Abstract
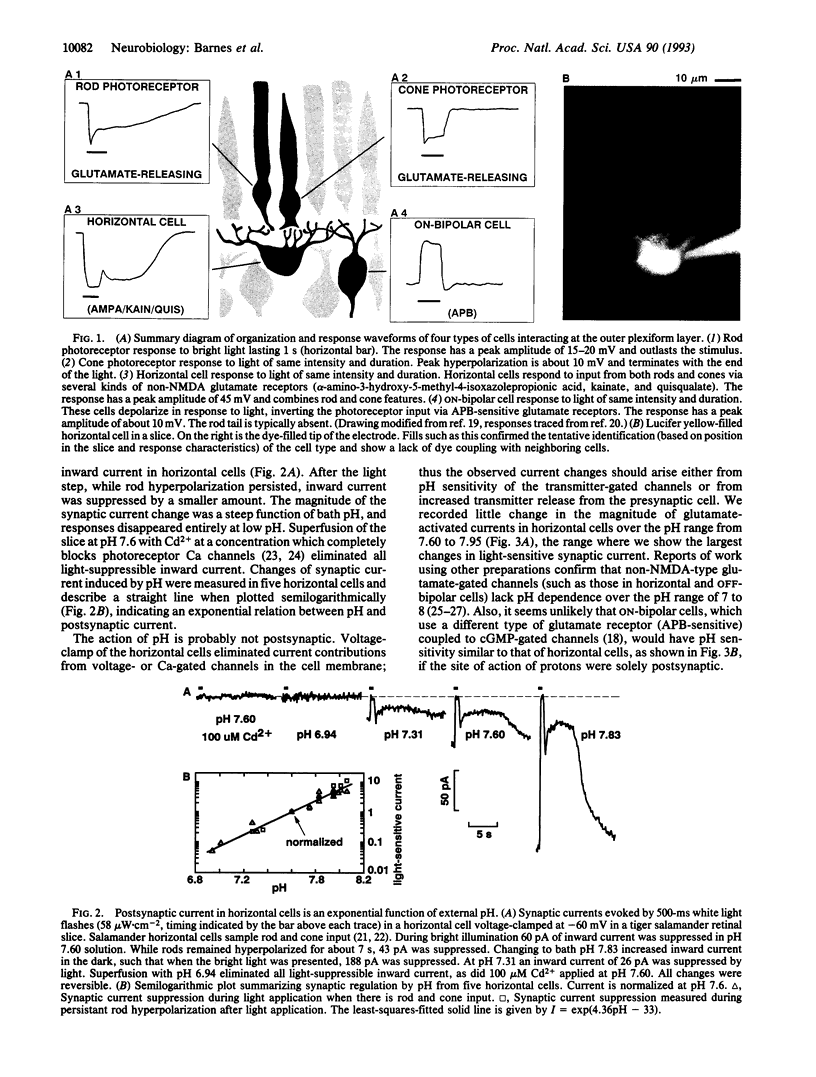
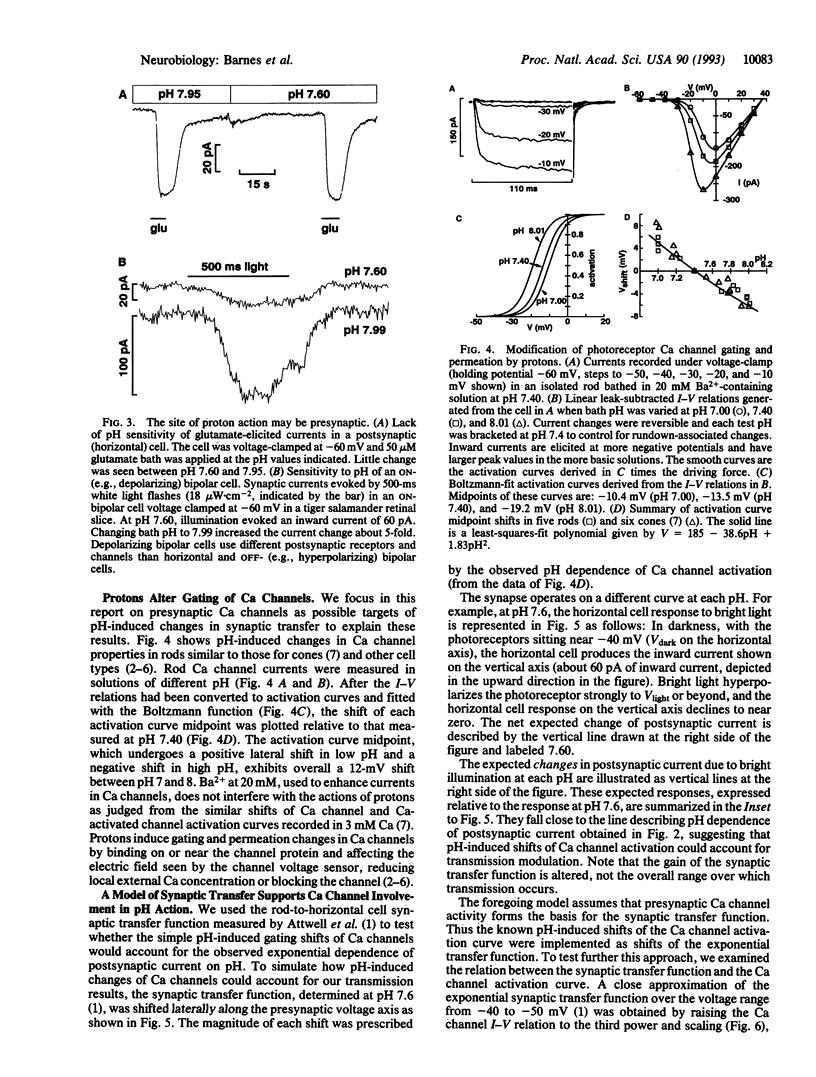
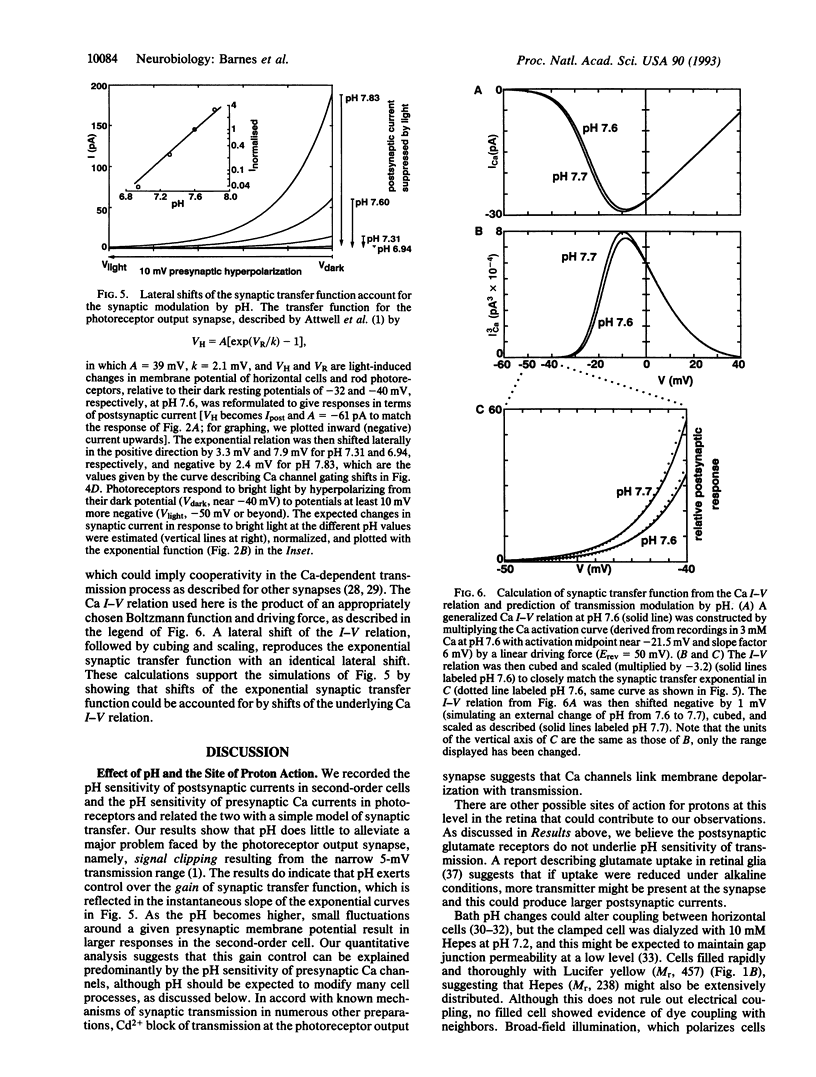
Synaptic transmission of the light response from photoreceptors to second-order cells of the retina was studied with the whole-cell patch-clamp technique in tiger salamander (Ambystoma tigrinum) retinal slices. Synaptic strength is modulated by extracellular pH in a striking manner: Light-sensitive postsynaptic currents in horizontal and bipolar cells were found to be exponential functions of pH, exhibiting an e-fold increase per 0.23 pH unit over the pH range from 7 to 8. Calcium channel currents in isolated photoreceptors were measured and also exhibited proton sensitivity. External alkalinization from pH 7 to 8 shifted the voltage dependence of channel activation negative by 12 mV. A model of the synaptic transfer function suggested that presynaptic Ca channels could be the primary sites of proton action. Increased Ca influx and transmitter release brought about by alkalinization give rise to larger postsynaptic currents. These results suggest that activity-dependent interstitial pH changes known to occur in the retina, while not alleviating signal clipping at this synapse, may provide an adaptative mechanism controlling gain at the photoreceptor output synapse.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Borges S., Wu S. M., Wilson M. Signal clipping by the rod output synapse. Nature. 1987 Aug 6;328(6130):522–524. doi: 10.1038/328522a0. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985 Oct;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S., Bui Q. Modulation of calcium-activated chloride current via pH-induced changes of calcium channel properties in cone photoreceptors. J Neurosci. 1991 Dec;11(12):4015–4023. doi: 10.1523/JNEUROSCI.11-12-04015.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S., Hille B. Ionic channels of the inner segment of tiger salamander cone photoreceptors. J Gen Physiol. 1989 Oct;94(4):719–743. doi: 10.1085/jgp.94.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Barnes S. Characterization of a voltage-gated K+ channel that accelerates the rod response to dim light. Neuron. 1989 Nov;3(5):573–581. doi: 10.1016/0896-6273(89)90267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges S., Wilson M. Structure of the receptive fields of bipolar cells in the salamander retina. J Neurophysiol. 1987 Dec;58(6):1275–1291. doi: 10.1152/jn.1987.58.6.1275. [DOI] [PubMed] [Google Scholar]
- Borgula G. A., Karwoski C. J., Steinberg R. H. Light-evoked changes in extracellular pH in frog retina. Vision Res. 1989;29(9):1069–1077. doi: 10.1016/0042-6989(89)90054-0. [DOI] [PubMed] [Google Scholar]
- Bouvier M., Szatkowski M., Amato A., Attwell D. The glial cell glutamate uptake carrier countertransports pH-changing anions. Nature. 1992 Dec 3;360(6403):471–474. doi: 10.1038/360471a0. [DOI] [PubMed] [Google Scholar]
- Christensen B. N., Hida E. Protonation of histidine groups inhibits gating of the quisqualate/kainate channel protein in isolated catfish cone horizontal cells. Neuron. 1990 Oct;5(4):471–478. doi: 10.1016/0896-6273(90)90086-u. [DOI] [PubMed] [Google Scholar]
- DeVries S. H., Schwartz E. A. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989 Jul;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsanyi K., Mangel S. C. Modulation of cone to horizontal cell transmission by calcium and pH in the fish retina. Vis Neurosci. 1993 Jan-Feb;10(1):81–91. doi: 10.1017/s0952523800003242. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nunn B. J. The effect of ions on sodium-calcium exchange in salamander rods. J Physiol. 1987 Oct;391:371–398. doi: 10.1113/jphysiol.1987.sp016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T., Ciani S., Hagiwara S. Effects of the external pH on Ca channels: experimental studies and theoretical considerations using a two-site, two-ion model. Proc Natl Acad Sci U S A. 1986 Feb;83(3):654–658. doi: 10.1073/pnas.83.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J. Signal transmission at the photoreceptor synapse. Role of calcium ions and protons. Ann N Y Acad Sci. 1991;635:468–470. doi: 10.1111/j.1749-6632.1991.tb36529.x. [DOI] [PubMed] [Google Scholar]
- Krafte D. S., Kass R. S. Hydrogen ion modulation of Ca channel current in cardiac ventricular cells. Evidence for multiple mechanisms. J Gen Physiol. 1988 May;91(5):641–657. doi: 10.1085/jgp.91.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Mueller P., Pugh E. N., Jr Protons suppress the dark current of frog retinal rods. J Physiol. 1984 Feb;347:85–110. doi: 10.1113/jphysiol.1984.sp015055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L. M., Werblin F. S. Synaptic transmission to the horizontal cells in the retina of the larval tiger salamander. J Physiol. 1978 Jun;279:321–346. doi: 10.1113/jphysiol.1978.sp012347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S., Jahr C. E. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990 Jul 19;346(6281):269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Negishi K., Sugawara K. Evidence for the anoxia sensitivity of the synaptic region at the outer plexiform layer in the fish retina. Vision Res. 1973 May;13(5):983–987. doi: 10.1016/0042-6989(73)90077-1. [DOI] [PubMed] [Google Scholar]
- Negishi K., Svaetichin G. Effects of anoxia, CO2 and NH3 on S-potential producing cells and on neurons. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;292(3):177–205. doi: 10.1007/BF00362735. [DOI] [PubMed] [Google Scholar]
- Oakley B., 2nd, Wen R. Extracellular pH in the isolated retina of the toad in darkness and during illumination. J Physiol. 1989 Dec;419:353–378. doi: 10.1113/jphysiol.1989.sp017876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977 May;267(2):429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Interactions of protons with single open L-type calcium channels. Location of protonation site and dependence of proton-induced current fluctuations on concentration and species of permeant ion. J Gen Physiol. 1989 Jul;94(1):23–42. doi: 10.1085/jgp.94.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillman A. J., Owen W. G., Fernandez H. R. The generation of the late receptor potential: an excitation-inhibition phenomenon. Vision Res. 1972 Sep;12(9):1519–1531. doi: 10.1016/0042-6989(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Skrzypek J., Werblin F. Lateral interactions in absence of feedback to cones. J Neurophysiol. 1983 Apr;49(4):1007–1016. doi: 10.1152/jn.1983.49.4.1007. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981 Feb 13;211(4483):712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Dichter M., Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis S. F., Cull-Candy S. G. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990 May 24;345(6273):347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. The control of sensitivity in the retina. Sci Am. 1973 Jan;228(1):70–79. doi: 10.1038/scientificamerican0173-70. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978 Jul;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. M. Synaptic connections between neurons in living slices of the larval tiger salamander retina. J Neurosci Methods. 1987 Jun;20(2):139–149. doi: 10.1016/0165-0270(87)90046-x. [DOI] [PubMed] [Google Scholar]
- Wu S. M. Synaptic transmission from rods to bipolar cells in the tiger salamander retina. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3944–3947. doi: 10.1073/pnas.82.11.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto F., Borgula G. A., Steinberg R. H. Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Exp Eye Res. 1992 May;54(5):685–697. doi: 10.1016/0014-4835(92)90023-l. [DOI] [PubMed] [Google Scholar]
- Yang X. L., Wu S. M. Coexistence and function of glutamate receptor subtypes in the horizontal cells of the tiger salamander retina. Vis Neurosci. 1991 Oct;7(4):377–382. doi: 10.1017/s0952523800004867. [DOI] [PubMed] [Google Scholar]




