Key points
Maternal protein restriction during pregnancy increases both maternal and offspring oxidative stress and leads to metabolic dysfunction.
Maternal low protein diet during pregnancy increases maternal and offspring corticosterone.
Resveratrol administration partially prevents both maternal and offspring adverse outcomes induced by maternal protein restriction during pregnancy.
Abstract
Protein restriction in pregnancy produces maternal and offspring metabolic dysfunction potentially as a result of oxidative stress. Data are lacking on the effects of inhibition of oxidative stress. We hypothesized that maternal resveratrol administration decreases oxidative stress, preventing, at least partially, maternal low protein‐induced maternal and offspring metabolic dysfunction. In the present study, pregnant wistar rats ate control (C) (20% casein) or a protein‐restricted (R) (10% casein) isocaloric diet. Half of each group received resveratrol orally, 20 mg kg−1 day−1, throughout pregnancy. Post‐delivery, mothers and offspring ate C. Oxidative stress biomarkers and anti‐oxidant enzymes were measured in placenta, maternal and fetal liver, and maternal serum corticosterone at 19 days of gestation (dG). Maternal (19 dG) and offspring (postnatal day 110) glucose, insulin, triglycerides, cholesterol, fat and leptin were determined. R mothers showed metabolic dysfunction, increased corticosterone and oxidative stress and reduced anti‐oxidant enzyme activity vs. C. R placental and fetal liver oxidative stress biomarkers and anti‐oxidant enzyme activity increased. R offspring showed higher male and female leptin, insulin and corticosterone, male triglycerides and female fat than C. Resveratrol decreased maternal leptin and improved maternal, fetal and placental oxidative stress markers. R induced offspring insulin and leptin increases were prevented and other R changes were offspring sex‐dependent. Resveratrol partially prevents low protein diet‐induced maternal, placental and sex‐specific offspring oxidative stress and metabolic dysfunction. Oxidative stress is one mechanism programming offspring metabolic outcomes. These studies provide mechanistic evidence to guide human pregnancy interventions when fetal nutrition is impaired by poor maternal nutrition or placental function.
Key points
Maternal protein restriction during pregnancy increases both maternal and offspring oxidative stress and leads to metabolic dysfunction.
Maternal low protein diet during pregnancy increases maternal and offspring corticosterone.
Resveratrol administration partially prevents both maternal and offspring adverse outcomes induced by maternal protein restriction during pregnancy.
Abbreviations
- C
control diet
- Cres
control diet + resveratrol
- dG
days of gestation
- GPx
glutathione peroxidase
- HOMA
homeostatic model assessment
- MDA
malondialdehyde
- PND
postnatal day
- R
restricted diet
- Rres
restricted diet + resveratrol
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Introduction
Poor fetal and neonatal nutritions result in developmental programming of multiple organ systems in humans and animals (Raghunath et al. 2009; Allen, 2012; Connor et al. 2012; Martin‐Gronert & Ozanne, 2013; Nathanielsz et al. 2013; Hanson & Gluckman, 2014; Chan et al. 2015). These alterations in life course phenotype are the result of developmental programming, which can be defined as ‘the response to a specific challenge to the mammalian organism during a critical developmental time window that alters the trajectory of development, modifying the organism's phenotype with effects on health that can persist throughout the life course’ (Wadhwa et al. 2009; Gallo et al. 2013; Nathanielsz et al. 2013). One of the commonest and earliest experimental challenges studied is maternal protein restriction in pregnant rats. Maternal low protein diets result in male and female offspring experiencing metabolic dysfunction (Holemans et al. 2003; Armitage et al. 2004; Zambrano et al. 2005 a; Langley‐Evans & Sculley, 2006; Martin‐Gronert & Ozanne, 2013), hypertension (Woods et al. 2001), as well as cardiovascular (Barros et al. 2015; Nascimento et al. 2014), behavioural (Almeida et al. 1996; Reyes‐Castro et al. 2012) and reproductive (Engelbregt et al. 2000; Zambrano et al. 2014 a) dysfunction.
Adequate perinatal nutrition is important because macro‐ and/or micronutrient deficiency during development increases oxidative stress by generation of reactive oxygen species (ROS) and decreases overall anti‐oxidant activity (Mistry & Williams, 2011) associated with offspring insulin resistance (Zambrano et al. 2005 a; Zambrano et al. 2006; Padmavathi et al. 2009). Under normal developmental conditions, ROS production is balanced by the removal of free radicals by anti‐oxidant mechanisms (Halliwell, 2006) and oxidative stress only occurs when ROS generation exceeds the scavenging capacity of cellular anti‐oxidant mechanisms as a result of excessive ROS production and/or inadequate anti‐oxidant intake or synthesis.
Oxidative stress has been proposed as a causative agent of maternal pregnancy problems (e.g. recurrent pregnancy loss, pre‐eclampsia and gestational diabetes) (Agarwal et al. 2012). There is also evidence to support the view that inadequate maternal nutrition and other challenges increase maternal and fetal oxidative stress, as well as ROS damage, and are also responsible for developmental programming of offspring disease (Giussani et al. 2012; Richter et al. 2012; Tarry‐Adkins et al. 2013; Vega et al. 2015). In adults, chronic metabolic disorders may result from ROS generation and can be improved with dietary anti‐oxidants (Willcox et al. 2004). Flavonoids and other polyphenols have powerful anti‐oxidant activities in vitro and in vivo, including the ability to scavenge a range of ROS (Heijnen et al. 2001; Santos & Mira, 2004). Resveratrol isolated from the oriental medicinal plant Polygonum capsidatum (Nonomura et al. 1963) exerts a variety of pharmacological effects, such as anticancer (Bhat & Pezzuto, 2002; Carter et al. 2014) and anti‐ inflammation actions (Jang et al. 1999). Resveratrol exerts a strong inhibitory effect on production of ROS and free radical scavenging properties in many experimental systems (Fremont, 2000; Martinez & Moreno, 2000; Bhat et al. 2001; Delmas et al. 2013; Cottart et al. 2014).
The effects of resveratrol on programming challenges include maternal hypoxia (Dolinsky et al. 2011; Bourque et al. 2012; Rueda‐Clausen et al. 2012), maternal alcohol exposure (Kumar et al. 2011), maternal lipopolysaccharide treatment (Rose et al. 2014), maternal valproic acid inducing abnormal neural development (Bambini‐Junior et al. 2014) and phthalates challenging male reproductive development (Botelho et al. 2009). Maternal resveratrol treatment has never been used to determine the effects of anti‐oxidant therapy in relation to programming by maternal low protein diets. In one study, lazaroid, a 21‐aminosteroid anti‐oxidant inhibitor of lipid peroxidation (Cambonie et al. 2007), was shown to prevent adult hypertension, vascular dysfunction and microvascular rarefaction in male offspring following fetal exposure to a low‐protein diet. No metabolic end‐points were determined
In a recent review, two leading researchers of oxidative stress as a mechanism underlying hypoxia and developmental origins of cardiovascular dysfunction state that mechanisms ‘remain elusive, precluding the identification of potential clinical therapy’ (Giussani & Davidge, 2013; Giussani et al. 2014). The same limitation to understanding the responsible mechanism is present in relation to programming by maternal undernutrition. One early study showed that in rat offspring, developmental programming by maternal low protein diets results from increased adrenocortical activity. Offspring adrenalectomy prevented programming of hypertension while administration of glucocorticoids to raise offspring corticosterone levels to those observed following fetal exposure to a low protein diet restored the hypertension (Gardner et al. 1997). We therefore determined changes in corticosterone in both mothers and offspring. Therefore, in the absence of any prior data evaluating resveratrol treatment on metabolic outcomes resulting from a low protein diet, the present study was based on the hypothesis that resveratrol administration during pregnancy decreases oxidative stress and prevents, at least partially, maternal, fetal and offspring metabolic outcomes resulting from low protein during pregnancy and improves offspring metabolism. We also hypothesized that there would be differences with respect to the sex of the offspring.
Methods
Care and maintenance of animals
All procedures described in the present study were approved by the Animal Experimentation Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición ‘Salvador Zubirán’, Mexico, and were conducted in accordance with the guidelines of Mexican law on animal protection (NOM‐062‐ZOO‐1999).
Details of maternal diet, breeding and management have been reported previously in detail (Zambrano et al. 2005 b). Female albino adult Wistar rats, aged 15–17 weeks, weighing 280 ± 3 g (mean ± SD), were fed a Purina 5001 rodent diet (LabDiet, St. Louis, MO, USA) and maintained under a 12:12 h light/dark cycle (lights on 07.00 h) at 22–23°C. Founder female rats were mated overnight with proven male breeders. Females recruited to the study underwent a daily vaginal swab and the day that spermatozoa were present in the vaginal smear was designated as the day of conception (day 0). Only rats that became pregnant within 5 days of introduction of a fertile male were studied. Pregnant rats were placed in individual cages and randomly allocated to eat either control (C: 20% casein; n = 32) or restricted isocaloric diet (R: 10% casein; n = 33) (Zambrano et al. 2005 b). Food and water were provided ad libitum. Pregnant rats were weighed daily during pregnancy and lactation. Food was provided as flat biscuits and food intake was measured daily. Half of the control rats were randomly assigned to receive resveratrol (Cres), as were half of the R mothers (Rres).
Maternal resveratrol intervention
Pharmacokinetic properties of resveratrol indicate that it cannot be dissolved in water, it has a short half‐life (Baur & Sinclair, 2006) and, once administered, it is rapidly metabolized in the body (Delmas et al. 2006). Because of these characteristics, resveratrol was dissolved in an aqueous solution of carboxymethylcellulose 0.5% that was also used as the vehicle (Das et al. 2008). The dose of resveratrol (Sigma‐Aldrich, St Louis, MO, USA) fed per rat was 20 mg (kg body weight)−1 day−1. The resveratrol or vehicle was administered orally by gavage from 0 days of gestation (dG) until delivery. At 19 dG, half of the females from each group were killed to obtain serum, maternal and fetal tissue. Six rats per group were allowed to deliver to provide offspring for studies at postnatal day (PND) 110. Post‐delivery mothers were fed with the C diet. Offspring were weaned at PND 21 and fed the Purina 5001 diet throughout the study.
Maternal phenotype, blood parameters and fetal characteristics at 19 dG
Serum and tissue collection
At 19 dG (chosen to avoid effects of parturition) at 6.00 h, food was removed from pregnant rats. Between 10.00 and 11.00 h, pregnant rats were rapidly killed by decapitation by experienced personnel who were trained to use the rodent guillotine (Thomas Scientific, Swedesboro, NJ, USA). To ensure homogeneity of study subjects, litters >14 or <10 pups were excluded. Trunk blood was collected, allowed to clot at 4°C for 1 h, centrifuged at 1500 g for 15 min at 4°C and serum stored at –75°C.
Through a midline abdominal incision, the maternal liver was removed, cleaned, weighed and frozen at –75°C. The uterine horns were exposed and fetuses were rapidly killed by decapitation, counted and weighed. Each placenta was cleaned, weighed and male and females from each litter pooled together as one sample for each sex. Fetal livers were isolated and weighed. All male livers from each litter were pooled to produce a single sample for the litter. Females were treated similarly. Livers were immediately frozen in liquid nitrogen.
Adult offspring phenotype
Offspring were studied at PND 110. One male and one female per litter (n = 6) were randomly chosen, fasted for 4 h and killed by decapitation, and the trunk blood and liver were obtained and stored at –75°C for later protein quantification, as well as for determination of biomarkers of oxidative stress (ROS and anti‐oxidant enzymes). Visceral fat depots located inside the thorax (mediastinal) and abdomen (omental, perirenal, retroperitoneal, epididymal, periovarian, perivescical and parametrial) were excised and weighed. The adiposity index was calculated as total adipose tissue (g) body weight (g)−1.
Biochemical analysis
Serum measurements
Glucose, triglycerides and cholesterol were determined enzymatically with a Synchron CX auto analyser (Beckman Coulter, Fullerton, CA, USA) and corticosterone, insulin and leptin were determined by radioimmunoassay (Diagnostic Products Corp, Los Angeles, CA, USA; Linco Research, St Charles, MO, USA) (Guzman et al. 2006; Zambrano et al. 2006). Homeostatic model assessment (HOMA) was calculated from HOMA = glucose (mmol−1) × insulin (μU ml−1)/22.5 (Zambrano et al. 2006; Rasmussen et al. 2009).
Tissue homogenates and protein determination
Next, 100 mg of frozen placenta and liver were homogenized in 1 ml of 0.9% saline solution at 4°C, and protein was quantified by the Bradford method (Bradford, 1976). Lipid peroxidation was determined at the time of tissue homogenization. All determinations were performed in duplicate and averaged for statistical analysis.
Lipid peroxidation assay
Lipid peroxidation was assessed by malondialdehyde (MDA) content (Buege & Aust, 1978), measured by a thiobarbituric acid spectrometry assay, as described previously (Vega et al. 2015) in serum and liver. Tissue MDA was normalized to protein concentration. Intra‐ and inter‐assay CVs were ≤3% and ≤5%, respectively.
ROS assay
ROS formation was estimated as reported previously (Vega et al. 2015), adapted for tissue homogenates and expressed as dichlorofluorescein formed [nmol (mg protein) –1 min–1].
Superoxide dismutase activity
Superoxide dismutase (SOD) activity was measured using a commercial kit (RANSOD by Randox Laboratories, Crumlin, UK; catalogue numbers SD125 and SD126) and normalized to protein concentration. Intra‐ and interassay CVs were ≤7% and ≤8%, respectively.
Glutathione peroxidase activity
Glutathione peroxidase (GPx) activity was measured using a commercial kit (RANSEL by Randox Laboratories; catalogue number RSD504). Data were normalized to protein concentration. Intra‐ and inter‐assay CVs were ≤3% and ≤5%, respectively.
Statistical analysis
For 19 dG studies, 10 C mothers, 10 Cres, 14 R and seven Rres mothers were studied. One animal of each sex from six different litters was studied per postnatal group. All data are reported as the mean ± SEM. Analysis of maternal diet effects and resveratrol intervention was by ANOVA with Tukey's post hoc test where appropriate. P < 0.05 was considered statistically significant.
Results
Maternal characteristics
Maternal body weight and food intake during gestation
Maternal body and liver weight at 19 dG and the end of pregnancy were similar among groups. At the end of gestation, maternal weight gain was lower in R than C. At the beginning and end of lactation, Cres mothers weighed more than R. Resveratrol administration did not modify food intake (Table 1).
Table 1.
Maternal, placenta and fetal characteristics
| C | Cres | R | Rres | P | |
|---|---|---|---|---|---|
| Maternal | |||||
| Body weight (g) at onset pregnancy | 280 ± 4 | 283 ± 5 | 278 ± 4 | 278 ± 2 | 0.62 |
| Maternal body weight (g) at 19 dG | 407 ± 10 | 394 ± 10 | 375 ± 8 | 391 ± 9 | 0.17 |
| Maternal liver weight (g) at 19 dG | 12.8 ± 0.6 | 12.6 ± 0.5 | 11.7 ± 0.3 | 11.2 ± 0.2 | 0.07 |
| Maternal body weight (g) at the end of pregnancy | 423 ± 11 | 409 ± 9 | 389 ± 9 | 407 ± 8 | 0.13 |
| Maternal weight gain (g) through the whole of gestation | 143 ± 7a | 127 ± 7ab | 113 ± 6b | 131 ± 8ab | 0.05 |
| Food intake (g) during gestation | 579 ± 10 | 581 ± 11 | 579 ± 7 | 580 ± 6 | 0.753 |
| Maternal body weight (g) at the beginning of lactation | 314 ± 7ab | 336 ± 7a | 295 ± 7b | 311 ± 9ab | 0.01 |
| Maternal body weight (g) at the end of lactation | 278 ± 6ab | 292 ± 5a | 268 ± 3b | 284 ± 6ab | 0.02 |
| Litter size | 11.7 ± 0.33 | 11.4 ± 0.27 | 11.3 ± 0.19 | 11.4 ± 0.3 | 0.707 |
| Male:female ratio | 1.1 ± 0.13 | 0.9 ± 0.08 | 1.2 ± 0.07 | 1.1 ± 0.08 | 0.318 |
| Placenta | |||||
| Male placenta weight (g) at 19 dG | 0.51 ± 0.01 | 0.52 ± 0.02 | 0.48 ± 0.02 | 0.46 ± 0.01 | 0.07 |
| Female placenta weight (g) at 19 dG | 0.51 ± 0.01 | 0.52± 0.02 | 0.49 ± 0.02 | 0.48 ± 0.02 | 0.38 |
| Fetuses | |||||
| Male body weight (g) at 19 dG | 3.1 ± 0.07a | 3.2 ± 0.05a | 2.4 ± 0.04b | 2.7 ± 0.08c | 0.001 |
| Male fetus:placenta ratio at 19 dG | 6.1 ± 0.15a | 6.1 ± 0.21a | 4.9 ± 0.19b | 5.8 ± 0.16a | 0.001 |
| Male liver weight (g) at 19 dG | 0.18 ± 0.006 | 0.19 ± 0.008 | 0.18 ± 0.008 | 0.18 ± 0.005 | 0.91 |
| Female body weight (g) at 19 dG | 3.1 ± 0.05a | 3.2 ± 0.03a | 2.5 ± 0.1b | 2.7 ± 0.05ab | 0.001 |
| Female fetus:placenta ratio at 19 dG | 5.9 ± 0.14ab | 6.2 ± 0.21a | 5.1 ± 0.33b | 5.6 ± 0.14ab | 0.023 |
| Female liver weight (g) at 19 dG | 0.17 ± 0.004a | 0.19 ± 0.01b | 0.14 ± 0.004c | 0.18 ± 0.004ab | 0.001 |
Data are the mean ± SEM. One‐way ANOVA, P < 0.05 for data not sharing at least one letter. Number per group: C = 10, Cres = 10, R = 14 and Rres = 7 at 19 dG. n = 6 per group during lactation.
Placenta and fetal measures
Litter size and sex ratio were similar among groups. Placental and fetal body and liver weight data are reported in Table 1. Male and female R fetal weights were lower than C fetuses and resveratrol administration partially prevented this decrease in males. For both sexes, the fetal:placental ratio was lower in the R group. This decrease was prevented in males by maternal resveratrol treatment. In resveratrol exposed females, the ratio did not differ from either controls or R offspring (Table 1). Female but not male liver fetal weight was lower in R and higher in Cres compared to C (Table 1).
Oxidative stress markers in mothers at 19dG
At 19 dG, maternal serum and liver MDA were higher in R compared to the C group (Fig. 1 A and B). At 19 dG, the low protein diet increased maternal liver ROS (Fig. 1 C). Resveratrol treatment in pregnancy decreased serum MDA and hepatic ROS in both Cres and Rres compared to the corresponding untreated groups, C and R (Fig. 1 A and C). Liver SOD activity was lower in Cres, R and Rres relative to C (Fig. 1 D). GPx activity was lower in R compared to C (Fig. 1 E).
Figure 1. Maternal oxidative stress biomarkers at 19 dG .
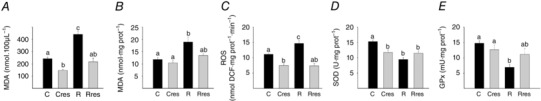
Serum MDA (A), liver MDA (B), liver ROS (C), liver SOD (D) and GPx (E) activities. Data are the mean ± SEM; P < 0.05 when not sharing at least one letter. Number per group: C = 10, Cres = 10, R = 14 and Rres = 7.
Maternal metabolic variables at 19dG
Maternal serum glucose was similar in C and R. Resveratrol reduced blood glucose in C mothers (Fig. 2 A). Serum insulin concentration and HOMA were higher in R than C. Although resveratrol significantly improved both of these key metabolic variables, the reductions were only by 17% and 22% (Fig. 2 B and C). Serum leptin levels were higher in R compared to C, Cres and Rres (Fig. 2 D). Serum triglyceride and cholesterol levels were similar in all four groups (Fig. 2 E and F).
Figure 2. Maternal metabolism parameters at 19 dG .
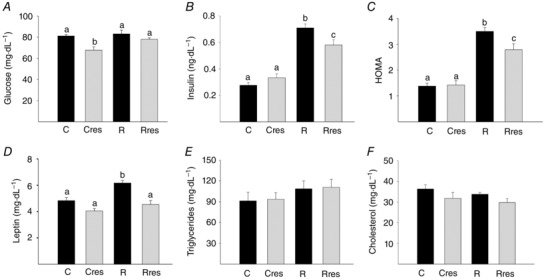
Glucose (A), insulin (B), HOMA (C), leptin (D), triglycerides (E) and cholesterol (F). Data are the mean ± SEM; P < 0.05 when not sharing at least one letter. Number per group: C = 10, Cres = 10, R = 14 and Rres = 7.
At 19 dG, maternal serum corticosterone was higher in R mothers than C. This increase was reduced by 20% after the administration of resveratrol (Fig. 3 A).
Figure 3. Serum corticosterone levels .
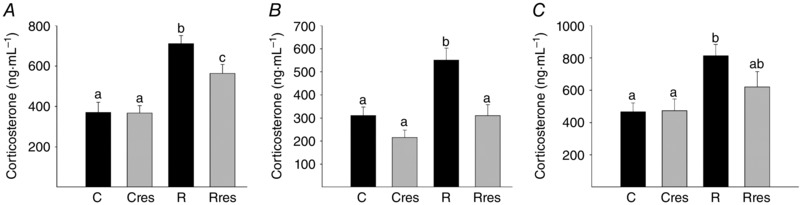
Maternal at 19 dG (A), male (B) and female (C) offspring at PND 110. Data are the mean ± SEM; P < 0.05 when not sharing at least one letter. Number per group: C = 10, Cres = 10, R = 14 and Rres = 7.
Placental oxidative stress markers
At 19 dG, male and female placental MDA was higher in R and lower in male Cres and Rres compared to C (Fig. 4 A and B). ROS levels were higher in both male and female R placentas compared to C placentas (Fig. 4 C and D). Placental SOD activity was higher in female R than C without any change in males (Fig. 4 E and F). Placental GPx activity was higher in male R compared to the C, Cres and Rres, and lower in female Cres and Rres compared to R (Fig. 4 G and H).
Figure 4. Placental oxidative stress biomarkers at 19 dG .
Male MDA (A), ROS (C), SOD (E), GPx (G) and female MDA (B), ROS (D), SOD (F) and GPx (H). Data are the mean ± SEM; P < 0.05 when not sharing at least one letter. Number per group: C = 10, Cres = 10, R = 14 and Rres = 7.
Fetal liver oxidative stress markers
At 19 dG, liver MDA and ROS levels were higher in male and female R fetuses compared to C (Fig. 5 A–D). ROS was lower in female Rres than the other three groups (Fig. 5 D). SOD activity was higher in male R compared to C, Cres and Rres. By contrast, female SOD activity was lower in R compared to C, whereas Rres showed the lowest value (Fig. 5 E and F). Male GPx liver activity was lower in Cres compared to C and Rres compared to R. Female GPx was higher in R compared to C (Fig. 5 G and H).
Figure 5. Liver oxidative stress in fetuses of 19 dG .
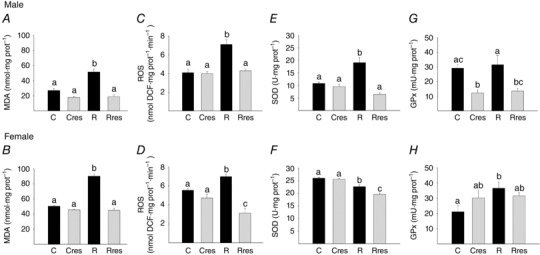
Male MDA (A), ROS (C), SOD (E), GPx (G) and female MDA (B), ROS (D), SOD (F) and GPx (H). Data are the mean ± SEM; P < 0.05 when not sharing at least one letter. Number per group: C = 10, Cres = 10, R = 14 and Rres = 7.
Adult offspring phenotype
At PND 110, Cres males weighed less than C (Fig. 6 A). Total fat and adiposity index were lower in male Cres compared to C (Fig. 6 B and C). Female body weight was higher in Rres compared to C and Cres, and total fat and adiposity index were higher in female R and Rres compared to C and Cres (Fig. 6 G–I). Fat depot distribution is shown in Table 2. Male offspring retroperitoneal and perirenal fat was decreased in Cres compared to C and R. Retroperitoneal and perirenal and parametrial fat were greater in R and Rres females compared to C and Cres. Male epidiymal fat was decreased in Cres compared to C and R and lower in Rres compared to R. Female periovarian and perivescical, male and female mediastinial and omental fat were similar among groups (Table 2). Male and female serum corticosterone levels were higher in the R group than C. In male offspring, maternal resveratrol administration prevented the R induced increase in corticosterone. In female offspring, corticosterone in Rres did not differ from either controls or R offspring (Fig. 3 B and C).
Figure 6. Offspring characteristics at PND 110 .
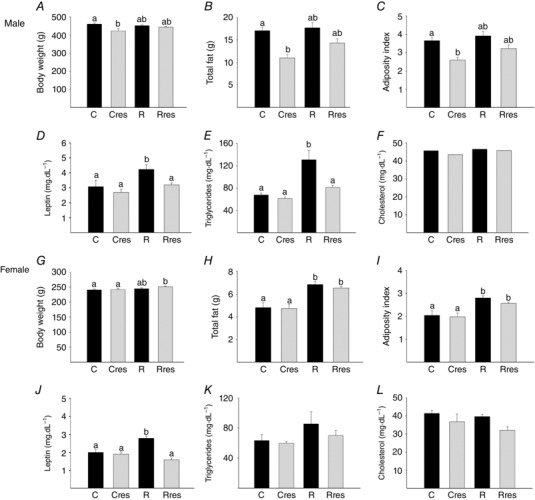
Male: body weight (A), total fat (B), adiposity index (C), leptin (D), triglycerides (E) and cholesterol (F). Female: body weight (G), total fat (H), adiposity index (I), leptin (J), triglycerides (K) and cholesterol (L). Data are the mean ± SEM; P < 0.05 when not sharing at least one letter. n = 6 per group.
Table 2.
Offspring (F1) male and female fat depots weight at PND 110
| Fat (g) | C | Cres | R | Rres | P |
|---|---|---|---|---|---|
| Male | |||||
| Mediastinial | 0.29 ± 0.02 | 0.23 ± 0.02 | 0.32 ± 0.04 | 0.36 ± 0.03 | 0.37 |
| Retroperitoneal and perirenal | 7.8 ± 0.4a | 4.8 ± 0.4b | 8.1 ± 0.7a | 6.6 ± 0.5ab | 0.001 |
| Omental | 0.8 ± 0.07 | 0.7 ± 0.1 | 0.7 ± 0.04 | 0.7 ± 0.04 | 0.12 |
| Epidydimal | 8.1 ± 0.4ac | 5.2 ± 0.4b | 8.6 ± 0.5a | 6.6 ± 0.5bc | 0.001 |
| Female | |||||
| Mediastinial | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.36 |
| Retroperitoneal and perirenal | 1.7 ± 0.2a | 1.7 ± 0.2a | 2.4 ± 0.1b | 2.4 ± 0.1b | 0.01 |
| Omental | 0.4 ± 0.06 | 0.4 ± 0.02 | 0.5 ± 0.08 | 0.4 ± 0.05 | 0.85 |
| Parametrial | 1.4 ± 0.1a | 1.4 ± 0.2a | 2.5 ± 0.1b | 2.1 ± 0.1b | 0.001 |
| Periovarian and Perivescical | 1.2 ± 0.08 | 1.1 ± 0.2 | 1.3 ± 0.1 | 1.5 ± 0.09 | 0.21 |
Data are the mean ± SEM. One‐way ANOVA, P < 0.05 for data not sharing at least one letter. n = 6 per group.
Serum leptin was higher in R males and females compared to all other groups (Fig. 6 D and J). Male serum triglycerides in R were higher than C, Cres and Rres, with no differences among female offspring groups (Fig. 6 E and K). Male and female serum cholesterol were similar among groups (Fig. 6 F and L).
For both sexes, serum glucose levels were similar among groups. Insulin and the HOMA index were higher in R in both sexes compared to all other groups (Fig. 7).
Figure 7. Offspring metabolism at PND 110 .
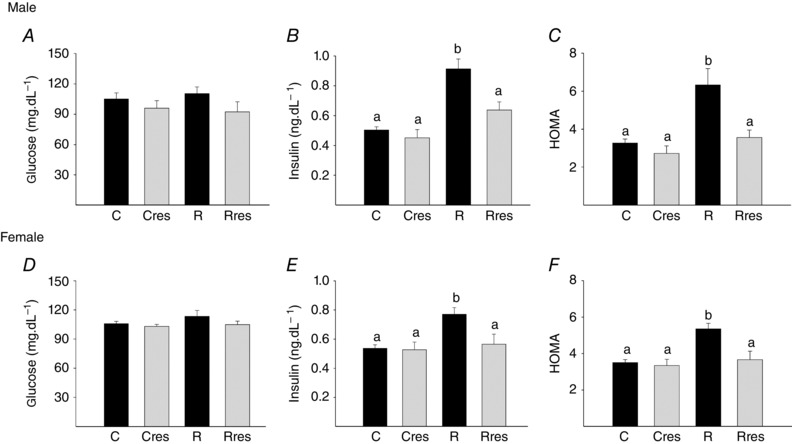
Male: glucose (A), insulin (B) and HOMA (C). Female: glucose (D), insulin (E) and HOMA (F). Data are the mean ± SEM; P < 0.05 when not sharing at least one letter. n = 6 per group.
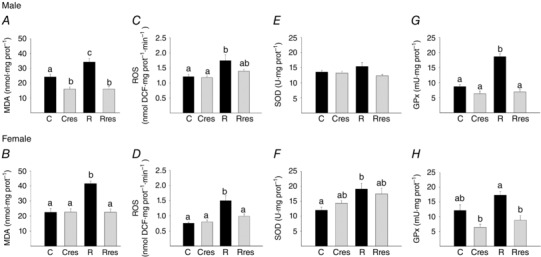
Offspring oxidative stress parameters at PND 110
Serum MDA was higher in R compared to C, Cres and Rres in both male and female offspring (Fig. 8 A and F). Liver MDA concentration in male offspring was lower in Cres compared to R and lower in Rres compared to C and R (Fig. 8 B), whereas, in female offspring, liver MDA was higher in R compared to C, Cres and Rres (Fig. 8 G). Liver ROS concentration was higher in R compared to the rest of the groups for male offspring, whereas female offspring groups were similar (Fig. 8 C and H). Liver SOD and GPx activities were lower in R male offspring compared to the other male groups (Fig. 8 D and E). In female offspring, SOD Cres was higher than R and Rres (Fig. 8 I), whereas GPx was higher than the other three groups and R was lower than C (Fig. 8 J).
Figure 8. Offspring oxidative stress biomarkers at PND 110 .
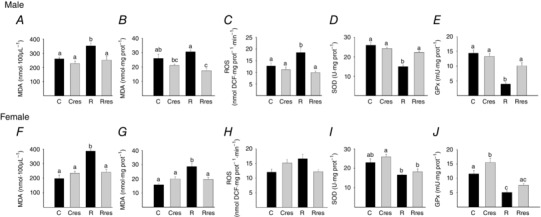
Male: serum MDA (A), liver: MDA (B), ROS (C), SOD (D) and GPx (E). Female: serum MDA (F), liver: MDA (G), ROS (H), SOD (I) and GPx (J). Data are the mean ± SEM; P < 0.05 when not sharing at least one letter. n = 6 per group.
Discussion
Compelling epidemiological evidence indicates that reduced maternal nutrition and poor fetal nutrient delivery have long‐term effects on offspring health. We studied a common anti‐oxidant intervention to determine the role of oxidative stress in programming the metabolic dysfunction seen in both sexes resulting from a maternal low protein diet in pregnancy. Pregnancy is accompanied by a high maternal energy and oxygen requirement to satisfy increasing maternal and fetal demands and therefore probably increases oxidative stress (Gitto et al. 2002). We observed that the low protein diet increased oxidative stress in pregnant mothers compared to mothers fed the control diet. The placenta is very sensitive to oxidative stress (Gupta et al. 2005). Placental changes in oxygen tension at the beginning of pregnancy help to sustain the increased metabolic rate that occurs during the rapid fetal growth phase (Burton, 2009) and are associated with both increased circulating ROS, as well as increased activity of enzymes such as catalase, GPx and SOD (Jauniaux et al. 2000), which are responses that help to protect against increased oxidative stress. The results of the present study show that maternal protein restriction increases oxidative stress markers in placentas of both male and female fetuses, whereas there are some fetal sex‐dependent differences in anti‐oxidant responses. Maternal nutrient deficiency alters placental function and increased oxidative damage may play a role in the observed reduction in the passage of nutrients from mother to fetus in the setting of reduced maternal nutrition and intra‐uterine growth restriction (Jones et al. 2013; Rebelato et al. 2013). The results of the present study show that resveratrol has no effect on placental efficiency, measured as the fetal:placenta ratio in controls, but does increase placental efficiency in placentas of restricted mothers to levels similar to controls. This improvement in placental efficiency probably represents a beneficial outcome.
Although some studies show increased placental oxidative stress in the presence of maternal protein restriction (Rebelato et al. 2013), we have been unable to find data on oxidative stress levels in fetal tissues in response to this challenge. However, other fetal programming models in the rat such as gestational diabetes have shown an increase in whole embryo lipid peroxidation and total thiols (Singh et al. 2011). In addition, hypoxic pregnancy promotes maternal and placental indices of oxidative stress, as well as enhanced aortic nitrotyrosine staining (Giussani et al. 2012; Richter et al. 2012). In the present study, hepatic lipid peroxidation and ROS production were increased in male and female fetuses by the maternal low protein diet. The changes in anti‐oxidant enzymes differed in male and female fetal liver tissues. Anti‐oxidant sex differences are present in adult rats. Female rats have higher hepatic and cardiac vitamin E concentrations than males but lower hepatic GPx and muscle vitamin C (Tiidus et al. 1999).
Because few studies report tissue oxidative stress changes or status during resveratrol treatment (Cambonie et al. 2007), our second aim was to determine the extent to which the anti‐oxidant resveratrol can prevent the oxidative stress changes resulting from maternal ingestion of a low protein isocaloric diet. Resveratrol has high anti‐oxidant activity (Martinez & Moreno, 2000; Bhat et al. 2001; Cottart et al. 2014), and modulates multiple signalling pathways, including phosphorylation of adenosine‐monophosphate‐activated protein kinase, adenylyl cyclase, sirtuin 1, cyclooxygenase, lipooxygenase and protein kinase C (Pirola & Frojdo, 2008). Bourque et al. (2012) showed that adverse fetal outcomes as a result of hypoxic pregnancies can be improved if pregnant rats consume resveratrol. They also showed that resveratrol crosses the placenta; however, it is uncertain whether the benefits of resveratrol are the result of its actions on the placenta or in the maternal–fetal system, or both (Bourque et al. 2012). Using transgenic mouse models, it was shown that AMPK and SIRT1 are key mediators of the metabolic health actions driven by resveratrol (Kulkarni & Canto, 2015). In the gestational diabetic rat, resveratrol improved maternal glucose and the lipid profile and prevented embryonic oxidative stress and apoptosis (Singh et al. 2013). However, no data exist on the ability of resveratrol to prevent unwanted effects on developmental programming mechanisms induced by maternal protein restriction in either the mother or her offspring. In addition, the resveratrol dose administered differs widely between studies (Table 3). In pregnant mice, a dose of 50 mg kg−1 day−1 resveratrol reduced the incidence of fetal malformations induced by 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin exposure in utero (Jang et al. 2008). In a prenatal pregnant rat restraint stress model, resveratrol administration of twice the mouse dose, 100 mg kg−1 day−1, inhibited mitochondrial loss in the offspring hippocampus (Cao et al. 2014). (Table 3). In the present study, maternal resveratrol administration at less than half the mouse dose (20 mg kg−1 day−1) had an overall beneficial effect by decreasing maternal MDA and ROS. Resveratrol also prevented the increase in placental and fetal oxidative stress markers. One resveratrol study in female Japanese macaques (Roberts et al. 2014) addressed the potential for beneficial effects of resveratrol administration in the setting of maternal obesity and fetal overnutrition. Mothers were maintained on a Western‐style diet and provided with resveratrol in the diet (0.37%) for 3 months before and during pregnancy. Because no data were provided on food intake, the dose of resveratrol consumed cannot be calculated. The findings of this interesting study indicated that resveratrol treatment improved uterine blood flow, reduced placental inflammation and improved fetal liver lipid deposition (Roberts et al. 2014). However, there was an unexpected enlargement of the fetal pancreas without a concomitant increase in islet mass, suggesting a potentially harmful direct action of resveratrol on the fetal pancreas (Roberts et al. 2014). These and other data raised some concerns regarding anti‐oxidant treatment in general and resveratrol in particular in pregnancy. However, Table 3 shows the widely different doses and routes of administration in different studies. Much more data are required to establish the potential cost–benefit of using resveratrol to prevent programming. In the present study, no adverse effects were observed potentially as a result of the lower dose.
Table 3.
Review of publications using resveratrol to determine effects of oxidative stress in response to programming challenges
| Species | Model | Dose | Period of intervention | Outcomes | Reference |
|---|---|---|---|---|---|
| Mouse C57BL/KsJ‐Lep (db/+) | Genetic gestational diabetes mellitus | Oral gavage (10 mg kg–1 day–1) | Prior to and throughout pregnancy | Resveratrol intervention improved maternal glucose metabolism, insulin tolerance and reproductive outcomes. Hepatic AMPK activation was enhanced in both the mother and the offspring. Glucose 6‐phosphatase production and activity were reduced | Yao et al. (2015) |
| Mouse C57BL/6J | Teratogenesis | Oral administration (50 mg kg–1 day–1) | Pregnancy 6 consecutive days from E8–E13 | Resveratrol treatment reduced the incidence of fetal malformations induced by 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin exposure in utero | Jang et al. (2008) |
| Wistar rats | Autism induced by valproic acid | Subcutaneous injections in DMSO (3.6 mg kg–1) | Pregnancy from E6.5 until E18.5 | Resveratrol prevented valproic acid induced signs of autism | Bambini‐Junior et al. (2014) |
| Wistar rat | Prenatal stress | Oral administration (10 mg kg–1 day–1) | Throughout pregnancy | Resveratrol treatment enhanced offspring number of newly born neurons and brain‐derived neurotrophic factor expression in the hippocampus | Madhyastha et al. (2013) |
| Wistar rat | Prenatal stress | Oral administration (10 mg kg–1 day–1) | Throughout pregnancy | Resveratrol administration improved offspring spatial learning and memory and enhanced brain Na+,K+‐ATPase activity | Sahu et al. (2013) |
| Wistar rats | Preeclampsia | Intragastric resveratrol administration 20 mg kg–1 day–1 | Pregnancy twice daily during the entire pregnancy | Resveratrol administration reduced placental MDA levels, increased SOD activity and inhibited apoptosis | Zou et al. (2014 ) |
| Sprague–Dawley | Diabetic Embryopathy | Resveratrol administered by gavage (100 mg kg–1 day–1) | Pregnancy from day E3–E12 | Embryos of diabetic rats treated with resveratrol showed normalized levels of retinoic acid receptors, and neuronal markers (GAP‐43, total tau and neurofilament B) | Singh et al. (2012) |
| Sprague–Dawley | Prenatal stress | Oral gavage (100 mg kg–1 day–1) | Pregnancy | Resveratrol administration inhibited mitochondrial loss in the offspring hippocampus and activated NrF2 and AMPK | Cao et al. (2014) |
| Mouse C57BL/6J, eNOS−/− and COMT−/− | Fetal growth restriction | Diet supplemented with resveratrol (4 g kg–1 diet) | Pregnancy between E0.5 and E18.5 | Resveratrol supplementation significantly increased uterine artery blood flow velocity and fetal weight in COMT−/− but not in eNOS−/− mice. No effects on litter size and placental weight | Poudel et al. (2013) |
| Sprague–Dawley | Breast cancer | 7ppm in diet | Pregnancy starting at E7 | Resveratrol administration exerted protective effects against breast tumour development | Papoutsis et al. (2015) |
| Sprague–Dawley | In utero LPS exposure induced dopaminergic deficits | 40 g of resveratrol‐enriched diet (120 mg of resveratrol per kg of rodent diet) | Pregnancy from E3–E22.5 | Dietary resveratrol intervention protected offspring against loss of striatial dopamine, dopamine metabolites and tyrosine hydroxylase expression | Rose et al. (2014) |
| Sprague–Dawley | Maternal hypoxia | 4 g kg–1 in high fat diet | Postnatal (offspring 3 weeks of age) for 9 weeks | Resveratrol improved cardiac recovery from ischaemia‐reperfusion injury and attenuated superoxide levels in male and female offspring | Shah et al. (2016) |
| Rat | Hypoxia | 4 g kg–1 diet | Pregnancy from E7–E21 | Resveratrol was detected in plasma of hypoxic dams and fetuses (performed by ultra‐performance liquid chromatography). Resveratrol administration reversed fetal death in hypoxic pregnancies | Bourque et al. (2012) |
| Japanese macaques | Maternal obesity | High fat diet containing 0.37% of resveratrol | Prior to and throughout pregnancy | Resveratrol administration stimulated placental docosahexaenoic acid uptake capacity, AMPK activation and transporter expression | O'Tierney‐Ginn et al. (2015) |
| Japanese macaques | Western style diet | Diet containing 0.37% of resveratrol | 3 months before breading season and pregnancy | Resveratrol administration caused a significant increase in islet capillary density | Pound et al. (2014) |
| Japanese macaques | Western style diet | Diet containing 0.37% of resveratrol | 3 months before breading season and pregnancy | Resveratrol administration reduced maternal weight by 30% and improved glucose tolerance, increased uterine artery volume blood flow and reduced placental inflammation and triglycerides deposition. Resveratrol administration also altered fetal pancreatic development (fetal pancreatic mass was enlarged by 42% with increased proliferation) | Roberts et al. (2014) |
Hypertrophy of pancreatic β‐cells and the heart is accompanied by the induction of anti‐oxidant enzymes that may contribute to cell survival (Rathore et al. 1998; Laybutt et al. 2002). The production of anti‐oxidant enzymes may increase or decrease in the setting of oxidative stress in ways that remain unexplained. One recent study showed that a low protein diet during gestation and lactation in rats increased the production of ROS and decreased anti‐oxidant activity levels in heart tissue associated with increased susceptibility to developing metabolic and cardiac diseases in adulthood (Nascimento et al. 2014). Thus, when cells are exposed to oxidative stress, they may increase the activity and expression of anti‐oxidant enzymes as a defence mechanism against free radicals. However, excessive free radical production may result in damage of the molecular machinery required to produce these enzymes (Rodriguez et al. 2004).
The final aim of the present study was to determine the extent to which resveratrol administration and the accompanying modifications in oxidative stress outcomes prevent the well‐documented offspring metabolic programming changes produced by maternal low protein diets. Our overall hypothesis was that resveratrol administration during pregnancy would at least partially prevent maternal oxidative stress increases, thereby improving adult offspring adverse metabolic outcomes. The lowering of glucose in controls in the presence of unchanged insulin and the lowering of HOMA in both restricted mothers at the end of gestation and their offspring indicates a beneficial effect of resveratrol on both maternal and offspring insulin sensitivity. One interesting observation was the effect of resveratrol in reducing serum MDA and liver ROS and serum glucose in Cres mothers without altering insulin. In male Cres offspring, body weight and fat were also reduced. These findings indicate that Res may have maternal metabolic effects that are insulin independent and also potential direct effects on offspring adipose tissue. The beneficial effects of maternal resveratrol treatment on both C and R offspring were sex‐dependent. For example, resveratrol decreased fat in C males and triglycerides in R males with no effects in females. Other studies have shown anti‐oxidant sex differences, and conclude that changes in a sex‐ and tissue‐specific manner are the result of changes in the post‐transcriptional mechanisms in some enzymes such as GPx (Riese et al. 2006).
The present study shows sex differences in the offspring response to the low protein diet and the effect of resveratrol. For example, the R diet increases triglycerides in male but not female offspring. Also, liver ROS increases in male but not female offspring of R mothers. Mitochondria are the major source of free radicals in cells. Mitochondria from female rats generate half the amount of hydrogen produced by males and have higher levels of mitochondrial reduced glutathione (Borras et al. 2007). Cellular levels of anti‐oxidant proteins, including catalase, glutathione peroxidase, peroxiredoxin, superoxide dismutase and thioredoxin, are regulated in sex‐dependent manner in metabolic tissues in ways that may result in females having a greater capacity to combat oxidative stress than males (Chaudhari et al. 2014). Further studies will need to be conducted to address these findings.
Carefully controlled interventions are essential for determining causative mechanisms in developmental programming with the aim of developing evidence based interventions (Nathanielsz et al. 2013). Recent studies have addressed potential benefits of pharmacological interventions in pregnancy on the short‐ and long‐term health of both the mother and child (Duque‐Guimaraes & Ozanne, 2013). For example, in rats, glycine (Jackson et al. 2002) and folic acid (Lillycrop et al. 2010) enrichment of maternal restricted diets prevent alterations in offspring liver metabolism and hypertension. In rodents, an improvement in offspring glucose homeostasis is observed when maternal low protein diets are supplemented with the amino acid taurine (Mortensen et al. 2010). Interventions with natural products, drugs or isolated compounds have shown beneficial results in offspring of mothers with poor nutrition. For example, maternal anti‐oxidant supplementation (vitamin A, E, C and selenium) in rats fed a Western diet partially prevented offspring adiposity and normalized glucose tolerance (Mortensen et al. 2010). Genistein supplementation in mice during gestation protects offspring from susceptibility to obesity (Dolinoy et al. 2006). The administration of the potent inhibitor of lipid peroxidation lazariod to protein‐restricted mothers reduced the cardiovascular changes in adult offspring (Cambonie et al. 2007). In our model, the anti‐oxidant compound resveratrol improved the maternal metabolism of protein‐restricted diets and, as a result, prevented the adverse effects on offspring metabolism in adulthood. The differences between male and female offspring merit further investigation.
Maternal undernutrition in the rat from the beginning of pregnancy (Zambrano et al. 2005 b; Guzman et al. 2006) or at late pregnancy increases maternal corticosterone levels (Lesage et al. 2001) In the present study, serum corticosterone was 91% higher in R compared to C mothers and resveratrol administration reduced this increase to 51%. Glucocorticoids play a major role in developmental programming, as shown by the beneficial effects of maternal adrenalectomy on the programming of hypertension by maternal low protein diets (Langley‐Evans, 1997). Glucocorticoids have been proposed as fundamental orchestrators of developmental programming (Zambrano et al. 2014 b). We propose that the 50% inhibition of the maternal corticosterone increase resulting from the low protein diet seen following resveratrol administration contributes to the prevention of offspring adverse outcomes independently of the direct effects of decreased ROS. Additional beneficial effects of resveratrol are probably independent of corticosterone‐mediated effects.
In summary, pregnancy leads to a physiological state of maternal oxidative stress that is increased by a maternal low protein diet. The present study demonstrates increased oxidative stress in offspring of low protein mothers. Maternal intervention with resveratrol during pregnancy decreased maternal and offspring oxidative stress and the resultant damage in a sex‐specific manner. The efficacy of this intervention supports a causative role for oxidative stress in the developmental programming of metabolic outcomes. The data reported in the present study indicate that oxidative stress is a major mechanism by which poor fetal nutrition leads to developmental programming of adult disease, indicating potential interventions when fetal nutrition is impaired either by a lack of maternal nutrition or impaired placental function.
Additional information
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
CCV, LAR, GLRG, CJB and AMVM researched data. CCV, FL, GACC, PWN and EZ were responsible for the study design, preparation and review of the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by CONACyT 155166.
Acknowledgements
LARC and GLRG are graduate students from Doctorado en Ciencias Biomédicas, Facultad de Medicina, Universidad Nacional Autónoma de México.
References
- Agarwal A, Aponte‐Mellado A, Premkumar BJ, Shaman A & Gupta S (2012). The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 10, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LH (2012). Global dietary patterns and diets in childhood: implications for health outcomes. Ann Nutr Metab 61 (Suppl 1), 29–37. [DOI] [PubMed] [Google Scholar]
- Almeida SS, Tonkiss J & Galler JR (1996). Prenatal protein malnutrition affects exploratory behavior of female rats in the elevated plus‐maze test. Physiol Behav 60, 675–680. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW & Poston L (2004). Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol 561, 355–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambini‐Junior V, Zanatta G, Della Flora Nunes G, Mueller de Melo G, Michels M, Fontes‐Dutra M, Nogueira Freire V, Riesgo R & Gottfried C (2014). Resveratrol prevents social deficits in animal model of autism induced by valproic acid. Neurosci Lett 583, 176–181. [DOI] [PubMed] [Google Scholar]
- Barros MA, De Brito Alves JL, Nogueira VO, Wanderley AG & Costa‐Silva JH (2015). Maternal low‐protein diet induces changes in the cardiovascular autonomic modulation in male rat offspring. Nutr Metab Cardiovasc Dis 25, 123–130. [DOI] [PubMed] [Google Scholar]
- Baur JA & Sinclair DA (2006). Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5, 493–506. [DOI] [PubMed] [Google Scholar]
- Bhat KP & Pezzuto JM (2002). Cancer chemopreventive activity of resveratrol. Ann NY Acad Sci 957, 210–229. [DOI] [PubMed] [Google Scholar]
- Bhat KPL, Kosmeder JW, 2nd & Pezzuto JM (2001). Biological effects of resveratrol. Antioxid Redox Signal 3, 1041–1064. [DOI] [PubMed] [Google Scholar]
- Borras C, Gambini J & Vina J (2007). Mitochondrial oxidant generation is involved in determining why females live longer than males. Front Biosci 12, 1008–1013. [DOI] [PubMed] [Google Scholar]
- Botelho GG, Bufalo AC, Boareto AC, Muller JC, Morais RN, Martino‐Andrade AJ, Lemos KR & Dalsenter PR (2009). Vitamin C and resveratrol supplementation to rat dams treated with di(2‐ethylhexyl)phthalate: impact on reproductive and oxidative stress end points in male offspring. Arch Environ Contam Toxicol 57, 785–793. [DOI] [PubMed] [Google Scholar]
- Bourque SL, Dolinsky VW, Dyck JR & Davidge ST (2012). Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta 33, 449–452. [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Buege JA & Aust SD (1978). Microsomal lipid peroxidation. Methods Enzymol 52, 302–310. [DOI] [PubMed] [Google Scholar]
- Burton GJ (2009). Oxygen, the Janus gas; its effects on human placental development and function. J Anat 215, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Le NL, Pladys P, Gauthier C, Lahaie I, Abran D, Lavoie JC & Nuyt AM (2007). Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low‐protein diet. Am J Physiol Regul Integr Comp Physiol 292, R1236–R1245. [DOI] [PubMed] [Google Scholar]
- Cao K, Zheng A, Xu J, Li H, Liu J, Peng Y, Long J, Zou X, Li Y, Chen C & Feng Z (2014). AMPK activation prevents prenatal stress‐induced cognitive impairment: modulation of mitochondrial content and oxidative stress. Free Radical Bio Med 75, 156–166. [DOI] [PubMed] [Google Scholar]
- Carter LG, D'Orazio JA & Pearson KJ (2014). Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer 21, R209–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KA, Tsoulis MW & Sloboda DM (2015). Early‐life nutritional effects on the female reproductive system. J Endocrinol 224, R45–62. [DOI] [PubMed] [Google Scholar]
- Chaudhari HN, Kim SW & Yun JW (2014). Gender‐dimorphic regulation of antioxidant proteins in response to high‐fat diet and sex steroid hormones in rats. Free Radical Res 48, 587–598. [DOI] [PubMed] [Google Scholar]
- Connor KL, Vickers MH, Beltrand J, Meaney MJ & Sloboda DM (2012). Nature, nurture or nutrition? Impact of maternal nutrition on maternal care, offspring development and reproductive function. J Physiol 590, 2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottart CH, Nivet‐Antoine V & Beaudeux JL (2014). Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol Nutr Food Res 58, 7–21. [DOI] [PubMed] [Google Scholar]
- Das S, Lin H‐S, Ho PC & Ng K‐Y (2008). The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharmaceut Res 25, 2593–2600. [DOI] [PubMed] [Google Scholar]
- Delmas D, Aires V, Colin DJ, Limagne E, Scagliarini A, Cotte AK & Ghiringhelli F (2013). Importance of lipid microdomains, rafts, in absorption, delivery, and biological effects of resveratrol. Ann NY Acad Sci 1290, 90–97. [DOI] [PubMed] [Google Scholar]
- Delmas D, Lancon A, Colin D, Jannin B & Latruffe N (2006). Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets 7, 423–442. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Weidman JR, Waterland RA & Jirtle RL (2006). Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect 114, 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinsky VW, Rueda‐Clausen CF, Morton JS, Davidge ST & Dyck JR (2011). Continued postnatal administration of resveratrol prevents diet‐induced metabolic syndrome in rat offspring born growth restricted. Diabetes 60, 2274–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque‐Guimaraes DE & Ozanne SE (2013). Nutritional programming of insulin resistance: causes and consequences. Trends Endocrinol Metab 24, 525–535. [DOI] [PubMed] [Google Scholar]
- Engelbregt MJ, Houdijk ME, Popp‐Snijders C & Delemarre‐van de Waal HA (2000). The effects of intra‐uterine growth retardation and postnatal undernutrition on onset of puberty in male and female rats. Pediatr Res 48, 803–807. [DOI] [PubMed] [Google Scholar]
- Fremont L (2000). Biological effects of resveratrol. Life Sci 66, 663–673. [DOI] [PubMed] [Google Scholar]
- Gallo LA, Tran M, Moritz KM & Wlodek ME (2013). Developmental programming: variations in early growth and adult disease. Clin Exp Pharmacol Physiol 40, 795–802. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Jackson AA & Langley‐Evans SC (1997). Maintenance of maternal diet‐induced hypertension in the rat is dependent on glucocorticoids. Hypertension 30, 1525–1530. [DOI] [PubMed] [Google Scholar]
- Gitto E, Reiter RJ, Karbownik M, Tan DX, Gitto P, Barberi S & Barberi I (2002). Causes of oxidative stress in the pre‐ and perinatal period. Biol Neonate 81, 146–157. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FB, Cross CM & Herrera EA (2012). Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PloS ONE 7, e31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA & Davidge ST (2013). Developmental programming of cardiovascular disease by prenatal hypoxia. J Dev Orig Health Dis 4, 328–337. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Niu Y, Herrera EA, Richter HG, Camm EJ, Thakor AS, Kane AD, Hansell JA, Brain KL, Skeffington KL, Itani N, Wooding FB, Cross CM & Allison BJ (2014). Heart disease link to fetal hypoxia and oxidative stress. Adv Exp Med Biol 814, 77–87. [DOI] [PubMed] [Google Scholar]
- Gupta S, Agarwal A & Sharma RK (2005). The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet Gynecol Surv 60, 807–816. [DOI] [PubMed] [Google Scholar]
- Guzman C, Cabrera R, Cardenas M, Larrea F, Nathanielsz PW & Zambrano E (2006). Protein restriction during fetal and neonatal development in the rat alters reproductive function and accelerates reproductive ageing in female progeny. J Physiol 572, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B (2006). Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141, 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA & Gluckman PD (2014). Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev 94, 1027–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen CGM, Haenen GRMM, van Acker FAA, van der Vijgh WJF & Bast A (2001). Flavonoids as peroxynitrite scavengers: the role of the hydroxyl groups. Toxicol in Vitro 15, 3–6. [DOI] [PubMed] [Google Scholar]
- Holemans K, Aerts L & Van Assche FA (2003). Fetal growth restriction and consequences for the offspring in animal models. J Soc Gynecol Investig 10, 392–399. [DOI] [PubMed] [Google Scholar]
- Jackson AA, Dunn RL, Marchand MC & Langley‐Evans SC (2002). Increased systolic blood pressure in rats induced by a maternal low‐protein diet is reversed by dietary supplementation with glycine. Clin Sci (Lond) 103, 633–639. [DOI] [PubMed] [Google Scholar]
- Jang DS, Kang BS, Ryu SY, Chang IM, Min KR & Kim Y (1999). Inhibitory effects of resveratrol analogs on unopsonized zymosan‐induced oxygen radical production. Biochem Pharmacol 57, 705–712. [DOI] [PubMed] [Google Scholar]
- Jang JY, Park D, Shin S, Jeon JH, Choi BI, Joo SS, Hwang SY, Nahm SS & Kim YB (2008). Antiteratogenic effect of resveratrol in mice exposed in utero to 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin. Eur J Pharmacol 591, 280–283. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN & Burton GJ (2000). Onset of maternal arterial blood flow and placental oxidative stress – a possible factor in human early pregnancy failure. Am J Pathol 157, 2111–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML, Mark PJ, Mori TA, Keelan JA & Waddell BJ (2013). Maternal dietary omega‐3 fatty acid supplementation reduces placental oxidative stress and increases fetal and placental growth in the rat. Biol Reprod 88, 37. [DOI] [PubMed] [Google Scholar]
- Kulkarni SS & Canto C (2015). The molecular targets of resveratrol. Biochim Biophys Acta 1852, 1114–1123. [DOI] [PubMed] [Google Scholar]
- Kumar A, Singh CK, Lavoie HA, Dipette DJ & Singh US (2011). Resveratrol restores Nrf2 level and prevents ethanol‐induced toxic effects in the cerebellum of a rodent model of fetal alcohol spectrum disorders. Mol Pharmacol 80, 446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley‐Evans SC (1997). Hypertension induced by foetal exposure to a maternal low‐protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens 15, 537–544. [DOI] [PubMed] [Google Scholar]
- Langley‐Evans SC & Sculley DV (2006). The association between birthweight and longevity in the rat is complex and modulated by maternal protein intake during fetal life. FEBS Lett 580, 4150–4153. [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Kaneto H, Hasenkamp W, Grey S, Jonas JC, Sgroi DC, Groff A, Ferran C, Bonner‐Weir S, Sharma A & Weir GC (2002). Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to beta‐cell survival during chronic hyperglycemia. Diabetes 51, 413–423. [DOI] [PubMed] [Google Scholar]
- Lesage J, Blondeau B, Grino M, Breant B & Dupouy JP (2001). Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo‐pituitary adrenal axis in the newborn rat. Endocrinology 142, 1692–1702. [DOI] [PubMed] [Google Scholar]
- Lillycrop KA, Rodford J, Garratt ES, Slater‐Jefferies JL, Godfrey KM, Gluckman PD, Hanson MA & Burdge GC (2010). Maternal protein restriction with or without folic acid supplementation during pregnancy alters the hepatic transcriptome in adult male rats. Br J Nutr 103, 1711–1719. [DOI] [PubMed] [Google Scholar]
- Madhyastha S, Sekhar S & Rao G (2013). Resveratrol improves postnatal hippocampal neurogenesis and brain derived neurotrophic factor in prenatally stressed rats. Int J Dev Neurosci 31, 580–585. [DOI] [PubMed] [Google Scholar]
- Martin‐Gronert MS & Ozanne SE (2013). Early life programming of obesity. Medycyna wieku rozwojowego 17, 7–12. [PubMed] [Google Scholar]
- Martinez J & Moreno JJ (2000). Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem Pharmacol 59, 865–870. [DOI] [PubMed] [Google Scholar]
- Mistry HD & Williams PJ (2011). The importance of antioxidant micronutrients in pregnancy. Oxid Med Cell Longev 2011, 841749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen OH, Olsen HL, Frandsen L, Nielsen PE, Nielsen FC, Grunnet N & Quistorff B (2010). Gestational protein restriction in mice has pronounced effects on gene expression in newborn offspring's liver and skeletal muscle; protective effect of taurine. Pediatr Res 67, 47–53. [DOI] [PubMed] [Google Scholar]
- Nascimento L, Freitas CM, Silva‐Filho R, Leite AC, Silva AB, da Silva AI, Ferreira DS, Pedroza AA, Maia MB, Fernandes MP & Lagranha C (2014). The effect of maternal low‐protein diet on the heart of adult offspring: role of mitochondria and oxidative stress. Appl Physiol Nutr Metab 39, 880–887. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW, Ford SP, Long NM, Vega CC, Reyes‐Castro LA & Zambrano E (2013). Interventions to prevent adverse fetal programming due to maternal obesity during pregnancy. Nutr Rev 71(Suppl 1), S78–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura S, Kanagawa H & Makimoto A (1963). [Chemical constituents of polygonaceous plants. I. Studies on the components of Ko‐J O‐Kon. (Polygonum cuspidatum Sieb. et Zucc.)]. Yakugaku Zasshi 83, 988–990. [PubMed] [Google Scholar]
- O'Tierney‐Ginn P, Roberts V, Gillingham M, Walker J, Glazebrook PA, Thornburg KL, Grove K & Frias AE (2015). Influence of high fat diet and resveratrol supplementation on placental fatty acid uptake in the Japanese macaque. Placenta 36, 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmavathi IJN, Kishore YD, Venu L, Ganeshan M, Harishankar N, Giridharan NV & Raghunath M (2009). Prenatal and perinatal zinc restriction: effects on body composition, glucose tolerance and insulin response in rat offspring. Exp Physiol 94, 761–769. [DOI] [PubMed] [Google Scholar]
- Papoutsis AJ, Selmin OI, Borg JL & Romagnolo DF (2015). Gestational exposure to the AhR agonist 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin induces BRCA‐1 promoter hypermethylation and reduces BRCA‐1 expression in mammary tissue of rat offspring: preventive effects of resveratrol. Mol Carcinog 54, 261–269. [DOI] [PubMed] [Google Scholar]
- Pirola L & Frojdo S (2008). Resveratrol: one molecule, many targets. IUBMB Life 60, 323–332. [DOI] [PubMed] [Google Scholar]
- Poudel R, Stanley JL, Rueda‐Clausen CF, Andersson IJ, Sibley CP, Davidge ST & Baker PN (2013). Effects of resveratrol in pregnancy using murine models with reduced blood supply to the uterus. PloS ONE 8, e64401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound LD, Comstock SM & Grove KL (2014). Consumption of a Western‐style diet during pregnancy impairs offspring islet vascularization in a Japanese macaque model. Am J Physiol Endocrinol Metab 307, E115–E123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunath M, Venu L, Padmavathi I, Kishore YD, Ganeshan M, Anand Kumar K, Sainath PB & Rao KR (2009). Modulation of macronutrient metabolism in the offspring by maternal micronutrient deficiency in experimental animals. Indian J Med Res 130, 655–665. [PubMed] [Google Scholar]
- Rasmussen KM, Catalano PM & Yaktine AL (2009). New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol 21, 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore N, John S, Kale M & Bhatnagar D (1998). Lipid peroxidation and antioxidant enzymes in isoproterenol induced oxidative stress in rat tissues. Pharmacol Res 38, 297–303. [DOI] [PubMed] [Google Scholar]
- Rebelato HJ, Esquisatto MA, Moraes C, Amaral ME & Catisti R (2013). Gestational protein restriction induces alterations in placental morphology and mitochondrial function in rats during late pregnancy. J Mol Histol 44, 629–637. [DOI] [PubMed] [Google Scholar]
- Reyes‐Castro LA, Rodriguez JS, Charco R, Bautista CJ, Larrea F, Nathanielsz PW & Zambrano E (2012). Maternal protein restriction in the rat during pregnancy and/or lactation alters cognitive and anxiety behaviors of female offspring. Int J Dev Neurosci 30, 39–45. [DOI] [PubMed] [Google Scholar]
- Richter HG, Camm EJ, Modi BN, Naeem F, Cross CM, Cindrova‐Davies T, Spasic‐Boskovic O, Dunster C, Mudway IS, Kelly FJ, Burton GJ, Poston L & Giussani DA (2012). Ascorbate prevents placental oxidative stress and enhances birth weight in hypoxic pregnancy in rats. J Physiol 590, 1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese C, Michaelis M, Mentrup B, Gotz F, Kohrle J, Schweizer U & Schomburg L (2006). Selenium‐dependent pre‐ and posttranscriptional mechanisms are responsible for sexual dimorphic expression of selenoproteins in murine tissues. Endocrinology 147, 5883–5892. [DOI] [PubMed] [Google Scholar]
- Roberts VH, Pound LD, Thorn SR, Gillingham MB, Thornburg KL, Friedman JE, Frias AE & Grove KL (2014). Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J 28, 2466–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V & Reiter RJ (2004). Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36, 1–9. [DOI] [PubMed] [Google Scholar]
- Rose KM, Parmar MS & Cavanaugh JE (2014). Dietary supplementation with resveratrol protects against striatal dopaminergic deficits produced by in utero LPS exposure. Brain Res 1573, 37–43. [DOI] [PubMed] [Google Scholar]
- Rueda‐Clausen CF, Morton JS, Dolinsky VW, Dyck JR & Davidge ST (2012). Synergistic effects of prenatal hypoxia and postnatal high‐fat diet in the development of cardiovascular pathology in young rats. Am J Physiol Regul Integr Comp Physiol 303, R418–R426. [DOI] [PubMed] [Google Scholar]
- Sahu SS, Madhyastha S & Rao GM (2013). Neuroprotective effect of resveratrol against prenatal stress induced cognitive impairment and possible involvement of Na(+), K(+)‐ATPase activity. Pharmacol Biochem Behav 103, 520–525. [DOI] [PubMed] [Google Scholar]
- Santos MR & Mira L (2004). Protection by flavonoids against the peroxynitrite‐mediated oxidation of dihydrorhodamine. Free Radical Res 38, 1013–1020. [DOI] [PubMed] [Google Scholar]
- Shah A, Reyes LM, Morton JS, Fung D, Schneider J & Davidge ST (2016). Effect of resveratrol on metabolic and cardiovascular function in male and female adult offspring exposed to prenatal hypoxia and a high‐fat diet. J Physiol 594, 1465–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CK, Kumar A, Hitchcock DB, Fan D, Goodwin R, LaVoie HA, Nagarkatti P, DiPette DJ & Singh US (2011). Resveratrol prevents embryonic oxidative stress and apoptosis associated with diabetic embryopathy and improves glucose and lipid profile of diabetic dam. Mol Nutr Food Res 55, 1186–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CK, Kumar A, LaVoie HA, DiPette DJ & Singh US (2012). Resveratrol prevents impairment in activation of retinoic acid receptors and MAP kinases in the embryos of a rodent model of diabetic embryopathy. Reprod Sci 19, 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CK, Kumar A, Lavoie HA, Dipette DJ & Singh US (2013). Diabetic complications in pregnancy: is resveratrol a solution? Exp Biol Med (Maywood) 238, 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarry‐Adkins JL, Martin‐Gronert MS, Fernandez‐Twinn DS, Hargreaves I, Alfaradhi MZ, Land JM, Aiken CE & Ozanne SE (2013). Poor maternal nutrition followed by accelerated postnatal growth leads to alterations in DNA damage and repair, oxidative and nitrosative stress, and oxidative defense capacity in rat heart. FASEB J 27, 379–390. [DOI] [PubMed] [Google Scholar]
- Tiidus PM, Bombardier E, Hidiroglou N & Madere R (1999). Gender and exercise influence on tissue antioxidant vitamin status in rats. J Nutr Sci Vitaminol (Tokyo) 45, 701–710. [DOI] [PubMed] [Google Scholar]
- Vega CC, Reyes‐Castro LA, Bautista CJ, Larrea F, Nathanielsz PW & Zambrano E (2015). Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int J Obes (Lond) 39, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Buss C, Entringer S & Swanson JM (2009). Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med 27, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox JK, Ash SL & Catignani GL (2004). Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr 44, 275–295. [DOI] [PubMed] [Google Scholar]
- Woods LL, Ingelfinger JR, Nyengaard JR & Rasch R (2001). Maternal protein restriction suppresses the newborn renin‐angiotensin system and programs adult hypertension in rats. Pediatr Res 49, 460–467. [DOI] [PubMed] [Google Scholar]
- Yao L, Wan J, Li H, Ding J, Wang Y, Wang X & Li M (2015). Resveratrol relieves gestational diabetes mellitus in mice through activating AMPK. Reprod Biol Endocrinol 13, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Bautista CJ, Deas M, Martinez‐Samayoa PM, Gonzalez‐Zamorano M, Ledesma H, Morales J, Larrea F & Nathanielsz PW (2006). A low maternal protein diet during pregnancy and lactation has sex‐ and window of exposure‐specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol 571, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Guzman C, Rodriguez‐Gonzalez GL, Durand‐Carbajal M & Nathanielsz PW (2014. a). Fetal programming of sexual development and reproductive function. Mol Cell Endocrinol 382, 538–549. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Martinez‐Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez‐Gonzalez GL, Guzman C, Larrea F & Nathanielsz PW (2005. a). Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol 566, 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Rodriguez‐Gonzalez GL, Guzman C, Garcia‐Becerra R, Boeck L, Diaz L, Menjivar M, Larrea F & Nathanielsz PW (2005. b). A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol 563, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Tuersunjiang, N , Long, N , Guo, Sun , K, Cox , L, Ford S , P Nathanielsz & Li C (2014. b). Increased central and peripheral glucocorticoid synthesis act as an orchestrator of developmental programming In Stress and Developmental Programming of Health and Disease: Beyond Phenomenology, eds. Zhang L. & Longo LD, pp. 464–485. Nova Science Publishers, Inc., New York. [Google Scholar]
- Zou Y, Zuo Q, Huang S, Yu X, Jiang Z, Zou S, Fan M & Sun L (2014). Resveratrol inhibits trophoblast apoptosis through oxidative stress in preeclampsia‐model rats. Molecules 19, 20570–20579. [DOI] [PMC free article] [PubMed] [Google Scholar]


