Abstract
Background
Intellectual Disability (ID) is characterized by deficits in intellectual functions such as reasoning, problem-solving, planning, abstract thinking, judgment, and learning. As new avenues are emerging for treatment of genetically determined ID (such as Down’s syndrome or Fragile X syndrome), it is necessary to identify objective reliable and sensitive outcome measures for use in clinical trials.
Objective
We developed a novel visual analogical reasoning paradigm, inspired by the Progressive Raven’s Matrices, but appropriate for Intellectually Disabled patients. This new paradigm assesses reasoning and inhibition abilities in ID patients.
Methods
We performed behavioural analyses for this task (with a reaction time and error rate analysis, Study 1) in 96 healthy controls (adults and typically developed children older than 4) and 41 genetically determined ID patients (Fragile X syndrome, Down syndrome and ARX mutated patients). In order to establish and quantify the cognitive strategies used to solve the task, we also performed an eye-tracking analysis (Study 2).
Results
Down syndrome, ARX and Fragile X patients were significantly slower and made significantly more errors than chronological age-matched healthy controls. The effect of inhibition on error rate was greater than the matrix complexity effect in ID patients, opposite to findings in adult healthy controls. Interestingly, ID patients were more impaired by inhibition than mental age-matched healthy controls, but not by the matrix complexity. Eye-tracking analysis made it possible to identify the strategy used by the participants to solve the task. Adult healthy controls used a matrix-based strategy, whereas ID patients used a response-based strategy. Furthermore, etiologic-specific reasoning differences were evidenced between ID patients groups.
Conclusion
We suggest that this paradigm, appropriate for ID patients and developmental populations as well as adult healthy controls, provides an objective and quantitative assessment of visual analogical reasoning and cognitive inhibition, enabling testing for the effect of pharmacological or behavioural intervention in these specific populations.
Introduction
Intellectual Disability (ID) is characterized by a significant limitation in both intellectual functioning (which refers to general mental capacities such as reasoning, problem-solving, planning, abstract thinking, judgment, learning from experience) and in adaptive behavior as expressed in conceptual, social and practical adaptive skills (AAIDD, [1]). This disability originates before the age of 18. Impairment of reasoning abilities is a core symptom in ID patients [2]. In addition, inhibition (one of the three components of executive functions) represents a significant factor in practical and conceptual adaptive skills in children with ID [3, 4]. Several theoretical approaches have been developed to describe cognitive impairment in ID: (i) the difference approach which seeks to identify specific cognitive profiles with strengths and weaknesses, (ii) the developmental approach comparing ID patients with chronological (CA HC) and mental (MA HC) age-matched healthy control groups (developmental delay / deviance) and more recently (iii) the neuroconstructivism approach which focuses on developmental trajectories resulting from dynamic multidirectional interactions between genes, brain, cognition and environment [5–7].
Genetic causes of ID include visible chromosomal anomalies (such as Down’s syndrome (DS) [8] which is the most frequent aneuploidy), chromosomal micro deletion and monogenic diseases (such as mutation of the ARX gene [9–11] or mutation of the FMR1 gene leading to Fragile X syndrome (FraX) [12]). DS and FraX are both well characterized syndromes including carefully detailed cognitive profiles, whereas ARX gene mutated patients (ARX) were more recently described [9–11]. The cognitive profile in DS is characterized by impairments in morphosyntax, verbal short-term memory, and explicit long-term memory; whereas visuospatial abilities, visuospatial short-term memory, and implicit long-term memory functions are relatively preserved [13–17]. The cognitive phenotype in FraX associates a relative strength in vocabulary capacity, visuo-perceptual abilities, with a weakness in verbal short-term memory, visuo-constructive abilities, visuo-spatial memory, linguistic processing, attention and executive functions [18–22].
Until very recently, treatments for ID have mainly focused on symptom management (including attention deficits and anxiety), and on minimizing complications related to comorbidities (e.g. epilepsy). New pharmacological treatment options that target the underlying defect related to each genetic mutation are currently being explored and may offer future opportunities to directly improve abstract reasoning [23]. The identification of reliable and sensitive outcome measures for use in clinical trials in Fragile X syndrome was recognized as a priority in the recent meeting convened by the National Institutes of Health [24]. Many therapeutic trials in ID patients use questionnaires completed by parents or caregivers, which rate the abnormal behaviors of patients [25]. Unfortunately, these tests are subjective, dependent on the judgment of the parents, and foregoing direct evaluation of the ID patients. In studies conducted by pharmaceutical companies, these questionnaires require selecting very impaired patients with pervasive behavioral problems in order to quantify improvement with the drug in question. This is a serious limitation if the effect of a new drug to improve specific cognitive abilities such as the use of more efficient reasoning strategies can be expected in less impaired patients.
In addition, most of the cognitive tests were developed to distinguish typically developing persons and ID patients, leading to a floor effect in the latter who systematically fail these tests. Therefore, these tests are not adapted to capture the potential effect of a drug within ID patients group.
To address the lack of a sensitive, reliable and simple outcome measure to assess non-verbal reasoning abilities (i.e. the core symptom in ID), we developed a novel visual analogical reasoning paradigm appropriate for ID patients, which was inspired by the Progressive Raven’s Matrices (PM). The PM test is very well correlated to the Intelligence Quotient. It is a non-verbal multiple choice measure of the reasoning component of Spearman's g factor, which is often referred to as general intelligence. In each test item, the subject is asked to identify the missing element that completes a pattern comprised of a 3x3 matrix. Several explanatory models have been proposed for analyzing cognitive abilities required in the PM test. Carpenter et al. [26] suggested that subject’s performances depended primarily upon their capacity to induce abstract relations and their working memory capacity. Other models evoked the speed of information processing [27] or the speed for hypothesis generation [28]. Finally, Bethell-Fox [29] introduced the idea that individuals may differ qualitatively because they use different strategies to solve the same task. Using eye-tracking analysis in healthy controls, Vigneau et al. were able to differentiate a “constructive matching strategy” (matrix-based strategy) involving the construction of an idealized answer which is then compared to the response alternatives of the response choice, from a “response elimination strategy” (response-based strategy) involving a process of feature comparison between elements of the problem and elements of the alternatives [29, 30]. Since ID patients uniformly fail on this Raven’s PM we developed a novel paradigm called “SimpleMatrices”. Furthermore, recent technological developments have enabled researchers to apply non-invasive eye-tracking technology to populations with ID [31] that enable directly testing the hypothesis that the deficit in ID performance is due to the use of inefficient strategies.
Before using this new task in ID patients, we validated this new paradigm in 96 healthy controls (adults and typically developed children older than 4). We then tested it in 41 genetically determined ID patients (FraX, DS and ARX patients). Two modalities were used to record the data: behavioral data (with a reaction time and error rate analysis, Study 1) and eye-tracking data (in order to establish and quantify the cognitive strategies used to solve the task, Study 2). For each group of patients, a comparison was performed with CA HC and MA HC. Thus, we were able to discriminate between developmental delay and specific syndrome related cognitive impairments. Mathematical modeling of the developmental trajectory for this task was also performed [7, 32].
In this report, we demonstrate that this novel task, “SimpleMatrices”, is appropriate for intellectually disabled patients. We show that objective and quantitative assessment of reasoning strategies can be performed in patients with moderate intellectual deficit. “SimpleMatrices” is a new tool that can be used as an outcome measure, enabling testing for a drug effect on reasoning in ID populations. Furthermore, specific deficits in reasoning abilities can also be identified between etiologically diverse ID patient groups.
Materials and Methods
Stimuli
As item difficulty is directly related to the number of response choices and the number of elements in an item [29], in our simplified paradigm matrix stimuli consist of four elements (instead of the nine cells in Raven’s), with two response choices (instead of the eight in Raven’s). Four parameters are involved in matrix creation: color (white, black or grey), form (round, square or triangle), number (one or two), and size (large or small). The number of relations between the items that need to be considered jointly to find the correct answer define the relational complexity of a matrix.
There are three levels of complexity:
identical (Fig 1A): the three elements of the matrix are the same;
“one-relation” (Fig 1B): a very simple reasoning that requires consideration of only one varying parameter;
“two-relations” (Fig 1D): variation in two parameters must be integrated to get the correct answer.
Fig 1. Example of the 5 different conditions included in the task (1a: Identical matrix (Id), 1b: One-relation matrix with neutral response (1R_Neu), 1c: One-relation matrix with “to be inhibited” false response (1R_Inhib), Two-relation matrix with neutral response (2R_Neu), Two-relation matrix with “to be inhibited” false response (2R_Inhib)).
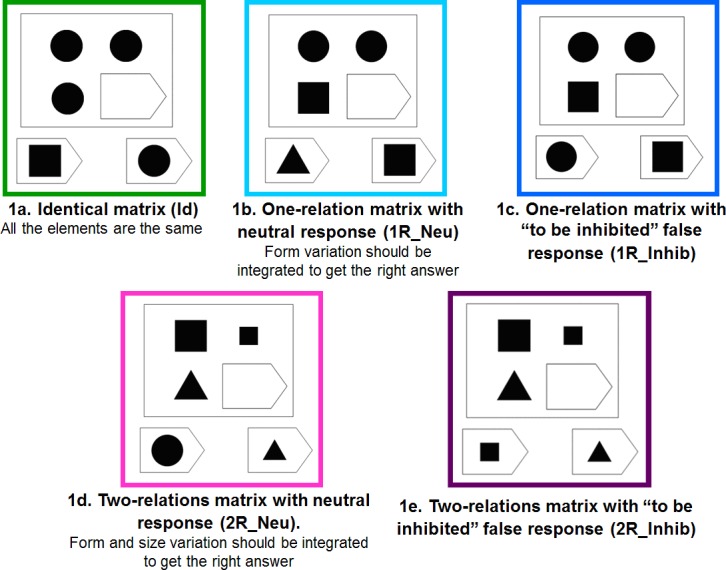
Furthermore, false-responses of two different types exist: a “neutral” response, which is a choice different from the items displayed in the matrix (Fig 1B and 1D) and a “to be inhibited” response, which is identical to one of the matrix items displayed (Fig 1C and 1E).
Thus, this task combines 5 different conditions: identical matrices (Id), one-relation matrices with neutral responses (1R_Neu), one-relation matrices with to be inhibited responses (1R_Inhib), two-relations matrices with neutral responses (2R_Neu) and two-relations matrices with to be inhibited responses (2R_Inhib).
Task Description
The participants were asked to identify the missing element that completes a pattern (Fig 1). The stimuli were displayed on a computer screen. Participants were told to find the best answer to fill in the missing “piece”. For the patients, immediate visual feedback via a happy or sad emoticon (in case of right or wrong answer, respectively) was given during the training to make the task easier to understand.
The paradigm consisted of four runs. Each matrix was preceded by a fixation cross. The subject then had to select the correct answer by pushing a button. The number of each type of condition was counterbalanced on each run, producing the same number of right and left answers. For each type of matrix, the type of variable relation (form, color, size, number) was also counterbalanced. The order of different conditions in the matrix display was randomized, as was the side of the correct answer. Each run consisted of 45 trials in the behavioral task and 15 trials in the eye-tracking task.
Procedure
Recruitment was accomplished through the primary care doctor of the patients or the specialist who asked for the molecular or cytogenetic testing. This study was approved by the Ethical Committee of French Public Hospitals (Comité de Protection des Personnes Lyon-Sud Est II, 2010-A00327-32, 09/22/2010). After we informed the patients and their parents or guardians about the aims of the study, all of them gave written informed consent before the study procedure started. Child healthy controls were recruited from kindergartens, elementary and middle schools in Lyon. The parents of each child included in the study signed an informed consent before the study procedure started. The data were acquired in the National Reference Center for Rare Causes of Intellectual Disability (L2C2, CNRS UMR 5304, Lyon, France).
Study 1: Behavioral Analysis
Participants (details in Table 1).
Table 1. Characteristics of the patients and healthy controls included in Study 1 and 2.
| Adult HC | Child HC | DS patients | ARX patients | FraX patients | |
|---|---|---|---|---|---|
| Study 1: Behavioral analysis | |||||
| Number of participants | n = 34 | n = 62 | n = 14 | n = 13 | n = 14 |
| Chronological age : mean | 27.7 years | [4–5 years] : n = 12 | 25.3 years | 22.2 years | 22.2 years |
| [range] | [18–43.7] | [6–7 years] : n = 11 | [15.6–37.4] | [13.2–40.2] | [14.5–31.5] |
| [8–9 years] : n = 11 | |||||
| [10–11 years]: n = 12 | |||||
| [12–13 years] : n = 9 | |||||
| [14–17 years] : n = 7 | |||||
| Sex ratio (% males) | 50% | 45% | 57% | 100% | 100% |
| Verbal IQ (VIQ) 1 | - | - | 49.1 | 50.2 | 52.0 |
| (SD 4.2) | (SD 4.8) | (SD 3.2) | |||
| Performance IQ (PIQ) 1 | - | - | 51.9 | 50.7 | 48.6 |
| (SD 5.3) | (SD 5.9) | (SD 8.8) | |||
| Total IQ (TIQ)1 | - | - | 47.9 | 47.3 | 49.7 |
| (SD 4.8) | (SD 8) | (SD 5.4) | |||
| Vocabulary Age2 | - | - | 10.1 years | 8.0 years | 10.7 years |
| (SD 6) | (SD 1.6) | (2.6) | |||
| Mental Age3 | - | - | 7.4 years | 7.1 years | 5.8 years |
| [5.75–9.5] | [4.25–11] | [4.25–7.5] | |||
| Study 2: Eye-tracking analysis | |||||
| Number of participants | n = 21 | n = 38 | n = 13 | - | n = 14 |
| Chronological age : mean, | 27.6 years | [4–7 years] : n = 11 | 24 years | - | 23.8 years |
| [range] | [18.3–41.8] | [8–11 years] : n = 15 | [14.2–38.7] | [13.4–31.6] | |
| [12–16 years] : n = 12 | |||||
| Sex ratio (% males) | 71% | 60% | 62% | 100% | 100% |
HC: Healthy Controls; DS: Down’s Syndrome; FraX: Fragile X syndrome; SD: Standard Deviation.
1Wechsler scales (the WAIS III in patients older than 16, the WISC IV in younger patients) were used for all patients but 6 Fragile X patients, for whom the Stanford Binet IV was used.
2Peabody Picture Vocabulary Test Revised (PPVT-R)
3Raven’s Coloured Progressive Matrices
Healthy controls (n = 96): 34 adult healthy controls aged 18 to 45 years, without any history of neurological or psychiatric disorder and 62 children older than 4 were included in Study 1. None of them met exclusion criteria: history of neurological or psychiatric disorder, repetition of a grade, learning disability requiring rehabilitation (speech therapy, psychomotor or oculomotor therapy).
Intellectually Disabled patients (n = 41): Three groups of ID patients were included in Study 1: 14 FraX patients having a full mutation (more than 200 CGG repeats) of the FMR1 gene located in Xq27.3; 14 DS patients having a whole 21st chromosome trisomy on karyotypic analysis; 13 ARX mutated patients having a mutation in the ARX gene (duplication of 24 bp (c.429-452 dup) in exon 2 second repeated PolyA tract, responsible for an insertion of 8 alanine residues in the ARX protein).
All ID patients had an IQ below 70 and the ability to perform the cognitive task. The 3 groups of ID patients did not differ significantly on Verbal IQ, Performance IQ, nor Total IQ. ARX and DS patients did not differ significantly on Raven’s PM. Nevertheless, since FraX patients had a significantly lower mental age on Raven’s PM than the other groups (p<0.05 and p<0.005 with ARX and DS patients respectively), specific mental age-matched groups were selected for each syndrome.
Data acquisition
The matrices were generated and displayed using Presentation software (http://www.neurobs.com). Each subject completed 4 runs. The task duration was around 15 minutes in healthy controls and 25 minutes in ID patients and young children. A break was allowed if necessary between two runs.
Data analysis
The raw data (logfiles from Presentation software) were analyzed automatically using Matlab 7.1. A Reaction Time (RT) and an Error Rate (ER) analysis were performed. The RT was defined as the time elapsing between the display of the matrix and the time when the subject pushed one of the two buttons to answer the problem. RT for the correct answers and ER were computed for each of the paradigm’s five conditions and for each subject included. Participants were excluded from the analysis if their ER for the two easiest conditions (‘Id’ and ‘1R_Neu’) was greater than the mean ER for all the 5 conditions from the group to which they belonged. This criterion was chosen to ensure that the task was well understood by all participants. This led us to exclude from the analysis: one DS patient, four ARX patients, three FraX patients, and one healthy child from the youngest group (4 to 5 years old).
Statistical analysis
Statistical analysis was performed using R software (http://www.r-project.org) on reaction time and error rate. The normality of the data distribution was first checked using the Shapiro and Wilk normality test. Then an ANOVA was applied in each group with two within group factors: complexity of the matrix (number of relations (0, 1, or 2) between items of the matrix needed to solve the problem) and inhibition (type of false response: either ‘neutral’ or ‘to be inhibited’). A significance level of p<0.05 was chosen. Post-hoc analysis was performed using the Scheffé test. The non-parametric Friedman test was applied when the data distribution was not normal. A between group analysis was also performed.
Each patient of each ID patient group (DS, ARX and FraX) was matched with both a CA HC subject and a MA HC subject. Group analyses were performed between each ID patient group and their respective chronological and mental age-matched control group.
Normative RT and ER data for this task were created in healthy controls and patients. Mathematical modelling of the developmental trajectory for this task in healthy controls was performed for RT and ER using “curvefit” toolbox in Matlab 7.1 (http://www.mathworks.fr).
Test-retest reliability (the degree to which test scores are consistent from one test administration to the next) was assessed within a session (between each of the four runs) in all healthy controls, DS and FraX patients using the Pearson correlation coefficient, while between session analysis was completed on the subset of participants who performed the task twice.
Study 2: Eye-Tracking Analysis
Participants (see Table 1)
The same inclusion and exclusion criteria as used in Study 1 were applied respectively for each group.
Healthy controls (HC): (n = 59) 21 adult healthy controls and 38 children older than 4 were included in Study 2. Ten out of the 21 adult healthy controls and 31 out of the 38 children were also included in Study 1.
Intellectually Disabled patients: (n = 27) Two groups of ID patients were included in Study 2: 14 FraX patients (12 out of the 14 patients also included in Study 1) and 13 DS patients (all also included in Study 1).
Data acquisition
Eye movements, fixations, and pupil diameters were acquired on the L2C2 CognitoScope platform (CNRS UMR5304) using a Tobii X120 eye-tracker, which is a remote binocular system with a temporal resolution of 60 Hz and a spatial resolution of 0.5 degree of visual angle. Thanks to the head movement compensation algorithms, no head restraints neede to be used. The eye-tracker, with an attached infrared light source to illuminate the pupil, was placed in front of the monitor where the stimuli were displayed. The device was below eye level, with an inclination of 19° relative to the horizontal and 70 cm from the participant. Each participant was seated on a comfortable and steady chair, with adjustable height to ensure good signal. Children were supplied footrests if needed. At the beginning of every eye-tracking session, we calibrated eye position for each participant. This calibration step was performed by having the participant look at colored dots in the center and four corners of the screen.
We used Tobii studio software to display the stimuli and record the eye-tracking data. In total, the analysis was performed on 12 matrices for each of the 5 conditions. During the task, an experimenter was present and controlled the quality of the eye-tracking data on a remote screen.
Data analysis
The eye-tracking data analysis was performed using Matlab 7.1 (http://www.mathworks.fr) (S1 File).
Three main Areas Of Interest (AOIs) were defined for each stimulus displayed: the Matrix and the two responses. Within the Matrix, four additional AOI were defined: element 1, element 2, element 3 and element 4 (Fig 2).
Fig 2. AOI (Area of Interest) defined for each stimuli of the eye-tracking task (the Matrix with its four elements and the two responses).
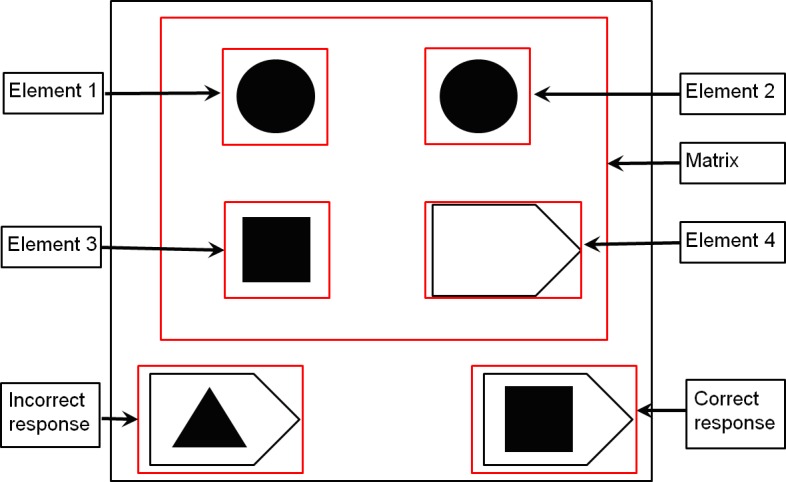
The following parameters were extracted and computed:
Number of transitions between the Matrix and the Responses (defined as the number of toggles from the matrix area to the response areas or vice versa);
Latency of the first transition between the Matrix and the responses;
Proportion of time on each AOI (defined as the time spent on a given AOI divided by the time spent to solve the corresponding trial);
Latency of first fixation on each AOI (defined as the time between the display of the stimulus on the screen and the first gaze fixation on each AOI).
The eye-tracking analysis was performed to characterize the cognitive strategy used to solve the reasoning task. If the participants used their peripheral vision from a fixed central gaze fixation, their eye-tracking data would not reflect the strategy they used. This led us to exclude from the analysis three adult healthy controls who spent 56.96%, 41.91% and 47.78% of the time respectively on element 4 (empty) for the identical condition.
Statistical analysis
Statistical analysis was performed using R software (http://www.r-project.org) on each of the parameters defined above. The normality of the data distribution was first checked using the Shapiro and Wilk normality test. Then an ANOVA was applied in each group with two within group factors: complexity of the matrix (number of relations (0, 1, or 2) between items of the matrix needed to solve the problem) and inhibition (type of false response: either neutral or to be inhibited). A significance level of p<0.05 was chosen. Post-hoc analysis was performed using the Scheffé test. The non-parametric Friedman test was applied if the data distribution was not normal. A between group analysis was also performed.
Each patient of each ID patient group (DS, and FraX) was matched with both a CA HC and a MA HC. Group analyses were performed between each ID patient group and their respective CA HC and MA HC groups.
Results
Study 1: Behavioral analysis
Results in Adult HC
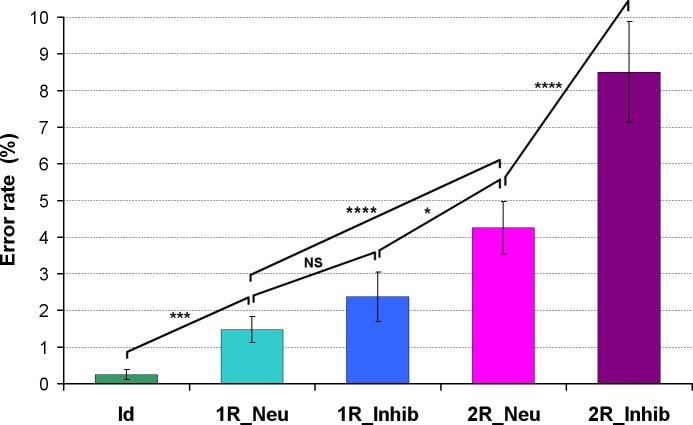
A significant effect of the matrix complexity was found on both RT and ER in adult HC (S1 Fig and Fig 3). They took significantly more time to solve the most complex matrices and made more errors on them. RT and ER were significantly greater for one relation compared to zero relation (p<0.001 and p<0.005 respectively) and greater for two relations compared to one (p<0.001 and p = 0.001 respectively).
Fig 3. Result of the ER in 34 adult healthy controls (*:p<0.05, ***:p<0.005, ****:p<0.001, NS: Non Significant).
There was no significant effect of inhibition on RT and ER for the easiest conditions (one relation) whereas a significant effect was observed for the most complex conditions (two relations) on both RT (p = 0.01, S1 Fig) and ER (p<0.001, Fig 3).
The interaction between matrix complexity (number of relations) and inhibition was statistically significant on both RT [F(1,33) = 20.48, p<0.001] and ER [F(1,33) = 8.18, p<0.01]: the effect of the matrix complexity was greater than the inhibition effect in adult HC.
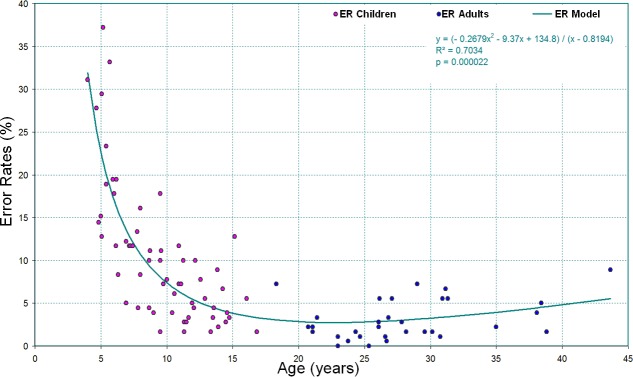
Developmental trajectory in Typically Developed (TD) children
We performed a mathematical modeling of the developmental trajectory of RT and ER in healthy controls (older than 4) with the “curvefit” toolbox in Matlab 7.1 (http://www.mathworks.fr). The mathematical equations are indicated on S2 Fig and Fig 4. The coefficient of determination R2 and the significance of the correlation were 0.71 and p<0.001, respectively for RT and 0.70 and p<0.001 respectively for ER.
Fig 4. Non-linear regression analysis of ER with chronological age in healthy controls, children older than 4 and adults.
Normative data of the RT and ER for this task were created in healthy controls (both children and adults) and are reported in S1 and S2 Tables respectively.
In TD children (grouped in 2 year increments), a significant effect of matrix complexity was found on RT (S3 Fig). TD children took significantly more time to solve the most complex matrices: RT was significantly greater for one relation compared to zero relation (p<0.005 for each group) and greater for two relations compared to one (p<0.005 for each group).
A significant effect of inhibition was found on ER in TD children (S4 Fig). Moreover, the effect of inhibition on ER was significantly greater than the effect of matrix complexity (number of relations) until they were 6 years old. Interestingly, the ER was significantly greater for the 1R_Inhib condition than for the 2R_Neu condition, despite the higher complexity of the later condition. The significant effect of inhibition on ER for the easiest condition (one relation) was not observed in TD children >10 years old.
The lack of inhibition effect observed in the two youngest groups of TD children (4–5 and 6–7 years) on RT (S3 Fig) is associated with more impulsivity, leading to an apparent “opposite effect”, as the 4–5 year old children were significantly quicker for the items with ‘to be inhibited responses’ than with ‘neutral responses’. The 4–5 year old children seemed to be “attracted” by the wrong answer that looked like the matrix with a high ER. TD children older than 10 years old had a pattern for the RT very similar to that of the HC adults (S3 Fig).
Results in ID patients (DS, ARX, FraX): comparison between ID patients and control groups
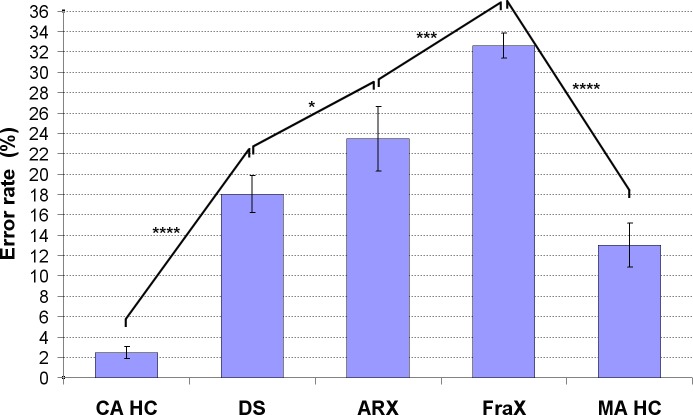
A highly significant group effect was found on both RT and ER comparing ID patients to their respective CA HC: DS, ARX and FraX patients were significantly slower and made significantly more errors than their respective CA HC groups (S5 Fig and Fig 5, Table 2).
Fig 5. Group effect between DS, ARX, FraX, MA HC and CA HC on ER (MA HC: Mental Age-matched Healthy Controls, CA HC: Chronological Age-matched Healthy Controls, *:p<0.05, ***: p<0.005, ****: p<0.001).
Table 2. Comparison between each group of patients and the respective CA HC and MA HC group for the behavioral data.
| Group comparison | Age | RT | ER | |||
|---|---|---|---|---|---|---|
| Patients | Controls | Group effect | Group effect | [Matrix complexity*group] Interaction | [Inhibition*group] Interaction | |
| DS / CA HC | 25.3 years | 25.4 years | p<0.001**** | p<0.001**** | p<0.001**** | p<0.001**** |
| [15.6–37.4] | [14.6–38.1] | |||||
| DS / MA HC | 7.4 years | 7.3 years | NS | p<0.01** | NS | NS |
| [5.75–9.5] | [5.4–9] | (p = 0.019* for 1R_Inhib) | ||||
| ARX / CA HC | 19.4 years | 19.0 years | p<0.001**** | p<0.001**** | p<0.05* | p = 0.001** |
| [13.2–39.1] | [13.6–38.8] | |||||
| ARX / MA HC | 7.5 years | 7.4 years | NS | p<0.001**** | NS | p<0.05* |
| [5.25–11] | [5–11.25] | |||||
| FraX / CA HC | 23.5 years | 23.1 years | p<0.001**** | p<0.001**** | p = 0.01* | p<0.001**** |
| [14.5–31.5] | [14.5–31.2] | |||||
| FraX / MA HC | 5.7 years | 5.9 years | NS | p<0.001**** | NS | p<0.001**** |
| [4.25–7] | [4–7.2] | |||||
CA HC: Chronological Age-matched Healthy Controls; MA HC: Mental Age-matched Healthy Controls; DS: Down’s Syndrome; FraX: Fragile X syndrome; ARX: ARX gene mutated patients; RT: Reaction Time; ER: Error Rate
*: p<0.05
**: p<0.01
****: p<0.001; NS: Non Significant.
We also performed a comparison between ID patients (DS, ARX and FraX patients) and their respective MA HC, allowing to distinguish what was related to a developmental delay in their performance and what could be considered as deviant.
When pooling all the conditions, no difference was observed on RT between ID patient groups and their respective MA HC (S5 Fig and Table 2). By contrast, ID patients made significantly more errors than their respective MA HC (Fig 5 and Table 2).
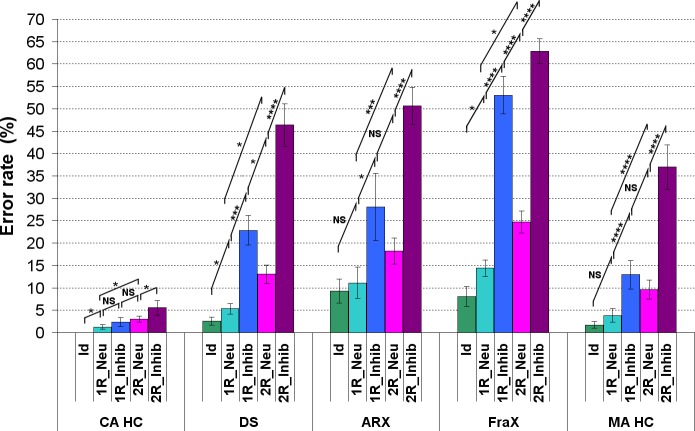
A significant effect of the matrix complexity was found on both RT and ER in DS, ARX and FraX patients (S6 Fig and Fig 6), as well as in MA HC and CA HC. It took significantly more time and more errors were made for the most complex matrices. The interaction between the matrix complexity (number of relations) and the group on ER was not significant for each patient group when compared to their respective MA HC: all groups (each patient group and their respective MA HC group) were similarly impaired by the increase of matrix complexity (Table 2).
Fig 6. ER for each of the five conditions for each group of patients (DS, FraX, ARX) and for both CA HC and MA HC.
(For display purposes and because the different chronological age-matched and mental age-matched control groups did not respectively differ significantly one from the other, the results of only one chronological age-matched control group and one mental age-matched control group are displayed).
Unlike the CA HC, there was a significant effect of inhibition observed on ER even for the easiest conditions (one relation) in DS, ARX and FraX patients (Fig 6). The ER was significantly greater for the 1R_Inhib condition than for the 2R_Neu condition, despite the higher complexity of the later condition (Fig 6). The interaction between matrix complexity (number of relations) and inhibition was statistically significant for the ID patient groups: unlike the CA HC, the effect of inhibition on ER was greater than the matrix complexity effect.
Results in ID patients (DS, ARX, FraX): specific cognitive profile related to ID etiology
When pooling all the conditions, no difference was observed on RT between the different ID patients groups (mean RT of 2.84s for DS, 2.92s for FraX, 2.89s for ARX; S5 Fig and Table 2). But there was a significant group effect on ER between patients groups: FraX patients made significantly more errors than ARX patients, who made significantly more errors than DS patients (Fig 5).
The sensitivity to inhibition, although true for all patients groups, was especially striking for FraX patients who made significantly more errors than DS and ARX patients for the 1R_Inhib condition. The interaction between inhibition and group was significant on ER between both ARX and FraX patients and their respective MA HC: both groups (ARX and FraX patient groups and their respective MA HC groups) made more errors for the ‘to be inhibited false response’ conditions, but ARX and Fragile X patients were more impaired by the inhibition than their MA HC. The interaction between inhibition and group was not significant when comparing DS patients to MA HC. However, DS patients made significantly more errors than their MA HC in the ‘1R_Inhib’ condition (Table 2). Thus, DS patients did differ from the MA HC for the easiest condition with inhibition but not for the most complicated one.
Correlations with the neuropsychological tests
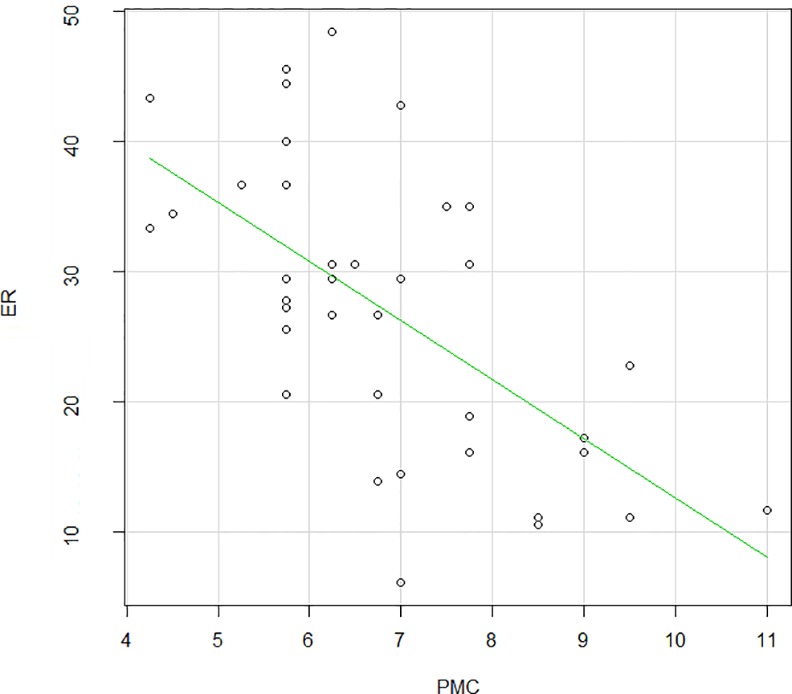
No correlation was found between RT and any of the cognitive measures (including Total IQ, Verbal and Performance IQ, Peabody Picture Vocabulary Test and Raven’s Coloured Progressive Matrices). But ER was strongly negatively correlated to the Raven’s Colored Progressive Matrices (Pearson's product-moment correlation = -0.62, p<0.001, Fig 7): the lower the ER was, the higher the mental age for the Raven’s.
Fig 7. Correlation between ER and the Raven’s Coloured Progressive Matrices in ID patients (Pearson's product-moment correlation = -0.62, p<0.0001).
Test-Retest reliability
Each participant performed four runs. We assessed the degree to which test scores were consistent from one test administration to the next (test-retest reliability) in adult healthy controls, DS and FraX patients. S7 Fig and Fig 8 displayed RT and ER for each of the three groups and for each run. There was no significant difference for any of the variables in the three groups (S8, S9 and S10 Figs). Pearson's product-moment correlation was computed between the 4 different runs for each group (S3 Table). These correlations, which were higher than 0.7 with p-values lower than 0.01, suggest good test-retest reliability.
Fig 8. ER for each run in Adult healthy controls, DS and FraX patients.
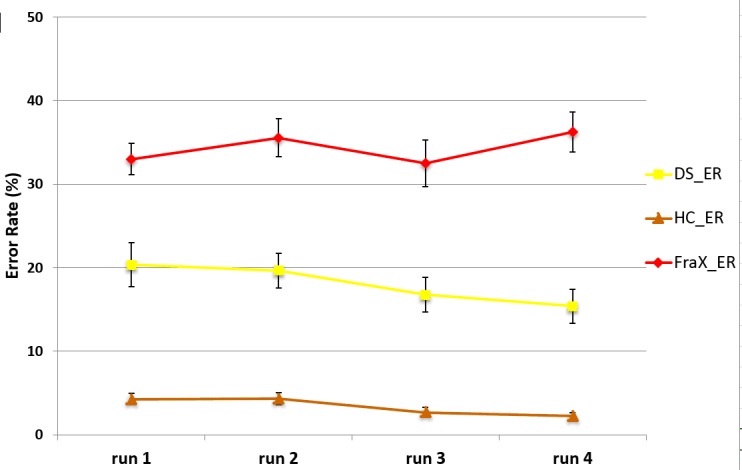
Furthermore, we performed the same analysis of the ER and RT on half of the task (run 1 and 2). We found the same results as in the analysis presented above and using the four runs.
Study 2: Eye-tracking analysis
Results in Adult HC
A significant effect of the matrix complexity (number of relations) was found on both the number of transitions (toggles) between the matrix and the responses and the latency of the first transition between the matrix and the responses in adult HC (S11 and S12 Figs). When the matrix became more difficult (increase in number of relations), adult HC made more transitions and spent more time on the matrix before switching to the responses, leading to a later occurrence of the first transition between the matrix and the responses.
There was no significant effect of inhibition on the number of transitions between the matrix and the responses in adult HC (S11 Fig).
Given that there were significant differences in RT between groups, we analyzed the proportional time on each AOI (defined as the time spent on a given AOI divided by the time spent to solve the corresponding trial) rather than the absolute time in order to allow the comparison of the different groups. In adult HC, the greatest proportional time was spent on the matrix. Moreover, they first looked at element 1, then at element 2, then at element 3 and then at the responses.
Results in TD children
There was a significant age effect on the number of transitions between the matrix and the responses. TD children older than 8 years were not statistically different from the adults, whereas children younger than 8 years made significantly more transitions. There was also a significant age effect on the latency of the first transition between the matrix and the responses: this latency increased as children got older. Children older than 12 years were not significantly different from adults.
A significant effect of the matrix complexity was found on the number of transitions between the matrix and the responses in TD children (S13 Fig). The number of transitions was significantly greater for the matrices with two than with one relation. The effect of the matrix complexity on the latency of the first transition between the matrix and the responses became significant only in children older than 12 years (S14 Fig).
Like for the adults, there was no significant effect of inhibition on the number of transitions between the matrix and the responses in TD children.
The greatest proportion of time was spent on the matrix in all groups of TD children. Adult HC spent significantly more time than any group of TD children on element 1, which reflects a full exploration of the matrix in order to deduce the correct answer.
Children 4 to 7 years old first looked at element 3, then at the responses, then at element 2, and then at element 1. Children 8 to 11 years old first looked at element 3, then at element 2, then at the responses, and then at element 1. Adolescents from 12 to 16 years old first looked at element 3, then at element 2, then at element 1, and then at the responses.
Results in ID patients: DS, ARX, FraX
There was a significant group effect on the number of transitions between the matrix and the responses: DS and FraX patients made more transitions than their respective CA HC (Table 3). The difference in number of transitions between the matrix and the responses between DS patients and FraX patients and their respective MA HC was not significant (Table 3).
Table 3. Comparison between each group of patients and the respective CA HC and MA HC group for the eye-tracking data.
| Group comparison | Age | Number of Transitions Matrix/Responses | Latency of the first Transition Matrix/Responses | Proportion of observational time | ||||
|---|---|---|---|---|---|---|---|---|
| Patients | Controls | Group effect | [Matrix complexity*group] Interaction | Group effect | [Matrix complexity*group] Interaction | Matrix | Responses | |
| DS / CA HC | 24 years | 24.3 years | p<0.001**** | NS | p<0.001**** | p<0.001**** | p<0.001**** | p<0.005*** |
| [14.2–38.7] | [14.3–37.6] | |||||||
| DS / MA HC | 7.1 years | 7.2 years | NS | NS | p<0.001**** | NS | p<0.001**** | p<0.05* |
| [5.7–9.5] | [5.3–9.5] | |||||||
| FraX / CA HC | 23.8 years | 23.2 years | p<0.001**** | NS | p<0.001**** | p<0.001**** | p<0.001**** | p<0.001**** |
| [13.4–31.6] | [12.3–31] | |||||||
| FraX / MA HC | 5.8 years | 6.4 years | NS | NS | p<0.001**** | NS | p<0.001**** | p<0.001**** |
| [4.3–7.5] | [4.7–8.1] | |||||||
CA HC: Chronological Age-matched Healthy Controls; MA HC: Mental Age-matched Healthy Controls; DS: Down’s Syndrome; FraX: Fragile X syndrome
*: p<0.05
***: p<0.005
****: p<0.001; NS: Non Significant.
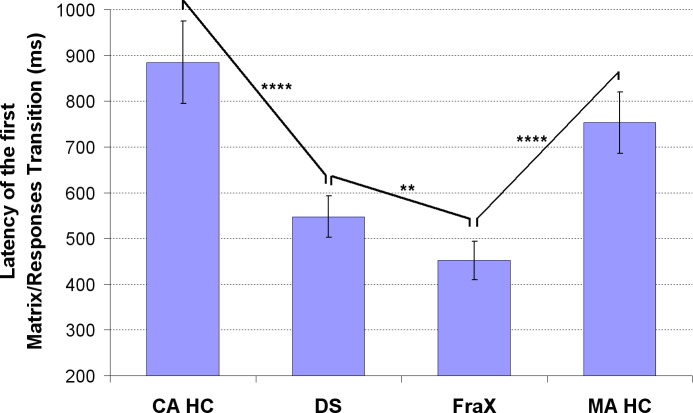
A significant group effect was also found on the latency of the first transition between the matrix and the responses: the first transition toward the responses occurred much earlier for the DS and FraX patients than for both CA HC and MA HC (Fig 9, Table 3). The healthy controls looked longer at the matrix before looking at the responses than the patients.
Fig 9. Analysis of the latency (ms) of the first transition between the matrix and the responses in DS, Fra X, and for both CA HC and MA HC.
(For display purposes and because the different chronological age-matched and mental age-matched control groups did not respectively differ significantly one from the other, the results of only one chronological age-matched control group and one mental age-matched control group are displayed).
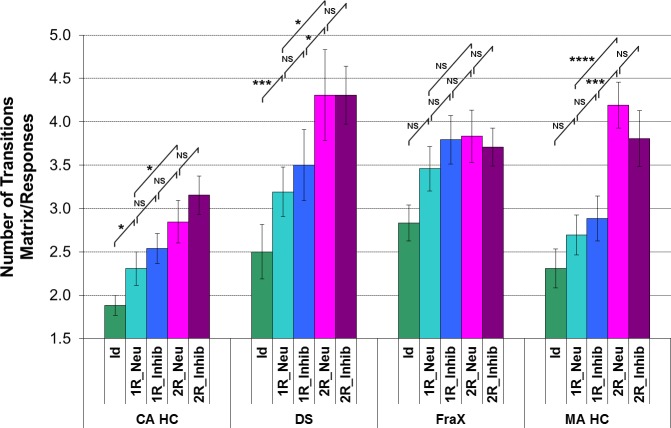
A significant effect of the matrix complexity (number of relations) on the number of transitions between the matrix and the responses was found in DS patients as well as in CA HC (Fig 10). Both groups made more transitions between the matrix and the responses for the conditions with two relations compared to the condition with one and for the conditions with one relation compared to the condition with zero. On the contrary, there was no significant effect of the matrix complexity on the number of transitions between the matrix and the responses in FraX patients.
Fig 10. Analysis of the number of transitions between the matrix and the responses for the DS, FraX, and for both CA HC and MA HC.
(For display purposes and because the different chronological age-matched and mental age-matched control groups did not respectively differ significantly one from the other, the results of only one chronological age-matched control group and one mental age-matched control group are displayed).
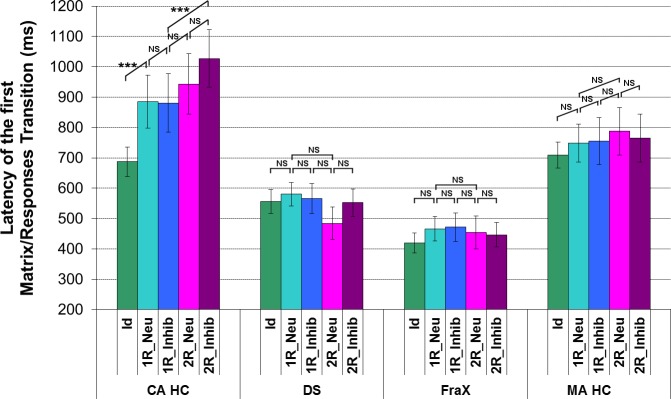
Unlike the CA HC, no effect of the matrix complexity was found on the latency of the first transition between the matrix and the responses in DS and in FraX patients: for all conditions, the latency of the first transition between the matrix and the responses was around 550ms and 400ms for the DS and FraX patients respectively (Fig 11).
Fig 11. Analysis of the latency (ms) of the first transition between the matrix and the responses for each of the 5 conditions in DS, Fra X, and for both CA HC and MA HC.
(For display purposes and because the different chronological age-matched and mental age-matched control groups did not respectively differ significantly one from the other, the results of only one chronological age-matched control group and one mental age-matched control group are displayed).
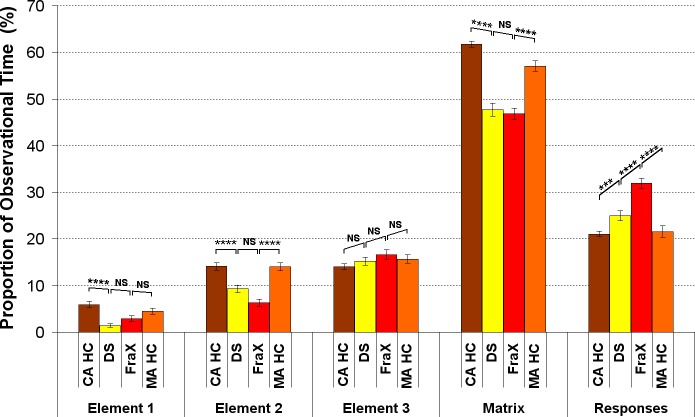
A significant group effect on the proportional time on each AOI was found in DS and FraX patients compared to their respective CA HC and MA HC: DS and FraX patients spent significantly less time on the matrix (elements 1 and 2), and more on the responses than their respective CA HC and MA HC (Fig 12). DS and FraX patients were the two groups spending the least time on element 1.
Fig 12. Analysis of the proportion of time on each AOI in DS, FraX, and for both CA HC and MA HC.
(For display purposes and because the different chronological age-matched and mental age-matched control groups did not respectively differ significantly one from the other, the results of only one chronological age-matched control group and one mental age-matched control group are displayed).
The analysis of the latency of the first fixation on each AOI revealed a different gaze pattern for DS, FraX patients and CA HC. DS patients looked first at element 3, then at the responses, then at element 2 and finally at element 1. FraX patients looked first at the responses, then at element 3, then element 2 and finally at element 1. The analysis of the latency of the first fixation on each AOI revealed that DS patients and MA HC used the same gaze pattern looking at the AOI in the same order, even if controls looked significantly later on the responses.
Discussion
We developed a novel visual analogical reasoning paradigm, inspired by the Progressive Raven’s Matrices, but appropriate for ID patients, enabling an objective and quantitative assessment of reasoning and inhibition abilities in this specific population.
The behavioral analysis (Study 1) showed in adult HC a significant effect of the matrix complexity (number of relations) on RT and ER, as well as an effect of inhibition but only for the most difficult conditions (two relations). They took more time to solve the matrix and made more errors with increasing matrix complexity and with the presence of inhibition. The effect of matrix complexity was much greater than the effect of inhibition in adult HC. Interestingly, we were able to quantify effects even in adult HC using this very simple paradigm. We did not observe any ceiling effect.
The developmental data showed that the effect of inhibition on ER was significantly greater than the effect of matrix complexity (number of relations) in TD children until they were 6 years old. The significant effect of inhibition on ER was not detected for the easiest condition (one relation) in TD children over the age of 10. This is in agreement with data from the literature about the time course of maturation of most executive functions [33–36]. Mathematical modeling of the developmental trajectory specific to this task (for RT and ER) was provided in HC [7, 32]. RT markedly decreased with age reaching a nadir at about 20 years of age in TD children then slowly increased again in adults. ER also strongly decreased with age. It would be interesting to include younger patients in order to determine whether this is also the case for ID patients.
In ID patients, as in adult HC, there was a significant effect of matrix complexity on RT and ER. But unlike in adult HC, the effect of inhibition was significant for the easiest conditions both on RT and ER in ID patients. They made more errors solving a simpler condition with inhibition than a more difficult condition without inhibition, showing that the effect of inhibition on ER was greater than the matrix complexity effect. DS, ARX and FraX patients were significantly slower and made significantly more errors than CA HC. ID patients made significantly more errors than their respective MA HC. ID patients and MA HC were similarly impaired when matrix complexity increased, but ID patients were more impaired than their respective MA HC by the inhibition. Executive inhibition deficit characterizes the three groups of ID patients we tested. The structure of executive functions with three components (working memory, inhibition and switching) seems to be similar in ID patients and healthy controls [3, 37].
The comparison between the three patient groups revealed that FraX patients made significantly more errors than ARX patients, who made significantly more errors than DS patients. FraX patients were very sensitive to inhibition even for the easiest conditions and made significantly more errors than DS and ARX patients for the easiest inhibition condition. Patients with FraX are known to exhibit attention deficit [18, 21, 22] and a deficit in executive functioning, especially in inhibition, working memory, cognitive flexibility and planning [20, 22, 38]. A broad impairment in executive functioning has also been suggested in DS patients, especially in cognitive flexibility, inhibition, working memory and planning [39]. Deficits in attention and executive functioning have both been found at a higher frequency in FraX patients in comparison with DS patients, especially inhibition deficits [21]. Our results are consistent with this finding. Very little is known about ARX patient neuropsychological profile. ARX patients seem to also have attention deficit [11]. It has been suggested in the literature that ID patients might have a specific profile of executive functioning with performance below their chronological age on all executive tasks; appropriate to their mental age in fluency or switching but below their mental age in inhibition, planning and non-verbal working memory [40, 41]. Our results suggest that this inhibition deficit is observed in ID patients, at a variable level according to the etiology of ID.
In order to analyze the cognitive strategies used to solve the task, an eye-tracking data analysis was also performed (Study 2). We adapted to our paradigm the parameters identified by Vigneau et al. to distinguish the cognitive strategies used to solve the reasoning task [30]. The eye-tracking analysis enabled us to differentiate a Matrix-based strategy involving the construction of an idealized answer which is then compared to the response alternatives of the response choice, from a Response-based strategy involving a process of feature comparison between elements of the problem and elements of the alternatives [29, 30], Table 4.
Table 4. Eye-tracking expected profiles for the matrix-based and the response-based strategies.
| Expected profiles for the two distinct cognitive strategies | ||
|---|---|---|
| Matrix-based strategy | Response-based strategy | |
| Number of transitions between the matrix and the responses | Few | Many |
| Proportion of time spent on the matrix | +++ | + |
| Proportion of time spent on the responses | + | +++ |
| Latency of the first transition between the matrix and the responses | increased | decreased |
| Latency of the first transition on the ‘responses’ | increases with matrix complexity | No effect of matrix complexity |
In Adult HC, We Identified a Matrix-Based Strategy
Adult HC spent most of their time looking at the matrix. They started by looking at the element 1 (reflecting an evenly distributed examination of the matrix cells), and finished by looking at the responses. When the matrix complexity increased, adult HC spent more time on the matrix before switching to the responses, and made more alternations between the matrix and the responses. This is consistent with Vigneau’s finding that higher ability subjects typically spent more time at the encoding phase of the process, especially when the number of rules increases, and demonstrates that our very simple paradigm was sensitive enough to reproduce these effects.
In DS and FraX Patients, We Identified a Response-Based Strategy (Table 4)
DS and FraX patients made more transitions than their respective CA HC. The first transition towards the responses occurred much earlier for DS and FraX patients than for their respective CA HC. The matrix complexity had no effect on the latency of the first transition between the matrix and the responses in DS and FraX patients. Moreover, DS and FraX patients spent significantly less time on the matrix, but more on the responses than CA HC. The gaze pattern in FraX patients was characterized by the fact that they first looked at the responses.
The developmental data showed a significant age effect on the number of transitions between the matrix and the responses, which decreases when children get older. There was also a significant age effect on the latency of the first transition between the matrix and the responses, which increases when children get older.
When DS and FraX patients were compared to their respective MA HC group MA HC looked longer at the matrix before switching to the responses. MA HC spent also proportionally more time on the matrix and less on the responses than both DS and FraX patients. The differences in gaze pattern between DS and FraX patients and their respective MA HC were striking. ID patients’ strategy is not only immature related to their delay, but also different from the typically developed children.
The eye-tracking analysis made it possible to identify the strategy used by participants to solve the task. ID patients are not able to explicitly explain the strategy they used to solve the task, but with eye-tracking analysis, we can understand how they performed the task, which is crucial information in order to help them improve their performance through remediation strategies.
It is interesting to note that the eye-tracking pattern allowed us to distinguish between DS and FraX: the first transition between matrix and responses occurred significantly later and FraX patients spent significantly more time on the responses than DS patients. Thus, the eye-tracking analysis of this new paradigm revealed not only differences between ID patients and controls, but also between the different etiologies of ID–a distinction that would not be possible using the Raven’s matrices [42].
Limitations and Future Perspectives
As our goal was to quantitatively assess visual analogical reasoning abilities in ID patients, we designed the paradigm to be as simple as possible. However, this meant limiting to only two response choices, leading to a high probability of correct answers. Despite this limitation, we observed significant differences between groups. Furthermore, we only acquired eye-tracking data in two ID populations (DS and FraX). It would be interesting to study other ID etiologies in the future (such as ARX, PQBP1 or Williams syndrome) to check if a specific eye-tracking pattern exists for each of them. Finally, we were able to determine the strategies that ID patients used to solve the task, but not the neural correlates associated with this cognitive strategy. Further study using fMRI would be needed in the future to determine these neural correlates.
We validated this novel visual analogical reasoning task, SimpleMatrices, in both ID patients and healthy controls. The test-retest reliability was assessed in patients and in healthy controls and showed high validity. We suggest that this paradigm, appropriate for ID patients and developmental populations, provides an objective and quantitative assessment of visual analogical reasoning and cognitive inhibition, and thus might be useful for the evaluation of pharmacological treatment or behavioural intervention in these specific populations.
Supporting Information
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(For display purposes and because the different chronological age-matched and mental age-matched control groups did not respectively differ significantly one from the other, the results of only one chronological age-matched control group and one mental age-matched control group are displayed).
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(PDF)
(DOC)
(DOC)
(DOC)
Acknowledgments
Authors are indebted to patients and their families and caregivers. Special thanks for the support of the French XLMR parents’ association “Xtraordinaire” and of the French Fragile X parents’ association “Le Goéland”. Financial support was provided from the French Ministry of Health (grant PHRC 2008, VDP), the CNRS / Hospice Civils de Lyon (research fellow post-graduate grant for AC), the Fondation Jérôme-Lejeune (grant for AC), the association “Xtraordinaire” (grant for AC), Novartis Pharmaceuticals and the ARRENNE association (grant for AC).
Abbreviations
- AOI
Area Of Interest
- ARX gene
Aristaless Related Homeobox gene
- ARX patient
ARX gene mutated patient
- CA HC
Chronological Age-matched Healthy Control
- DS
Down’s syndrome
- ER
Error Rate
- FMR1 gene
Fragile X Mental Retardation 1 gene
- FraX
Fragile X syndrome
- HC
Healthy Control
- ID
Intellectual Disability
- IQ
Intelligence Quotient
- MA HC
Mental Age-matched Healthy Control
- PM
Progressive Raven’s Matrices
- RT
Reaction Time
- TD
Typically Developed
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Financial support was provided from the French Ministry of Health (grant PHRC 2008, VDP), the CNRS / Hospice Civils de Lyon (research fellow post-graduate grant for AC), the Fondation Jérôme-Lejeune (grant for AC), the Xtraordinaire Association (grant for AC), Novartis Pharmaceuticals and the ARRENNE association (grant for AC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Authors received funding from Novartis Pharmaceuticals. But Novartis was not involved in the study design, collection, analysis, and interpretation of data, writing of the paper nor decision to submit for publication.
References
- 1.Schalock, R.L., S. Borthwick-Duffy, V. Bradley, W.H. Buntinx, and D.L. Coulter, Intellectual Disability: Definition, Classification, and Systems of Supports (Eleventh edition). Washington, DC., 2010.
- 2.Greenspan S. and Woods G.W., Intellectual disability as a disorder of reasoning and judgement: the gradual move away from intelligence quotient-ceilings. Curr Opin Psychiatry, 2015. 27(2): p. 110–6. [DOI] [PubMed] [Google Scholar]
- 3.Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., and Wager T.D., The unity and diversity of executive functions and their contributions to complex "Frontal Lobe" tasks: a latent variable analysis. Cogn Psychol, 2000. 41(1): p. 49–100. [DOI] [PubMed] [Google Scholar]
- 4.Gligorovic M. and Buha Ethurovic N., Inhibitory control and adaptive behaviour in children with mild intellectual disability. J Intellect Disabil Res, 2014. 58(3): p. 233–42. 10.1111/jir.12000 [DOI] [PubMed] [Google Scholar]
- 5.Ellis N.R. and Cavalier A.R., Research perspectives in mental retardation. Lawrence Erlbaum Associates Publishers, 1982: p. 121–152. [Google Scholar]
- 6.Zigler E. and Balla D., Mental retardation: the developmental-difference controversy Lawrence Erlbaum Associates Publishers, 1982. [Google Scholar]
- 7.Karmiloff-Smith A., Nativism versus neuroconstructivism: rethinking the study of developmental disorders. Dev Psychol, 2009. 45(1): p. 56–63. 10.1037/a0014506 [DOI] [PubMed] [Google Scholar]
- 8.Bittles A.H., Bower C., Hussain R., and Glasson E.J., The four ages of Down syndrome. Eur J Public Health, 2007. 17(2): p. 221–5. [DOI] [PubMed] [Google Scholar]
- 9.Bienvenu T., Poirier K., Friocourt G., Bahi N., Beaumont D., Fauchereau F., et al. , ARX, a novel Prd-class-homeobox gene highly expressed in the telencephalon, is mutated in X-linked mental retardation. Hum Mol Genet, 2002. 11(8): p. 981–91. [DOI] [PubMed] [Google Scholar]
- 10.Stromme P., Mangelsdorf M.E., Shaw M.A., Lower K.M., Lewis S.M., Bruyere H., et al. , Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nat Genet, 2002. 30(4): p. 441–5. [DOI] [PubMed] [Google Scholar]
- 11.Curie A., Nazir T., Brun A., Paulignan Y., Reboul A., Delange K., et al. , The c.429_452 duplication of the ARX gene: a unique developmental-model of limb kinetic apraxia. Orphanet J Rare Dis, 2014. 9(1): p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberle I., Rousseau F., Heitz D., Kretz C., Devys D., Hanauer A., et al. , Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science, 1991. 252(5009): p. 1097–102. [DOI] [PubMed] [Google Scholar]
- 13.Vicari S., Implicit versus explicit memory function in children with Down and Williams syndrome. Downs Syndr Res Pract, 2001. 7(1): p. 35–40. [DOI] [PubMed] [Google Scholar]
- 14.Vicari S., Motor development and neuropsychological patterns in persons with Down syndrome. Behav Genet, 2006. 36(3): p. 355–64. [DOI] [PubMed] [Google Scholar]
- 15.Lott I.T. and Dierssen M., Cognitive deficits and associated neurological complications in individuals with Down's syndrome. Lancet Neurol, 2010. 9(6): p. 623–33. 10.1016/S1474-4422(10)70112-5 [DOI] [PubMed] [Google Scholar]
- 16.Jarrold C., Baddeley A.D., and Phillips C., Long-term memory for verbal and visual information in Down syndrome and Williams syndrome: performance on the Doors and People test. Cortex, 2007. 43(2): p. 233–47. [DOI] [PubMed] [Google Scholar]
- 17.Frenkel S. and Bourdin B., Verbal, visual, and spatio-sequential short-term memory: assessment of the storage capacities of children and teenagers with Down's syndrome. J Intellect Disabil Res, 2009. 53(2): p. 152–60. 10.1111/j.1365-2788.2008.01139.x [DOI] [PubMed] [Google Scholar]
- 18.Cornish K.M., Munir F., and Cross G., Spatial cognition in males with Fragile-X syndrome: evidence for a neuropsychological phenotype. Cortex, 1999. 35(2): p. 263–71. [DOI] [PubMed] [Google Scholar]
- 19.Van der Molen M.J.W., Huizenga M., Huizenga H.M., Ridderinkhof K.R., Van der Molen M.W., Hamel B., et al. , Profiling Fragile X syndrome in males: Strengths and weaknesses in cognitive abilities. Res Dev Disabil, 2010. 31: p. 426–439. 10.1016/j.ridd.2009.10.013 [DOI] [PubMed] [Google Scholar]
- 20.Hooper S.R., Hatton D., Sideris J., Sullivan K., Hammer J., Schaaf J., et al. , Executive functions in young males with fragile X syndrome in comparison to mental age-matched controls: baseline findings from a longitudinal study. Neuropsychology, 2008. 22(1): p. 36–47. 10.1037/0894-4105.22.1.36 [DOI] [PubMed] [Google Scholar]
- 21.Munir F., Cornish K.M., and Wilding J., A neuropsychological profile of attention deficits in young males with fragile X syndrome. Neuropsychologia, 2000. 38(9): p. 1261–70. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan K., Hatton D.D., Hammer J., Sideris J., Hooper S., Ornstein P.A., et al. , Sustained attention and response inhibition in boys with fragile X syndrome: measures of continuous performance. Am J Med Genet B Neuropsychiatr Genet, 2007. 144B(4): p. 517–32. [DOI] [PubMed] [Google Scholar]
- 23.Picker J.D. and Walsh C.A., New innovations: therapeutic opportunities for intellectual disabilities. Ann Neurol, 2013. 74(3): p. 382–90. 10.1002/ana.24002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry-Kravis E., Hessl D., Abbeduto L., Reiss A.L., Beckel-Mitchener A., and Urv T.K., Outcome measures for clinical trials in fragile X syndrome. J Dev Behav Pediatr, 2013. 34(7): p. 508–22. 10.1097/DBP.0b013e31829d1f20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacquemont S., Curie A., des Portes V., Torrioli M.G., Berry-Kravis E., Hagerman R.J., et al. , Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med, 2011. 3(64): p. 64ra1 10.1126/scitranslmed.3001708 [DOI] [PubMed] [Google Scholar]
- 26.Carpenter P.A., Just M.A., and Shell P., What one intelligence test measures: a theoretical account of the processing in the Raven Progressive Matrices Test. Psychol Rev, 1990. 97(3): p. 404–31. [PubMed] [Google Scholar]
- 27.Jensen A.R., Individual differences in the Hick paradigm. Speed of information processing and intelligence, Edition Vernon, 1987: p. 101–175. [Google Scholar]
- 28.Verguts A., De Boek P., and Maris E., Generation speed in Raven's Progressive Matrices test. Intelligence, 2000. 27: p. 329–345. [Google Scholar]
- 29.Bethell-Fox C.E., Lohman D.F., and Snow R.E., Adaptive reasoning: componential and eye movement analysis of geometric analogy performance. Intelligence, 1984. 8: p. 205–238. [Google Scholar]
- 30.Vigneau F., Caissie A.F., and Bors D.A., Eye-movement analysis demonstrates strategic influences on intelligence. Intelligence, 2006. 34: p. 262–272. [Google Scholar]
- 31.Karatekin C., Eye tracking studies of normative and atypical development. Developmental review., 2007. 27: p. 283–348. [Google Scholar]
- 32.Thomas M.S., Annaz D., Ansari D., Scerif G., Jarrold C., and Karmiloff-Smith A., Using developmental trajectories to understand developmental disorders. J Speech Lang Hear Res, 2009. 52(2): p. 336–58. 10.1044/1092-4388(2009/07-0144) [DOI] [PubMed] [Google Scholar]
- 33.Paus T., Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci, 2005. 9(2): p. 60–8. [DOI] [PubMed] [Google Scholar]
- 34.Adleman N.E., Menon V., Blasey C.M., White C.D., Warsofsky I.S., Glover G.H., et al. , A developmental fMRI study of the Stroop color-word task. Neuroimage, 2002. 16(1): p. 61–75. [DOI] [PubMed] [Google Scholar]
- 35.Tamm L., Menon V., and Reiss A.L., Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry, 2002. 41(10): p. 1231–8. [DOI] [PubMed] [Google Scholar]
- 36.Durston S., Tottenham N.T., Thomas K.M., Davidson M.C., Eigsti I.M., Yang Y., et al. , Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry, 2003. 53(10): p. 871–8. [DOI] [PubMed] [Google Scholar]
- 37.Willner P., Bailey R., Parry R., and Dymond S., Evaluation of executive functioning in people with intellectual disabilities. J Intellect Disabil Res, 2010. 54(4): p. 366–79. 10.1111/j.1365-2788.2010.01249.x [DOI] [PubMed] [Google Scholar]
- 38.Woodcock K.A., Oliver C., and Humphreys G.W., Task-switching deficits and repetitive behaviour in genetic neurodevelopmental disorders: data from children with Prader-Willi syndrome chromosome 15 q11-q13 deletion and boys with Fragile X syndrome. Cogn Neuropsychol, 2009. 26(2): p. 172–94. 10.1080/02643290802685921 [DOI] [PubMed] [Google Scholar]
- 39.Lanfranchi S., Jerman O., Dal Pont E., Alberti A., and Vianello R., Executive function in adolescents with Down Syndrome. J Intellect Disabil Res, 2010. 54(4): p. 308–19. 10.1111/j.1365-2788.2010.01262.x [DOI] [PubMed] [Google Scholar]
- 40.Danielsson H., Henry L., Ronnberg J., and Nilsson L.G., Executive functions in individuals with intellectual disability. Res Dev Disabil, 2010. 31(6): p. 1299–304. 10.1016/j.ridd.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 41.Danielsson H., Henry L., Messer D., and Ronnberg J., Strengths and weaknesses in executive functioning in children with intellectual disability. Res Dev Disabil, 2012. 33(2): p. 600–7. 10.1016/j.ridd.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 42.Vakil E. and Lifshitz-Zehavi H., Solving the Raven Progressive Matrices by adults with intellectual disability with/without Down syndrome: different cognitive patterns as indicated by eye-movements. Res Dev Disabil, 2012. 33(2): p. 645–54. 10.1016/j.ridd.2011.11.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(For display purposes and because the different chronological age-matched and mental age-matched control groups did not respectively differ significantly one from the other, the results of only one chronological age-matched control group and one mental age-matched control group are displayed).
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(PDF)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.