Key points
Fetal heart rate variability and changes in the ST segment of the electrocardiogram are used clinically during labour to identify fetuses at risk of severe metabolic acidosis or death.
Sympathetic nervous system activity contributes to heart rate variability in healthy normoxic fetuses, and is critical for the rapid haemodynamic adaptations to repeated episodes of asphyxia induced by brief complete umbilical cord occlusions at rates consistent with active labour.
We now show that chemical sympathectomy did not alter fetal heart rate variability between episodes of brief repeated asphyxia or elevation of the ST segment during asphyxia.
The lack of influence of the sympathetic system on fetal heart rate variability between episodes of brief asphyxia suggests that measures of fetal heart rate variability are unlikely to help monitor changes in sympathetic nervous system activity during active labour.
Abstract
Changes in fetal heart rate variability (FHRV) and ST segment elevation (measured as the T/QRS ratio) are used to evaluate fetal adaptation to labour. The sympathetic nervous system (SNS) is an important contributor to FHRV under healthy normoxic conditions, and is critical for rapid support of blood pressure during brief labour‐like asphyxia. However, although it has been assumed that SNS activity contributes to FHRV during labour; this has never been tested, and it is unclear whether the SNS contributes to the rapid increase in T/QRS ratio during brief asphyxia. Thirteen chronically instrumented fetal sheep at 0.85 of gestation received either chemical sympathectomy with 6‐hydroxydopamine (6‐OHDA; n = 6) or sham treatment (control; n = 7), followed 4–5 days later by 2 min episodes of complete umbilical cord occlusion repeated every 5 min for up to 4 h, or until mean arterial blood pressure fell to <20 mmHg for two successive occlusions. FHRV was decreased before occlusions in the 6‐OHDA group (P < 0.05) and 2–4.5 h during recovery after occlusions (P < 0.05) compared to the control group. During each occlusion there was a rapid increase in T/QRS ratio. Between successive occlusions the T/QRS ratio rapidly returned to baseline, and FHRV increased above baseline in both groups (P < 0.05), with no significant effect of sympathectomy on FHRV or T/QRS ratio. In conclusion, these data show that SNS activity does not mediate the increase in FHRV between repeated episodes of brief umbilical cord occlusion or the transient increase in T/QRS ratio during occlusions.
Key points
Fetal heart rate variability and changes in the ST segment of the electrocardiogram are used clinically during labour to identify fetuses at risk of severe metabolic acidosis or death.
Sympathetic nervous system activity contributes to heart rate variability in healthy normoxic fetuses, and is critical for the rapid haemodynamic adaptations to repeated episodes of asphyxia induced by brief complete umbilical cord occlusions at rates consistent with active labour.
We now show that chemical sympathectomy did not alter fetal heart rate variability between episodes of brief repeated asphyxia or elevation of the ST segment during asphyxia.
The lack of influence of the sympathetic system on fetal heart rate variability between episodes of brief asphyxia suggests that measures of fetal heart rate variability are unlikely to help monitor changes in sympathetic nervous system activity during active labour.
Abbreviations
- 6‐OHDA
6‐hydroxydopamine
- FHR
fetal heart rate
- FHRV
fetal heart rate variability
- MAP
mean arterial pressure
- RMSSD
root mean square of successive RR intervals
- SDNN
standard deviation of RR intervals
- SNS
sympathetic nervous system
- STV
short‐term variation
Introduction
Early prediction of fetal compromise during human labour is crucial to the prevention of long‐term neurodevelopmental disability. However, despite decades of use, the physiology underlying key clinical indices of fetal wellbeing such as fetal heart rate variability (FHRV) remains poorly understood (Westgate et al. 2007). More recently some centres have begun analysing ST segment morphology as an adjunct to standard fetal heart rate (FHR) monitoring (Amer‐Wahlin et al. 2007). Regulation of FHRV and the ST segment is multifactorial, and complicated by fetal responses to asphyxia that can alter the systems that regulate both FHRV and the morphology of the ST segment.
Normal fetal heart rate variability represents the integration of the sympathetic nervous system (SNS), parasympathetic activity and the intrinsic pacemaker rhythms of the sino‐atrial node (Jensen et al. 2009; Papaioannou et al. 2013), with some evidence of non‐neural components related in part to fetal body and breathing movements (Dalton et al. 1977; Visser et al. 1982; Dalton et al. 1983). Clinically and experimentally, FHRV is initially increased between the brief periods of asphyxia that are characteristic of established labour (Westgate et al. 1999; Siira et al. 2005; van Laar et al. 2008). The SNS is a key afferent component of the fetus’ protective chemoreflex (Giussani et al. 1993), and we have recently demonstrated that sympathectomy impaired fetal reflex adaptation during repeated exposure to episodes of brief asphyxia, resulting in impaired blood pressure support, although overall fetal survival was not impaired (Galinsky et al. 2014). Clinically, there is indirect evidence from changes in the specific frequency bands of FHRV during labour that developing fetal acidosis is associated with impairment of SNS activity that may be reflected in changes in FHRV (Siira et al. 2005; van Laar et al. 2008); however, this possibility has never been directly investigated.
An elevated ST segment, measured relative to the QRS complex (the T/QRS ratio) occurs during asphyxia in association with intrapartum decelerations (Westgate et al. 1992), and, in combination with intrapartum cardiotocographic monitoring, can improve identification of fetal hypoxia (Amer‐Wahlin et al. 2001). Similarly, experimental studies have consistently reported a rapid increase in T/QRS ratio during asphyxia and hypoxia that resolves rapidly after return of normoxia or release of umbilical cord occlusion (Greene et al. 1982; Westgate et al. 2001 a; Wibbens et al. 2005; Wassink et al. 2014). This increase is thought to primarily reflect alterations in cellular ionic currents associated with anaerobic cardiac metabolism (Hokegard et al. 1981), with a contribution from β‐adrenergic stimulation (Hokegard et al. 1979; Rosen et al. 1984). No studies have investigated whether SNS‐mediated β‐adrenergic stimulation contributes to this ST segment elevation during asphyxia, although the timing of the increase is at least broadly consistent with the reported time course of renal SNS activation during asphyxia (Booth et al. 2012).
In the present study we sought to address these fundamental questions and hypothesised that sympathetic activation during asphyxia contributes to both the regulation of FHRV and ST segment elevation during perinatal asphyxia. To test our hypothesis we performed chemical sympathectomy with the neurotoxin 6‐hydroxydopamine (6‐OHDA) on healthy fetal sheep at 0.85 of gestation (Galinsky et al. 2014), followed by exposure to repeated brief umbilical cord occlusions at a frequency consistent with active labour (Westgate et al. 1999). The sympathetic system includes both neural and adrenal elements; infusion of 6‐OHDA destroys sympathetic nerve endings while leaving the adrenal medulla structurally intact (Lewis et al. 1984; Jensen & Lang, 1992; Jensen et al. 2009). The fetal adrenal medulla can release catecholamines in response to hypoxia independently of neural input, with little effect of splanchnic nerve stimulation until 125 days of gestation (term is 147 days) (Comline & Silver, 1961). Confirming a key role for non‐neural control of the adrenal response, we have shown in the present cohort that the considerable increase in circulating catecholamine levels during repeated brief umbilical cord occlusions was unchanged by sympathectomy (Galinsky et al. 2014). Thus, in the present study we specifically investigated the contribution of the SNS to FHRV.
Methods
Fetal surgery
All procedures were approved by the Animal Ethics Committee of the University of Auckland. Thirteen Romney/Suffolk fetal sheep were surgically instrumented at 116–122 days of gestation under sterile conditions (Jensen et al. 2009). Food, but not water, was withdrawn 18 h before surgery. Anaesthesia was induced by intravenous injection of alphaxalone and alphadolone (3 mg kg−1, Schering‐Plough Animal Health Ltd, Wellington, New Zealand), and general anaesthesia was maintained using 2–3% isoflurane in oxygen. A midline incision was made to expose the uterus, and the fetus was partially exteriorised for instrumentation. Polyvinyl catheters were placed in a fetal femoral artery to measure arterial blood pressure, a right axillary artery for pre‐ductal sampling and the amniotic sac to measure amniotic pressure. A pair of electrodes was placed subcutaneously over the right shoulder and at the level of the left fifth intercostal space to measure the fetal electrocardiogram. An inflatable silicone occluder was placed around the umbilical cord to facilitate umbilical cord occlusions (In Vivo Metric, Healdsburg, CA, USA).
All fetal leads were exteriorised through the maternal flank and a maternal long saphenous vein was catheterised to provide access for post‐operative care and euthanasia. Antibiotics (80 mg gentamicin, Pfizer New Zealand Ltd, Auckland, New Zealand) were administered into the amniotic sac prior to closure of the uterus. Ewes were given 5 ml of Streptopen (procaine penicillin (250000 IU ml−1) and dihydrostreptomycin (250 mg ml−1), Pitman‐Moore, Wellington, New Zealand) intramuscularly for prophylaxis 30 min before the start of surgery.
Postoperative care
Following surgery, ewes were housed together in separate metabolic cages with ad libitum access to food and water. Rooms were temperature and humidity controlled (16 ± 1°C, humidity 50 ± 10%) with a 12 h light/dark cycle (light 0600 to 1800 h). A period of four to six days post‐operative recovery was allowed before the experiments, during which time antibiotics were administered daily for 4 days intravenously to the ewe (80 mg gentamicin, Pfizer, and 600 mg benzylpencillin sodium, Novartis Ltd., Auckland, New Zealand). Fetal catheters were maintained patent by continuous infusion of heparinised isotonic saline (20 U ml−1 at 0.2 ml h−1).
Fetal recordings
Fetal physiological parameters were recorded continuously for offline analysis using customised data acquisition programs (LabView for Windows, National Instruments Inc., Austin, TX, USA). The raw fetal electrocardiogram was analogue filtered between 0.05 and 80 Hz and digitised at 512 Hz and from this continuous RR intervals were extracted for the calculation of FHR and FHRV as described below (Westgate et al. 1999).
Sympathectomy
Fetuses were randomly assigned to either saline (control; n = 7) or sympathectomy (6‐OHDA; n = 7). One fetus from the 6‐OHDA group had marked electrocardiogram artefact and was excluded from this study (thus the final n = 6). Fetal sympathectomy was performed 1 day after surgery using an intravenous infusion of 6‐OHDA (20 mg ml−1) or saline at a rate of 2.5 ml h−1 over 3 h (Jensen & Lang, 1992). To confirm that the sympathectomy was effective, a tyramine bolus (2 μg) was administered intravenously after 48 h. Tyramine raises blood pressure by stimulating the release of noradrenaline from peripheral nerve endings (Jensen et al. 2009). During the tyramine challenge the mean increase in mean arterial pressure (MAP) was 13.5 ± 1.6 mmHg in controls (P < 0.05) vs. 2.3 ± 1.4 mmHg in the 6‐OHDA group, confirming effective sympathectomy.
Experimental protocol
Experiments were conducted at 120–126 days gestation, when neural development approximates that of term human infants (McIntosh et al. 1979). Umbilical cord occlusions were performed by inflating the occluder with a volume of saline known to completely occlude the umbilical cord and released after 2 min. This procedure was repeated 3 min later and the process continued for up to 4 h or until either MAP fell below 20 mmHg during two successive occlusions or failed to recover to baseline when the next occlusion was due. The ewes and fetuses were killed by an overdose of pentobarbital sodium intravenously to the ewe (9 g Pentobarb 300, Chemstock International, Christchurch, New Zealand).
Data analysis
Offline analysis of the physiological data was performed using customised Labview programs (National Instruments Inc.). The experimental period comprised a 24 h baseline period, the repeated occlusion period and a 12 h recovery period. All physiological signals were recorded continuously for the entire experimental period.
FHRV was assessed continuously during baseline, the second and third inter‐occlusion minute during the occlusion series (i.e. the second and third minute after the end of each occlusion) and the recovery period. The first minute of the inter‐occlusion period was omitted from analysis due to the presence of an overshoot tachycardia after release of each occlusion. FHRV was assessed as the root mean squared of successive RR intervals (RMSSD), the skewness of the RR intervals, the standard deviation of RR intervals (SDNN) (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996) and short‐term variation (STV). STV was calculated by first calculating the average RR interval of successive 3.75 s (1/16 min) periods and then calculating the mean difference between successive periods over 1 min (Street et al. 1991). All measures of FHRV were assessed as 1 min epochs to ensure that values were comparable across all time points. Changes in FHRV over time during the baseline and recovery periods were averaged as medians before the mean across the entire group was calculated as 6‐OHDA administration was associated with a marked increase in the skewness of the RR intervals during the baseline period.
FHR was assessed continuously during baseline, the occlusion series and the recovery period. T/QRS ratio was calculated as previously described (Wassink et al. 2014) and was assessed for the occlusion and inter‐occlusion periods. The electrocardiogram waveform was averaged with respect to the S wave over 5 s intervals. For each averaged waveform the amplitude of both the QRS complex and the T wave (measured relative to the PQ interval) was calculated. Finally the ratio between these two amplitudes (the T/QRS ratio) was calculated for every averaged waveform as illustrated in Fig. 1. Visual assessment by a blinded observer was performed for all time points to confirm correct software identification of the T wave. The T/QRS ratio is shown as changes relative to the immediate baseline period, taken to be the three minutes immediately before the start of the occlusion series.
Figure 1. A representative example of an averaged electrocardiogram waveform taken from the baseline period of a control fetus .
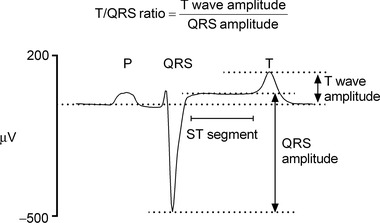
The T/QRS ratio is calculated by division of the T wave amplitude by the QRS complex amplitude.
The number of occlusions varied between fetuses. To allow direct comparison, the occlusion series was divided into three phases: the first phase consisted of the first three occlusions, the middle phase, the median three occlusions, and last phase, the final three occlusions. One fetus from the control group did not recover from the final occlusion, and so was excluded from the recovery analysis.
Statistical analysis
Statistical analysis was performed using SPSS (v22, SPSS Inc., Chicago, IL, USA) and SigmaPlot (v12.5, Systat Software, Washington, IL, USA). The effects of sympathectomy were evaluated by MANOVA, with time treated as a repeated measure, group as the independent factor and baseline as a covariate where appropriate. Phase and repetition were additionally treated as independent factors during FHR and T/QRS ratio analysis. Holm‐Sidak post hoc tests were performed when an overall significant effect was found. Comparisons to baseline were performed using the Dunnett's test. Statistical significance was accepted when P < 0.05. Data are presented as means ± SEM.
Results
Fetal blood gases, cortisol, catecholamine and cardiovascular responses during the occlusion series have previously been reported (Galinsky et al. 2014). Briefly, repeated occlusions were associated with progressive development of severe mixed respiratory and metabolic acidosis that was no different between groups (data not shown). All fetuses initially responded with an increase in MAP during the first three occlusions, and then progressively developed severe hypotension in subsequent occlusions with recovery to baseline during the inter‐occlusion period. Once hypotension developed during occlusion, sympathectomy was associated with a more rapid fall in MAP during the first minute of occlusion compared to controls (P < 0.05, data not shown). Despite this impaired cardiovascular adaptation, fetal survival was not impaired by sympathectomy and the number of occlusions before termination of the experiment was not significantly affected by sympathectomy (16.1 ± 2.2 vs. control 18.7 ± 2.3 occlusions) (Galinsky et al. 2014).
FHR and FHRV during the baseline period
FHR patterns were markedly different between groups during the 24 h baseline period. The control group showed a mixture of accelerations and small decelerations (Fig. 2) whereas the 6‐OHDA group showed a predominance of decelerations, with a significant reduction in accelerations. This was associated with increased skewness of the RR intervals in the 6‐OHDA group during the baseline period (2.62 ± 0.06 vs. 0.46 ± 0.04 control, P < 0.005) and a significant decrease in SDNN and STV (P < 0.05, Fig. 5) but not RMSSD, and a trend to lower FHR (P = 0.06) compared to the control group.
Figure 2. Examples of baseline fetal heart rate and corresponding RR interval histograms in fetal sheep after saline (control) or 6‐OHDA .

A, a representative example of baseline fetal heart rate (left) and the corresponding RR interval histogram (right) from a control fetus showing a predominance of accelerations with small decelerations 1 h before the start of the occlusion series. B and C, examples of the two common baseline fetal heart rate patterns and the corresponding RR interval histograms observed in the 6‐OHDA group from 1 h before the start of the occlusion series. The pattern displayed in B shows an overall reduction in heart rate variability while the pattern displayed in C shows a reduction in accelerations and a predominance of more severe decelerations, resulting in an increase in skewness and heart rate variability.
Figure 5. Time sequence of changes in fetal heart rate variability between episodes of brief complete umbilical cord occlusion in the saline control and 6‐OHDA groups .
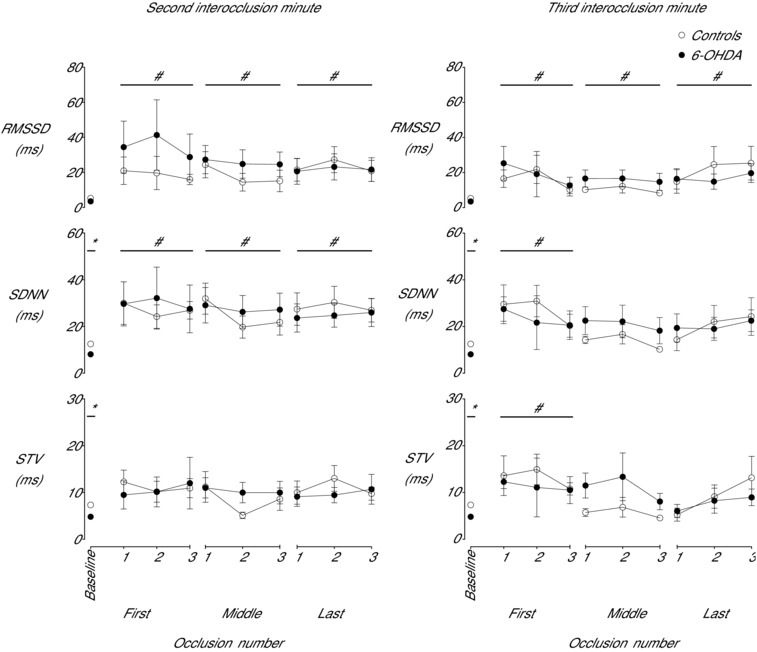
Time sequence of changes in fetal heart rate variability during the second and third inter‐occlusion minute as measured by the root mean square of successive RR intervals (RMSSD, ms), the standard deviation of RR intervals (SDNN, ms) and short‐term variability (STV, ms) from the baseline period and from three successive inter‐occlusion periods during the first, middle and final phase of the occlusion series in the control and 6‐OHDA groups. Data are means ± SEM. *P < 0.05, control vs. 6‐OHDA; # P < 0.05, both groups vs. baseline.
FHR, FHRV and T/QRS ratio during the occlusion series
FHR was significantly lower in the 6‐OHDA group compared to the control group in the 3 min immediately preceding the start of the occlusion series (P < 0.05, Fig. 3). Each occlusion, over the entire occlusion period, was associated with a rapid fall in FHR. The initial bradycardic response became attenuated by the third occlusion in the 6‐OHDA group compared to controls (P < 0.05). Release of occlusion was associated with rapid recovery of FHR, with a brief ‘overshoot’ tachycardia. This overshoot became progressively attenuated over the occlusion series but the degree of attenuation was greater in the control group (P < 0.05). Mean FHR during the inter‐occlusion periods was significantly higher overall in the 6‐OHDA group than controls, from the third occlusion onward (P < 0.05).
Figure 3. Time sequence of changes in fetal heart rate (FHR) from three successive occlusions during the first, middle and final phase of the occlusion series in the control and 6‐OHDA groups .
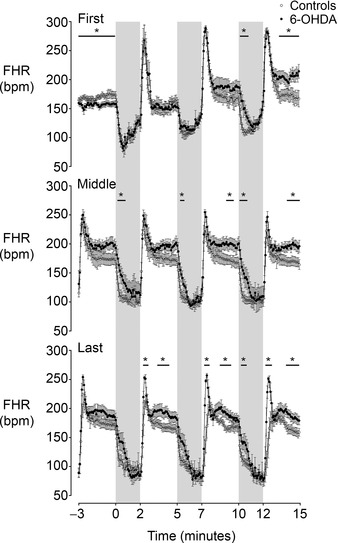
The shaded region denotes the period of asphyxia. Data are 5 s means ± SEM. *P < 0.05, control vs. 6‐OHDA.
The T/QRS ratio rose during each occlusion, and then rapidly returned to baseline after release of occlusion. There was no effect of 6‐OHDA on these dynamic changes in T/QRS ratio. The rise in T/QRS ratio during occlusions became progressively more rapid over the course of the experiment with T/QRS ratio increasing more rapidly in the middle phase compared to the first phase (P < 0.05, Fig. 4) and even more rapidly in the final phase compared to both the first and middle phases (P < 0.05). The maximal increase in T/QRS ratio occurred earlier during the last phase than in the first and middle phases (81.5 ± 4.8 vs. 105.3 ± 3.0 and 97.4 ± 4.0 s of occlusion respectively, P < 0.05). Further, inter‐occlusion T/QRS ratio was also significantly greater in the last phase than the first and middle phases (P < 0.05).
Figure 4. Time sequence of changes in the T/QRS ratio from three successive occlusions during the first, middle and final phase of the occlusion series in the control and 6‐OHDA groups .
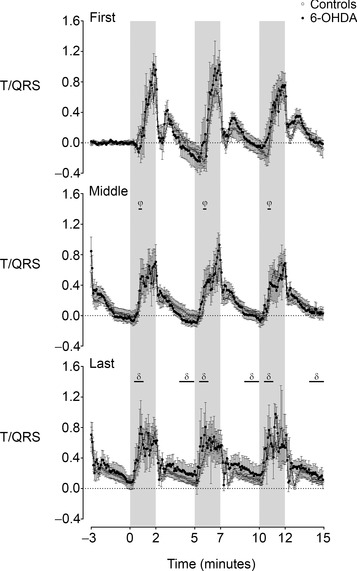
Note there was no significant effect of group on T/QRS ratio. The shaded region denotes the period of asphyxia. Data are 5 s means ± SEM. φ P < 0.05, middle phase vs. first phase; δ P < 0.05, final phase vs. first and middle phase.
During the inter‐occlusion phase there was a marked increase in FHRV across all measures (Figs 5 and 6), which was not affected by 6‐OHDA treatment. During the second inter‐occlusion minute (i.e. 60–120 s after occlusion), there was a significant increase in RMSSD (P < 0.001) and SDNN (P < 0.005) but not STV compared to baseline levels. Similarly, during the third inter‐occlusion minute (i.e. 120–180 s after occlusion) occlusion was associated with increased RMSSD throughout the whole occlusion series (P < 0.05) and increased SDNN (P < 0.05) and STV (P < 0.05) during the first phase. There was no overall change in inter‐occlusion FHRV between the phases of occlusion (Figs 5 and 6), with marked variation in the pattern of changes between individual animals.
Figure 6. Examples of fetal heart rate patterns from the middle phase in the control and 6‐OHDA groups .
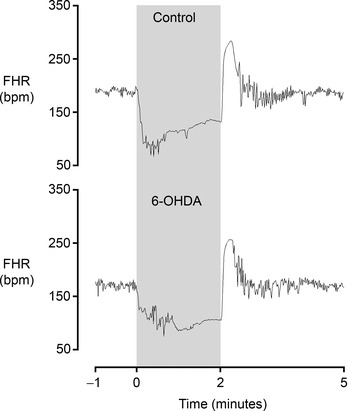
Note that fetal heart rate variability during the inter‐occlusion period is no different between groups. The shaded region denotes the period of asphyxia. Data are 1 s averages.
FHR and FHRV during the recovery period
Both occlusion groups developed a marked tachycardia after the end of the last occlusion (Fig. 7). This resolved more rapidly in the 6‐OHDA group, such that FHR was lower than in the saline control group from 1.5 to 8 h after the end of occlusions (P < 0.05). Further, FHRV (RMSSD, SDNN and STV) was significantly lower in the 6‐OHDA group compared to controls from 2 to 4.5 h after the end of occlusions (P < 0.05).
Figure 7. Time sequence of changes in fetal heart rate and variability after the period of repeated umbilical cord occlusions in the saline control and 6‐OHDA groups .
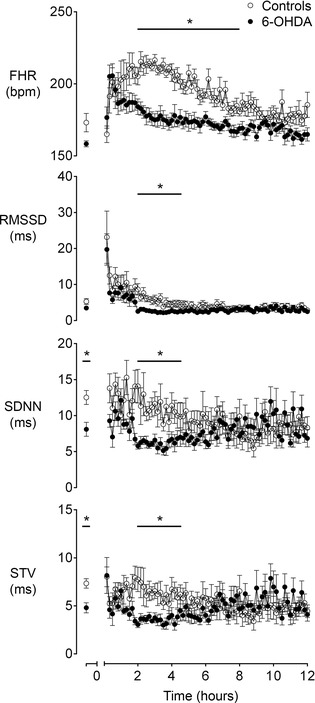
Time sequence of changes during the recovery period after repeated umbilical cord occlusions in fetal heart rate (FHR, beats min–1) and heart rate variability as measured by the root mean square of successive RR intervals (RMSSD, ms), the standard deviation of RR intervals (SDNN, ms) and short‐term variability (STV, ms) from the baseline period until 12 h after the end of the occlusion series in the control and 6‐OHDA groups. Data are 10 min means ± SEM. *P < 0.05, control vs. 6‐OHDA.
Discussion
The present study shows that exposure to asphyxia alters the regulation of fetal heart rate variability such that sympathetic neural activity has no influence on FHRV between repeated brief umbilical occlusions. This loss of SNS‐mediated FHRV is in contrast with marked peripheral sympathetic activation during prolonged mild to moderate fetal hypoxia and periods of intermittent asphyxia (Iwamoto et al. 1983; Jensen & Lang, 1992; Giussani et al. 1993; Booth et al. 2012; Galinsky et al. 2014). This finding suggests that myocardial SNS activity is attenuated during the immediate recovery from episodes of brief but intense asphyxia and illustrates that assumptions derived from studies in healthy, normoxic fetuses are not always valid during major stressors such as asphyxia. Additionally, we have shown for the first time that SNS activation does not contribute to T wave elevation during fetal asphyxia.
Fetal heart rate variability during the occlusion series
FHRV between episodes of brief umbilical cord occlusion increased in both groups across the entire occlusion series, similar to previous findings (Westgate et al. 1999). The increase primarily consisted of higher frequency fluctuations in heart rate as shown by increased RMSSD, which is a measure of high frequency heart rate changes. It has previously been shown that FHRV is increased during stable mild hypoxaemia in both the human (Thaler et al. 1985) and sheep fetus (Yu et al. 1998). In the fetal sheep, this increase was abolished by atropine but unaffected by non‐specific β‐adrenergic inhibition with propranolol, suggesting that cardiac sympathetic activity was suppressed during hypoxaemia (Yu et al. 1998). Additionally we have previously shown that although renal SNS activation occurs rapidly after the start of prolonged severe asphyxia, this activity becomes attenuated after the first few minutes (Booth et al. 2012).
Such paradigms of sustained hypoxaemia or asphyxia, however, are very different from the intermittent asphyxia that is most characteristic of labour, as previously reviewed (Westgate et al. 2007). We have recently shown that during brief intermittent umbilical cord occlusion sympathectomy was associated with attenuation of the initial rise in MAP during umbilical cord occlusions, and after the onset of hypotension, a markedly more rapid fall of MAP to the nadir. This shows that in the intact fetus, the SNS is able to be repeatedly activated and support arterial blood pressure during repeated short episodes of asphyxia (Galinsky et al. 2014). This is in contrast to our findings that sympathectomy did not impair fetal arterial blood pressure or alter FHRV in between the 2 min periods of complete umbilical cord occlusion. This strongly suggests that the SNS is acutely activated during such labour‐like insults to provide rapid blood pressure support, but is then profoundly attenuated during recovery between occlusions. During this inter‐occlusion interval blood pressure support is presumptively primarily maintained by humoral factors.
The most likely mechanism is feedback inhibition of SNS activity by the marked increase in circulating catecholamines during repeated occlusions. As previously reported (Galinsky et al. 2014), sympathectomy did not alter the fetal glucocorticoid or catecholamine responses in the present study, and plasma adrenaline levels in both groups rose during repeated occlusions (from 0.1 ± 0.0 and 0.2 ± 0.0 nmol l−1 to a peak of 80 ± 12 and 132 ± 41 nmol l−1 in the control and 6‐OHDA groups respectively), with comparable increases in plasma noradrenaline levels. The fetal adrenal can secrete catecholamines in response to hypoxia by a direct, non‐neurogenic mechanism (Comline & Silver, 1961; Adams et al. 1996). Thus, the maintained adrenal responses in the 6‐OHDA group strongly confirms a primary role for this direct adrenal response during repeated brief asphyxia, at this gestation. Although our hypothesis that catecholamines are able to suppress SNS activity has not been directly tested in the fetus, it is highly consistent with the finding that infusion of noradrenaline in healthy adults suppresses low frequency heart rate variability and muscle sympathetic activity (Tulppo et al. 1996). Moreover, situations of generalised increases in sympathetic activity such as heart failure and vigorous exercise are associated with decreased low frequency heart rate variability, reflecting reduced SNS modulation of heart rate variability (Tulppo et al. 1996; van de Borne et al. 1997). Alternatively, there is some evidence that SNS outflow is less variable under maximal tonic stimulation (Malik & Camm, 1994), and so maximal stimulation could potentially explain a lack of sympathetic control over FHRV in the present study. However, given that arterial blood pressure was similarly increased at this time in both intact and sympathectomised fetuses (Galinsky et al. 2014), this is unlikely.
In contrast to our findings, van Laar and colleagues reported that fetal acidosis during labour is clinically associated with a relative increase in low frequency spectral FHRV, which they interpreted as indirect evidence of increased sympathetic activity (van Laar et al. 2010). However, other clinical studies have shown a decrease in absolute low frequency activity during fetal distress (Siira et al. 2005; van Laar et al. 2008), consistent with the adult data discussed above. These contrasting results may in part reflect evidence from near‐term fetal sheep that both the sympathetic and parasympathetic systems contribute to low frequency FHRV, probably preventing low frequency activity from being a reliable measure of sympathetic activity (Koome et al. 2014). Moreover, changes in normalised as opposed to absolute low frequency activity may be due to changes in total FHRV.
Taken as a whole, the findings of the present study strongly suggest that increased parasympathetic activity is the key mediator of increased FHRV between brief episodes of asphyxia. The precise mechanism for increased parasympathetic activity is unknown. Speculatively, it could, at least in part, represent dysfunction of parasympathetic nuclei as neurons recover from hypoxic inhibition, leading to oscillations in vagal tone (Harris et al. 1982; Westgate et al. 2001 b). Although potentially increased inter‐occlusion FHRV could represent pacemaker dysfunction, as seen at the end of a period of 30 min of umbilical cord occlusion in preterm fetal sheep (George et al. 2004), in the present study there were no significant periods of asystole or other abnormal rhythms on the electrocardiogram.
In further support of the concept of increased parasympathetic activity between occlusions, we found that RMSSD, a measure of higher frequency FHRV that interrogates individual beat‐to‐beat differences (Frasch et al. 2009), was increased in the inter‐occlusion phase whereas baseline RMSSD was not significantly affected by 6‐OHDA. In adults, high frequency heart rate activity is predominantly mediated by parasympathetic input (Akselrod et al. 1985) and thus RMSSD can provide an indirect measure of parasympathetic activity (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). However, it is important not to over‐interpret these findings in the fetus. Even near‐term fetuses show relatively little high frequency heart rate activity compared to adults. This probably partly explains why both sympathectomy and parasympathetic blockade in fetal sheep were associated with significant reductions in overall spectral power, without significantly changing the distribution of spectral power (Koome et al. 2014). Thus, although RMSSD appeared to provide a robust measure of parasympathetic‐mediated FHRV in the present study, this should not be taken to mean that it would provide a reliable, direct measure of parasympathetic activity under all situations.
Although we found no effect of sympathectomy on any measure of inter‐occlusion FHRV, there were some differences in their pattern of changes that are likely to be due to their relative abilities to detect high frequency fluctuations. Whereas RMSSD is a measure of beat‐to‐beat differences, STV measures the difference between the average RR intervals from successive 3.75 s epochs. This equates to the average RR interval of approximately 10–14 beats during the inter‐occlusion period. Thus, STV has lower resolution than RMSSD and hence was less able to detect the rapid heart rate fluctuations during the inter‐occlusion period. In contrast, SDNN provides a measure of the overall distribution of RR intervals, which includes all rhythms with a period less than the analysed epoch (in this case 1 min) (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). SDNN detected increased FHRV during the second inter‐occlusion minute, but less consistently identified the smaller changes during the third inter‐occlusion minute.
Fetal heart rate patterns during the occlusion series
The development of overshoot tachycardia immediately after reperfusion was not attenuated by sympathectomy, but instead was maintained throughout the occlusion series in the 6‐OHDA group. Previously it has been shown that atropine produces or exacerbates overshoot tachycardia in both humans (Hon & Lee, 1963; Caldeyro‐Barcia et al. 1966) and fetal sheep (Harris et al. 1982; Itskovitz et al. 1982). This was abolished by co‐administration of propranolol which blocks both neural and catecholamine induced sympathetic tone (Saito et al. 1988). Together these studies demonstrate that overshoot tachycardia results from the combination of withdrawal of vagal tone and β‐adrenergic stimulation. The present study has now shown that this β‐adrenergic stimulation is not due to SNS stimulation, and so must be mediated by increased circulating catecholamines (Galinsky et al. 2014).
The initial fall in FHR during each occlusion was slower from the third occlusion onwards in the 6‐OHDA group. Given that 6‐OHDA was associated with loss of peripheral vascular resistance, and earlier onset of fetal hypotension during occlusions, it is possible that this reflects a baroreflex‐mediated response to help maintain combined ventricular output and to help support blood pressure. Against this hypothesis, we have previously shown that in intact animals worsening hypotension and acidosis was associated with increased inter‐occlusion FHR and an increased slope of the initial chemoreflex response (Bennet et al. 2005). Alternatively, the sympathectomised fetuses were found to be have impaired maintenance of blood pressure during occlusions but not between occlusions, suggesting that other circulating vasoactive and inotropic factors were up‐regulated. The sympathetic neuropeptide neuropeptide Y, for example, inhibits release of acetylcholine, and so might antagonise the vagal response (Herring, 2014). However, 6‐OHDA significantly reduces cardiac neuropeptide Y levels (Lundberg et al. 1985). Thus, potentially other humoral factors may have impaired the vagally mediated bradycardia at the onset of occlusion. In contrast, 6‐OHDA administration did not affect the final depth of each deceleration (Galinsky et al. 2014), consistent with considerable evidence that after the initial chemoreflex‐mediated fall in FHR, bradycardia is maintained primarily by hypoxic myocardial depression (Barcroft, 1946; Westgate et al. 2007).
T/QRS ratio during the occlusion series
Despite its clinical use in many countries (Amer‐Wahlin et al. 2007), the fundamental physiology behind changes in the ST segment during labour remains poorly understood but has been shown to have a close relationship with the progressive consumption of myocardial glycogen reserves (Hokegard et al. 1981). Furthermore, Hokegard and colleagues found that β‐adrenergic activation by isoprenaline induced T wave elevation during normoxia, while the non‐selective β‐adrenergic antagonist propranolol prevented T wave augmentation during mild hypoxia, but not during severe hypoxia (Hokegard et al. 1979), suggesting that severe hypoxia directly stimulates cardiac glycogenolysis independently of adrenergic stimulation. The present study supports this and illustrates that β‐adrenergic stimulation through cardiac SNS activation does not contribute to ST segment changes during severe asphyxia.
T wave elevation during occlusions developed more rapidly, over the course of the occlusion series. Speculatively, this may reflect more rapid activation of anaerobic metabolism over time. Additionally, fetal T waves remained moderately elevated during the inter‐occlusion period in the final phase of occlusion. Potentially, this may reflect evolving reversible myocardial injury (Gunn et al. 2000), or simply that myocardial ionic concentrations were no longer able to fully normalise due to more severe myocardial hypoxia–ischemia related to progressive cardiovascular decompensation and the development of intermittent hypotension during occlusions (Galinsky et al. 2014).
Baseline and recovery heart rate patterns
Chemical sympathectomy was associated with a marked reduction of FHRV before the start of the occlusion series as measured by SDNN and STV, but not RMSSD. This reduction was associated with a change in the pattern of the underlying FHR. The control group showed a predominance of accelerations with small decelerations. In contrast, the 6‐OHDA group showed an asymmetric heart rate trace with fewer accelerations and a shift towards a greater predominance of decelerations across the entire group as shown by a skewed distribution of RR intervals. Similar to previous findings, these decelerations were often seen despite suppression of the background FHRV (Jensen et al. 2009). At times these decelerations also became exacerbated and were of markedly greater magnitude than those seen in the control group and often led to transient increases in calculated FHRV. Intriguingly, this was predominantly seen during high voltage sleep, when sympathetic tone is known to increase in the intact fetus (Baust & Bohnert, 1969; Booth et al. 2011). This finding suggests that sympathetic tone has a role in preventing decelerations in the physiological state and additionally highlights that calculated FHRV is only a surrogate measure of altered patterns and may not necessarily detect a shift in the pattern of the heart rate.
During recovery from occlusions the control group showed increased FHR and FHRV, which were partially attenuated by 6‐OHDA starting approximately 2 h after the end of the occlusion series. This suggests that cardiac SNS activity made a progressively greater contribution to FHR as circulating catecholamines levels fell after the occlusions (Galinsky et al. 2014). Further, although all measures of FHRV showed essentially the same overall pattern, quantitatively the reduction in RMSSD after sympathectomy was less than for SDNN or STV, consistent with a small (but not zero) contribution of SNS to the higher frequencies of FHRV that are measured by RMSSD. Again, this is consistent with the proposal that there is considerable overlap between the frequency of sympathetic and parasympathetic activity before birth (Koome et al. 2014).
Conclusions
This study has revealed that while SNS activity was an important mediator of FHRV and FHR both in the baseline period and during recovery from brief repeated umbilical cord occlusions, SNS activity was suppressed between occlusions. These data, combined with the marked increase in RMSSD, strongly infer that the predominant mediator of increased FHRV between episodes of brief intermittent asphyxia was increased parasympathetic activity. Although the exact mechanism of SNS suppression is not known, it is highly likely to involve feedback inhibition of the SNS from the increase in circulating catecholamines. In turn, this is consistent with the concept that the SNS is primarily an effector of rapid adaptations such as the immediate vasoconstrictive response at the onset of asphyxia and that catecholamines and other humoral factors thereafter provide ongoing haemodynamic support.
Additional information
Competing interests
None declared.
Author contributions
These experiments were conducted in the Fetal Physiology and Neuroscience Group laboratory, at the University of Auckland. J.A.W., L.B. and A.J.G. conceived the hypotheses, experimental design and analysis protocols. C.A.L., R.G., J.A.W., L.B. and A.J.G. were responsible for data collection. C.A.L., R.G., G.W., M.J.C., J.O.D. and J.A.W. were responsible for the analysis. C.A.L. drafted the manuscript. All authors were involved in data interpretation and in the editing and revision of the manuscript. All authors qualify for authorship on the paper, are listed on the paper and approved the final version of the manuscript.
Funding
This study was funded by grants from the Health Research Council of New Zealand (grant number 12/613 and 14/216), the Auckland Medical Research Foundation (grant number 1108004) and the Lotteries Board of New Zealand (grant number 209214 and 340855). C.A.L. was supported by an Auckland Medical Research Foundation Doctoral Scholarship (grant number 1213003).
Translational perspective
The paradigm of two minute occlusions of the umbilical cord every five minutes is a severe insult, that leads to progressive development of hypotension and hypoperfusion (Galinsky et al. 2014), broadly consistent with the observed impaired intracerebral oxygenation during frequent contractions in human labour (Peebles et al. 1994). The increase in FHRV between occlusions in this setting occurred immediately after the start of occlusions, well before development of severe metabolic acidaemia or hypotension. Clinically, reduced antenatal STV was associated with higher risk of subsequent acidaemia in labour and of intrauterine growth restriction (IUGR) in a retrospective cohort (Serra et al. 2008). In a multicentre randomised trial of antenatal monitoring of IUGR, STV monitoring tended to reduce mortality compared with Doppler measures, but was less effective in reducing morbidity (Lees et al. 2015). In contrast, in a retrospective cohort study IUGR was not associated with minimal FHRV in labour (Epplin et al. 2015). Multiple factors may contribute to these complex clinical findings; however, the present preclinical findings raise the possibility that this discrepancy may be related in part to increased parasympathetic‐mediated FHRV in between severe contractions that would mask any preceding antenatal suppression of FHRV. Further, the present study shows that SNS activity can be suppressed in a setting of high fetal stress, with a high risk of metabolic acidaemia. This finding probably contributes to the rather variable results of studies of FHRV in labour.
References
- Adams MB, Simonetta G & McMillen IC (1996). The non‐neurogenic catecholamine response of the fetal adrenal to hypoxia is dependent on activation of voltage sensitive Ca2+ channels. Brain Res Dev Brain Res 94, 182–189. [DOI] [PubMed] [Google Scholar]
- Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC & Cohen RJ (1985). Hemodynamic regulation: investigation by spectral analysis. Am J Physiol Heart Circ Physiol 249, H867–H875. [DOI] [PubMed] [Google Scholar]
- Amer‐Wahlin I, Arulkumaran S, Hagberg H, Marsal K & Visser GH (2007). Fetal electrocardiogram: ST waveform analysis in intrapartum surveillance. BJOG 114, 1191–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer‐Wahlin I, Hellsten C, Noren H, Hagberg H, Herbst A, Kjellmer I, Lilja H, Lindoff C, Mansson M, Martensson L et al (2001). Cardiotocography only versus cardiotocography plus ST analysis of fetal electrocardiogram for intrapartum fetal monitoring: a Swedish randomised controlled trial. Lancet 358, 534–538. [DOI] [PubMed] [Google Scholar]
- Barcroft J. (1946). Researches in Prenatal Life Blackwell Scientific Publications Ltd, London and Oxford. [Google Scholar]
- Baust W & Bohnert B (1969). The regulation of heart rate during sleep. Exp Brain Res 7, 169–180. [DOI] [PubMed] [Google Scholar]
- Bennet L, Westgate JA, Lui YC, Wassink G & Gunn AJ (2005). Fetal acidosis and hypotension during repeated umbilical cord occlusions are associated with enhanced chemoreflex responses in near‐term fetal sheep. J Appl Physiol 99, 1477–1482. [DOI] [PubMed] [Google Scholar]
- Booth LC, Bennet L, Guild SJ, Barrett CJ, May CN, Gunn AJ & Malpas SC (2011). Maturation related changes in the pattern of renal sympathetic nerve activity from in utero to adulthood. Exp Physiol 96, 85–93. [DOI] [PubMed] [Google Scholar]
- Booth LC, Malpas SC, Barrett CJ, Guild SJ, Gunn AJ & Bennet L (2012). Renal sympathetic nerve activity during asphyxia in fetal sheep. Am J Physiol, Regul Integr Comp Physiol 303, R30–R38. [DOI] [PubMed] [Google Scholar]
- Caldeyro‐Barcia R, Medez‐Bauer C, Poseiro J, Escarcena L, Pose S, Bieniarz J, Arnt I, Gulin L & Althabe O. (1966). Control of the human fetal heart rate during labour In The Heart and Circulation in the Newborn and Infant, ed. Cassels D, pp. 7–36. Grune & Stratton, New York. [Google Scholar]
- Comline RS & Silver M (1961). The release of adrenaline and noradrenaline from the adrenal glands of the foetal sheep. J Physiol 156, 424–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KJ, Dawes GS & Patrick JE (1977). Diurnal, respiratory, and other rhythms of fetal heart rate in lambs. Am J Obstet Gynecol 127, 414–424. [DOI] [PubMed] [Google Scholar]
- Dalton KJ, Dawes GS & Patrick JE (1983). The autonomic nervous system and fetal heart rate variability. Am J Obstet Gynecol 146, 456–462. [DOI] [PubMed] [Google Scholar]
- Epplin KA, Tuuli MG, Odibo AO, Roehl KA, Macones GA & Cahill AG (2015). Effect of growth restriction on fetal heart rate patterns in the second stage of labor. Am J Perinatol Epub Jan 21. DOI: 10.1055/s‐0034‐1543954 [DOI] [PubMed] [Google Scholar]
- Frasch MG, Muller T, Hoyer D, Weiss C, Schubert H & Schwab M (2009). Nonlinear properties of vagal and sympathetic modulations of heart rate variability in ovine fetus near term. Am J Physiol Regul Integr Comp Physiol 296, R702–R707. [DOI] [PubMed] [Google Scholar]
- Galinsky R, Jensen EC, Bennet L, Mitchell CJ, Gunn ER, Wassink G, Fraser M, Westgate JA & Gunn AJ (2014). Sustained sympathetic nervous system support of arterial blood pressure during repeated brief umbilical cord occlusions in near‐term fetal sheep. Am J Physiol Regul Integr Comp Physiol 306, R787–R795. [DOI] [PubMed] [Google Scholar]
- George S, Gunn AJ, Westgate JA, Brabyn C, Guan J & Bennet L (2004). Fetal heart rate variability and brainstem injury after asphyxia in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol 287, R925–R933. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ, Bennet L & Hanson MA (1993). Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol 461, 431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene KR, Dawes GS, Lilja H & Rosen KG (1982). Changes in the ST waveform of the fetal lamb electrocardiogram with hypoxemia. Am J Obstet Gynecol 144, 950–958. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Maxwell L, de Haan HH, Bennet L, Williams CE, Gluckman PD & Gunn TR (2000). Delayed hypotension and subendocardial injury after repeated umbilical cord occlusion in near‐term fetal lambs. Am J Obstet Gynecol 183, 1564–1572. [DOI] [PubMed] [Google Scholar]
- Harris JL, Krueger TR & Parer JT (1982). Mechanisms of late decelerations of the fetal heart rate during hypoxia. Am J Obstet Gynecol 144, 491–496. [DOI] [PubMed] [Google Scholar]
- Herring N (2014). Autonomic control of the heart: going beyond the classical neurotransmitters. Exp Physiol 100, 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokegard KH, Eriksson BO, Kjellmer I, Magno R & Rosen KG (1981). Myocardial metabolism in relation to electrocardiographic changes and cardiac function during graded hypoxia in the fetal lamb. Acta Physiol Scand 113, 1–7. [DOI] [PubMed] [Google Scholar]
- Hokegard KH, Karlsson K, Kjellmer I & Rosen KG (1979). ECG‐changes in the fetal lamb during asphyxia in relation to beta‐adrenoceptor stimulation and blockade. Acta Physiol Scand 105, 195–203. [DOI] [PubMed] [Google Scholar]
- Hon EH & Lee ST (1963). Electronic evaluation of the fetal heart rate. VIII. Patterns preceding fetal death, further observations. Am J Obstet Gynecol 87, 814–826. [PubMed] [Google Scholar]
- Itskovitz J, Goetzman BW & Rudolph AM (1982). The mechanism of late deceleration of the heart rate and its relationship to oxygenation in normoxemic and chronically hypoxemic fetal lambs. Am J Obstet Gynecol 142, 66–73. [DOI] [PubMed] [Google Scholar]
- Iwamoto HS, Rudolph AM, Mirkin BL & Keil LC (1983). Circulatory and humoral responses of sympathectomized fetal sheep to hypoxemia. Am J Physiol Heart Circ Physiol 245, H767–H772. [DOI] [PubMed] [Google Scholar]
- Jensen A & Lang U (1992). Foetal circulatory responses to arrest of uterine blood flow in sheep: effects of chemical sympathectomy. J Dev Physiol 17, 75–86. [PubMed] [Google Scholar]
- Jensen EC, Bennet L, Guild SJ, Booth LC, Stewart J & Gunn AJ (2009). The role of the neural sympathetic and parasympathetic systems in diurnal and sleep state related cardiovascular rhythms in the late gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol 297, R998–R1008. [DOI] [PubMed] [Google Scholar]
- Koome ME, Bennet L, Booth LC, Davidson JO, Wassink G & Gunn AJ (2014). Ontogeny and control of the heart rate power spectrum in the last third of gestation in fetal sheep. Exp Physiol 99, 80–88. [DOI] [PubMed] [Google Scholar]
- Lees CC, Marlow N, van Wassenaer‐Leemhuis A, Arabin B, Bilardo CM, Brezinka C, Calvert S, Derks JB, Diemert A, Duvekot JJ et al (2015). 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet Epub Mar. doi: 10.1016/S0140‐6736(14)62049‐3 [DOI] [PubMed] [Google Scholar]
- Lewis AB, Wolf WJ & Sischo W (1984). Fetal cardiovascular and catecholamine responses to hypoxemia after chemical sympathectomy. Pediatr Res 18, 318–322. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Saria A, Franco‐Cereceda A, Hokfelt T, Terenius L & Goldstein M (1985). Differential effects of reserpine and 6‐hydroxydopamine on neuropeptide Y (NPY) and noradrenaline in peripheral neurons. Naunyn Schmiedebergs Arch Pharmacol 328, 331–340. [DOI] [PubMed] [Google Scholar]
- McIntosh GH, Baghurst KI, Potter BJ & Hetzel BS (1979). Foetal brain development in the sheep. Neuropathol Appl Neurobiol 5, 103–114. [DOI] [PubMed] [Google Scholar]
- Malik M & Camm AJ (1994). Heart rate variability and clinical cardiology. Br Heart J 71, 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou VE, Verkerk AO, Amin AS & de Bakker JM (2013). Intracardiac origin of heart rate variability, pacemaker funny current and their possible association with critical illness. Curr Cardiol Rev 9, 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles DM, Spencer JA, Edwards AD, Wyatt JS, Reynolds EO, Cope M & Delpy DT (1994). Relation between frequency of uterine contractions and human fetal cerebral oxygen saturation studied during labour by near infrared spectroscopy. Br J Obstet Gynaecol 101, 44–48. [DOI] [PubMed] [Google Scholar]
- Rosen KG, Dagbjartsson A, Henriksson BA, Lagercrantz H & Kjellmer I (1984). The relationship between circulating catecholamines and ST waveform in the fetal lamb electrocardiogram during hypoxia. Am J Obstet Gynecol 149, 190–195. [DOI] [PubMed] [Google Scholar]
- Saito J, Okamura K, Akagi K, Tanigawara S, Shintaku Y, Watanabe T, Akiyama N, Endo C, Sato A & Yajima A (1988). Alteration of FHR pattern associated with progressively advanced fetal acidemia caused by cord compression. Nippon Sanka Fujinka Gakkai Zasshi 40, 775–780. [PubMed] [Google Scholar]
- Serra V, Moulden M, Bellver J & Redman CW (2008). The value of the short‐term fetal heart rate variation for timing the delivery of growth‐retarded fetuses. BJOG 115, 1101–1107. [DOI] [PubMed] [Google Scholar]
- Siira SM, Ojala TH, Vahlberg TJ, Jalonen JO, Valimaki IA, Rosen KG & Ekholm EM (2005). Marked fetal acidosis and specific changes in power spectrum analysis of fetal heart rate variability recorded during the last hour of labour. BJOG 112, 418–423. [DOI] [PubMed] [Google Scholar]
- Street P, Dawes GS, Moulden M & Redman CW (1991). Short‐term variation in abnormal antenatal fetal heart rate records. Am J Obstet Gynecol 165, 515–523. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996). Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J 17, 354–381. [PubMed] [Google Scholar]
- Thaler I, Timor‐Tritsch IE & Blumenfeld Z (1985). Effect of acute hypoxia on human fetal heart rate. The significance of increased heart rate variability. Acta Obstet Gynecol Scand 64, 47–50. [DOI] [PubMed] [Google Scholar]
- Tulppo MP, Makikallio TH, Takala TE, Seppanen T & Huikuri HV (1996). Quantitative beat‐to‐beat analysis of heart rate dynamics during exercise. Am J Physiol Heart Circ Physiol 271, H244–H252. [DOI] [PubMed] [Google Scholar]
- van de Borne P, Montano N, Pagani M, Oren R & Somers VK (1997). Absence of low‐frequency variability of sympathetic nerve activity in severe heart failure. Circulation 95, 1449–1454. [DOI] [PubMed] [Google Scholar]
- van Laar JO, Peters CH, Vullings R, Houterman S, Bergmans JW & Oei SG (2010). Fetal autonomic response to severe acidaemia during labour. BJOG 117, 429–437. [DOI] [PubMed] [Google Scholar]
- van Laar JO, Porath MM, Peters CH & Oei SG (2008). Spectral analysis of fetal heart rate variability for fetal surveillance: review of the literature. Acta Obstet Gynecol Scand 87, 300–306. [DOI] [PubMed] [Google Scholar]
- Visser GH, Goodman JD, Levine DH & Dawes GS (1982). Diurnal and other cyclic variations in human fetal heart rate near term. Am J Obstet Gynecol 142, 535–544. [DOI] [PubMed] [Google Scholar]
- Wassink G, Galinsky R, Drury PP, Gunn ER, Bennet L & Gunn AJ (2014). Does maturity affect cephalic perfusion and T/QRS ratio during prolonged umbilical cord occlusion in fetal sheep? Obstet Gynecol Int 2014, 314159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgate JA, Bennet L, Brabyn C, Williams CE & Gunn AJ (2001. a). ST waveform changes during repeated umbilical cord occlusions in near‐term fetal sheep. Am J Obstet Gynecol 184, 743–751. [DOI] [PubMed] [Google Scholar]
- Westgate JA, Bennet L, de Haan HH & Gunn AJ (2001. b). Fetal heart rate overshoot during repeated umbilical cord occlusion in sheep. Obstet Gynecol 97, 454–459. [DOI] [PubMed] [Google Scholar]
- Westgate JA, Bennet L & Gunn AJ (1999). Fetal heart rate variability changes during brief repeated umbilical cord occlusion in near term fetal sheep. Br J Obstet Gynaecol 106, 664–671. [DOI] [PubMed] [Google Scholar]
- Westgate JA, Harris M, Curnow JS & Greene KR (1992). Randomised trial of cardiotocography alone or with ST waveform analysis for intrapartum monitoring. Lancet 340, 194–198. [DOI] [PubMed] [Google Scholar]
- Westgate JA, Wibbens B, Bennet L, Wassink G, Parer JT & Gunn AJ (2007). The intrapartum deceleration in center stage: a physiological approach to interpretation of fetal heart rate changes in labor. Am J Obstet Gynecol 197, e1–e11.236. [DOI] [PubMed] [Google Scholar]
- Wibbens B, Westgate JA, Bennet L, Roelfsema V, de Haan HH, Hunter CJ & Gunn AJ (2005). Profound hypotension and associated ECG changes during prolonged cord occlusion in the near term fetal sheep. Am J Obstet Gynecol 193, 803–810. [DOI] [PubMed] [Google Scholar]
- Yu ZY, Lumbers ER, Gibson KJ & Stevens AD (1998). Effects on hypoxaemia on foetal heart rate, variability and cardiac rhythm. Clin Exp Pharmacol Physiol 25, 577–584. [DOI] [PubMed] [Google Scholar]


