Key points
Erythropoietin is a neuroprotectant undergoing clinical trial for brain injury in term and preterm infants.
This is the first experimental study to assess the acute effects of erythropoietin on the cerebral white matter in preterm ventilated lambs.
Administration of erythropoietin within minutes of injurious ventilation onset amplified pro‐inflammatory cytokine gene expression in both the periventricular and subcortical white matter compared to the ventilation alone group; erythropoietin had no effect on the area of microglial aggregations in white matter regions with a reduction in cellular density of aggregations in the subcortical white matter only.
Administration of erythropoietin in conjunction with injurious ventilation increased gene expression of tight junction proteins and reduced protein extravasation from blood vessels in the cerebral white matter.
Given the increase in inflammation after erythropoietin, we recommend further investigation into its use as a treatment for ventilated preterm babies prior to clinical translation.
Abstract
Inadvertently injurious ventilation of preterm neonates in the delivery room can cause cerebral white matter (WM) inflammation and injury. We investigated the impact of an early high dose of recombinant human erythropoietin (EPO) on ventilation‐induced WM changes in preterm lambs. Injurious ventilation, targeting a V T of 15 ml kg−1 with no positive end‐expiratory pressure, was initiated for 15 min in preterm lambs (0.85 gestation). Conventional ventilation was continued for a further 105 min. Lambs received either 5000 IU kg−1 of EPO (EPREX®; Vent+EPO; n = 6) or vehicle (Vent; n = 8) via an umbilical vein at 4 ± 2 min. Markers of WM injury and inflammation were assessed using quantitative real‐time PCR (qPCR) and immunohistochemistry and compared to a group of unventilated controls (UVC; n = 4). In Vent+EPO lambs compared to Vent lambs: (i) interleukin (IL)‐1β and IL‐6 mRNA levels in the periventricular WM and IL‐8 mRNA levels in the subcortical WM were higher (P < 0.05 for all); (ii) the density of microglia within the aggregations was not different in the periventricular WM and was lower in the subcortical WM (P = 0.001); (iii) the density of astrocytes was lower in the subcortical WM (P = 0.002); (iv) occludin and claudin‐1 mRNA levels were higher in the periventricular WM (P < 0.02 for all) and (vi) the number of blood vessels with protein extravasation was lower (P < 0.05). Recombinant human EPO had variable regional effects within the WM when administered during injurious ventilation. The adverse short‐term outcomes discourage the use of early high dose EPO administration in preterm ventilated babies.
Key points
Erythropoietin is a neuroprotectant undergoing clinical trial for brain injury in term and preterm infants.
This is the first experimental study to assess the acute effects of erythropoietin on the cerebral white matter in preterm ventilated lambs.
Administration of erythropoietin within minutes of injurious ventilation onset amplified pro‐inflammatory cytokine gene expression in both the periventricular and subcortical white matter compared to the ventilation alone group; erythropoietin had no effect on the area of microglial aggregations in white matter regions with a reduction in cellular density of aggregations in the subcortical white matter only.
Administration of erythropoietin in conjunction with injurious ventilation increased gene expression of tight junction proteins and reduced protein extravasation from blood vessels in the cerebral white matter.
Given the increase in inflammation after erythropoietin, we recommend further investigation into its use as a treatment for ventilated preterm babies prior to clinical translation.
Abbreviations
- CSF
cerebrospinal fluid
- EPO
recombinant human erythropoietin
- GFAP
glial fibrillary acidic protein
- Iba‐1
ionized calcium‐binding adapter molecule‐1
- IL
interleukin
- IVH
intraventricular haemorrhage
- VT
tidal volume
- UVC
unventilated control
- WM
white matter
Introduction
There are two pathways key to preterm brain injury: the instigation of a cerebral inflammatory response (which can trigger a cascade of pathways that can ultimately amplify white matter (WM) injury) and cerebral haemodynamic instability (Del Toro et al. 1991; Khwaja & Volpe, 2008). Preterm infants are already vulnerable to both cerebral inflammation and haemodynamic instability, and thus any neonatal intervention with the potential to exacerbate these pathways may increase the risk of chronic disability. Due to the immaturity of a preterm infant's lungs, many will require some form of respiratory support (Chow, 2011). However, initiation of respiratory support in the delivery room can be injurious due to the inadvertently high tidal volumes (V T) delivered (ranging from 0–31 ml kg−1) (Schmölzer et al. 2010). We have previously demonstrated that the initiation of ventilation of preterm lambs with high V T activates the pathways involved in preterm brain injury by altering cerebral haemodynamic stability and initiating a cerebral inflammatory response (Polglase et al. 2012; Skiöld et al. 2014).
Erythropoietin (EPO) is a glycoprotein hormone with extensive neuroprotective effects acting through anti‐apoptotic, anti‐oxidative stress and anti‐inflammatory pathways (Van Der Kooij et al. 2008). These pathways are involved in preterm brain injury (Volpe, 2003; Khwaja & Volpe, 2008) and are exacerbated by ventilation (Polglase et al. 2012; Barton et al. 2014); thus, EPO may reduce the progression of white matter injury characteristic of chronic disability such as cerebral palsy (Counsell et al. 2003). Indeed, clinical trials demonstrate that EPO is well tolerated and improves neurodevelopmental outcomes in preterm infants when administered up to the first 24 h of life (He et al. 2008; Juul et al. 2008). A recent clinical trial has shown that high dose EPO (3000 IU/kg) administered to preterm infants before 3 h of age at birth, at 12–18 h and at 36–42 h after birth improves WM development (O'Gorman et al. 2015). However, early treatment with EPO, particularly in ventilated preterm infants, remains relatively unexplored and deserves further validation given EPOs rapid translation into the clinical setting. Indeed, our recent study of early high dose EPO (5000 IU/kg) in preterm ventilated lambs found increased lung inflammation and injury compared to ventilated controls (Polglase et al. 2014 a), which suggests that early high dose EPO may amplify ventilation‐induced lung injury, and may subsequently lead to brain inflammation and injury.
In this study we aimed to assess the short‐term effects of acute high dose EPO on WM inflammation and injury, and cerebral haemodynamics using our model of injurious ventilation in the preterm lamb. This is the first study to explore the effects of EPO administration after only 2 h, rendering this study important in characterizing the immediate neonatal response (physiological and neuropathological) to an acute high dose of EPO using a clinically relevant preterm model of ventilation. Given our previous findings in the lungs (Polglase et al. 2014 a), we hypothesized that early high dose EPO administered 4 min after the initiation of high V T ventilation would exacerbate ventilation‐induced brain injury.
Methods
Experiments were approved by the Monash Medical Centre Animal Ethics Committee A, Monash University, and were conducted in accordance with the National Health and Medical Research Council of Australia's guidelines.
Instrumentation and delivery
At 126 ± 1 (mean ± SD) days of gestation, unventilated controls (UVC; n = 4) were humanely killed (sodium pentobarbitone; > 100 mg kg−1, i.v.) without surgical instrumentation, anaesthesia or ventilation. For the ventilated groups, the ewe was anaesthetized via intravenous injection of thiopentone sodium (20 mg kg−1), followed by tracheal intubation and delivery of inhalational anaesthesia (isoflurane 1.5–2.5% in oxygen; Bomac Animal Health, NSW, Australia). The fetuses were delivered by caesarean section, dried, weighed and placed under a radiant heater for ventilation. The ewe was immediately killed after delivery of the fetuses (sodium pentobarbitone; > 100 mg kg−1, i.v.). Polyvinyl catheters (ID 0.86 mm, OD 1.52 mm, Dural Plastics, Australia) were inserted into both the umbilical artery and vein to monitor mean arterial pressure and maintain anaesthesia, respectively. Anaesthesia was maintained for the entirety of the experiment to prevent spontaneous breathing (Alfaxane i.v. 5–15 mg kg−1 h−1; Jurox, East Tamaki, Auckland, New Zealand). Ventilation was initiated with a high tidal volume targeting 12–15 ml kg−1 (peak inspiratory pressure was adjusted with an upper limit of 50 cmH2O) with no positive‐end expiratory pressure (PEEP) to cause ventilation‐induced brain pathology as described previously (Barton et al. 2015). After 15 min, lambs were ventilated in volume‐guarantee mode (tidal volume 7 ml kg−1, PEEP 5 cmH2O, inspiratory time 0.5 s and expiratory time 0.5 s) for a further 105 min. Ventilation was conducted using warmed, humidified air with the fraction of inspired oxygen initially set at 0.4 but adjusted to maintain arterial oxygen saturation () between 88 and 95%. Well‐being of the newborn preterm lamb was monitored by regular blood gas analysis (ABL30, Radiometer, Copenhagen, Denmark) and pre‐ductal transcutaneous oxyhaemoglobin saturation (data not shown). At the conclusion of ventilation, the lambs were humanely killed (sodium pentobarbitone; > 100 mg kg−1, i.v.) and samples collected (see below).
Treatment
Immediately following ventilation onset (4 ± 2 min), lambs were randomized to receive a bolus infusion of 5000 IU kg−1 of recombinant human (rh) EPO (Vent+EPO; n = 6; EPREX® Janssen, North Ryde, NSW, Australia) or an equivalent volume of vehicle (Vent; n = 8; pH neutral phosphate buffered saline) via the umbilical vein catheter. Blood samples were collected regularly during ventilation to monitor the lamb's well‐being.
Haemodynamic measurements
Spatially resolved spectroscopy (SRS, NIRO 200 Spectrophotometer; Hamamatsu Photonics K.K., Hamatsu City, Japan) was used to measure cerebral tissue oxygen saturation in real time as an indication of overall cerebral oxygenation (Murkin & Avango, 2009).
Tissue and cerebral spinal fluid collection
At autopsy, cerebral spinal fluid (CSF; 1.5 ml) was collected via the cisterna magna; EPO concentration was assessed using a human erythropoietin ELISA kit (STEMCELL Technologies, Melbourne, Australia). The brain was removed from the skull and the cerebrum halved along the midline. The periventricular WM and subcortical WM were dissected from the left cerebral hemisphere at the level of the lateral ventricle and snap‐frozen in liquid nitrogen. The right cerebral hemisphere was immersed in 4% paraformaldehyde (PFA) for 24 h. The entire forebrain was then cut coronally into blocks 5 mm thick (8–9 blocks per animal), post‐fixed in 4% PFA for 6 days and embedded in paraffin. Serial, 8 μm‐thick sections were cut from one block each at the level of the frontal and parietal lobes to include the periventricular and subcortical WM for immunohistochemical analysis. The lungs of these lambs were collected for a separate series of investigations with findings published previously (Polglase et al. 2014 a).
Real‐time PCR
The periventricular and subcortical WM from the left hemisphere were separately homogenized, and total mRNA was isolated from each region (RNeasy Midi Kit, Qiagen) and reverse‐transcribed into cDNA (SuperScript III reverse transcriptase, Invitrogen). Genes of interest were measured by qRT‐PCR using an Applied Biosystems 7900HT Fast Real‐Time PCR system. Relative mRNA expression of key pro‐inflammatory interleukins (IL‐1β, IL‐6 and IL‐8), tight junction proteins (occludin, claudin‐1 and claudin‐5) and markers of cell death (p53 and caspase‐3; subcortical WM only) were measured (see Table 1 for primer details). The expression of all genes was normalized to the 18S rRNA for each sample using the cycle threshold (ΔΔC T) method of analysis and was expressed relative to the UVC group.
Table 1.
Primer sequences for quantitative real time PCR
| Gene | Species | Accession no. | Primer sequence | Amplicon length (nt) |
|---|---|---|---|---|
| 18S | Rat | X01117 | 5′‐GTAACCCGTTGAACCCCATT‐3′ | 105 |
| 5′‐CCATCCAATCGGTAGTAGCG‐3′ | ||||
| IL‐1β | Sheep | NM_001009465 | 5′‐CGATGAGCTTCTGTGTGATG‐3′ | 120 |
| 5′‐CTGTGAGAGGAGGTGGAGAG‐3′ | ||||
| IL‐6 | Sheep | NM_001009392 | 5′‐CGCAAAGGTTATCATCATCC‐3′ | 107 |
| 5′‐CCCAGGAACTACCACAATCA‐3′ | ||||
| IL‐8 | Sheep | NM_001009401 | 5′‐CCTCAGTAAAGATGCCAATGA‐3′ | 82 |
| 5′‐TGACAACCCTACACCAGACC‐3′ | ||||
| Occludin | Cow | NM_001082433 | 5′‐GCAGGAAGTGCCTTTGGTAG‐3′ | 68 |
| 5′‐CCATAGCCATAACCGTAGCC‐3′ | ||||
| Claudin‐5 | Sheep | XM_004023107 | 5′‐CCTGGACCACAACATCGTGA‐3′ | 75 |
| 5′‐AGCACCGAGTCGTACACTTT‐3′ | ||||
| Claudin‐1 | Sheep | NM_001185016 | 5′‐GACGACGAGGCACAGAAGAT‐3′ | 108 |
| 5′‐CAAAACAGCCAGACCTGAAA‐3′ | ||||
| p53 | Sheep | NM_001009403 | 5′‐CAATGGAAGAATCGCAGGCA‐3′ | 143 |
| 5′‐TGAGTACGGGAGCAGGTCA‐3′ | ||||
| Caspase‐3 | Sheep | XM_004021690 | 5′‐ACCTCACCACGGAACTTTGT‐3′ | 56 |
| 5′‐GCAGGACTTGTTCTTCGGGA‐3′ |
Immunohistochemistry
Coronal sections from equivalent sites from the frontal and parietal lobes were stained with rabbit anti‐ionized calcium‐binding adapter molecule‐1 (Iba‐1; 1:1500, WAKO Pure Chemical Industries, Osaka, Japan) to identify microglia, rabbit anti‐sheep serum (1:1000, Sigma, USA) to identify altered blood–brain barrier permeability, rabbit anti‐glial fibrillary acidic protein (GFAP; 1:1000, DAKO, Glostrup, Denmark) to identify reactive astrocytes and rabbit anti‐caspase‐8 (1:100, Biorbyt Ltd, UK) to identify cells entering the apoptotic pathway. Prior to incubation with anti‐Iba‐1, ‐GFAP and ‐caspase‐8, sections were pre‐treated with citrate buffer (pH 6.0) in a microwave oven. Sections were incubated with appropriate biotinylated secondary antibodies (1:200) and reacted using the Vectastain Elite AB Complex kit (Vector Laboratories, Burlingame, CA, USA). Sections reacted with anti‐Iba‐1 and anti‐sheep serum were counterstained with 0.1% thionin. For each antibody, sections from each cohort were simultaneously reacted to reduce staining variability. When the primary antibody was replaced with phosphate buffered saline (pH 7.4) as a negative control, immunoreactivity was not observed.
Quantitative immunohistochemical analysis
Analyses were conducted at equivalent sites within the cerebral WM on one section from each of the frontal and parietal lobes from each lamb. Slides were coded and the observer was blinded to treatment.
The proportion of the periventricular WM and subcortical WM area occupied by Iba‐1‐positive microglial aggregations was assessed using ImageScope software (Aperio Technologies, Vista, CA, USA). The areas of individual aggregations were summed and divided by the total subcortical and periventricular WM area of the section; data are expressed as a percentage (%). The areal coverage (%) of Iba‐1‐immunoreactivity within an aggregation was also assessed using a set intensity threshold (ImageJ, NIH Image, Bethesda, MD, USA). For each parameter, the mean was calculated for each WM region and for each animal, and a mean of means determined for each treatment group.
Areal density (cells mm−2) of Iba‐1‐positive microglia, GFAP‐positive astrocytes and caspase 8‐positive cells were counted in two random non‐overlapping fields in the periventricular WM and in six random non‐overlapping fields in the subcortical WM from three separate gyri (avoiding microglial aggregations) using ImageJ. Microglia were distinguished based on morphology (e.g. ramified microglia were characterized by long branching processes; amoeboid microglia by large, densely stained soma with retracted processes)(Atik et al. 2014; Van Den Heuij et al. 2014). The ratio of amoeboid‐to‐total microglia was also determined and expressed as a percentage. For each immunostain, the mean cell density was determined for each animal and for each WM region, and then collectively averaged for each treatment group.
Blood vessel profiles with serum extravasation were counted in the cerebral WM (periventricular and subcortical combined) and expressed as number of vessel profiles with protein extravasation within the total WM area. Given the acute nature of the study, the total number of blood vessels is unlikely to vary between groups, therefore the number of vessels showing serum extravasation reflects the incidence of protein extravasation. A mean value was calculated for each animal for comparison between groups.
Statistical analysis
Two‐way repeated measures ANOVA was used to compare the serial V T and NIRS data between groups and over time (Sigmaplot, Systat Software Inc.). All fetal, molecular and immunohistochemical data were compared using a one‐way ANOVA (for parametric data) with a Holms–Sidak test for post hoc comparisons, or Kruskal–Wallis ANOVA on ranks (for non‐parametric data) with Dunn's test for post hoc comparisons. Linear regression analysis was conducted to determine if there was a correlation between the concentration of EPO within the CSF and the molecular and immunohistochemical data for each Vent+EPO lamb. Data are presented as means ± SEM. Values of P < 0.05 were considered statistically significant.
Results
Lamb well‐being
Body weights, sex, birth order and fetal pH, , and have been published previously (Polglase et al. 2014 a), and were not different between groups.
Cerebrospinal fluid rhEPO concentration
All Vent+EPO lambs had detectable levels of rhEPO in CSF 2 h following administration, with a mean concentration of 292.5 ± 52.2 mU ml−1 (Fig. 1; the normal mean CSF concentration of rhEPO in CSF of human neonates is 7.6 ± 1.4 mU ml−1; Juul et al. 1997).
Figure 1. rhEPO concentration in cerebrospinal fluid .
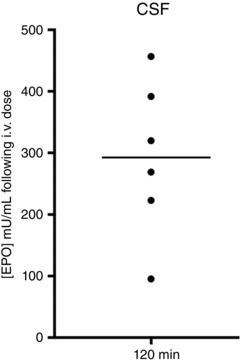
EPO concentration (mU ml−1) measured in cerebrospinal fluid (CSF) collected at the conclusion of ventilation (120 min) in each Vent+EPO lamb (black line represents mean concentration). All Vent+EPO lambs (n = 6) had a measureable EPO concentration in their CSF ∼120 min after i.v. administration.
Ventilation, oxygenation and physiology
Delivered V T during the initial 15 min of ventilation was not different between groups, nor was it different thereafter. Vent+EPO lambs did show higher variability between animals in delivered V T compared to Vent lambs (Fig. 2 A). The cerebral tissue oxygenation index was significantly higher in the Vent+EPO lambs at 6–13 min compared to Vent lambs, but there was no difference for the remainder of the study (Fig. 2 B).
Figure 2. Ventilator and oxygenation parameters .

A, tidal volume (V T) delivered did not differ between Vent and Vent+EPO lambs for the duration of ventilation. B, tissue oxygenation index (TOI; measured using NIRS) was higher in Vent+EPO lambs compared to Vent lambs between 6 and 13 min during high V T delivery but there was no difference thereafter. Data are presented as means ± SEM. #Significant difference (P < 0.05) between Vent and Vent+EPO groups.
Cerebral inflammation
IL‐1β mRNA levels in the periventricular WM were higher in Vent+EPO lambs compared to both UVC and Vent groups (P < 0.001), but there were no differences observed in the subcortical WM (P = 0.534; Fig. 3 A).
Figure 3. Pro‐inflammatory cytokine gene expression in the cerebral WM .
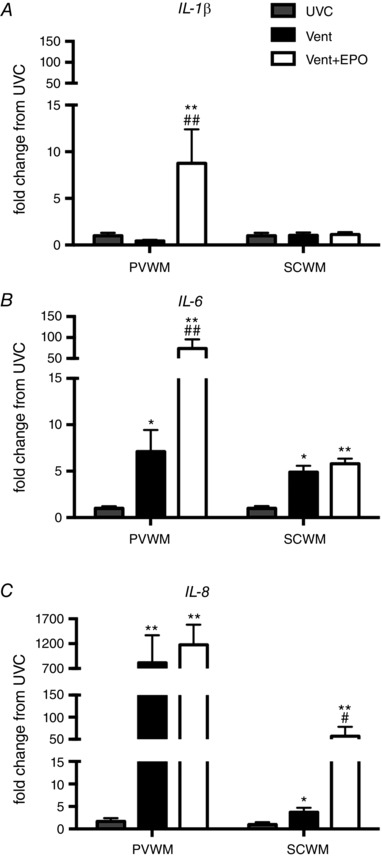
A, IL‐1β mRNA levels were increased in Vent+EPO compared to UVC and Vent in the PVWM; there was no difference between groups in the SCWM. B, IL‐6 mRNA levels was increased in Vent compared to UVC in the PVWM and further increased in Vent+EPO; in the SCWM Vent and Vent+EPO had increased levels compared to UVC. C, IL‐8 mRNA levels were increased in Vent and Vent+EPO compared to UVC in the PVWM; in the SCWM IL‐8 mRNA levels were increased in Vent compared to UVC and further increased in Vent+EPO compared to both UVC and Vent. *P < 0.05 and **P < 0.001 compared to UVC; # P < 0.05 and ## P < 0.001 compared to Vent.
IL‐6 mRNA levels in the periventricular WM were higher in Vent lambs compared to UVC (P = 0.001) and were further amplified in Vent+EPO lambs compared to both Vent (P < 0.001) and UVC (P < 0.001). In the subcortical WM, IL‐6 mRNA levels were higher in Vent (P = 0.001) and Vent + EPO (P < 0.001) lambs compared to UVC, but there was no difference between the two ventilation groups (Fig. 3 B).
IL‐8 mRNA levels in the periventricular WM were higher in both Vent and Vent+EPO lambs compared to UVC (P < 0.001 for both), but there was no difference between Vent groups. IL‐8 mRNA levels in the subcortical WM were higher in Vent lambs compared to UVC (P = 0.003) and were greater in Vent+EPO lambs compared to Vent (P = 0.004) and UVC (P < 0.001; Fig. 3 C).
The area of periventricular WM occupied by microglial aggregations tended to be higher in Vent lambs compared to UVC (P = 0.092), and was higher in Vent+EPO lambs compared to UVC (P = 0.033; Fig. 4 A). In the subcortical WM, the area of WM occupied by microglial aggregations was higher in the Vent group compared to UVC (P = 0.005), but tended to be lower in the Vent+EPO group compared to the Vent group (P = 0.051; Fig. 4 A). The areal coverage of Iba‐1‐immunoreactivity in the periventricular WM within aggregations was not significantly different between groups (P = 0.079; Fig. 4 B). The areal coverage of Iba‐1‐immunoreactivity within aggregations in the subcortical WM was significantly higher in Vent compared to UVC lambs (P < 0.001) and Vent+EPO lambs had lower areal coverage within aggregations than Vent lambs (P = 0.001; Fig. 4 B).
Figure 4. Iba‐1‐positive microglia in the cerebral WM .
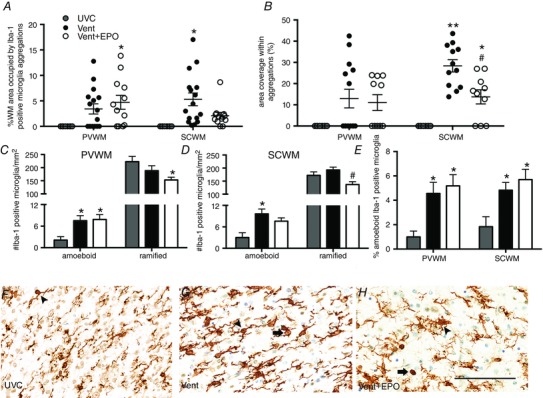
A, the percentage of WM occupied by microglial aggregations increased in Vent+EPO compared to UVC in the periventricular WM (PVWM); the percentage of WM occupied by microglial aggregations increased in Vent compared to UVC in the subcortical WM (SCWM). B, the area coverage (%) of Iba‐1‐immunoreactivity in aggregations was not different between groups in the PVWM; in the SCWM it was increased in Vent and Vent+EPO compared to UVC; Vent+EPO had reduced area coverage (%) compared to Vent. C, the density of amoeboid microglia in the PVWM was higher in the Vent and Vent+EPO groups compared to UVC; there was a reduction in the density of ramified microglia in the Vent+EPO group compared to UVC in the PVWM. D, the density of amoeboid microglia in the SCWM was increased in the Vent group compared to UVC; the density of ramified microglia in the SCWM was decreased in the Vent+EPO group compared to Vent alone. E, this resulted in a significant increase in % amoeboid microglia in the Vent and Vent+EPO groups compared to UVC in both PVWM and SCWM. F–H, representative images from the SCWM with arrowheads indicating ramified microglia and arrows indicating amoeboid microglia. Scale bar = 100 μm. *P < 0.05 and **P < 0.001 compared to UVC; # P < 0.05 and ## P < 0.001 compared to Vent.
The density of Iba‐1‐positive amoeboid microglia in the periventricular WM was higher in both the Vent and Vent+EPO groups compared to UVC (P = 0.034 for both); there was no difference between ventilation groups. The density of ramified microglia in the periventricular WM was lower in the Vent+EPO group compared to UVC (P = 0.048) but there was no difference between UVC and Vent groups (Fig. 4 C). In the subcortical WM, the density of Iba‐1‐positive amoeboid microglia was higher in the Vent group compared to UVC (P = 0.005), and tended to be higher in the Vent+EPO group than UVC (P = 0.058). The density of ramified microglia was not different between UVC and Vent groups, but was lower in the Vent+EPO group compared to Vent (P = 0.003; Fig. 4 D and F–H). The ratio of amoeboid to total microglia in both the periventricular and subcortical WM was higher in the Vent group (P = 0.030 and P = 0.026, respectively) and Vent+EPO group (P = 0.021 and P = 0.009, respectively) compared to UVC; there was no difference between ventilated groups (Fig. 4 E).
Astrogliosis and apoptosis
The density of GFAP‐positive astrocytes in the periventricular WM was not different between groups (P = 0.102; Fig. 5 A). The density of GFAP‐positive astrocytes in the subcortical WM tended to be higher in Vent compared to UVC lambs (P = 0.053) and was lower in Vent+EPO lambs compared to Vent lambs (P = 0.002; Fig. 5 A–D).
Figure 5. Astrocyte density .
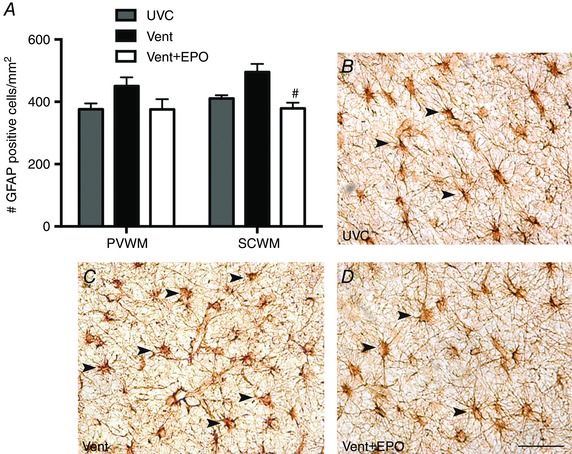
A, the density of GFAP‐positive astrocytes in the subcortical WM (SCWM) was reduced in Vent+EPO compared to Vent lambs; there was no change in the periventricular WM (PVWM). This is demonstrated by comparing images of the SCWM from UVC (B), Vent (C) and Vent+EPO (D) lambs. Arrowheads indicate GFAP‐positive astrocytes. Scale bar = 50 μm. # P < 0.05 compared to Vent.
In the periventricular WM, p53 mRNA levels were higher in Vent+EPO (P = 0.023) but not Vent (P = 0.241) lambs when compared to UVC lambs (Fig. 6 A). In the subcortical WM, p53 mRNA levels were higher in the Vent and Vent+EPO lambs compared to UVC (P < 0.001; Fig. 6 A), but there was no difference between Vent groups (P > 0.05; Fig. 6 A). Caspase‐3 mRNA levels in the subcortical WM were higher in the Vent (P = 0.011) and Vent+EPO (P = 0.002) lambs compared to UVC (Fig. 6 B); however, there was no difference between Vent groups. The density of caspase‐8 positive cells was not different between groups in the periventricular WM (P = 0.296) or subcortical WM (P = 0.115; Fig. 6 C).
Figure 6. Early indicators of apoptosis .
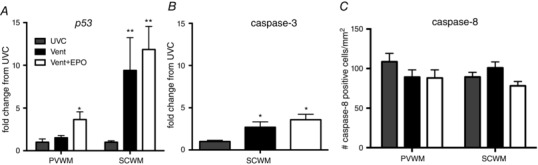
A, p53 gene expression was increased in Vent+EPO compared to UVC and Vent groups in the periventricular WM (PVWM) and in Vent and Vent+EPO groups compared to UVC in the subcortical WM (SCWM). B, caspase‐3 gene expression was increased in Vent and Vent+EPO compared to UVC in the SCWM. C, there was no difference in density of caspase‐8 positive cells in the PVWM or SCWM between groups. *P < 0.05 and **P < 0.001 compared to UVC.
Blood–brain barrier integrity and permeability
Occludin mRNA levels in the periventricular WM were higher in Vent+EPO lambs compared to both UVC and Vent lambs (P < 0.001 for both); there was no difference between groups in the subcortical WM (Fig. 7 A). Claudin‐5 mRNA levels in the periventricular and subcortical WM were not different between groups (Fig. 7 B). Claudin‐1 mRNA levels in the periventricular WM were higher in Vent and Vent+EPO lambs compared to UVC (P < 0.001); expression was increased in Vent+EPO lambs compared to Vent lambs (P = 0.018; Fig. 7 C). In the subcortical WM, claudin‐1 mRNA levels were lower in both Vent and Vent+EPO groups compared to UVC (P < 0.001); however, there was no difference between Vent groups (Fig. 7 C).
Figure 7. Blood–brain barrier integrity and permeability .
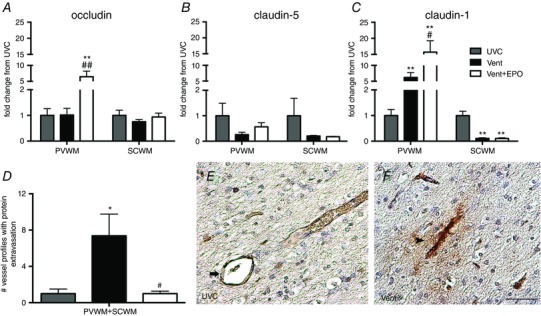
A, occludin mRNA levels were increased in the Vent+EPO group compared to both UVC and Vent in the periventricular WM (PVWM); there was no difference in the subcortical WM (SCWM). B, claudin‐5 mRNA levels were not different between groups in the PVWM and SCWM. C, claudin‐1 mRNA levels were higher in the Vent group compared to UVC and further increased in Vent+EPO compared to Vent in the PVWM; claudin‐1 mRNA levels in the SCWM were decreased in the Vent and Vent+EPO groups compared to UVC. D, the number of blood vessel profiles with protein extravasation in the PVWM and SCWM combined was increased in Vent compared to UVC; EPO treatment reduced this increase to control levels. E and F, representative image of a vessel profile with an intact vascular wall (indicated by arrow) from a UVC animal (E) and a vessel profile with protein extravasation (indicated by arrowhead) from a Vent animal (F). Scale bar = 50 μm. *P < 0.05 and **P < 0.001 compared to UVC; # P < 0.05 and ## P < 0.001 compared to Vent.
The total number of blood vessel profiles with protein extravasation in the WM (periventricular and subcortical WM combined) was higher in Vent lambs compared to UVC (P < 0.05) and was lower in Vent+EPO compared to Vent lambs (P = 0.002; Fig. 7 D–F).
Correlation between EPO concentration in CSF and neuropathology
There was a negative correlation between CSF EPO concentration and the areal coverage of Iba‐1‐immunoreactivity in the subcortical WM (r 2 = −0.9332, P = 0.0065) but no correlation with any other molecular or immunohistochemical findings.
Discussion
This is the first study to assess the acute effects of early high dose EPO treatment on the cerebral WM of preterm lambs. The key findings from our study are as follows: a single i.v. high dose of rhEPO administered to ventilated preterm lambs was sufficient to cross the blood–brain barrier; rhEPO increased pro‐inflammatory cytokine expression in subcortical and periventricular WM above that of UVC and Vent groups; and rhEPO reduced gliosis and preserved integrity of the blood–brain barrier in the subcortical WM but not the periventricular WM after only 2 h. This study suggests that an early high dose of EPO administered to ventilated preterm lambs has regional short‐term effects within the cerebral WM.
The initiation of ventilation is known to instigate a profound pulmonary and systemic inflammatory cascade (Hillman et al. 2007; Polglase et al. 2008) and alter pulmonary and cardiovascular haemodynamics, which lead to brain inflammation and injury (Barton et al. 2014; Polglase et al. 2014 b; Skiöld et al. 2014). We previously demonstrated in the lungs from the same lambs used in this study that early EPO administration amplified lung inflammation and injury above that of ventilation alone (Polglase et al. 2014 a). We proposed that the amplification in lung inflammation and injury would migrate systemically to the brain to instigate a localized cerebral response. Indeed, in the current study Vent+EPO lambs had significantly elevated pro‐inflammatory cytokine mRNA in the cerebral WM compared to Vent lambs thus supporting this hypothesis and indicating a potentially harmful effect, particularly in the periventricular WM. However, despite these adverse changes, EPO treatment ameliorated characteristic ventilation‐induced injury markers in the subcortical WM including increased microgliosis and blood–brain barrier permeability and, importantly, significantly improved cerebral oxygenation. Given that studies have shown a prolonged TOI < 55% is positively correlated with poorer neurological outcome (Hagino et al. 2005), the increased TOI in the Vent+EPO lambs appears to indicate a neuroprotective effect; assessment of oxidative stress markers at a later time point will clarify whether the increased TOI is protective or injurious. Thus, it appears EPO has the capacity to act through both haemodynamic and inflammatory pathways involved in ventilation‐induced brain injury, although there are differential effects in the periventricular and subcortical WM.
In our current study, EPO treatment in ventilated preterm lambs had regional effects on cerebral inflammation. Within the periventricular WM, there were profound increases in pro‐inflammatory cytokine gene expression after EPO administration in ventilated lambs. This was coupled with microglial activation and aggregation, although neither were increased above that of the Vent alone group. Similarly, there was no difference in area of microglial aggregations between Vent and Vent+EPO groups in the periventricular WM. It is plausible that we have assessed this area prior to cytokine changes manifesting into inflammatory cell activation, aggregation and recruitment, and subsequent overt injury. We postulate that the inflammatory changes within the periventricular WM are a downstream effect of the amplified lung and systemic inflammation as a result of EPO administration (Polglase et al. 2014 a); that is, cytokines migrate from the lungs across the blood–brain barrier (Threlkeld et al. 2010) to generate a localized response. This hypothesis is supported by our findings of no correlation between the concentration of EPO in the CSF and the pro‐inflammatory response within the periventricular WM. We suggest that low dose EPO, or EPO treatment subsequent to the peak pro‐inflammatory response in the lungs (i.e. delayed administration), may mitigate the changes we observed in the periventricular WM.
Pro‐inflammatory cytokine gene expression increased in the subcortical WM in Vent+EPO lambs, although not to the same extent as in the periventricular WM. Despite this increase, EPO resulted in a decrease in cellular density of microglia within aggregations and in the areal density of astrocytes in the subcortical WM. Additionally, in the subcortical WM, we found that the concentration of EPO measured in the CSF of Vent+EPO lambs was negatively correlated with the areal coverage of microglial within aggregations in the subcortical WM (i.e. the higher the concentration of EPO in the CSF of Vent+EPO lambs, the lower the areal coverage of microglia within aggregations) suggesting a direct anti‐inflammatory effect of EPO within the subcortical WM.
In addition to its anti‐inflammatory effects, EPO is also a potent anti‐apoptotic agent (Van Der Kooij et al. 2008). In the current study, ventilation in preterm lambs increased p53 and caspase‐3 gene expression in the subcortical WM after only 2 h and surprisingly EPO treatment exacerbated this increase. Despite the increase in gene expression, we found no effect of ventilation or EPO treatment on the density of caspase‐8 positive cells; however, this may be due to the acute nature of our study. A limitation of the present study is that we did not assess p53 and caspase‐3, or the pro‐inflammatory cytokines at a protein level and instead assessed cell density of apoptotic cells and microglia, respectively, to indicate if the pathways were activated.
Clinical trials using high dose EPO in preterm infants have reported conflicting findings. Trials have either reported a trend towards a reduction in the overall incidence of intraventricular haemorrhage (IVH), with no short‐term benefits for Grade III or IV IVH (Juul et al. 2008), or an increase in severe IVH (Ohls et al. 2004; Fauchere et al. 2008). In the current study we found that EPO treatment increased mRNA levels of tight junction proteins, and reduced protein extravasation from blood vessels. Our findings are consistent with a protective effect of EPO on the bovine blood–brain barrier in vitro (Martínez‐Estrada et al. 2003) and in rodents (Liu et al. 2013). These data suggest that EPO provides protection against haemodynamic‐related ventilation‐induced changes, which to date has only been suggested in adults with cardiac arrest (Grmec et al. 2009) and is yet to be explored in the adult or neonatal brain. Thus, due to the disparate clinical findings, and the indication that EPO is protective against both critical pathways involved in ventilation‐induced brain injury, further investigation of the impact of EPO on the preterm brain is warranted.
Clinical studies have demonstrated the safety, ease of use and reparative and regenerative properties of EPO given hours, or up to days, after birth (McPherson et al. 2007; Brown et al. 2009; Zhu et al. 2009; Neubauer et al. 2010; McAdams et al. 2013). Administering EPO immediately following birth is being introduced clinically (Fauchere et al. 2008; Leuchter et al. 2014; O'Gorman et al. 2015) despite a lack of pre‐clinical data to support this. It has been shown that in neonatal rats, EPO reduces infarct size and neuronal apoptosis (Aydin et al. 2003; Kumral et al. 2003) following hypoxia–ischaemia, and improves spatial memory long‐term (Kumral et al. 2004) when administered 24 h prior to the insult. To our knowledge, our study is the first to use a clinically relevant large animal model to investigate the impact of early EPO administration in conjunction with a known injurious insult, ventilation. Our previous study showed that lung inflammation and injury resultant from ventilation in preterm lambs was amplified by EPO administration (Polglase et al. 2014 a). Another study reported that a bolus low dose of EPO (300 IU kg−1 per dose) given after endotoxin infusion increased serum TNF‐α, IL‐6, and IL‐1β with amplified injury in the liver, kidneys, lungs and small intestine in rats (Wu et al. 2009). Taken together, these studies, along with the findings of the current study, highlight that caution needs to be taken before translating the use of early administration of high dose EPO into the clinic, particularly given the inflammation we found in the lungs and liver previously (Polglase et al. 2014 a) and in the periventricular WM in the current study.
Here we have administered a high dose of EPO (5000 IU/kg), previously used in ovine studies (Juul et al. 2004; Rees et al. 2010), to preterm lambs at 0.85 gestation. This regimen resulted in highly elevated (yet neuroprotective; Juul et al. 2004) EPO concentrations in the CSF. In light of this, lower doses of EPO should be explored to investigate whether inflammation is prevented in the cerebral WM, and whether a more clinically relevant systemic EPO concentration could be achieved (Dame et al. 2001). This is of particular importance given the potential U‐shaped dosing curve (Juul, 2012) as well as the differing clearance rates in term compared to preterm infants (Juul et al. 2008; Juul & Ferriero, 2014). Interestingly, despite lambs receiving the same dose of EPO, the EPO concentration in the CSF varied and these were not correlated to the majority of neuropathological outcomes measured. At 0.85 gestation in sheep, the lungs are developmentally equivalent to those of a 25–27 week preterm infant (Alcorn et al. 1981), while the brain, in regard to white matter development, is near term with 0.9 gestation in the sheep being equivalent to term in human infants (Back et al. 2006). However, even at this relatively mature stage of development, the ovine brain is still vulnerable to white matter damage (Polglase et al. 2012; Skiöld et al. 2014), which we show can be affected by early high dose EPO.
Conclusions
In conclusion, we found that early high dose administration of EPO within the first few minutes of injurious ventilation in preterm lambs has differential effects in the periventricular and subcortical WM. In the subcortical WM, EPO reduces the glial cell response and blood vessel permeability in the cerebral WM likely via increased blood–brain barrier integrity. This suggests that EPO administration has the capacity to protect against early markers of preterm brain injury caused by haemodynamic and inflammatory mechanisms. However, despite these favourable effects in the subcortical WM, EPO administration had adverse effects within the periventricular WM indicating that EPO could have potentially neurotoxic effects on this region of WM when administered in conjunction with ventilation onset. Thus, we conclude that further studies exploring alternative dosing regimens are essential prior to EPO being used clinically as an early treatment for ventilation‐induced brain injury.
Additional information
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
S.B. participated in the design, experiments, data collection, statistical analysis and analysis and writing the manuscript. T.M., G.P. and M.T. designed the study and coordinated the experiments, provided assistance and technical expertise for data collection and analysis as well as drafting the manuscript. A.M. and V.Z. provided assistance and technical expertise for molecular and immunohistochemical protocols and data collection and helped draft the manuscript. J.M., T.L. and K.C. participated in the experiments and tissue collection and helped draft the manuscript. All authors approved the final version of the manuscript and all persons designated as authors qualify for authorship; all those who qualify for authorship are listed.
Funding
This research project was supported by an AVANT Innovative Research Grant awarded by the Research Foundation of Cerebral Palsy Alliance, NH&MRC Research Fellowships (G.R.P.: 1026890 and T.J.M.: 10043294) and Project grant (1021702), a Rebecca L. Cooper Medical Research Foundation Fellowship (G.R.P.) and the Victorian Government's Operational Infrastructure Support Program.
Acknowledgements
The authors gratefully acknowledge the expert technical assistance of Dr Ilias Nitsos and Karyn Rodgers and the facilities and scientific and technical assistance of the Histology Facility, Hudson Institute of Medical Research.
G. R. Polglase and M. Tolcos contributed equally to this work.
References
- Alcorn DG, Adamson TM, Maloney JE & Robinson PM (1981). A morphologic and morphometric analysis of fetal lung development in the sheep. Anat Rec 201, 655–667. [DOI] [PubMed] [Google Scholar]
- Atik A, Cheong JLY, Harding R, Rees SM, De Matteo R & Tolcos M (2014). Impact of daily high‐dose caffeine exposure on developing white matter of the immature ovine brain. Pediatr Res 76, 54–63. [DOI] [PubMed] [Google Scholar]
- Aydin A, Genc K, Akhisaroglu M, Yorukoglu K, Gokmen N & Gonullu E (2003). Erythropoietin exerts neuroprotective effect in neonatal rat model of hypoxic‐ischemic brain injury. Brain Dev 25, 494–498. [DOI] [PubMed] [Google Scholar]
- Back SA, Riddle A & Hohimer AR (2006). Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white‐matter injury. J Child Neurol 21, 582–589. [DOI] [PubMed] [Google Scholar]
- Barton SK, Melville JM, Tolcos M, Polglase GR, McDougall ARA, Azhan A, Crossley KJ, Jenkin G & Moss TJM (2015). Human amnion epithelial cells modulate ventilation‐induced white matter pathology in preterm lambs. Dev Neurosci 37, 338–348. [DOI] [PubMed] [Google Scholar]
- Barton SK, Moss TJ, Hooper SB, Crossley KJ, Gill AW, Kluckow M, Zahra VA, Wong FY, Pichler G, Galinsky R, Miller SL, Tolcos M & Polglase GR (2014). Protective ventilation of preterm lambs exposed to acute chorioamnionitis does not reduce ventilation‐induced lung or brain injury. PLoS One 9, e112402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Eichorst D, LaLa‐Black B & Gonzalez R (2009). Higher cumulative doses of erythropoietin and developmental outcomes in preterm infants. Pediatrics 124, e681–e687. [DOI] [PubMed] [Google Scholar]
- Chow SSW (2011). Report of the Australian and New Zealand Neonatal Network. ANZNN, Sydney.
- Counsell SJ, Rutherford MA, Cowan FM & Edwards AD (2003). Magnetic resonance imaging of preterm injury. Arch Dis Child Fetal Neonatal Ed 88, F269–F274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame C, Juul SE & Christensen RD (2001). The biology of erythropoietin in the central nervous system and its neurotrophic and neuroprotective potential. Biol Neonate 79, 228–235. [DOI] [PubMed] [Google Scholar]
- Del Toro J, Louis PT & Goddard‐Finegold J (1991). Cerebrovascular regulation and neonatal brain injury. Pediatr Neurol 7, 3–12. [DOI] [PubMed] [Google Scholar]
- Fauchere JC, Dame C, Vonthein R, Koller B, Arri S, Wolf M & Bucher HU (2008). An approach to using recombinant erythropoietin for neuroprotection in very preterm infants. Pediatrics 122, 375–382. [DOI] [PubMed] [Google Scholar]
- Grmec Š, Strnad M, Kupnik D, Sinkovič A & Gazmuri R (2009). Erythropoietin facilitates the return of spontaneous circulation and survival in victims of out‐of hospital cardiac arrest. Resuscitation 80, 631–637. [DOI] [PubMed] [Google Scholar]
- Hagino I, Anttila V, Zurakowski D, Duebener LF, Lidov HGW & Jonas RA (2005). Tissue oxygenation index is a useful monitor of histologic and neurologic outcome after cardiopulmonary bypass in piglets. J Thorac Cardiovasc Surg 130, 384–392. [DOI] [PubMed] [Google Scholar]
- He JS, Huang ZL, Yang H, Weng KZ & Zhu SB (2008). Early use of recombinant human erythropoietin promotes neurobehavioural development in preterm infants. Zhongguo Dang Dai Er Ke Ka Zhi 10, 586–588. [PubMed] [Google Scholar]
- Hillman NH, Moss TJM, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, Nitsos I, Kramer BW & Jobe AH (2007). Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med 176, 575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul SE (2012). Neuroprotective role of erythropoietin in neonates. J Matern Fetal Neonatal Med 25, 105–107. [DOI] [PubMed] [Google Scholar]
- Juul SE & Ferriero DM (2014). Pharmacologic neuroprotective strategies in neonatal brain injury. Clin Perinatol 41, 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul SE, Harcum J, Li Y & Christensen RD (1997). Erythropoietin is present in the cerebrospinal fluid of neonates. J Pediatr 130, 428–430. [DOI] [PubMed] [Google Scholar]
- Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA & Mayock DE (2008). A phase I/II trial of high‐dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics 122, 383–391. [DOI] [PubMed] [Google Scholar]
- Juul SE, McPherson RJ, Farrell FX, Jolliffe L, Ness DJ & Gleason CA (2004). Erythropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high‐dose recombinant erythropoietin. Biol Neonate 85, 138–144. [DOI] [PubMed] [Google Scholar]
- Khwaja O & Volpe JJ (2008). Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed 93, F153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumral A, Ozer E, Yilmaz O, Akhisaroglu M, Gokmen N, Duman N, Ulukus C, Genc S & Ozkan H (2003). Neuroprotective effect of erythropoietin on hypoxic‐ischemic brain injury in neonatal rats. Biol Neonate 83, 224–228. [DOI] [PubMed] [Google Scholar]
- Kumral A, Uysal N, Tugyan K, Sonmez A, Yilmaz O, Gokmen N, Kiray M, Genc S, Duman N, Koroglu TF, Ozkan H & Genc K (2004). Erythropoietin improves long‐term spatial memory deficits and brain injury following neonatal hypoxia‐ischemia in rats. Behav Brain Res 153, 77–86. [DOI] [PubMed] [Google Scholar]
- Leuchter RH, Gui L, Poncet A, Hagmann C, Lodygensky GA, Martin E, Koller B, Darqué A, Bucher HU & Hüppi PS (2014). Association between early administration of high‐dose erythropoietin in preterm infants and brain MRI abnormality at term‐equivalent age. JAMA 312, 817–824. [DOI] [PubMed] [Google Scholar]
- Liu K, San T, Wang P, Liu YH, Zhang LW & Xue YX (2013). Effects of erythropoietin on blood‐brain barrier tight junctions in ischemia‐reperfusion rats. J Mol Neurosci 49, 369–379. [DOI] [PubMed] [Google Scholar]
- Martínez‐Estrada OM, Rodriguez‐Millán E, González‐De Vicente E, Reina M, Vilaró S & Fabre M (2003). Erythropoietin protects the in vitro blood‐brain barrier against VEGF‐induced permeability. Eur J Neurosci 18, 2538–2544. [DOI] [PubMed] [Google Scholar]
- McAdams RM, McPherson RJ, Mayock DE & Juul SE (2013). Outcomes of extremely low birth weight infants given early high‐dose erythropoietin. J Perinatol 33, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson RJ, Demers EJ & Juul SE (2007). Safety of high‐dose recombinant erythropoietin in neonatal rat model. Neonatology 91, 36–43. [DOI] [PubMed] [Google Scholar]
- Murkin JM & Avango M (2009). Near‐infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 103, 3–13. [DOI] [PubMed] [Google Scholar]
- Neubauer AP, Voss W, Wachtendorf M & Jungmann T (2010). Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol 67, 657–666. [DOI] [PubMed] [Google Scholar]
- O'Gorman RL, Bucher HU, Held U, Koller BM, Huppi PS & Hagmann CF (2015). Tract‐based spatial statistics to assess the neuroprotective effect of early erythropoietin on white matter development in preterm infants. Brain 138, 388–397. [DOI] [PubMed] [Google Scholar]
- Ohls RK, Ehrenkranz RA, Das A, Dusick AM, Yolton K, Romano E, Delaney‐Black V, Papile LA, Simon NP, Steichen JJ & Lee KG (2004). Neurodevelopmental outcome and growth at 18 to 22 months’ corrected age in extremely low birth weight infants treated with early erythropoietin and iron. Pediatrics 114, 1287–1291. [DOI] [PubMed] [Google Scholar]
- Polglase G, Barton SK, Melville JM, Zahra VA, Wallace MJ, Siew ML, Tolcos M & Moss TJM (2014. a). Prophylactic erythropoietin exacerbates ventilation‐induced lung inflammation and injury in preterm lambs. J Physiol 592, 1993–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polglase G, Miller SL, Barton SK, Baburamani AA, Wong FY, Aridas JDS, Gill AW, Moss TJ, Tolcos M, Kluckow M & Hooper SB (2012). Initiation of resuscitation with high tidal volumes causes cerebral hemodynamic disturbance, brain inflammation and injury in preterm lambs. PLoS One 7, e39535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polglase GR, Hillman NH, Pillow JJ, Cheah FC, Nitsos I, Moss TJM, Kramer BW, Ikegami M, Kallapur SG & Jobe AH (2008). Positive end‐expiratory pressure and tidal volume during initial ventilation of preterm lambs. Pediatr Res 64, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polglase GR, Miller SL, Barton SK, Kluckow M, Gill AW, Hooper SB & Tolcos M (2014. b). Respiratory support for premature neonates in the delivery room: effects on cardiovascular function and the development of brain injury. Pediatr Res 75, 682–688. [DOI] [PubMed] [Google Scholar]
- Rees SM, Hale N, De Matteo R, Cardamone L, Tolcos M, Loeliger M, Mackintosh A, Shields A, Probyn ME, Greenwood D & Harding R (2010). Erythropoietin is neuroprotective in a preterm ovine model of endotoxin‐induced brain injury. J Neuropathol Exp Neurol 69, 306–319. [DOI] [PubMed] [Google Scholar]
- Schmölzer GM, Kamlin OCOF, O'Donnell CPF, Dawson JA, Morley CJ & Davis PG (2010). Assessment of tidal volume and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed 95, F393–F397. [DOI] [PubMed] [Google Scholar]
- Skiöld B, Wu Q, Hooper SB, Davis PG, McIntyre R, Tolcos M, Pearson J, Vreys R, Egan GF, Barton SK, Cheong JLY & Polglase G (2014). Early detection of ventilation‐induced brain injury using magnetic resonance spectroscopy and diffusion tensor imaging: an in vivo study in preterm lambs. PLoS One 9, e95804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlkeld SW, Lynch JL, Lynch KM, Sadowska GB, Banks WA & Stonestreet BS (2010). Ovine proinflammatory cytokines cross the murine blood‐brain barrier by a common saturable transport mechanism. Neuroimmunomodulation 17, 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuij LG, Mathai S, Davidson JO, Lear CA, Booth LC, Fraser M, Gunn AJ & Bennet L (2014). Synergistic white matter protection with acute‐on‐chronic endotoxin and subsequent asphyxia in preterm fetal sheep. J Neuroinflammation 16, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Kooij MA, Groenendaal F, Kavelaars A, Heijnen CJ & Van Bel F (2008). Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res Rev 59, 22–33. [DOI] [PubMed] [Google Scholar]
- Volpe JJ (2003). Cerebral white matter injury of the premature infant—more common than you think. Pediatrics 112, 176–180. [DOI] [PubMed] [Google Scholar]
- Wu WT, Hu TM, Lin NT, Subeg YM, Lee RP & Hsu BG (2009). Low‐dose erythropoietin aggravates endotoxin‐induced organ damage in conscious rats. Cytokine 49, 155–162. [DOI] [PubMed] [Google Scholar]
- Zhu C, Kang W & Xu F (2009). Erythropoietin improved neurologic outcomes in newborns with hypoxic‐ischemic encephalopathy. Pediatrics 124, e218–226. [DOI] [PubMed] [Google Scholar]


