Abstract
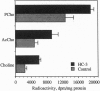
To identify the metabolic pathway that generates choline (Cho) for acetylcholine (AcCho) from its storage pool in membrane phosphatidylcholine (PtdCho), human neuronal cells (LA-N-2) were radioisotopically labeled with 1-O-hexadecyl-2-hydroxy-sn-glycero(3)phospho[14C]choline. The compound was efficiently taken up by the cells and metabolically labelled PtdCho, Cho, AcCho, and phosphocholine pools. In pulse-chase experiments, the specific radioactivities of the metabolites of 1-O-hexadecyl-2-hydroxy-sn-glycero(3)-phospho[14C]choline indicated that it was rapidly acylated to Ptd-Cho and then hydrolyzed first to free Cho and not to phosphocholine or glycerophosphocholine. This Cho was subsequently converted to AcCho and to phosphocholine. In the absence of exogenous Cho, at least 15% of the total cellular AcCho pool was synthesized by this pathway in 1 h. The data demonstrate that the liberation of the free Cho precursor for AcCho synthesis from PtdCho can be accomplished in a one-step process, indicating the involvement of a phospholipase D-type enzyme. In the presence of hemicholinium-3, which inhibits Cho transport, the amount of intracellular [14C]Cho metabolites that accumulated during the chase period was higher than in control cells, indicating that PtdCho hydrolysis liberated Cho directly into the cytoplasm. These data show that cholinergic cells are characterized by an intracellular pathway, catalyzed by a phospholipase D, that generates Cho for AcCho synthesis from PtdCho. Abnormalities in the regulation of this pathway may contribute to selective vulnerability of cholinergic neurons in certain neurodegenerative diseases, e.g., Alzheimer disease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blusztajn J. K., Liscovitch M., Richardson U. I. Synthesis of acetylcholine from choline derived from phosphatidylcholine in a human neuronal cell line. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5474–5477. doi: 10.1073/pnas.84.15.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blusztajn J. K., Lopez Gonzalez-Coviella I., Logue M., Growdon J. H., Wurtman R. J. Levels of phospholipid catabolic intermediates, glycerophosphocholine and glycerophosphoethanolamine, are elevated in brains of Alzheimer's disease but not of Down's syndrome patients. Brain Res. 1990 Dec 17;536(1-2):240–244. doi: 10.1016/0006-8993(90)90030-f. [DOI] [PubMed] [Google Scholar]
- Blusztajn J. K., Wurtman R. J. Choline and cholinergic neurons. Science. 1983 Aug 12;221(4611):614–620. doi: 10.1126/science.6867732. [DOI] [PubMed] [Google Scholar]
- Bowen D. M., Smith C. B., White P., Davison A. N. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976 Sep;99(3):459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- Bárány M., Chang Y. C., Arús C., Rustan T., Frey W. H., 2nd Increased glycerol-3-phosphorylcholine in post-mortem Alzheimer's brain. Lancet. 1985 Mar 2;1(8427):517–517. doi: 10.1016/s0140-6736(85)92114-2. [DOI] [PubMed] [Google Scholar]
- Chalifa V., Möhn H., Liscovitch M. A neutral phospholipase D activity from rat brain synaptic plasma membranes. Identification and partial characterization. J Biol Chem. 1990 Oct 15;265(29):17512–17519. [PubMed] [Google Scholar]
- Collier B., Poon P., Salehmoghaddam S. The formation of choline and of acetylcholine by brain in vitro. J Neurochem. 1972 Jan;19(1):51–60. doi: 10.1111/j.1471-4159.1972.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Dennis E. A., Rhee S. G., Billah M. M., Hannun Y. A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991 Apr;5(7):2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Frenkel R. A., Johnston J. M. Metabolic conversion of platelet-activating factor into ethanolamine plasmalogen in an amnion-derived cell line. J Biol Chem. 1992 Sep 25;267(27):19186–19191. [PubMed] [Google Scholar]
- Hattori H., Kanfer J. N., Massarelli R. Stimulation of phospholipase D activity and indication of acetylcholine synthesis by oleate in rat brain synaptosomal preparations. Neurochem Res. 1987 Aug;12(8):687–692. doi: 10.1007/BF00970523. [DOI] [PubMed] [Google Scholar]
- Hii C. S., Edwards Y. S., Murray A. W. Phorbol ester-stimulated hydrolysis of phosphatidylcholine and phosphatidylethanolamine by phospholipase D in HeLa cells. Evidence that the basal turnover of phosphoglycerides does not involve phospholipase D. J Biol Chem. 1991 Oct 25;266(30):20238–20243. [PubMed] [Google Scholar]
- Holbrook P. G., Pannell L. K., Murata Y., Daly J. W. Molecular species analysis of a product of phospholipase D activation. Phosphatidylethanol is formed from phosphatidylcholine in phorbol ester- and bradykinin-stimulated PC12 cells. J Biol Chem. 1992 Aug 25;267(24):16834–16840. [PubMed] [Google Scholar]
- Kanoh H., Kanaho Y., Nozawa Y. Pertussis toxin-insensitive G protein mediates carbachol activation of phospholipase D in rat pheochromocytoma PC12 cells. J Neurochem. 1992 Nov;59(5):1786–1794. doi: 10.1111/j.1471-4159.1992.tb11011.x. [DOI] [PubMed] [Google Scholar]
- Kanoh H., Ohbayashi H., Matsuda Y., Nonomura Y., Nozawa Y. Enhancing effect of wortmannin on muscarinic stimulation of phospholipase D in rat pheochromocytoma PC12 cells. Biochem Biophys Res Commun. 1992 Oct 30;188(2):510–515. doi: 10.1016/0006-291x(92)91085-5. [DOI] [PubMed] [Google Scholar]
- Lefresne P., Guyenet P., Glowinski J. Acetylcholine synthesis from (2- 14 C)pyruvate in rat striatal slices. J Neurochem. 1973 Apr;20(4):1083–1097. doi: 10.1111/j.1471-4159.1973.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Liscovitch M., Blusztajn J. K., Freese A., Wurtman R. J. Stimulation of choline release from NG108-15 cells by 12-O-tetradecanoylphorbol 13-acetate. Biochem J. 1987 Jan 1;241(1):81–86. doi: 10.1042/bj2410081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M., Chalifa V., Danin M., Eli Y. Inhibition of neural phospholipase D activity by aminoglycoside antibiotics. Biochem J. 1991 Oct 1;279(Pt 1):319–321. doi: 10.1042/bj2790319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M., Freese A., Blusztajn J. K., Wurtman R. J. High-performance liquid chromatography of water-soluble choline metabolites. Anal Biochem. 1985 Nov 15;151(1):182–187. doi: 10.1016/0003-2697(85)90069-7. [DOI] [PubMed] [Google Scholar]
- Liscovitch M. Signal-dependent activation of phosphatidylcholine hydrolysis: role of phospholipase D. Biochem Soc Trans. 1991 Apr;19(2):402–407. doi: 10.1042/bst0190402. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M., Wonnacott S., Rubio M. A. The effect of acetylcholine release on choline fluxes in isolated synaptic terminals. J Neurochem. 1981 Feb;36(2):379–393. doi: 10.1111/j.1471-4159.1981.tb01605.x. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G., Suzuki J., Dolman C. E., Nagai T. Aging, Alzheimer's disease, and the cholinergic system of the basal forebrain. Neurology. 1984 Jun;34(6):741–745. doi: 10.1212/wnl.34.6.741. [DOI] [PubMed] [Google Scholar]
- Möhn H., Chalifa V., Liscovitch M. Substrate specificity of neutral phospholipase D from rat brain studied by selective labeling of endogenous synaptic membrane phospholipids in vitro. J Biol Chem. 1992 Jun 5;267(16):11131–11136. [PubMed] [Google Scholar]
- Nitsch R. M., Blusztajn J. K., Pittas A. G., Slack B. E., Growdon J. H., Wurtman R. J. Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1671–1675. doi: 10.1073/pnas.89.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai J. K., Siegel M. I., Egan R. W., Billah M. M. Phospholipase D catalyzes phospholipid metabolism in chemotactic peptide-stimulated HL-60 granulocytes. J Biol Chem. 1988 Sep 5;263(25):12472–12477. [PubMed] [Google Scholar]
- Parducz A., Joó F., Toldi J. Formation of synaptic vesicles in the superior cervical ganglion of cat: choline dependency. Exp Brain Res. 1986;63(1):221–224. doi: 10.1007/BF00235667. [DOI] [PubMed] [Google Scholar]
- Pepitoni S., Mallon R. G., Pai J. K., Borkowski J. A., Buck M. A., McQuade R. D. Phospholipase D activity and phosphatidylethanol formation in stimulated HeLa cells expressing the human m1 muscarinic acetylcholine receptor gene. Biochem Biophys Res Commun. 1991 Apr 15;176(1):453–458. doi: 10.1016/0006-291x(91)90945-4. [DOI] [PubMed] [Google Scholar]
- Pettegrew J. W. Molecular insights into Alzheimer's disease. Ann N Y Acad Sci. 1989;568:5–28. doi: 10.1111/j.1749-6632.1989.tb12486.x. [DOI] [PubMed] [Google Scholar]
- Pettegrew J. W., Panchalingam K., Moossy J., Martinez J., Rao G., Boller F. Correlation of phosphorus-31 magnetic resonance spectroscopy and morphologic findings in Alzheimer's disease. Arch Neurol. 1988 Oct;45(10):1093–1096. doi: 10.1001/archneur.1988.00520340047010. [DOI] [PubMed] [Google Scholar]
- Potter P. E., Meek J. L., Neff N. H. Acetylcholine and choline in neuronal tissue measured by HPLC with electrochemical detection. J Neurochem. 1983 Jul;41(1):188–194. doi: 10.1111/j.1471-4159.1983.tb13668.x. [DOI] [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Platelet-activating factor. J Biol Chem. 1990 Oct 15;265(29):17381–17384. [PubMed] [Google Scholar]
- Párducz A., Kiss Z., Joó F. Changes of the phosphatidylcholine content and the number of synaptic vesicles in relation to the neurohumoral transmission in sympathetic ganglia. Experientia. 1976 Dec 15;32(12):1520–1521. doi: 10.1007/BF01924428. [DOI] [PubMed] [Google Scholar]
- Qian Z., Drewes L. R. A novel mechanism for acetylcholine to generate diacylglycerol in brain. J Biol Chem. 1990 Mar 5;265(7):3607–3610. [PubMed] [Google Scholar]
- Qian Z., Reddy P. V., Drewes L. R. Guanine nucleotide-binding protein regulation of microsomal phospholipase D activity of canine cerebral cortex. J Neurochem. 1990 May;54(5):1632–1638. doi: 10.1111/j.1471-4159.1990.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Quastel J. H., Tennenbaum M., Wheatley A. H. Choline ester formation in, and choline esterase activities of, tissues in vitro. Biochem J. 1936 Sep;30(9):1668–1681. doi: 10.1042/bj0301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson U. I., Liscovitch M., Blusztajn J. K. Acetylcholine synthesis and secretion by LA-N-2 human neuroblastoma cells. Brain Res. 1989 Jan 9;476(2):323–331. doi: 10.1016/0006-8993(89)91253-5. [DOI] [PubMed] [Google Scholar]
- Saito M., Kanfer J. Phosphatidohydrolase activity in a solubilized preparation from rat brain particulate fraction. Arch Biochem Biophys. 1975 Jul;169(1):318–323. doi: 10.1016/0003-9861(75)90346-x. [DOI] [PubMed] [Google Scholar]
- Sandmann J., Peralta E. G., Wurtman R. J. Coupling of transfected muscarinic acetylcholine receptor subtypes to phospholipase D. J Biol Chem. 1991 Apr 5;266(10):6031–6034. [PubMed] [Google Scholar]
- Singh I. N., McCartney D. G., Sorrentino G., Massarelli R., Kanfer J. N. Phorbol esters modulate phospholipid metabolism in a human cholinergic cell line, LA-N-2: a possible role for the base exchange enzymes. J Neurosci Res. 1992 Aug;32(4):583–592. doi: 10.1002/jnr.490320414. [DOI] [PubMed] [Google Scholar]
- Singh I. N., Sorrentino G., Massarelli R., Kanfer J. N. Oleoylamine and sphingosine stimulation of phosphatidylserine synthesis by LA-N-2 cells is protein kinase C independent. FEBS Lett. 1992 Jan 20;296(2):166–168. doi: 10.1016/0014-5793(92)80371-m. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Strum J. C., Emilsson A., Wykle R. L., Daniel L. W. Conversion of 1-O-alkyl-2-acyl-sn-glycero-3-phosphocholine to 1-O-alk-1'-enyl-2-acyl-sn-glycero-3-phosphoethanolamine. A novel pathway for the metabolism of ether-linked phosphoglycerides. J Biol Chem. 1992 Jan 25;267(3):1576–1583. [PubMed] [Google Scholar]
- Ulus I. H., Wurtman R. J., Mauron C., Blusztajn J. K. Choline increases acetylcholine release and protects against the stimulation-induced decrease in phosphatide levels within membranes of rat corpus striatum. Brain Res. 1989 Apr 10;484(1-2):217–227. doi: 10.1016/0006-8993(89)90364-8. [DOI] [PubMed] [Google Scholar]