei identification is obtained with TOPRO3; white bar, 40 μm. D, The graph shows the relative levels of GLUT4 receptor in the muscle of HT and WT mice. A total of 300 fibers were counted per group, and a significant expression of GLUT4 was observed in mice fed CD WT vs HT, data represent the mean ± SEM; *, P < .05. A similar significant expression of Glut4 in mice fed HFD between WT and HT; **, P < .001.
There was a significantly higher density of Glut4 transporter on muscle cells in HT mice (Figure 3C) compared with WT on both diets (Figure 3D).
Changes in the expression of transcription factors regulating metabolic response in skeletal muscle
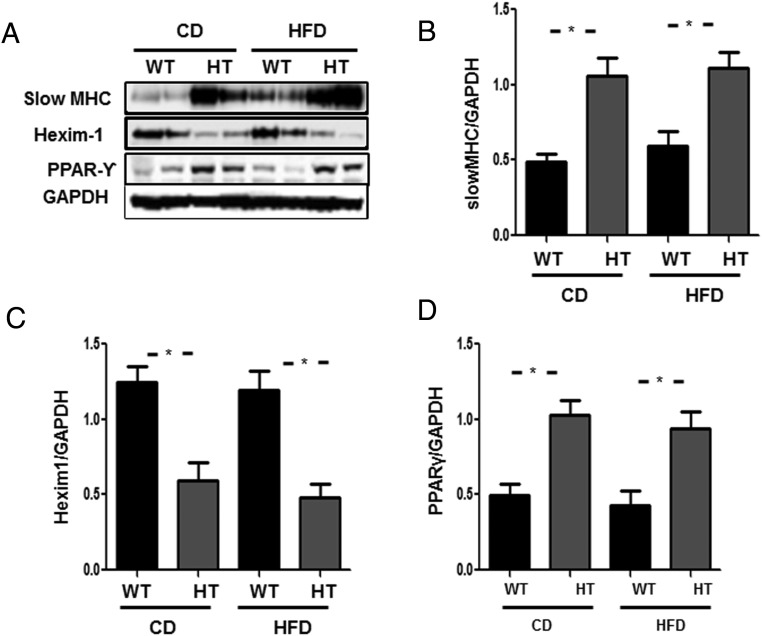
We observed increased expression of sMHC in gastrocnemius muscle of HT mice in both diets (Figure 4A, upper panel). Lower Hexim1 expression was confirmed in the HT group, unaffected by diet (Figure 4A). Similar protein extract analysis revealed significantly increased expression of PPARγ (Figure 4A), and quantified in Figure 4, B–D. We also observed decreased expression of C/EBPβ (Supplemental Figure 3) in the HT mice in both diets.
Figure 4.
Changes in the expression of transcription factors regulating metabolic response in gastrocnemius muscle of HT mice. A, Western blotting of gastrocnemius muscle total protein extract (from quadruplicate experiments) of WT, and heterozygous (HT) fed chow or HFD for 14 weeks. B, Graphs showing data quantification of sMHC to GAPDH ratio in WT vs HT fed chow or HFD showing increased expression of sMHC in the HT background in both diets. C, Graphs showing data quantification of Hexim1 to GAPDH ratio in WT vs HT fed chow or HFD showing decreased expression of Hexim1 in the HT background in both diets. D, Graphs showing data quantification of PPARγ to GAPDH ratio in WT vs HT fed chow or HFD showing increased expression of PPARγ in both diets. Data quantification is represented by the mean ± SEM; *, P < .05 obtained by one-way ANOVA followed by Bonferroni's post hoc test.
Leptin signaling in the hypothalamus is under Hexim1 control
Leptin treatment caused a significant increase in pY705-Stat3 level in hypothalamus of HT mice compared with WT mice (Figure 5, A and B). Hexim1 haploinsufficiency was confirmed by lower expression of Hexim1 protein in HT mice (Figure 5A, middle panel). We observed a significant increase in SOCS3 level in leptin treated WT mice not seen in the HT mice (Figure 5, C and D). Double-immunofluorescence staining showed colocalization of Hexim1 with leptin activated pY705-Stat3 in the arcuate nucleus. The panel Hexim1-TOPRO-pYStat3 shows that Hexim1 and pY705-Stat3 have a nuclear distribution (Figure 5E). Compared with WT after leptin treatment, there was an increase in pY705-Stat3 in HT vs WT mice (Figure 5F).
Figure 5.
Enhanced leptin signaling in the arcuate nucleus of HT mice. A, pYStat3 expression in the hypothalamus from WT or HT mice (upper panel), Hexim1 expression (middle panel), and GAPDH expression used as a loading control. B, Graphs showing data quantification of pY705Stat3 phosphorylation to GAPDH ratio after leptin treatment showing increased expression of pY705Stat3 in WT and HT mice at basal level and after 2 of leptin treatment. Data represent the mean ± SEM; *, P < .05. We also observed a significant increase in pY705Stat3 phosphorylation to GAPDH ratio in HT mice as compared with WT at 2 and 4 hours. Data represent the mean ± SEM; **, P < .001. C, SOCS3 expression, and GAPDH used as a loading control. D, Graphs showing data quantification of SOCS3 to GAPDH ratio in WT vs HT after leptin treatment showing decreased expression of SOCS3 in HT mice after 2 and 4 hours of leptin treatment. Data represent the mean ± SEM; *, P < .05. E, Confocal microscopy from hypothalamic sections from WT and HT mice after administering leptin 1 μg/g of body weight via ip for 4 hours showing the expression of Hexim1 (red), identified with antimouse 594, and pY705Stat3 (green), detected using antirabbit 488, in the arcuate nucleus. Yellow arrows indicate a lack of nuclear colocalization between Hexim1 and pY705Stat3 expression. A third panel shows overlapping Hexim1/TOPRO3 (nuclei)/pY705Stat3 signal; white bar, 40 μm. Quantitation of pYStat3 expression was obtained between WT and HT by determining the percentage of expression of pYStat3 vs 250 nuclei stained (TOPRO3) in 3 independent experiments. F, pY705Stat3 expression was significantly enhanced in the hypothalamus of HT mice after 4 hours of leptin treatment. Mean ± SEM; WT vs HT; *, P < .0001 paired t test. Mice were administered leptin 1 μg/g of body weight, given via ip for 2 and 4 hours.
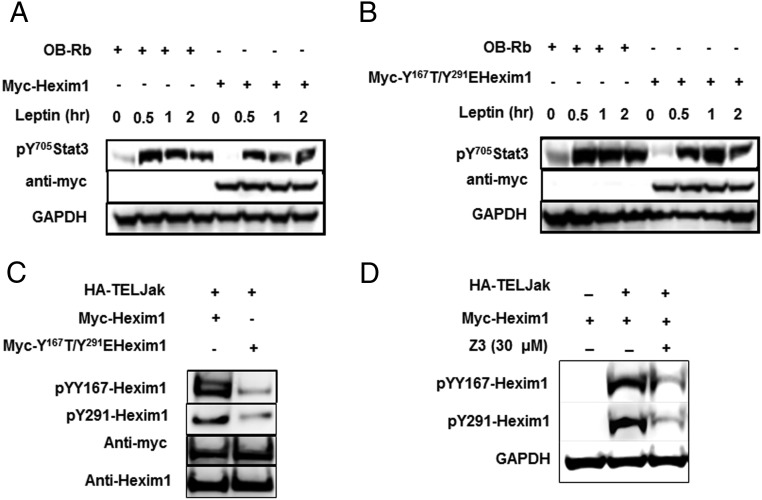
Hexim1 inhibits leptin mediated activation of pY705Stat3
Upon leptin treatment in transfected Ob-Rb in HEK293, Ob-Rb alone showed the induction of pY705-Stat3. Levels of induced pY705-Stat3 phosphorylation in leptin-treated cells decreased when cells coexpressed myc-Hexim1 (Figure 6A). However, when Ob-Rb was cotransfected with a substitution mutation on the conserved YXXL motif of Hexim1 (myc-YY161TG/Y291EHexim1), phosphorylation of pY705Stat3 in leptin-treated cells was restored to the level seen in cells expressing only Ob-Rb (Figure 6B) (Data quantitation are shown in Supplemental Figure 4). Initial evidence suggesting that Hexim1 might be a substrate of activated Jak2 kinase was demonstrated using the constitutively activated TELJak2 kinase that triggered tyrosine phosphorylation of myc-Hexim1 at residues pY167Hexim1 and pY291Hexim1. Cotransfection of a plasmid carrying a cDNA expressing substitution mutations of both YXXL motifs (pY167T/pY291EHexim1) showed a significant reduction in TELJak2 mediated tyrosine phosphorylation (Figure 6C). Inhibition of TELJak2 using the Jak2 kinase inhibitor Z3 (21) showed decreased Hexim1 tyrosine phosphorylation at residues pY167Hexim1 and pY291Hexim1 (Figure 6D).
Figure 6.
Inhibition of leptin/Jak2 signaling pathway through conserved Hexim1-YXXL motifs. A, Leptin mediated induction of pY705-Stat3 after coexpression by transient transfection assay on HEK-293 cells of HA-leptin receptor Ob-Rb with myc-Hexim1. B, Leptin induction of pY705-Stat3 after cotransfection of HA-Ob-Rb with myc-pY167T/pY291EYXXLHexim1. Myc and GAPDH expression were detected as internal loading control. C, Coimmunoprecipitation and Western blot analysis of whole-cell extracts from HEK293 cells transiently transfected with myc-Hexim1 and the constitutively active HA-TELJAK2. Immunoprecipitation was performed with antimyc followed by Western blotting with affinity-purified rabbit anti-Y167Hexim1 and anti-pY291Hexim1. As loading control antimyc and anti-Hexim1 were detected. D, Jak2-mediated tyrosine phosphorylation of Hexim1 was inhibited by the Jak2 inhibitor Z3 (30μM). Tyrosine phosphorylation of Hexim1 was detected using anti-pY167Hexim1 and pY291Hexim1 antibodies. GAPDH was used as a loading control.
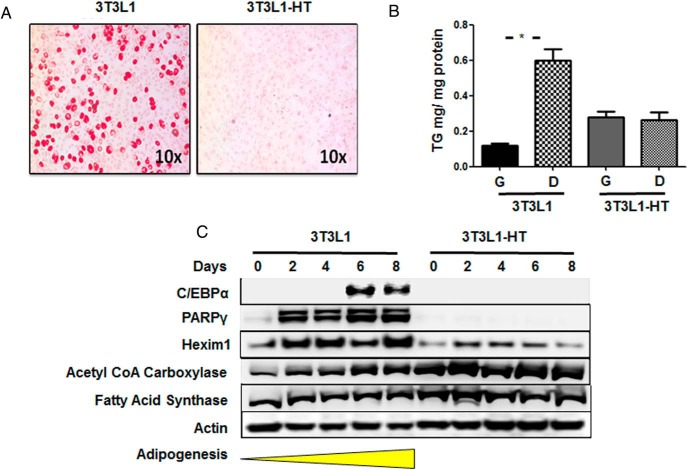
Hexim1 expression regulates adipogenesis
To investigate the role of Hexim1 in adipogenesis, we used 3T3L1 cells, a cell line that known to differentiate into adipocytes (22). To lower Hexim1 levels in 3T3LI cells, we used gene recombination using a vector carrying a Neo-Hexim1 cassette (10). 3T3L1 and 3T3L1-HT were induced to differentiate and adipogenesis was evaluated by lipid accumulation (Figure 7A), triglyceride levels (Figure 7B), and the expression pattern of C/EBPα, PPARγ, as well as the de novo lipogenesis enzymes, acetyl CoA carboxylase and fatty acid synthase. 3T3L1-HT failed to undergo adipogenesis as evidenced by a complete absence of C/EBPα, PPARγ. However, de novo lipogenesis enzymes were detectable (Figure 7C).
Figure 7.
Hexim1 regulates adipogenesis. A, Red-O staining of 3T3L1 and 3T3L1-HT cell lines after 8 days of differentiation. B, Triglycerides levels in 3T3L1 and 3T3L1-HT cells in growth (G) and differentiation (D) medium. C, Western blot analysis showing the expression of C/EBPα and PPARγ in 3T3L1 cell lines only, whereas acetyl CoA carboxylase and fatty acid synthase are observed in both cell lines. Analysis of Hexim1 expression shows heterozycity in 3T3L1-HT. Actin is detected as loading control.
Discussion
This report describes the phenotypic and metabolic changes observed in HT mice subjected to HFD and identifies Hexim1 as an important modulator of leptin signaling activity in the hypothalamus. Our findings show that Hexim1 inhibits leptin-mediated pY705-Stat3 signaling and selectively modulates C/EBPα, PPARγ, and SOCS3 transcription in metabolically significant tissues such as the hypothalamus, skeletal muscle, and adipose tissue during HFD.
HT mice are resistant to obesity and diabetes
Lower weight gain observed in HT mice vs WT mice fed HFD might be due to higher endogenous plasma leptin levels in the HT background (Table 1). Our findings of increased levels of adiponectin in HT mice (Table 1) are consistent with the known synergism between leptin and adiponectin counteracting adiposity and insulin resistance (23). Although adipose tissue is the primary source of adiponectin, the increased expression of PPARγ observed in skeletal muscle likely contributes to enhanced expression and secretion of adiponectin and Glut4 expression (24).
Leptin treatment of fasted WT and HT mice triggered a mild pY705Stat3 phosphorylation in WT mice as compared with HT mice. Indeed, we observed a significant increase of pY705Stat3 phosphorylation following leptin treatment in HT mice likely due to increased plasma leptin levels during fasting state detected in HT mice. The enhanced posttranslational response triggered by leptin in the HT background provided the first evidence that Hexim1 inhibits tyrosine signaling mediated by leptin/Jak2 axis. Additionally, and besides the established inhibition of transcription elongation (25), Hexim1 expression is essential for inducible SOCS3 and C/EBPs (C/EBPα and C/EBPβ) protein expression. Gene expression analysis using Affymetrix in the HT background revealed decreased mRNA expression of SOCS3, C/EBPα, C/EBPβ, and C/EBPδ (data not shown). These observations are consistent with previous reports where decreased expression of C/EBPs were associated with lower inducible expression of SOCS3 (26, 27), and suggest a mechanism whereby Hexim1 regulation of C/EBP (α, β, and δ) expression, might determine the inducible expression of the SOCS3 promoter in the hypothalamus during metabolic stress.
Hexim1 has also been shown to inhibit NF-κB and glucocorticoid signaling (9). This suggests that haploinsufficiency of Hexim1 in mice might enhance the inflammatory response (28). Consistent with this, chow-fed mice had higher plasma leptin levels, which are known to increase proinflammatory plasma free fatty acids (29), that activate Toll like receptor 4 and induce IL-6 production and release (30).
HT mice had higher type I slow muscle fiber content and higher Glut4 level than WT mice. This phenotype is consistent with other reports where higher type I slow muscle fiber (31, 32) and is also characterized by increased adiponectin levels and insulin sensitivity (33, 34). We also observed increased PPARγ expression in HT mice that increases expression and secretion of adiponectin (24). Indeed, skeletal muscle is a key target of adiponectin which modulates insulin activity (Glut 4 translocation), and glucose and fatty acid metabolism (35). Our findings also agree with previous reports showing that muscle-specific deletion of PPARγ causes insulin resistance (36) and that, in the absence of C/EBPα, Glut4 is up-regulated (37). HT mice had significantly lower levels of C/EBPα and C/EBPβ, which increases insulin sensitivity (38).
Hexim1 inhibits the Leptin/Jak2 axis
We hypothesize that Hexim1 might be functionally and structurally linked to the leptin/Jak2/Stat3/SOCS3 axis, because we observed that Hexim1 contains conserved YXXL motifs that are important functional features of Jak2 kinase activity spanning the pseudo kinase and kinase domains (15). Our data suggests that a possible role for Hexim1 is to oppose the function of the YXXL-containing SH2B1 protein that enhances Jak2 kinase activity (39), and lack of SH2B1 promotes obesity and type 2 diabetes (40). Hexim1 inhibited the Leptin/Jak2/pY705Stat3 signaling pathways, and substitution mutation of the YXXL motif reversed the inhibitory effect. We are currently trying to determine the “cross talk” between Jak2, Hexim1, and SH2B1 in this process.
Whether Hexim1 inhibited leptin/Jak2 signaling through direct interaction with the Jak2 kinase is not yet clear. Contrary to Hexim1, Jak2 is commonly distributed in the cytoplasm (41). Although recent reports indicate that Jak2 also resides in the nucleus (42), our findings suggest a role in which Hexim1's mediated inhibition of Jak2 are pivotal during leptin signaling. Previous report revealed a significant cytoplasmic and nuclear distribution of Hexim1 suggesting a less-understood cytoplasmic role for Hexim1 (43).
Hexim1 expression regulates adipogenesis
Smaller adipocyte size with lower tissue triglyceride content in HFD HT mice may be due to enhanced fatty acid oxidation (44), known to be associated with increased plasma leptin levels and leptin-mediated lipolysis (29). Given the role of C/EBPα, C/EBPβ, and C/EBPδ during adipocyte development (7) and the decreased expression of C/EBPα, C/EBPβ, and C/EBPδ in the HT background, it was not unexpected to see a blockade of adipogenesis in 3T3L1 HT cells, as well as in primary mouse embryonic fibroblasts from HT and Hexim1 null mice (data not shown). Further studies are needed to establish the transcriptional mechanism(s) that mediates Hexim1-dependent expression of C/EBPs.
Our results reveal at least 3 transcriptional mechanisms by which Hexim1 modulates leptin function and glucose disposal. First, Hexim1 regulates the leptin/Jak2 axis via conserved YXXL motifs resulting in inhibition of tyrosine phosphorylation of Y705Stat3 that will likely trigger a reduced pY705Stat3 nuclear translocation and transcription of Stat3-dependent genes. Second, decreased Hexim1 expression should have triggered enhanced expression of SOCS3 in the HT background. However, our whole-genome analysis revealed a second transcriptional mechanism by which Hexim1 can control SOCS3 expression, namely through selective impairment of SOCS3 inducible expression. Third, by a yet unknown mechanism, Hexim1 regulates the expression of C/EBPs that are essential for promoting adipogenesis and inducing SOCS3 expression.
In conclusion, we have shown that Hexim1 regulates the expression of transcription factors known to modulate obesity and glucose disposal including SOCS3, Stat3, C/EBPs (C/EBPα and C/EBPβ) and the PPARγ. The finding that Hexim1 regulates the expression of such an array of transcription factors in the hypothalamus, skeletal muscle and adipocytes suggests that Hexim1 plays a central role in the maintenance of whole-body energy balance.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank Dr Jahangir Iqbal, PhD at State University of New York (SUNY) Downstate Medical School for analyzing plasma cholesterol and triglycerides, Dr Anthony Sclafani PhD at Brooklyn College New York for consultation and mouse eatometers, and Dr Michael Wagner PhD at SUNY Downstate Medical School for editing the manuscript.
This study was partially funded by National Institutes of Health Grant R15CA169984 (to M.D.-M.) and Faculty Development Grant at SUNY Downstate Medical School (to E.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- C/EBP
- CCAAT/enhancer-binding protein
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- Hexim1
- hexamethylene bis-acetamide inducible-1
- HFD
- high-fat diet
- HT
- Hexim1 heterozygous
- Jak2
- Janus kinase 2
- PPAR
- peroxisome proliferator-activated receptor
- SH2B1
- Src Homology 2B adaptor protein 1
- sMHC
- slow myosin heavy chain
- SOCS
- suppressor of cytokine signaling 3
- Stat3
- signal transduction and activator of transcription 3
- WT
- wild type.
References
- 1. Martí A, Berraondo B, Martínez JA. Leptin: physiological actions. J Physiol Biochem. 1999;55(1):43–49. [PubMed] [Google Scholar]
- 2. Villanueva EC, Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes. 2008;32(suppl 7):S8–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robertson SA, Leinninger GM, Myers MG., Jr Molecular and neural mediators of leptin action. Physiol Behav. 2008;94(5):637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjørbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10(7):734–738. [DOI] [PubMed] [Google Scholar]
- 5. Yang Z, Hulver M, McMillan RP, et al. Regulation of insulin and leptin signaling by muscle suppressor of cytokine signaling 3 (SOCS3). PLoS One. 7(10):e47493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006;17(9):365–371. [DOI] [PubMed] [Google Scholar]
- 7. Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12(11):722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo L, Li X, Tang QQ. Transcriptional regulation of adipocyte differentiation: a central role for CCAAT/enhancer-binding protein (C/EBP) β. J Biol Chem. 2015;290(2):755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou Q, Yik JH. The Yin and Yang of P-TEFb regulation: implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol Mol Biol Rev. 2006;70(3):646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang F, Wagner M, Siddiqui MA. Ablation of the CLP-1 gene leads to down-regulation of the HAND1 gene and abnormality of the left ventricle of the heart and fetal death. Mech Dev. 2004;121(6):559–572. [DOI] [PubMed] [Google Scholar]
- 11. Mascareno EJ, Belashov I, Siddiqui MA, Liu F, Dhar-Mascareno M. Hexim-1 modulates androgen receptor and the TGF-β signaling during the progression of prostate cancer. Prostate.72(9):1035–1044. [DOI] [PubMed] [Google Scholar]
- 12. Mascareno E, Galatioto J, Rozenberg I, et al. Cardiac lineage protein-1 (CLP-1) regulates cardiac remodeling via transcriptional modulation of diverse hypertrophic and fibrotic responses and angiotensin II-transforming growth factor β (TGF-β1) signaling axis. J Biol Chem. 287(16):13084–13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Espinoza-Derout J, Wagner M, Salciccioli L, et al. Positive transcription elongation factor b activity in compensatory myocardial hypertrophy is regulated by cardiac lineage protein-1. Circ Res. 2009;104(12):1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwak H, Lis JT. Control of transcriptional elongation. Annu Rev Genet. 2013;47:483–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maures TJ, Kurzer JH, Carter-Su C. SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab. 2007;18(1):38–45. [DOI] [PubMed] [Google Scholar]
- 16. Galic S, Sachithanandan N, Kay TW, Steinberg GR. Suppressor of cytokine signalling (SOCS) proteins as guardians of inflammatory responses critical for regulating insulin sensitivity. Biochem J. 2014;461(2):177–188. [DOI] [PubMed] [Google Scholar]
- 17. Rosen ED, Hsu CH, Wang X, et al. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev. 2002;16(1):22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iqbal J, Parks JS, Hussain MM. Lipid absorption defects in intestine-specific microsomal triglyceride transfer protein and ATP-binding cassette transporter A1-deficient mice. J Biol Chem. 2013;288(42):30432–30444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belouzard S, Delcroix D, Rouillé Y. Low levels of expression of leptin receptor at the cell surface result from constitutive endocytosis and intracellular retention in the biosynthetic pathway. J Biol Chem. 2004;279(27):28499–28508. [DOI] [PubMed] [Google Scholar]
- 20. Monni R, Santos SC, Mauchauffe M, et al. The TEL-Jak2 oncoprotein induces Socs1 expression and altered cytokine response in Ba/F3 cells. Oncogene. 2001;20(7):849–858. [DOI] [PubMed] [Google Scholar]
- 21. Sayyah J, Magis A, Ostrov DA, Allan RW, Braylan RC, Sayeski PP. Z3, a novel Jak2 tyrosine kinase small-molecule inhibitor that suppresses Jak2-mediated pathologic cell growth. Mol Cancer Ther. 2008;7(8):2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kallen CB, Lazar MA. Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 1996;93(12):5793–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–946. [DOI] [PubMed] [Google Scholar]
- 24. Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91(1):258S–261S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dey A, Chao SH, Lane DP. HEXIM1 and the control of transcription elongation: from cancer and inflammation to AIDS and cardiac hypertrophy. Cell Cycle. 2007;6(15):1856–1863. [DOI] [PubMed] [Google Scholar]
- 26. Yarwood SJ, Borland G, Sands WA, Palmer TM. Identification of CCAAT/enhancer-binding proteins as exchange protein activated by cAMP-activated transcription factors that mediate the induction of the SOCS-3 gene. J Biol Chem. 2008;283(11):6843–6853. [DOI] [PubMed] [Google Scholar]
- 27. Cui TX, Lin G, LaPensee CR, et al. C/EBPβ mediates growth hormone-regulated expression of multiple target genes. Mol Endocrinol. 25(4):681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Z, Gan L, Zhou Z, Jin W, Sun C. SOCS3 promotes inflammation and apoptosis via inhibiting JAK2/STAT3 signaling pathway in 3T3-L1 adipocyte. Immunobiology. 2015;220(8):947–953. [DOI] [PubMed] [Google Scholar]
- 29. Shen J, Tanida M, Niijima A, Nagai K. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci Lett. 2007;416(2):193–197. [DOI] [PubMed] [Google Scholar]
- 30. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song XM, Ryder JW, Kawano Y, Chibalin AV, Krook A, Zierath JR. Muscle fiber type specificity in insulin signal transduction. Am J Physiol. 1999;277(6 pt 2):R1690–R1696. [DOI] [PubMed] [Google Scholar]
- 32. Lillioja S, Young AA, Culter CL, et al. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987;80(2):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaster M, Staehr P, Beck-Nielsen H, Schrøder HD, Handberg A. GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes. 2001;50(6):1324–1329. [DOI] [PubMed] [Google Scholar]
- 34. Armoni M, Harel C, Karnieli E. Transcriptional regulation of the GLUT4 gene: from PPAR-γ and FOXO1 to FFA and inflammation. Trends Endocrinol Metab. 2007;18(3):100–107. [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Sweeney G. Adiponectin action in skeletal muscle. Best Pract Res Clin Endocrinol Metab. 2014;28(1):33–41. [DOI] [PubMed] [Google Scholar]
- 36. Hevener AL, He W, Barak Y, et al. Muscle-specific Pparg deletion causes insulin resistance. Nat Med. 2003;9(12):1491–1497. [DOI] [PubMed] [Google Scholar]
- 37. Wu Z, Xie Y, Morrison RF, Bucher NL, Farmer SR. PPARγ induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPα during the conversion of 3T3 fibroblasts into adipocytes. J Clin Invest. 1998;101(1):22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang L, Shao J, Muhlenkamp P, et al. Increased insulin receptor substrate-1 and enhanced skeletal muscle insulin sensitivity in mice lacking CCAAT/enhancer-binding protein β. J Biol Chem. 2000;275(19):14173–14181. [DOI] [PubMed] [Google Scholar]
- 39. Rui L, Carter-Su C. Identification of SH2-bβ as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc Natl Acad Sci USA. 1999;96(13):7172–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Song W, Ren D, Li W, et al. SH2B regulation of growth, metabolism, and longevity in both insects and mammals. Cell Metab. 2010;11(5):427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Valentino L, Pierre J. JAK/STAT signal transduction: regulators and implication in hematological malignancies. Biochem Pharmacol. 2006;71(6):713–721. [DOI] [PubMed] [Google Scholar]
- 42. Zouein FA, Duhe RJ, Booz GW. JAKs go nuclear: emerging role of nuclear JAK1 and JAK2 in gene expression and cell growth. Growth Factors. 29(6):245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ouchida R, Kusuhara M, Shimizu N, et al. Suppression of NF-κB-dependent gene expression by a hexamethylene bisacetamide-inducible protein HEXIM1 in human vascular smooth muscle cells. Genes Cells. 2003;8(2):95–107. [DOI] [PubMed] [Google Scholar]
- 44. Yu YH, Ginsberg HN. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res. 2005;96(10):1042–1052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.