The epidemic of obesity and diabetes has focused attention on adipose tissue and molecular mechanisms underlying energy homeostasis. Adipose tissue is often classified as “white” adipose tissue (WAT) or “brown” adipose tissue (BAT) (1). WAT is composed of unilocular adipocytes specialized for triglyceride storage. WAT secretes adipokines and other factors that mediate the regulation of feeding, fuel metabolism, neuroendocrine, and immune functions. BAT is composed of multilocular adipocytes with abundant mitochondria, and expression of uncoupling protein 1 (UCP1) that diminishes the proton gradient by uncoupling cellular respiration and mitochondrial ATP synthesis (2). Activation of UCP1 increases glucose and free fatty acid oxidation and heat production (2). The thermogenic capacity of BAT makes it a plausible therapeutic target for inducing weight loss. Recent discoveries concerning the development of BAT, and identification of functional BAT in adult humans have generated enormous excitement in the fields of obesity and diabetes (2–4).
The epidemic of obesity and diabetes has focused attention on adipose tissue and molecular mechanisms underlying energy homeostasis. Adipose tissue is often classified as “white” adipose tissue (WAT) or “brown” adipose tissue (BAT). The thermogenic capacity of BAT makes it a plausible therapeutic target for inducing weight loss. Recent discoveries concerning the development of BAT, and identification of functional BAT in adult humans have generated enormous excitement in the fields of obesity and diabetes.
Discovery of Beige Fat
In mice, UCP1+ brown adipocytes are typically clustered within distinct depots in the interscapular and perirenal regions. However, several studies have identified UCP1-positive cells in the sc WAT after chronic cold exposure or β-adrenergic agonist (2–4). Both classical (brown) and inducible (beige) adipocytes share structural similarities in abundant mitochondria and multiple lipid droplets but they have distinct developmental lineages. A key discovery was the observation that classical brown adipocytes are derived from a MYF5+ lineage shared with skeletal muscle, whereas beige adipocytes originate from a MYF5− lineage (4). Beige adipocytes express low levels of UCP1 and other thermogenic genes in the basal unstimulated state, but they have the capacity of fully inducing a thermogenic response equivalent to classical brown adipocytes when treated with appropriate hormones. Apart from sympathetic nerve stimulation, other factors associated with browning of adipose tissue in mice include exercise, environmental enrichment, cancer cachexia, hormones and other circulating factors, eg, fibroblast growth factor-21, bone morphogenic protein (BMP4), BMP7, BMP8b, growth differentiation factor-5, vascular endothelial growth factor, natriuretic peptides, and prostaglandins (2–4).
The role of BAT-mediated thermogenesis has been investigated in mice. Ucp1 knockout mice housed under thermoneutral conditions display impaired diet-induced thermogenesis and become obese (5). Transplantation of BAT increases energy expenditure, improves glucose homeostasis, and reduces lipids. The transcriptional regulator PRD1-BF-1-RIZ1 homologous domain-containing protein-16 (PRDM16) is highly enriched in BAT and virtually undetectable in visceral WAT. Forced expression of PRDM16 induces thermogenic gene expression and represses muscle gene expression (2–4, 6). Importantly, PRDM16 and its binding partner, CCAT/enhancer binding protein, promote differentiation of fibroblasts into brown adipocytes. Ablation of PRDM16 from all adipocytes did not significantly affect the classical BAT. However, the beige adipocytes in sc WAT were severely reduced, and the beige adipose-deficient mice developed a massively increased sc WAT, hepatic steatosis, and insulin resistance (6). On the other hand, a targeted ablation of PRDM16 in MYF5+ cells has no effect in young mice and a minimal effect on brown adipocyte development in older mice, whereas ablation of both PRDM16 and a closely related protein, PRDM3, results in a severe loss of thermogenic gene expression in brown adipocytes (3, 4, 6). Other transcriptional regulators of brown and beige adipocytes include early B cell factor-2, which acts upstream of PRDM16 to promote perixome proliferator-activated receptor γ-mediated transcription of brown adipocyte specific genes, EHMT1, a histone lysine methyltransferase associated with PRDM16 transcriptional complex, and transducin-like enhancer protein, a cofactor that represses thermogenesis in brown and beige adipocytes (2–4).
BAT in Humans
Until recently, it was widely believed that BAT was present in significant amounts only in human infants and regressed in adults (1). However, in 2009, there were various reports of BAT activity in the supraclavicular and paraspinal regions in adult humans based on 18F-fluorodeoxyglucose (FDG) Positron Emission Tomography Scan, (PET) imaging (7–9). The BAT signal was inversely associated with body mass index and increased in response to cold exposure. Tissue biopsies confirmed the presence of UCP1+ cells, and various molecular markers consistent with BAT (2, 9). Research publications describing human BAT location and functions have increased tremendously in the past 5 years (Figure 1).
Figure 1.
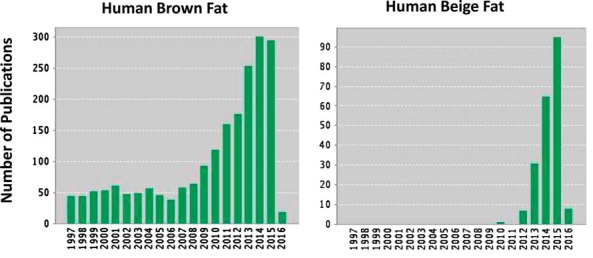
Number of publications on human brown and beige fat listed in Web of Science (Thomson-Reuters).
Although some studies have attempted to establish specific gene expression profiles of human brown and beige adipocytes, the results are likely to be confounded by multiple factors such as tissue heterogeneity, obesity vs leanness, aging, and ambient temperature (2–4, 10). The metabolic role of BAT has been examined under various conditions in humans using 18F-FDG-PET/CT imaging. The level of BAT activity induced by cold or diet is correlated with energy expenditure. Plasma glucose and lipid levels are inversely related to BAT activity in some population studies (11). Cold exposure increases glucose disposal specifically in BAT, and this response seems to be blunted in obese individuals. The effect of cold exposure on glucose homeostasis was evaluated with hyperinsulinemic-euglycemic clamp (12). Whole-body glucose disposal, glucose oxidation, and insulin sensitivity were enhanced in individuals with significant amounts of BAT, whereas the cold exposure did not affect glucose metabolism in those who did not have detectable BAT activity (12). Together, these results support the notion that BAT serves an important role in modulating risk of diabetes.
Therapeutic Potential of BAT Thermogenesis
The concept of inducing thermogenesis as a therapeutic strategy for obesity has been around for many decades. For example, the uncoupling agent, dinitrophenol, was shown to be effective for weight loss, but the drug was discontinued due to hyperthermia and other severe complications. Thyroxine and catecholamines induce brown and beige adipose but the side effects of these compounds limits their use in obesity treatment. A β3-selective adrenergic agonist, CL 316243, potently activates brown and beige adipose and reduces adiposity in mice but the drug is ineffective in humans. The task ahead is to develop more specific uncoupling agents or activators of UCP1 that provide metabolic benefits by enhancing fat oxidation, reducing body fat and improving glucose homeostasis, with minimum adverse effects. Because cold activates brown and beige adipocytes, it is possible that lowering the ambient temperature may be useful in treating obesity (13). However, there are questions about the level and duration of cold exposure and whether people would be willing to give up their preference for thermal comfort. Putative drug targets for promoting BAT thermogenesis include BMP7, BMP8b, cyclooxygenase-2, natriuretic peptides and fibroblast growth factor-21, but their pleotropic actions will limit the therapeutic use.
Despite remarkable progress on BAT biology, much remains to be done in order to translate basic research into clinical practice. Better understanding of specific signaling pathways in human brown and beige adipocytes will provide new targets for drug development. As discussed earlier, BAT affects glucose and lipid metabolism, hence a deeper knowledge about interactions of BAT, brain, skeletal muscle and liver would help uncover novel roles for BAT in diabetes and metabolic dysfunction. Another area that demands further research is the development of new technologies to assess the mass of brown and beige adipose tissue in humans. Although the BAT activity can be reasonably measured with 18F-FDG-PET/CT scans, this technique does not measure BAT mass and the study subjects are also exposed to ionizing radiation. Thus, the availability of less invasive imaging techniques and new biomarkers for brown and beige-adipocytes would advance clinical research into thermogenic therapies for obesity, diabetes, and other metabolic diseases.
Acknowledgments
This work was supported by the American Diabetes Association Grant 7-13-BS-004 and National Institutes of Health Grants R01-NS084965 and P01-DK049210.
Disclosure Summary: The author has nothing to disclose.
Footnotes
- BAT
- “brown” adipose tissue
- BMP
- bone morphogenic protein
- FDG
- fluorodeoxyglucose
- PET
- Positron Emission Tomography Scan
- PRDM16
- PRD1-BF-1-RIZ1 homologous domain-containing protein-16
- UCP1
- uncoupling protein 1
- WAT
- “white” adipose tissue.
References
- 1. Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112(pt 1):35–39. [PMC free article] [PubMed] [Google Scholar]
- 2. Kajimura S, Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol. 2014;76:225–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–1263. [DOI] [PubMed] [Google Scholar]
- 4. Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11(4):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9(2):203–209. [DOI] [PubMed] [Google Scholar]
- 6. Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–1508. [DOI] [PubMed] [Google Scholar]
- 8. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–1525. [DOI] [PubMed] [Google Scholar]
- 10. Cypess AM, White AP, Vernochet C, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19(5):635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chondronikola M, Volpi E, Børsheim E, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63(12):4089–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ouellet V, Routhier-Labadie A, Bellemare W, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96(1):192–199. [DOI] [PubMed] [Google Scholar]


