Abstract
The steroid hormones 17β-estradiol and progesterone are critical regulators of endometrial stromal cell differentiation, known as decidualization, which is a prerequisite for successful establishment of pregnancy. The present study using primary human endometrial stromal cells (HESCs) addressed the role of estrogen receptor-α (ESR1) in decidualization. Knockdown of ESR1 transcripts by RNA interference led to a marked reduction in decidualization of HESCs. Gene expression profiling at an early stage of decidualization indicated that ESR1 negatively regulates several cell cycle regulatory factors, thereby suppressing the proliferation of HESCs as these cells enter the differentiation program. ESR1 also controls the expression of WNT4, FOXO1, and progesterone receptor (PGR), well-known mediators of decidualization. Whereas ESR1 knockdown strongly inhibited the expression of FOXO1 and WNT4 transcripts within 24 hours of the initiation of decidualization, PGR expression remained unaffected at this early time point. Our study also revealed a major role of cAMP signaling in influencing the function of ESR1 during decidualization. Using a proteomic approach, we discovered that the cAMP-dependent protein kinase A (PKA) phosphorylates Mediator 1 (MED1), a subunit of the mediator coactivator complex, during HESC differentiation. Using immunoprecipitation, we demonstrated that PKA-phosphorylated MED1 interacts with ESR1. The PKA-dependent phosphorylation of MED1 was also correlated with its enhanced recruitment to estrogen-responsive elements in the WNT4 gene. Knockdown of MED1 transcripts impaired the expression of ESR1-induced WNT4 and FOXO1 transcripts and blocked decidualization. Based on these findings, we conclude that modulation of ESR1-MED1 interactions by cAMP signaling plays a critical role in human decidualization.
In women and other mammals, the ovarian steroid hormones, 17β-estradiol (E) and progesterone (P), orchestrate waves of cell proliferation, differentiation and remodeling in the endometrium to prepare it for embryo implantation and establishment of pregnancy (1–3). Decidualization is a steroid hormone-regulated differentiation process during which fibroblastic endometrial stromal cells are transformed into decidual cells that exhibit a morphologically distinct, secretory phenotype and acquire the ability to support embryonic growth and development during implantation and early phases of placentation (1–3). Impaired decidualization is associated with reproductive dysfunction, such as infertility, recurrent pregnancy loss, and endometriosis (4, 5). A better understanding of the mechanisms by which the steroid hormones regulate the decidualization process might help address the deficiencies underlying these endometrial disorders.
E functions through its intracellular receptors, estrogen receptor-α (ESR1) and estrogen receptor-β, whereas P acts through the progesterone receptor (PGR). In the uterus, ESR1 and PGR exhibit a dynamic pattern of expression in both epithelial and stromal compartments during the reproductive cycle and pregnancy (6–9). Studies using mice lacking the Pgr gene have established unequivocally that PGR plays a critical role in regulating the differentiation of stromal cells into decidual cells (10, 11). Similar studies using a global Esr1-null mouse model, (12–14), showed that the uteri of these mice are unable to support embryo implantation but are able to mount a partial decidual response when subjected to an experimentally induced decidual stimulation, leading investigators to conclude that ESR1 is essential for embryo attachment to uterine epithelium but dispensable for stromal differentiation. However, later reports indicated that these mutant mice still expressed a truncated ESR1, which retained DNA binding, hormone binding, and partial transcription regulatory functions, raising the possibility that this truncated receptor might have mediated the decidual response observed in the mutant mice (15, 16). These reports called for a reassessment of the role of ESR1 in the regulation of decidualization.
To address this issue, our laboratory recently developed a conditional knockout mouse model in which the Esr1 gene was deleted from both epithelial and stromal cells of the uterus (17). These mutant mice failed to execute experimentally induced decidualization, revealing, for the first time to our knowledge, a critical role of ESR1 in this differentiation process. After this observation, we developed another mouse model in which Esr1 was conditionally ablated in the uterine luminal and glandular epithelia but was retained in the stroma (17). Interestingly, this epithelium-specific deletion of Esr1 also severely compromised the decidual response, indicating that epithelial ESR1 contributes to endometrial stromal differentiation. Our study revealed that epithelial ESR1 directs an intricate network of paracrine signals that mediate communications between different endometrial cells. First, glandular ESR1 controls the production of the leukemia inhibitory factor (LIF) in uterine glands. LIF signaling via its receptors present on the luminal epithelium produces Indian hedgehog (IHH), which acts in a paracrine manner on Patch1 receptors in stromal cells to induce their differentiation. Although these findings brought to light the mechanisms via which epithelial ESR1 controls decidualization, the role, if any, of stromal ESR1 during this process remained unclear.
Currently no mouse model exists to test the stroma-specific role of ESR1 in the endometrium. To address the functional role of stromal ESR1 during decidualization, we used a well-established in vitro system in which primary human endometrial stromal cells (HESCs) are induced to undergo decidualization in response to a hormonal cocktail containing E, P, and a cAMP analog. Importantly, based on biochemical and morphological criteria, these primary cultures retain the biological characteristics of endometrial stromal cells and, upon differentiation, exhibit the decidual phenotype that is remarkably similar to the one seen in vivo (18–20). By knocking down ESR1 transcripts with RNA interference (RNAi), we established that stromal ESR1 is essential for human decidualization and identified the downstream pathways that mediate its function. Furthermore, we identified Mediator 1 (MED1), a subunit of the mediator complex, as a coregulatory factor that plays a critical role by functionally linking ESR1 with cAMP signaling during decidualization.
Materials and Methods
HESC culture conditions
Our studies involving primary HESC cultures follow the regulations stated for the protection of human subjects participating in clinical research and are approved by the institutional review boards of Emory University, Wake Forest University (Winston-Salem, North Carolina), and the University of Illinois at Urbana-Champaign (UIUC). Endometrial samples from the early proliferative stage of the menstrual cycle were obtained by Pipelle biopsy at Emory University and Wake Forest Medical Centers from fertile, regularly cycling volunteers with no sign of uterine abnormality, providing written informed consent as described previously (21). Cells were cultured in DMEM/F-12 medium (Invitrogen) supplemented with 5% (vol/vol) fetal bovine serum (Hyclone), 50 μg/mL penicillin, and 50 μg/mL streptomycin (Invitrogen). For in vitro differentiation, the cells were treated with differentiation cocktail composed of 0.5 mM 8-bromoadenosine-cAMP (Sigma), 1 μM progesterone (Sigma), and 10 nM E (Sigma) in DMEM/F-12 medium (Invitrogen) supplemented with 2% (vol/vol) charcoal dextran-stripped fetal bovine serum. The medium/differentiation cocktail was refreshed every 48 hours.
Small interfering RNA (siRNA) transfection
HESCs were transfected with siRNA targeting ESR1 (Ambion), MED1 (Dharmacon), or nontargeting control (scrambled) siRNA (Ambion/Dharmacon), at 20 nM concentration, following the manufacturer's protocol (SilentFect; Bio-Rad Laboratories). Forty-eight hours after the transfection, the siRNA was removed, and the cells were washed with PBS and treated with differentiation cocktail for time periods as indicated in the figure legends.
Cell cycle synchronization by double-thymidine block
Double-thymidine block was used to synchronize HESCs during cell proliferation studies, with minor modifications (22). Briefly, when cells reached 60% confluency, they were incubated in 2 mM thymidine containing medium for 12–14 hours. To release the thymidine block; HESCs were washed with PBS and incubated in fresh medium for 12 hours. The siRNA was added to the cells at this stage, and the transfection was carried out for 24–36 hours concurrent with the second thymidine block. Thymidine and siRNA were removed and cells were washed with PBS followed by the addition of medium containing the differentiation cocktail. 5-Bromo-2′-deoxyuridine (BrdU) was added to the medium during the last 6–24 hours of differentiation. Cells were fixed in 4% formalin solution (Sigma) for 10 minutes, followed by PBS washes. The cells were permeabilized with 0.1% Triton X-100 in PBS for 15 minutes and incubated with 10% serum for 1 hour. Anti-BrdU antibody was used to stain cells that incorporated BrdU. 4′,6-diamidino-2-phenylindole (DAPI; 1 μg/mL) was used to stain DNA.
Microarray sample preparation and data analysis
HESCs were transfected with siRNA targeting ESR1 or nontargeting control siRNA as explained above. Total RNA was isolated by Trizol reagent (Invitrogen) and further purified using RNeasy columns (QIAGEN) following the manufacturer's instructions. RNA samples were processed at the UIUC Biotechnology Center. RNA integrity was verified using an Agilent 2100 Bioanalyzer. Each RNA sample was processed to generate labeled cRNA following established protocols for hybridization to Affymetrix Human Genome HG-U133 A2.0 arrays or GeneChip Human Gene 1.0 ST arrays. The resulting data files were analyzed by Affymetrix GeneChip Expression Console software using the Robust Multi-array Average (RMA) algorithm. Data were analyzed from three independent experiments. The microarray data have been deposited in National Center for Biotechnology Information Gene Expression Omnibus (GEO) and can be accessed through GEO accession number GSE73550. Genes with a minimum of 1.2-fold-change in the same direction in all three independent microarrays were considered to be differentially regulated. Canonical pathways analysis identified the most significantly enriched pathways from the Ingenuity Pathways Analysis library of canonical pathways (Ingenuity Systems).
RNA isolation and real-time RT-PCR analysis
Total RNA was extracted from cultured HESCs using Trizol and converted to cDNA using the Stratagene cDNA reverse transcription kit, following the manufacturer's instructions. cDNA samples were subjected to real-time quantitative PCR using gene-specific primers. 36B4 was used as the reference gene. For gene expression analysis after the siRNA mediated knockdown of MED1, Calnexin was used as the reference gene. Fold changes in gene expression were calculated using the delta delta cycle threshold method (23). The real-time quantitative PCR results are expressed as mean ± SEM of independent experiments. Statistical significance was determined using the Student's t test, and a value of P < .05 was considered significant.
Adenovirus transduction
In some experiments HESCs were transduced with the adenovirus expressing FLAG-tagged ESR1 for 24 hours before the hormone treatments. The FLAG-tagged, ESR1-expressing adenovirus was kindly provided by the laboratory of Professor Benita Katzenellenbogen at UIUC.
Western blot analysis
Primary HESCs were subjected to different treatments for various periods of time as indicated in the figure legends. Whole-cell extracts were prepared and equal amounts of proteins were analyzed by SDS-PAGE and transferred to PVDF membrane, and specific proteins were detected by Western blotting using antibodies against calnexin (Santa Cruz Biotechnology), ESR1 (Novocastra), MED1 (A300-793A; Bethyl Laboratories; sc8998, Santa Cruz Biotechnology), and the phosphorylated protein kinase A (PKA) substrate motif RRXS*/T* (100G7E; Cell Signaling; number 9624). The antibody against the phospho-PKA substrate recognizes proteins containing the RRXS*/T* motif when it is phosphorylated at serine or threonine residue with arginine at the −3 and −2 positions. This antibody does not recognize the nonphosphorylated RRXS*/T* motif (24).
Immunoprecipitation and silver staining
HESCs were subjected to decidualization in vitro using differentiation cocktail and harvested at different times as indicated in the figure legends. Whole-cell extracts were prepared and equal amounts of proteins were used for immunoprecipitation (IP). IP was performed using Direct IP kit (Thermo Scientific) following the manufacturer's instructions. Immune complexes were eluted and separated by SDS-PAGE. The gel was silver stained using the Silver Quest staining kit (Invitrogen), and specific bands cut from the gel were submitted to the UIUC Proteomics Center for liquid chromatography and mass spectrometry analysis for protein identification.
Chromatin immunoprecipitation (ChIP)
HESCs were seeded on 150-mm dishes. After the cells were attached, the medium was switched to 2% (vol/vol) charcoal dextran-stripped fetal bovine serum containing medium and transduced with adenovirus carrying FLAG-tagged ESR1 for 24 hours. The following day, cells were treated with either E+P or E+P + 8-bromoadenosine-cAMP for 30 minutes. ChIP assays were performed using the EZ-ChIP kit (Millipore) according to the manufacturer's instructions with minor modifications. Anti-FLAG M2 affinity gel (Sigma; A2220) and anti-MED1 antibody (Santa Cruz Biotechnology; sc-8998) were used overnight at 4°C to immunoprecipitate FLAG-ESR1 and MED1, respectively. Normal mouse IgG (Santa Cruz Biotechnology; sc-2027) immunoprecipitation served as a negative control. For ChIP, PCR primers were designed to amplify ESR1 binding elements (ERE) in the regulatory regions of the WNT4 gene and a primer pair flanking an ERE-free sequence was used as a negative control. Primer sequences are available upon request. The resulting signals were normalized to input DNA and relative fold enrichment was calculated as the enrichment in experimental samples over that of negative control ChIP.
Results
ESR1 promotes HESC differentiation
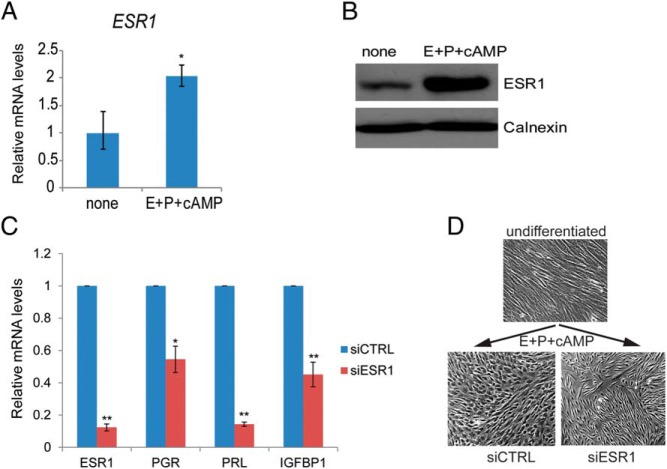
In vivo studies have shown that ESR1 is expressed in HESCs during the proliferative and secretory phases of the menstrual cycle (6–9). When we monitored ESR1 expression in primary HESC cultures, we detected both mRNA and protein in proliferating HESC (Figure 1, A and B, none), corresponding to approximately 28 400 [3H]E binding sites/cell as determined by Scatchard analysis (25). Addition of the differentiation cocktail to these cells led to a marked increase in ESR1 mRNA and protein levels within 24 hours of treatment (Figure 1, A and B, E+P+cAMP). To test the functional role of ESR1 in HESC differentiation, its expression was suppressed by administering siRNA targeting the ESR1 transcripts. Compared with HESCs treated with control siRNA, interference of ESR1 transcripts led to significantly reduced expression of transcripts corresponding to PGR and the well-known decidualization markers, PRL and IGFBP1, when measured 72 hours after the addition of the decidualization cocktail (Figure 1C). Furthermore, in the absence of ESR1 transcripts, we failed to observe the fibroblastic to epitheloid morphological change that is typically associated with decidualization (Figure 1D). Collectively these results suggested that ESR1 plays a key regulatory role during HESC differentiation.
Figure 1.
A, HESCs were either untreated or treated with differentiation cocktail. Cells were harvested 24 hours after treatment. Total RNA was isolated; cDNAs were prepared and subjected to real-time PCR using gene-specific primers for 36B4 and ESR1. PCR data are normalized to 36B4 mRNA levels. Relative fold changes were normalized with respect to the untreated sample. Graphs represent means ± SEM of three experiments. The Student's t test was used for statistical analysis. *, P ≤ .05. B, HESCs were either untreated or treated with a differentiation cocktail as indicated on the figure. Cells were lysed at 24 hours after treatment, analyzed by SDS-PAGE, and subjected to Western blotting using antibodies directed against ESR1 and calnexin. Calnexin immunoblotting serves as a loading control. The figure is the representative of three independent experiments. C, HESCs were transfected with siRNA targeting ESR1 or scrambled siRNA (control). Forty-eight hours after transfection, HESCs were treated with a differentiation cocktail to initiate in vitro differentiation. Cells were harvested at 72 hours after a differentiation cocktail treatment. Total RNA was isolated and cDNAs were prepared. cDNAs were subjected to real-time PCR using gene-specific primers for 36B4, ESR1, PGR, PRL, and IGFBP1. PCR data are normalized to 36B4 mRNA levels. Relative fold changes were normalized with respect to control nontargeting siRNA treatment. Graphs represent means ± SEM from three independent experiments. The Student's t test was used for statistical analysis. **, P ≤ .01; *, P ≤ .05. D, HESCs were transfected with siRNA targeting ESR1 or control siRNA (siCTRL). Forty-eight hours after transfection, cells were incubated with a differentiation cocktail for 72 hours. Cells were visualized under a bright-field microscope to analyze decidual morphology.
ESR1 controls cell cycle progression during decidualization
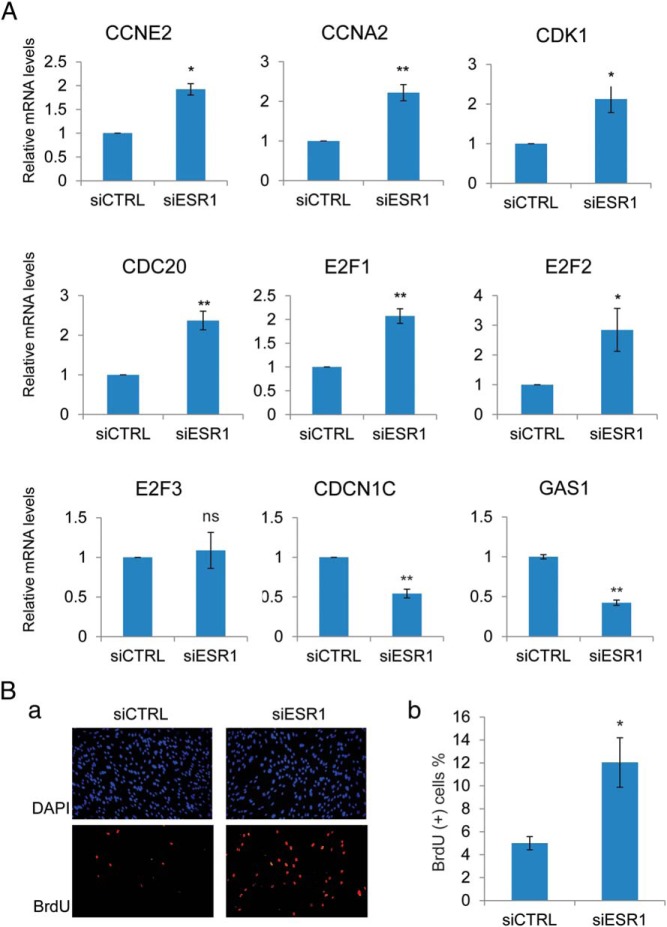
To identify the downstream pathways regulated by ESR1 during HESC differentiation, we compared the gene expression profiles of HESCs transfected with siRNA targeting ESR1 transcripts or control (scrambled) siRNA and differentiated for 24 hours. Suppression of ESR1 expression led to the up-regulation (>1.2-fold) of 194 genes and the down-regulation (<0.8-fold) of 318 genes (GEO accession number GSE73550). Genes with altered regulation were classified according to their known biological functions using Ingenuity Pathway Analysis (Supplemental Tables 1–3). Prominent among these genes were multiple pathways involved in cell cycle regulation (Supplemental Table 4). Well-known cell cycle regulatory factors, such as Cyclin (CCN)-E2, CCNA2, cell division cycle, CDC20, cyclin-dependent kinase (CDK)-1, E2F transcription factor 1 (E2F1), and E2F transcription factor 2 (E2F2), which are primarily involved in G1-to-S transition of the cell cycle, were paradoxically elevated in ESR1-depleted HESCs. Additionally, the expression of Cyclin-dependent kinase inhibitor 1C (CDKN1C) and Growth arrest-specific 1 (GAS1) transcripts, which are involved in cell cycle suppression, was repressed (Figure 2A). These results indicated that ESR1 normally inhibits cell cycle progression during HESC differentiation. Loss of ESR1 expression upon siRNA administration releases this inhibition, leading to increased HESC proliferation. We tested this hypothesis by silencing ESR1 expression in HESCs synchronized at the G1-S boundary, using the double-thymidine block method (22). Our results showed a significant increase in BrdU incorporation in HESCs depleted of ESR1 compared with the cells containing normal ESR1 concentrations (Figure 2B). These results suggested that ESR1 inhibits cell proliferation during HESC differentiation by suppressing the G1-to-S transition.
Figure 2.
A, HESCs were transfected with siRNA targeting ESR1 or scrambled siRNA (control [siCTRL]). Forty-eight hours after transfection, HESCs were treated with a differentiation cocktail to initiate in vitro differentiation. Cells were harvested at 24 hours after a differentiation cocktail treatment. Total RNA was isolated and cDNAs were prepared. cDNAs were subjected to real-time PCR using gene-specific primers for 36B4, CCNE2, CCNA2, CDK1, CDC20, E2F1, E2F2, E2F3, CDKN1C, and GAS1. PCR data are normalized to 36B4 mRNA levels. Relative fold changes were normalized with respect to control nontargeting siRNA treatment. Mean ± SEM of at least three independent experiments are shown. The Student's t test was used for statistical analysis. **, P ≤ .01; *, P ≤ .05. E, HESCs were synchronized at the G1/S stage by double-thymidine block and transfected with siRNA targeting ESR1 or scrambled siRNA (control). Twenty-four hours after transfection, HESCs were treated with differentiation cocktail to initiate in vitro differentiation. At 18 hours after a differentiation cocktail treatment, BrdU was added to the medium (100 μM). At 6 hours after BrdU addition (24 h of differentiation), cells were fixed and subjected to immunofluorescence to detect BrdU-positive cells, which are indicated in red. 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei are shown in blue. a, Images are the representatives of three independent experiments. b, The graph shows the mean values of percentages of BrdU-positive cells calculated from five fields in three different experiments. The Student's t test was used for statistical analysis. *, P ≤ .05.
cAMP signaling is necessary for ESR1-dependent gene expression during decidualization
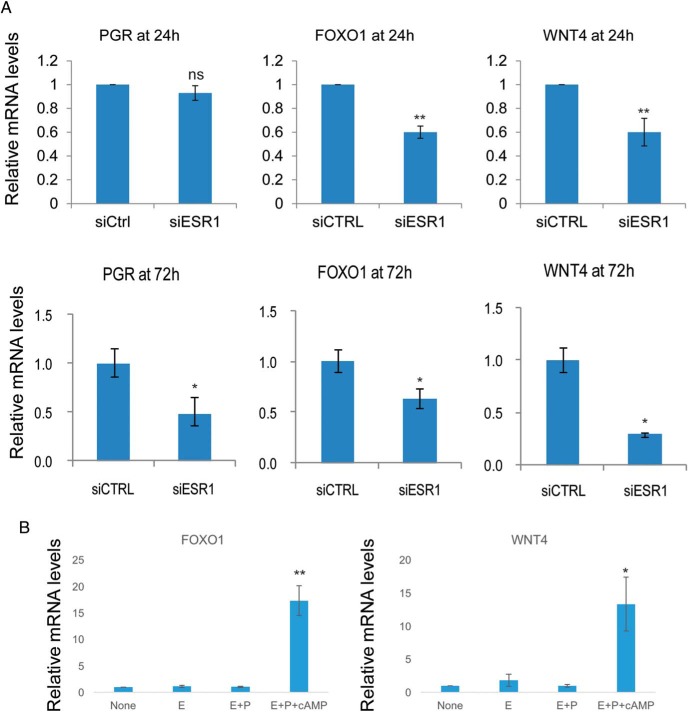
Prominent among the many genes whose expression was down-regulated in HESCs after the attenuation of ESR1 expression were two important regulators of HESC differentiation: Forkhead box protein O1 (FOXO1) and wingless-type mouse mammary tumor virus integration site family member 4 (WNT4). Previous reports indicated that these genes play critical roles in HESC differentiation (21, 26–28). To validate the results of the microarray analysis, we used real-time RT-PCR. Our results indicated that expression of FOXO1 and WNT4 is down-regulated in ESR1-depleted HESC at 24 hours during the differentiation process, whereas that of PGR remained unaffected at this early time point (Figure 3A, upper panels). However, the knockdown of ESR1 led to the down-regulation of FOXO1 and WNT4 as well as PGR at 72 hours (Figure 3A, lower panels).
Figure 3.
A, HESCs were transfected with siRNA targeting ESR1 or scrambled siRNA (control [siCTRL]). Forty-eight hours after transfection, HESCs were treated with a differentiation cocktail to initiate in vitro differentiation. Cells were harvested at 24 and 72 hours after the differentiation cocktail treatment. Total RNA was isolated and cDNAs were prepared. cDNAs were subjected to real-time PCR using gene-specific primers for 36B4, PGR, FOXO1, and WNT4. PCR data are normalized to 36B4 mRNA levels. Relative fold changes were normalized with respect to control nontargeting siRNA treatment. Mean ± SEM of three independent experiments are shown. The Student's t test was used for statistical analysis. **, P ≤ .01; *, P ≤ .05; ns, nonsignificant. B, HESCs were hormone starved in 2% charcoal-stripped serum containing medium for 48 hours. Then cells were either untreated or treated with E alone, E+P, or E+P+cAMP for 24 hours. Total RNA was isolated and cDNAs were prepared. cDNAs were subjected to real-time PCR using gene-specific primers for 36B4, FOXO1, and WNT4. PCR data are normalized to 36B4 mRNA levels. The figure shows mean ± SEM of three independent experiments. A one-way ANOVA followed by a Tukey honest significant difference test was used for statistical analysis. **, P ≤ .01; *, P ≤ .05; ns, nonsignificant.
We next monitored whether the expression of FOXO1 and WNT4 downstream of ESR1 is regulated in response to E or P treatment. To our surprise, we did not detect any significant up-regulation of these genes when HESCs were treated with E alone or E+P. However, with the addition of cAMP along with E+P, we observed a robust increase in FOXO1 and WNT4 expression (Figure 3B). Interestingly, addition of cAMP alone also increased the expression of WNT4 and FOXO1 genes, although to a lesser extent than the induction observed when all three components (E+P+cAMP) of the differentiation cocktail were present (data not shown). Collectively these data suggested that cAMP signaling has a major impact on ESR1-dependent expression of genes involved in decidualization.
Coactivator MED1 is a target of phosphorylation by cAMP-dependent protein kinase during HESC differentiation
It is well established that cAMP binds to the regulatory subunits of PKA, resulting in the activation of the catalytic subunits of this enzyme (29). Active PKA phosphorylates regulatory factors that play important roles in transcriptional control. To identify the downstream targets of PKA-dependent phosphorylation, we used a monoclonal antibody that recognizes phosphorylated PKA substrates containing the consensus motif RRXS*/T* (where Ser and/or Thr residues are phosphorylated) mimicking substrates of PKA (24, 30).
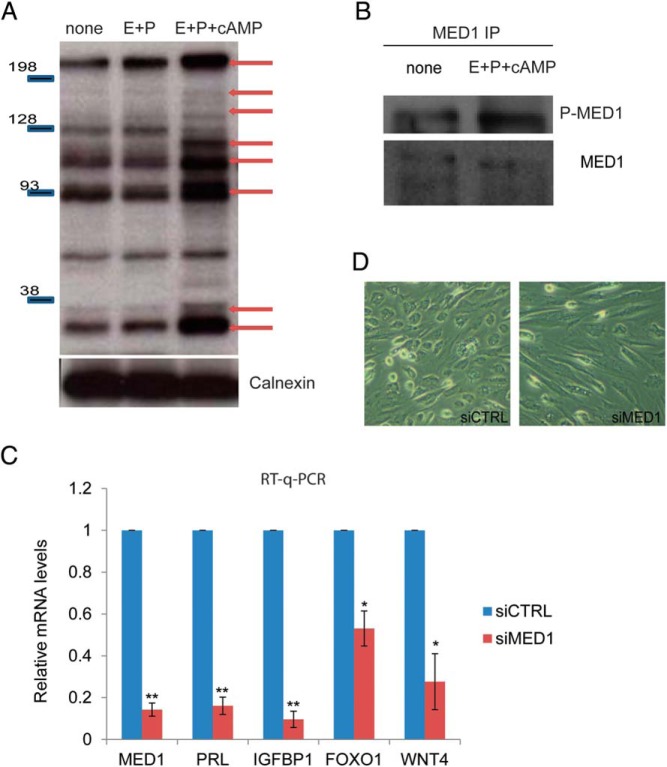
In this experiment, HESCs were treated with vehicle, E+P, or E+P+cAMP. To identify the proteins that are targets of phosphorylation by PKA, we performed Western blotting using the phospho-PKA substrate antibody. This antibody recognized several proteins in soluble extracts of vehicle-treated HESCs (Figure 4A, none). We did not detect any significant change in the pattern of the phosphorylated proteins in extracts of HESC treated with E+P (Figure 4A, E+P). However, several additional proteins were detected by this antibody in the extracts of HESCs treated with the complete cocktail containing E+P+cAMP (Figure 4A). These results indicated that cAMP-activated PKA phosphorylates multiple proteins as HESCs undergo differentiation in response to E+P+cAMP.
Figure 4.
A, HESCs were untreated or treated with E+P or E+P+cAMP. Cells were lysed at 3 hours after treatment, analyzed by SDS-PAGE, and subjected to Western blotting using phospho-PKA substrate antibody. Data are representative of six independent experiments. B, HESCs were either untreated or treated with differentiation cocktail, composed of E+P+cAMP for 24 hours. Whole-cell lysates from HESCs were immunoprecipitated with a MED1 antibody. The immunocomplexes were then probed by a Western blot using a MED1 antibody and phospho-PKA substrate antibody as indicated in the figure. The figure is the representative of two independent experiments. C, HESCs were transfected with siRNA targeting MED1 or nontargeting scrambled siRNA for 48 hours. After siRNA treatment, cells were treated with differentiation cocktail composed of E+P+cAMP for 72 hours. Real-time quantitative PCR (RT-q-PCR) was performed to analyze gene expression using specific primers for MED1, PRL, IGFBP1, FOXO1, and WNT4; values are normalized to control siRNA treatment. Figure shows mean ± SEM of three different experiments The Student's t test was used for statistical analysis. **, P ≤ .01; *, P ≤ .05. D, HESCs were transfected with siRNA targeting MED1 or control siRNA (control [siCTRL]). Forty-eight hours after transfection, HESCs were treated with a differentiation cocktail to initiate in vitro differentiation. At 72 hours of differentiation, cells were visualized under a bright-field microscope to monitor early changes in decidual morphology. Data are representative of four experiments.
To further analyze the proteins that are phosphorylated by PKA in differentiating HESCs, we prepared soluble extracts from cells treated with or without E+P+cAMP and performed immunoprecipitation using the phospho-PKA substrate antibody. The immunoprecipitated proteins were analyzed by SDS-PAGE and visualized by silver staining (data not shown). Consistent with the results shown in Figure 4A, we detected several polypeptides that were present in the immunoprecipitate obtained from the extracts prepared from HESCs treated with E+P+cAMP but were absent in the extracts obtained from cells treated with E and P only. In addition, we detected several protein bands whose intensity increased markedly in immunoprecipitates from the extracts of HESCs treated with E+P+cAMP compared with those obtained from extracts of HESCs treated with E+P. These polypeptide bands were excised from the gel and subjected to mass spectrometric analysis. Peptide sequences were obtained from several of these polypeptides, and their identities were established (Supplemental Table 5). Interestingly, we identified MED1, a subunit of the Mediator complex that functions as a coactivator by bridging the transcription factors and the general transcription machinery (31), as a potential target of phosphorylation by PKA.
To ascertain that MED1 is indeed phosphorylated by PKA in differentiating HESCs, we immunoprecipitated MED1 from extracts of HESC treated with or without E+P+cAMP and performed Western blotting with the phospho-PKA substrate antibody. Consistent with our mass spectrometric analysis, we observed a markedly increased PKA-dependent phosphorylation of MED1 in extracts of HESCs treated with E+P+cAMP (Figure 4B). This specific modification of MED1 during decidualization suggested that it is potentially involved in a regulatory role during this process.
MED1 is critical for HESC differentiation
To address the role of MED1 in HESC differentiation, we used RNAi. Knockdown of MED1 transcripts by siRNA did not affect HESC proliferation (data not shown) but drastically reduced the expression of transcripts encoding the decidualization markers prolactin (PRL) and IGF binding protein 1 (IGFBP1) (Figure 4C). We also observed that the lack of MED1 expression in HESCs prevented the characteristic morphological transformation of the fibroblastic stromal cells into epitheloid decidual cells (Figure 4D). Additionally, the down-regulation of MED1 inhibited the expression of FOXO1 and WNT4, which operate downstream of ESR1. These results indicated a critical role of MED1 in ESR1-dependent gene expression during HESC differentiation.
cAMP signaling promotes MED1 recruitment at genomic target sites of ESR1 during HESC differentiation
To address the mechanisms by which MED1 regulates ESR1-dependent gene transcription, we first analyzed whether MED1 interacts with ESR1 in differentiating HESC. As shown in Figure 5A, immunoprecipitation of MED1 from HESC extracts also pulled down ESR1. Similarly, immunoprecipitation of ESR1 from HESC extracts coprecipitated MED1 (Figure 5B), confirming strong interactions between ESR1 and MED1. Furthermore, we observed that cAMP signaling, which results in the phosphorylation of MED1, markedly enhanced the interaction between MED1 and ESR1 (Figure 5C).
Figure 5.
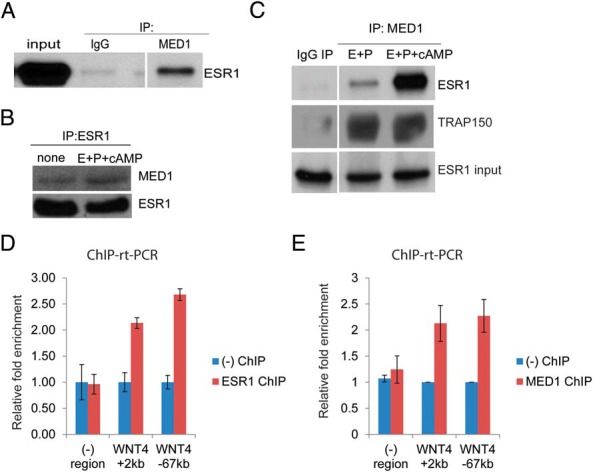
A, HESCs were transduced with recombinant adenovirus-expressing FLAG-ESR1. The next day, whole-cell protein lysates were immunoprecipitated with MED1antibody or IgG control. Immunoprecipitated protein complexes were probed with ESR1 antibody. B, HESCs were transduced with recombinant adenovirus-expressing FLAG-ESR1 for 24 hours. The next day, cells were treated with E+P or E+P+cAMP for 3 hours. Whole-cell lysates were immunoprecipitated with anti-FLAG beads. Immunoprecipitated complexes were probed using antibodies against MED1 and ESR1. The experiment was repeated three times and representative data are shown. C, HESCs were transduced with recombinant adenovirus harboring FLAG-tagged ESR1 for 24 hours. Samples were treated with E+P+cAMP for 3 hours. Whole-cell lysates were immunoprecipitated with a MED1 antibody. The immunoprecipitated complexes were probed by Western blot using antibodies against ESR1 and thyroid hormone receptor associated protein 150 (TRAP150) as indicated in the figure. The TRAP150 polypeptide, an integral component of the mediator complex, serves as a control for immunoprecipitation. The figure is the representative of two independent experiments. D, HESCs were transduced with recombinant adenovirus harboring FLAG-tagged ESR1 for 24 hours. Samples were treated with E+P+cAMP for 1 hour. ChIP was performed as described in Material and Methods. Chromatin enrichment was quantified by real-time PCR (ChIP-rt-PCR) using primers flanking potential ESR1 binding sites upstream of (−67 kb) and within (+2 kb) the WNT4 gene. The experiment was repeated twice and representative data are shown. E, ChIP was performed under similar conditions to immunoprecipitate MED1 bound chromatin as explained in Material and Methods. Primers flanking potential ESR1 bound regulatory regions indicated as WNT4 +2kb and WNT4 −67kb, were used to quantify the chromatin enrichment. The experiment was repeated twice and representative data are shown.
These results predicted that MED1 is a critical coregulatory factor recruited by ESR1 to EREs of target genes in the genome. To test this possibility, we performed ChIP experiments using ESR1 and MED1 antibodies to verify the recruitment of these factors at potential ESR1 binding sites previously identified in the regulatory regions of WNT4 (32). As shown in Figure 5, D and E, we detected recruitment of ESR1 and MED1 to these sites when HESCs were treated with a differentiation cocktail that induces PKA-dependent phosphorylation of the coactivator. Depletion of ESR1 drastically reduced the recruitment of MED1 to the WNT4 ERE (Supplemental Figure 1). Collectively our results are consistent with the hypothesis that MED1 is a key mediator of cAMP regulation during HESC differentiation. This coactivator, phosphorylated by PKA, is recruited by ESR1 occupying genomic binding sites and mediates the expression of important downstream targets of ESR1, such as WNT4, during decidualization.
Discussion
Recent studies in mice have revealed that molecular communication between the epithelial and stromal compartments is critical for acquisition of endometrial receptivity and functional interactions with the embryo (33). The ESR1 protein exhibits a dynamic pattern of expression in both epithelial and stromal compartments of the uterus during the reproductive cycle and pregnancy (6–9). We have recently shown that epithelial ESR1 controls stromal differentiation via a paracrine mechanism in the mouse (17). The preovulatory surge of E promotes production of LIF by ESR1 in the glandular epithelium during days 1 and 2 of pregnancy (34). The secreted LIF acts on the luminal epithelium to activate ERK1/2, which induces the expression of IHH on day 3 (17). IHH then acts on the stromal cells via the patched 1 receptor to activate the chicken ovalbumin upstream promoter transcription factor II pathway and promote uterine receptivity and stromal differentiation (17). In rodents, it is well documented that a transient rise of E occurs on day 4, immediately prior to implantation. It acts via epithelial ESR1 to induce a second phase of LIF production in the glandular epithelium (35). This latter rise in LIF leads to an activation of the Janus kinase-signal transducer and activator of transcription 3 pathway, which facilitates epithelial remodeling and allows embryo attachment during implantation (36).
Although these findings provided an insight into the paracrine mechanisms by which endometrial epithelial ESR1 functions during early pregnancy, the functional role of endometrial stromal ESR1 remained unclear. There is evidence that, during the reproductive cycle, stromal ESR1 regulates epithelial growth via mechanisms involving the production of paracrine growth factors, such as IGFs and FGFs (37–39). However, as P levels rise in the preimplantation phase, epithelial growth ceases and decidualization ensues. ESR1 expression in stromal cells remains strong during decidualization, indicating that stromal ESR1 is likely to play a role in this differentiation process. The present study, using primary cultures of HESCs and a loss-of-function approach using RNAi, establishes that ESR1 indeed controls stromal proliferation as well as differentiation during decidualization.
In human endometrium, ESR1 is expressed in both proliferative and secretory phases of the menstrual cycle. The expression of ESR1 is prominent in glandular epithelial and stromal cells of the basalis and functionalis regions during the proliferative, periovulatory (d 14 and 15), and early secretory phases of the cycle (40, 41). ESR1 is relatively less abundant in functionalis epithelium and stroma during the mid- and late secretory phases. Interestingly, stromal cells adjacent to the secretory glands in the basalis retain a strong expression of ESR1 in the midsecretory phase, coinciding with the onset of predecidualization (42). As human endometrium transitions from the proliferative to secretory phase, a sharp drop in the mitotic activity in both epithelial and stromal compartments indicates a cell cycle blockade. It is well documented that several cell cycle-regulatory factors are differentially expressed in secretory phase endometrium when compared with the proliferative phase. Specifically, transcripts of CCNA2, CCNE2, CDC20, and E2F1, which are all involved in cell cycle progression, are reported to be decreased significantly in the secretory phase (43, 44). Conversely, the transcripts of CDKN1C and GAS1, which are involved in inhibition of the cell cycle, are increased. Strikingly, our gene expression profiling analysis indicated that the down-regulation of ESR1 expression led to the up-regulation of CCNA2, CCNE2, CDC20, and E2F1, and the down-regulation of CDKN1C and GAS1 (Supplemental Table 4 and Figure 2A). These results indicated that ESR1 suppresses the cell cycle as the endometrium undergoes differentiation during decidualization. In agreement with this prediction, we demonstrated that HESC proliferation, as measured by BrdU incorporation, was indeed elevated upon siRNA-mediated depletion of ESR1 transcripts (Figure 2B). Collectively our results are consistent with the hypothesis that ESR1 controls the cell cycle during the transition from the proliferative to secretory endometrium.
Our study also supports a central role for ESR1 in regulating HESC differentiation. The microarray results revealed that WNT4 and FOXO1, known regulators of HESC differentiation, operate downstream of ESR1 (Figure 3A). FOXO1 is involved in cell cycle inhibition, defense against oxidative stress, DNA repair, and apoptosis (45, 46). We have previously shown that the loss of WNT4 expression prevents HESC differentiation (28). Depletion of ESR1 transcripts in HESCs led to a marked down-regulation of FOXO1 and WNT4 transcripts within 24 hours of the decidualization program. It is important to note that during this early phase, we did not see any alteration in PGR transcripts. It is therefore possible that this early regulation of FOXO1 and WNT4 by ESR1 occurs independently of PGR. However, we cannot rule out the possibility that the PGR that already exists in the stromal cells may cooperate with ESR1 to regulate the expression of these genes. Interestingly, we did observe a marked decline in PGR expression in HESCs lacking ESR1 by 72 hours in the decidualization program. These results indicated that the effects of ESR1 during the later phases of decidualization could be mediated via the downstream regulation of PGR.
According to a recent genome-wide ESR1 binding study in the mouse uterus, Foxo1 and Wnt4 both harbor prototypic EREs (47). The Wnt4 ERE, located in the first intron, is functionally responsive to E treatment (47). These findings, combined with the results of Figures 3A and 5D, are consistent with the hypothesis that ESR1 may directly regulate FOXO1 and WNT4 during HESC proliferation and differentiation. Interestingly, the suppression of FOXO1 transcripts in HESCs under differentiation conditions was shown to promote proliferation (27). Several cell cycle-related genes, reported to be regulated by FOXO1, such as CDKN1C, BUB1, NEK2, and PLK4, were also found to be downstream of ESR1 in our gene expression analyses (27). It is conceivable that some of the inhibitory effects of ESR1 on the cell cycle progression of HESCs are mediated by FOXO1.
We observed an important role of cAMP signaling in modulating ESR1-dependent gene transcription during decidualization. Numerous previous studies have documented that cAMP-induced signaling pathways are important for decidualization (18, 48, 49). Intracellular cAMP levels were reported to be elevated in stromal cells during decidualization in vivo (50, 51). Under in vitro conditions, P alone or in combination with E is a weak inducer of HESC differentiation (52). Addition of a cAMP analog, such as dibutyryl-cAMP or 8-bromo-cAMP, augments the differentiation process by activating PKA (53). Although these cAMP analogs are widely used in HESCs in vitro decidualization protocols, the molecular targets of active PKA during the differentiation process are mostly unknown.
Our study revealed that the expression of WNT4 and FOXO1 downstream of ESR1 during HESC differentiation is dependent on the presence of cAMP (Figure 3B), suggesting an influence of PKA signaling on ESR1-dependent gene transcription. An unbiased approach, using an antibody that recognizes the phosphorylated PKA substrate motif, RRXS*/T*, identified several PKA-mediated phosphopeptides during HESC differentiation (Supplemental Table 5). Interestingly, neither ESR1 nor PGR was identified as a direct target of PKA. The PKA targets detected by mass spectrometry included the transcriptional coactivator MED1 and several cytoskeletal proteins including nonmuscle myosin, vimentin, and tropomyosin. Phosphorylation of cytoskeletal proteins by PKA is likely to be important for decidualization because this process involves major cytoskeletal remodeling and changes in cell architecture. To understand how PKA modulates steroid receptor-dependent transcription during decidualization, we focused on the role of MED1, which interacts with a variety of nuclear receptors to bridge their activity with the general transcription machinery (31, 54–57).
We observed that the depletion of MED1 severely impaired the decidualization of HESCs, confirming its requirement during the differentiation process. MED1 was previously reported to be a substrate for PKA phosphorylation (58). Our study showed that MED1 interacts with ESR1 in HESCs, and this interaction is markedly enhanced upon phosphorylation of MED1 by PKA. We further noted that MED1iss recruited to ESR1 binding sites in the regulatory regions of WNT4 gene in a ESR1-dependent manner(Figure 5E and Supplemental Figure 1). These results provide a plausible mechanism by which the phosphorylation of MED1 by PKA influences ESR1-dependent gene transcription during decidualization. Further mutagenesis analysis is required to firmly establish that phosphorylation of MED1 is obligatory for its enhanced binding to ESR1. It should be noted that MED1 serves as a cofactor of several other nuclear receptors, including retinoic acid receptors and peroxisomal proliferator-activated receptor, whose transcriptional activity might also be affected by the posttranslational modification of MED1 (59, 60). It is of interest that we failed to detect any interaction between PGR and phosphorylated MED1 in coimmunoprecipitation experiments using extracts of HESCs treated with E+P+cAMP (data not shown). Based on this finding and the existing literature, it is tempting for us to propose that PGR-dependent gene transcription during decidualization proceeds primarily via the participation of other coactivators, such as the steroid receptor coactivator family of proteins, rather than MED1. There is ample evidence that the steroid receptor coactivator family coactivators are posttranslationally modified by PKA (61). This modification might form the molecular basis of the cooperation between cAMP and PGR-dependent pathways during decidualization that has been reported by many groups (62, 63).
In summary, our study revealed that ESR1 plays two major roles during human decidualization. First, it suppresses proliferation of HESCs by modulating the expression of several cell cycle regulatory factors. Second, ESR1 promotes differentiation of HESCs by inducing the expression of important regulators of the decidualization process, namely FOXO1 and WNT4. Most importantly, we identified MED1 as a functional link between active PKA and ESR1-dependent gene transcription, providing a mechanism by which cAMP signaling acts in concert with ESR1 to drive downstream gene expression during the vital decidualization program.
Additional material
Supplementary data supplied by authors.
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through Cooperative Agreement U54 HD055787 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to R.N.T., I.C.B., and M.K.B.) and Grant R21 HD078983 (to M.K.B. and I.C.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- 5-bromo-2′-deoxyuridine
- CCN
- cyclin
- CDK
- cyclin-dependent kinase
- ChIP
- chromatin immunoprecipitation
- DAPI
- 4′,6-diamidino-2-phenylindole
- E
- 17β-estradiol
- ERE
- ESR1 binding element
- ESR1
- estrogen receptor-α
- FOXO1
- Forkhead box protein O1
- GEO
- Gene Expression Omnibus
- HESC
- human endometrial stromal cell
- IGFBP1
- IGF binding protein 1
- IHH
- Indian hedgehog
- IP
- immunoprecipitation
- LIF
- leukemia inhibitory factor
- MED1
- Mediator 1
- P
- progesterone
- PGR
- P receptor
- PKA
- protein kinase A
- PRL
- prolactin
- RNAi
- RNA interference
- siRNA
- small interfering RNA
- UIUC
- University of Illinois at Urbana-Champaign
- WNT4
- wingless-type mouse mammary tumor virus integration site family member 4.
References
- 1. Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185–199. [DOI] [PubMed] [Google Scholar]
- 2. Carson DD, Bagchi I, Dey SK, et al. Embryo implantation. Dev Biol. 2000;223(2):217–237. [DOI] [PubMed] [Google Scholar]
- 3. Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salker M, Teklenburg G, Molokhia M, et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5(4):e10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25(6):445–453. [DOI] [PubMed] [Google Scholar]
- 6. Snijders MP, de Goeij AF, Debets-Te Baerts MJ, Rousch MJ, Koudstaal J, Bosman FT. Immunocytochemical analysis of oestrogen receptors and progesterone receptors in the human uterus throughout the menstrual cycle and after the menopause. J Reprod Fertil. 1992;94(2):363–371. [DOI] [PubMed] [Google Scholar]
- 7. Perrot-Applanat M, Deng M, Fernandez H, Lelaidier C, Meduri G, Bouchard P. Immunohistochemical localization of estradiol and progesterone receptors in human uterus throughout pregnancy: expression in endometrial blood vessels. J Clin Endocrinol Metab. 1994;78(1):216–224. [DOI] [PubMed] [Google Scholar]
- 8. Mylonas I, Jeschke U, Shabani N, et al. Immunohistochemical analysis of estrogen receptor α, estrogen receptor β and progesterone receptor in normal human endometrium. Acta Histochem. 2004;106(3):245–252. [DOI] [PubMed] [Google Scholar]
- 9. Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140(11):5310–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lydon JP, DeMayo FJ, Funk CR, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. [DOI] [PubMed] [Google Scholar]
- 11. Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol. 2008;19(2):178–186. [DOI] [PubMed] [Google Scholar]
- 12. Curtis Hewitt S, Goulding EH, Eddy EM, Korach KS. Studies using the estrogen receptor α knockout uterus demonstrate that implantation but not decidualization-associated signaling is estrogen dependent. Biol Reprod. 2002;67(4):1268–1277. [DOI] [PubMed] [Google Scholar]
- 13. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90(23):11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paria BC, Huet-Hudson YM, Dey SK. Blastocyst's state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci USA. 1993;90(21):10159–10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. [DOI] [PubMed] [Google Scholar]
- 16. Hewitt SC, Kissling GE, Fieselman KE, Jayes FL, Gerrish KE, Korach KS. Biological and biochemical consequences of global deletion of exon 3 from the ER α gene. FASEB J. 2010;24(12):4660–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pawar S, Laws MJ, Bagchi IC, Bagchi MK. Uterine epithelial estrogen receptor-α controls decidualization via a paracrine mechanism. Mol Endocrinol. 2015;29(9):1362–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178(3):357–372. [DOI] [PubMed] [Google Scholar]
- 19. Klemmt PAB, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85(3):564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod. 2009;80(1):105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Q, Kannan A, Wang W, et al. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007;282(43):31725–31732. [DOI] [PubMed] [Google Scholar]
- 22. Bostock CJ, Prescott DM, Kirkpatrick JB. An evaluation of the double thymidine block for synchronizing mammalian cells at the G1-S border. Exp Cell Res. 1971;68(1):163–168. [DOI] [PubMed] [Google Scholar]
- 23. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 24. Soulard A, Cremonesi A, Moes S, Schütz F, Jenö P, Hall MN. The rapamycin-sensitive phosphoproteome reveals that TOR controls protein kinase A toward some but not all substrates. Mol Biol Cell. 2010;21(19):3475–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78(3):642–649. [DOI] [PubMed] [Google Scholar]
- 26. Franco HL, Dai D, Lee KY, et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011;25(4):1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takano M, Lu Z, Goto T, et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol. 2007;21(10):2334–2349. [DOI] [PubMed] [Google Scholar]
- 28. Li Q, Kannan A, Das A, et al. WNT4 acts downstream of BMP2 and functions via β-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154(1):446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway. differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci. 1997;2:d331–d342. [DOI] [PubMed] [Google Scholar]
- 30. Moir RD, Lee J, Haeusler RA, Desai N, Engelke DR, Willis IM. Protein kinase A regulates RNA polymerase III transcription through the nuclear localization of Maf1. Proc Natl Acad Sci USA. 2006;103(41):15044–15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malik S, Guermah M, Yuan CX, Wu W, Yamamura S, Roeder RG. Structural and functional organization of TRAP220, the TRAP/mediator subunit that is targeted by nuclear receptors. Mol Cell Biol. 2004;24(18):8244–8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carroll JS, Meyer CA, Song J, et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38(11):1289–1297. [DOI] [PubMed] [Google Scholar]
- 33. Pawar S, Hantak AM, Bagchi IC, Bagchi MK. Minireview: steroid-regulated paracrine mechanisms controlling implantation. Mol Endocrinol. 2014;28(9):1408–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shen MM, Leder P. Leukemia inhibitory factor is expressed by the preimplantation uterus and selectively blocks primitive ectoderm formation in vitro. Proc Natl Acad Sci USA. 1992;89(17):8240–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141(12):4365–4372. [DOI] [PubMed] [Google Scholar]
- 36. Pawar S, Starosvetsky E, Orvis GD, Behringer RR, Bagchi IC, Bagchi MK. STAT3 regulates uterine epithelial remodeling and epithelial-stromal crosstalk during implantation. Mol Endocrinol. 2013;27(12):1996–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu L, Pollard JW. Estradiol-17β regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci USA. 2007;104(40):15847–15851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Q, DeMayo FJ, Lydon JP, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331(6019):912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hewitt SC, Collins J, Grissom S, Deroo B, Korach KS. Global uterine genomics in vivo: microarray evaluation of the estrogen receptor α-growth factor cross talk mechanism. Mol Endocrinol. 2005;19(3):657–668. [DOI] [PubMed] [Google Scholar]
- 40. Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67(2):334–340. [DOI] [PubMed] [Google Scholar]
- 41. Lessey BA, Palomino WA, Apparao KB, Young SL, Lininger RA. Estrogen receptor-α (ER-α) and defects in uterine receptivity in women. Reprod Biol Endocrinol. 2006;4(suppl 1):S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsuzaki S, Fukaya T, Suzuki T, Murakami T, Sasano H, Yajima A. Oestrogen receptor alpha and beta mRNA expression in human endometrium throughout the menstrual cycle. Mol Hum Reprod. 1999;5(6):559–564. [DOI] [PubMed] [Google Scholar]
- 43. Talbi S, Hamilton AE, Vo KC, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097–1121. [DOI] [PubMed] [Google Scholar]
- 44. Groothuis PG, Dassen HH, Romano A, Punyadeera C. Estrogen and the endometrium: lessons learned from gene expression profiling in rodents and human. Hum Reprod Update. 2007;13(4):405–417. [DOI] [PubMed] [Google Scholar]
- 45. Kops GJ, Medema RH, Glassford J, et al. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol Cell Biol. 2002;22(7):2025–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kajihara T, Jones M, Fusi L, et al. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol Endocrinol. 2006;20(10):2444–2455. [DOI] [PubMed] [Google Scholar]
- 47. Hewitt SC, Li L, Grimm SA, et al. Research resource: whole-genome estrogen receptor α binding in mouse uterine tissue revealed by ChIP-seq. Mol Endocrinol. 2012;26(5):887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Telgmann R, Maronde E, Taskén K, Gellersen B. Activated protein kinase A is required for differentiation-dependent transcription of the decidual prolactin gene in human endometrial stromal cells. Endocrinology. 1997;138(3):929–937. [DOI] [PubMed] [Google Scholar]
- 49. Jones MC, Fusi L, Higham JH, et al. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci USA. 2006;103(44):16272–16277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pansini F, Bergamini CM, Bettocchi S, Jr, et al. Sex steroid hormones influence the cAMP content in human endometrium during the menstrual cycle. Gynecol Obstet Invest. 1984;18(4):174–177. [DOI] [PubMed] [Google Scholar]
- 51. Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J. 2008;55(5):795–810. [DOI] [PubMed] [Google Scholar]
- 52. Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140(10):4809–4820. [DOI] [PubMed] [Google Scholar]
- 53. Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci. 2000;5:D678–D693. [DOI] [PubMed] [Google Scholar]
- 54. Yuan CX, Ito M, Fondell JD, Fu ZY, Roeder RG. The TRAP220 component of a thyroid hormone receptor-associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc Natl Acad Sci USA. 1998;95(14):7939–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang X, Krutchinsky A, Fukuda A, et al. MED1/TRAP220 exists predominantly in a TRAP/Mediator subpopulation enriched in RNA polymerase II and is required for ER-mediated transcription. Mol Cell. 2005;19(1):89–100. [DOI] [PubMed] [Google Scholar]
- 56. Warnmark A, Almlöf T, Leers J, Gustafsson JA, Treuter E. Differential recruitment of the mammalian mediator subunit TRAP220 by estrogen receptors ERα and ERβ. J Biol Chem. 2001;276(26):23397–23404. [DOI] [PubMed] [Google Scholar]
- 57. Kang YK, Guermah M, Yuan CX, Roeder RG. The TRAP/Mediator coactivator complex interacts directly with estrogen receptors α and β through the TRAP220 subunit and directly enhances estrogen receptor function in vitro. Proc Natl Acad Sci USA. 2002;99(5):2642–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Misra P, Owuor ED, Li W, et al. Phosphorylation of transcriptional coactivator peroxisome proliferator-activated receptor (PPAR)-binding protein (PBP). Stimulation of transcriptional regulation by mitogen-activated protein kinase. J Biol Chem. 2002;277(50):48745–48754. [DOI] [PubMed] [Google Scholar]
- 59. Brar AK, Kessler CA, Meyer AJ, Cedars MI, Jikihara H. Retinoic acid suppresses in vitro decidualization of human endometrial stromal cells. Mol Hum Reprod. 1996;2(3):185–193. [DOI] [PubMed] [Google Scholar]
- 60. Chang HJ, Lee JH, Hwang KJ, Kim MR, et al. Transforming growth factor (TGF)-β1-induced human endometrial stromal cell decidualization through extracellular signal-regulated kinase and Smad activation in vitro: peroxisome proliferator-activated receptor γ acts as a negative regulator of TGF-β1. Fertil Steril. 2008;90(suppl 4):1357–1365. [DOI] [PubMed] [Google Scholar]
- 61. Li S, Shang Y. Regulation of SRC family coactivators by post-translational modifications. Cell Signal. 2007;19(6):1101–1112. [DOI] [PubMed] [Google Scholar]
- 62. Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology. 2000;141(9):3510–3513. [DOI] [PubMed] [Google Scholar]
- 63. Kaya HS, Hantak AM, Stubbs LJ, Taylor RN, Bagchi IC, Bagchi MK. Roles of progesterone receptor A and B isoforms during human endometrial decidualization. Mol Endocrinol. 2015;29(6):882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.