Abstract
Key points
The in vivo fetal cardiovascular defence to chronic hypoxia has remained by and large an enigma because no technology has been available to induce significant and prolonged fetal hypoxia whilst recording longitudinal changes in fetal regional blood flow as the hypoxic pregnancy is developing.
We introduce a new technique able to maintain chronically instrumented maternal and fetal sheep preparations under isobaric chronic hypoxia for most of gestation, beyond levels that can be achieved by high altitude and of relevance in magnitude to the human intrauterine growth‐restricted fetus.
This technology permits wireless recording in free‐moving animals of longitudinal maternal and fetal cardiovascular function, including beat‐to‐beat alterations in pressure and blood flow signals in regional circulations.
The relevance and utility of the technique is presented by testing the hypotheses that the fetal circulatory brain sparing response persists during chronic fetal hypoxia and that an increase in reactive oxygen species in the fetal circulation is an involved mechanism.
Abstract
Although the fetal cardiovascular defence to acute hypoxia and the physiology underlying it have been established for decades, how the fetal cardiovascular system responds to chronic hypoxia has been comparatively understudied. We designed and created isobaric hypoxic chambers able to maintain pregnant sheep for prolonged periods of gestation under controlled significant (10% O2) hypoxia, yielding fetal mean levels (11.5 ± 0.6 mmHg) similar to those measured in human fetuses of hypoxic pregnancy. We also created a wireless data acquisition system able to record fetal blood flow signals in addition to fetal blood pressure and heart rate from free moving ewes as the hypoxic pregnancy is developing. We determined in vivo longitudinal changes in fetal cardiovascular function including parallel measurement of fetal carotid and femoral blood flow and oxygen and glucose delivery during the last third of gestation. The ratio of oxygen (from 2.7 ± 0.2 to 3.8 ± 0.8; P < 0.05) and of glucose (from 2.3 ± 0.1 to 3.3 ± 0.6; P < 0.05) delivery to the fetal carotid, relative to the fetal femoral circulation, increased during and shortly after the period of chronic hypoxia. In contrast, oxygen and glucose delivery remained unchanged from baseline in normoxic fetuses. Fetal plasma urate concentration increased significantly during chronic hypoxia but not during normoxia (Δ: 4.8 ± 1.6 vs. 0.5 ± 1.4 μmol l−1, P<0.05). The data support the hypotheses tested and show persisting redistribution of substrate delivery away from peripheral and towards essential circulations in the chronically hypoxic fetus, associated with increases in xanthine oxidase‐derived reactive oxygen species.
Key points
The in vivo fetal cardiovascular defence to chronic hypoxia has remained by and large an enigma because no technology has been available to induce significant and prolonged fetal hypoxia whilst recording longitudinal changes in fetal regional blood flow as the hypoxic pregnancy is developing.
We introduce a new technique able to maintain chronically instrumented maternal and fetal sheep preparations under isobaric chronic hypoxia for most of gestation, beyond levels that can be achieved by high altitude and of relevance in magnitude to the human intrauterine growth‐restricted fetus.
This technology permits wireless recording in free‐moving animals of longitudinal maternal and fetal cardiovascular function, including beat‐to‐beat alterations in pressure and blood flow signals in regional circulations.
The relevance and utility of the technique is presented by testing the hypotheses that the fetal circulatory brain sparing response persists during chronic fetal hypoxia and that an increase in reactive oxygen species in the fetal circulation is an involved mechanism.
Abbreviations
- HIF
hypoxia‐inducible factor
- IUGR
intrauterine growth restriction
- NO
nitric oxide
- •O2−
superoxide anion
- ROS
reactive oxygen species
- XO
xanthine oxidase
Introduction
The phrase ‘Everest in utero’ was coined by Sir Joseph Barcroft to highlight that the fetus develops under conditions of relative hypoxia compared with the oxygenation of the adult individual (Barcroft et al. 1933). Low oxygen tension in fetal life is essential for normal placental development as well as appropriate formation and maturation of the fetal cardiovascular system (Compernolle et al. 2003; Burton, 2009). However, reductions in fetal oxygenation below baseline can be harmful to the developing fetus unless appropriate compensatory responses are triggered. Short term, acute episodes of fetal hypoxia are common in late gestation, such as those occurring during transient compression of the umbilical cord (Giussani et al. 1997) or those resulting from myometrial contractions during labour and delivery (Huch et al. 1977). A sustained reduction from baseline in fetal oxygenation or chronic fetal hypoxia is associated with conditions of increased placental vascular resistance, leading to impaired uteroplacental blood flow. Chronic fetal hypoxia is therefore associated with pre‐eclampsia (Kingdom & Kaufmann, 1997), placental insufficiency (Pardi et al. 1993), chorioamnionitis (Maberry et al. 1990), gestational diabetes (Escobar et al. 2013) and even maternal obesity (Hayes et al. 2012; Kaplan‐Sturk et al. 2013). Chronic fetal hypoxia may also occur during impaired maternal oxygenation, as in maternal smoking (Longo, 1976), maternal respiratory diseases (Katz & Scheiner, 2008), maternal severe anaemia (Davis et al. 2005) or pregnancy at high altitude (Makowski et al. 1968; Giussani et al. 2001; Tissot van Patot et al. 2012; Soria et al. 2013). Acute episodes of severe fetal hypoxia may result in marked fetal acidosis and cardiovascular compromise with subsequent hypoxic–ischaemic encephalopathy, which is predictive of developing cerebral palsy and cognitive disability later in life (Low et al. 1985; Gunn & Bennet, 2009). Chronic fetal hypoxia can lead to fetal growth restriction, compromise the development of key organs and systems, and trigger an increased susceptibility to disease in later life (see Giussani & Davidge, 2013 for a review). Therefore, the fetal defence responses to acute and chronic hypoxia are essential to protect against significant morbidity and mortality in offspring. Not surprisingly, elucidation of the fetal compensatory responses to acute and chronic hypoxia and the mechanisms mediating them remains at the forefront of perinatal science and obstetric practice today.
The sheep fetus has long been the experimental model of choice for investigating fetal hypoxia in vivo. The fetal defence to acute hypoxia is contingent on the fetal cardiovascular system (Rudolph, 1984; Giussani et al. 1994). The fetal cardiovascular defence to acute hypoxia and the physiology underlying it are well established and characterised (see Giussani, 2015 for a review). In response to acute hypoxia, the fetus elicits bradycardia and redistributes its cardiac output (Cohn et al. 1974). Parallel measurement of continuous changes in carotid and femoral blood flow show cerebral vasodilatation and peripheral vasoconstriction, demonstrating in vivo the haemodynamics of the fetal brain sparing effect (Giussani et al. 1993). The bradycardia and peripheral vasoconstriction are triggered exclusively by a carotid chemoreflex (Giussani et al. 1993; Bartelds et al. 1993). The peripheral vasoconstriction is then maintained by the release of constrictor hormones into the fetal circulation (Jones & Robinson, 1975; Fletcher et al. 2006) as well as alterations in local factors, including the generation of reactive oxygen species (ROS) (Thakor et al. 2010, 2015; Kane et al. 2012, 2014). Therefore, the physiology underlying the fetal cardiovascular defence to acute episodes of hypoxia involves carotid chemoreflex and endocrine components as well as a local oxidant tone acting at the level of the fetal vasculature (see Giussani, 2016).
In marked contrast, the fetal in vivo haemodynamic responses to chronic fetal hypoxia and the mechanisms mediating them are not well characterised or understood. Progress in this field has been hampered in part by the inability to record continuous cardiovascular function in the fetus, including measurement of regional blood flow, as the chronic fetal hypoxia is actually occurring. Therefore, the objectives of this work were to introduce to the field a new technique for physiological research and to show the utility of the technique by investigating the fetal in vivo haemodynamic responses to significant chronic hypoxia in real time. We aimed to design and create isobaric hypoxic chambers able to maintain pregnant sheep for prolonged periods of gestation under controlled long‐term hypoxia, yielding fetal levels similar to those measured in human intrauterine growth restriction (IUGR) pregnancy. Our second aim was to establish a wireless data acquisition system able to record fetal blood flow signals in addition to fetal blood pressure and fetal heart rate from free moving ewes as the hypoxic pregnancy was developing. Third, we wanted to use this system to determine in vivo in real time fetal cardiovascular function, including parallel measurement of fetal carotid and femoral blood flow and regional oxygen and glucose delivery during long‐term significant hypoxia in late gestation fetal sheep. We propose that alterations in the fetal vascular oxidant tone contribute to the fetal redistribution of blood flow away from the periphery during chronic fetal hypoxia. Therefore, the fourth aim of this work was to test the inter‐related hypotheses that the fetal circulatory brain sparing response does persist during significant chronic fetal hypoxia and that an increase in ROS in the fetal circulation is an involved mechanism. In vivo generation of ROS in the maternal and the fetal circulation was determined by measurement of changes in plasma urate and ascorbate concentrations, two of the few accepted biomarkers of ROS generation within the circulation in vivo (Halliwell & Gutteridge, 2004).
Methods
Ethical approval
All procedures were performed under the UK Animals (Scientific Procedures) Act 1986 and were approved by the Ethical Review Committee of the University of Cambridge.
Surgical preparation, connection to CamDAS and post‐operative care
Twelve Welsh Mountain pregnant ewes and their singleton fetuses were surgically instrumented using strict aseptic techniques at 116 ± 1 days of gestational age (term is approximately 145 days), as described in detail (Fletcher et al. 2000, 2006). In brief, food but not water was withheld from the pregnant ewe for 24 h prior to surgery. On the day of surgery, the ewe was transferred to the preoperative room, where the neck fleece was clipped and anaesthesia was induced by injection of Alfaxan (1.5–2.5 mg kg−1 alfaxalone; Jurox Ltd, Worcestershire, UK) into the jugular vein. The ewe was then placed on her back and intubated (Portex cuffed endotracheal tube; Smiths Medical International Ltd, Ashford, UK) with the aid of a laryngoscope. Pre‐operative anaesthesia was maintained by spontaneous inhalation of 1.5% isoflurane in O2 (2 l min−1; IsoFlo; Abbott Laboratories Ltd, Maidenhead, UK) and the abdomen, flanks and medial surfaces of the hind limbs were shaved and cleaned.
The ewe was then transferred to the surgical suite operating table and the shaved and cleaned surfaces were scrubbed with alcohol in water, followed by a spray of hibitane solution (Hibitane Plus in alcohol and water; 5% chlorohexidine gluconate; Regent Medical Ltd, Manchester, UK) and another spray of concentrated iodine solution (Povidone‐Iodine; Seton Healthcare Group PLC, Oldham, UK). General anaesthesia (1.5–2.0% isoflurane in 60:40 O2/N2O) was maintained using positive pressure ventilation in a non‐rebreathing circuit (Datex‐Ohmeda Ltd, Hatfield, UK). Antibiotics (30 mg kg−1 i.m. procaine benzylpenicillin; Depocillin; Intervet UK Ltd, Milton Keynes, UK) and an analgesic agent (1.4 mg kg−1 s.c. carprofen; Rimadyl; Pfizer Ltd, Sandwich, UK) were administered immediately before the start of surgery. The animal was covered with a plastic sterile drape (Buster Opcover; Buster, Kruuse, Denmark) and with sterile surgical linen drapes on top, such that only the midline incision site was left exposed. Midline abdominal and uterine incisions were then made, the fetal hind limbs were exteriorised minimising amniotic fluid loss and, on one side, fetal femoral arterial (i.d. 0.86 mm, o.d. 1.52 mm; Critchly Electrical Products, Kingsgrove, NSW, Australia) and venous (i.d. 0.56 mm, o.d. 0.96 mm) catheters were inserted. The catheter tips were advanced to the descending aorta and inferior vena cava, respectively. Another catheter was anchored onto the fetal hind limb for recording of the reference amniotic pressure. A Transonic flow probe was positioned around the contralateral femoral artery (MC2RS‐JSF‐WC120‐CS12‐GCP, Transonic Systems, Ithaca, NY, USA). The fetal skin incisions were closed with thin linen suture and the uterine incision was closed in layers (3‐0 Dexon II Bi‐colour; Sherwood, Davis & Geck, Gosport, UK). The dead space of the catheters was filled with heparinised saline (80 i.u. heparin ml−1 in 0.9% NaCl) and the catheter ends were plugged with sterile brass pins. The fetal head was then palpated and exteriorised through a second uterine incision. The fetal carotid arteries were isolated and on one side a catheter was inserted with the tip remaining in the ascending aorta. A second Transonic flow probe (MC2RS‐JSF‐WC120‐CS12‐GCP) was positioned around the contralateral carotid artery (Giussani et al. 1993) and the fetal skin incision and the second uterine incision were closed as before. All catheters were then exteriorised via a keyhole incision in the maternal flank on the ewe's right side whilst the flow probe leads were exteriorised through a keyhole incision on the ewe's left flank. The maternal peritoneum was then closed in three segments with thick linen suture, and the maternal abdominal skin incision was sewn together (Ethilon 2‐0; Ethicon Ltd, Edinburgh, UK). A Teflon catheter (i.d. 1.0 mm, o.d. 1.6 mm; Altec, St Austell, UK) was then inserted into the maternal femoral artery and placed in the descending aorta, and a maternal venous catheter placed in the inferior vena cava (i.d. 0.86 mm, o.d. 1.52 mm; Critchly Electrical Products). These catheters were exteriorised through the same keyhole on the ewe's right side flank.
A custom made jacket designed to house the bespoke wireless Cambridge Data Acquisition System (CamDAS, Maastricht Instruments, Maastricht, The Netherlands) was then fitted to the ewe. The CamDAS contained a pressure box and a flow box able to record simultaneously four pressure and four flow signals, respectively (Fig. 1). It was powered by lithium batteries which were also housed within the jacket. The catheters were then connected to pressure transducers (COBE; Argon Division, Maxxim Medical, Athens, TX, USA) within the pressure box and the flow probes were connected to the flow box. Heart rate was triggered from the blood pressure and flow waveforms. Recordings of fetal arterial blood pressure and fetal heart rate, amniotic pressure, fetal carotid blood flow and fetal femoral blood flow could then be continuously transmitted wirelessly via Bluetooth technology onto a laptop computer. At this time the anaesthetic was turned off and the ewe was ventilated until spontaneous respiratory movements were observed. The ewe was extubated when spontaneous breathing returned and the animal was allowed to recover in a floor pen with free access to food and water.
Figure 1. Isobaric hypoxic chambers and the CamDAS system .
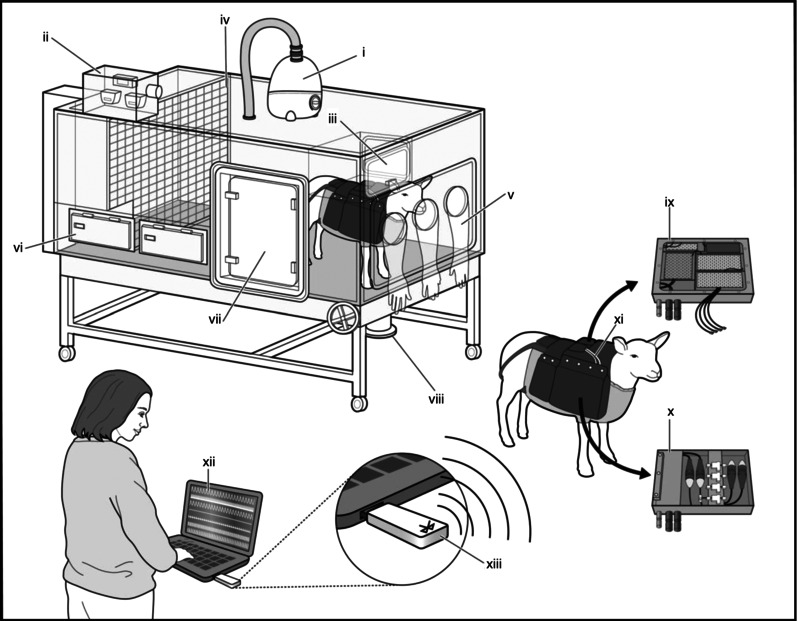
Each chamber was equipped with an electronic servo‐controlled humidity cool steam injection system to return the appropriate humidity to the inspirate (i). Ambient , , humidity and temperature within each chamber were monitored via sensors (ii). For experimental procedures, each chamber had a double transfer port (iii) to internalise material and a manually operated sliding panel (iv) to bring the ewe into a position where daily sampling of blood could be achieved through glove compartments (v). Each chamber incorporated a drinking bowl on continuous water supply and a rotating food compartment (vi) for determining food intake. A sealed transfer isolation cart could be attached to a side exit (vii) to couple chambers together for cleaning. Waste was disposed off via a sealable waste pipe (viii). The CamDAS system was contained in a custom‐made sheep jacket able to hold a box containing the Transonic flow probe connectors on one side (ix) and the pressure acquisition system box (x) on the other. Cables (xi) connected the two boxes together and also linked to two battery packs able to power the system for 24 hours. Measurements made using the data acquisition were transmitted wirelessly to a laptop kept outside the chamber room (xii) via Bluetooth (xiii), thereby making it possible to view continuous recordings of the maternal and fetal cardiovascular data.
Ewes wearing jackets with the CamDAS were housed in individual pens in rooms with a 12:12 h light–dark cycle where they had free access to hay and water and were fed concentrates twice daily (100 g sheep nuts no. 6; H & C Beart Ltd, Kings Lynn, UK). Antibiotics were administered daily to the ewe (0.20–0.25 mg kg−1 i.m. depocillin; Mycofarm, Cambridge, UK) for the first 3 days of recovery and daily to the fetus i.v. and into the amniotic cavity (600 mg in 2 ml 0.9% NaCl, benzylpenicillin; Crystapen, Schering‐Plough, Animal Health Division, Welwyn Garden City, UK). Generally, normal feeding patterns were restored within 24–48 h of recovery. Ewes were then randomly allocated to one of two experimental groups: normoxia (n = 6) or chronic hypoxia (n = 6).
Chronic hypoxia protocol
Ewes allocated to chronic hypoxia were housed in one of four bespoke isobaric hypoxic chambers (Telstar Ace, Dewsbury, UK; Fig. 1). These chambers were supplied with variable amounts of nitrogen and air provided via nitrogen generators and air compressors, respectively, from a custom designed nitrogen generating system (Domnick Hunter Gas Generation, Gateshead, UK). The system operated continuously, automatically switching between adsorption beds of two nitrogen generators (Domnick Hunter N2MAX112X2) to ensure a constant provision of pure nitrogen gas. The purity of the nitrogen was monitored to ensure only gas of the required purity reached the application. Compressed air and compressed nitrogen were then piped to the laboratory containing the hypoxic chambers and gases were blended to requirements. The inspirate air mixture underwent a minimum of 12 changes per hour in each chamber and the incoming air mixture was passed via silencers able to reduce noise levels within the hypoxic chamber laboratory (76 dB(A)) and inside each chamber (63 dB(A)) to values lower than those necessary to abide by the Control of Noise at Work Regulations. This not only complied with human health and safety and animal welfare regulations but also provided a tranquil environment for the animal inside each chamber. All chambers were equipped with an electronic automatic humidity cool steam injection system (1100‐03239 HS‐SINF; Masalles, Barcelona, Spain) to ensure appropriate humidity in the inspirate (55 ± 10%). Ambient PO2, , humidity and temperature within each chamber were monitored via sensors, displayed and values recorded continuously via the Trends Building Management System of the University of Cambridge through a secure Redcare intranet. In this way, the percentage of oxygen in the isolators could be controlled with precision continuously over long periods of time. For experimental procedures, each chamber had a double transfer port to internalise material and a manually operated sliding panel to encourage the ewe into a position where daily sampling of blood could be achieved through glove compartments (Fig. 1). Each chamber incorporated a drinking bowl on continuous water supply and a rotating food compartment which could be removed for determining food intake. The chambers were transparent, allowing ewes to visualise each other. A transfer isolation cart could couple two chambers together, allowing ewes to move transiently to an adjacent chamber maintained at the same oxygen environment. This was necessary for cleaning the chambers, which occurred once per week. Therefore, all experimental and maintenance procedures could be carried out without interruption of the hypoxic exposure.
Pregnancies assigned to the chronic hypoxia group were placed inside the chambers under normoxic conditions (11 l s −1 air, equating to 39.6 m3 h−1) for 2 days. On the fifth post‐operative day at 121 ± 1 days of gestation, pregnancies assigned to chronic hypoxia were exposed to ca 10% O2 by altering the incoming gas mixture to 5 l s−1 air/6 l s−1 N2. The induction of hypoxia was gradual, achieving 10% O2 over 24 h. Following 11 days of chronic hypoxia exposure, the pregnancies were returned to breathing normoxic air again within the chambers. Cardiovascular data were transmitted wirelessly via Bluetooth technology and recorded onto a laptop kept outside the hypoxic chamber laboratory. This permitted continuous in vivo recordings of the maternal and fetal cardiovascular data without disturbing the animal's environment.
Pregnancies allocated to the normoxia group were housed in a barn in floor pens with the same floor area as that of the hypoxic chambers. Both the normoxia and the chronic hypoxia groups of ewes were fed daily the same bespoke maintenance diet made up of concentrate pellets and hay (40 g nuts kg–1 and 3 g hay kg–1; Manor Farm Feeds Ltd, Oakham, UK) to facilitate the monitoring of food intake.
Blood sampling regimen and analysis
Samples (0.3 ml) of ascending and descending aortic fetal as well as descending aortic maternal blood were taken daily for measurement of fetal and maternal blood gas, acid base and metabolic status. Arterial blood gas and acid base values were measured using an ABL5 blood gas analyser (Radiometer, Copenhagen, Denmark; maternal measurements corrected to 38 °C, fetal measurements corrected to 39.5 °C). Values for percentage saturation of haemoglobin with oxygen (SatHb) and for the concentration of haemoglobin in blood ([Hb]) were determined using a haemoximeter (OSM3; Radiometer). Blood glucose and lactate concentrations were measured using an automated analyser (Yellow Springs 2300 Stat Plus Glucose/Lactate Analyser; YSI Ltd, Farnborough, UK). Values for haematocrit were obtained in duplicate using a microhaematocrit centrifuge (Hawksley, Lancing, UK). An additional 1 ml of maternal arterial and fetal arterial blood was taken during baseline (24 h prior to chronic hypoxia) and at +1 h, +6 h, +1 day, +5 days and +10 days of chronic hypoxia and at +2 days following chronic hypoxia or at equivalent times in normoxic animals for determination of plasma vitamin C and urate concentrations.
Determination of plasma urate and vitamin C
Plasma concentrations of urate were measured using an automated Siemens Dimension RxL analyser (Dimension RxL Max integrated Chemistry system, Siemens, UK, Core Biochemical Assay Laboratory, Cambridge, UK). In brief, urate in the plasma (taken from previously unthawed heparin‐treated sample aliquots) is converted to allantoin by the action of uricase (urate oxidase). As urate is able to absorb light at 293 nm but allantoin is not, the change in absorbance at 293 nm is directly proportional to the urate concentration in the sample. Additional dilutions of low calibrator solutions were used to improve the reproducibility of low analyte concentrations. The inter‐assay coefficients of variation were 5.0% at 200 μmol l−1 and 2.7% at 560 μmol l−1. The lower limit of detection of the assay was 6 μmol l−1.
Plasma concentrations of ascorbic acid were measured by a fluorimetric technique using a centrifugal analyser with a fluorescence attachment, according to the method of Vuilleumier & Keck (1989; Core Biochemical Assay Laboratory, Cambridge, UK). In brief, aliquots of maternal and fetal plasma (acidified 1:1 with ice‐cold 10% metaphosphoric acid) were centrifuged and the supernatant stored at −80 °C. They were then loaded in duplicate onto a black microtitre plate with standards and quality controls. Addition of ascorbate oxidase converts any vitamin C in the sample to dehydroascorbic acid, which is then condensed with 1,2‐phenyldiamine to form a fluorescent quinoxaline derivative. The fluorescence is measured on a Fluoroskan Ascent FL (Fluoroskan Ascent FL Microplate Fluorometer and Luminometer, Thermo Fisher Scientific, Basingstoke, UK) and is proportional to the vitamin C concentration in the sample. The inter‐assay coefficients of variation were 7.9% at 27.1 μmol l−1 and 5.0% at 89.7 μmol l−1; the lower limit of detection of the assay was 10 μmol l−1.
Data and statistical analyses
All data are expressed as mean ± SEM. For the cardiovascular data, minute by minute average values were downloaded continuously throughout the experiment and imported into an Excel spreadsheet.
Values during 2 h morning (10.00–12.00 h) and night‐time (22.00–24.00 h) epochs were then averaged for each day. Fetal arterial blood oxygen content (O2 content) was calculated using eqn (1):
| (1) |
where [Hb] (g dl−1) is the blood concentration of haemoglobin, SatHb is the percentage oxygen saturation of haemoglobin and where 1 molecule of Hb (mol. wt 64 450) binds 4 molecules of oxygen. The contribution of oxygen dissolved in plasma is regarded as negligible (Owens et al. 1987). Values for oxygen and for glucose delivery to the fetal ascending and descending aorta were then calculated using eqns (2) and (3), respectively:
| (2) |
| (3) |
For statistical analysis, cardiovascular data were analysed comparing the effect of treatment, time and interactions between treatment and time using two‐way repeated measures ANOVA with Tukey's post hoc test. Where relevant, area under the curve or slopes were analysed to better summarise the data. Comparison of slopes was performed using the Student's t test for unpaired data. For all comparisons, statistical significance was accepted at P < 0.05.
Results
Maternal food consumption, arterial blood gas, acid base and metabolic status
Basal maternal daily food consumption was not different between groups (normoxic (N): 1.3 ± 0.9 vs. hypoxic (H): 1.1 ± 0.4 kg day−1). Exposure to chronic hypoxia did not affect maternal daily food intake (N: 1.5 ± 0.9 vs. H: 1.5 ± 0.9 kg day−1). Basal values for maternal arterial blood gas, acid base and metabolic status were not different between groups and were within the normal range for Welsh Mountain ewes at the appropriate time of gestation prior to experimentation (Fletcher et al. 2002, 2006 Fig. 2). Ewes exposed to chronic hypoxia had a significant reduction in the partial pressure of arterial oxygen (105.7 ± 3.7 to 42.0 ± 1.2 mmHg) and in oxygen saturation (103.5 ± 0.5 to 78.6 ± 5.7%) compared to controls (: 104.2 ± 1.9 and Sat[Hb]: 92.36 ± 1.5) and their own baseline (P < 0.05, Fig. 2). Furthermore, ewes exposed to chronic hypoxia had significantly elevated haematocrit by the end of exposure relative to controls (33.9 ± 1.0 vs. 28.5 ± 0.9%). These changes occurred without significant alteration from baseline or between groups in arterial pH, partial pressure of arterial carbon dioxide, acid base excess, blood glucose or blood lactate concentrations (Fig. 2). Maternal blood lactate concentrations showed a transient 24 h increase following the onset of hypoxia, but these alterations did not reach significance. While values for partial pressure of arterial oxygen and oxygen saturation returned towards basal levels, values for haematocrit remained significantly elevated in the chronic hypoxia ewes following re‐oxygenation.
Figure 2. Maternal blood gas, acid base and metabolic status .
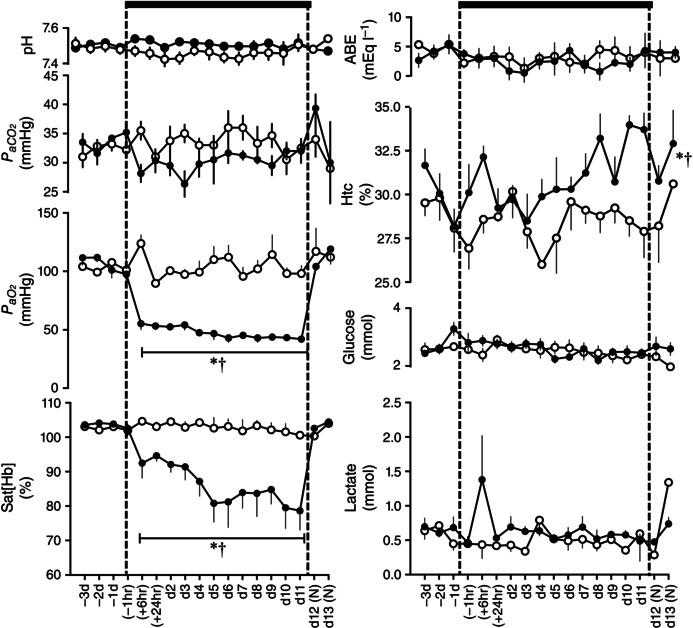
Values are mean ± SEM for pregnant sheep undergoing normoxic (◯, n = 6) or chronic hypoxic ( , n = 6) pregnancy. Maternal blood gas values were corrected to 38 °C. pH, arterial pH; , arterial CO2 partial pressure; , arterial O2 partial pressure; Sat[Hb], percentage saturation of haemoglobin; ABE, acid base excess; Htc, haematocrit; Glucose, blood glucose concentration; Lactate, blood lactate concentration; (N), normoxic recovery. The x‐axis shows time in hours (hr) and days (d). Significant differences (P < 0.05): *differences indicating a significant main effect of time compared with baseline; †differences indicating a significant main effect of treatment compared with normoxic pregnancy (two‐way repeated‐measures ANOVA + Tukey test). For Htc, the comparison of slopes was achieved with the Student's t test for unpaired data.
, n = 6) pregnancy. Maternal blood gas values were corrected to 38 °C. pH, arterial pH; , arterial CO2 partial pressure; , arterial O2 partial pressure; Sat[Hb], percentage saturation of haemoglobin; ABE, acid base excess; Htc, haematocrit; Glucose, blood glucose concentration; Lactate, blood lactate concentration; (N), normoxic recovery. The x‐axis shows time in hours (hr) and days (d). Significant differences (P < 0.05): *differences indicating a significant main effect of time compared with baseline; †differences indicating a significant main effect of treatment compared with normoxic pregnancy (two‐way repeated‐measures ANOVA + Tukey test). For Htc, the comparison of slopes was achieved with the Student's t test for unpaired data.
Fetal arterial blood gas, acid base and metabolic status
Basal values for descending aortic fetal arterial blood gas, acid base and metabolic status were similar between groups and were within the normal range for Welsh Mountain singleton sheep fetuses at this stage of gestation (Fletcher et al. 2002, 2006, Fig. 3). Fetuses exposed to chronic hypoxia had a significant reduction from baseline in the partial pressure of arterial oxygen (20.9 ± 0.5 to 11.5 ± 0.6 mmHg) and oxygen saturation (63.0 ± 1.9 to 24.6 ± 2.9%, P < 0.05, Fig. 3). Fetuses exposed to chronic hypoxia also had significantly elevated haematocrit by the end of exposure relative to controls (36.1 ± 1.3 vs. 28.0 ± 0.5, P < 0.05). Chronically hypoxic fetuses also showed a transient increase in arterial pH and reductions in acid base excess by the end of the hypoxic period, and sustained falls in the partial pressure of arterial carbon dioxide and increases in blood lactate concentrations during the hypoxic exposure (Fig. 3). These effects occurred without significant alteration from baseline in blood glucose concentrations. While values for all altered variables returned towards baseline, values for haematocrit remained significantly elevated in the chronic hypoxia fetuses following re‐oxygenation.
Figure 3. Fetal blood gas, acid base and metabolic status .
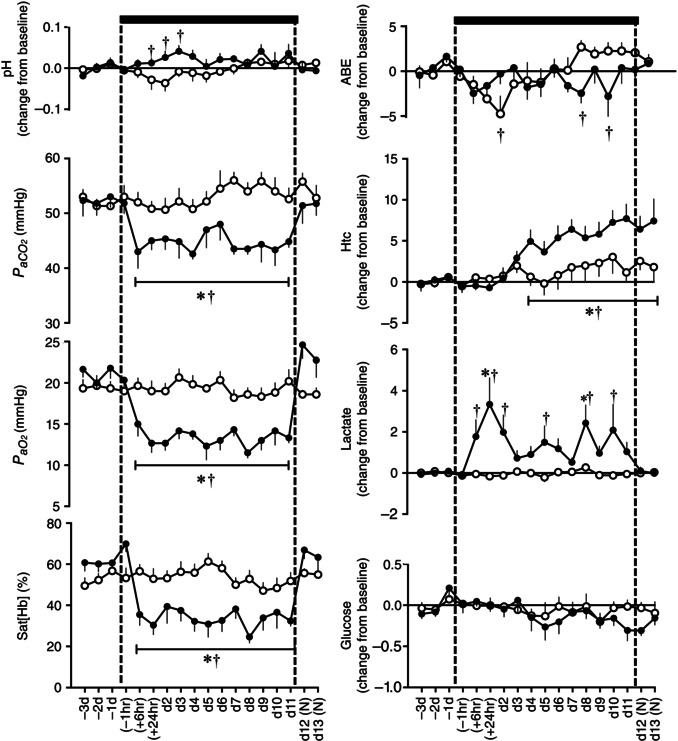
Values are mean ± SEM for fetal sheep undergoing normoxic (◯, n = 6) or chronic hypoxic ( , n = 6) pregnancy. Fetal blood gas values were corrected to 39.5 °C. pH, arterial pH; , arterial CO2 partial pressure; , arterial O2 partial pressure; Sat[Hb], percentage saturation of haemoglobin; ABE, acid base excess; Htc, haematocrit; Glucose, blood glucose concentration; Lactate, blood lactate concentration; (N), normoxic recovery. The x‐axis shows time in hours (hr) and days (d). Significant differences (P < 0.05): *differences indicating a significant main effect of time compared with baseline; †differences indicating a significant main effect of treatment compared with normoxic pregnancy (two‐way repeated‐measures ANOVA + Tukey test).
, n = 6) pregnancy. Fetal blood gas values were corrected to 39.5 °C. pH, arterial pH; , arterial CO2 partial pressure; , arterial O2 partial pressure; Sat[Hb], percentage saturation of haemoglobin; ABE, acid base excess; Htc, haematocrit; Glucose, blood glucose concentration; Lactate, blood lactate concentration; (N), normoxic recovery. The x‐axis shows time in hours (hr) and days (d). Significant differences (P < 0.05): *differences indicating a significant main effect of time compared with baseline; †differences indicating a significant main effect of treatment compared with normoxic pregnancy (two‐way repeated‐measures ANOVA + Tukey test).
Fetal cardiovascular responses
Basal values for fetal descending aortic blood pressure (41.1 ± 0.6 vs. 39.2 ± 0.5 mmHg), fetal heart rate (186.1 ± 2.0 vs. 177.3 ± 1.8 beats min−1), carotid blood flow (73.3 ± 3.0 vs. 75.7 ± 1.9 ml min−1) and femoral blood flow (32.3 ± 1.1 vs. 35.5 ± 1.5 ml min−1) were not different between normoxic and chronic hypoxic groups and were within the normal range for Welsh Mountain singleton sheep fetuses at this stage of gestation (Giussani et al. 1993; Jellyman et al. 2005, 2009). Fetuses undergoing normoxic pregnancy showed progressive increases in arterial blood pressure (41.1 ± 0.6 to 50.1 ± 2.3 mmHg), carotid blood flow (73.3 ± 3.0 to 92.3 ± 7.4 ml min−1) and femoral blood flow (32.3 ± 1.1 to 35.9 ± 2.5 ml min−1) and progressive decreases in heart rate (186.1 ± 2.0 to 163.3 ± 7.0 beats min−1) with advancing gestational age (P < 0.05, Fig. 4). In contrast, in fetuses exposed to chronic hypoxia, the increment in fetal arterial blood pressure with advancing gestation was significantly diminished and the decrement in fetal heart rate occurred much later following the onset of hypoxia but reaching similar levels by the end of the period of exposure (Fig. 4). Furthermore, fetuses exposed to chronic hypoxia showed sustained elevations in both carotid and femoral blood flow during exposure (Table 1 and Fig. 4). While values for carotid and femoral blood flow returned towards basal levels, values for arterial blood pressure and for heart rate remained significantly altered from baseline in the chronic hypoxia fetuses following re‐oxygenation.
Figure 4. Fetal cardiovascular responses to chronic hypoxia .
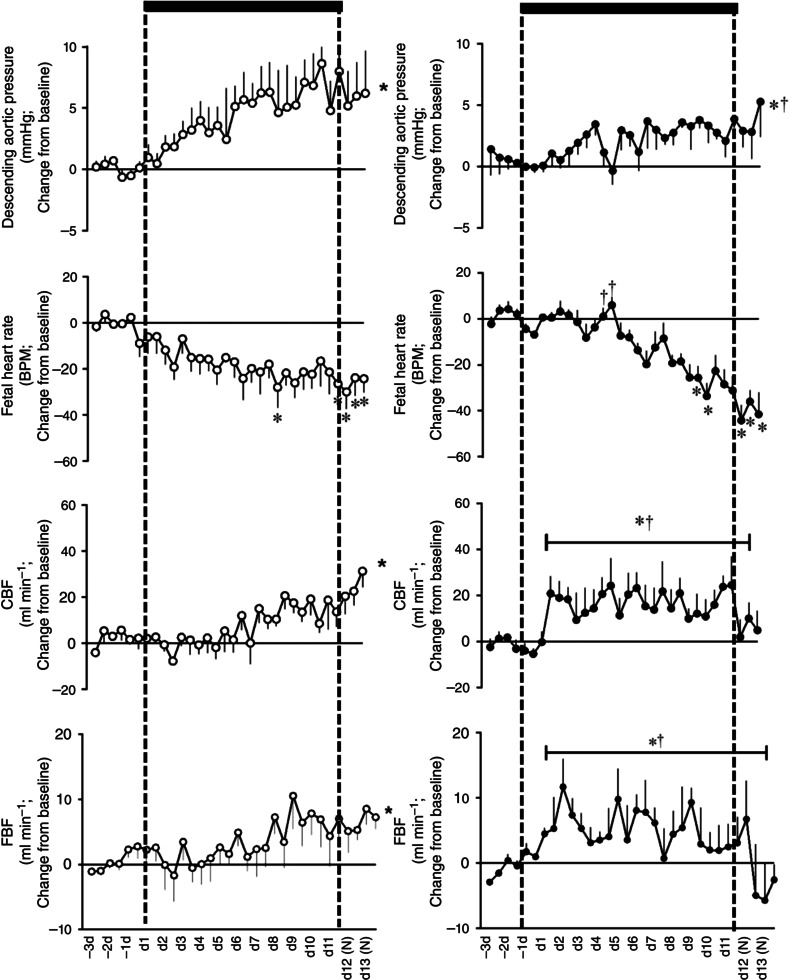
Values are mean ± SEM for the change from baseline in cardiovascular variables in fetal sheep undergoing normoxic (◯, n = 6, left) or chronic hypoxic ( , n = 6, right) pregnancy. CBF, carotid blood flow; FBF, femoral blood flow; BPM, beats per minute; (N), normoxic recovery. The x‐axis shows time in days (d). Significant differences (P < 0.05): *differences indicating a significant main effect of time compared with baseline; †differences indicating a significant main effect of treatment compared with normoxic pregnancy (two‐way repeated‐measures ANOVA + Tukey test). For descending aortic pressure, the two‐way ANOVA represents a comparison of slopes. For FBF and CBF, the two‐way ANOVA represents a comparison of areas under the curve.
, n = 6, right) pregnancy. CBF, carotid blood flow; FBF, femoral blood flow; BPM, beats per minute; (N), normoxic recovery. The x‐axis shows time in days (d). Significant differences (P < 0.05): *differences indicating a significant main effect of time compared with baseline; †differences indicating a significant main effect of treatment compared with normoxic pregnancy (two‐way repeated‐measures ANOVA + Tukey test). For descending aortic pressure, the two‐way ANOVA represents a comparison of slopes. For FBF and CBF, the two‐way ANOVA represents a comparison of areas under the curve.
Table 1.
Variables used to calculate oxygen and glucose delivery in the chronically hypoxic fetus
| Descending aortic | Descending aortic | Ascending aortic | Ascending aortic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O2 content | blood glucose | O2 content | blood glucose | Carotid blood flow | Femoral blood flow | |||||||
| (mmol l −1) | (mmol l−1) | (mmol l−1) | (mmol l−1) | (ml min−1) | (ml min−1) | |||||||
| N | H | N | H | N | H | N | H | N | H | N | H | |
| Experi‐ | ||||||||||||
| mental | ||||||||||||
| day | ||||||||||||
| −3 | 2.81 ± 0.08 | 3.20 ± 0.24 | 0.89 ± 0.17 | 0.86 ± 0.03 | 3.17 ± 0.13 | 3.90 ± 0.19 | 0.97 ± 0.26 | 0.91 ± 0.04 | 68.8 ± 13.8 | 69.7 ± 2.4 | 30.8 ± 4.6 | 32.0 ± 2.9 |
| −2 | 2.93 ± 0.22 | 3.17 ± 0.19 | 0.90 ± 0.11 | 0.89 ± 0.08 | 3.23 ± 0.18 | 3.25 ± 0.05 | 0.83 ± 0.20 | 0.85 ± 0.05 | 71.7 ± 10.4 | 81.2 ± 4.0 | 29.6 ± 3.8 | 36.1 ± 3.4 |
| −1 | 3.14 ± 0.12 | 3.30 ± 0.07 | 0.83 ± 0.15 | 1.20 ± 0.12 | 3.17 ± 0.14 | 3.59 ± 0.07 | 0.98 ± 0.12 | 1.17 ± 0.12 | 72.8 ± 9.1 | 74.3 ± 5.3 | 28.5 ± 0.6 | 36.1 ± 3.4 |
| (−1 h) | 2.89 ± 0.44 | 3.42 ± 0.20 | 0.72 ± 0.13 | 0.98 ± 0.11 | 3.17 ± 0.38 | 3.65 ± 0.22 | 0.83 ± 0.07 | 0.96 ± 0.11 | 69.7 ± 10.8 | 81.4 ± 7.1 | 35.9 ± 0.0 | 37.3 ± 1.9 |
| (+6 h) | 2.89 ± 0.41 | 1.82 ± 0.24*† | 0.78 ± 0.10 | 1.02 ± 0.07 | 3.39 ± 0.47 | 1.91 ± 0.21*† | 0.79 ± 0.09 | 1.04 ± 0.10 | 68.2 ± 12.3 | 104.1 ± 7.5* | 35.5 ± 0.4 | 44.3 ± 4.1 |
| (+24 h) | 2.93 ± 0.50 | 1.63 ± 0.31*† | 0.76 ± 0.16 | 0.97 ± 0.08 | 3.34 ± 0.43 | 1.89 ± 0.32*† | 0.73 ± 0.10 | 0.98 ± 0.10 | 64.5 ± 8.9 | 96.8 ± 9.9 | 33.1 ± 1.2 | 47.1 ± 3.2* |
| 2 | 2.81 ± 0.19 | 2.08 ± 0.38* | 0.82 ± 0.14 | 0.92 ± 0.08 | 3.21 ± 0.15 | 2.01 ± 0.26*† | 0.76 ± 0.11 | 0.86 ± 0.09 | 76.9 ± 11.8 | 90.9 ± 10.7† | 35.8 ± 1.1 | 40.8 ± 3.0*† |
| 3 | 3.05 ± 0.17 | 2.25 ± 0.37* | 0.79 ± 0.09 | 1.03 ± 0.15 | 3.54 ± 0.15 | 2.51 ± 0.27*† | 0.82 ± 0.10 | 0.96 ± 0.14 | 78.2 ± 8.2 | 86.2 ± 12.1† | 33.8 ± 3.0 | 36.2 ± 1.1† |
| 4 | 3.03 ± 0.22 | 2.17 ± 0.30*† | 0.77 ± 0.18 | 0.98 ± 0.03 | 3.49 ± 0.19 | 2.56 ± 0.24*† | 0.70 ± 0.15 | 0.89 ± 0.05 | 74.8 ± 7.7 | 104.4 ± 11.7* | 36.0 ± 3.6 | 45.6 ± 3.6† |
| 5 | 3.22 ± 0.41 | 1.89 ± 0.39*† | 0.67 ± 0.16 | 0.84 ± 0.08 | 3.52 ± 0.20 | 2.01 ± 0.32*† | 0.72 ± 0.12 | 0.79 ± 0.07 | 69.3 ± 6.0 | 96.4 ± 11.6* | 35.4 ± 4.3 | 43.3 ± 1.5 |
| 6 | 3.01 ± 0.41 | 2.16 ± 0.35* | 0.61 ± 0.09 | 0.92 ± 0.04 | 3.45 ± 0.29 | 2.44 ± 0.38*† | 0.73 ± 0.09 | 0.96 ± 0.06 | 77.3 ± 7.1 | 88.2 ± 11.5* | 35.9 ± 5.1 | 38.8 ± 2.3† |
| 7 | 2.74 ± 0.25 | 2.64 ± 0.35* | 0.72 ± 0.15 | 0.89 ± 0.08 | 3.03 ± 0.10 | 2.81 ± 0.35 | 0.73 ± 0.16 | 0.91 ± 0.11 | 82.2 ± 10.0 | 103.7 ± 14.6 | 38.9 ± 4.8 | 40.8 ± 1.9 |
| 8 | 3.20 ± 0.38 | 1.62 ± 0.17*† | 0.82 ± 0.10 | 0.90 ± 0.11 | 3.33 ± 0.42 | 1.79 ± 0.12*† | 0.77 ± 0.15 | 0.92 ± 0.11 | 81.9 ± 7.8 | 101.4 ± 7.7* | 44.2 ± 1.2 | 43.2 ± 1.9 |
| 9 | 2.86 ± 0.09 | 2.27 ± 0.40* | 0.80 ± 0.11 | 0.78 ± 0.11 | 3.29 ± 0.16 | 2.67 ± 0.20* | 0.69 ± 0.13 | 0.84 ± 0.09 | 93.0 ± 12.7 | 82.4 ± 11.6* | 45.4 ± 3.3* | 35.7 ± 1.7 |
| 10 | 2.73 ± 0.11 | 2.56 ± 0.54* | 0.72 ± 0.15 | 0.81 ± 0.07 | 3.40 ± 0.04 | 2.92 ± 0.61* | 0.85 ± 0.16 | 0.84 ± 0.08 | 90.2 ± 10.1 | 94.9 ± 8.3 | 37.0 ± 1.2* | 39.5 ± 1.8 |
| 11 | 3.11 ± 0.21 | 2.30 ± 0.16*† | 0.62 ± 0.10 | 0.79 ± 0.04 | 3.26 ± 0.27 | 2.69 ± 0.29* | 0.80 ± 0.15 | 0.82 ± 0.07 | 82.5 ± 5.4 | 101.3 ± 12.6* | 36.2 ± 1.3 | 39.9 ± 7.2 |
| 12(N) | 3.03 ± 0.20 | 4.40 ± 0.19† | 0.69 ± 0.11 | 0.62 ± 0.04 | 3.35 ± 0.17 | 4.58 ± 0.18† | 0.86 ± 0.17 | 0.65 ± 0.05 | 83.2 ± 10.3 | 85.3 ± 7.2* | 36.8 ± 1.0 | 29.7 ± 3.7† |
| 13(N) | 2.64 ± 0.17 | 4.35 ± 0.38† | 0.69 ± 0.12 | 0.80 ± 0.06 | 2.70 ± 0.12 | 4.82 ± 0.25 | 0.66 ± 0.13 | 0.76 ± 0.03 | 101.8 ± 16.2* | 92.9 ± 9.7* | 37.1 ± 2.3 | 30.1 ± 0.7 |
Values are mean ± SEM variables required to calculate oxygen and glucose delivery throughout the experimental protocol. Data are shown for fetal ascending and descending arterial oxygen content, blood glucose and flows in normoxic (N) and hypoxic (H) fetuses (n = 6 both groups). Significant differences (P < 0.05): *differences indicating a significant main effect of time compared with baseline; †differences indicating a significant main effect of treatment compared with normoxic pregnancy (two‐way repeated‐measures ANOVA + Tukey test).
Fetal ascending and descending aortic oxygen and glucose delivery
Values for oxygen and glucose delivery to the ascending and descending aortic circulations were calculated using the values for oxygen content, blood glucose and blood flow in the relevant circulations shown in Table 1. Fetuses exposed to chronic hypoxia showed significantly reduced values for oxygen delivery to both ascending (227.0 ± 9.8 vs. 256.7 ± 7.9 μmol min−1) and descending (90.6 ± 4.7 vs. 110.7 ± 4.7 μmol min−1) aortic circulations relative to normoxic fetuses (Fig. 5). However, when oxygen delivery was expressed as a ratio between vascular beds, there was a significant increase in the oxygen delivery to the ascending relative to the descending aortic circulation during chronic hypoxia (P < 0.05, Fig. 5). In contrast, glucose delivery to either ascending or descending aorta was unaltered from baseline in fetuses exposed to chronic hypoxia. However, a significant increase in glucose delivery to the ascending aorta was calculated when expressed as a ratio relative to values for the descending aorta by the end of the experimental protocol (Fig. 5).
Figure 5. Fetal carotid and femoral arterial oxygen and glucose delivery in the chronically hypoxic fetus .
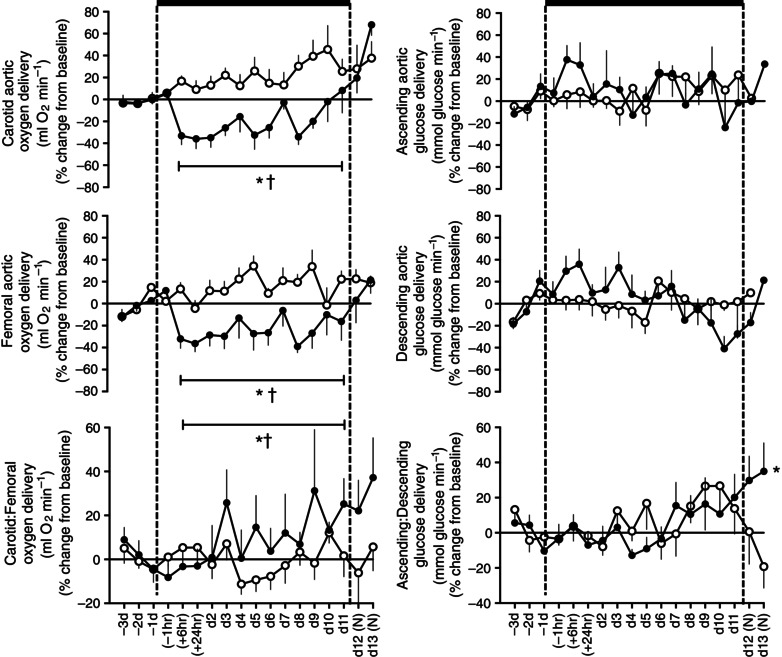
Values are mean ± SEM for the change from baseline in oxygen and glucose delivery in the ascending and the descending aorta and the ratio of these values in fetal sheep undergoing normoxic (◯, n = 6) or chronic hypoxic ( , n = 6) pregnancy. (N), normoxic recovery. The x‐axis shows time in hours (hr) and days (d). Significant differences (P < 0.05): *differences indicating a significant main effect of time compared with baseline; †differences indicating a significant main effect of treatment compared with normoxic pregnancy (two‐way repeated‐measures ANOVA + Tukey test). For the ratio of ascending/descending oxygen delivery, the two‐way ANOVA represents an analysis of the area under the curve.
, n = 6) pregnancy. (N), normoxic recovery. The x‐axis shows time in hours (hr) and days (d). Significant differences (P < 0.05): *differences indicating a significant main effect of time compared with baseline; †differences indicating a significant main effect of treatment compared with normoxic pregnancy (two‐way repeated‐measures ANOVA + Tukey test). For the ratio of ascending/descending oxygen delivery, the two‐way ANOVA represents an analysis of the area under the curve.
Maternal and fetal plasma urate and vitamin C concentrations
Values for basal urate concentrations were significantly higher in the fetal (N: 25.5 ± 1.7 μmol l−1 and H: 21.3 ± 2.1 μmol l−1) than in the maternal (N: 7.5 ± 0.8 μmol l−1 and H: 6.6 ± 0.7 μmol l−1) circulation in both normoxic and hypoxic pregnancy (P < 0.05). While fetal plasma urate concentrations increased from baseline in fetuses undergoing chronic hypoxia, plasma urate remained unchanged from baseline in fetuses undergoing normoxic pregnancy and in mothers of normoxic or hypoxic pregnancy (Fig. 6). In contrast, values for basal vitamin C concentrations were similar in the fetal (N: 30.4 ± 4.8 μmol l−1 and H: 31.8 ± 3.5 μmol l−1) and in the maternal (N: 31.4 ± 4.2 μmol l−1 and H: 33.9 ± 1.1 μmol l−1) circulation in both normoxic and hypoxic pregnancy. However, while fetal levels of vitamin C remained unchanged from baseline, there was a progressive increase in maternal plasma vitamin C with advancing gestation in both normoxic and hypoxic pregnancy (Fig. 6).
Figure 6. Fetal and maternal vitamin C and urate levels in the chronically hypoxic fetus .
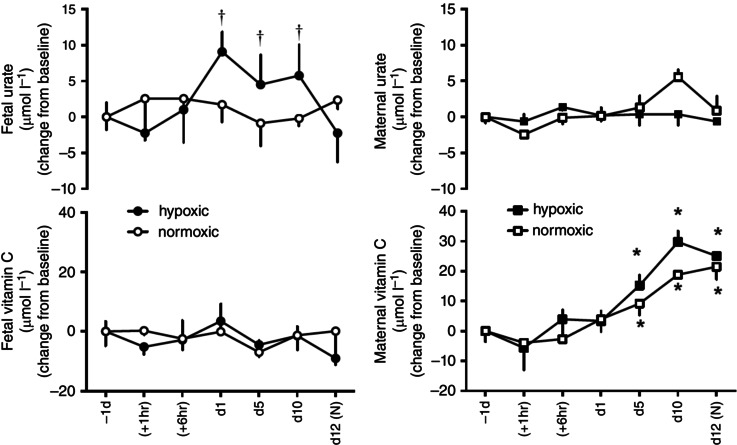
Values are mean ± SEM for the change from baseline in vitamin C and urate in pregnant ewes and fetal sheep undergoing normoxic (◯, fetus; □, ewe; n = 6) or chronic hypoxic ( , fetus; ■, ewe; n = 6) pregnancy. (N), normoxic recovery. The x‐axis shows time in hours (hr) and days (d). Significant differences (P < 0.05): *differences indicating a significant main effect of time compared with baseline; †differences indicating a significant main effect of treatment compared with normoxic pregnancy (two‐way repeated‐measures ANOVA + Tukey test). For the fetal urate levels the two‐way ANOVA represents an analysis of the area under the curve.
, fetus; ■, ewe; n = 6) pregnancy. (N), normoxic recovery. The x‐axis shows time in hours (hr) and days (d). Significant differences (P < 0.05): *differences indicating a significant main effect of time compared with baseline; †differences indicating a significant main effect of treatment compared with normoxic pregnancy (two‐way repeated‐measures ANOVA + Tukey test). For the fetal urate levels the two‐way ANOVA represents an analysis of the area under the curve.
Discussion
The data show that exposure of pregnant ewes in late gestation to chronic hypoxia in isobaric chambers led to sustained reductions in fetal to mean levels of ca 11 mmHg and therefore similar to those measured in human infants of hypoxic IUGR pregnancy (Hecher et al. 1995) whilst not affecting maternal food intake. Chronic fetal hypoxia of this magnitude was accompanied by sustained reductions in fetal , progressive increases in fetal haematocrit and variable increases in fetal blood lactate levels. Chronically hypoxic fetuses showed an impaired ontogenic increase in arterial blood pressure and a delayed ontogenic fall in fetal heart rate with advancing gestation. Parallel recording of carotid and femoral blood flow revealed sustained increases during the period of chronic hypoxia in chronically hypoxic fetuses, which were greater than those measured in normoxic fetuses with advancing gestation. The ratio of oxygen and glucose delivery to the fetal carotid circulation relative to the femoral circulation increased significantly and progressively in the chronically hypoxic fetus. Basal plasma urate concentrations were higher in the fetus than in the mother and plasma urate increased significantly in the chronically hypoxic fetus. Conversely, basal plasma ascorbic acid concentrations were similar in the mother and fetus and plasma ascorbic acid increased to similar extents only in the maternal circulation in normoxic and hypoxic pregnancy. These data support the hypotheses tested that the fetal brain sparing response persists during significant chronic fetal hypoxia and that an increase in ROS in the fetal circulation is an involved mechanism.
Several compensatory responses to hypoxia are regulated at least in part by the hypoxia‐inducible factor (HIF) family of transcription factors (Semenza, 2004). These coordinate intracellular responses to hypoxia by regulating the expression of hundreds of genes, including erythropoietin or EPO. The increased expression of the glycoprotein erythropoietin leads to increased red blood cell production, which can be measured as an elevation in packed red cell volume or the haematocrit. The present data confirm that this level of chronic hypoxia led to significant activation of the HIF‐regulated gene product erythropoietin, as the fetal and to a lesser extent maternal haematocrit increased progressively during and immediately following the period of chronic hypoxia. Additional blood gas data in the present study show that chronic hypoxia was accompanied by significant and sustained hypocapnia in the fetal but not in the maternal circulation. Fetal hypocapnia could be due to a shift in the fetal oxidative metabolism, decreasing fetal oxygen consumption and thereby fetal CO2 production and/or faster clearance of CO2 from the fetal to the maternal circulation. The sustained elevation in fetal rather than maternal blood lactate concentration during chronic hypoxia in the present study indicates an increase in fetal anaerobic metabolism, as has been previously suggested during hypoxic pregnancy (Lueder et al. 1995; Thompson, 2003). Bacon et al. (1984) also reported changes in placental barrier thickness and/or blood flow in chronically hypoxic guinea pig pregnancy.
Several studies have reported ontogenic increases in fetal arterial blood pressure and fetal peripheral blood flow and decreases in fetal heart rate with advancing gestation in several species (Reeves et al. 1972; Boddy et al. 1974; Dawes et al. 1980; MacDonald et al. 1983; Kitanaka et al. 1989; Forhead et al. 2000; Giussani et al. 2005). The present data are the first to report ontogenic increases in carotid blood flow with advancing gestation in control fetal sheep. One previous study reported lower mean values for fetal arterial blood pressure in chronically hypoxic fetuses of placentally restricted pregnancies (Edwards et al. 1999). However, others using the same placentally restricted model or in hypoxic fetuses from ovine pregnancies exposed to mild chronic hypoxia have reported similar fetal blood pressure between control and experimental animals (Kitanaka et al. 1989; Danielson et al. 2005; Pulgar et al. 2006, 2007, 2009; Poudel et al. 2015). By comparison, elevated basal values and alterations in the developmental decline of fetal heart rate with advancing gestation have been more consistently reported for the chronically hypoxic sheep fetus (Kitanaka et al. 1989; Pulgar et al. 2006). These findings are in keeping with a sympathetic dominant influence on cardiovascular control in the chronically hypoxic fetus (Kitanaka et al. 1989; Edwards et al. 1999). That blood flow to the fetal cerebral vascular bed increases in a sustained manner in response to chronic hypoxia has been established for many years (Richardson et al. 1993; Richardson & Bocking, 1998). In contrast, it has been generally assumed but widely accepted that blood flow to the peripheral circulations is decreased in the chronically hypoxic fetus and that this sustained redistribution of blood flow away from the periphery contributes to the repeatedly reported asymmetric growth restriction in the chronically hypoxic fetus (see Barker, 1996; Giussani, 2016). Two studies support lower basal values for femoral (Poudel et al. 2015) and carcass (Kamitomo et al. 1993) blood flow in the chronically hypoxic fetus, with single time point measurements with microspheres or acute recordings of femoral blood flow for 2 h. In this paper, we report that continuous longitudinal measurement of fetal femoral blood flow reveals a sustained increase during chronic hypoxia, akin to the peripheral dilatator response to hypoxia in the adult individual or to the enhanced basal femoral blood flow in adult offspring of chronically hypoxic pregnancy (Coney & Marshall, 2010). However, when calculating the actual delivery of oxygen and glucose to regional circulations, the ratio of substrate delivery to the carotid relative to the femoral circulation in the fetus shows a progressive increase as the chronic hypoxia develops. The latter provides first‐hand evidence for persistent brain sparing and continued redistribution of oxygen delivery away from peripheral circulations and towards the brain in the chronically hypoxic fetus.
The use of in vivo models to address questions regarding ROS generation comes with complications, as free radicals, by their very nature, are difficult to measure in these preparations. This problem is further compounded in the present study due to relative inaccessibility of the fetus within the intrauterine environment within a hypoxic chamber. Nevertheless, of all the techniques available, dynamic changes in urate and ascorbate concentrations in plasma constitute two of the few accepted biomarkers of ROS generation within the circulation in vivo (Halliwell & Gutteridge, 2004). Plasma urate concentration is an established marker of the activation of the xanthine oxidase (XO) pathway and, hence, of superoxide anion (•O2 −) generation (Berry & Hare, 2004). In sheep, ascorbate forms part of the endogenous antioxidant defence as ovine species possess the enzyme gulonolactone oxidase, which promotes the de novo synthesis of ascorbate via the hexuronic acid pathway of the liver and/or kidney (Banhegyi et al. 1997). It is established that plasma ascorbate concentrations also increase throughout gestation in several species, consistent with a functional role for this antioxidant in prenatal life (Kolb et al. 1991). We have previously reported the discovery that enhanced ROS generation contributes to the fetal peripheral vasoconstrictor response to an episode of acute hypoxia, part of the fetal brain sparing effect (Thakor et al. 2010, 2015; Kane et al. 2012, 2014). ROS do so by quenching NO and promoting a vascular oxidant tone that complements carotid chemoreflex and endocrine constrictor mechanisms, aiding the redistribution of blood flow away from peripheral circulations (Giussani, 2016). Data from the present study suggest that there may be tonic activation of the XO pathway during basal conditions in the fetus relative to the mother, and that the XO pathway in the fetus is more sensitive to chronic hypoxia than the mother. As basal is about one‐quarter lower in the fetal than in the maternal arterial circulation, it is tempting to speculate that the XO pathway is more active during basal conditions and more responsive to chronic hypoxia in fetal than in adult life, purely by virtue of this difference in oxygenation (Everest in utero; Barcroft et al. 1933). The significant increase in plasma urate concentrations in the circulation of the chronically hypoxic fetus is consistent with sustained activation of the XO pathway and continued excess ROS generation. Sustained XO‐derived ROS generation may thus contribute to the vascular oxidant tone of the fetal peripheral circulations, aiding in the shift in the delivery of oxygenated blood away from the fetal femoral and towards the fetal cerebral circulation in chronically hypoxic pregnancy of this magnitude. However, it is also possible that differences in circulating urate concentrations between mother and fetus reflect, in part, different rates of protein degradation and/or differences in renal clearance in the ewe and offspring.
Historically, there have been seminal investigations which have induced chronic fetal hypoxia by impairing uteroplacental blood flow by carunclectomy (Robinson et al. 1979; Poudel et al. 2015), placental embolisation (Block et al. 1984; Boyle et al. 1984; Gagnon et al. 1997), restriction of uterine blood flow (Richardson & Bocking, 1998; Stein et al. 1999; Lang et al. 2000), single umbilical artery ligation (Oyama et al. 1992; Supramaniam et al. 2006) and umbilical cord compression (Itskovitz et al. 1987; Giussani et al. 1997). However, all of these experimental manipulations reduce nutrient as well as oxygen delivery to the fetal circulation, preventing elucidation of the effects of chronic hypoxia on fetal cardiovascular function in isolation. Other equally important contributions have included description of fetal or neonatal cardiovascular function at the conclusion of the chronic hypoxic exposure (Rouwet et al. 2002; Sharma et al. 2006; Herrera et al. 2008, 2012; Tintu et al. 2009; Camm et al. 2010; Lindgren & Altimiras 2013; Iversen et al. 2014). A cluster of studies has investigated the effects of chronic hypoxia on fetal cardiovascular function in vivo, but only reported effects on fetal arterial blood pressure, heart rate and ventricular output (Alonso et al. 1989; Kitanaka et al. 1989; Kamitomo et al. 1994; Pulgar et al. 2006, 2007, 2009; Tissot van Patot et al. 2012). Another significant series of investigations has exploited the natural hypobaric hypoxia of high altitude to study the effects on fetal cardiovascular function of long‐term hypoxic gestation (Kamitomo et al. 1992, 1993, 2002; Browne et al. 1997 a,b; Onishi et al. 2003). While these studies have provided highly important contributions to the field of knowledge, exposure of pregnant ewes to altitudes between 3000 and 4500 m above sea level yields late gestation fetal arterial levels between 15 and 19 mmHg (Kamitomo et al. 1992, 1993, 2002; Browne et al. 1997 a,b; Onishi et al. 2003; Tissot van Patot et al. 2012). These values are much milder than those measured in the umbilical cord of the human hypoxic fetus in IUGR pregnancy, which are closer to 10–12 mmHg (Hecher et al. 1995). Investigation of this level of significant chronic fetal hypoxia using high altitude would involve exposure at 6500–7000 m above sea level (Gallagher & Hackett, 2004). Therefore, in summary, the work presented has introduced to the field of study a new technique for physiological research able to maintain chronically instrumented maternal and fetal sheep for prolonged periods of gestation under significant and controlled isolated chronic fetal hypoxia beyond levels that can be achieved by habitable high altitude. This technology also permits real time wireless recording in free moving animals of in vivo continuous maternal and fetal cardiovascular function, including alterations in regional blood flow signals as the hypoxic pregnancy is developing. Bioethically, the technology not only improves the physiological quality of the maternal and fetal in vivo data but it also improves animal welfare. This is the first time that this has been possible.
Additional Information
Competing interests
DAG, EAH and ADK worked with Telstar ACE to design the hypoxic chambers and with Maastricht Instruments to design and create the data acquisition system.
Author contributions
The experiments in this study were performed in the Department of Physiology, Development and Neuroscience, University of Cambridge. BJA, KLB, YN, ADK, EAH, AST, KJB, CMC, NI, KLS, CB and DAG conceived and designed the experiments. BJA, KLB, YN, ADK, EAH, AST, KJB, CMC, NI, KLS, CB and DAG collected, analysed and interpreted the experimental data. BJA, KLB, YN, ADK, EAH, AST, KJB, CMC, NI, KLS, CB and DAG drafted the article and revised it critically for important intellectual content.
Disclosure
License agreement 100395 CamDAS: Technology for simultaneous wireless recording of arterial blood pressure and blood flow in large animals. Giussani, D.A., Maatricht Instruments and Cambridge Enterprise.
Acknowledgements
This work was supported by the British Heart Foundation. Dino Giussani is Professor of Developmental Cardiovascular Physiology & Medicine at the Department of Physiology Development & Neuroscience at the University of Cambridge, Professorial Fellow and Director of Studies in Medicine at Gonville & Caius College, a Lister Institute Fellow and a Royal Society Wolfson Research Merit Award Holder. We thank Professor Abigail L. Fowden for continuous encouragement and insightful scientific discussion.
[The copyright line for this article was changed on 23rd September 2016 after original online publication]
References
- Alonso JG, Okai T, Longo LD & Gilbert RD (1989). Cardiac function during long‐term hypoxemia in fetal sheep. Am J Physiol 257, H581–H589. [DOI] [PubMed] [Google Scholar]
- Bacon BJ, Gilbert RD, Kaufmann P, Smith AD, Trevino FT & Longo LD (1984). Placental anatomy and diffusing capacity in guinea pigs following long‐term maternal hypoxia. Placenta 5, 475–487. [DOI] [PubMed] [Google Scholar]
- Banhegyi G, Braun L, Csala M, Puskas F & Mandl J (1997). Ascorbate metabolism and its regulation in animals. Free Radic Biol Med 23, 793–803. [DOI] [PubMed] [Google Scholar]
- Barcoft J, Herkel W & Hill S (1933). The rate of blood flow and gaseous metabolism of the uterus during pregnancy. J Physiol 77, 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP (1998). Mothers, Babies, and Disease in Later Life. Churchill Livingstone, Edinburgh. [Google Scholar]
- Barker DJP (1996). Growth in utero and coronary heart disease. Nutr Rev. 54(2 Pt 2), S1–7. [DOI] [PubMed] [Google Scholar]
- Bartelds B, van Bel F, Teitel DF & Rudolph AM (1993). Carotid, not aortic, chemoreceptors mediate the fetal cardiovascular response to acute hypoxemia in lambs. Pediatr Res 34, 51–55. [DOI] [PubMed] [Google Scholar]
- Berry C & Hare M (2004). Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 555, 589–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block BS, Llanos AJ & Creasy RK (1984). Responses of the growth‐retarded fetus to acute hypoxemia. Am J Obstet Gynecol 148, 878–885. [DOI] [PubMed] [Google Scholar]
- Boddy K, Dawes GS, Fisher R, Pinter S & Robinson JS (1974). Foetal respiratory movements electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. J Physiol 243, 599–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JW, Lotgering FK & Longo LD (1984). Acute embolization of the uteroplacental circulation: uterine blood flow and placental CO diffusing capacity. J Dev Physiol 6, 377–386. [PubMed] [Google Scholar]
- Browne VA, Stiffel VM, Pearce WJ, Longo LD & Gilbert RD (1997. a). Activator calcium and myocardial contractility in fetal sheep exposed to long‐term high‐altitude hypoxia. Am J Physiol 272, H1196–H1204. [DOI] [PubMed] [Google Scholar]
- Browne VA, Stiffel VM, Pearce WJ, Longo LD & Gilbert RD (1997. b). Cardiac β‐adrenergic receptor function in fetal sheep exposed to long‐term high‐altitude hypoxemia. Am J Physiol 273, R2022–R2031. [DOI] [PubMed] [Google Scholar]
- Burton GJ (2009). Oxygen, the Janus gas; its effects on human placental development and function. J Anat 215, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camm EJ, Hansell JA, Kane AD, Herrera EA, Lewis C, Wong S, Morrell NW & Giussani DA (2010). Partial contributions of developmental hypoxia and undernutrition to prenatal alterations in somatic growth and cardiovascular structure and function. Am J Obstet Gynecol 203, 495.e24–e34. [DOI] [PubMed] [Google Scholar]
- Cohn HE, Sacks EJ, Heymann MA & Rudolph AM (1974). Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol 120, 817–824. [DOI] [PubMed] [Google Scholar]
- Compernolle V, Brusselmans K, Franco D, Moorman A, Dewerchin M, Collen D & Carmeliet P (2003). Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia‐inducible factor‐1α. Cardiovasc Res 60, 569–579. [DOI] [PubMed] [Google Scholar]
- Coney AM1 & Marshall JM (2010). Effects of maternal hypoxia on muscle vasodilatation evoked by acute systemic hypoxia in adult rat offspring: changed roles of adenosine and A1 receptors. J Physiol 588, 5115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson L, McMillen IC, Dyer JL & Morrison JL (2005). Restriction of placental growth results in greater hypotensive response to α‐adrenergic blockade in fetal sheep during late gestation. J Physiol 563, 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L, Thornburg KL & Giraud GD (2005). The effects of anaemia as a programming agent in the fetal heart. J Physiol 565, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes GS, Johnston BM & Walker DW (1980). Relationship of arterial pressure and heart rate in fetal, new‐born and adult sheep. J Physiol 309, 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LJ, Simonetta G, Owens JA, Robinson JS & McMillen IC (1999). Restriction of placental and fetal growth in sheep alters fetal blood pressure responses to angiotensin II and captopril. J Physiol 515, 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar J, Teramo K, Stefanovic V, Andersson S, Asensi MA, Arduini A, Cubells E, Sastre J & Vento M (2013). Amniotic fluid oxidative and nitrosative stress biomarkers correlate with fetal chronic hypoxia in diabetic pregnancies. Neonatology 103, 193–198. [DOI] [PubMed] [Google Scholar]
- Fletcher AJ, Goodfellow MR, Forhead AJ, Gardner DS, McGarrigle HH, Fowden AL & Giussani DA (2000). Low doses of dexamethasone suppress pituitary‐adrenal function but augment the glycemic response to acute hypoxemia in fetal sheep during late gestation. Pediatr Res 47, 684–691. [DOI] [PubMed] [Google Scholar]
- Fletcher AJ, McGarrigle HH, Edwards CM, Fowden AL & Giussani DA (2002). Effects of low dose dexamethasone treatment on basal cardiovascular and endocrine function in fetal sheep during late gestation. J Physiol 545, 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AJ, Gardner DS, Edwards CM, Fowden AL & Giussani DA (2006). Development of the ovine fetal cardiovascular defense to hypoxemia towards full term. Am J Physiol Heart Circ Physiol 291, H3023–H3034. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Broughton Pipkin F, Taylor PM, Baker K, Balouzet V, Giussani DA & Fowden AL (2000). Developmental changes in blood pressure and the renin‐angiotensin system in Pony fetuses during the second half of gestation. J Reprod Fert Suppl. 56, 693–703. [PubMed] [Google Scholar]
- Gallagher SA & Hackett PH (2004). High‐altitude illness. Emerg Med Clin North Am 22, 329–355, viii. [DOI] [PubMed] [Google Scholar]
- Gagnon R, Murotsuki J, Challis JR, Fraher L & Richardson BS (1997). Fetal sheep endocrine responses to sustained hypoxemic stress after chronic fetal placental embolization. Am J Physiol 272, E817–E823. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ, Bennet L & Hanson MA (1993). Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol 461, 431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA & Hanson MA (1994). Fetal cardiovascular reflex responses to hypoxia. Fetal Matern Med Rev 6, 17–37. [Google Scholar]
- Giussani DA, Unno N, Jenkins SL, Wentworth RA, Derks JB, Collins JH & Nathanielsz PW (1997). Dynamics of cardiovascular responses to repeated partial umbilical cord compression in late‐gestation sheep fetus. Am J Physiol. 273, H2351–H2360. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Phillips PS, Anstee S & Barker DJ (2001). Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res 49, 490–494. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Forhead AJ & Fowden AL (2005). Development of cardiovascular function in the horse fetus. J Physiol 565, 1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA. (2016). The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol 594, 1215–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA & Davidge ST (2013). Developmental programming of cardiovascular disease by prenatal hypoxia. J Dev Orig Health Dis 4, 328–337. [DOI] [PubMed] [Google Scholar]
- Gunn AJ & Bennet L (2009). Fetal hypoxia insults and patterns of brain injury: insights from animal models. Clin Perinatol 36, 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B & Gutteridge JMC (2004). Free Radicals in Biology and Medicine. Oxford University Press, Oxford. [Google Scholar]
- Hayes EK, Lechowicz A, Petrik JJ, Storozhuk Y, Paez‐Parent S, Dai Q, Samjoo IA, Mansell M, Gruslin A, Holloway AC & Raha S (2012). Adverse fetal and neonatal outcomes associated with a life‐long high fat diet: role of altered development of the placental vasculature. PLoS One 7, e33370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecher K, Snijders R, Campbell S & Nicolaides K (1995). Fetal venous, intracardiac, and arterial blood flow measurements in intrauterine growth retardation: Relationship with fetal blood gases. Am J Obstet Gynecol 173, 10–15. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Reyes RV, Giussani DA, Riquelme RA, Sanhueza EM, Ebensperger G, Casanello P, Méndez N, Ebensperger R, Sepúlveda‐Kattan E, Pulgar VM, Cabello G, Blanco CE, Hanson MA, Parer JT & Llanos AJ (2008). Carbon monoxide: a novel pulmonary artery vasodilator in neonatal llamas of the Andean altiplano. Cardiovasc Res 77, 197–201. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Camm EJ, Cross CM, Mullender JL, Wooding FB & Giussani DA (2012). Morphological and functional alterations in the aorta of the chronically hypoxic fetal rat. J Vasc Res 49, 50–58. [DOI] [PubMed] [Google Scholar]
- Huch A, Huch R, Schneider H & Rooth G (1977). Continuous transcutaneous monitoring of fetal oxygen tension during labour. Br J Obstet Gynaecol 84(Suppl 1), 1–39. [DOI] [PubMed] [Google Scholar]
- Itskovitz J, LaGamma EF & Rudolph AM (1987). Effects of cord compression on fetal blood flow distribution and O2 delivery. Am J Physiol 252, H100–H109. [DOI] [PubMed] [Google Scholar]
- Iversen NK1, Wang T, Baatrup E & Crossley DA 2nd (2014). The role of nitric oxide in the cardiovascular response to chronic and acute hypoxia in White Leghorn chicken (Gallus domesticus). Acta Physiol 211, 346–357. [DOI] [PubMed] [Google Scholar]
- Jellyman JK, Gardner DS, Edwards CM, Fowden AL & Giussani DA (2005). Fetal cardiovascular, metabolic and endocrine responses to acute hypoxaemia during and following maternal treatment with dexamethasone in sheep. J Physiol 567, 673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellyman JK, Gardner DS, McGarrigle HH, Fowden AL & Giussani DA (2009). Antenatal glucocorticoid therapy increases glucose delivery to cerebral circulations during acute hypoxemia in fetal sheep during late gestation. Am J Obstet Gynecol 201, 82.e1–e8. [DOI] [PubMed] [Google Scholar]
- Jones CT & Robinson RO (1975). Plasma catecholamines in foetal and adult sheep. J Physiol 248, 15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitomo M, Longo LD & Gilbert RD (1992). Right and left ventricular function in fetal sheep exposed to long‐term high‐altitude hypoxemia. Am J Physiol 262, H399–H405. [DOI] [PubMed] [Google Scholar]
- Kamitomo M, Alonso JG, Okai T, Longo LD & Gilbert RD (1993). Effects of long‐term, high‐altitude hypoxemia on ovine fetal cardiac output and blood flow distribution. Am J Obstet Gynecol 169, 701–707. [DOI] [PubMed] [Google Scholar]
- Kamitomo M, Longo LD & Gilbert RD (1994). Cardiac function in fetal sheep during two weeks of hypoxemia. Am J Physiol 266, R1778–R1785. [DOI] [PubMed] [Google Scholar]
- Kamitomo M, Onishi J, Gutierrez I, Stiffel VM & Gilbert RD (2002). Effects of long‐term hypoxia and development on cardiac contractile proteins in fetal and adult sheep. J Soc Gynecol Invest 9, 335–341. [PubMed] [Google Scholar]
- Kane AD, Herrera EA, Hansell JA & Giussani DA (2012). Statin treatment depresses the fetal defence to acute hypoxia via increasing nitric oxide bioavailability. J Physiol 590, 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane AD, Hansell JA, Herrera EA, Allison BJ, Niu Y, Brain KL, Kaandorp JJ, Derks JB & Giussani DA (2014). Xanthine oxidase and the fetal cardiovascular defence to hypoxia in late gestation ovine pregnancy. J Physiol 592, 475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan‐Sturk R, Åkerud H, Volgsten H, Hellström‐Westas L & Wiberg‐Itzel E (2013). Outcome of deliveries in healthy but obese women: obesity and delivery outcome. BMC Res Notes 6, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz O & Sheiner E (2008). Asthma and pregnancy: a review of two decades. Expert Rev Respir Med 2, 97–107. [DOI] [PubMed] [Google Scholar]
- Kingdom JC & Kaufmann P (1997). Oxygen and placental villous development: origins of fetal hypoxia. Placenta 18, 613–621. [DOI] [PubMed] [Google Scholar]
- Kitanaka T, Alonso JG, Gilbert RD, Siu BL, Clemons GK & Longo LD (1989). Fetal responses to long‐term hypoxemia in sheep. Am J Physiol 256, R1348–R1354. [DOI] [PubMed] [Google Scholar]
- Kolb E, Wahren M, Leo M, Siebert P, Erices J, Gollnitz L & Volker L (1991). [Ascorbic acid concentration in plasma, in amniotic and allantoic fluids, in the placenta and in 13 tissues of sheep fetuses and newborn lambs]. Deutsch Tierarztl Wochenschr 98, 424–427. [PubMed] [Google Scholar]
- Lang U, Baker RS, Khoury J & Clark KE (2000). Effects of chronic reduction in uterine blood flow on fetal and placental growth in the sheep. Am J Physiol Regul Integr Comp Physiol 279, R53–R59. [DOI] [PubMed] [Google Scholar]
- Lindgren I & Altimiras J (2013). Prenatal hypoxia programs changes in beta‐adrenergic signaling and postnatal cardiac contractile dysfunction. Am J Physiol Regul Integr Comp Physiol 305, R1093–R1101. [DOI] [PubMed] [Google Scholar]
- Longo LD (1976). Carbon monoxide: effects on oxygenation of the fetus in utero. Science 194, 523–525. [DOI] [PubMed] [Google Scholar]
- Low JA, Galbraith RS, Muir DW, Killen HL, Pater EA & Karchmar EJ (1985). The relationship between perinatal hypoxia and newborn encephalopathy. Am J Obstet Gynecol 152, 256–260. [DOI] [PubMed] [Google Scholar]
- Lueder FL, Kim SB, Buroker CA, Bangalore SA & Ogata ES (1995). Chronic maternal hypoxia retards fetal growth and increases glucose utilization of select fetal tissues in the rat. Metabolism 44, 532–537. [DOI] [PubMed] [Google Scholar]
- Maberry MC, Ramin SM, Gilstrap LC 3rd, Leveno KJ & Dax JS (1990). Intrapartum asphyxia in pregnancies complicated by intra‐amniotic infection. Obstet Gynecol 76, 351–354. [PubMed] [Google Scholar]
- Macdonald AA, Colenbrander B & Wensing CJG (1983). The effects of gestational age and chronic fetal decapitation on arterial blood pressure in the fetus. Eur J Obstet Gynaecol Reprod Biol 16, 63–70. [DOI] [PubMed] [Google Scholar]
- Makowski EL, Battaglia FC, Meschia G, Behrman RE, Schruefer J, Seeds AE & Bruns PD (1968). Effect of maternal exposure to high altitude upon fetal oxygenation. Am J Obstet Gynecol 100, 852–856. [DOI] [PubMed] [Google Scholar]
- Marshall JM (1999). The Joan Mott Prize Lecture. The integrated response to hypoxia: from circulation to cells. Exp Physiol 84, 449–470. [PubMed] [Google Scholar]
- Onishi J, Kamitomo M, Stiffel VM & Gilbert RD (2003). Effects of long‐term high‐altitude hypoxia on myocardial protein kinase A activity and troponin I isoforms in fetal and nonpregnant sheep. J Soc Gynecol Invest 10, 189–193. [DOI] [PubMed] [Google Scholar]
- Oyama K, Padbury J, Chappell B, Martinez A, Stein H & Humme J (1992). Single umbilical artery ligation‐induced fetal growth retardation: effect on postnatal adaptation. Am J Physiol 263, E575–E583. [DOI] [PubMed] [Google Scholar]
- Owens JA, Falconer J and Robinson JS (1987). Effect of restriction of placental growth on oxygen delivery to and consumption by the pregnant uterus and fetus. J Dev Physiol 9, 137–150. [PubMed] [Google Scholar]
- Pardi G, Cetin I, Marconi AM, Lanfranchi A, Bozzetti P, Ferrazzi E, Buscaglia M & Battaglia FC (1993). Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med 328, 692–696. [DOI] [PubMed] [Google Scholar]
- Poudel R, McMillen IC, Dunn SL, Zhang S & Morrison JL (2015). Impact of chronic hypoxemia on blood flow to the brain, heart, and adrenal gland in the late‐gestation IUGR sheep fetus. Am J Physiol Regul Integr Comp Physiol 308, R151–R162. [DOI] [PubMed] [Google Scholar]
- Pulgar VM, Zhang J, Massmann GA & Figueroa JP (2006). Prolonged mild hypoxia alters fetal sheep electrocorticogram activity. J Soc Gynecol Investig 13, 404–411. [DOI] [PubMed] [Google Scholar]
- Pulgar VM, Zhang J, Massmann GA & Figueroa JP (2007). Mild chronic hypoxia modifies the fetal sheep neural and cardiovascular responses to repeated umbilical cord occlusion. Brain Res 1176, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulgar VM, Hong JK, Jessup JA, Massmann AG, Diz DI & Figueroa JP (2009). Mild chronic hypoxemia modifies expression of brain stem angiotensin peptide receptors and reflex responses in fetal sheep. Am J Physiol Regul Integr Comp Physiol 297, R446–R452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves JT, Daoud FS & Gentry M (1972). Growth of the fetal calf and its arterial pressure blood gases and hematologic data. J Appl Physiol 32, 240–244. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Carmichael L, Homan J & Patrick JE (1993). Cerebral oxidative metabolism in fetal sheep with prolonged and graded hypoxemia. J Dev Physiol 19, 77–83. [PubMed] [Google Scholar]
- Richardson BS & Bocking AD (1998). Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp Biochem Physiol A Mol Integr Physiol 119, 717–723. [DOI] [PubMed] [Google Scholar]
- Robinson JS, Kingston EJ, Jones CT & Thorburn GD (1979). Studies on experimental growth retardation in sheep. The effect of removal of a endometrial caruncles on fetal size and metabolism. J Dev Physiol 1, 379–398. [PubMed] [Google Scholar]
- Rouwet EV, Tintu AN, Schellings MW, van Bilsen M, Lutgens E, Hofstra L, Slaaf DW, Ramsay G & Le Noble FA (2002). Hypoxia induces aortic hypertrophic growth, left ventricular dysfunction, and sympathetic hyperinnervation of peripheral arteries in the chick embryo. Circulation 105, 2791–2796. [DOI] [PubMed] [Google Scholar]
- Rudolph AM (1984). The fetal circulation and its response to stress. J Dev Physiol 6, 11–19. [PubMed] [Google Scholar]
- Semenza GL (2004). Hydroxylation of HIF‐1: oxygen sensing at the molecular level. Physiology (Bethesda) 19, 176–182. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Lucitti JL, Nordman C, Tinney JP, Tobita K & Keller BB (2006). Impact of hypoxia on early chick embryo growth and cardiovascular function. Pediatr Res 59, 116–120. [DOI] [PubMed] [Google Scholar]
- Soria R, Julian CG, Vargas E, Moore LG & Giussani DA (2013). Graduated effects of high‐altitude hypoxia and highland ancestry on birth size. Pediatr Res 74, 633–638. [DOI] [PubMed] [Google Scholar]
- Stein P, White SE, Homan J, Hanson MA & Bocking AD (1999). Altered fetal cardiovascular responses to prolonged hypoxia after sinoaortic denervation. Am J Physiol 276, R340–R346. [DOI] [PubMed] [Google Scholar]
- Supramaniam VG, Jenkin G, Loose J, Wallace EM & Miller SL (2006). Chronic fetal hypoxia increases activin A concentrations in the late‐pregnant sheep. Br J f Obstet Gynaecol 113, 102–109. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Richter HG, Kane AD, Dunster C, Kelly FJ, Poston L & Giussani DA (2010). Redox modulation of the fetal cardiovascular defence to hypoxaemia. J Physiol 588, 4235–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakor AS, Allison BJ, Niu Y, Botting KJ, Serón‐Ferré M, Herrera EA & Giussani DA (2015). Melatonin modulates the fetal cardiovascular defense response to acute hypoxia. J Pineal Res 59, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LP (2003). Effects of chronic hypoxia on fetal coronary responses. High Alt Med Biol 4, 215–224. [DOI] [PubMed] [Google Scholar]
- Tintu A, Rouwet E, Verlohren S, Brinkmann J, Ahmad S, Crispi F, van Bilsen M, Carmeliet P, Staff AC, Tjwa M, Cetin I, Gratacos E, Hernandez‐Andrade E, Hofstra L, Jacobs M, Lamers WH, Morano I, Safak E, Ahmed A & le Noble F (2009). Hypoxia induces dilated cardiomyopathy in the chick embryo: mechanism, intervention, and long‐term consequences. PloS One 4, e5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot van Patot MC, Ebensperger G, Gassmann M & Llanos AJ (2012). The hypoxic placenta. High Alt Med Biol 13, 176–184. [DOI] [PubMed] [Google Scholar]
- Vuilleumier J & Keck E (1989). Fluorometric assay of vitamin C in biological materials using a centrifugal analyser with fluroescence attachment. J Micronutrient Analysis 5, 25–34. [Google Scholar]


